Hydrogel-Based Treatment of Diabetic Wounds: From Smart Responsive to Smart Monitoring
Abstract
1. Introduction
2. Pathophysiological Characteristics of Diabetic Wounds
2.1. Effects of Hyperglycemia
2.2. Abnormal Oxidative Stress and Inflammatory Response
2.3. Bacterial Infection
2.4. Angiogenesis Disorders
3. Smart Response of Hydrogels to Wound Microenvironment Modulation
3.1. pH Response
3.2. Enzyme Response
3.3. ROS Response
3.3.1. Responsive Degradation and Drug Release
3.3.2. Efficient ROS Scavenging Activity
3.4. Glucose Response
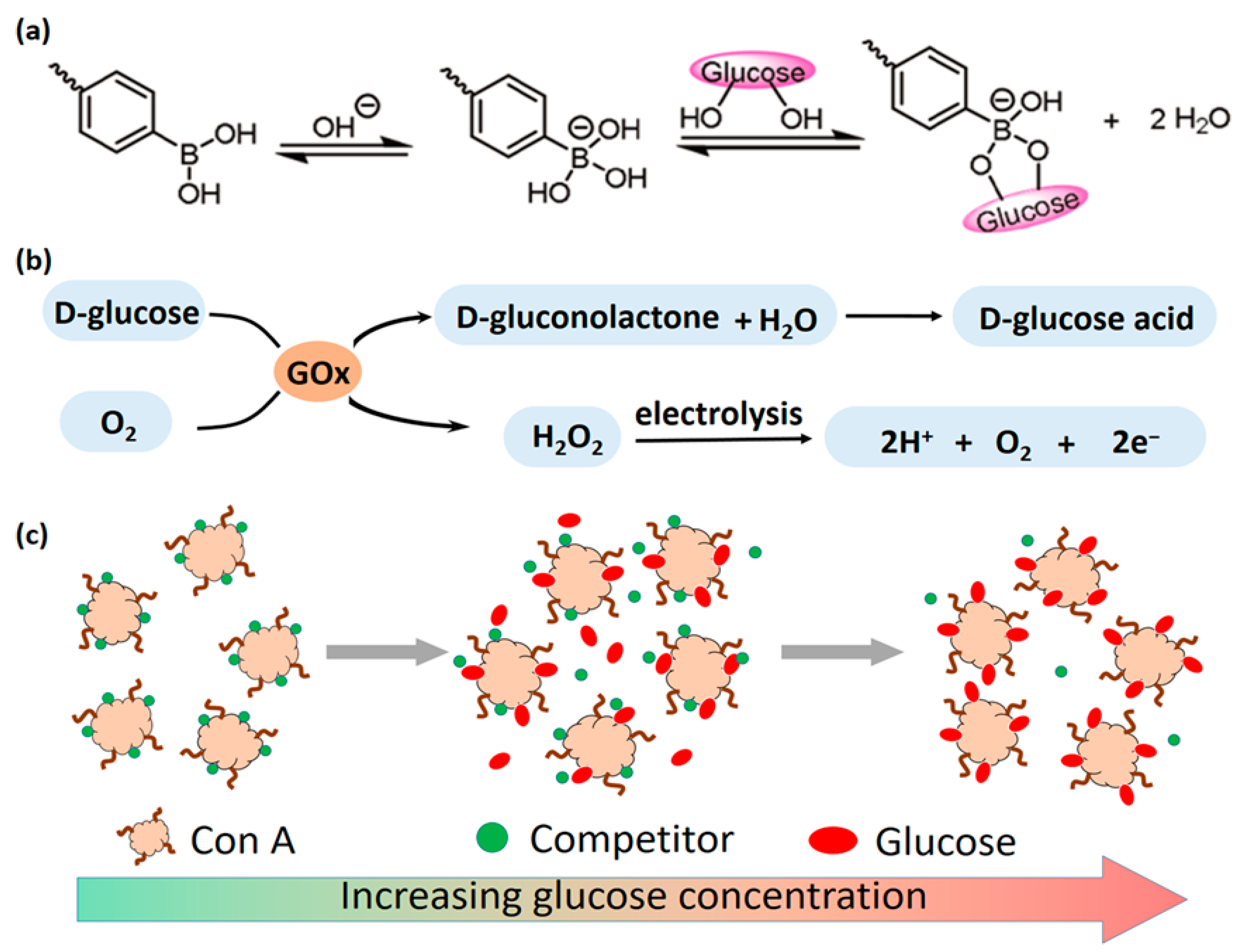
3.4.1. GOx Catalytic Mechanism
3.4.2. ConA Binding Mechanism
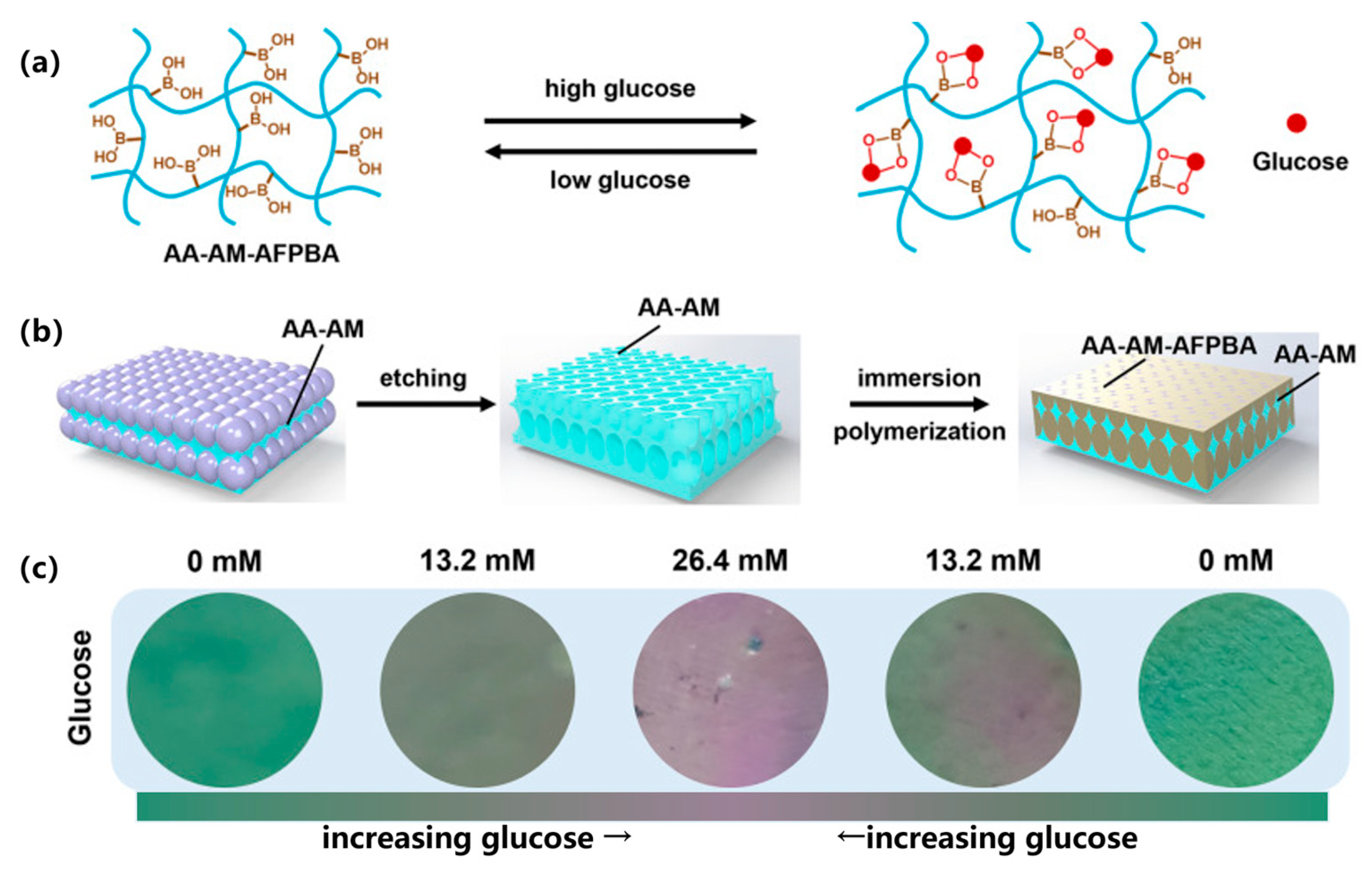
3.4.3. Competitive Binding Mechanism of Phenylboronic Acid
4. Smart Monitoring-Based Integration Strategies for Hydrogel Biosensors
4.1. Glucose-Responsive Integrated Smart Monitoring Strategy
4.1.1. Optical Sensors
Photonic Crystal (PC)/Structural Color Sensors
Fluorescence Sensors
Noble Metal Nanoparticle Sensors
Holographic Sensors
4.1.2. Electrochemical Sensors
Enzyme-Catalyzed Electrochemical Glucose Sensors
Non-Enzymatic Catalytic Electrochemical Glucose Sensors
4.2. pH-Responsive Integrated Smart Monitoring Strategy
4.3. Multiple Signal Monitoring Strategy
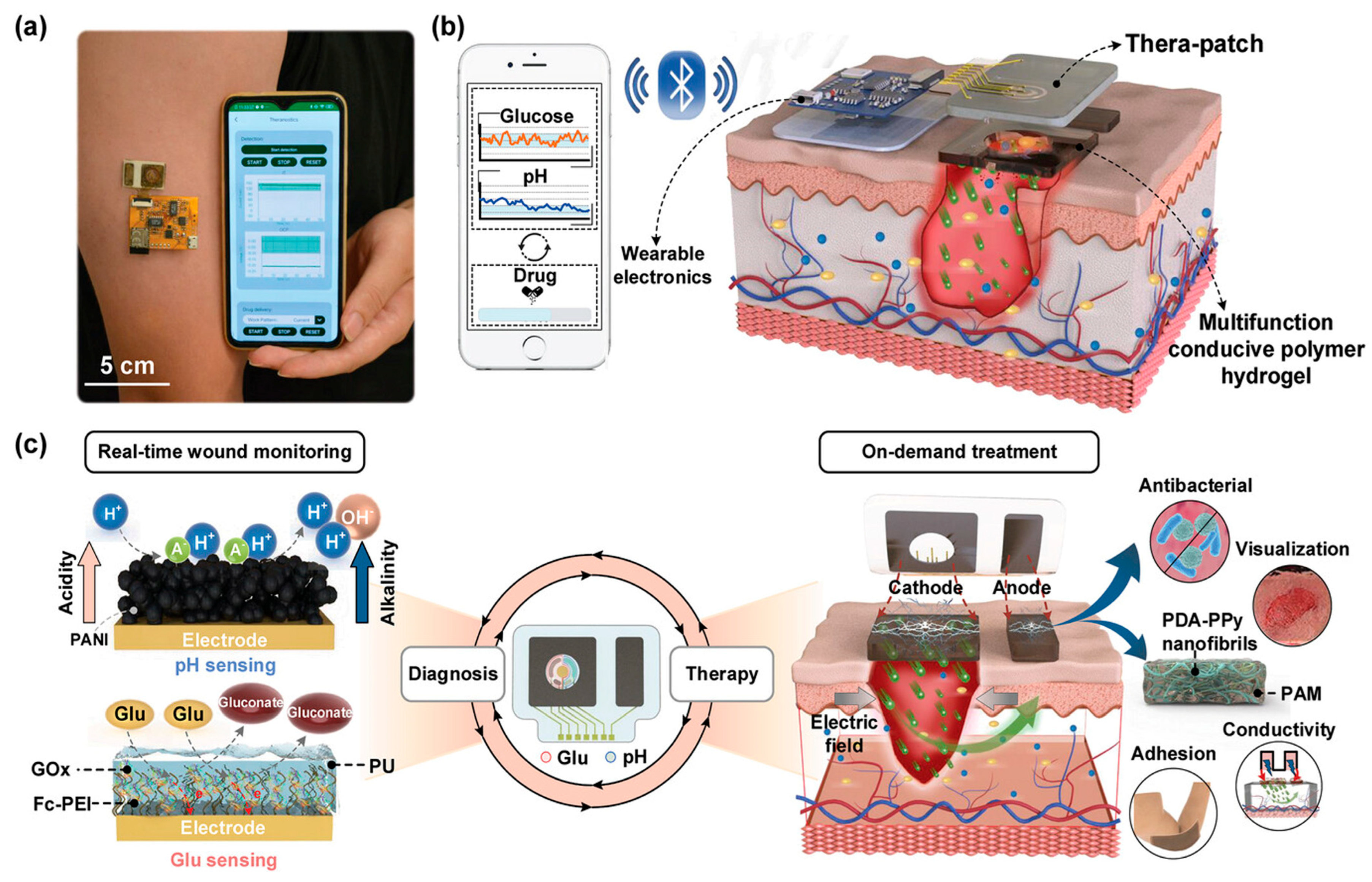
4.4. Integrated Diagnosis and Treatment Strategy
5. Conclusions and Prospects
5.1. Conclusions
5.2. Challenges
5.3. Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3-APBA/3-AAPB | 3-acrylamidophenylboronic acid |
| AA | Acrylic acid |
| AFPBA | (4-((2-acrylamidoethyl)carbamoyl)-3-fluorophenyl)boronic acid |
| AGEs | Advanced glycation end-products |
| AgNPs | Silver nanoparticles |
| AM | Acrylamide |
| BSA | Bovine albumin |
| CD | Carbon dots |
| CDT | Chemodynamic therapy |
| CGM | Continuous Glucose Monitoring |
| CMC-Eu-EDTA | Carboxymethyl cellulose ethylenediaminetetraacetic acid |
| Con A | Concanavalin A |
| Dex-MA | Dextran methacrylate |
| ECM | Extracellular matrix |
| FAD-GDH | Flavin adenine dinucleotide-dependent glucose dehydrogenase |
| FRET | Fluorescence Resonance Energy Transfer |
| GOx | Glucose Oxidase |
| GSH-Px | Glutathione peroxidase |
| HAP | Hyaluronic acid |
| IL | Interference layer |
| ISF | Interstitial fluid |
| LSPR | Localized Surface Plasmon Resonance |
| MeHA | Methacrylated Hyaluronic Acid |
| MMP-9 | Matrix metallopeptidase 9 |
| NDs | Nanodiamonds |
| NIR | Near Infrared |
| ODex | Oxidized dextran |
| OECT | Organic electrochemical transistors |
| PBA | Phenylboronic acid |
| PC | Photonic Crystal |
| PCHs | Photonic crystal hydrogels |
| PEGDA | Poly(ethylene glycol) diacrylate |
| PET | Photoinduced Electron Transfer |
| PLQ | phenanthroline quinone |
| PMH | Pt metal hydrogel |
| PVA | Polyvinyl alcohol |
| QDs | Quantum Dots |
| Reg3α | Regenerating family protein 3α |
| RGO | Reduced Graphene Oxide |
| RM | Responsive matrix |
| ROS | Reactive oxygen species |
| SERS | Surface-Enhanced Raman Scattering |
| SF | Silk Fibroin |
| SOD | Superoxide dismutase |
| SPCE | Screen-printed carbon electrode |
| TA | Tannic acid |
References
- Huang, K.; Mi, B.; Xiong, Y.; Fu, Z.; Zhou, W.; Liu, W.; Liu, G.; Dai, G. Angiogenesis during diabetic wound repair: From mechanism to therapy opportunity. Burn Trauma 2025, 13, tkae052. [Google Scholar] [CrossRef]
- Parker, E.D.; Lin, J.; Mahoney, T.; Ume, N.; Yang, G.; Gabbay, R.A.; ElSayed, N.A.; Bannuru, R.R. Economic costs of diabetes in the U.S. in 2022. Diabetes Care 2024, 47, 26–43. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic wound-healing science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Tan, T.-W.; Boulton, A.J.M.; Bus, S.A. Diabetic foot ulcers: A review. JAMA 2023, 330, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Berlanga-Acosta, J.; Schultz, G.S.; López-Mola, E.; Guillen-Nieto, G.; García-Siverio, M.; Herrera-Martínez, L. Glucose toxic effects on granulation tissue productive cells: The diabetics’ impaired healing. BioMed Res. Int. 2013, 2013, 256043. [Google Scholar] [CrossRef]
- Huang, F.; Lu, X.; Yang, Y.; Yang, Y.; Li, Y.; Kuai, L.; Li, B.; Dong, H.; Shi, J. Microenvironment-based diabetic foot ulcer nanomedicine. Adv. Sci. 2022, 10, e2203308. [Google Scholar] [CrossRef]
- Moura, L.I.F.; Dias, A.M.A.; Carvalho, E.; de Sousa, H.C. Recent advances on the development of wound dressings for diabetic foot ulcer treatment—A review. Acta Biomater. 2013, 9, 7093–7114. [Google Scholar] [CrossRef]
- Zhou, W.; Duan, Z.; Zhao, J.; Fu, R.; Zhu, C.; Fan, D. Glucose and MMP-9 dual-responsive hydrogel with temperature sensitive self-adaptive shape and controlled drug release accelerates diabetic wound healing. Bioact. Mater. 2022, 17, 1–17. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.; Li, Q.; Yang, L.; Liu, H.; Yan, R.; Xiao, L.; Liu, H.; Wang, J.; Yang, B.; et al. Transparent conductive supramolecular hydrogels with stimuli-responsive properties for on-demand dissolvable diabetic foot wound dressings. Macromol. Rapid Commun. 2020, 41, e2000441. [Google Scholar] [CrossRef]
- Ge, T.; Jin, J.; Feng, K.; Gu, X.; Ye, G.; Shi, T.; Li, J.; Wang, H.; Wang, H.; Chen, M. Synthesis and characterization of a novel photothermal hydrogel composite with combined osteogenic and antibacterial activities. Biomed. Mater. 2025, 20, 015037. [Google Scholar] [CrossRef]
- Arabpour, Z.; Abedi, F.; Salehi, M.; Baharnoori, S.M.; Soleimani, M.; Djalilian, A.R. Hydrogel-Based Skin Regeneration. Int. J. Mol. Sci. 2024, 25, 1982. [Google Scholar] [CrossRef]
- Jin, S.; Newton, M.A.A.; Cheng, H.; Zhang, Q.; Gao, W.; Zheng, Y.; Lu, Z.; Dai, Z.; Zhu, J. Progress of Hydrogel Dressings with Wound Monitoring and Treatment Functions. Gels 2023, 9, 694. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; Zhao, W.; Zhu, Z.; Pei, X. Recent advances of hydrogels as smart dressings for diabetic wounds. J. Mater. Chem. B 2024, 12, 1126–1148. [Google Scholar] [CrossRef]
- Liang, Y.; Li, M.; Yang, Y.; Qiao, L.; Xu, H.; Guo, B. pH/Glucose Dual Responsive Metformin Release Hydrogel Dressings with Adhesion and Self-Healing via Dual-Dynamic Bonding for Athletic Diabetic Foot Wound Healing. ACS Nano 2022, 16, 3194–3207. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wu, Y.; Li, W.; Wang, Y.; Kong, Q. Development of a Microenvironment-Responsive Hydrogel Promoting Chronically Infected Diabetic Wound Healing through Sequential Hemostatic, Antibacterial, and Angiogenic Activities. ACS Appl. Mater. Interfaces 2022, 14, 30480–30492. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Z.; Wang, J.; Li, R.; Li, T.; Chang, M.; Yan, F.; Wang, Y. Encapsulation of Curcumin Nanoparticles with MMP9-Responsive and Thermos-Sensitive Hydrogel Improves Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 16315–16326. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Peng, L.; Lin, J.; Li, Y.; Luo, Q.; Jiang, S.; Tian, H.; Zhang, Y.; Liu, X.; Liu, J. Enzyme hybrid virus-like hollow mesoporous CuO adhesive hydrogel spray through glucose-activated cascade reaction to efficiently promote diabetic wound healing. Chem. Eng. J. 2021, 415, 128901. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Long, L.; Yang, L.; Fu, D.; Hu, C.; Kong, Q.; Wang, Y. Inflammation-Responsive Drug-Loaded Hydrogels with Sequential Hemostasis, Antibacterial, and Anti-Inflammatory Behavior for Chronically Infected Diabetic Wound Treatment. ACS Appl. Mater. Interfaces 2021, 13, 33584–33599. [Google Scholar] [CrossRef]
- Yang, L.; Liang, F.; Zhang, X.; Jiang, Y.; Duan, F.; Li, L.; Ren, F. Remodeling microenvironment based on MOFs-Hydrogel hybrid system for improving diabetic wound healing. Chem. Eng. J. 2022, 427, 131506. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, H.; Zhou, L.; Cheng, F.; Liu, Z.; Zhang, H.; Zhang, Q. Injectable redox and light responsive MnO2 hybrid hydrogel for simultaneous melanoma therapy and multidrug-resistant bacteria-infected wound healing. Biomaterials 2020, 260, 120314. [Google Scholar] [CrossRef]
- Sugawara, A.; Asoh, T.A.; Takashima, Y.; Harada, A.; Uyama, H. Mechano-Responsive Hydrogels Driven by the Dissociation of a Host-Guest Complex. ACS Macro Lett. 2021, 10, 971–977. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, C.; Deng, D.; Gu, Y.; Wang, H.; Zhong, Q. Multiple Stimuli-Responsive MXene-Based Hydrogel as Intelligent Drug Delivery Carriers for Deep Chronic Wound Healing. Small 2022, 18, e2104368. [Google Scholar] [CrossRef]
- Contardi, M.; Summa, M.; Lenzuni, M.; Miracoli, L.; Bertora, F.; Mendez, M.D.; Athanassiou, A.; Bertorelli, R. Combining Alginate/PVPI-Based Film with Frequency Rhythmic Electrical Modulation System (FREMS) Technology as an Advanced Strategy for Diabetic Wounds. Macromol. Biosci. 2024, 24, e2300349. [Google Scholar] [CrossRef]
- Zheng, X.; Yao, J.; Yao, J.; Wang, P.; Liu, W.; Fan, D.; Hui, J. Multifunctional hydrogel combined with electrical stimulation therapy for promoting diabetic wound healing. Nano Res. 2024, 17, 9942–9953. [Google Scholar] [CrossRef]
- Xie, M.; Wang, L.; Guo, B.; Wang, Z.; Chen, Y.E.; Ma, P.X. Ductile electroactive biodegradable hyperbranched polylactide copolymers enhancing myoblast differentiation. Biomaterials 2015, 71, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Dworzański, J.; Strycharz-Dudziak, M.; Kliszczewska, E.; Kiełczykowska, M.; Dworzańska, A.; Drop, B.; Polz-Dacewicz, M. Glutathione peroxidase (GPx) and superoxide dismutase (SOD) activity in patients with diabetes mellitus type 2 infected with Epstein-Barr virus. PLoS ONE 2020, 15, e0230374. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Hamad, K.; Al Shibitini, A.; Juma, S.; Sharifi, S.; Gould, L.; Mahmoudi, M. Investigating Inflammatory Markers in Wound Healing: Understanding Implications and Identifying Artifacts. ACS Pharmacol. Transl. Sci. 2024, 7, 18–27. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Tao, S.; Wang, Q.; Ma, P.Q.; Li, Z.B.; Wu, Y.L.; Li, D.W. Research advances in smart responsive-hydrogel dressings with potential clinical diabetic wound healing properties. Mil. Med. Res. 2023, 10, 37. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Zhang, M.; Ran, M.; Xing, M.; Li, X.; Han, S.; Ren, K.; Wang, Y. A fully biodegradable and self-regulating injectable silk fibroin hydrogel for glucose-responsive insulin delivery. Appl. Mater. Today 2025, 44, 102700. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, H.Q.; Chao, X.; Xu, W.X.; Liu, Y.; Ling, G.X.; Zhang, P. Multimodal therapy strategies based on hydrogels for the repair of spinal cord injury. Mil. Med. Res. 2022, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Min, T.; Bian, X.; Dong, Y.; Zhang, P.; Wen, Y. Rational design of intelligent and multifunctional dressing to promote acute/chronic wound healing. ACS Appl. Bio Mater. 2022, 5, 4055–4085. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Song, S.; Zeng, H.; Ge, Z.; Liu, B.; Fan, Z. 3D printing MOF nanozyme hydrogel with dual enzymatic activities and visualized glucose monitoring for diabetic wound healing. Chem. Eng. J. 2023, 471, 144649. [Google Scholar] [CrossRef]
- Liu, K.; Kang, Y.; Dong, X.; Li, Q.; Wang, Y.; Wu, X.; Yang, X.; Chen, Z.; Dai, H. A simple yet effective hydrogel dressing for advanced microenvironmental management of diabetic wounds with intrinsic regulation. Chem. Eng. J. 2023, 470, 143987. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Zhao, M.; Liu, G.; Wu, J. Advances of hydrogel dressings in diabetic wounds. Biomater. Sci. 2021, 9, 1530–1546. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, Z.; Lei, S.; Zhou, J.; Liu, Y.; Yu, X.; Wang, J.; Wan, D.; Shi, J.; Wang, S. A temperature and pH dual-responsive injectable self-healing hydrogel prepared by chitosan oligosaccharide and aldehyde hyaluronic acid for promoting diabetic foot ulcer healing. Int. J. Biol. Macromol. 2023, 253, 127213. [Google Scholar] [CrossRef]
- Yang, H.; Lv, D.; Qu, S.; Xu, H.; Li, S.; Wang, Z.; Cao, X.; Rong, Y.; Li, X.; Wu, H.; et al. A ROS-responsive lipid nanoparticles release multifunctional hydrogel based on microenvironment regulation promotes infected diabetic wound healing. Adv. Sci. 2024, 11, e2403219. [Google Scholar] [CrossRef]
- Hao, J.; Liu, C.; Zhou, L.; Wu, N.; Sun, M.; Kuang, J.; Pan, H.; Lian, Y.; Li, J.; Dong, Y.; et al. Enhancing diabetic wound healing with a pH/glucose dual-responsive hydrogel for ROS clearance and antibacterial activity. Int. J. Biol. Macromol. 2024, 272, 132935. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y.; Wang, H.; Meng, Y.; Dai, X.; Du, L.; Li, L. Construction of pH-sensitive multifunctional hydrogel with synergistic anti-inflammatory effect for treatment of diabetic wounds. Pharmaceutics 2025, 17, 644. [Google Scholar] [CrossRef]
- Meng, H.; Su, J.; Shen, Q.; Hu, W.; Li, P.; Guo, K.; Liu, X.; Ma, K.; Zhong, W.; Chen, S.; et al. A smart MMP-9-responsive hydrogel releasing M2 macrophage-derived exosomes for diabetic wound healing. Adv. Healthc. Mater. 2025, 14, e2404966. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, S.; Liu, J.; Ren, L.; Luo, C.; Chen, G.; Yu, L. Regenerating family protein 3alpha-loaded responsive hydrogel accelerates infected diabetic wound healing. Adv. Healthc. Mater. 2025, 14, e2500403. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, J.; Zhao, M.; Luo, H.; Lin, M.; Luo, Y.; Li, Y.; He, H.; Wu, J. Adhesive, injectable, and ROS-responsive hybrid polyvinyl alcohol (PVA) hydrogel co-delivers metformin and fibroblast growth factor 21 (FGF21) for enhanced diabetic wound repair. Front. Bioeng. Biotechnol. 2022, 10, 968078. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, M.; Li, J.; Liu, Z.; Lyu, L.; Xiao, Y.; Yang, G.; Liu, J.; Wang, Q.; Ding, X.; et al. Adhesive and antioxidant hydrogel with glucose/ROS dual-responsive drug release for diabetic oral mucosal wound healing. ACS Biomater. Sci. Eng. 2025, 11, 2321–2337. [Google Scholar] [CrossRef]
- Ni, Z.; Yu, H.; Wang, L.; Huang, Y.; Lu, H.; Zhou, H.; Liu, Q. Multistage ROS-responsive and natural polyphenol-driven prodrug hydrogels for diabetic wound healing. ACS Appl. Mater. Interfaces 2022, 14, 52643–52658. [Google Scholar] [CrossRef]
- Qian, X.; Ko, A.; Li, H.; Liao, C. Flexible non-enzymatic glucose strip for direct non-invasive diabetic management. Microchem. J. 2024, 197, 109818. [Google Scholar] [CrossRef]
- Bercea, M.; Lupu, A. Recent insights into glucose-responsive concanavalin A-based smart hydrogels for controlled insulin delivery. Gels 2024, 10, 260. [Google Scholar] [CrossRef]
- Morariu, S. Advances in the design of phenylboronic acid-based glucose-sensitive hydrogels. Polymers 2023, 15, 582. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Meng, Z.; Guan, L.; Liu, A.; Li, L.; Nešić, M.D.; Yang, B.; Qu, W.; Lin, Q. Glucose-responsive hydrogel optimizing Fenton reaction to eradicate multidrug-resistant bacteria for infected diabetic wound healing. Chem. Eng. J. 2024, 487, 150545. [Google Scholar] [CrossRef]
- Khattak, S.; Ullah, I.; Sohail, M.; Akbar, M.U.; Rauf, M.A.; Ullah, S.; Shen, J.; Xu, H.T. Endogenous/exogenous stimuli-responsive smart hydrogels for diabetic wound healing. Aggregate 2024, 6, e688. [Google Scholar] [CrossRef]
- Xu, K.; Shan, W.; Hu, N.; Wang, J.; Zhou, W.; Muller-Buschbaum, P.; Zhong, Q. High efficiency of in-situ cross-linking and acid triggered drug delivery by introducing tobramycin into injectable and biodegradable hydrogels. Colloids Surf. B Biointerfaces 2022, 218, 112756. [Google Scholar] [CrossRef]
- Zhang, T.; Meng, Z.; Yu, H.; Ding, P.; Kai, T. An intelligent and conductive hydrogel with multiresponsive and ROS scavenging properties for infection prevention and anti-inflammatory treatment assisted by electrical stimulation for diabetic wound. Adv. Sci. 2025, 12, e2500696. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Dai, F.; Zhao, S.; Li, Z.; Liang, H.; Wang, X.; Zhao, L.; Tan, H. pH and glucose dual-responsive hydrogels promoted diabetic wound healing by remodeling the wound microenvironment. Adv. Healthc. Mater. 2025, 14, e2500810. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Zwolanek, D.; Keene, D.R.; Schulz, J.N.; Blumbach, K.; Heinegård, D.; Zaucke, F.; Paulsson, M.; Krieg, T.; Koch, M.; et al. Collagen XII and XIV, new partners of cartilage oligomeric matrix protein in the skin extracellular matrix suprastructure. J. Biol. Chem. 2012, 287, 22549–22559. [Google Scholar] [CrossRef]
- Martino, M.M.; Briquez, P.S.; Maruyama, K.; Hubbell, J.A. Extracellular matrix-inspired growth factor delivery systems for bone regeneration. Adv. Drug Deliv. Rev. 2015, 94, 41–52. [Google Scholar] [CrossRef]
- Broverman, R.L.; Nguyen, K.H.; da Silveira, A.; Brinkley, L.L.; Macauley, S.P.; Zeng, T.; Yamamoto, H.; Tarnuzzer, R.W.; Schultz, G.S.; Kerr, M.; et al. Changes in the expression of extracellular matrix (ECM) and matrix metalloproteinases (MMP) of proliferating rat parotid acinar cells. J. Dent. Res. 1998, 77, 1504–1514. [Google Scholar] [CrossRef]
- Alvarez, O.M.; Markowitz, L.; Onumah, N.; Wendelken, M. Swift Downregulation of Gelatinases (MMP-2, MMP-9) in Neuropathic Diabetic Foot Ulcers Treated With Total Contact Cast. Wounds 2019, 31, E39–E41. [Google Scholar]
- Corbel, M.; Boichot, E.; Lagente, V. Role of gelatinases MMP-2 and MMP-9 in tissue remodeling following acute lung injury. Braz. J. Med. Biol. Res. 2000, 33, 749–754. [Google Scholar] [CrossRef]
- Swetha, R.; Gayen, C.; Kumar, D.; Singh, T.D.; Modi, G.; Singh, S.K. Biomolecular basis of matrix metallo proteinase-9 activity. Future Med. Chem. 2018, 10, 1093–1112. [Google Scholar] [CrossRef]
- Tang, S.; Feng, K.; Yang, R.; Cheng, Y.; Shi, N.; Zhang, H.; Wei, Z.; Ma, Y. A dual-action strategy: Wound microenvironment responsive hydrogel and exosome-mediated glucose regulation enhance inside-out diabetic wound repair. J. Control Release 2025, 382, 113716. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, K.; Xiao, C.; Chen, X. Guanosine-driven hyaluronic acid-based supramolecular hydrogels with peroxidase-like activity for chronic diabetic wound treatment. Acta Biomater. 2023, 172, 206–217. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, M.; Ou, J.; Li, K.; Ju, X.; Tian, Y.; Niu, Z. Multi-responsive sodium hyaluronate/tannic acid hydrogels with ROS scavenging ability promote the healing of diabetic wounds. Int. J. Biol. Macromol. 2024, 278, 134896. [Google Scholar] [CrossRef]
- Pu, M.; Cao, H.; Zhang, H.; Wang, T.; Li, Y.; Xiao, S.; Gu, Z. ROS-responsive hydrogels: From design and additive manufacturing to biomedical applications. Mater. Horiz. 2024, 11, 3721–3746. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhang, Y.; Chan, S.Y.; Du, Z.; Yan, Y.; Wang, T.; Li, P.; Huang, W. Hydrogel-based flexible materials for diabetes diagnosis, treatment, and management. npj Flex. Electron. 2021, 5, 26. [Google Scholar] [CrossRef]
- Yu, S.; Xian, S.; Ye, Z.; Pramudya, I.; Webber, M.J. Glucose-Fueled Peptide Assembly: Glucagon Delivery via Enzymatic Actuation. J. Am. Chem. Soc. 2021, 143, 12578–12589. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhu, Y.; Si, J.; Zhang, R.; Ji, Y.; Fan, J.; Dong, Y. Glucose-activated nanozyme hydrogels for microenvironment modulation via cascade reaction in diabetic wound. Chin. Chem. Lett. 2025, 36, 110012. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, W.; Xu, P.; Fu, X.; Yu, X.; Chen, L.; Leng, F.; Yu, C.; Yang, Z. Glucose-responsive multifunctional metal-organic drug-loaded hydrogel for diabetic wound healing. Acta Biomater. 2022, 140, 206–218. [Google Scholar] [CrossRef]
- Dong, D.; Cheng, Z.; Wang, T.; Wu, X.; Ding, C.; Chen, Y.; Xiong, H.; Liang, J. Acid-degradable nanocomposite hydrogel and glucose oxidase combination for killing bacterial with photothermal augmented chemodynamic therapy. Int. J. Biol. Macromol. 2023, 234, 123745. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Y.; Zhang, R.; Yu, Q.; Liu, Y.; Liu, Y. Glucose-Activated Nanoconfinement Supramolecular Cascade Reaction in Situ for Diabetic Wound Healing. ACS Nano 2022, 16, 9929–9937. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, Y.Q.; Liu, P.; Cao, J.; Shen, A.G.; Chu, P.K. Blood-Glucose-Depleting Hydrogel Dressing as an Activatable Photothermal/Chemodynamic Antibacterial Agent for Healing Diabetic Wounds. ACS Appl. Mater. Interfaces 2023, 15, 24162–24174. [Google Scholar] [CrossRef]
- Kanokpaka, P.; Chang, Y.H.; Chang, C.C.; Rinawati, M.; Wang, P.C.; Chang, L.Y.; Yeh, M.H. Enabling glucose adaptive self-healing hydrogel based triboelectric biosensor for tracking a human perspiration. Nano Energy 2023, 112, 108513. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int. J. Biol. Macromol. 2019, 122, 137–148. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, Z.; Zhang, T.; Zhang, W.; Chen, W.; Cao, Y.; Chen, M.; Zhou, X. Orange-Emissive Carbon Quantum Dots: Toward Application in Wound pH Monitoring Based on Colorimetric and Fluorescent Changing. Small 2019, 15, e1902823. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Saroj, S.; Saha, S.; Rakshit, T.; Pal, S. In Situ-Forming Protein-Polymer Hydrogel for Glucose-Responsive Insulin Release. ACS Appl. Bio Mater. 2023, 6, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.-N.; Ju, X.-J.; Pu, X.-Q.; Xie, R.; Wang, W.; Liu, Z.; Chu, L.-Y. Injectable Temperature/Glucose Dual-Responsive Hydrogels for Controlled Release of Insulin. Ind. Eng. Chem. Res. 2021, 60, 8147–8158. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, H.; Wang, L.; Shen, D.; Liu, J. Preparation of phenylboronic acid-based glucose-responsive hydrogels and microneedles for regulated delivery of insulin. Eur. Polym. J. 2023, 192, 112061. [Google Scholar] [CrossRef]
- Shen, D.; Yu, H.; Wang, L.; Chen, X.; Feng, J.; Li, C.; Xiong, W.; Zhang, Q. Glucose-responsive hydrogel-based microneedles containing phenylborate ester bonds and N-isopropylacrylamide moieties and their transdermal drug delivery properties. Eur. Polym. J. 2021, 148, 110348. [Google Scholar] [CrossRef]
- Ali, A.; Saroj, S.; Saha, S.; Gupta, S.K.; Rakshit, T.; Pal, S. Glucose-Responsive Chitosan Nanoparticle/Poly(vinyl alcohol) Hydrogels for Sustained Insulin Release In Vivo. ACS Appl. Mater. Interfaces 2023, 15, 32240–32250. [Google Scholar] [CrossRef]
- Maity, B.; Moorthy, H.; Govindaraju, T. Glucose-Responsive Self-Regulated Injectable Silk Fibroin Hydrogel for Controlled Insulin Delivery. ACS Appl. Mater. Interfaces 2023, 15, 49953–49963. [Google Scholar] [CrossRef]
- He, J.; Li, Z.; Chen, J.; Wang, J.; Qiao, L.; Guo, B.; Hu, J. NIR/glucose stimuli-responsive multifunctional smart hydrogel wound dressing with NO/O2 dual gas-releasing property promotes infected diabetic wound healing. Chem. Eng. J. 2024, 492, 152249. [Google Scholar] [CrossRef]
- Fujita, H.; Yamagishi, K.; Zhou, W.; Tahara, Y.; Huang, S.Y.; Hashimoto, M.; Fujie, T. Design and fabrication of a flexible glucose sensing platform toward rapid battery-free detection of hyperglycaemia. J. Mater. Chem. C 2021, 9, 7336–7344. [Google Scholar] [CrossRef]
- Bai, J.; Liu, D.; Tian, X.; Wang, Y.; Cui, B.; Yang, Y.; Dai, S.; Lin, W.; Zhu, J.; Wang, J.; et al. Coin-sized, fully integrated, and minimally invasive continuous glucose monitoring system based on organic electrochemical transistors. Sci. Adv. 2024, 10, eadl1856. [Google Scholar] [CrossRef]
- Li, Q.-F.; Chen, X.; Wang, H.; Liu, M.; Peng, H.-L. Pt/MXene-Based Flexible Wearable Non-Enzymatic Electrochemical Sensor for Continuous Glucose Detection in Sweat. ACS Appl. Mater. Interfaces 2023, 15, 13290–13298. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, Y.; Wang, Z.; Huang, X.; Huang, W. Hydrogel-based composites: Unlimited platforms for biosensors and diagnostics. View 2021, 2, 20200165. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Y.; Lee, J.; Hoover, A.; King, S.A.; Chen, L.; Zhao, J.; Lin, Q.; Yu, C.; Zhu, L.; et al. Continuous Glucose Monitoring Enabled by Fluorescent Nanodiamond Boronic Hydrogel. Adv. Sci. 2023, 10, e2203943. [Google Scholar] [CrossRef]
- Williams, T.J.; Jeevarathinam, A.S.; Jivan, F.; Baldock, V.; Kim, P.; McShane, M.J.; Alge, D.L. Glucose biosensors based on Michael addition crosslinked poly(ethylene glycol) hydrogels with chemo-optical sensing microdomains. J. Mater. Chem. B 2023, 11, 1749–1759. [Google Scholar] [CrossRef]
- Li, Y.; Luo, S.; Gui, Y.; Wang, X.; Tian, Z.; Yu, H. Difunctional Hydrogel Optical Fiber Fluorescence Sensor for Continuous and Simultaneous Monitoring of Glucose and pH. Biosensors 2023, 13, 287. [Google Scholar] [CrossRef]
- Pradhan, R.; Chimene, D.; Ko, B.S.; Goncharov, A.; Ozcan, A.; McShane, M.J. Insertable Biomaterial-Based Multianalyte Barcode Sensor toward Continuous Monitoring of Glucose and Oxygen. ACS Sens. 2024, 9, 6060–6070. [Google Scholar] [CrossRef]
- Liu, W.; Qiu, Y.; Zhao, W.; Zhu, A.; Zhang, G.; Wu, Z. Core/shell structure photonic crystal microneedle patch for painless, in situ and visualization in glucose monitoring. Colloids Surf. A Physicochem. Eng. Asp. 2024, 687, 133487. [Google Scholar] [CrossRef]
- Tai, Y.T.; Wei, C.Y.; Ko, F.H. Hydrogel-based colorimetric power-saving sensors for on-site detection of chloride ions and glucose in sweat. Biosens. Bioelectron. 2025, 271, 117041. [Google Scholar] [CrossRef]
- Yang, X.; Chai, L.; Huang, Z.; Zhu, B.; Liu, H.; Shi, Z.; Wu, Y.; Guo, L.; Xue, L.; Lei, Y. Smart photonic crystal hydrogels for visual glucose monitoring in diabetic wound healing. J. Nanobiotechnol. 2024, 22, 618. [Google Scholar] [CrossRef]
- Ahmed, I.; Elsherif, M.; Ali, M.; Al Ghaferi, A.; Mohammad, B.; Butt, H. Photonic hydrogel for continuous glucose monitoring using smartphone readout. Mater. Des. 2023, 231, 112065. [Google Scholar] [CrossRef]
- Chu, Z.; Xue, C.; Shao, K.; Xiang, L.; Zhao, X.; Chen, C.; Pan, J.; Lin, D. Photonic Crystal-Embedded Molecularly Imprinted Contact Lenses for Controlled Drug Release. ACS Appl. Bio Mater. 2022, 5, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Luo, W.; Pan, J.; Li, G.; Pu, Y.; Si, L.; Shi, G.; Shao, Y.; Ma, H.; Guan, J. Glucose-Sensing Photonic Nanochain Probes with Color Change in Seconds. Adv. Sci. 2022, 9, e2105239. [Google Scholar] [CrossRef] [PubMed]
- Maity, D.; Guha Ray, P.; Fussenegger, M. Glucose-Operated Widget (GLOW) for Closed-Loop Optogenetic Glycemic Control. Adv. Mater. 2024, 36, e2408537. [Google Scholar] [CrossRef]
- Deng, M.; Song, G.; Zhong, K.; Wang, Z.; Xia, X.; Tian, Y. Wearable fluorescent contact lenses for monitoring glucose via a smartphone. Sens. Actuators B Chem. 2022, 352, 131067. [Google Scholar] [CrossRef]
- Ahmed, I.; El Turk, S.; Al Ghaferi, A.; Samad, Y.A.; Butt, H. Nanocomposite Hydrogel-Based Optical Fiber Probe for Continuous Glucose Sensing. Small Sci. 2024, 4, 2300189. [Google Scholar] [CrossRef]
- Yang, X.; Duan, X.; Zhu, L.; Zhao, X.; Zhang, S.; Ni, S.; Wu, S.; Han, Z.; Deng, W.; Sun, D.; et al. Carbon-Dot-Zn2+ Assembly Hybrid Multifunctional Hydrogels for Synergistically Monitoring Local Glucose and Repairing Diabetic Wound. ACS Appl. Mater. Interfaces 2025, 17, 38874–38889. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, D.; Ma, X.; Huang, R.; Xu, J.; Xu, X.; Cai, L.; Xu, L. Surface enhanced Raman scattering active substrate based on hydrogel microspheres for pretreatment-free detection of glucose in biological samples. Talanta 2023, 260, 124657. [Google Scholar] [CrossRef]
- Wang, J.; Ye, J.; Li, Z.; Li, X.; Luo, Y.; Zhou, Z.; Liu, C.; Xu, T.; Zhang, X. An Integrated Janus Bioelectronic Bandage for Unidirectional Pumping and Monitoring of Wound Exudate. Nano Lett. 2025, 25, 5156–5164. [Google Scholar] [CrossRef]
- Davies, S.; Hu, Y.; Blyth, J.; Jiang, N.; Yetisen, A.K. Reusable Dual-Photopolymerized Holographic Glucose Sensors. Adv. Funct. Mater. 2023, 33, 2214197. [Google Scholar] [CrossRef]
- Lu, D.; Li, H.; Xiao, N.; Jiang, M.; Zuna, Y.; Feng, S.; Li, Z.; Long, J.; Marty, J.L.; Zhu, Z. Salivary glucose detection based on platinum metal hydrogel prepared mouthguard electrochemical sensor. Talanta 2025, 283, 127197. [Google Scholar] [CrossRef] [PubMed]
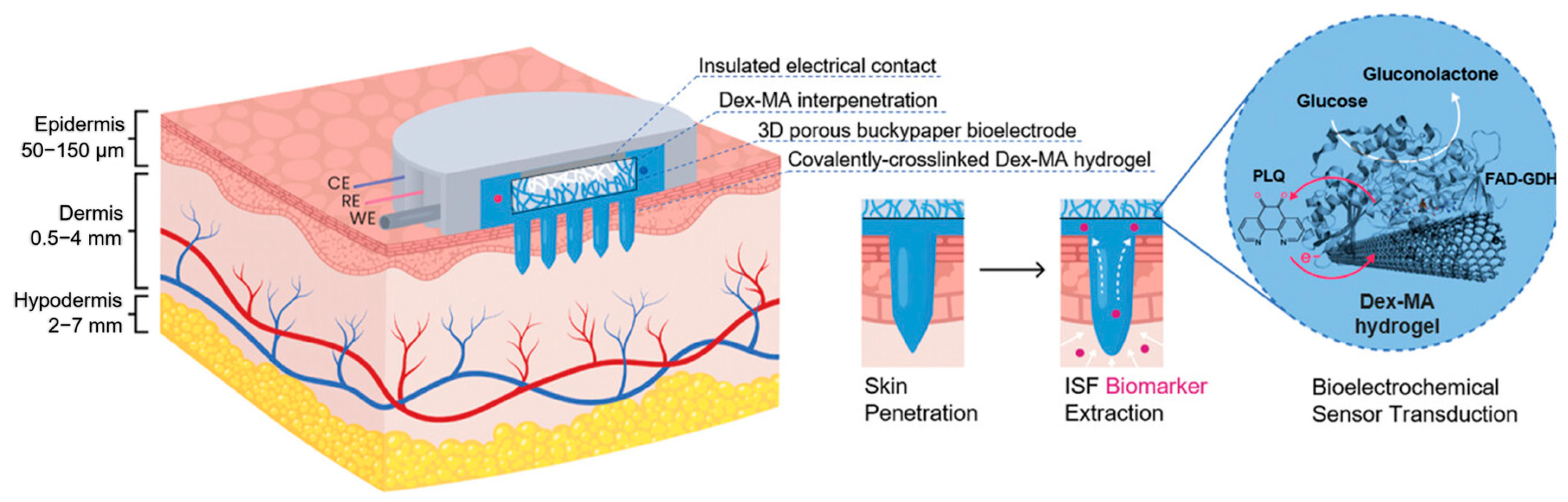
- Darmau, B.; Sacchi, M.; Texier, I.; Gross, A.J. Self-Extracting Dextran-Based Hydrogel Microneedle Arrays with an Interpenetrating Bioelectroenzymatic Sensor for Transdermal Monitoring with Matrix Protection. Adv. Healthc. Mater. 2025, 14, e2403209. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Jiang, D.; Ge, Y.; Huang, L.; Xiao, Y.; Ren, X.; Liu, X.; Zhang, Q.; Wang, Y. A PEDOT:PSS conductive hydrogel incorporated with Prussian blue nanoparticles for wearable and noninvasive monitoring of glucose. Chem. Eng. J. 2022, 431, 134109. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, J.; Liu, H.; Ran, R.; Zhang, T.; Li, Z. Enzyme-chitosan hydrogels for high sensitivity flexible silk-based electrochemical glucose sensor. Microchem. J. 2024, 200, 110306. [Google Scholar] [CrossRef]
- Man, T.; Yu, G.; Zhu, F.; Huang, Y.; Wang, Y.; Su, Y.; Deng, S.; Pei, H.; Li, L.; Ye, H.; et al. Antidiabetic Close Loop Based on Wearable DNA-Hydrogel Glucometer and Implantable Optogenetic Cells. JACS Au 2024, 4, 1500–1508. [Google Scholar] [CrossRef]
- Yan, J.; Wang, J.; Wu, Z.; Li, Z.; Li, Z.; Hu, Z. Bioelectronic sutures with electrochemical glucose-sensing for real-time wound monitoring. Anal. Chim. Acta 2025, 1370, 344320. [Google Scholar] [CrossRef]
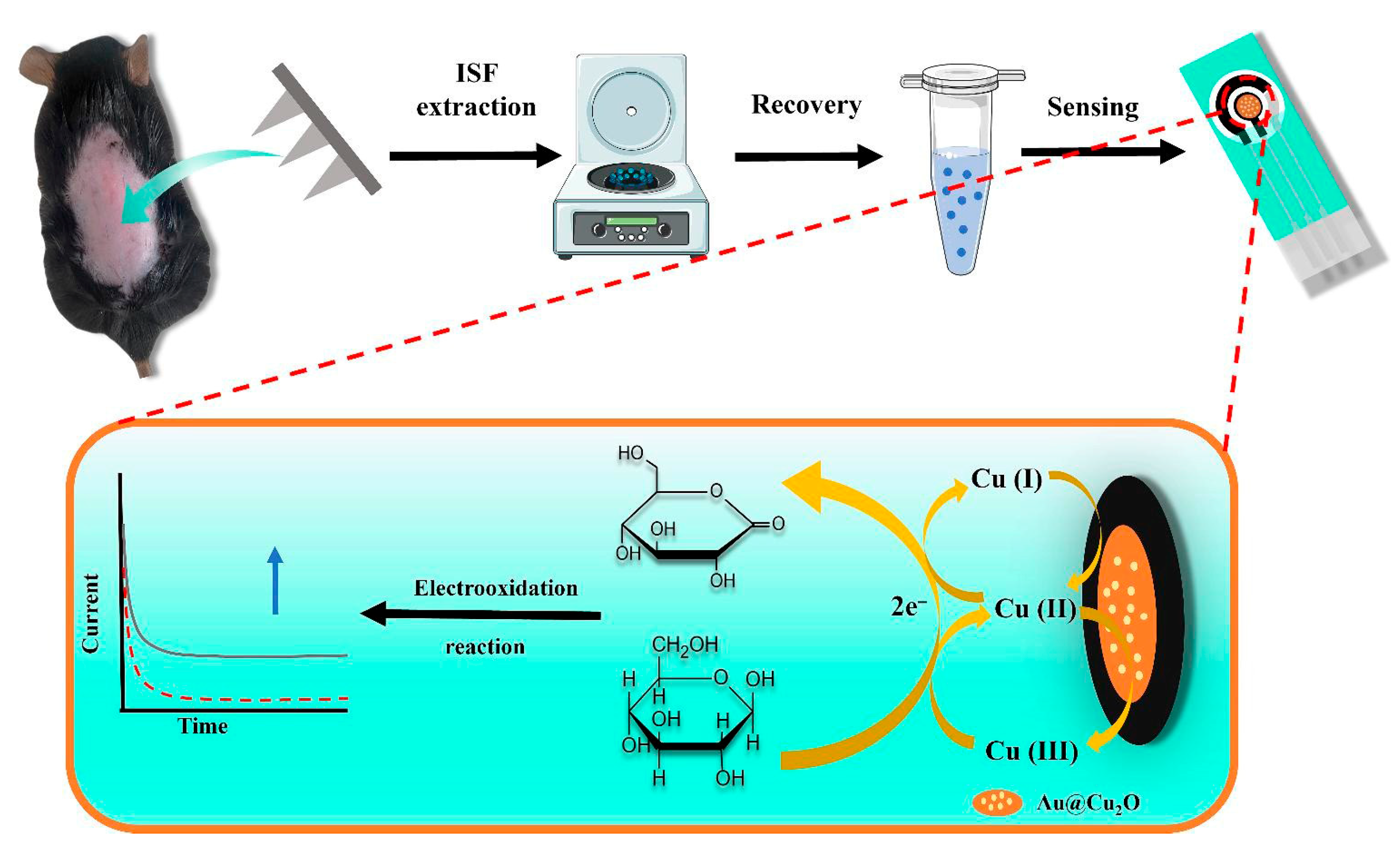
- Jin, D.; Xu, Z.; Zhao, H.; Deng, S.; Qu, Z.; Dou, R.; Liu, W. A minimally invasive sensing system based on hydrogel microneedle patches and Au/Cu2O nanospheres modified screen-printed carbon electrode for glucose monitoring in interstitial skin fluid. Microchem. J. 2024, 205, 111367. [Google Scholar] [CrossRef]
- Mirani, B.; Pagan, E.; Currie, B.; Siddiqui, M.A.; Hosseinzadeh, R.; Mostafalu, P.; Zhang, Y.S.; Ghahary, A.; Akbari, M. An Advanced Multifunctional Hydrogel-Based Dressing for Wound Monitoring and Drug Delivery. Adv. Healthc. Mater. 2017, 6, 1700718. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Y.; Sun, M.; Chen, Y.; Tu, W.; Zhou, Y.; Li, X.; Hu, T. Multifunctional Carbon-Based Nanocomposite Hydrogels for Wound Healing and Health Management. Gels 2025, 11, 345. [Google Scholar] [CrossRef]
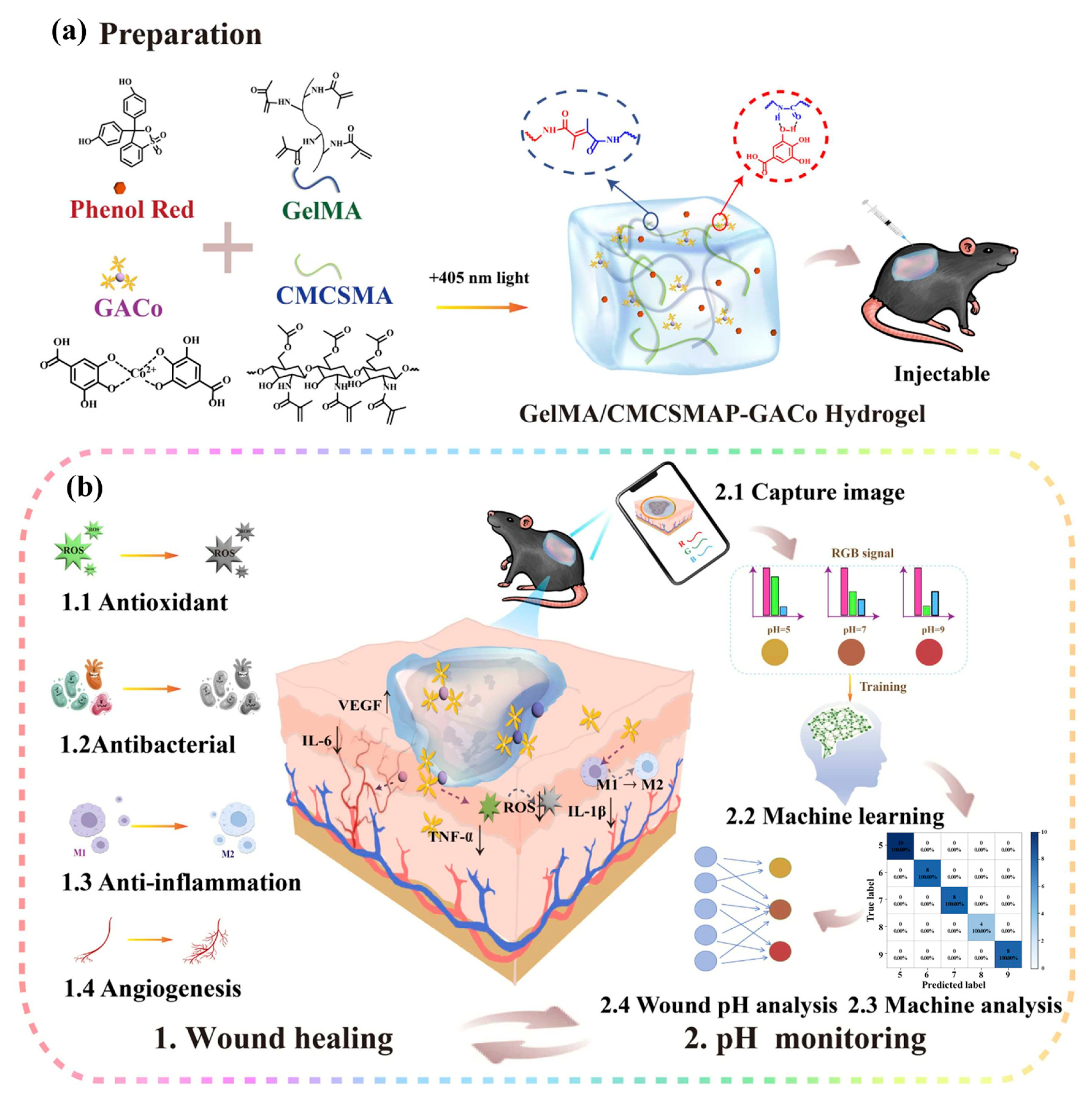
- Deng, D.; Liang, L.; Su, K.; Gu, H.; Wang, X.; Wang, Y.; Shang, X.; Huang, W.; Chen, H.; Wu, X.; et al. Smart hydrogel dressing for machine learning-enabled visual monitoring and promote diabetic wound healing. Nano Today 2025, 60, 102559. [Google Scholar] [CrossRef]
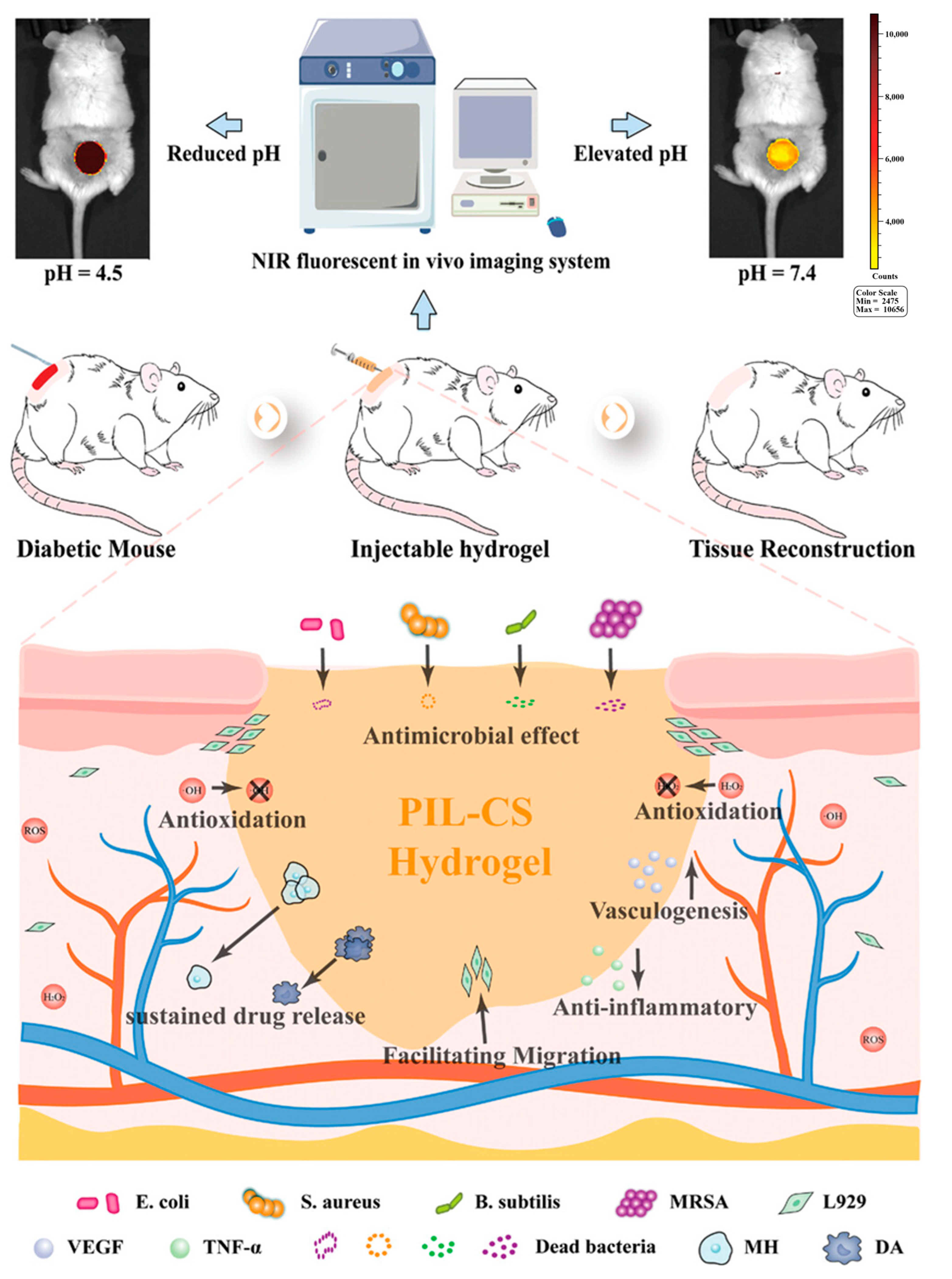
- Zong, Y.; Zong, B.; Zha, R.; Zhang, Y.; Li, X.; Wang, Y.; Fang, H.; Wong, W.L.; Li, C. An Antibacterial and Anti-Oxidative Hydrogel Dressing for Promoting Diabetic Wound Healing and Real-Time Monitoring Wound pH Conditions with a NIR Fluorescent Imaging System. Adv. Healthc. Mater. 2023, 12, e2300431. [Google Scholar] [CrossRef]
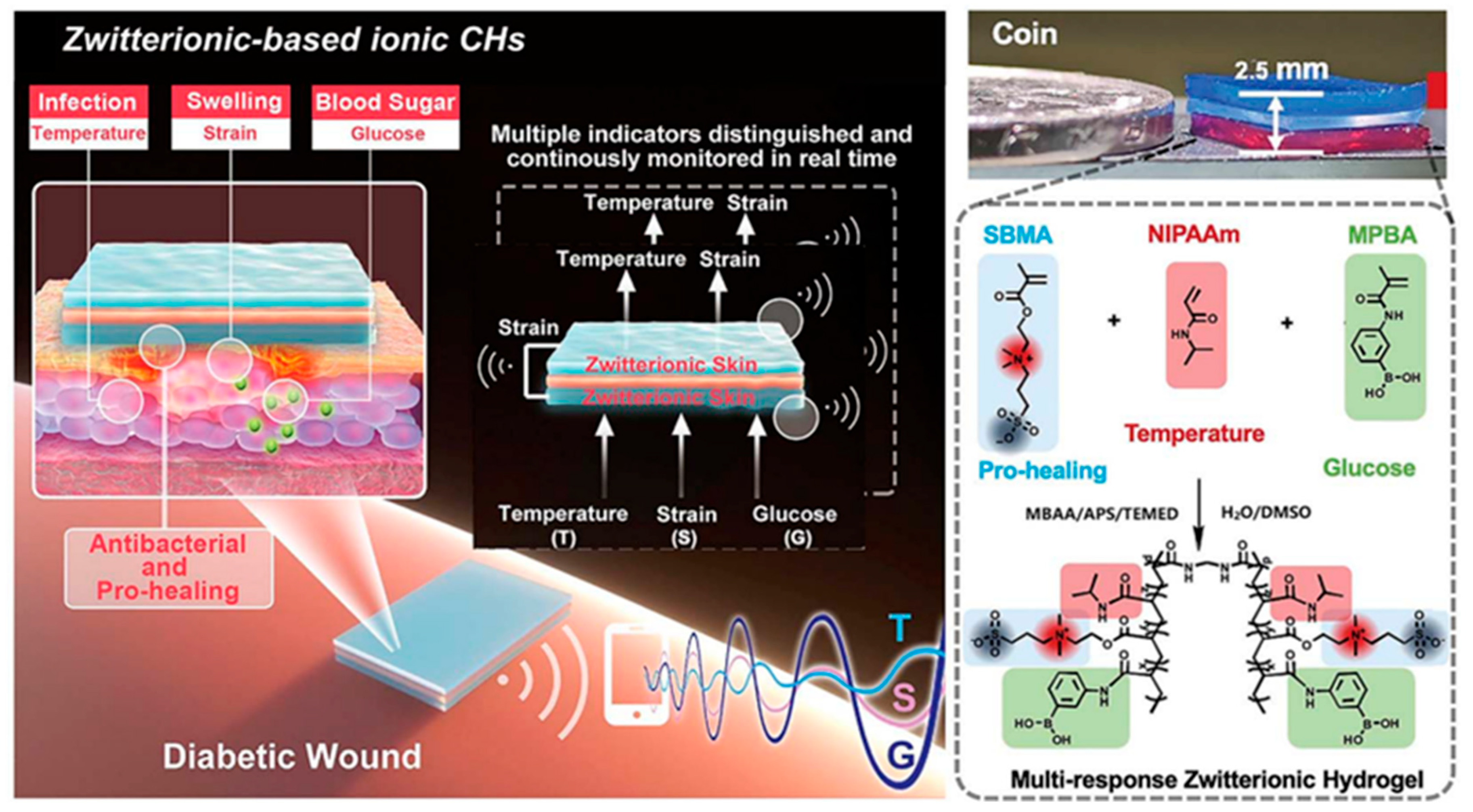
- Zhu, Y.; Zhang, J.; Song, J.; Yang, J.; Du, Z.; Zhao, W.; Guo, H.; Wen, C.; Li, Q.; Sui, X.; et al. A Multifunctional Pro-Healing Zwitterionic Hydrogel for Simultaneous Optical Monitoring of pH and Glucose in Diabetic Wound Treatment. Adv. Funct. Mater. 2019, 30, 1905493. [Google Scholar] [CrossRef]
- Huang, D.; Du, J.; Luo, F.; He, G.; Zou, M.; Wang, Y.; Lin, Z.; Wu, D.; Weng, Z. Injectable Hydrogels with Integrated Ph Probes and Ultrasound-Responsive Microcapsules as Smart Wound Dressings for Visual Monitoring and On-Demand Treatment of Chronic Wounds. Adv. Healthc. Mater. 2024, 13, e2303379. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; Guo, Q.; Jia, L.; Yin, T.; Huang, W.; Zhang, X.; Zhou, S. Multifunctional Hydrogels for the Healing of Diabetic Wounds. Adv. Healthc. Mater. 2024, 13, e2301885. [Google Scholar] [CrossRef] [PubMed]
- Sen, R.K.; Prabhakar, P.; Shruti; Verma, P.; Vikram, A.; Mishra, A.; Dwivedi, A.; Gowri, V.S.; Chaurasia, J.P.; Mondal, D.P.; et al. Smart Nanofibrous Hydrogel Wound Dressings for Dynamic Infection Diagnosis and Control: Soft but Functionally Rigid. ACS Appl. Bio Mater. 2024, 7, 999–1016. [Google Scholar] [CrossRef]
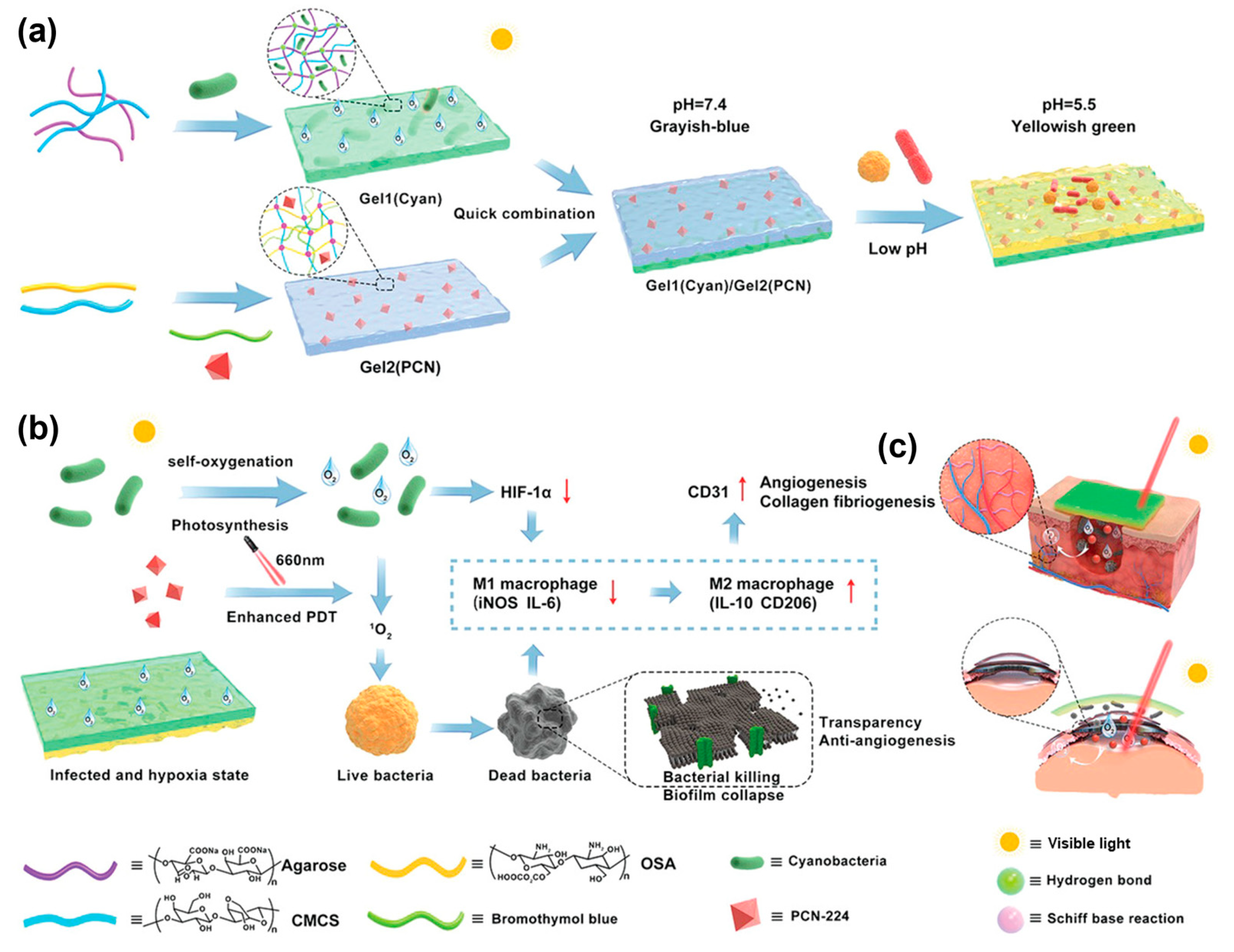
- Zhu, Z.; Wang, L.; Peng, Y.; Chu, X.; Zhou, L.; Jin, Y.; Guo, H.; Gao, Q.; Yang, J.; Wang, X.; et al. Continuous Self-Oxygenated Double-Layered Hydrogel under Natural Light for Real-Time Infection Monitoring, Enhanced Photodynamic Therapy, and Hypoxia Relief in Refractory Diabetic Wounds Healing. Adv. Funct. Mater. 2022, 32, 2201875. [Google Scholar] [CrossRef]
- Zheng, X.T.; Zhong, Y.; Chu, H.E.; Yu, Y.; Zhang, Y.; Chin, J.S.; Becker, D.L.; Su, X.; Loh, X.J. Carbon Dot-Doped Hydrogel Sensor Array for Multiplexed Colorimetric Detection of Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 17675–17687. [Google Scholar] [CrossRef]
- Xie, H.; Wang, Z.; Wang, R.; Chen, Q.; Yu, A.; Lu, A. Self-Healing, Injectable Hydrogel Dressing for Monitoring and Therapy of Diabetic Wound. Adv. Funct. Mater. 2024, 34, 2401209. [Google Scholar] [CrossRef]
- Wang, L.; Fan, L.; Filppula, A.M.; Wang, Y.; Bian, F.; Shang, L.; Zhang, H. Dual physiological responsive structural color hydrogel particles for wound repair. Bioact. Mater. 2025, 46, 494–502. [Google Scholar] [CrossRef]
- Shen, K.; Liu, Z.; Xie, R.; Zhang, Y.; Yang, Y.; Zhao, X.; Zhang, Y.; Yang, A.; Cheng, Y. Nanocomposite conductive hydrogels with Robust elasticity and multifunctional responsiveness for flexible sensing and wound monitoring. Mater. Horiz. 2023, 10, 2096–2108. [Google Scholar] [CrossRef]
- Zeng, Q.; Peng, Q.; Wang, F.; Shi, G.; Haick, H.; Zhang, M. Tailoring Food Biopolymers into Biogels for Regenerative Wound Healing and Versatile Skin Bioelectronics. Nanomicro Lett. 2023, 15, 153. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Bai, M.; Zhu, Y.; Liu, X.; Tian, S.; Long, Y.; Ma, Y.; Wen, C.; Li, Q.; Yang, J.; et al. Pro-Healing Zwitterionic Skin Sensor Enables Multi-Indicator Distinction and Continuous Real-Time Monitoring. Adv. Funct. Mater. 2021, 31, 2106406. [Google Scholar] [CrossRef]
- Ge, Z.; Guo, W.; Tao, Y.; Sun, H.; Meng, X.; Cao, L.; Zhang, S.; Liu, W.; Akhtar, M.L.; Li, Y.; et al. Wireless and Closed-Loop Smart Dressing for Exudate Management and On-Demand Treatment of Chronic Wounds. Adv. Mater. 2023, 35, e2304005. [Google Scholar] [CrossRef]
- Zhao, C.; Li, Y.; Zhao, J.; Li, H.; Xu, J.; Gao, Z.; Ding, C.; Song, Y.Y. A “Test-to-Treat” Pad for Real-Time Visual Monitoring of Bacterial Infection and On-Site Performing Smart Therapy Strategies. ACS Nano 2023, 17, 13296–13309. [Google Scholar] [CrossRef]
- Gong, X.; Yang, J.; Zheng, Y.; Chen, S.; Duan, H.; Gao, J.; Haick, H.; Yi, C.; Jiang, L. Polymer Hydrogel-Based Multifunctional Theranostics for Managing Diabetic Wounds. Adv. Funct. Mater. 2024, 34, 2315564. [Google Scholar] [CrossRef]



| Response Type | Response Mechanism | Composition | Application | Advantage | Ref. |
|---|---|---|---|---|---|
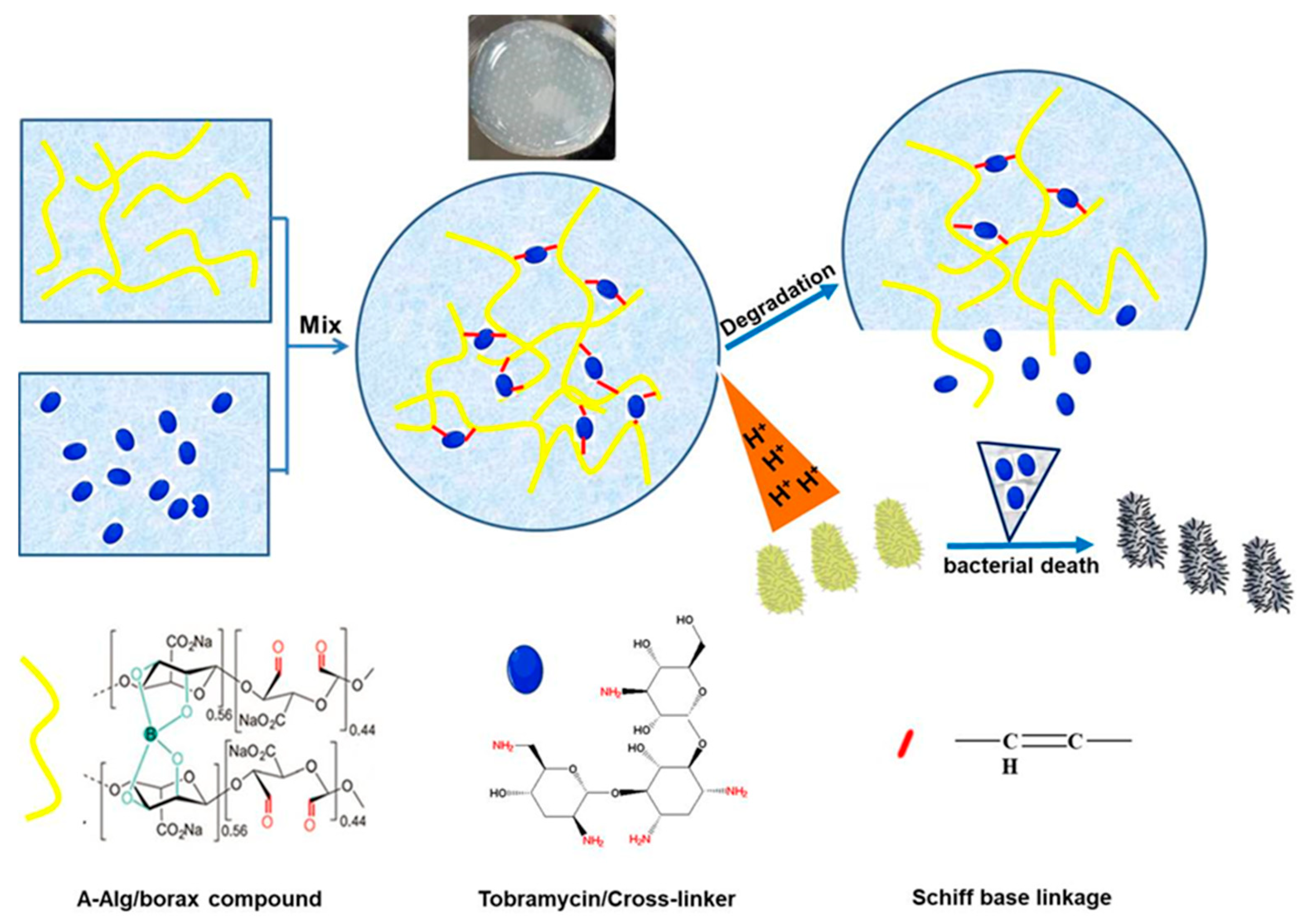
| pH Response | 1. Solvation or contraction of ionizable groups (-COOH, -NH2) of polymers; 2. Acid-sensitive dynamic covalent bond degradation | Alginate, chitosan, carboxymethyl cellulose, oxidized dextran, bovine serum albumin (BSA) | pH-responsive degradation of ODex/BSA-Zn hydrogels via Schiff base bonding | Dynamically adapts to the acidic environment of the wound for precise and targeted drug delivery; some hydrogels can actively regulate pH to promote macrophage polarization | [36,37,38,39] |
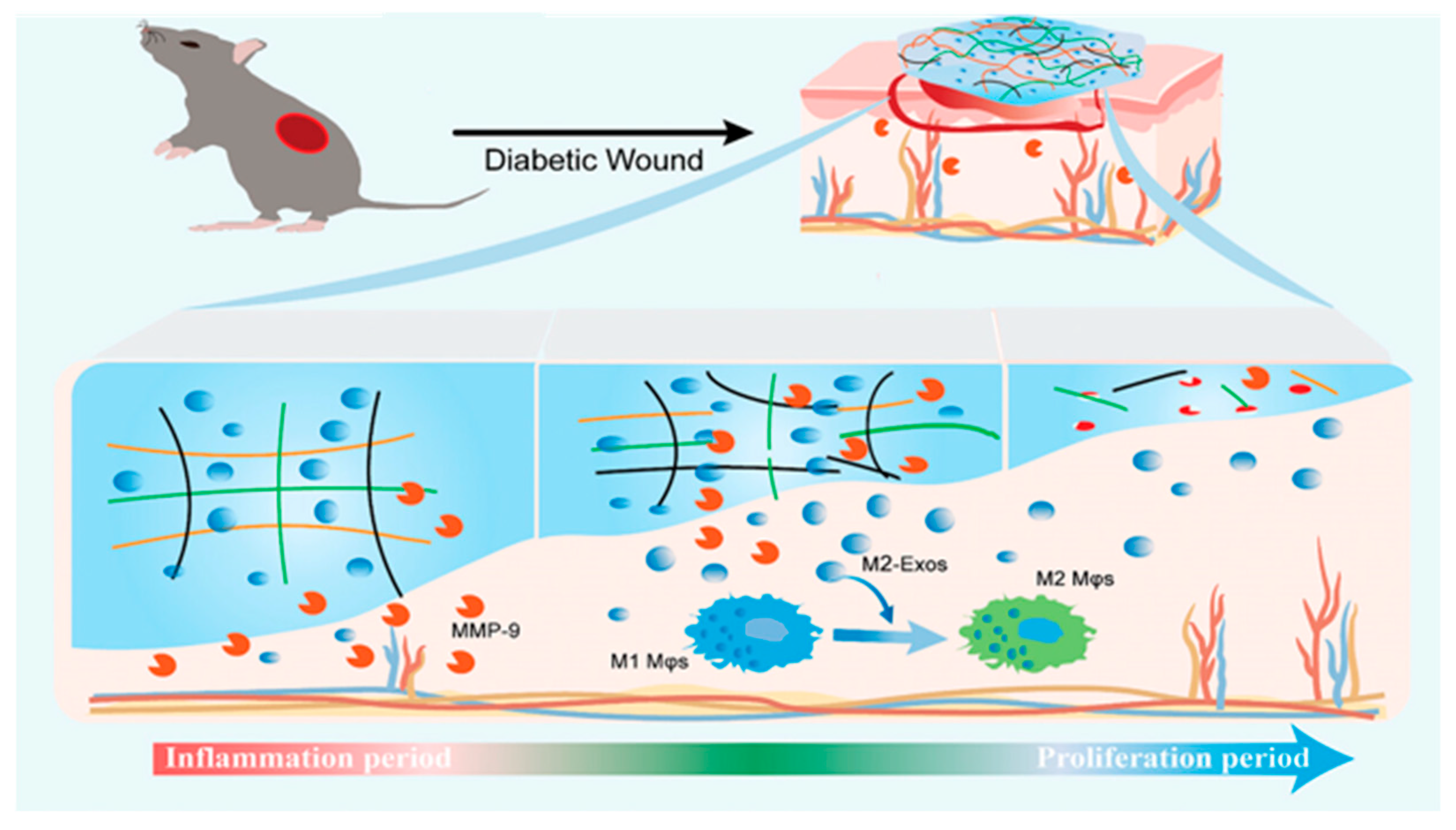
| enzyme Response | Use of protease-sensitive peptide chains, such as MMP-9, as cross-linking units to trigger hydrogel degradation or drug release after enzymatic digestion | Oxidized dextran, carboxymethyl chitosan, MMP-9-sensitive peptide chain | MMP-9 releases M2-type macrophage exosomes in response to hydrogels and promotes M1→M2 polarization to accelerate healing | Specific targeting of inflammatory sites, regulating abnormal enzyme activity, and promoting wound repair | [40] |
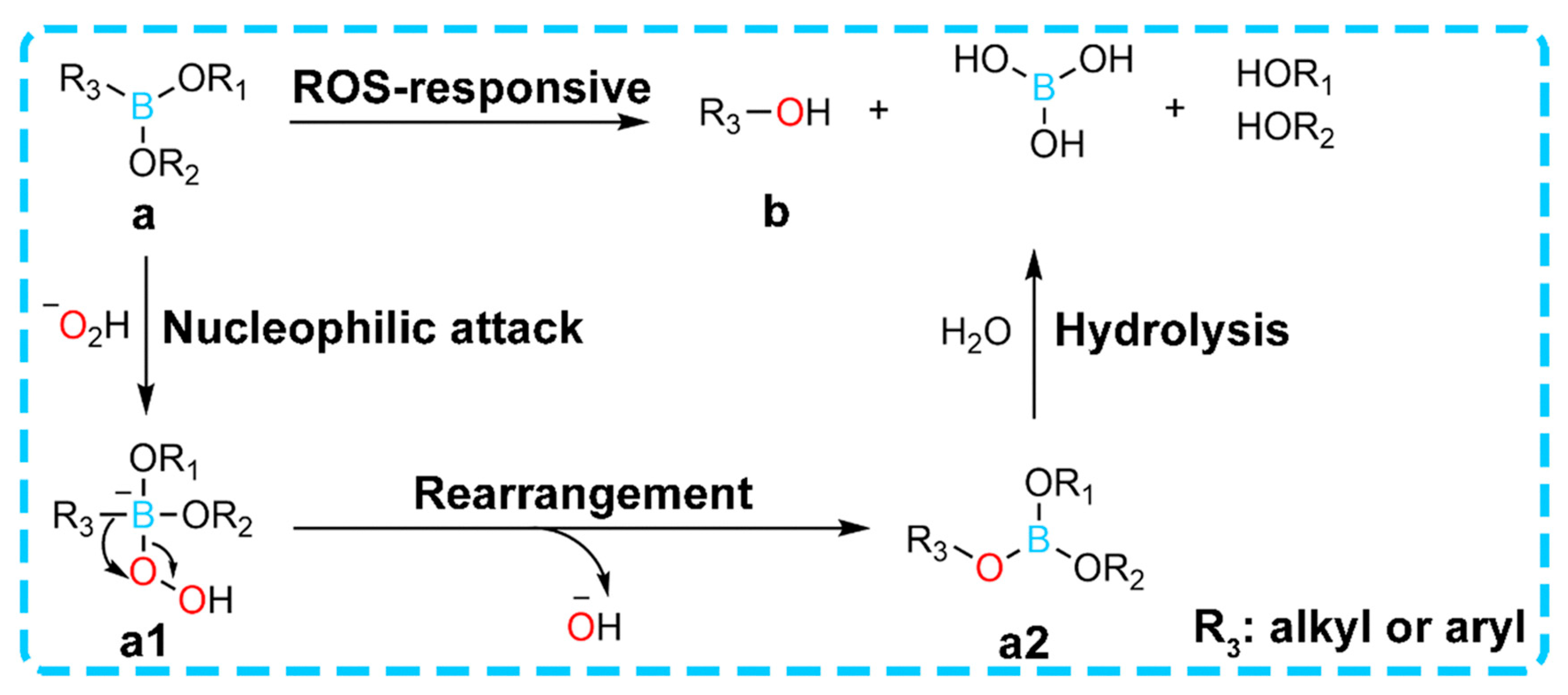
| ROS Response | ROS-sensitive chemical bond (thioketone, borate) breakage triggers degradation or drug release | Tannic acid (TA), polyvinyl alcohol (PVA), cerium dioxide (CeO2) nanoenzymes, phenylborate crosslinker | 1. HAP-PVA/Reg3α hydrogel degrades and releases the drug in a high hydrogen peroxide (H2O2) environment; 2. PPBA-TA-PVA hydrogel cascade scavenges ROS | Dual function: responsive drug release + direct removal of oxidative stress to improve the wound microenvironment | [41,42,43,44] |
| Glucose Response | 1. Glucose oxidase (GOx) catalyzes the production of gluconic acid and H2O2 from glucose; 2. Concanavalin A (ConA) binds competitively to glucose; 3. Phenylboronic acid (PBA) reversibly binds to glucose | GOx, ConA, PBA | 1. GOx hydrogel triggers insulin release via pH/ROS changes; 2. PBA hydrogel binds glucose to regulate solubility | Real-time monitoring of blood glucose levels, on-demand drug release, can be integrated with other response mechanisms to build a multi-functional system | [45,46,47] |
| Sensor Type | Principle | Composition | Preparation Techniques | Advantage | Limitations | Refs. |
|---|---|---|---|---|---|---|
| PC | Structural color changes due to Bragg diffraction | Hydrogel + PBA/AFPBA + nanostructures | Nano self-assembly, template imprinting, in situ polymerization | Visual color change, reusable, microneedle integration | Slow response (minutes), visible light observation required | [90,91] |
| Luminous | Change in fluorophore signal (intensity/wavelength) | Hydrogel + fluorophore (NDs/QDs etc.) + enzyme/aptamer/PBA | Hydrogel polymerization of doped fluorophores, enzyme fixation | Continuous monitoring, biocompatibility, high specificity, self-healing capability | An external light source is required for excitation, and long-term stability needs to be improved | [87] |
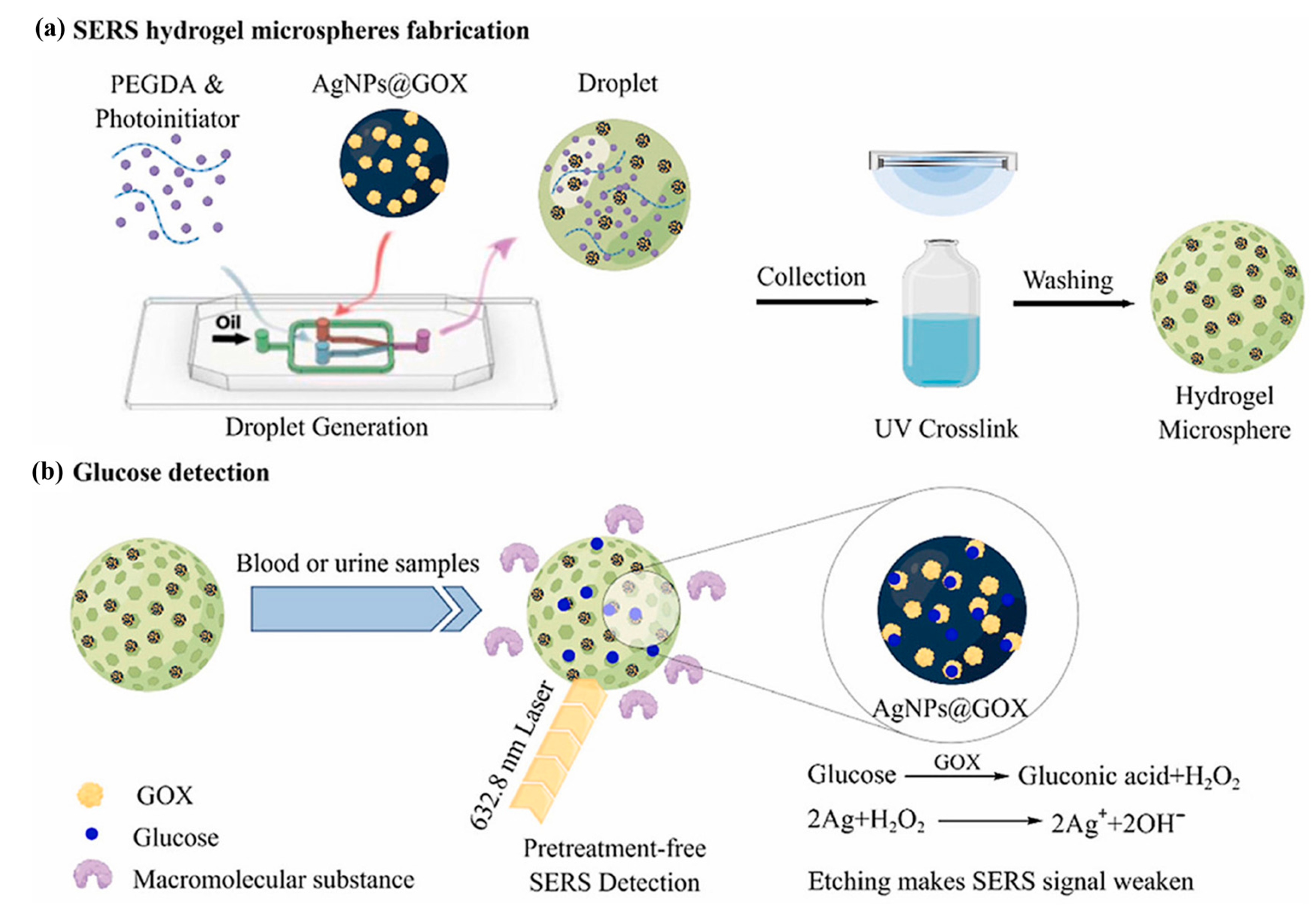
| SERS | Precious metal nanoparticles enhance Raman scattering signals | Hydrogel + Ag/Au nanoparticles | Nanoparticle integration into hydrogel microspheres/substrates | Ultra-high sensitivity, possible pre-process-free detection | High cost and signal stability are affected by the environment | [95,98] |
| LSPR | Localized surface plasmon resonance effects on noble metal nanoparticles | Hydrogel + PBA + Au nanoparticles | Covalent immobilization of nanoparticles, fibre tip polymerization | Fast response (seconds), quantitative monitoring, flexible, high sensitivity | Dependent on nanoparticle uniformity, it requires precision optical inspection equipment | [86] |
| Holographic | Holographic grating period change induces reflection wavelength change | Hydrogel + PBA + dual photopolymerization layer | Preparation of periodic hydrogel films by two-photopolymerization | Reusable, adjustable sensitivity, instant visualization of readings | Temperature-dependent response time, complex preparation process | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Wei, Y.; Xu, K. Hydrogel-Based Treatment of Diabetic Wounds: From Smart Responsive to Smart Monitoring. Gels 2025, 11, 647. https://doi.org/10.3390/gels11080647
He X, Wei Y, Xu K. Hydrogel-Based Treatment of Diabetic Wounds: From Smart Responsive to Smart Monitoring. Gels. 2025; 11(8):647. https://doi.org/10.3390/gels11080647
Chicago/Turabian StyleHe, Xinghan, Yongyi Wei, and Ke Xu. 2025. "Hydrogel-Based Treatment of Diabetic Wounds: From Smart Responsive to Smart Monitoring" Gels 11, no. 8: 647. https://doi.org/10.3390/gels11080647
APA StyleHe, X., Wei, Y., & Xu, K. (2025). Hydrogel-Based Treatment of Diabetic Wounds: From Smart Responsive to Smart Monitoring. Gels, 11(8), 647. https://doi.org/10.3390/gels11080647







