Phase-Conversion Stiffened Dual-Network Hydrogel for Fracture Plugging in Oil-Based Drilling Fluid
Abstract
1. Introduction
2. Results and Discussion
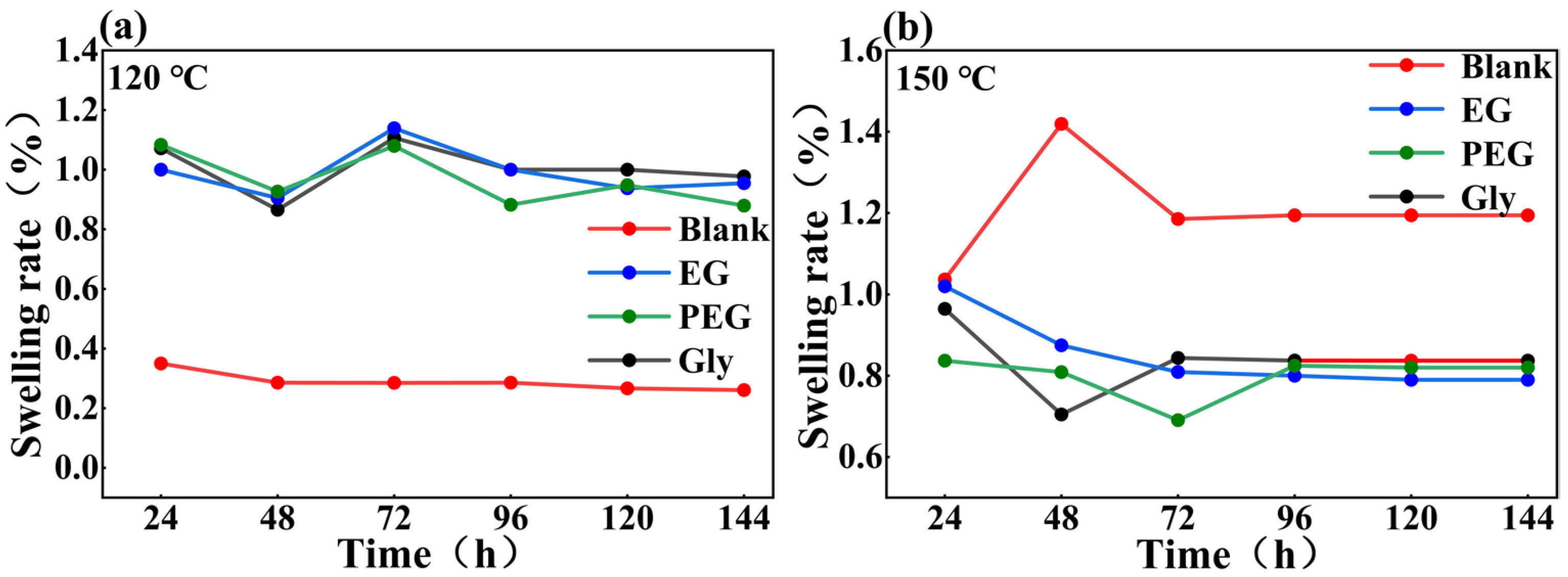
2.1. Swelling Property
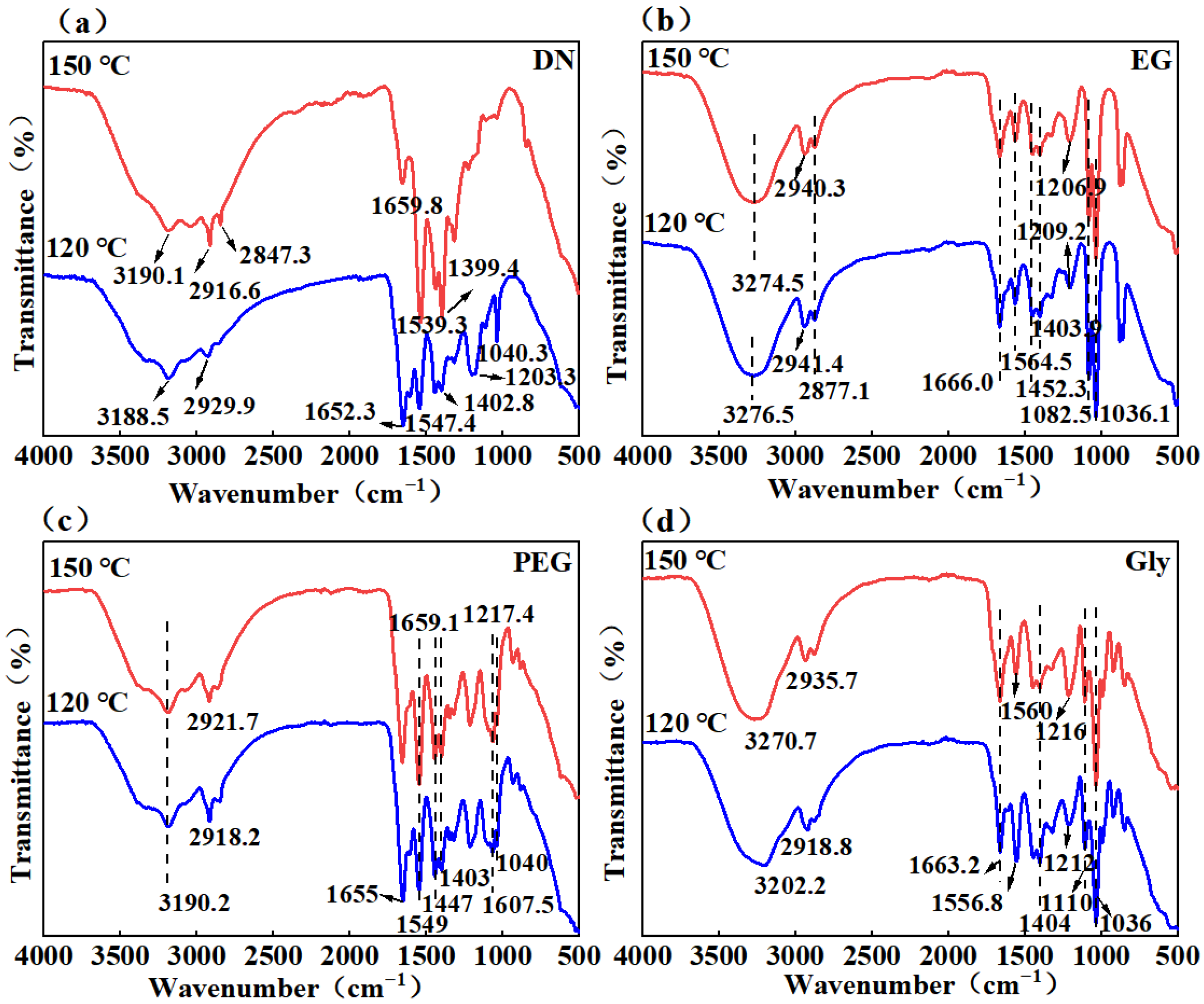
2.2. ATR-FTIR Spectra Analysis
2.3. Thermal Stability of Hydrogels
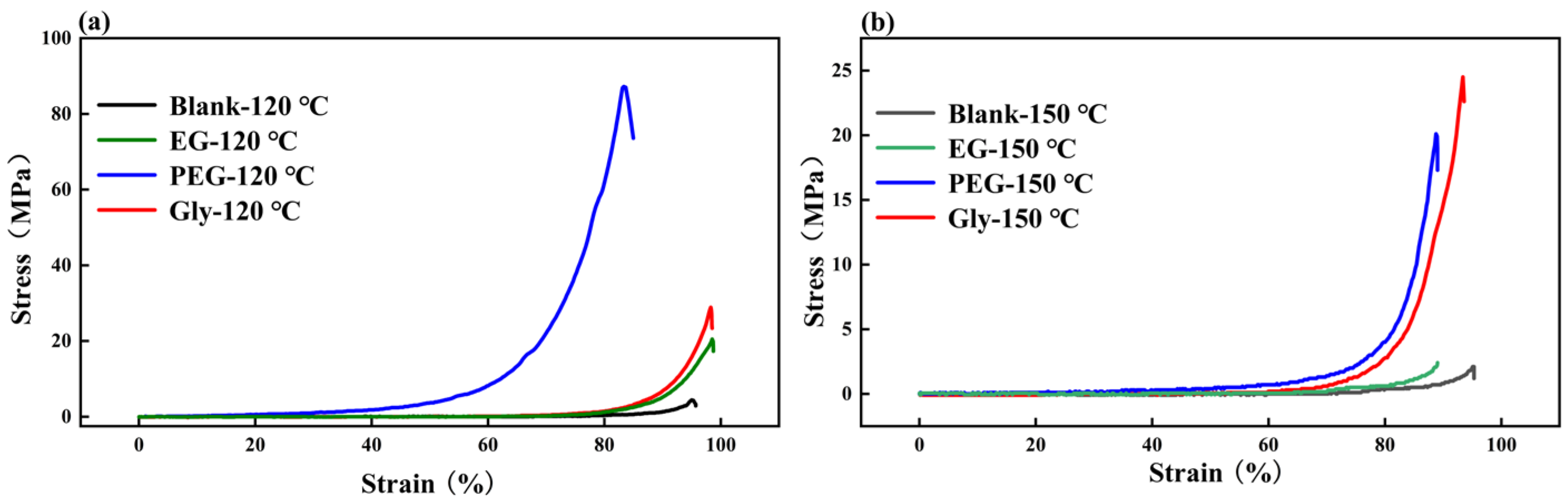
2.4. Compression Strength and Breakthrough Pressure Test
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Development of Dual-Network Hydrogel
4.3. Phase-Conversion Induction and Characterization of Gel Samples
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sayindla, S.; Lund, B.; Ytrehus, J.D.; Saasen, A. Hole-cleaning performance comparison of oil-based and water-based drilling fluids. J. Pet. Sci. Eng. 2017, 159, 49–57. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, J.; Liu, F.; Cheng, R.; Bai, Y.; Wang, R.; Wang, J.; Geng, Y.; Jin, Y.; Ma, Z. Micro-nano polymer microspheres as a plugging agent in oil-based drilling fluid. Colloids Surf. A Physicochem. Eng. Asp. 2023, 673, 131808. [Google Scholar] [CrossRef]
- Ma, B.; Wang, R.; Ni, H.; Wang, K. Experimental study on harmless disposal of waste oil based mud using supercritical carbon dioxide extraction. Fuel 2019, 252, 722–729. [Google Scholar] [CrossRef]
- Rana, A.; Khan, I.; Saleh, T.A. Advances in Carbon Nanostructures and Nanocellulose as Additives for Efficient Drilling Fluids: Trends and Future Perspective—A Review. Energy Fuels 2021, 35, 7319–7339. [Google Scholar] [CrossRef]
- Yu, H.; Zhao, Z.; Taleghani, A.D.; Lian, Z.; Zhang, Q. Modeling thermal-induced wellhead growth through the lifecycle of a well. Geoenergy Sci. Eng. 2024, 241, 213098. [Google Scholar] [CrossRef]
- Deng, R.; Dong, J.; Dang, L. Numerical simulation and evaluation of residual oil saturation in waterflooded reservoirs. Fuel 2025, 384, 134018. [Google Scholar] [CrossRef]
- Mirabbasi, S.M.; Ameri, M.J.; Alsaba, M.; Karami, M.; Zargarbashi, A. The evolution of lost circulation prevention and mitigation based on wellbore strengthening theory: A review on experimental issues. J. Pet. Sci. Eng. 2022, 211, 110149. [Google Scholar] [CrossRef]
- Pu, L.; Xu, P.; Xu, M.; Song, J.; He, M. Lost circulation materials for deep and ultra-deep wells: A review. J. Pet. Sci. Eng. 2022, 214, 110404. [Google Scholar] [CrossRef]
- Jia, H.; Wu, J.; Wu, S.; Liang, Y.; Wang, M.; Wan, X.; Li, P. New insights into the DPR mechanism of elastic energy released by polymer gel for enhanced oil recovery. Petroleum 2024, 10, 539–547. [Google Scholar] [CrossRef]
- Xiong, Z.; Fu, F.; Zou, Z.; Li, X.; Tao, S.; Li, Y. Development and application of guar gum crosslinked gel with adjustable gelation time for total loss treatment. Petroleum 2023, 9, 621–628. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Fu, Y.; Wang, N.; Liu, Q.; Zhao, S.; Yang, H.Y.; Liu, C. Fabrication of temperature and pH dual-sensitive semi-interpenetrating network hydrogel with enhanced adhesion and antibacterial properties. Polymer 2025, 326, 128343. [Google Scholar] [CrossRef]
- Ayari, M.; Osfouri, S.; Azin, R.; Rostami, A. An environmentally friendly and low-cost alginate-based gel for water management in petroleum reservoirs: Characterization and efficacy investigation. Petroleum 2025, 11, 248–258. [Google Scholar] [CrossRef]
- Bai, Y.-R.; Dai, L.-Y.; Sun, J.-S.; Jiang, G.-C.; Lv, K.-H.; Cheng, R.-C.; Shang, X.-S. Plugging performance and mechanism of an oil-absorbing gel for lost circulation control while drilling in fractured formations. Pet. Sci. 2022, 19, 2941–2958. [Google Scholar] [CrossRef]
- Du, L.; Xiao, Y.-Y.; Jiang, Z.-C.; Xu, H.; Zeng, H.; Li, H. A high temperature-resistant, strong, and self-healing double-network hydrogel for profile control in oil recovery. J. Colloid Interface Sci. 2025, 679, 490–502. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, X.; Li, L.; Zhang, J.; Shi, X.; Hu, G. Research Progress of High-Temperature Resistant Functional Gel Materials and Their Application in Oil and Gas Drilling. Gels 2022, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ma, J.; Chang, X.; Wu, Y.; Jiang, G.; Qiu, S.; Moukoko, A.D.K. Novel polymeric organic gelator as lost circulation material for oil-based drilling fluids. Geoenergy Sci. Eng. 2023, 231, 212414. [Google Scholar] [CrossRef]
- Tavakoli, P.; Shadizadeh, S.R.; Hayati, F.; Fattahi, M. Effects of synthesized nanoparticles and Henna-Tragacanth solutions on oil/water interfacial tension: Nanofluids stability considerations. Petroleum 2020, 6, 293–303. [Google Scholar] [CrossRef]
- Fuchs, S.; Shariati, K.; Ma, M. Specialty Tough Hydrogels and Their Biomedical Applications. Adv. Healthc. Mater. 2019, 9, 1901396. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Wang, C.; Sun, J.; Long, Y.; Wang, R.; Qu, Y.; Peng, L.; Ren, H.; Gao, S. A re-crosslinkable composite gel based on curdlan for lost circulation control. J. Mol. Liq. 2023, 371, 121010. [Google Scholar] [CrossRef]
- Wang, Z.; Qiu, W.; Zhang, Q. Constructing phase separation in polymer gels: Strategies, functions and applications. Prog. Polym. Sci. 2024, 154. [Google Scholar] [CrossRef]
- Cui, W.; Cai, Y.; Zheng, Y.; Ran, R. Mechanical enhancement of hydrophobically associating hydrogels by solvent-regulated phase separation. Polymer 2020, 210, 123042. [Google Scholar] [CrossRef]
- Wang, D.-M.; Venault, A.; Lai, J.-Y. Fundamentals of nonsolvent-induced phase separation of Chapter 2. In Hollow Fiber Membranes: Fabrication and Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 13–56. [Google Scholar] [CrossRef]
- Hua, M.; Wu, S.; Ma, Y.; Zhao, Y.; Chen, Z.; Frenkel, I.; Strzalka, J.; Zhou, H.; Zhu, X.; He, X. Strong tough hydrogels via the synergy of freeze-casting and salting out. Nature 2021, 590, 594–599. [Google Scholar] [CrossRef]
- Zheng, X.; Duan, Z.; Zhuang, Y.; Zhang, S.; Cui, X.; Qin, D. Application of reagent-Assisted Dual-Network Hydrogel in Water-Based Drilling Fluid for Lost Circulation Treatment in Fractured Formation. ACS Omega 2023, 9, 1166–1173. [Google Scholar] [CrossRef]
- Li, X.; Cui, K.; Sun, T.L.; Meng, L.; Yu, C.; Li, L.; Creton, C.; Kurokawa, T.; Gong, J.P. Mesoscale bicontinuous networks in self-healing hydrogels delay fatigue fracture. Proc. Natl. Acad. Sci. USA 2020, 117, 7606–7612. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H.; Li, D.; Long, S.; Zhang, G.; Wu, Z. Dual Ionically Cross-linked Double-Network Hydrogels with High Strength, Toughness, Swelling Resistance, and Improved 3D Printing Processability. ACS Appl. Mater. Interfaces 2018, 10, 31198–31207. [Google Scholar] [CrossRef]
- Liang, M.; Ge, X.; Dai, J.; Ren, P.; Wei, D.; Xu, L.; Zhang, Q.; He, C.; Lu, Z.; Zhang, T. High-Strength Hydrogel Adhesive Formed via Multiple Interactions for Persistent Adhesion under Saline. ACS Appl. Bio Mater. 2021, 4, 5016–5025. [Google Scholar] [CrossRef]
- Savari, S.; Whitfill, D.L.; Jamison, D.E.; Kumar, A. A Method to Evaluate Lost Circulation MaterialsInvestigation of Effective Wellbore Strengthening Applications. SPE Drill. Complet. 2014, 29, 329–333. [Google Scholar] [CrossRef]




| Sample | Stage | Tonset (℃) | Tpeak (℃) | Tendset (℃) | W (%) | Residue (%) |
|---|---|---|---|---|---|---|
| DN-120 °C | I | 40 | 245.62 | 273.33 | 21 | 23.54 |
| II | 273.33 | 320.94 | 350 | 14.06 | ||
| III | 350 | 428.99 | 468 | 41.4 | ||
| DN-150 °C | I | 40 | 258.74 | 294.6 | 33.9 | 18.06 |
| II | 294.6 | 322.03 | 359.1 | 11 | ||
| III | 359.12 | 427.39 | 503.3 | 37.04 | ||
| EG-120 °C | I | 40 | 144.31 | 266.8 | 55 | 21.29 |
| II | 266.8 | 283.61 | 329 | 6.7 | ||
| III | 329 | 391.41 | 487 | 17.01 | ||
| EG-150 °C | I | 40 | 138.71 | 271 | 74 | 7.20 |
| II | 271 | 282.21 | 332 | 5.8 | ||
| III | 332 | 389.31 | 471 | 13 | ||
| PEG-120 °C | I | 40 | 238.35 | 286 | 27 | 16.27 |
| II | 286 | 320.74 | 363 | 23 | ||
| III | 363 | 417.09 | 529 | 33.73 | ||
| PEG-150 °C | I | 40 | 240.14 | 289 | 28 | 16.26 |
| II | 289 | 317.23 | 366 | 21 | ||
| III | 366 | 421.34 | 559 | 34.74 | ||
| GLY-120 °C | I | 40 | 186.32 | 269 | 45 | 12.05 |
| II | 269 | 289.92 | 351 | 20.05 | ||
| III | 351 | 388.62 | 480 | 22.9 | ||
| GLY-150 °C | I | 40 | 207.12 | 270 | 43 | 12.04 |
| II | 270 | 289.72 | 354 | 22.06 | ||
| III | 354 | 387.72 | 468 | 22.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, X.; Wang, C.; Huang, W.; Zhang, S.; Chen, H.; Wu, B. Phase-Conversion Stiffened Dual-Network Hydrogel for Fracture Plugging in Oil-Based Drilling Fluid. Gels 2025, 11, 635. https://doi.org/10.3390/gels11080635
Cui X, Wang C, Huang W, Zhang S, Chen H, Wu B. Phase-Conversion Stiffened Dual-Network Hydrogel for Fracture Plugging in Oil-Based Drilling Fluid. Gels. 2025; 11(8):635. https://doi.org/10.3390/gels11080635
Chicago/Turabian StyleCui, Xinying, Chengwen Wang, Weian Huang, Shifeng Zhang, Haiqun Chen, and Bo Wu. 2025. "Phase-Conversion Stiffened Dual-Network Hydrogel for Fracture Plugging in Oil-Based Drilling Fluid" Gels 11, no. 8: 635. https://doi.org/10.3390/gels11080635
APA StyleCui, X., Wang, C., Huang, W., Zhang, S., Chen, H., & Wu, B. (2025). Phase-Conversion Stiffened Dual-Network Hydrogel for Fracture Plugging in Oil-Based Drilling Fluid. Gels, 11(8), 635. https://doi.org/10.3390/gels11080635





