A Stability Study of [Cu(I)(dmby)2]TFSI in Biopolymer-Based Aqueous Quasi-Solid Electrolytes
Abstract
1. Introduction
2. Results and Discussion
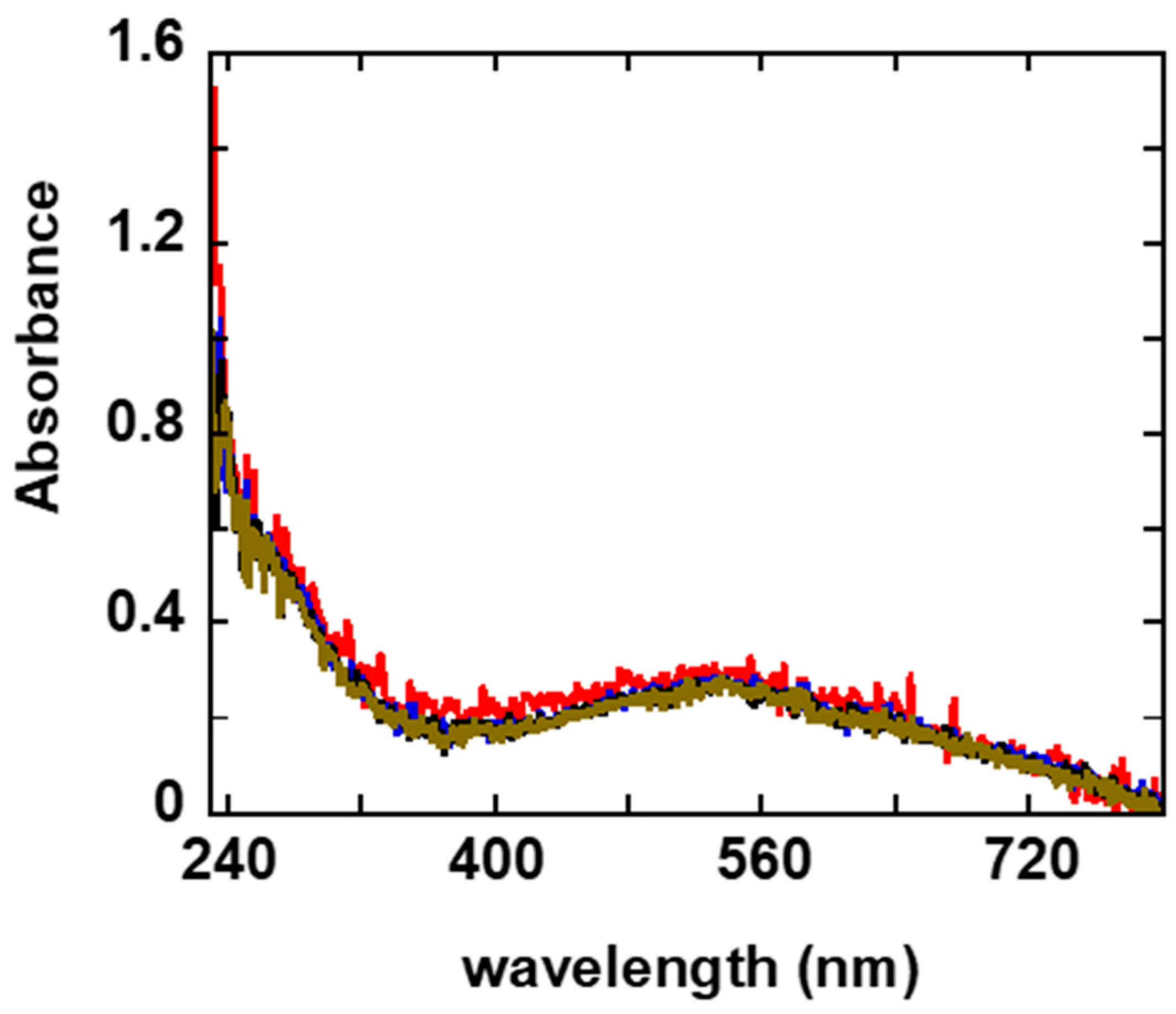
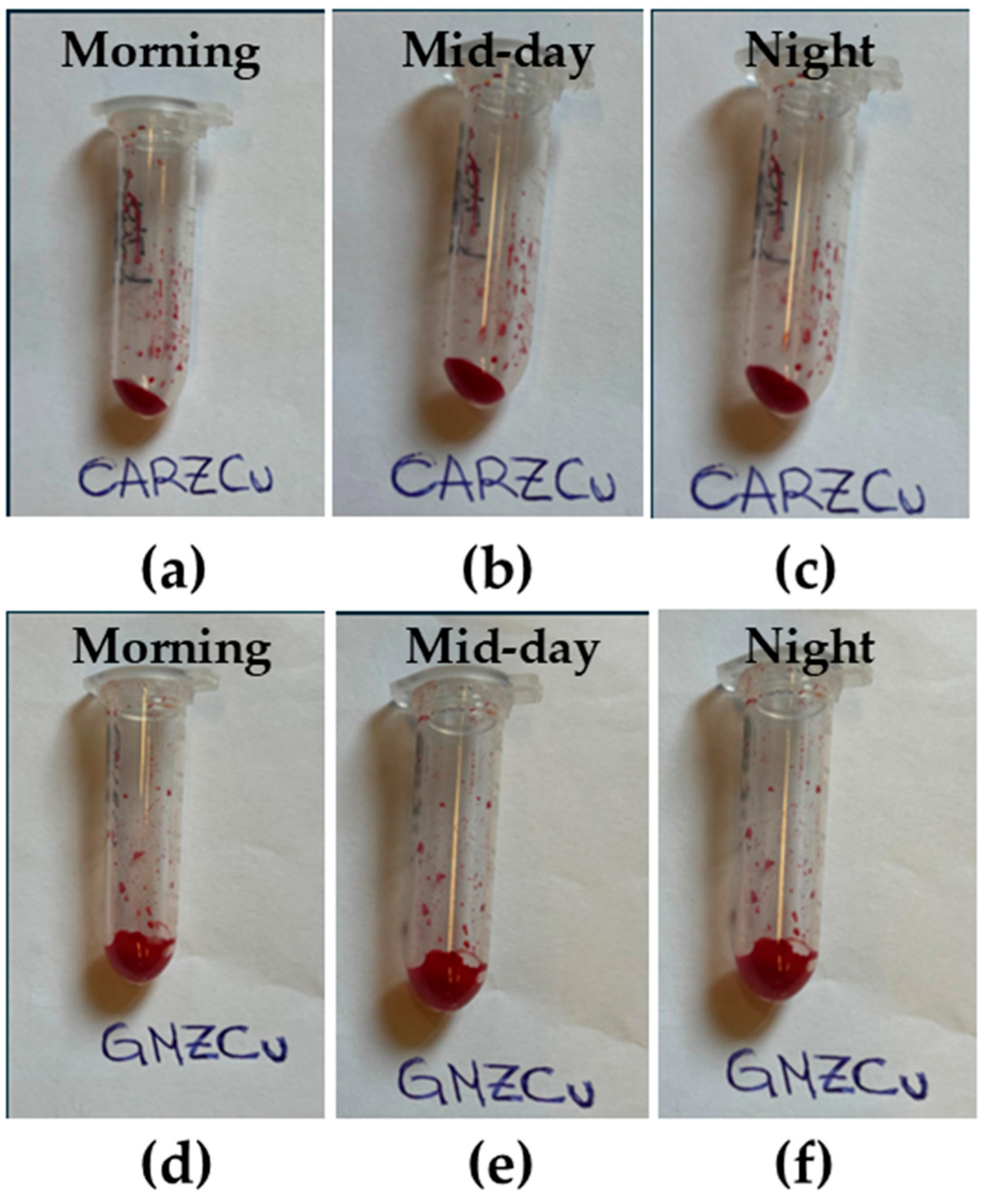
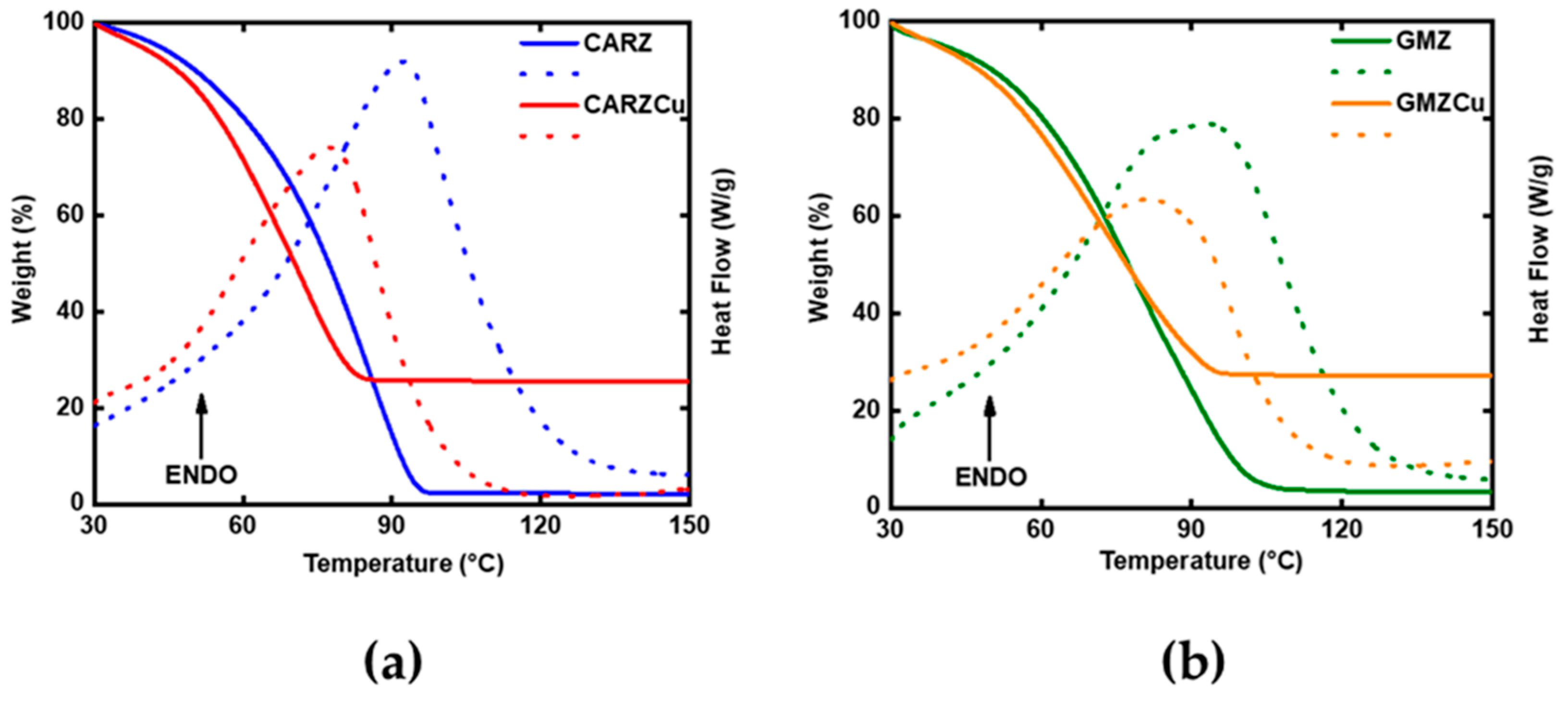
2.1. Temporal and Thermal Stability
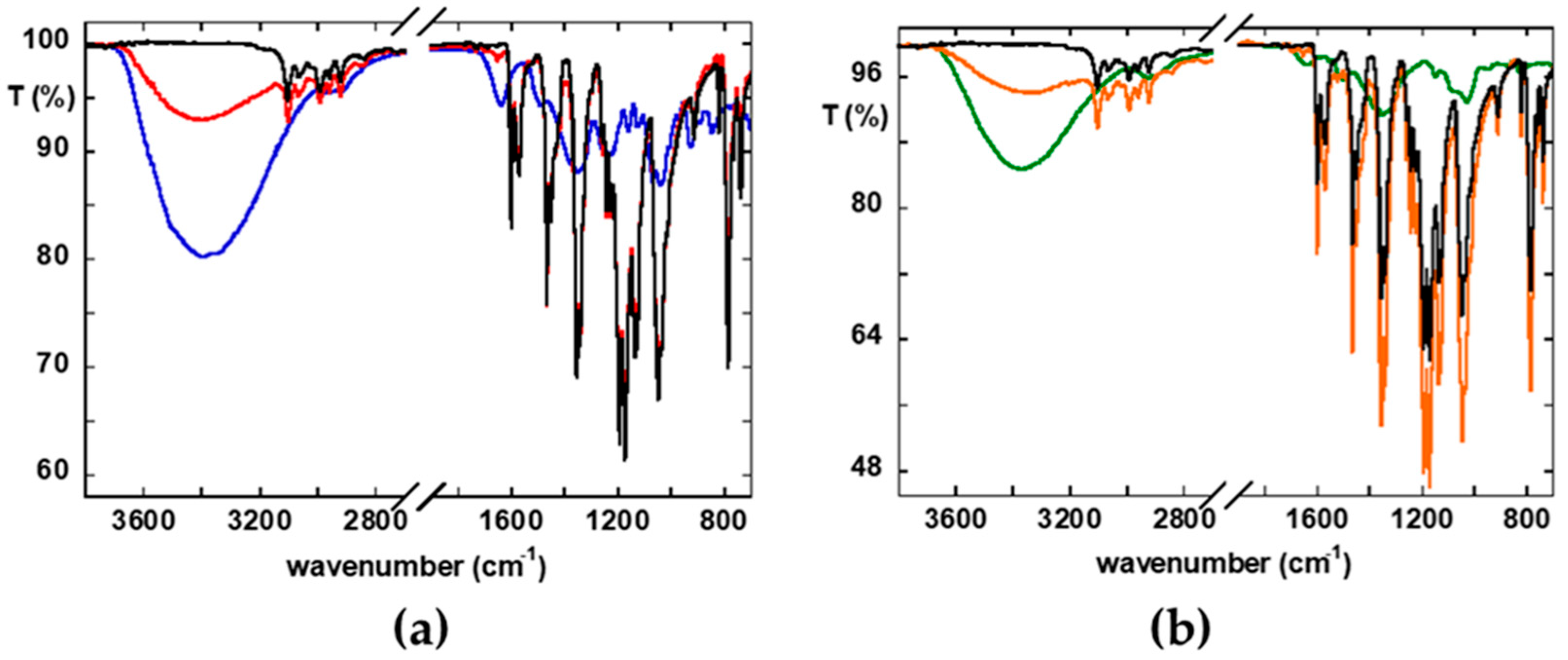
2.2. Characterization
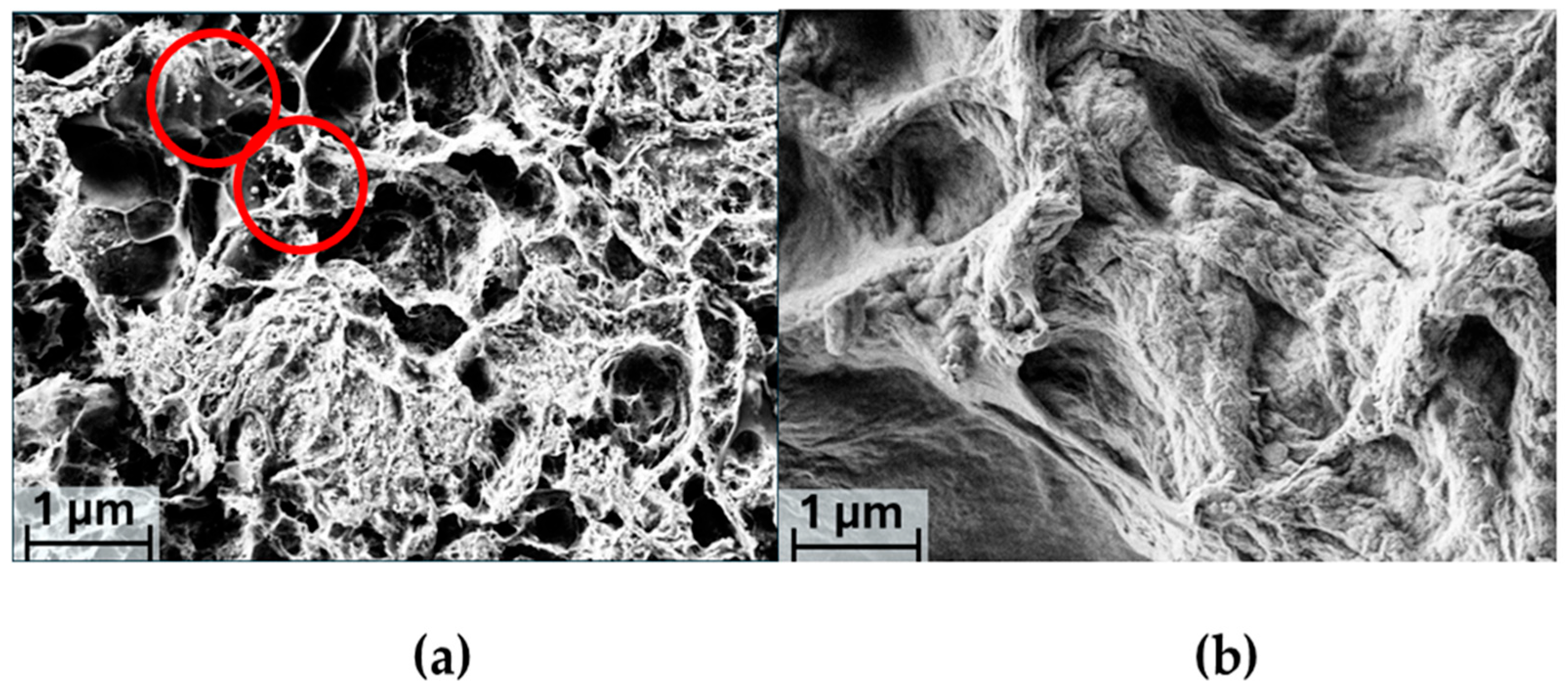
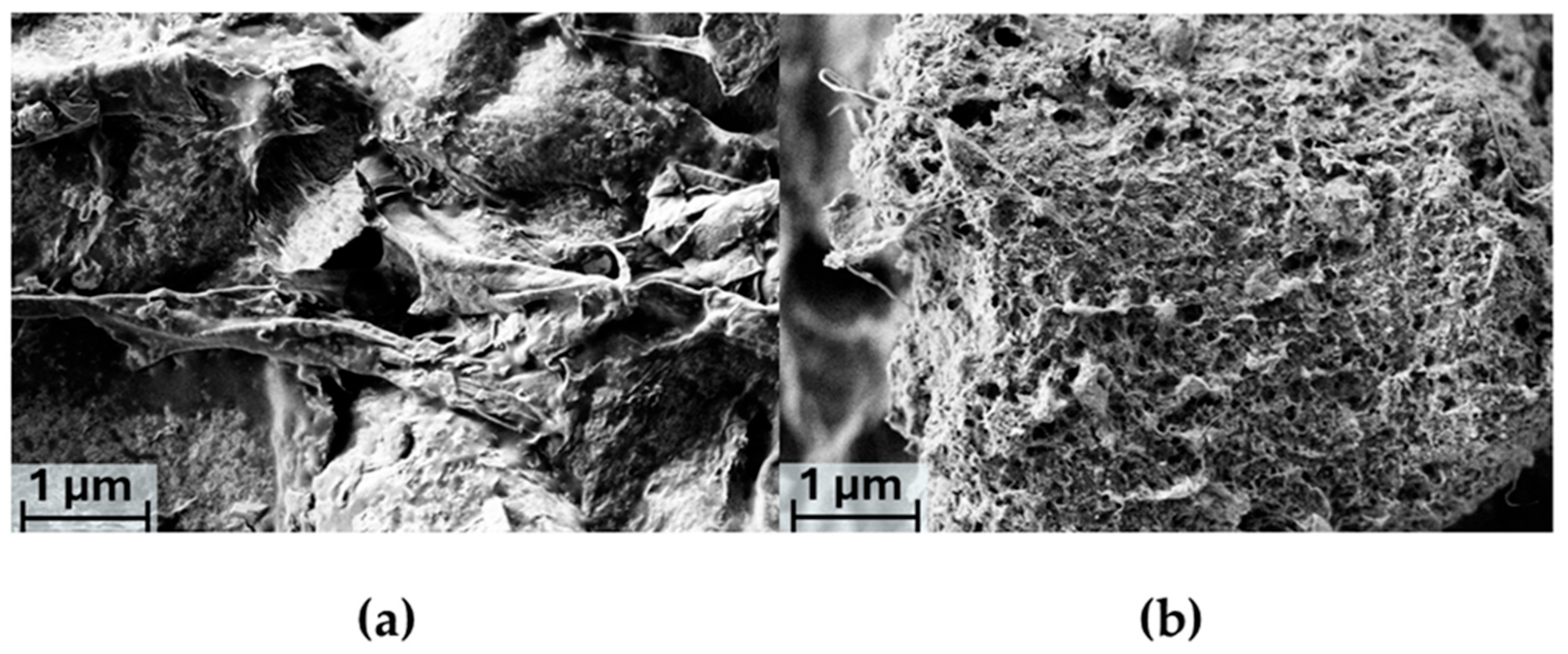
2.3. SEM Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Gels Synthesis
4.3. [Cu(I)(dmby)2]TFSI Incorporation
4.4. Temporal and Thermal Stability Analysis
4.5. Structural and Morphological Characterization
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, Y.S.; Jin, Q.Q.; Ren, T.Z. Expanded Graphite/Pencil-Lead as Counter Electrode for Dye-Sensitized Solar Cells. Solid State Electron. 2011, 63, 76–82. [Google Scholar] [CrossRef]
- Wu, J.; Lan, Z.; Lin, J.; Huang, M.; Li, P. Effect of Solvents in Liquid Electrolyte on the Photovoltaic Performance of Dye-Sensitized Solar Cells. J. Power Sources 2007, 173, 585–591. [Google Scholar] [CrossRef]
- Mosconi, E.; Selloni, A.; De Angelis, F. Solvent Effects on the Adsorption Geometry and Electronic Structure of Dye-Sensitized TiO 2: A First-Principles Investigation. J. Phys. Chem. C 2012, 116, 5932–5940. [Google Scholar] [CrossRef]
- Keuleers, R.; Desseyn, H.O.; Rousseau, B.; Van Alsenoy, C. Vibrational Analysis of Urea. J. Phys. Chem. A 1999, 103, 4621–4630. [Google Scholar] [CrossRef]
- Wang, P.; Zakeeruddin, S.M.; Moser, J.E.; Nazeeruddin, M.K.; Sekiguchi, T.; Grätzel, M. A Stable Quasi-Solid-State Dye-Sensitized Solar Cell with an Amphiphilic Ruthenium Sensitizer and Polymer Gel Electrolyte. Nat. Mater. 2003, 2, 402–407. [Google Scholar] [CrossRef]
- Venkatesan, S.; Liu, I.P.; Li, C.W.; Tseng-Shan, C.M.; Lee, Y.L. Quasi-Solid-State Dye-Sensitized Solar Cells for Efficient and Stable Power Generation under Room Light Conditions. ACS Sustain. Chem. Eng. 2019, 7, 7403–7411. [Google Scholar] [CrossRef]
- Önen, T.; Karakuş, M.Ö.; Coşkun, R.; Çetin, H. Reaching Stability at DSSCs with New Type Gel Electrolytes. J. Photochem. Photobiol. A Chem. 2019, 385, 112082. [Google Scholar] [CrossRef]
- Storck, J.L.; Grothe, T.; Dotter, M.; Adabra, S.; Surjawidjaja, M.; Brockhagen, B. Long-Term Stability Improvement of Non-Toxic Dye-Sensitized Solar Cells via Poly(Ethylene Oxide) Gel Electrolytes for Future Textile-Based Solar Cells. Polymers 2020, 12, 3035. [Google Scholar] [CrossRef]
- Grätzel, M. Recent Advances in Sensitized Mesoscopic Solar Cells. Acc. Chem. Res. 2009, 42, 1788–1798. [Google Scholar] [CrossRef]
- Imahori, H.; Umeyama, T.; Ito, S. Large π-Aromatic Molecules as Potential Sensitizers for Highly Efficient Dye-Sensitized Solar Cells. Acc. Chem. Res. 2009, 42, 1809–1818. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Hasan, M.M.; Islam, M.D.; Rashid, T.U. Biopolymer-Based Electrolytes for Dye-Sensitized Solar Cells: A Critical Review. Energy Fuels 2020, 34, 15634–15671. [Google Scholar] [CrossRef]
- Liao, K.Y.; Li, W.C.; Wen, T.C. Constructing Supercapacitors with Biopolymer Bearing Zwitterion as Hydrogel Electrolyte and Binder for Superior Performance at −40 °C. J. Power Sources 2024, 598, 234191. [Google Scholar] [CrossRef]
- Cevik, E.; Gunday, S.T.; Bozkurt, A.; Iqbal, A.; Asiri, S.; Alqarni, A.; Almofeh, A. Scalable Quasi-Solid-State Bio-polymer Hydrogel Electrolytes for High-Performance Supercapacitor Application. ACS Sustain. Chem. Eng. 2022, 10, 10839–10848. [Google Scholar] [CrossRef]
- Karakuş, M.Ö.; Yakışıklıer, M.E.; Delibaş, A.; Çetin, H. A Roadmap for Hydrogel-Based Quasi-Solid Electrolyte Preparation for Use in Dye-Sensitized Solar Cell. Electrochim. Acta 2022, 427, 140841. [Google Scholar] [CrossRef]
- Galliano, S.; Bella, F.; Bonomo, M.; Viscardi, G.; Gerbaldi, C.; Boschloo, G.; Barolo, C. Hydrogel Electrolytes Based on Xanthan Gum: Green Route towards Stable Dye-Sensitized Solar Cells. Nanomaterials 2020, 10, 1585. [Google Scholar] [CrossRef] [PubMed]
- Galliano, S.; Bella, F.; Piana, G.; Giacona, G.; Viscardi, G.; Gerbaldi, C.; Grätzel, M.; Barolo, C. Finely Tuning Electrolytes and Photoanodes in Aqueous Solar Cells by Experimental Design. Sol. Energy 2018, 163, 251–255. [Google Scholar] [CrossRef]
- Kandhasamy, M.; Shanmugam, G.; Kamaraj, S.; Selvaraj, B.; Gunasekeran, A.; Sambandam, A. Effect of D-Limonene Additive in Copper Redox-Based Quasi-Solid-State Electrolytes on the Performance of Dye-Sensitized Solar Cells. Mater. Today Commun. 2023, 35, 105505. [Google Scholar] [CrossRef]
- Torres, F.G.; De-la-Torre, G.E. Algal-Based Polysaccharides as Polymer Electrolytes in Modern Electrochemical Energy Conversion and Storage Systems: A Review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100023. [Google Scholar] [CrossRef]
- Torres, F.G.; Arroyo, J.; Alvarez, R.; Rodriguez, S.; Troncoso, O.; López, D. Carboxymethyl κ/ι-Hybrid Carrageenan Doped with NH4I as a Template for Solid Bio-Electrolytes Development. Mater. Chem. Phys. 2019, 223, 659–665. [Google Scholar] [CrossRef]
- Tian, H.; Sun, L. Iodine-Free Redox Couples for Dye-Sensitized Solar Cells. J. Mater. Chem. 2011, 21, 10592–10601. [Google Scholar] [CrossRef]
- Yella, A.; Lee, H.-W.; Nok Tsao, H.; Yi, C.; Kumar Chandiran, A.; Nazeeruddin, M.; Wei-Guang Diau, E.; Yeh, C.-Y.; Zakeeruddin, S.M.; Grätzel, M. Porphyrin-Sensitized Solar Cells with Cobalt (II/III)-Based Redox Electrolyte Exceed 12 Percent Efficiency. Science 2011, 334, 629–634. [Google Scholar] [CrossRef]
- Feldt, S.M.; Gibson, E.A.; Gabrielsson, E.; Sun, L.; Boschloo, G.; Hagfeldt, A. Design of Organic Dyes and Cobalt Polypyridine Redox Mediators for High-Efficiency Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2010, 132, 16714–16724. [Google Scholar] [CrossRef]
- Hattori, S.; Wada, Y.; Yanagida, S.; Fukuzumi, S. Blue Copper Model Complexes with Distorted Tetragonal Geometry Acting as Effective Electron-Transfer Mediators in Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2005, 127, 9648–9654. [Google Scholar] [CrossRef]
- Law, C.; Pathirana, S.C.; Li, X.; Anderson, A.Y.; Barnes, P.R.F.; Listorti, A.; Ghaddar, T.H.; ORegan, B.C. Water-Based Electrolytes for Dye-Sensitized Solar Cells. Adv. Mater. 2010, 22, 4505–4509. [Google Scholar] [CrossRef]
- Saygili, Y.; Söderberg, M.; Pellet, N.; Giordano, F.; Cao, Y.; Munoz-García, A.B.; Zakeeruddin, S.M.; Vlachopoulos, N.; Pavone, M.; Boschloo, G.; et al. Copper Bipyridyl Redox Mediators for Dye-Sensitized Solar Cells with High Photovoltage. J. Am. Chem. Soc. 2016, 138, 15087–15096. [Google Scholar] [CrossRef]
- Segura Zarate, A.Y.; Gontrani, L.; Galliano, S.; Bauer, E.M.; Donia, D.T.; Barolo, C.; Bonomo, M.; Carbone, M. Green Zinc/Galactomannan-Based Hydrogels Push up the Photovoltage of Quasi Solid Aqueous Dye Sensitized Solar Cells. Sol. Energy 2024, 272, 112460. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.W. Properties and Characterization of Bionanocomposite Films Prepared with Various Biopolymers and ZnO Nanoparticles. Carbohydr. Polym. 2014, 106, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Bouttier-Figueroa, D.C.; Sotelo-Lerma, M. Fabrication and Characterization of an Eco-Friendly Antibacterial Nanocomposite of Galactomannan/ZnO by in Situ Chemical Co-Precipitation Method. Compos. Interfaces 2019, 26, 83–95. [Google Scholar] [CrossRef]
- Chang, W.C.; Sie, S.Y.; Yu, W.C.; Lin, L.Y.; Yu, Y.J. Preparation of Nano-Composite Gel Electrolytes with Metal Oxide Additives for Dye-Sensitized Solar Cells. Electrochim. Acta 2016, 212, 333–342. [Google Scholar] [CrossRef]
- Spadaro, D.; Barichello, J.; Citro, I.; Calogero, G. Environmentally Friendly Water-Based Electrolyte for Dye-Sensitized Solar Cells: Future Prospective and Outlook. Solar 2023, 3, 229–252. [Google Scholar] [CrossRef]
- Conradie, J. Polypyridyl Copper Complexes as Dye Sensitizer and Redox Mediator for Dye-Sensitized Solar Cells. Electrochem. Commun. 2022, 134, 107182. [Google Scholar] [CrossRef]
- Masud; Zhou, H.; Kim, H.K. Effective Redox Shuttles for Polymer Gel Electrolytes-Based Quasi-Solid-State Dye-Sensitized Solar Cells in Outdoor and Indoor Applications: Comprehensive Comparison and Guidelines. Mater. Today Energy 2023, 34, 101299. [Google Scholar] [CrossRef]
- Wong, X.W.; Lee, Y.H.; Venkatesan, S.; Ho, K.C.; Teng, H.; Lee, Y.L. Quasi-Solid-State Dye-Sensitized Solar Cells with over 36% Efficiency Achieved by a Direct-Contact Cell Structure and In Situ Gelation of Copper-Based Electrolytes. ACS Sustain. Chem. Eng. 2025, 13, 3432–3440. [Google Scholar] [CrossRef]
- Wardhana, Y.W.; Aanisah, N.; Sopyan, I.; Hendriani, R.; Chaerunisaa, A.Y. Gelling Power Alteration on Kappa-Carrageenan Dispersion through Esterification Method with Different Fatty Acid Saturation. Gels 2022, 8, 752. [Google Scholar] [CrossRef] [PubMed]
- Chrissafis, K.; Lalia-Kantouri, M.; Aslanidis, P. Kinetic Analysis of the Thermal Decomposition of Copper (I) Complexes with Heterocyclic Thiones. J. Therm. Anal. Calorim. 2011, 104, 1045–1050. [Google Scholar] [CrossRef]
- Popescu, E.C.; Boscornea, C. Structure and Properties of Carragenan. Polym. Chem. 2007, 2007, 27–32. [Google Scholar]
- Shibayama, M.; Mizutani, S.-Y.; Nomura, S. Thermal Properties of Copolymer Gels Containing N-Isopropylacrylamide. Macromolecules 1996, 29, 2019–2024. [Google Scholar] [CrossRef]
- Helen, P.A.; Selvin, P.C.; Sakthivel, P. Quasi-Solid-State Plasticized Chitosan Biopolymer Electrolyte with Enhanced Mg2+ Ion Mobility for next-Generation Mg Ion Battery. J. Solid. State Electrochem. 2024, 28, 3147–3161. [Google Scholar] [CrossRef]
- Kandhasamy, M.; Shanmugam, G.; Duvaragan, B.K.; Kamaraj, S. Unleashing the Potential of Cu1+/2+ Blended Quasi-Solid-State Electrolytes for Long-Term Stability of Dye-Sensitized Solar Cells. Sol. Energy 2024, 277, 112733. [Google Scholar] [CrossRef]
- Seyfi, S.; Alizadeh, R.; Ganji, M.D.; Amani, V. Molecular, Electronic Structure and Spectroscopic Properties of 6,6′-Dimethyl-2,2′-Bipyridine and HgI2 Complex: Experimental and DFT Investigations. Vacuum 2017, 139, 9–22. [Google Scholar] [CrossRef]
- Lin, J.; Qin, B.; Fang, Z. Nickel Bipyridine (Ni(bpy)3Cl2) Complex Used as Molecular Catalyst for Photocatalytic CO2 Reduction. Catal. Lett. 2019, 149, 25–33. [Google Scholar] [CrossRef]
- Rey, I.; Johansson, P.; Lindgren, J.; Lassè, J.C.; Grondin, J.; Servant, L. Spectroscopic and Theoretical Study of (CF3SO2)2N- (TFSI-) and (CF3SO2)2NH (HTFSI). J. Phys. Chem. A 1998, 102, 3249–3258. [Google Scholar] [CrossRef]
- Tranquilan-Aranilla, C.; Nagasawa, N.; Bayquen, A.; Dela Rosa, A. Synthesis and Characterization of Carboxymethyl Derivatives of Kappa-Carrageenan. Carbohydr. Polym. 2012, 87, 1810–1816. [Google Scholar] [CrossRef]
- Munshi, M.U.; Martens, J.; Berden, G.; Oomens, J. Gas-Phase Infrared Ion Spectroscopy Characterization of Cu(II/I)Cyclam and Cu(II/I)2,2′-Bipyridine Redox Pairs. J. Phys. Chem. A 2019, 123, 4149–4157. [Google Scholar] [CrossRef]
- Millane, R.P.; Chandrasekaran, R.; Arnott, S. The molecular structure of kappa-carrageenan and comparison with iota-carrageenan. Carbohydr. Res. 1988, 182, 1–17. [Google Scholar] [CrossRef]
- Hennink, W.E.; Van Nostrum, C.F. Novel Crosslinking Methods to Design Hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Mihaila, S.M.; Gaharwar, A.K.; Reis, R.L.; Marques, A.P.; Gomes, M.E.; Khademhosseini, A. Photocrosslinkable Kappa-Carrageenan Hydrogels for Tissue Engineering Applications. Adv. Healthc. Mater. 2013, 2, 895–907. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Malyar, Y.N.; Ghatfaoui, S.; Issaoui, N.; Al-Dossary, O.; Wojcik, M.J.; Kazachenko, A.S.; Miroshnikova, A.V.; Berezhnaya, Y.D. A Density Functional Theory Calculations of Infrared Spectra of Galactomannan Butyl Ether. J. Mol. Struct. 2022, 1251, 131998. [Google Scholar] [CrossRef]
- Shimizu, G.; Vaidhyanathan, R.; Taylor, J. Phosphonate and Sulfonate Metal Organic Frameworks. Chem. Soc. Rev. 2009, 38, 1430–1449. [Google Scholar] [CrossRef]
- Zheng, Z.; Xu, P.; Jiang, Y.; Liang, Y.J.; Li, J.X. “Soft–hard” strategy to construct a pyrazine sulfonic acid copper(II) supramolecular structure and a study of its fluorescent property. J. Struct. Chem. 2021, 62, 292–299. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; da Silva, D.B.; Carvalho, I. Carrageenans: Biological Properties, Chemical Modifications and Structural Analysis—A Review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Liu, F.X.; Jing, X.; Yang, J.; Mi, H.Y.; Feng, F.Y.; Liu, Y.J. Recent Progress in Low Hysteresis Gels: Strategies, Applications, and Challenges. Nano Today 2025, 61, 102601. [Google Scholar] [CrossRef]
- Song, B.-K. Molecular Architecture of Galactomannans. Ph.D. Thesis, Polytechnic University, New York, NY, USA, 1987. Available online: https://www.proquest.com/docview/2885585093?pq-origsite=gscholar&fromopenview=true&sourcetype=Dissertations%20&%20Theses (accessed on 10 August 2025).
- Yang, Z.; Yang, H.; Yang, H. Characterisation of Rheology and Microstructures of κ-Carrageenan in Ethanol-Water Mixtures. Food Res. Int. 2018, 107, 738–746. [Google Scholar] [CrossRef]
- Crumbliss, A.L.; Perine, S.C.; Edwards, A.K.; Rillema, D.P. Characterization of Carrageenan Hydrogel Electrode Coating with Immobillzed Cationic Metal Complex Redox Couples. J. Phys. Chem. 1992, 96, 1388–1394. [Google Scholar] [CrossRef]
- Kavan, L.; Saygili, Y.; Freitag, M.; Zakeeruddin, S.M.; Hagfeldt, A.; Grätzel, M. Electrochemical Properties of Cu(II/I)-Based Redox Mediators for Dye-Sensitized Solar Cells. Electrochim. Acta 2017, 227, 194–202. [Google Scholar] [CrossRef]
| Sample Label | ZnO Concentration | GM% | CAR% |
|---|---|---|---|
| CARZ | 0.025 M | X | 1 |
| GMZ | 0.025 M | 1 | X |
| Sample Label | ZnO Concentration | GM% | CAR% | Cu Complex |
|---|---|---|---|---|
| CARZCu | 0.025 M | X | 1 | 0.07 g |
| GMZCu | 0.025 M | 1 | X | 0.07 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bracchini, G.A.; Bauer, E.M.; Mazzuca, C.; Carbone, M. A Stability Study of [Cu(I)(dmby)2]TFSI in Biopolymer-Based Aqueous Quasi-Solid Electrolytes. Gels 2025, 11, 645. https://doi.org/10.3390/gels11080645
Bracchini GA, Bauer EM, Mazzuca C, Carbone M. A Stability Study of [Cu(I)(dmby)2]TFSI in Biopolymer-Based Aqueous Quasi-Solid Electrolytes. Gels. 2025; 11(8):645. https://doi.org/10.3390/gels11080645
Chicago/Turabian StyleBracchini, Giulia Adriana, Elvira Maria Bauer, Claudia Mazzuca, and Marilena Carbone. 2025. "A Stability Study of [Cu(I)(dmby)2]TFSI in Biopolymer-Based Aqueous Quasi-Solid Electrolytes" Gels 11, no. 8: 645. https://doi.org/10.3390/gels11080645
APA StyleBracchini, G. A., Bauer, E. M., Mazzuca, C., & Carbone, M. (2025). A Stability Study of [Cu(I)(dmby)2]TFSI in Biopolymer-Based Aqueous Quasi-Solid Electrolytes. Gels, 11(8), 645. https://doi.org/10.3390/gels11080645










