Tuning Nanostructure of Gels: From Structural and Functional Controls to Food Applications
Abstract
1. Introduction
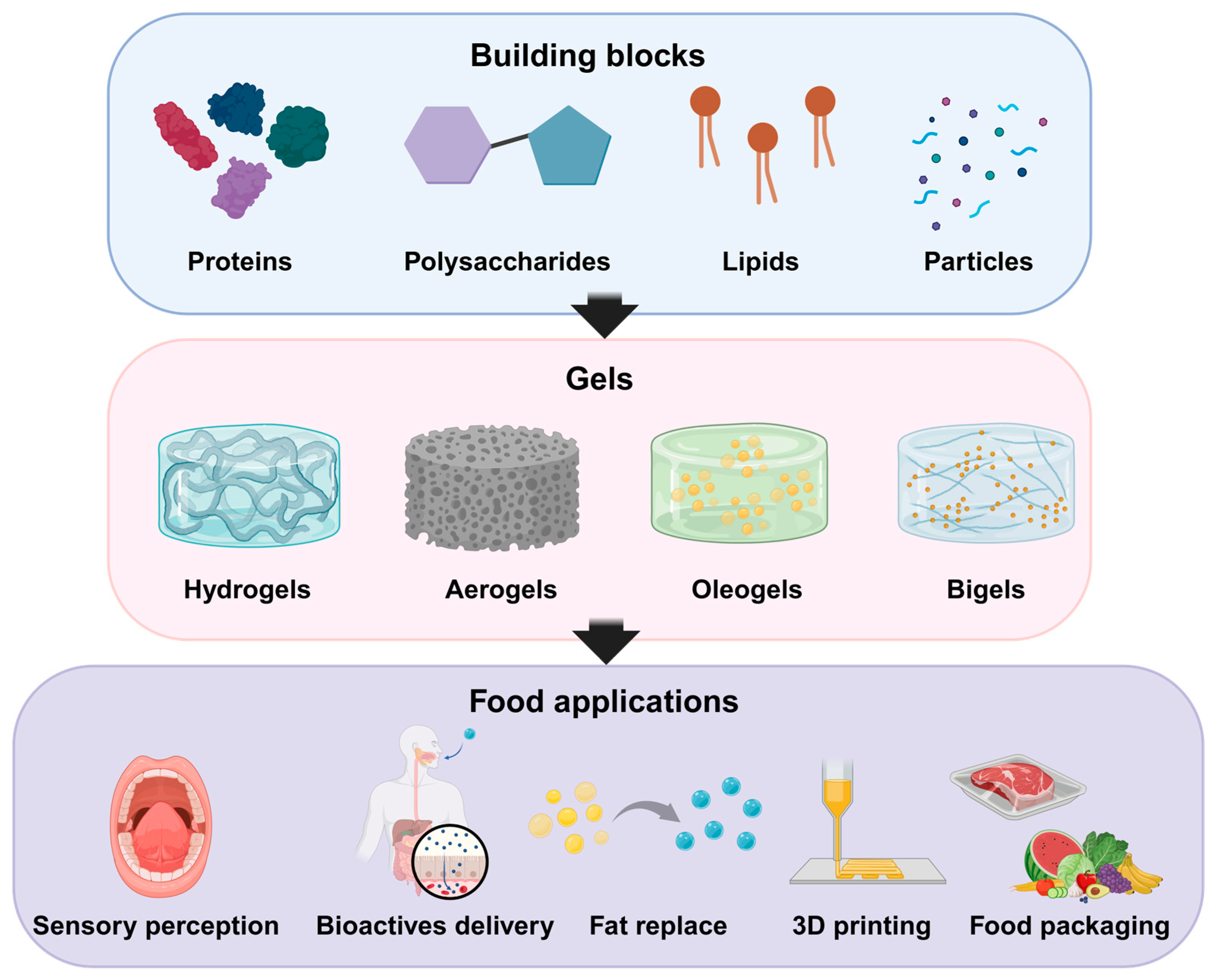
2. Building Blocks and Gelation Mechanisms
2.1. Structuring Agents
2.1.1. Proteins
2.1.2. Polysaccharides
2.1.3. Lipids
2.1.4. Particles
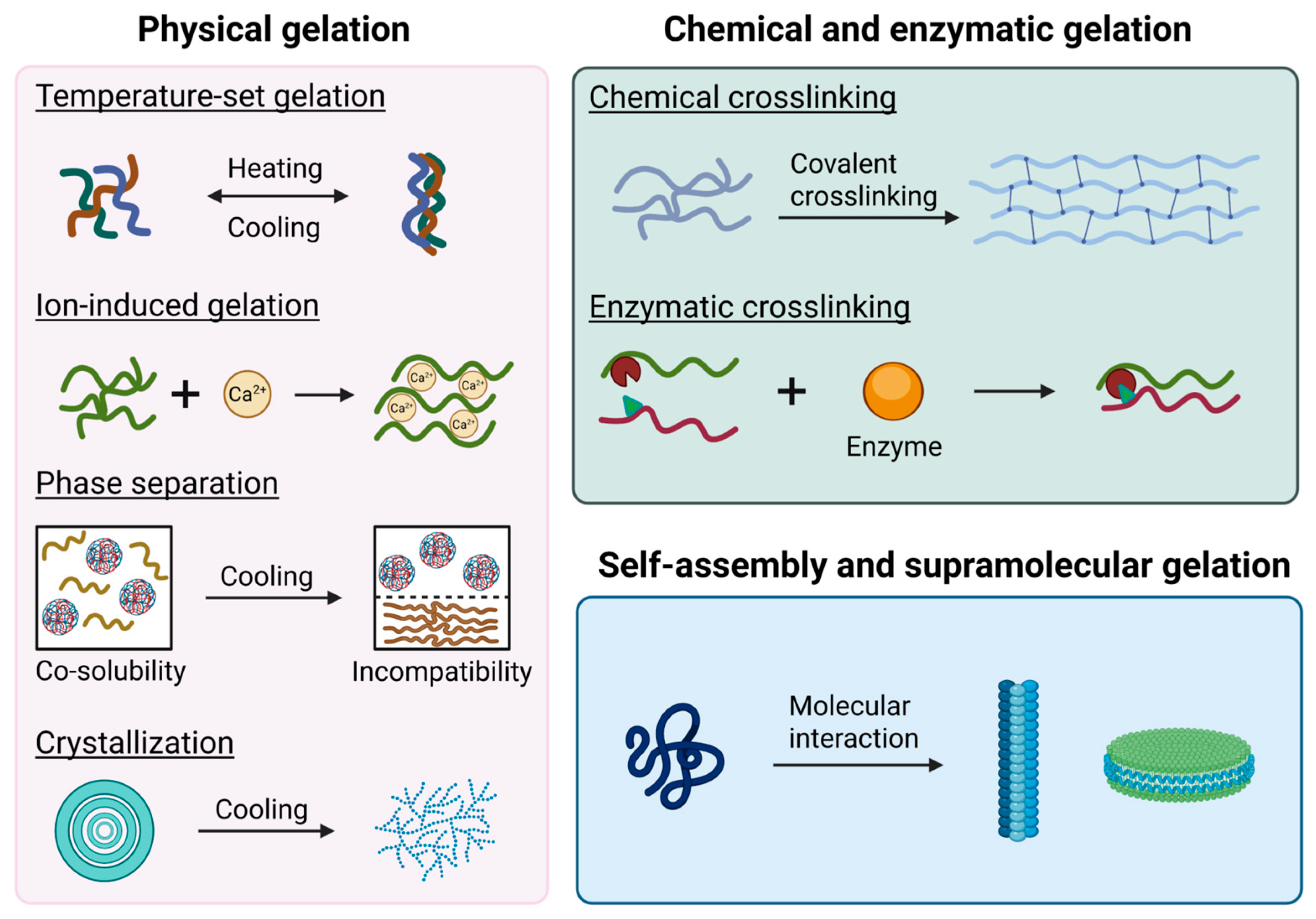
2.2. Gelation Mechanisms and Nanostructure Genesis
2.2.1. Physical Gelation
- Heat/Cool-Driven Conformational Transitions. Heat-driven conformational transition occurs when heating unfolds proteins or specific polysaccharides (like konjac glucomannan), promoting aggregation and network formation through hydrophobic interactions, hydrogen bonds, and sometimes disulfide bonds, often facilitated by additional factors like alkali treatment; conversely [18,35]. Cool-driven conformational transition occurs upon cooling, allowing polymers such as gelatin or amylose in starches to reassociate and form stable junction zones and networks, where the cooling process critically determines the resulting gel’s texture and firmness [67].
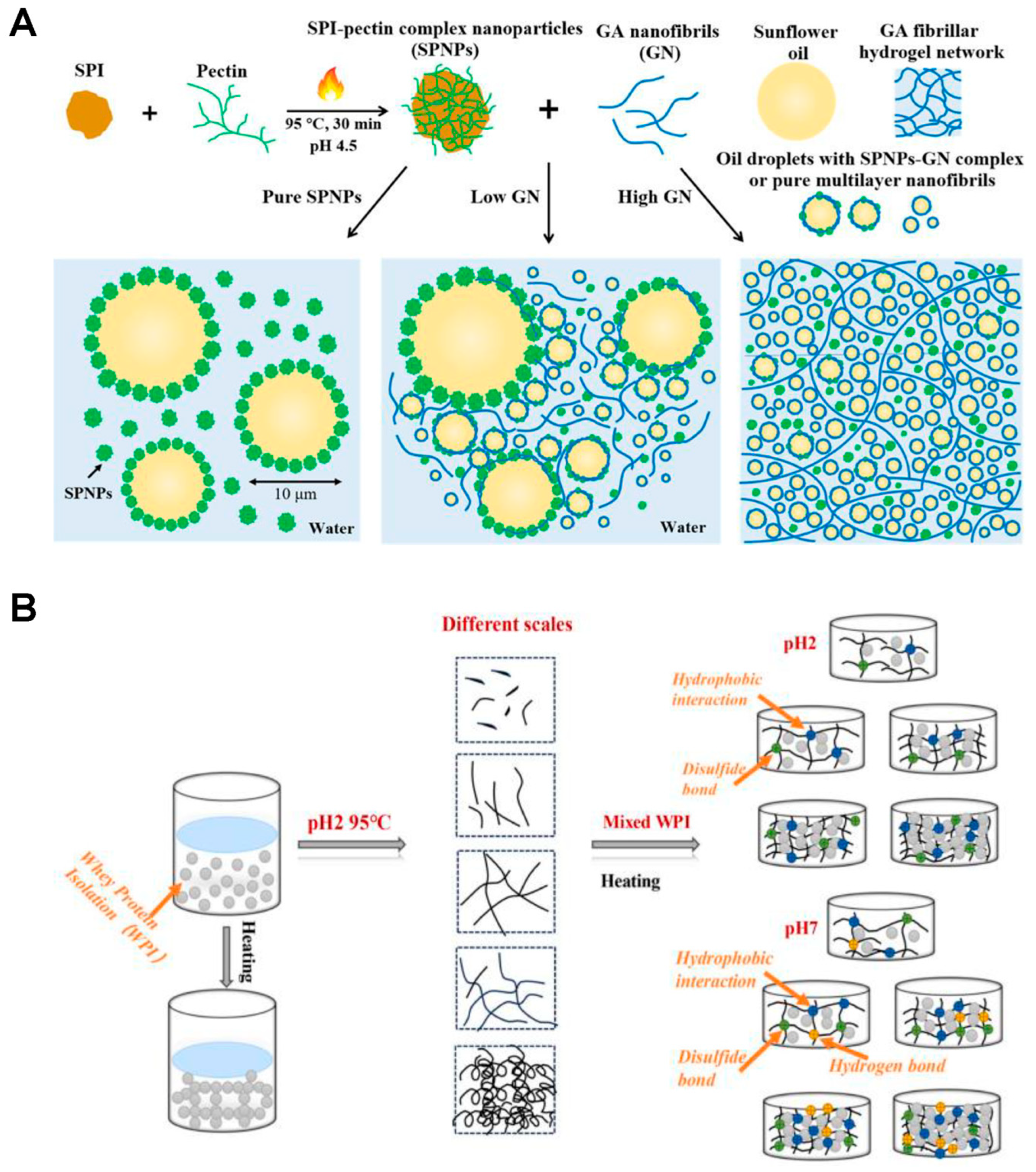
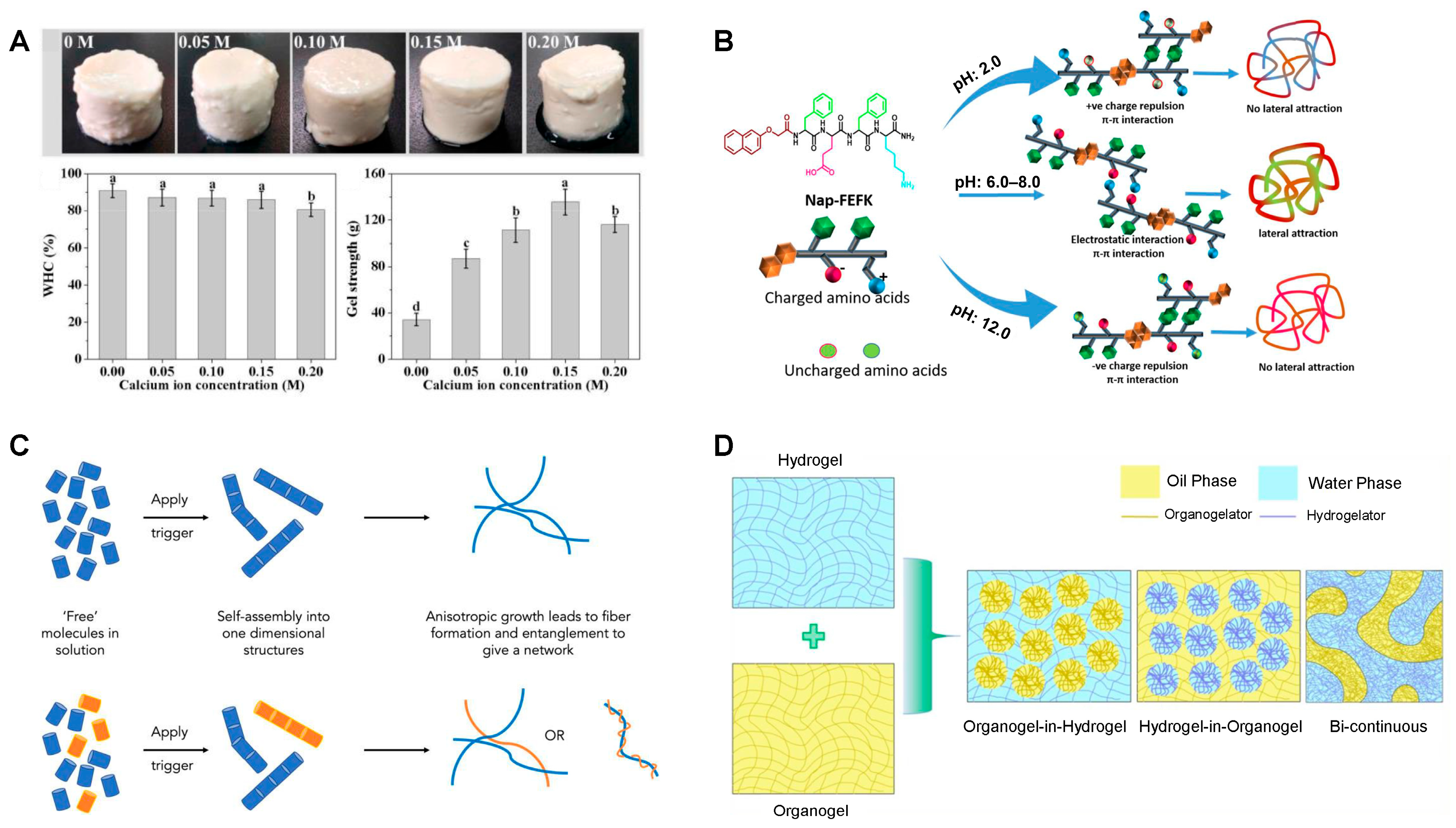
- Ion-Induced Gelation. Addition of ions (e.g., Ca2+) to protein or polysaccharide systems (such as alginate or pectin) promotes conformational changes (e.g., α-helix to β-sheet in proteins), enhances hydrophobic interactions, and leads to the formation of stable, ordered 3D networks, as shown in Figure 3A. Excessive ion concentration also causes irregular aggregates and coarser structures [68].
- Crystallization. In starch and polysaccharide gels, particularly starch-based systems, crystallization is a key mechanism where linear polymer chains (like amylose) align and associate during cooling, forming stable crystalline junction zones that significantly reinforce the gel network structure; the degree of this crystallization critically determines the gel’s firmness, stability, and overall textural quality in the final product [10,67].
2.2.2. Chemical and Enzymatic Gelation
- Chemical Gelation. Food gels are mainly stabilized by non-covalent interactions such as hydrogen bonds, electrostatic forces, Van der Waals forces, and hydrophobic interactions, which enable the formation of three-dimensional networks without the need for covalent cross-linking [33,34]. However, disulfide bonds serve as an important exception, providing covalent cross-linking that enhances stability and rigidity in protein gels [15,71]. The resulting gel network’s strength and structure are strongly influenced by factors including temperature, pH, ionic strength, the concentration of gelling agents, and the presence of small molecules such as sugars, acids, and salts [33,67,72].
- Enzymatic Gelation. The formation of covalent bonds or modify polymer structures in enzymatic gelation are catalyzed by enzymes [73], where transglutaminase and specific proteases induce protein gelation by cross-linking molecules, creating networks with unique textures [73,74], while in polysaccharide systems (e.g., carrageenan, agar, alginate), enzymes like epimerases, desulfatases, and lyases alter the carbohydrate backbone to tailor gel characteristics [74].
2.2.3. Self-Assembly and Supramolecular Gelation
3. Characterization of Nano-/Microstructures in Food Gels
3.1. Microscopy Techniques
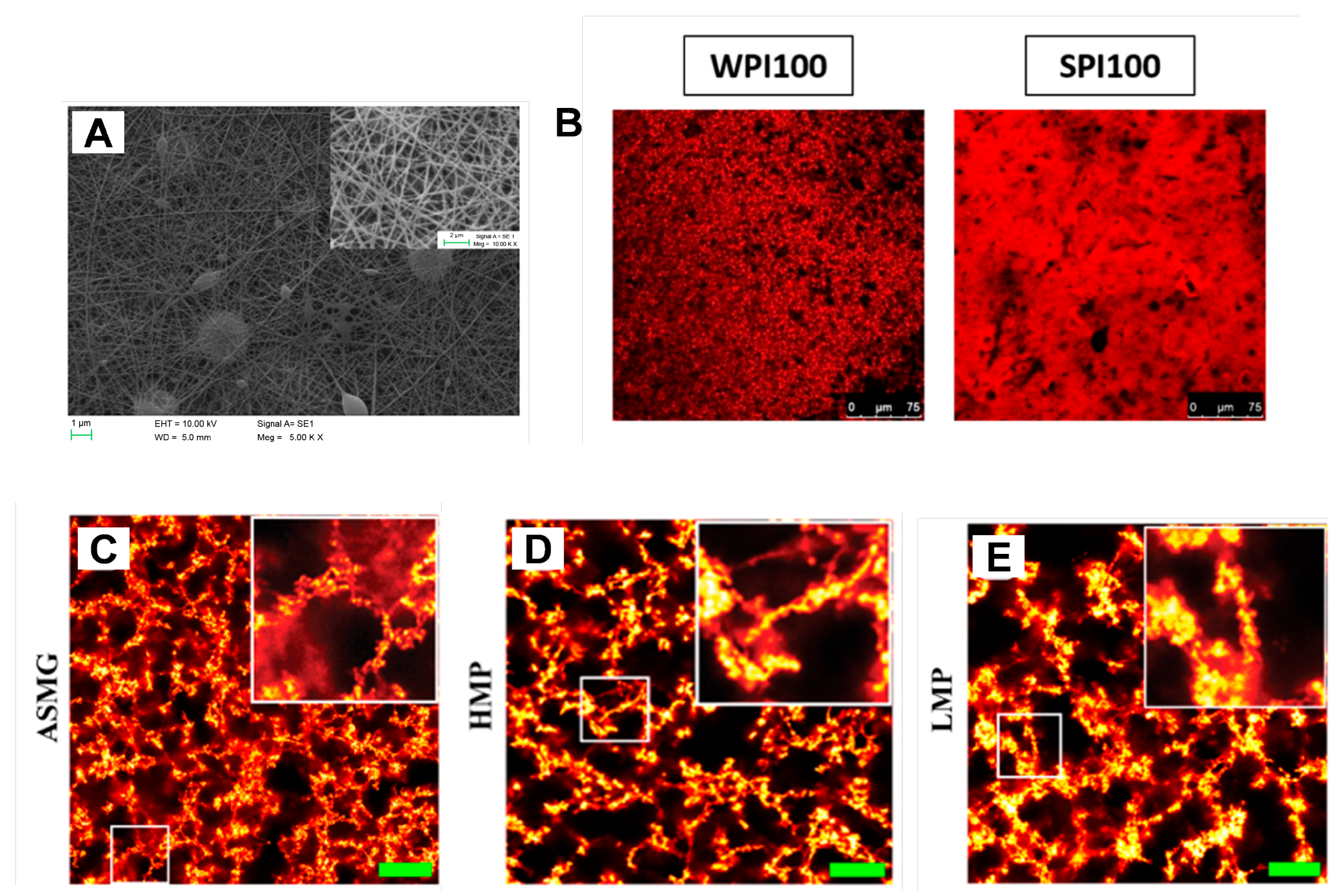
3.2. Scattering Techniques
3.3. Spectroscopy
3.4. Rheology and Microrheology
3.5. Technical Challenges
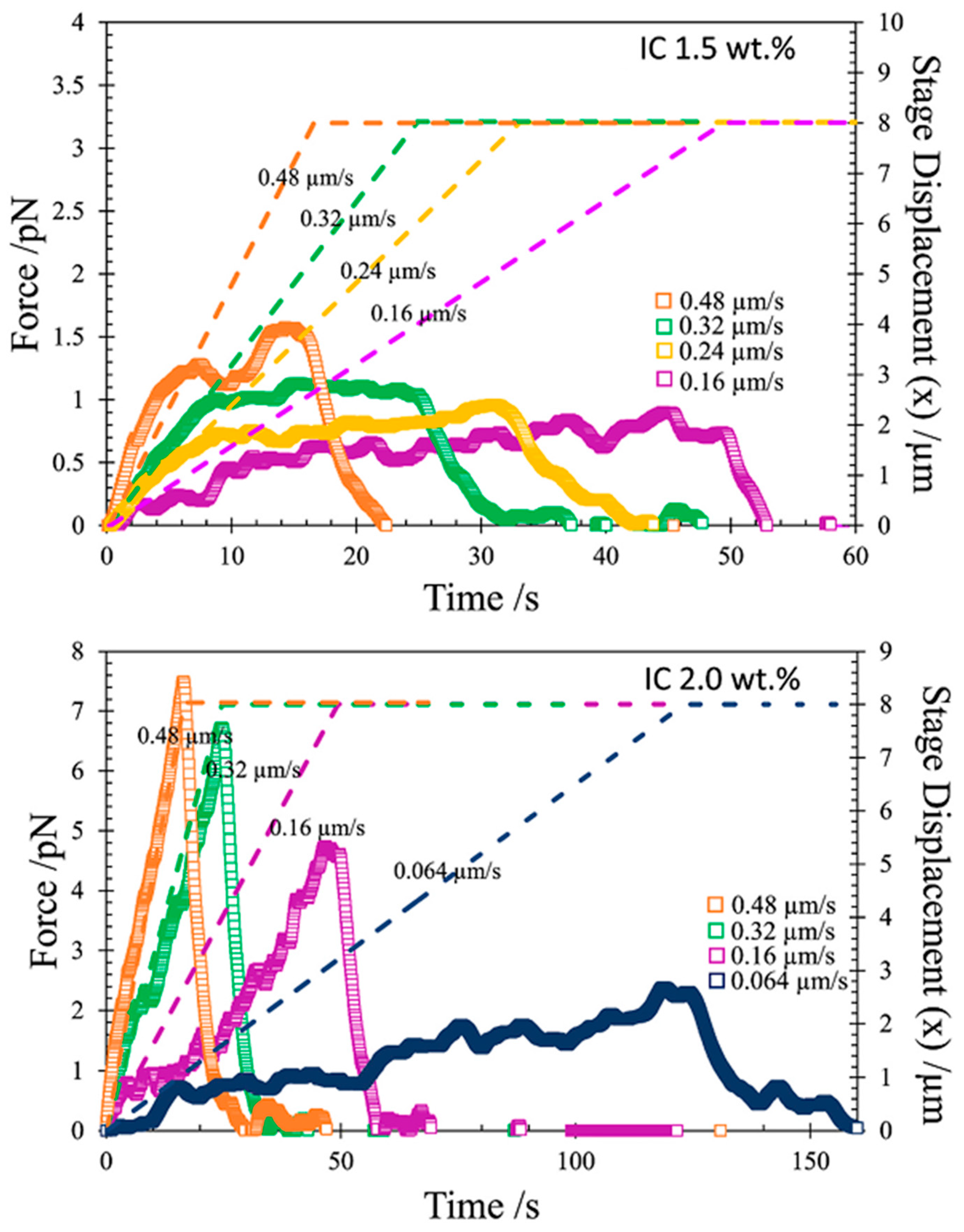
4. Strategies for Tuning Nanostructure of Gels
4.1. Chemical Composition
4.1.1. Concentration
4.1.2. pH and Ionic Strength
4.1.3. Co-Solutes/Sugars
4.1.4. Molecular Weight and Chain Flexibility
4.1.5. Blending of Components
4.2. Physical Control
4.2.1. Temperature Control
4.2.2. Shear/Flow Fields
4.2.3. Pressure
4.2.4. Ultrasound
| Strategy | Mechanism | Examples | Impact on Nanostructure | Ref. |
|---|---|---|---|---|
| Concentration | Increased concentration enhances molecular proximity | Alginate gels; κ-carrageenan gels | Promotes more frequent junction zone formation, leading to denser and stronger networks | [154,155] |
| pH adjustment | Modulates charge distribution and gelation behavior of biopolymers | Acid/alkaline-induced casein, pea, or pectin gels | Alters network density, pore size, and aggregation behavior | [156,157,158] |
| Ionic strength and Ion type | Ionic cross-linking or shielding modulates gelation and structure | Ca2+-induced alginate gel; K+-induced κ-carrageenan gel; Na+ effect on protein gels | Controls gel stiffness, porosity, and nano-fibrillar structure | [159,160,161,162,163] |
| Solvent Quality/Polarity | Affects molecular interactions and phase separation | Ethanol or sugar concentration to promote gelation | Changes gel network compactness and aggregation state | [164,165,166] |
| Co-gelling or composite systems | Combines multiple gelling agents or nanofibrils to form hybrid structures | Alginate–gellan gum, protein–polysaccharide blends | Enhances hierarchical structure and multi-scale network architecture | [167,168,169,170,171] |
| Thermal treatment | Induces denaturation or conformational changes that promote gelation | Alginate gels; Heat-set whey protein gels; protein–polysaccharide gels; gelatin melting and reformation | Modulates fibril size, network junctions, and WHC | [172,173,174,175] |
| Shear processing | Aligns, disrupts, or restructures gel network during processing | Homogenization, extrusion, or whipping of gels | Controls anisotropy, fibrillar orientation, and nanopore structure | [176,177,178] |
| Pressure | Induces protein unfolding and aggregation via non-thermal means | High-pressure-treated starch or protein gels | Increases WHC and creates uniform, dense nanostructures | [143,144,147,179] |
| Ultrasound | Improves interaction between phases in composite gels | Protein, protein–polysaccharide, or emulsion-filled gels | Promotes finer emulsions and more uniform microphase distribution at the nanoscale | [39,180,181,182] |
4.3. Engineering Multifunctional Gel Matrices Through Nanostructure Integration
4.3.1. Modulating Gel Properties via Nanoparticles as Functional Modifiers
4.3.2. Reinforcing Gel Networks Through Nanofiber-Based Structural Integration
4.3.3. Safety and Regulatory Concerns of Nanomaterials in Food Products
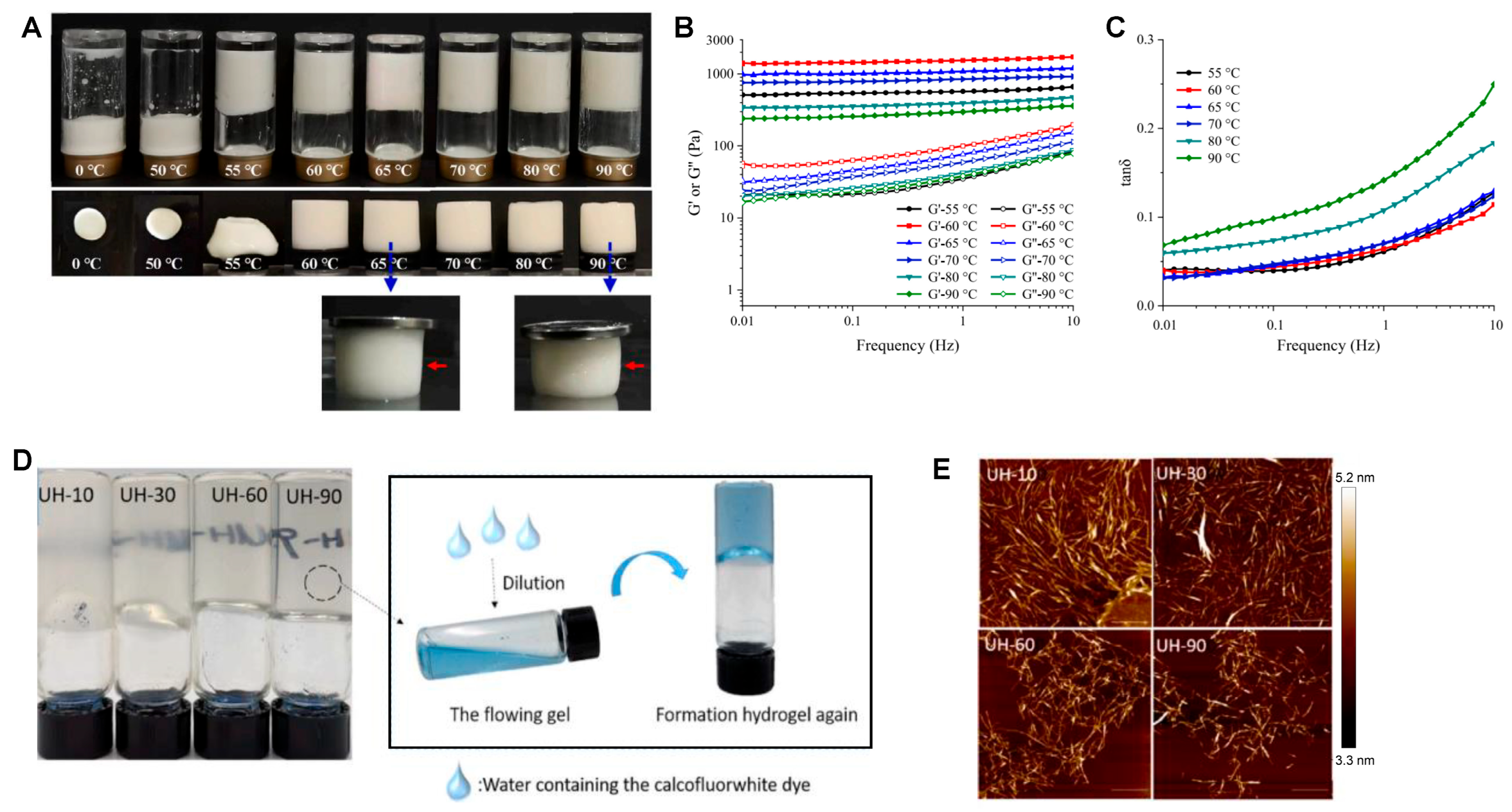
5. Nanostructural Modulation for Function Control in Food Gels
5.1. Texture Customization Through Controlled Mechanics of Nanostructures
5.2. Controlling WHC and Stability via Nanostructure-Mediated Water Confinement
5.3. Modulating Light Transmission, Scattering, and Absorption via Nanostructured Matrices
5.4. Programming Bioactive Release Through Engineered Nanostructural Barriers/Diffusion
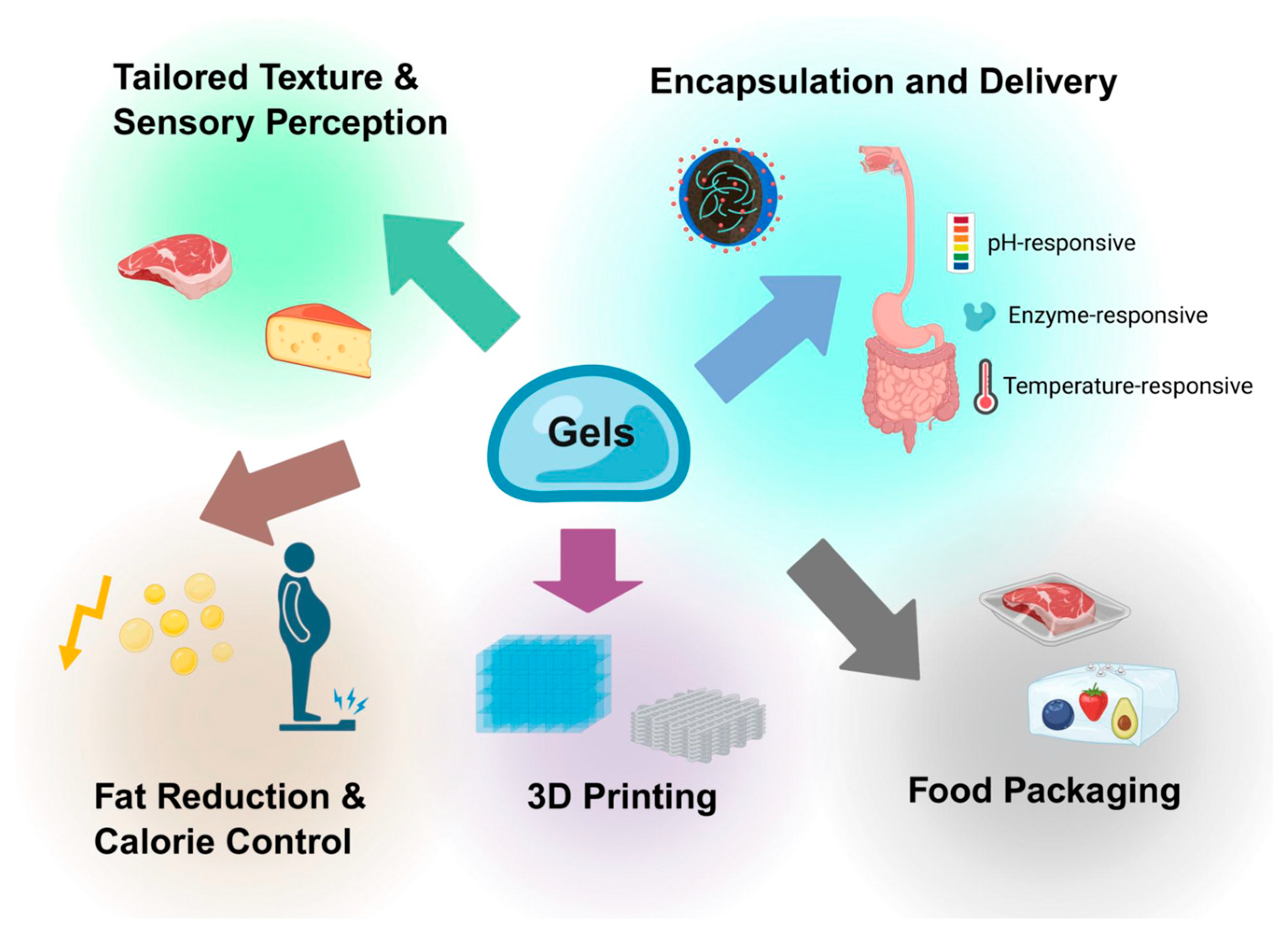
6. Applications Enabled by Tuning Nanostructure of Food Gels
6.1. Tailored Texture and Sensory Perception
6.2. Encapsulation and Delivery of Bioactives
6.3. Fat Reduction and Calorie Control
6.4. Food Packaging with Improved Shelf-Life and Smart Responsiveness
6.5. Novel Food Structures and 3D Printing
7. Challenges and Perspectives
7.1. Current Challenges
7.2. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WHC | Water-Holding Capacity |
| 3D | Three-Dimensional |
| IDDSI | International Dysphagia Diet Standardization Initiative |
| Cryo-SEM | Cryo-Scanning Electron Microscopy |
| TEM | Transmission Electron Microscopy |
| AFM | Atomic Force Microscopy |
| STED | Stimulated Emission Depletion |
| FLIM | Fluorescence Lifetime Imaging Microscopy |
| LM | Light Microscopy |
| PLM | Polarized Light Microscopy |
| SAXS | Small-Angle X-Ray Scattering |
| SANS | Small-Angle Neutron Scattering |
| USANS | Ultra-Small-Angle Neutron Scattering |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| NMR | Nuclear Magnetic Resonance |
| LMWGs | Low-Molecular-Weight Gelators |
| ICP-ASE | Inductively Coupled Plasma Atomic Emission Spectroscopy |
| CNC | Cellulose Nanocrystals |
| HPH | High-Pressure Homogenization |
References
- Liu, B.; Yang, H.; Zhu, C.; Xiao, J.; Cao, H.; Simal-Gandara, J.; Li, Y.; Fan, D.; Deng, J. A comprehensive review of food gels: Formation mechanisms, functions, applications, and challenges. Crit. Rev. Food Sci. Nutr. 2024, 64, 760–782. [Google Scholar] [CrossRef]
- Abdullah; Liu, L.; Javed, H.U.; Xiao, J. Engineering Emulsion Gels as Functional Colloids Emphasizing Food Applications: A Review. Front. Nutr. 2022, 9, 890188. [Google Scholar] [CrossRef]
- Dickinson, E. Emulsion gels: The structuring of soft solids with protein-stabilized oil droplets. Food Hydrocoll. 2012, 28, 224–241. [Google Scholar] [CrossRef]
- Fu, K.; Wang, H.; Pan, T.; Cai, Z.; Yang, Z.; Liu, D.; Wang, W. Gel-forming polysaccharides of traditional gel-like foods: Sources, structure, gelling mechanism, and advanced applications. Food Res. Int. 2024, 198, 115329. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, Z.; Lai, B.; Wang, C.; Wu, H. Recent advances in marine-derived protein/polysaccharide hydrogels: Classification, fabrication, characterization, mechanism and food applications. Trends Food Sci. Technol. 2024, 151, 104637. [Google Scholar] [CrossRef]
- Klein, M.; Poverenov, E. Natural biopolymer-based hydrogels for use in food and agriculture. J. Sci. Food Agric. 2020, 100, 2337–2347. [Google Scholar] [CrossRef]
- Miao, W.; Jiang, H.; Li, X.; Sang, S.; Jiang, L.; Lin, Q.; Zhang, Z.; Chen, L.; Long, J.; Jiao, A.; et al. Recent advances in natural gums as additives to help the construction and application of edible biopolymer gels: The example of hydrogels and oleogels. Crit. Rev. Food Sci. Nutr. 2024, 64, 12702–12719. [Google Scholar] [CrossRef]
- Chen, L.; Lin, S.; Sun, N. Food gel-based systems for efficient delivery of bioactive ingredients: Design to application. Crit. Rev. Food Sci. Nutr. 2023, 64, 13193–13211. [Google Scholar] [CrossRef]
- Francavilla, A.; Corradini, M.G.; Joye, I.J. Bigels as Delivery Systems: Potential Uses and Applicability in Food. Gels 2023, 9, 648. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Lu, Y.; Cui, M.; Miao, S.; Gao, Y. Design of gel structures in water and oil phases for improved delivery of bioactive food ingredients. Crit. Rev. Food Sci. Nutr. 2020, 60, 1651–1666. [Google Scholar] [CrossRef] [PubMed]
- Faber, T.J.; Van Breemen, L.C.A.; McKinley, G.H. From firm to fluid—Structure-texture relations of filled gels probed under Large Amplitude Oscillatory Shear. J. Food Eng. 2017, 210, 1–18. [Google Scholar] [CrossRef]
- Min, C.; Ma, W.; Kuang, J.; Huang, J.; Xiong, Y.L. Textural properties, microstructure and digestibility of mungbean starch–flaxseed protein composite gels. Food Hydrocoll. 2022, 126, 107482. [Google Scholar] [CrossRef]
- Macias-Rodriguez, B.A.; Gouzy, R.; Coulais, C.; Velikov, K.P. Thermoresponsive oil-continuous gels based on double-interpenetrating colloidal-particle networks. Soft Matter 2024, 20, 3033–3043. [Google Scholar] [CrossRef]
- Li, A.; Gong, T.; Yang, X.; Guo, Y. Interpenetrating network gels with tunable physical properties: Glucono-δ-lactone induced gelation of mixed Alg/gellan sol systems. Int. J. Biol. Macromol. 2020, 151, 257–267. [Google Scholar] [CrossRef]
- Cao, Y.; Mezzenga, R. Design principles of food gels. Nat. Food 2020, 1, 106–118. [Google Scholar] [CrossRef]
- Wang, C.; Yan, R.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Wang, J.; Qiu, C.; et al. Development of emulsion-based edible inks for 3D printing applications: Pickering emulsion gels. Food Hydrocoll. 2023, 138, 108482. [Google Scholar] [CrossRef]
- Edwards, C.E.R.; Mai, D.J.; Tang, S.; Olsen, B.D. Molecular anisotropy and rearrangement as mechanisms of toughness and extensibility in entangled physical gels. Phys. Rev. Mater. 2020, 4, 015602. [Google Scholar] [CrossRef]
- Foegeding, E.A. Food Biophysics of Protein Gels: A Challenge of Nano and Macroscopic Proportions. Food Biophys. 2006, 1, 41–50. [Google Scholar] [CrossRef]
- Vecchio, D.A.; Hammig, M.D.; Xiao, X.; Saha, A.; Bogdan, P.; Kotov, N.A. Spanning Network Gels from Nanoparticles and Graph Theoretical Analysis of Their Structure and Properties. Adv. Mater. 2022, 34, 2201313. [Google Scholar] [CrossRef]
- Datta, S.; Bhattacharya, S. Multifarious facets of sugar-derived molecular gels: Molecular features, mechanisms of self-assembly and emerging applications. Chem. Soc. Rev. 2015, 44, 5596–5637. [Google Scholar] [CrossRef]
- Yang, T.; Skirtach, A.G. Nanoarchitectonics of Sustainable Food Packaging: Materials, Methods, and Environmental Factors, Materials. Materials 2025, 18, 1167. [Google Scholar] [CrossRef]
- Martins, J.T.; Bourbon, A.I.; Pinheiro, A.C.; Fasolin, L.H.; Vicente, A.A. Protein-Based Structures for Food Applications: From Macro to Nanoscale. Food Syst. 2018, 2, 77. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, T.; Liu, Y.; Li, B.; Li, L.; Zhang, X. Enhanced foamability and stability of aqueous foams through novel pickering fat globules formulated with solid lipid particles. Food Hydrocoll. 2024, 150, 109684. [Google Scholar] [CrossRef]
- Rusch, P.; Zámbó, D.; Bigall, N.C. Control over Structure and Properties in Nanocrystal Aerogels at the Nano-, Micro-, and Macroscale. Accounts Chem. Res. 2020, 53, 2414–2424. [Google Scholar] [CrossRef]
- Cao, L.; Huang, Y.; Parakhonskiy, B.; Skirtach, A.G. Nanoarchitectonics beyond perfect order—Not quite perfect but quite useful. Nanoscale 2022, 14, 15964–16002. [Google Scholar] [CrossRef] [PubMed]
- Sherman, Z.M.; Green, A.M.; Howard, M.P.; Anslyn, E.V.; Truskett, T.M.; Milliron, D.J. Colloidal Nanocrystal Gels from Thermodynamic Principles. Accounts Chem. Res. 2021, 54, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Ossai, C.I.; Raghavan, N. Nanostructure and nanomaterial characterization, growth mechanisms, and applications, Nanotechnology Reviews. Nanotechnol. Rev. 2018, 7, 209–231. [Google Scholar] [CrossRef]
- Li, X.; Lou, L.; Song, W.; Zhang, Q.; Huang, G.; Hua, Y.; Zhang, H.-T.; Xiao, J.; Wen, B.; Zhang, X. Controllably Manipulating Three-Dimensional Hybrid Nanostructures for Bulk Nanocomposites with Large Energy Products. Nano Lett. 2017, 17, 2985–2993. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Wang, T.; Liu, M. Tuning Soft Nanostructures in Self-assembled Supramolecular Gels: From Morphology Control to Morphology-Dependent Functions. Small 2015, 11, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Liu, Y.; Qiao, C.; Sun, Y.; Zhao, Z.; Liu, J.; Zhu, L.; Zeng, H. Nano-fibrous biopolymers as building blocks for gel networks: Interactions, characterization, and applications. Adv. Colloid Interface Sci. 2025, 338, 103398. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Y.; Yue, W.; Qin, W.; Dong, H.; Vasanthan, T. Nanostructures of protein-polysaccharide complexes or conjugates for encapsulation of bioactive compounds. Trends Food Sci. Technol. 2021, 109, 169–196. [Google Scholar] [CrossRef]
- Tan, Y.; Zi, Y.; Peng, J.; Shi, C.; Zheng, Y.; Zhong, J. Gelatin as a bioactive nanodelivery system for functional food applications. Food Chem. 2023, 423, 136265. [Google Scholar] [CrossRef] [PubMed]
- Nishinari, K. Gelling Properties. In Food Hydrocolloids: Functionalities and Applications; Fang, Y., Zhang, H., Nishinari, K., Eds.; Springer: Singapore, 2021; pp. 119–170. [Google Scholar]
- Banerjee, S.; Bhattacharya, S. Food Gels: Gelling Process and New Applications. Crit. Rev. Food Sci. Nutr. 2012, 52, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, T. Gelation of food protein-protein mixtures. Adv. Colloid Interface Sci. 2019, 270, 147–164. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, T.; Tian, Y.; You, L.; Huang, Y.; Wang, S. Novel self-assembling peptide hydrogel with pH-tunable assembly microstructure, gel mechanics and the entrapment of curcumin. Food Hydrocoll. 2022, 124, 107338. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Zheng, L. Soy protein-based protein composite system: Gelation, application, and challenges—A review. Eur. Food Res. Technol. 2025, 251, 311–325. [Google Scholar] [CrossRef]
- Lv, Y.; Xu, L.; Su, Y.; Chang, C.; Gu, L.; Yang, Y.; Li, J. Effect of soybean protein isolate and egg white mixture on gelation of chicken myofibrillar proteins under salt/-free conditions. LWT 2021, 149, 111871. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Yan, Z.; Zhang, R.; Du, Z.; Shang, X.; Zhang, T.; Liu, X. Mechanism of ultrasound-induced soybean/egg white composite gelation: Gel properties, morphological structure and co-aggregation kinetics. Int. J. Biol. Macromol. 2024, 266, 131267. [Google Scholar] [CrossRef]
- Manzoor, A.; Dar, A.H.; Pandey, V.K.; Shams, R.; Khan, S.; Panesar, P.S.; Kennedy, J.F.; Fayaz, U.; Khan, S.A. Recent insights into polysaccharide-based hydrogels and their potential applications in food sector: A review. Int. J. Biol. Macromol. 2022, 213, 987–1006. [Google Scholar] [CrossRef]
- Hu, X.; Jiang, Q.; Du, L.; Meng, Z. Edible polysaccharide-based oleogels and novel emulsion gels as fat analogues: A review. Carbohydr. Polym. 2023, 322, 121328. [Google Scholar] [CrossRef]
- Kong, D.; Zhang, M.; Mujumdar, A.S.; Yu, D. New drying technologies for animal/plant origin polysaccharide-based future food processing: Research progress, application prospects and challenges. Food Biosci. 2023, 56, 103315. [Google Scholar] [CrossRef]
- Xiang, Q.; Hao, Y.; Xia, Z.; Liao, M.; Rao, X.; Lao, S.; He, Q.; Ma, C.; Liao, W. Biomedical Applications and Nutritional Value of Specific Food-Derived Polysaccharide-Based Hydrogels. Adv. Nutr. Int. Rev. J. 2024, 15, 100309. [Google Scholar] [CrossRef]
- Yang, X.; Li, A.; Li, D.; Guo, Y.; Sun, L. Applications of mixed polysaccharide-protein systems in fabricating multi-structures of binary food gels—A review. Trends Food Sci. Technol. 2021, 109, 197–210. [Google Scholar] [CrossRef]
- Ren, S.; Zhang, G.; Wang, Z.; Sun, F.; Cheng, T.; Wang, D.; Yang, H.; Wang, Z.; Guo, Z. Potentially texture-modified food for dysphagia: Gelling, rheological, and water fixation properties of rice starch–soybean protein composite gels in various ratios. Food Hydrocoll. 2024, 153, 110025. [Google Scholar] [CrossRef]
- Du, L.; Guo, Y.; Meng, Z. Organogels, O/W and W/O emulsion gels structured by monoglycerides: The study on the gelation behavior and crystal network. Eur. Food Res. Technol. 2025, 251, 165–177. [Google Scholar] [CrossRef]
- Shi, Z.; Shi, Z.; Wu, M.; Shen, Y.; Li, G.; Ma, T. Fabrication of emulsion gel based on polymer sanxan and its potential as a sustained-release delivery system for β-carotene. Int. J. Biol. Macromol. 2020, 164, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Funami, T.; Ishihara, S.; Maeda, K.; Nakauma, M. Review paper: Recent development in Pickering emulsion gel technology for food and beverage applications. Food Hydrocoll. 2025, 162, 110901. [Google Scholar] [CrossRef]
- Yang, T.; Zan, S.; Li, B.; Li, L.; Zhang, X. Interfacial adsorption dynamics of solid lipid particles at oil/water interfaces through QCM-D technique. Food Hydrocoll. 2024, 148, 109431. [Google Scholar] [CrossRef]
- Gomes, A.; Furtado, G.d.F.; Cunha, R.L. Bioaccessibility of Lipophilic Compounds Vehiculated in Emulsions: Choice of Lipids and Emulsifiers. J. Agric. Food Chem. 2018, 67, 13–18. [Google Scholar] [CrossRef]
- Zhang, R.; Li, B.; Song, Y.; Li, L.; Zhang, X. Tailoring stability in oil-in-water high internal phase Pickering emulsions (HIPPEs) through surface modification of beeswax-based solid lipid particles (SLPs) with various surfactants. Food Hydrocoll. 2024, 156, 110264. [Google Scholar] [CrossRef]
- Ferro, A.C.; Okuro, P.K.; Badan, A.P.; Cunha, R.L. Role of the oil on glyceryl monostearate based oleogels. Food Res. Int. 2019, 120, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Meng, Z. Engineering surfactant-free pickering double emulsions gels with different structures as low-calorie fat analogues: Tunable oral perception, inhibiting lipid digestion, and potent co-delivery for lycopene and epigallocatechin gallate. Food Chem. 2025, 463, 141378. [Google Scholar] [CrossRef]
- Patel, A.R.; Dewettinck, K. Comparative evaluation of structured oil systems: Shellac oleogel, HPMC oleogel, and HIPE gel. Eur. J. Lipid Sci. Technol. 2015, 117, 1772–1781. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Song, Z.; Tian, W.; Abdullah; Huang, Q.; Chen, M.; Huang, Y.; Xiao, H.; Xiao, J. Advancements in lipid-based delivery systems for functional foods: A comprehensive review of literature and patent trends. Crit. Rev. Food Sci. Nutr. 2025, 65, 2456–2472. [Google Scholar] [CrossRef]
- Dille, M.; Draget, K.; Hattrem, M. 9—The effect of filler particles on the texture of food gels. In Modifying Food Texture; Chen, J., Rosenthal, A., Eds.; Woodhead Publishing: London, UK, 2015; pp. 183–200. [Google Scholar]
- Ghebremedhin, M.; Seiffert, S.; Vilgis, T.A. Molecular behavior of fluid gels—The crucial role of edges and particle surface in macroscopic properties. Food Funct. 2022, 13, 6902–6922. [Google Scholar] [CrossRef]
- Song, J.; Vikulina, A.S.; Parakhonskiy, B.V.; Skirtach, A.G. Hierarchy of hybrid materials. Part-II: The place of organics-on-inorganics in it, their composition and applications. Front. Chem. 2023, 11, 1078840. [Google Scholar] [CrossRef]
- Gravelle, A.J.; Nicholson, R.A.; Barbut, S.; Marangoni, A.G. Considerations for readdressing theoretical descriptions of particle-reinforced composite food gels. Food Res. Int. 2019, 122, 209–221. [Google Scholar] [CrossRef]
- Abalymov, A.; Van der Meeren, L.; Saveleva, M.; Prikhozhdenko, E.; Dewettinck, K.; Parakhonskiy, B.V.; Skirtach, A.G. Cells-Grab-on Particles: A Novel Approach to Control Cell Focal Adhesion on Hybrid Thermally Annealed Hydrogels. ACS Biomater. Sci. Eng. 2020, 6, 3933–3944. [Google Scholar] [CrossRef]
- Cao, L.; Lewille, B.; Dewettinck, K.; Willaert, R.G.; Tan, M.; Skirtach, A.G.; Parakhonskiy, B.V. Emulsion-filled bulk gels based on alginate and gellan gum: The fabrication, characterization, curcumin delivery, and antioxidative properties. Chem. Eng. J. 2024, 501, 157649. [Google Scholar] [CrossRef]
- Zou, Y.; Guo, J.; Yin, S.-W.; Wang, J.-M.; Yang, X.-Q. Pickering Emulsion Gels Prepared by Hydrogen-Bonded Zein/Tannic Acid Complex Colloidal Particles. J. Agric. Food Chem. 2015, 63, 7405–7414. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, H.; Lu, W.; Nishinari, K.; Fang, Y. New insights into food hydrogels with reinforced mechanical properties: A review on innovative strategies. Adv. Colloid Interface Sci. 2020, 285, 102278. [Google Scholar] [CrossRef]
- Huang, R.; Liu, L.; Cai, M.; Sun, X.; Feng, G.; Zeng, M. Fabrication of emulsion gels with oyster protein particles through depletion attraction for 3D printing. Food Hydrocoll. 2024, 155, 110150. [Google Scholar] [CrossRef]
- Torres, O.; Tena, N.M.; Murray, B.; Sarkar, A. Novel starch based emulsion gels and emulsion microgel particles: Design, structure and rheology. Carbohydr. Polym. 2017, 178, 86–94. [Google Scholar] [CrossRef]
- Moschakis, T. Microrheology and particle tracking in food gels and emulsions. Curr. Opin. Colloid Interface Sci. 2013, 18, 311–323. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Ai, Y. Gelation mechanisms of granular and non-granular starches with variations in molecular structures. Food Hydrocoll. 2022, 129, 107658. [Google Scholar] [CrossRef]
- Xiao, Y.; Kang, S.; Liu, Y.; Guo, X.; Li, M.; Xu, H. Effect and mechanism of calcium ions on the gelation properties of cellulose nanocrystals-whey protein isolate composite gels. Food Hydrocoll. 2021, 111, 106401. [Google Scholar] [CrossRef]
- Hou, X.; Lin, L.; Li, K.; Jiang, F.; Qiao, D.; Zhang, B.; Xie, F. Towards superior biopolymer gels by enabling interpenetrating network structures: A review on types, applications, and gelation strategies. Adv. Colloid Interface Sci. 2024, 325, 103113. [Google Scholar] [CrossRef]
- Jin, W.; Xu, W.; Ge, H.; Li, J.; Li, B. Coupling process of phase separation and gelation in konjac glucomannan and gelatin system. Food Hydrocoll. 2015, 51, 188–192. [Google Scholar] [CrossRef]
- Totosaus, A.; Montejano, J.G.; A Salazar, J.; Guerrero, I. A review of physical and chemical protein-gel induction. Int. J. Food Sci. Technol. 2002, 37, 589–601. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, X.; Cheng, Y.; Zhao, G.; Zhou, Y. Gelation mechanism of alkali induced heat-set konjac glucomannan gel. Trends Food Sci. Technol. 2021, 116, 244–254. [Google Scholar] [CrossRef]
- Huyst, A.M.; Deleu, L.J.; Luyckx, T.; Van der Meeren, L.; Housmans, J.A.; Grootaert, C.; Monge-Morera, M.; Delcour, J.A.; Skirtach, A.G.; Rousseau, F.; et al. Impact of heat and enzymatic treatment on ovalbumin amyloid-like fibril formation and enzyme-induced gelation. Food Hydrocoll. 2022, 131, 107784. [Google Scholar] [CrossRef]
- Rhein-Knudsen, N.; Meyer, A.S. Chemistry, gelation, and enzymatic modification of seaweed food hydrocolloids. Trends Food Sci. Technol. 2021, 109, 608–621. [Google Scholar] [CrossRef]
- Kalab, M.; Harwalkar, V. Milk Gel Structure. I. Application of Scanning Electron Microscopy to Milk and Other Food Gels. J. Dairy Sci. 1973, 56, 835–842. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Sun, Z.; Liu, F.; Du, L.; Wang, D. Preparation and characterization of gelatin/chitosan/3-phenylacetic acid food-packaging nanofiber antibacterial films by electrospinning. Int. J. Biol. Macromol. 2021, 169, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mascaraque, L.G.; Pinho, S.C. Microstructural Analysis of Whey/Soy Protein Isolate Mixed Gels Using Confocal Raman Microscopy. Foods 2021, 10, 2179. [Google Scholar] [CrossRef]
- Mishra, V.S.; Via, M.A.; Andersen, U.; Møller, F.; Brewer, J.R.; Simonsen, A.C. Quantifying microstructure and molecular dynamics in plant-dairy based model gels using FLIM and STED microscopy. Food Hydrocoll. 2025, 163, 111086. [Google Scholar] [CrossRef]
- Li, R.; Ebbesen, M.F.; Glover, Z.J.; Jæger, T.C.; Rovers, T.A.; Svensson, B.; Brewer, J.R.; Simonsen, A.C.; Ipsen, R.; Hougaard, A.B. Discriminating between different proteins in the microstructure of acidified milk gels by super-resolution microscopy. Food Hydrocoll. 2023, 138, 108468. [Google Scholar] [CrossRef]
- Błaszczak, W.; Lewandowicz, G. Light Microscopy as a Tool to Evaluate the Functionality of Starch in Food. Foods 2020, 9, 670. [Google Scholar] [CrossRef] [PubMed]
- De Witte, F.; Penagos, I.A.; Moens, K.; Skirtach, A.G.; Van Bockstaele, F.; Dewettinck, K. Multiscale assessment of the effect of a stearic-palmitic sucrose ester on the crystallization of anhydrous milk fat. Food Res. Int. 2024, 197, 115243. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Zheng, Y.; Fu, X.; Zeng, D.; Liu, H. A novel characterization of starch gelatinization using microscopy observation with deep learning methodology. J. Food Eng. 2022, 327, 111057. [Google Scholar] [CrossRef]
- Ventura, I.; Jammal, J.; Bianco-Peled, H. Insights into the nanostructure of low-methoxyl pectin–calcium gels. Carbohydr. Polym. 2013, 97, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, F.; Seiffert, S. Nanostructural heterogeneity in polymer networks and gels. Polym. Chem. 2015, 6, 5515–5528. [Google Scholar] [CrossRef]
- Olakanmi, S.; Karunakaran, C.; Jayas, D. Applications of X-ray micro-computed tomography and small-angle X-ray scattering techniques in food systems: A concise review. J. Food Eng. 2023, 342, 111355. [Google Scholar] [CrossRef]
- Bayrak, M.; Whitten, A.E.; Mata, J.P.; Conn, C.E.; Floury, J.; Logan, A. Logan, Real-time monitoring of casein gel microstructure during simulated gastric digestion monitored by small-angle neutron scattering. Food Hydrocoll. 2023, 144, 108919. [Google Scholar] [CrossRef]
- Bayrak, M.; Mata, J.P.; Whitten, A.E.; Conn, C.E.; Floury, J.; Logan, A. Logan, Physical disruption of gel particles on the macroscale does not affect the study of protein gel structure on the micro or nanoscale. Colloid Interface Sci. Commun. 2022, 46, 100574. [Google Scholar] [CrossRef]
- McDowall, D.; Adams, D.J.; Seddon, A.M. Using small angle scattering to understand low molecular weight gels. Soft Matter 2022, 18, 1577–1590. [Google Scholar] [CrossRef]
- Tomchuk, O.V.; Avdeev, M.V.; Aleksenskii, A.E.; Vul, A.Y.; Ivankov, O.I.; Ryukhtin, V.V.; Füzi, J.; Garamus, V.M.; Bulavin, L.A. Sol–Gel Transition in Nanodiamond Aqueous Dispersions by Small-Angle Scattering. J. Phys. Chem. C 2019, 123, 18028–18036. [Google Scholar] [CrossRef]
- Bourbon, A.I.; Pereira, R.N.; Pastrana, L.M.; Vicente, A.A.; Cerqueira, M.A. Protein-Based Nanostructures for Food Applications. Gels 2019, 5, 9. [Google Scholar] [CrossRef]
- Hassan, N.; Ahmad, T.; Zain, N.M.; Awang, S.R. Identification of bovine, porcine and fish gelatin signatures using chemometrics fuzzy graph method. Sci. Rep. 2021, 11, 9793. [Google Scholar] [CrossRef] [PubMed]
- Lshaq, A.; Rahman, U.U.; Sahar, A.; Perveen, R.; Deering, A.J.; Khalil, A.A.; Aadil, R.M.; Hafeez, M.A.; Khaliq, A.; Siddique, U. Potentiality of analytical approaches to determine gelatin authenticity in food systems: A review. LWT 2020, 121, 108968. [Google Scholar] [CrossRef]
- Martin, B.; Skirtach, A.G.; Boon, N.; De Troch, M. Lipid Quantification and Determination of Astaxanthin Conjugation by Raman Microscopy on Individual Copepod Eggs. J. Raman Spectrosc. 2025, 56, 472–480. [Google Scholar] [CrossRef]
- Marvizadeh, M.M.; Oladzadabbasabadi, N.; Nafchi, A.M.; Jokar, M. Preparation and characterization of bionanocomposite film based on tapioca starch/bovine gelatin/nanorod zinc oxide. Int. J. Biol. Macromol. 2017, 99, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Barasinski, M.; Jasper, V.; Görke, M.; Garnweitner, G. In Situ Tracking of Nanoparticles During Electrophoresis in Hydrogels Using a Fiber-Based UV-Vis System. Powders 2025, 4, 3. [Google Scholar] [CrossRef]
- Rahman, N.; Ahmad, I. Coordination polymer gel mediated spectrophotometric, ICP-AES and spectrofluorimetric methods for trace As(III) determination in water and food samples. Chemosphere 2024, 351, 141272. [Google Scholar] [CrossRef]
- Fan, Z.; Cheng, P.; Zhang, P.; Zhang, G.; Han, J. Rheological insight of polysaccharide/protein based hydrogels in recent food and biomedical fields: A review. Int. J. Biol. Macromol. 2022, 222, 1642–1664. [Google Scholar] [CrossRef] [PubMed]
- Ilyin, S.O. Structural Rheology in the Development and Study of Complex Polymer Materials. Polymers 2024, 16, 2458. [Google Scholar] [CrossRef]
- Krajina, B.A.; Tropini, C.; Zhu, A.; DiGiacomo, P.; Sonnenburg, J.L.; Heilshorn, S.C.; Spakowitz, A.J. Dynamic Light Scattering Microrheology Reveals Multiscale Viscoelasticity of Polymer Gels and Precious Biological Materials. ACS Central Sci. 2017, 3, 1294–1303. [Google Scholar] [CrossRef]
- Geonzon, L.C.; Kobayashi, M.; Tassieri, M.; Bacabac, R.G.; Adachi, Y.; Matsukawa, S. Microrheological properties and local structure of ι-carrageenan gels probed by using optical tweezers. Food Hydrocoll. 2023, 137, 108325. [Google Scholar] [CrossRef]
- He, S.; Caggioni, M.; Lindberg, S.; Schultz, K.M. Gelation phase diagrams of colloidal rod systems measured over a large composition space. RSC Adv. 2022, 12, 12902–12912. [Google Scholar] [CrossRef]
- Schmidt, R.F.; Kiefer, H.; Dalgliesh, R.; Gradzielski, M.; Netz, R.R. Nanoscopic Interfacial Hydrogel Viscoelasticity Revealed from Comparison of Macroscopic and Microscopic Rheology. Nano Lett. 2024, 24, 4758–4765. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Wang, Y.; Jin, Y.; Guo, X.; Liu, Y.; Qi, X.; Lei, H.; Xu, H. Heat-induced whey protein isolate gels improved by cellulose nanocrystals: Gelling properties and microstructure. Carbohydr. Polym. 2020, 231, 115749. [Google Scholar] [CrossRef] [PubMed]
- Qu, R.-J.; Wang, Y.; Li, D.; Wang, L.-J. Rheological behavior of nanocellulose gels at various calcium chloride concentrations. Carbohydr. Polym. 2021, 274, 118660. [Google Scholar] [CrossRef]
- Feng, X.; Dai, H.; Ma, L.; Fu, Y.; Yu, Y.; Zhou, H.; Guo, T.; Zhu, H.; Wang, H.; Zhang, Y. Properties of Pickering emulsion stabilized by food-grade gelatin nanoparticles: Influence of the nanoparticles concentration. Colloids Surf. B Biointerfaces 2020, 196, 111294. [Google Scholar] [CrossRef]
- Yao, W.; Huang, X.; Li, C.; Kong, B.; Xia, X.; Sun, F.; Liu, Q.; Cao, C. Underlying the effect of soybean oil concentration on the gelling properties of myofibrillar protein-based emulsion gels: Perspective on interfacial adsorption, rheological properties and protein conformation. Food Hydrocoll. 2025, 162, 110935. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, L.; Lee, S.J.; Yang, Z. Formation and characterisation of concentrated emulsion gels stabilised by faba bean protein isolate and its applications for 3D food printing. Colloids Surf. A Physicochem. Eng. Asp. 2023, 671, 131622. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Teng, A.; Liu, A. Mechanical reinforcement of gelatin hydrogel with nanofiber cellulose as a function of percolation concentration. Int. J. Biol. Macromol. 2017, 103, 226–233. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Y.; Li, Y.; Xie, X.-A.; Li, P.; Du, B.; Li, L. Effect of different valence metal cations on the gel characteristics and microstructure of Inca peanut albumin gels. Food Hydrocoll. 2024, 151, 109783. [Google Scholar] [CrossRef]
- Kaur, H.; Sharma, P.; Patel, N.; Pal, V.K.; Roy, S. Accessing Highly Tunable Nanostructured Hydrogels in a Short Ionic Complementary Peptide Sequence via pH Trigger. Langmuir 2020, 36, 12107–12120. [Google Scholar] [CrossRef]
- Adams, D.J. Personal Perspective on Understanding Low Molecular Weight Gels. J. Am. Chem. Soc. 2022, 144, 11047–11053. [Google Scholar] [CrossRef]
- Shakeel, A.; Farooq, U.; Gabriele, D.; Marangoni, A.G.; Lupi, F.R. Bigels and multi-component organogels: An overview from rheological perspective. Food Hydrocoll. 2021, 111, 106190. [Google Scholar] [CrossRef]
- Raak, N.; Leonhardt, L.; Rohm, H.; Jaros, D. Size Modulation of Enzymatically Cross-Linked Sodium Caseinate Nanoparticles via Ionic Strength Variation Affects the Properties of Acid-Induced Gels. Dairy 2021, 2, 148–164. [Google Scholar] [CrossRef]
- Yan, X.; Jia, Y.; Man, H.; Liu, L.; Sun, S.; Qi, B.; Li, Y. Intermolecular interactions and gel properties of composite agglomerative networks based on oppositely charged polymers: Effects of pH and ionic strength. Food Hydrocoll. 2023, 139, 108557. [Google Scholar] [CrossRef]
- Sosa, E.I.F.; Chaves, M.G.; Peyrano, F.; Quiroga, A.V.; Avanza, M.V. Thermal Gelation of Proteins from Cajanus cajan Influenced by pH and Ionic Strength. Plant Foods Hum. Nutr. 2023, 78, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Q.; Chen, X.; McClements, D.J.; Zou, L.; Liu, W. Pickering-stabilized emulsion gels fabricated from wheat protein nanoparticles: Effect of pH, NaCl and oil content. J. Dispers. Sci. Technol. 2018, 39, 826–835. [Google Scholar] [CrossRef]
- Malektaj, H.; Drozdov, A.D.; Fini, E.; Christiansen, J.D. The Effect of pH on the Viscoelastic Response of Alginate–Montmorillonite Nanocomposite Hydrogels. Molecules 2024, 29, 244. [Google Scholar] [CrossRef] [PubMed]
- Ghebremedhin, M.; Seiffert, S.; Vilgis, T.A. Effects of sugar molecules on the rheological and tribological properties and on the microstructure of agarose-based fluid gels. Front. Soft Matter 2024, 4, 1363898. [Google Scholar] [CrossRef]
- Wu, C.-L.; Liao, J.-S.; Wang, J.-M.; Qi, J.-R. Gelation behavior and mechanism of low methoxyl pectin in the presence of erythritol and sucrose: The role of co-solutes. Int. J. Biol. Macromol. 2024, 271, 132261. [Google Scholar] [CrossRef]
- Wang, R.; Hartel, R.W. Confectionery gels: Gelling behavior and gel properties of gelatin in concentrated sugar solutions. Food Hydrocoll. 2022, 124, 107132. [Google Scholar] [CrossRef]
- Liu, Z.; Ruan, M.; Ha, S.; Chitrakar, B.; Li, H.; Hu, L.; Mo, H. Effect of curcumin/cyclodextrin composite on the gelation and 3D printability of k-carrageenan in the sucrose co-solute field. J. Food Eng. 2025, 391, 112441. [Google Scholar] [CrossRef]
- Chivers, P.R.A.; Smith, D.K. Shaping and structuring supramolecular gels. Nat. Rev. Mater. 2019, 4, 463–478. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Liu, C.; Zheng, X.; Tang, K. Tuning structure and properties of gelatin edible films through pullulan dialdehyde crosslinking. LWT 2021, 138, 110607. [Google Scholar] [CrossRef]
- Montero, M.P.; Tovar, C.A.; Alemán, A.; Gómez-Guillén, M.C. Characterisation of desolvation-produced high-molecular-weight gelatin fractions and their use for nanoparticles synthesis. J. Food Eng. 2024, 370, 111965. [Google Scholar] [CrossRef]
- Yue, B.; Shi, Y.; Yang, F.; Ye, D.; Jin, J.; Zhang, P.; Song, S.; Xu, Y.; Lin, H.; Zhu, S.; et al. Smartly tuning supramolecular chirality in polymer gels via photoexcitation-induced cooperative self-assembly. Sci. China Chem. 2025, 68, 1126–1135. [Google Scholar] [CrossRef]
- Derkach, S.R.; Ilyin, S.O.; Maklakova, A.A.; Kulichikhin, V.G.; Malkin, A.Y. The rheology of gelatin hydrogels modified by κ-carrageenan. LWT 2015, 63, 612–619. [Google Scholar] [CrossRef]
- Maroufi, L.Y.; Ghorbani, M.; Tabibiazar, M.; Mohammadi, M.; Pezeshki, A. Advanced properties of gelatin film by incorporating modified kappa-carrageenan and zein nanoparticles for active food packaging. Int. J. Biol. Macromol. 2021, 183, 753–759. [Google Scholar] [CrossRef]
- Luangapai, F.; Iwamoto, S. Influence of blending and layer-by-layer assembly methods on chitosan–gelatin composite films enriched with curcumin nanoemulsion. Int. J. Biol. Macromol. 2023, 249, 126061. [Google Scholar] [CrossRef]
- Li, J.; Parakhonskiy, B.V.; Skirtach, A.G. A decade of developing applications exploiting the properties of polyelectrolyte multilayer capsules. Chem. Commun. 2022, 59, 807–835. [Google Scholar] [CrossRef]
- Hirst, A.R.; Smith, D.K.; Feiters, M.C.; Geurts, H.P.M.; Wright, A.C. Two-Component Dendritic Gels: Easily Tunable Materials. J. Am. Chem. Soc. 2003, 125, 9010–9011. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.G.; Hirst, A.R.; Smith, D.K.; Brennan, C.; Ashworth, I. Controlling the materials properties and nanostructure of a single-component dendritic gel by adding a second component. Chem. Commun. 2004, 3, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiang, Y.; Chen, Y.; Wang, Y.; Dong, W.; Liu, Y.; Qi, X.; Shen, J. A Natural Eumelanin-Assisted Pullulan/Chitosan Hydrogel for the Management of Diabetic Oral Ulcers. Macromol. Biosci. 2024, 25, 2400526. [Google Scholar] [CrossRef]
- Gudmundsson, T.A.; Kuppadakkath, G.; Ghosh, D.; Ruether, M.; Seddon, A.; Ginesi, R.E.; Doutch, J.; Adams, D.J.; Gunnlaugsson, T.; Damodaran, K.K. Nanoscale assembly of enantiomeric supramolecular gels driven by the nature of solvents. Nanoscale 2024, 16, 8922–8930. [Google Scholar] [CrossRef]
- Zhao, X.; Li, D.; Wang, L.-J.; Wang, Y. Role of gelation temperature in rheological behavior and microstructure of high elastic starch-based emulsion-filled gel. Food Hydrocoll. 2023, 135, 108208. [Google Scholar] [CrossRef]
- Cheng, L.-C.; Vehusheia, S.L.K.; Doyle, P.S. Tuning Material Properties of Nanoemulsion Gels by Sequentially Screening Electrostatic Repulsions and Then Thermally Inducing Droplet Bridging. Langmuir 2020, 36, 3346–3355. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.-C.; Hashemnejad, S.M.; Zarket, B.; Muthukrishnan, S.; Doyle, P.S. Thermally and pH-responsive gelation of nanoemulsions stabilized by weak acid surfactants. J. Colloid Interface Sci. 2020, 563, 229–240. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, J.; Deng, Y.; Zhou, P.; Li, P.; Zhao, Z.; Liu, G.; Zhang, M. Regulating heat-induced fibrous whey protein-wheat starch composite emulsion gels as dysphagia food by preheating temperature: Insights from protein-starch interactions. Food Hydrocoll. 2025, 159, 110621. [Google Scholar] [CrossRef]
- Moghimi, E.; Jacob, A.R.; Koumakis, N.; Petekidis, G. Colloidal gels tuned by oscillatory shear. Soft Matter 2017, 13, 2371–2383. [Google Scholar] [CrossRef]
- Koumakis, N.; Moghimi, E.; Besseling, R.; Poon, W.C.K.; Brady, J.F.; Petekidis, G. Tuning colloidal gels by shear. Soft Matter 2015, 11, 4640–4648. [Google Scholar] [CrossRef]
- Alfaro-Rodríguez, M.-C.; García, M.C.; Prieto-Vargas, P.; Muñoz, J. Rheological Properties and Physical Stability of Aqueous Dispersions of Flaxseed Fibers. Gels 2024, 10, 787. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Petekidis, G. Shear induced tuning and memory effects in colloidal gels of rods and spheres. J. Chem. Phys. 2022, 157, 234902. [Google Scholar] [CrossRef]
- Sudreau, I.; Manneville, S.; Servel, M.; Divoux, T. Shear-induced memory effects in boehmite gels. J. Rheol. 2021, 66, 91–104. [Google Scholar] [CrossRef]
- Zhang, S.; Han, J.; Chen, L. Fabrication of pea protein gels with modulated rheological properties using high pressure processing. Food Hydrocoll. 2023, 144, 109002. [Google Scholar] [CrossRef]
- Peyrano, F.; de Lamballerie, M.; Avanza, M.V.; Speroni, F. Gelation of cowpea proteins induced by high hydrostatic pressure. Food Hydrocoll. 2021, 111, 106191. [Google Scholar] [CrossRef]
- Renggli, D.; Doyle, P.S. Thermogelation of nanoemulsions stabilized by a commercial pea protein isolate: High-pressure homogenization defines gel strength. Soft Matter 2025, 21, 652–669. [Google Scholar] [CrossRef]
- Heidary, A.; Soltanizadeh, N. The Effects of High-Pressure Homogenization on Physicochemical and Functional Properties of Gelatin. Food Bioprocess Technol. 2024, 17, 100–122. [Google Scholar] [CrossRef]
- Liu, Y.; Chao, C.; Yu, J.; Wang, S.; Wang, S.; Copeland, L. New insights into starch gelatinization by high pressure: Comparison with heat-gelatinization. Food Chem. 2020, 318, 126493. [Google Scholar] [CrossRef]
- Lv, P.; Wang, D.; Dai, L.; Wu, X.; Gao, Y.; Yuan, F. Pickering emulsion gels stabilized by high hydrostatic pressure-induced whey protein isolate gel particles: Characterization and encapsulation of curcumin. Food Res. Int. 2020, 132, 109032. [Google Scholar] [CrossRef]
- Ni, Y.; Wu, J.; Jiang, Y.; Li, J.; Fan, L.; Huang, S. High-internal-phase pickering emulsions stabilized by ultrasound-induced nanocellulose hydrogels. Food Hydrocoll. 2022, 125, 107395. [Google Scholar] [CrossRef]
- Song, Y.; Xiao, J.; Li, L.; Wan, L.; Li, B.; Zhang, X. Ultrasound treatment of crystalline oil-in-water emulsions stabilized by sodium caseinate: Impact on emulsion stability through altered crystallization behavior in the oil globules. Ultrason. Sonochem. 2024, 106, 106897. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, S.-B. Structural and functional properties of self-assembled peanut protein nanoparticles prepared by ultrasonic treatment: Effects of ultrasound intensity and protein concentration. Food Chem. 2023, 413, 135626. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Tao, R.; Sun, Y.; Wang, L.; Li, Y.; Fan, B.; Wang, F. Enhancing the Gelation Behavior of Transglutaminase-Induced Soy Protein Isolate(SPI) through Ultrasound-Assisted Extraction. Foods 2024, 13, 738. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.V.; Huynh, P.X.; Kha, T.C. Ultrasound-Induced Modification of Durian Starch (Durio zibethinus) for Gel-Based Applications: Physicochemical and Thermal Properties. Gels 2025, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Guo, C.; Li, X.; Yuan, K.; Yang, X.; Guo, Y.; Yang, X. Preparation and structural characteristics of composite alginate/casein emulsion gels: A microscopy and rheology study. Food Hydrocoll. 2021, 118, 106792. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, D.; Guo, Q.; Liu, C. Textural and structural properties of a κ-carrageenan–konjac gum mixed gel: Effects of κ-carrageenan concentration, mixing ratio, sucrose and Ca2+ concentrations and its application in milk pudding. J. Sci. Food Agric. 2020, 101, 3021–3029. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Z.; Yu, S.; Guo, X.; Ai, C.; Tang, X.; Chen, H.; Lin, J.; Zhang, X.; Meng, H. Effects of pH and temperature on the structure, rheological and gel-forming properties of sugar beet pectins. Food Hydrocoll. 2021, 116, 106646. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, Q.; Wang, X.; Bai, Z.; Xu, X.; Ma, J. Casein-based hydrogels: Advances and prospects. Food Chem. 2024, 447, 138956. [Google Scholar] [CrossRef] [PubMed]
- Tanger, C.; Müller, M.; Andlinger, D.; Kulozik, U. Influence of pH and ionic strength on the thermal gelation behaviour of pea protein. Food Hydrocoll. 2022, 123, 106903. [Google Scholar] [CrossRef]
- Hu, C.; Lu, W.; Sun, C.; Zhao, Y.; Zhang, Y.; Fang, Y. Gelation behavior and mechanism of alginate with calcium: Dependence on monovalent counterions. Carbohydr. Polym. 2022, 294, 119788. [Google Scholar] [CrossRef]
- Mousavi, S.M.R.; Rafe, A.; Yeganehzad, S. Structure-rheology relationships of composite gels: Alginate and Basil seed gum/guar gum. Carbohydr. Polym. 2020, 232, 115809. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Mujmer, K.; Aswal, V.K.; Gupta, S.; Thareja, P. Structure, rheology, and 3D printing of salt-induced κ-carrageenan gels. Mater. Today Commun. 2023, 35, 105807. [Google Scholar] [CrossRef]
- Guo, R.; Liu, L.; Huang, Y.; Lv, M.; Zhu, Y.; Wang, Z.; Zhu, X.; Sun, B. Effect of Na+ and Ca2+ on the texture, structure and microstructure of composite protein gel of mung bean protein and wheat gluten. Food Res. Int. 2023, 172, 113124. [Google Scholar] [CrossRef]
- Lei, Y.; Ouyang, H.; Peng, W.; Yu, X.; Jin, L.; Li, S. Effect of NaCl on the Rheological, Structural, and Gelling Properties of Walnut Protein Isolate-κ-Carrageenan Composite Gels. Gels 2022, 8, 259. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.; Yoo, B.; Lim, S.-T. Effects of sugars and sugar alcohols on thermal transition and cold stability of corn starch gel. Food Hydrocoll. 2004, 18, 133–142. [Google Scholar] [CrossRef]
- Andreadis, M.; Moschakis, T. Effect of ethanol on gelation and microstructure of whey protein gels in the presence of NaCl. Food Hydrocoll. 2023, 134, 107985. [Google Scholar] [CrossRef]
- Jiang, W.-X.; Qi, J.-R.; Liao, J.-S.; Yang, X.-Q. Acid/ethanol induced pectin gelling and its application in emulsion gel. Food Hydrocoll. 2021, 118, 106774. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Li, L. Formation of pH-responsive hydrogel beads and their gel properties: Soybean protein nanofibers and sodium alginate. Carbohydr. Polym. 2024, 329, 121748. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Li, J.; Parakhonskiy, B.; Skirtach, A.G. Intestinal-specific oral delivery of lactoferrin with alginate-based composite and hybrid CaCO3-hydrogel beads. Food Chem. 2024, 451, 139205. [Google Scholar] [CrossRef]
- Le, X.T.; Rioux, L.-E.; Turgeon, S.L. Formation and functional properties of protein–polysaccharide electrostatic hydrogels in comparison to protein or polysaccharide hydrogels. Adv. Colloid Interface Sci. 2017, 239, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Tang, Y.; Mao, J.; Zhou, Y.; Chen, T.; Zhu, X. Cellulose nanofibril as a crosslinker to reinforce the sodium alginate/chitosan hydrogels. Int. J. Biol. Macromol. 2021, 189, 890–899. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, J.; Qin, S.; Pei, Y.; Zheng, X.; Tang, K. High mechanical strength gelatin composite hydrogels reinforced by cellulose nanofibrils with unique beads-on-a-string morphology. Int. J. Biol. Macromol. 2020, 164, 1776–1784. [Google Scholar] [CrossRef]
- Jeong, C.; Kim, S.; Lee, C.; Cho, S.; Kim, S.-B. Changes in the Physical Properties of Calcium Alginate Gel Beads under a Wide Range of Gelation Temperature Conditions. Foods 2020, 9, 180. [Google Scholar] [CrossRef]
- Yan, J.; Li, S.; Chen, G.; Ma, C.; McClements, D.J.; Liu, X.; Liu, F. Formation, physicochemical properties, and comparison of heat- and enzyme-induced whey protein-gelatin composite hydrogels. Food Hydrocoll. 2023, 137, 108384. [Google Scholar] [CrossRef]
- Ryu, J.; McClements, D.J. Impact of heat-set and cold-set gelling polysaccharides on potato protein gelation: Gellan gum, agar, and methylcellulose. Food Hydrocoll. 2024, 149, 109535. [Google Scholar] [CrossRef]
- Obas, F.-L.; Thomas, L.C.; Terban, M.W.; Schmidt, S.J. Characterization of the thermal behavior and structural properties of a commercial high-solids confectionary gel made with gelatin. Food Hydrocoll. 2024, 148, 109432. [Google Scholar] [CrossRef]
- Jia, R.; He, X.; McClements, D.J.; Qin, Y.; Xiong, L.; Dai, L.; Sun, Q. Control of starch gel properties by shear-induced disruption of gelatinized swollen starch granule integrity. Food Hydrocoll. 2025, 168, 111528. [Google Scholar] [CrossRef]
- Fang, F.; Martinez, M.M.; Campanella, O.H.; Hamaker, B.R. Long-term low shear-induced highly viscous waxy potato starch gel formed through intermolecular double helices. Carbohydr. Polym. 2020, 232, 115815. [Google Scholar] [CrossRef]
- Toprakcioglu, Z.; Knowles, T.P.J. Shear-mediated sol-gel transition of regenerated silk allows the formation of Janus-like microgels. Sci. Rep. 2021, 11, 6673. [Google Scholar] [CrossRef]
- Larrea-Wachtendorff, D.; Sousa, I.; Ferrari, G. Starch-Based Hydrogels Produced by High-Pressure Processing (HPP): Effect of the Starch Source and Processing Time. Food Eng. Rev. 2021, 13, 622–633. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, L.; Wang, H.; Zhou, B.; Jiang, L.; Zhu, X. Effect of pH-shifting and ultrasound on soy/potato protein structure and gelation. Food Hydrocoll. 2025, 159, 110672. [Google Scholar] [CrossRef]
- Mozafarpour, R.; Koocheki, A.; Sani, M.A.; McClements, D.J.; Mehr, H.M. Ultrasound-modified protein-based colloidal particles: Interfacial activity, gelation properties, and encapsulation efficiency. Adv. Colloid Interface Sci. 2022, 309, 102768. [Google Scholar] [CrossRef]
- Li, R.; Ma, C.; Wang, N.; Wang, J.; Yang, X. Exploring the potential of ultrasound to improve the physicochemical properties of protein-based emulsion gels: By ultrasonically treating emulsion (pre-emulsification, post-emulsification) and substrate solution respectively. Food Hydrocoll. 2025, 168, 111492. [Google Scholar] [CrossRef]
- Li, Q.; He, Q.; Xu, M.; Li, J.; Liu, X.; Wan, Z.; Yang, X. Food-Grade Emulsions and Emulsion Gels Prepared by Soy Protein–Pectin Complex Nanoparticles and Glycyrrhizic Acid Nanofibrils. J. Agric. Food Chem. 2020, 68, 1051–1063. [Google Scholar] [CrossRef]
- Hao, Y.; Li, S.; Guo, X.; Fang, M.; Liu, X.; Gong, Z. Preparation of shellac nanoparticles-chitosan complexes stabilized Pickering emulsion gels and its application in β-carotene delivery. Int. J. Biol. Macromol. 2024, 281, 136583. [Google Scholar] [CrossRef]
- Yang, T.; Jia, H.; Song, Y.; Xu, D.; Li, B.; Li, L.; Skirtach, A.; Zhang, X. Natural shellac nanoparticles embedded porous chitosan microgel as a stability-enhanced Pickering interfacial biocatalyst. Int. J. Biol. Macromol. 2025, 321, 146213. [Google Scholar] [CrossRef]
- Momtaz, M.; Momtaz, E.; Mehrgardi, M.A.; Momtaz, F.; Narimani, T.; Poursina, F. Preparation and characterization of gelatin/chitosan nanocomposite reinforced by NiO nanoparticles as an active food packaging. Sci. Rep. 2024, 14, 519. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Ji, N.; Wang, Y.; Dai, L.; Xiong, L.; Sun, Q. Starch-based nanoparticles: Stimuli responsiveness, toxicity, and interactions with food components. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1075–1100. [Google Scholar] [CrossRef]
- Gong, H.; Zi, Y.; Kan, G.; Li, L.; Shi, C.; Wang, X.; Zhong, J. Preparation of food-grade EDC/NHS-crosslinked gelatin nanoparticles and their application for Pickering emulsion stabilization. Food Chem. 2024, 436, 137700. [Google Scholar] [CrossRef]
- Liu, H.; Liu, C.; McClements, D.J.; Xu, X.; Bai, C.; Sun, Q.; Xu, F.; Dai, L. Reinforcement of heat-set whey protein gels using whey protein nanofibers: Impact of nanofiber morphology and pH values. Food Hydrocoll. 2024, 153, 109954. [Google Scholar] [CrossRef]
- Mendoza, L.; Batchelor, W.; Tabor, R.F.; Garnier, G. Gelation mechanism of cellulose nanofibre gels: A colloids and interfacial perspective. J. Colloid Interface Sci. 2018, 509, 39–46. [Google Scholar] [CrossRef]
- Ghorani, B.; Emadzadeh, B.; Rezaeinia, H.; Russell, S. Improvements in gelatin cold water solubility after electrospinning and associated physicochemical, functional and rheological properties. Food Hydrocoll. 2020, 104, 105740. [Google Scholar] [CrossRef]
- Onyeaka, H.; Passaretti, P.; Miri, T.; Al-Sharify, Z.T. The safety of nanomaterials in food production and packaging. Curr. Res. Food Sci. 2022, 5, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Störmer, A.; Bott, J.; Kemmer, D.; Franz, R. Critical review of the migration potential of nanoparticles in food contact plastics. Trends Food Sci. Technol. 2017, 63, 39–50. [Google Scholar] [CrossRef]
- Kaphle, A.; Navya, P.N.; Umapathi, A.; Daima, H.K. Nanomaterials for agriculture, food and environment: Applications, toxicity and regulation. Environ. Chem. Lett. 2018, 16, 43–58. [Google Scholar] [CrossRef]
- Qi, X.; Li, Y.; Xiang, Y.; Chen, Y.; Shi, Y.; Ge, X.; Zeng, B.; Shen, J. Hyperthermia-enhanced immunoregulation hydrogel for oxygenation and ROS neutralization in diabetic foot ulcers. Cell Biomater. 2025, 1, 100020. [Google Scholar] [CrossRef]
- Gupta, R.K.; Guha, P.; Srivastav, P.P. Investigating the toxicological effects of nanomaterials in food packaging associated with human health and the environment. J. Hazard. Mater. Lett. 2024, 5, 100125. [Google Scholar] [CrossRef]
- Rahman, J.M.H.; Shiblee, N.I.; Ahmed, K.; Khosla, A.; Kawakami, M.; Furukawa, H. Rheological and mechanical properties of edible gel materials for 3D food printing technology. Heliyon 2020, 6, e05859. [Google Scholar] [CrossRef] [PubMed]
- Beikzadeh, S.; Hosseini, S.M.; Mofid, V.; Ramezani, S.; Ghorbani, M.; Ehsani, A.; Mortazavian, A.M. Electrospun ethyl cellulose/poly caprolactone/gelatin nanofibers: The investigation of mechanical, antioxidant, and antifungal properties for food packaging. Int. J. Biol. Macromol. 2021, 191, 457–464. [Google Scholar] [CrossRef]
- Chen, J.; Luo, L.; Cen, C.; Liu, Y.; Li, H.; Wang, Y. The nano antibacterial composite film carboxymethyl chitosan/gelatin/nano ZnO improves the mechanical strength of food packaging. Int. J. Biol. Macromol. 2022, 220, 462–471. [Google Scholar] [CrossRef]
- Piao, X.; Li, J.; Zhao, Y.; Guo, L.; Zheng, B.; Zhou, R.; Ostrikov, K. Oxidized cellulose nanofibrils-based surimi gel enhancing additives: Interactions, performance and mechanisms. Food Hydrocoll. 2022, 133, 107893. [Google Scholar] [CrossRef]
- Riquelme, N.; Savignones, C.; López, A.; Zúñiga, R.N.; Arancibia, C. Effect of Gelling Agent Type on the Physical Properties of Nanoemulsion-Based Gels. Colloids Interfaces 2023, 7, 49. [Google Scholar] [CrossRef]
- Xu, W.; Ning, Y.; Wang, M.; Zhang, S.; Sun, H.; Yin, Y.; Li, N.; Li, P.; Luo, D. Construction of astaxanthin loaded Pickering emulsions gel stabilized by xanthan gum/lysozyme nanoparticles with konjac glucomannan from structure, protection and gastrointestinal digestion perspective. Int. J. Biol. Macromol. 2023, 252, 126421. [Google Scholar] [CrossRef]
- Alqarni, L.S.; Alghamdi, A.M.; Elamin, N.Y.; Rajeh, A. Enhancing the optical; electrical, dielectric properties and antimicrobial activity of chitosan/gelatin incorporated with Co-doped ZnO nanoparticles: Nanocomposites for use in energy storage and food packaging. J. Mol. Struct. 2024, 1297, 137011. [Google Scholar] [CrossRef]
- Shipovskaya, A.B.; Ushakova, O.S.; Volchkov, S.S.; Shipenok, X.M.; Shmakov, S.L.; Gegel, N.O.; Burov, A.M. Chiral Nanostructured Glycerohydrogel Sol–Gel Plates of Chitosan L- and D-Aspartate: Supramolecular Ordering and Optical Properties. Gels 2024, 10, 427. [Google Scholar] [CrossRef]
- Seiffert, S. Origin of nanostructural inhomogeneity in polymer-network gels. Polym. Chem. 2017, 8, 4472–4487. [Google Scholar] [CrossRef]
- Araújo, J.F.; Bourbon, A.I.; Simões, L.S.; Vicente, A.A.; Coutinho, P.J.G.; Ramos, O.L. Physicochemical characterisation and release behaviour of curcumin-loaded lactoferrin nanohydrogels into food simulants. Food Funct. 2020, 11, 305–317. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, X.; Hu, N.; Zhao, C.; Wu, Y.; Liu, J. Study on properties of TGase-induced pea protein–zein complex gels. J. Food Eng. 2023, 354, 111578. [Google Scholar] [CrossRef]
- Cortés, N.M.; Montoto, S.S.; Ruiz, M.E.; Califano, A.N.; Zaritzky, N.; Lorenzo, G. Rheological properties and microstructure of thermodynamically stable microemulsions as factors influencing the release rate of liposoluble vitamins. Food Hydrocoll. 2023, 141, 108699. [Google Scholar] [CrossRef]
- Zhou, H.; Hu, X.; Xiang, X.; McClements, D.J. Modification of textural attributes of potato protein gels using salts, polysaccharides, and transglutaminase: Development of plant-based foods. Food Hydrocoll. 2023, 144, 108909. [Google Scholar] [CrossRef]
- Cheng, Y.; Meng, Y.; Liu, S. Diversified Techniques for Restructuring Meat Protein-Derived Products and Analogues. Foods 2024, 13, 1950. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, H.; Ren, Y.; Sun, M.; Zhang, T.; Li, H.; Liu, X. Functionality and application of emulsion gels in fat replacement strategies for dairy products. Trends Food Sci. Technol. 2024, 152, 104673. [Google Scholar] [CrossRef]
- Ren, Y.; Huang, L.; Zhang, Y.; Li, H.; Zhao, D.; Cao, J.; Liu, X. Application of Emulsion Gels as Fat Substitutes in Meat Products. Foods 2022, 11, 1950. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.C.; Shin, D.-M.; Yune, J.H.; Jeong, C.H.; Han, S.G. Evaluation of gels formulated with whey proteins and sodium dodecyl sulfate as a fat replacer in low-fat sausage. Food Chem. 2021, 337, 127682. [Google Scholar] [CrossRef]
- Milićević, N.; Sakač, M.; Hadnađev, M.; Škrobot, D.; Šarić, B.; Hadnađev, T.D.; Jovanov, P.; Pezo, L. Physico-chemical properties of low-fat cookies containing wheat and oat bran gels as fat replacers. J. Cereal Sci. 2020, 95, 103056. [Google Scholar] [CrossRef]
- Li, S.; Yan, J.; Yang, J.; Chen, G.; McClements, D.J.; Ma, C.; Liu, X.; Liu, F. Modulating peppermint oil flavor release properties of emulsion-filled protein gels: Impact of cross-linking method and matrix composition. Food Res. Int. 2024, 185, 114277. [Google Scholar] [CrossRef]
- Kim, Y.M.; Lee, K.; Lee, Y.; Yang, K.; Choe, D.; Roh, Y.H. Thermoresponsive semi-interpenetrating gelatin-alginate networks for encapsulation and controlled release of scent molecules. Int. J. Biol. Macromol. 2022, 208, 1096–1105. [Google Scholar] [CrossRef]
- Keum, D.H.; Han, J.H.; Kwon, H.C.; Park, S.M.; Kim, H.Y.; Han, S.G. Enhancing the flavor of plant-based meat analogues using flavor-capturing alginate/β-cyclodextrin hydrogel beads. Int. J. Biol. Macromol. 2025, 309, 142930. [Google Scholar] [CrossRef]
- Liao, P.; Dai, S.; Lian, Z.; Tong, X.; Yang, S.; Chen, Y.; Qi, W.; Peng, X.; Wang, H.; Jiang, L. The Layered Encapsulation of Vitamin B2 and β-Carotene in Multilayer Alginate/Chitosan Gel Microspheres: Improving the Bioaccessibility of Vitamin B2 and β-Carotene. Foods 2022, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Davachi, S.M.; Ravanfar, R.; Dadmohammadi, Y.; Deisenroth, T.W.; Pho, T.V.; Odorisio, P.A.; Darji, R.H.; Abbaspourrad, A. Improvement of vitamin C stability in vitamin gummies by encapsulation in casein gel. Food Hydrocoll. 2021, 113, 106414. [Google Scholar] [CrossRef]
- Kuo, C.-C.; Clark, S.; Qin, H.; Shi, X. Development of a shelf-stable, gel-based delivery system for probiotics by encapsulation, 3D printing, and freeze-drying. LWT 2022, 157, 113075. [Google Scholar] [CrossRef]
- Zhang, S.; Leidy, H.J.; Vardhanabhuti, B. Protein Beverage vs. Protein Gel on Appetite Control and Subsequent Food Intake in Healthy Adults. Nutrients 2015, 7, 8700–8711. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, T.; Zhang, S.; Leng, J.; Li, L.; Zhao, W. β-Glucan-based superabsorbent hydrogel ameliorates obesity-associated metabolic disorders via delaying gastric emptying, improving intestinal barrier function, and modulating gut microbiota. Int. J. Biol. Macromol. 2025, 304, 140846. [Google Scholar] [CrossRef] [PubMed]
- Lenie, M.D.R.; Ahmadzadeh, S.; Van Bockstaele, F.; Ubeyitogullari, A. Development of a pH-responsive system based on starch and alginate-pectin hydrogels using coaxial 3D food printing. Food Hydrocoll. 2024, 153, 109989. [Google Scholar] [CrossRef]
- Yu, J.; Wang, X.-Y.; Li, D.; Wang, L.-J.; Wang, Y. Development of soy protein isolate emulsion gels as extrusion-based 3D food printing inks: Effect of polysaccharides incorporation. Food Hydrocoll. 2022, 131, 107824. [Google Scholar] [CrossRef]
- Chen, J.; Wu, A.; Yang, M.; Ge, Y.; Pristijono, P.; Li, J.; Xu, B.; Mi, H. Characterization of sodium alginate-based films incorporated with thymol for fresh-cut apple packaging. Food Control. 2021, 126, 108063. [Google Scholar] [CrossRef]
- Gheorghita, R.; Gutt, G.; Amariei, S. The Use of Edible Films Based on Sodium Alginate in Meat Product Packaging: An Eco-Friendly Alternative to Conventional Plastic Materials. Coatings 2020, 10, 166. [Google Scholar] [CrossRef]
- Sagiri, S.S.; Poverenov, E. Oleogel-Based Nanoemulsions for Beverages: Effect of Self-Assembled Fibrillar Networks on Stability and Release Properties of Emulsions. Foods 2024, 13, 680. [Google Scholar] [CrossRef]
- Ozorio, L.; Passerini, A.B.; Silva, A.P.; Braga, A.R.; Perrechil, F. Designing Plant-Based Foods: Biopolymer Gelation for Enhanced Texture and Functionality. Foods 2025, 14, 1645. [Google Scholar] [CrossRef]
- Salahi, M.R.; Mohebbi, M.; Razavi, S.M.A. Analyzing the effects of aroma and texture interactions on oral processing behavior and dynamic sensory perception: A case study on cold-set emulsion-filled gels containing limonene and menthol. Food Hydrocoll. 2024, 154, 110128. [Google Scholar] [CrossRef]
- Raj, A.S.; Rahul, R.; Karthik, P. Nanoorganogels for Encapsulating Food Bioactive Compounds. Food Bioprocess Technol. 2025, 18, 129–149. [Google Scholar] [CrossRef]
- Mohammadian, M.; Waly, M.I.; Moghadam, M.; Emam-Djomeh, Z.; Salami, M.; Moosavi-Movahedi, A.A. Nanostructured food proteins as efficient systems for the encapsulation of bioactive compounds. Food Sci. Hum. Wellness 2020, 9, 199–213. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, F.; Chen, Y.; Shen, Q.; Feng, S.; Liang, L. Co-encapsulation of bioactive components using protein-based various assemblies: Necessary, assembling structure, location and partition. Food Hydrocoll. 2024, 148, 109492. [Google Scholar] [CrossRef]
- Lai, W.-F.; Wong, W.-T. Property-Tuneable Microgels Fabricated by Using Flow-Focusing Microfluidic Geometry for Bioactive Agent Delivery. Pharmaceutics 2021, 13, 787. [Google Scholar] [CrossRef]
- Chen, H.; Pan, S.; Regenstein, J.M.; Huang, J.; Wang, L. Characteristics of composite gels composed of citrus insoluble nanofiber and amylose and their potential to be used as fat replacers. Food Chem. 2023, 409, 135269. [Google Scholar] [CrossRef]
- Cui, S.; McClements, D.J.; Shi, J.; Xu, X.; Ning, F.; Liu, C.; Zhou, L.; Sun, Q.; Dai, L. Fabrication and characterization of low-fat Pickering emulsion gels stabilized by zein/phytic acid complex nanoparticles. Food Chem. 2023, 402, 134179. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Huang, Y.; Zhou, S.; Regenstein, J.M.; Wang, L. A composite gel formed by konjac glucomannan together with Nano-CF obtained by FeCl3-citric acid hydrolysis as a potential fat substitute. Int. J. Biol. Macromol. 2024, 268, 131618. [Google Scholar] [CrossRef] [PubMed]
- DeLoid, G.M.; Sohal, I.S.; Lorente, L.R.; Molina, R.M.; Pyrgiotakis, G.; Stevanovic, A.; Zhang, R.; McClements, D.J.; Geitner, N.K.; Bousfield, D.W.; et al. Reducing Intestinal Digestion and Absorption of Fat Using a Nature-Derived Biopolymer: Interference of Triglyceride Hydrolysis by Nanocellulose. ACS Nano 2018, 12, 6469–6479. [Google Scholar] [CrossRef] [PubMed]
- Riahi, Z.; Hong, S.J.; Rhim, J.-W.; Shin, G.H.; Kim, J.T. High-performance multifunctional gelatin-based films engineered with metal-organic frameworks for active food packaging applications. Food Hydrocoll. 2023, 144, 108984. [Google Scholar] [CrossRef]
- Ahari, H.; Naeimabadi, M. Employing Nanoemulsions in Food Packaging: Shelf Life Enhancement. Food Eng. Rev. 2021, 13, 858–883. [Google Scholar] [CrossRef]
- Babu, P.J. Nanotechnology mediated intelligent and improved food packaging. Int. Nano Lett. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Moustafa, H.; Hemida, M.H.; Nour, M.A.; Abou-Kandil, A.I. Intelligent packaging films based on two-dimensional nanomaterials for food safety and quality monitoring: Future insights and roadblocks. J. Thermoplast. Compos. Mater. 2024, 38, 1208–1230. [Google Scholar] [CrossRef]
- Koirala, P.; Sagar, N.A.; Thuanthong, A.; Al-Asmari, F.; Jagtap, S.; Nirmal, N. Revolutionizing seafood packaging: Advancements in biopolymer smart nano-packaging for extended shelf-life and quality assurance. Food Res. Int. 2025, 203, 115826. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Li, Z.; Huang, X.; Du, L.; Li, W.; Gao, P.; Chen, Z.; Zhang, J.; Guo, Z.; Li, Z.; et al. Advances in 3D and 4D Printing of Gel-Based Foods: Mechanisms, Applications, and Future Directions. Gels 2025, 11, 94. [Google Scholar] [CrossRef]
- Barekat, S.; Ubeyitogullari, A. Maximizing sorghum proteins printability: Optimizing gel formulation and 3D-printing parameters to develop a novel bioink. Int. J. Biol. Macromol. 2025, 300, 140245. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, M.; Sun, Y.; Phuhongsung, P. Improving 3D/4D printing characteristics of natural food gels by novel additives: A review. Food Hydrocoll. 2022, 123, 107160. [Google Scholar] [CrossRef]
- Li, X.; Liuping, F.; Yuanfa, L. New insights into food O/W emulsion gels: Strategies of reinforcing mechanical properties and outlook of being applied to food 3D printing. Crit. Rev. Food Sci. Nutr. 2021, 63, 1564–1586. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Xu, T.; Liu, Y.; Li, H.; Luo, J.; Zou, Y.; Zhong, Y.; Li, Y.; Lu, B. Investigation of the mechanism of casein protein to enhance 3D printing accuracy of cassava starch gel. Carbohydr. Polym. 2022, 295, 119827. [Google Scholar] [CrossRef] [PubMed]
| Application | Function | Examples | Key Benefits | Ref. |
|---|---|---|---|---|
| Textural modification | Modify mouthfeel, texture, and rheology | Restructured meat, plant-based foods | Improves sensory appeal and consumer acceptability | [45,209,210] |
| Fat replacement | Mimic fat mouthfeel with lower calorie content | Protein or polysaccharide-based gels fat mimetics | Reduces calorie content while maintaining creamy texture | [211,212,213,214] |
| Flavor encapsulation | Trap and release volatile aroma/flavor compounds | Emulsion-filled gels, hydrogels with flavor compounds | Controlled flavor release and protection from oxidation | [215,216,217] |
| Nutrient delivery | Controlled release and protection of bioactives | Gels encapsulating vitamins, probiotics, polyphenols | Enhances stability, bioavailability, and targeted release | [218,219,220] |
| Satiety enhancement | Induce gastric retention or swelling to promote fullness | β-Glucan, or protein gels for appetite regulation | Supports weight management and satiety | [221,222] |
| 3D food printing | Serve as printable bio-ink or scaffold for customized food shapes | Alginate, pectin, starch, or protein-based printable gels | Enables designable textures and personalized nutrition | [223,224] |
| Edible coatings/films | Serve as sustainable food packaging materials | Gelatin or alginate-based films on fruits or meat | Enhances shelf-life and appearance | [225,226] |
| Water or oil structuring | Structure liquids into gels for functional or sensory improvement | Oleogels, hydrogel particles in beverages | Stabilizes emulsions and improves mouthfeel in reduced-fat products | [227] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Cao, L.; Song, J.; Skirtach, A.G. Tuning Nanostructure of Gels: From Structural and Functional Controls to Food Applications. Gels 2025, 11, 620. https://doi.org/10.3390/gels11080620
Yang T, Cao L, Song J, Skirtach AG. Tuning Nanostructure of Gels: From Structural and Functional Controls to Food Applications. Gels. 2025; 11(8):620. https://doi.org/10.3390/gels11080620
Chicago/Turabian StyleYang, Tangyu, Lin Cao, Junnan Song, and Andre G. Skirtach. 2025. "Tuning Nanostructure of Gels: From Structural and Functional Controls to Food Applications" Gels 11, no. 8: 620. https://doi.org/10.3390/gels11080620
APA StyleYang, T., Cao, L., Song, J., & Skirtach, A. G. (2025). Tuning Nanostructure of Gels: From Structural and Functional Controls to Food Applications. Gels, 11(8), 620. https://doi.org/10.3390/gels11080620







