Impact of Water Conductivity on the Structure and Swelling Dynamics of E-Beam Cross-Linked Hydrogels
Abstract
1. Introduction
2. Results and Discussion
2.1. Network Parameters
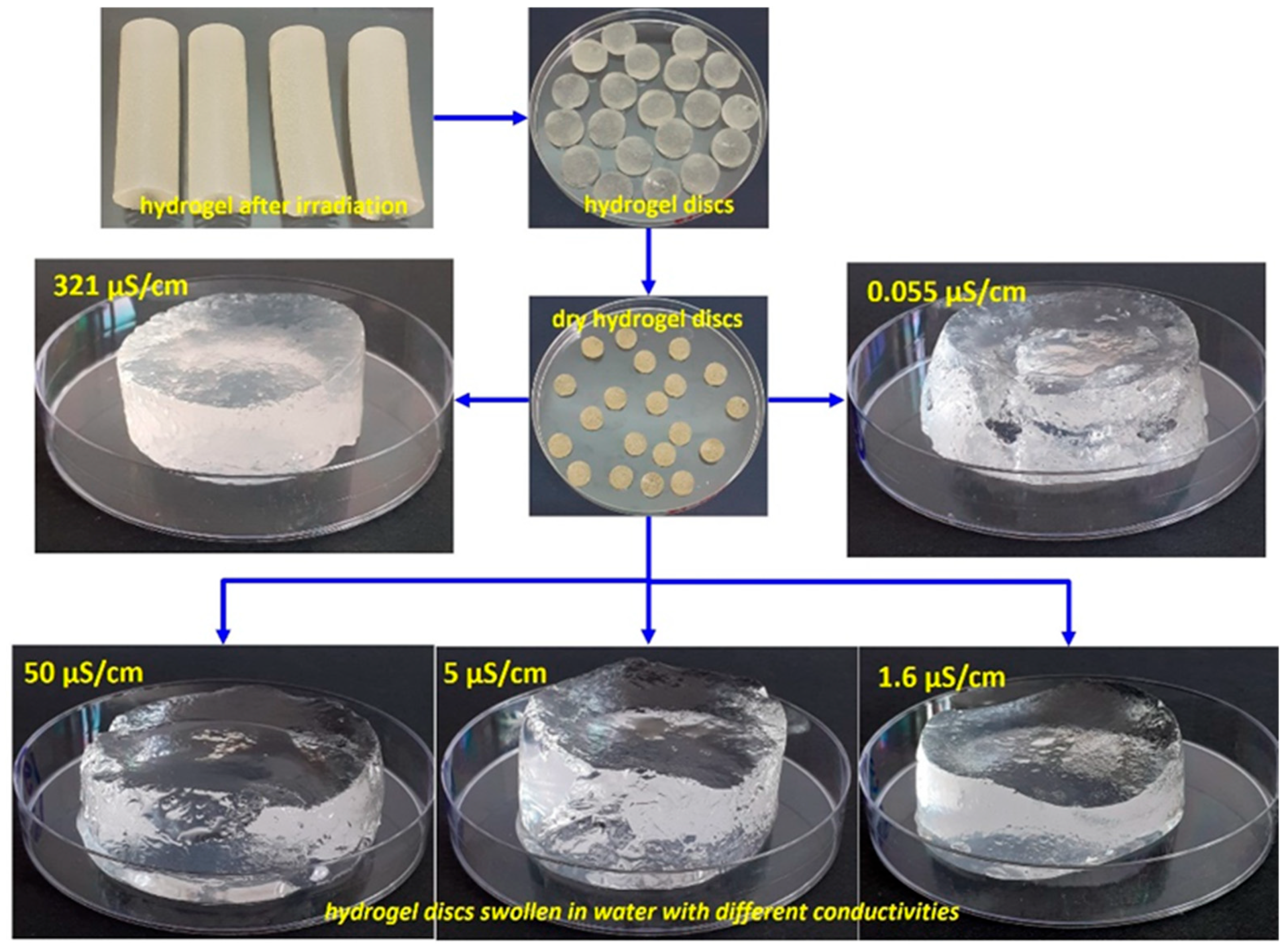
2.2. Swelling Degree of Hydrogels in Aqueous Media with Varying Electrical Conductivities
2.3. Swelling Kinetics vs. Water Electrical Conductivity
2.4. FTIR Spectral Analysis of Hydrogels Treated with Water of Varying Electrical Conductivity
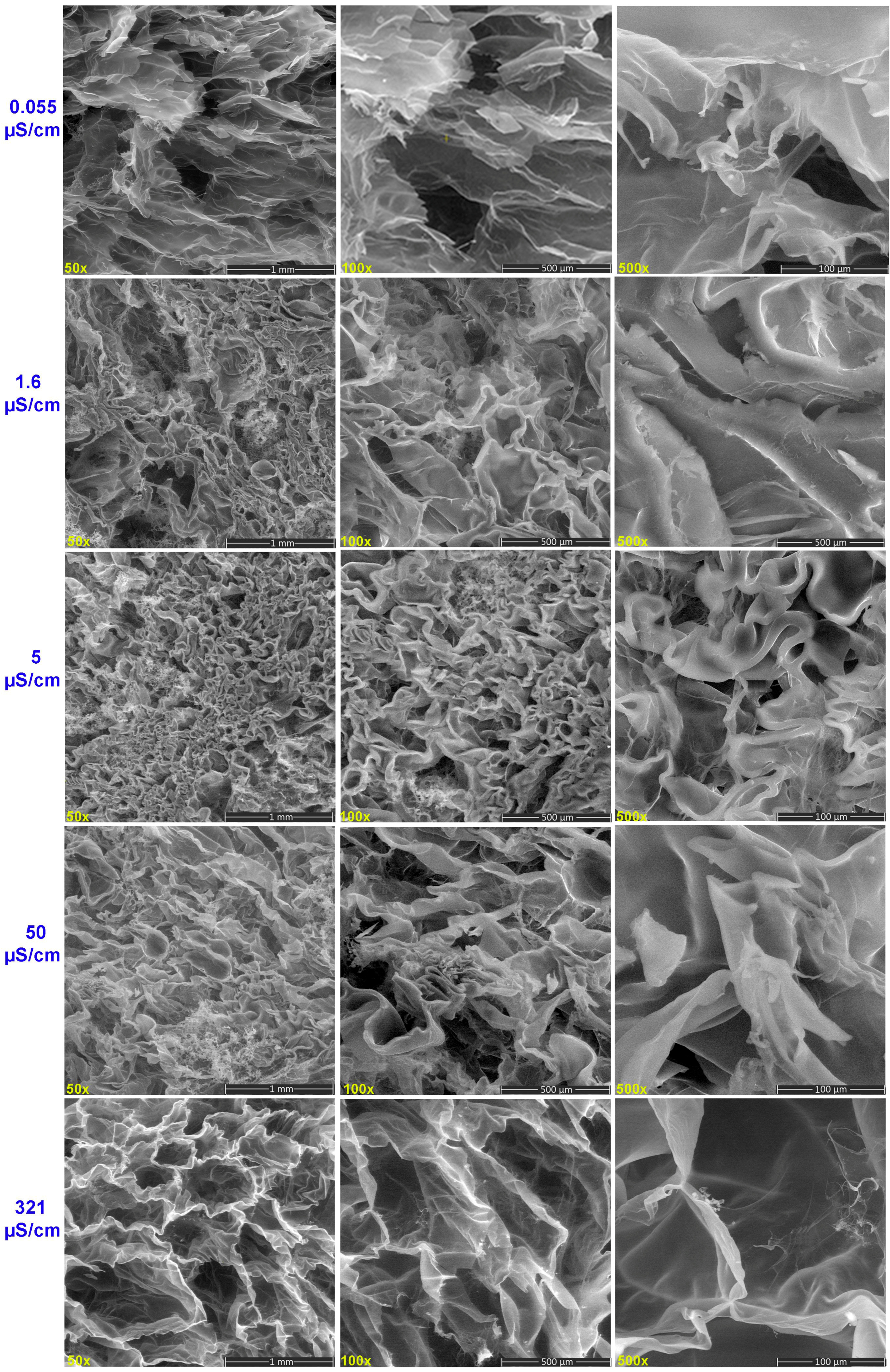
2.5. SEM Morphological Analysis of Hydrogels Treated with Water of Varying Electrical Conductivity
3. Conclusions
4. Materials and Methods
4.1. Materials
- (a)
- Deionized water (conductivity: 0.055 μS/cm; pH = 6.7) produced in the laboratory using TKA Pacific UP/UPW6 (Thermo Fisher Scientific, Niederelbert, Germany).
- (b)
- Distilled water (conductivity: 1.6 μS/cm; pH = 6.05; ionic concentrations: Ca2+ < 0.09 mg/L, Na+ < 1 mg/L, Fe3+ < 0.01 mg/L, SO42− < 1 mg/L, NO2− < 0.02 mg/L, NO3− < 1 mg/L), also obtained in the laboratory using a GFL 2304 glass water still (LAUDA DR. R. WOBSER GMBH & CO. KG, Laudaplatz 1, Germany).
- (c)
- Commercially demineralized water with medium-grade purity (conductivity: 5 μS/cm; pH = 5.9; ionic concentrations: Na+ < 0.01 mg/L, Fe3+ < 0.03 mg/L, CaCO3− < 0.02 mg/L, SiO2 ≤ 1 mg/L), procured from Laborex S.R.L., Prahova, Romania.
- (d)
- Commercially demineralized water (conductivity: 50 μS/cm; pH = 7; ionic concentrations: Na+ < 0.05 mg/L, Cl– = 0.248 mg/L, Fe3+ < 0.03 mg/L, CaCO3− < 0.05 mg/L, SiO2 ≤ 1 mg/L), procured from Laborex S.R.L., Prahova, Romania.
- (e)
- Tap water (conductivity: 321 μS/cm; pH = 7.66; ionic concentrations: NH4+ < 0.05 mg/L, Ca2+ = 58.44 mg/L, Mg2+ = 35.44 mg/L, NO2− < 0.033 mg/L, NO3− = 5.02 mg/L), from the municipal distribution network of Măgurele, Romania.
4.2. E-Beam Radiation Synthesis of Hydrogels
4.3. Analysis of Network Parameters
4.4. Swelling Behavior and Kinetics of the Hydrogels
4.5. Evaluation of Chemical Structure and Morphological Features of the Hydrogel
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Essawy, H.A.; Ghazy, M.B.M.; El-Hai, F.A.; Mohamed, M.F. Superabsorbent Hydrogels via Graft Polymerization of Acrylic Acid from Chitosan-Cellulose Hybrid and Their Potential in Controlled Release of Soil Nutrients. Int. J. Biol. Macromol. 2016, 89, 144–151. [Google Scholar] [CrossRef]
- Albalasmeh, A.A.; Mohawesh, O.; Gharaibeh, M.A.; Alghamdi, A.G.; Alajlouni, M.A.; Alqudah, A.M. Effect of Hydrogel on Corn Growth, Water Use Efficiency, and Soil Properties in a Semi-Arid Region. J. Saudi Soc. Agric. Sci. 2022, 21, 518–524. [Google Scholar] [CrossRef]
- Qin, C.; Wang, H.; Zhao, Y.; Qi, Y.; Wu, N.; Zhang, S.; Xu, W. Recent Advances of Hydrogel in Agriculture: Synthesis, Mechanism, Properties and Applications. Eur. Polym. J. 2024, 219, 113376. [Google Scholar] [CrossRef]
- He, M.; Shi, L.; Wang, G.; Cheng, Z.; Han, L.; Zhang, X.; Wang, C.; Wang, J.; Zhou, P.; Wang, G. Biocompatible and Biodegradable Chitosan/Sodium Polyacrylate Polyelectrolyte Complex Hydrogels with Smart Responsiveness. Int. J. Biol. Macromol. 2020, 155, 1245–1251. [Google Scholar] [CrossRef]
- Ma, Q.; Xia, J.; Xu, W.; Hashan, D.; Zhen, Q.; She, D. Optimizing Soil Remediation with Multi-Functional L-PH Hydrogel: Enhancing Water Retention and Heavy Metal Stabilization in Farmland Soil. Sci. Total. Environ. 2025, 959, 178154. [Google Scholar] [CrossRef]
- Su, W.; Xiao, L. Manganese-Doped Ferrihydrite/Cellulose/Polyvinyl Alcohol Composite Membrane: Easily Recyclable Adsorbent for Simultaneous Removal of Arsenic and Cadmium from Soil. Sci. Total. Environ. 2022, 815, 152748. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kar, A.K.; Singh, D.; Verma, R.; Shraogi, N.; Zehra, A.; Gautam, K.; Anbumani, S.; Ghosh, D.; Patnaik, S. PH-Responsive Eco-Friendly Chitosan Modified Cenosphere/Alginate Composite Hydrogel Beads as Carrier for Controlled Release of Imidacloprid towards Sustainable Pest Control. Chem. Eng. J. 2022, 427, 131215. [Google Scholar] [CrossRef]
- Liaudat, J.; Muraro, S. Drying of Silty Soil Treated with Superabsorbent Hydrogels: Retention Behaviour and Cracking. Eng. Geol. 2024, 331, 107433. [Google Scholar] [CrossRef]
- Di Martino, A.; Khan, Y.A.; Durpekova, S.; Sedlarik, V.; Elich, O.; Cechmankova, J. Ecofriendly Renewable Hydrogels Based on Whey Protein and for Slow Release of Fertilizers and Soil Conditioning. J. Clean. Prod. 2021, 285, 124848. [Google Scholar] [CrossRef]
- Rizwan, M.; Rubina Gilani, S.; Iqbal Durani, A.; Naseem, S. Materials Diversity of Hydrogel: Synthesis, Polymerization Process and Soil Conditioning Properties in Agricultural Field. J. Adv. Res. 2021, 33, 15–40. [Google Scholar] [CrossRef]
- Han, D.; Meng, F.; Li, J.; Liu, H.; Cao, J.; Song, X.; Chen, H.; Xu, W. Synthesis, Properties and Application of Pesticides Encapsulated Hydrogels. Eur. Polym. J. 2024, 215, 113196. [Google Scholar] [CrossRef]
- Zheng, L.; Seidi, F.; Wu, W.; Pan, Y.; Xiao, H. Dual-Functional Lignin-Based Hydrogels for Sustained Release of Agrochemicals and Heavy Metal Ion Complexation. Int. J. Biol. Macromol. 2023, 235, 123701. [Google Scholar] [CrossRef]
- Guo, Y.; Bae, J.; Fang, Z.; Li, P.; Zhao, F.; Yu, G. Hydrogels and Hydrogel-Derived Materials for Energy and Water Sustainability. Chem. Rev. 2020, 120, 7642–7707. [Google Scholar] [CrossRef]
- Tariq, Z.; Iqbal, D.N.; Rizwan, M.; Ahmad, M.; Faheem, M.; Ahmed, M. Significance of Biopolymer-Based Hydrogels and Their Applications in Agriculture: A Review in Perspective of Synthesis and Their Degree of Swelling for Water Holding. RSC Adv. 2023, 13, 24731–24754. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Aouada, F.A.; Fajardo, A.R.; Martins, A.F.; Paulino, A.T.; Davi, M.F.T.; Rubira, A.F.; Muniz, E.C. Superabsorbent Hydrogels Based on Polysaccharides for Application in Agriculture as Soil Conditioner and Nutrient Carrier: A Review. Eur. Polym. J. 2015, 72, 365–385. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; Amin, M.A.; Ismail, M.A.; Nowwar, A.I.; El-diehy, M.A.; Gayed, H.M. Radiation Cross-Linked Ultra-Absorbent Hydrogel to Rationalize Irrigation Water and Fertilizer for Maize Planting in Drought Conditions. Int. J. Biol. Macromol. 2023, 252, 126467. [Google Scholar] [CrossRef] [PubMed]
- Sennakesavan, G.; Mostakhdemin, M.; Dkhar, L.K.; Seyfoddin, A.; Fatihhi, S.J. Acrylic Acid/Acrylamide Based Hydrogels and Its Properties—A Review. Polym. Degrad. Stab. 2020, 180, 109308. [Google Scholar] [CrossRef]
- El-Rehim, H.A.A.; Hegazy, E.A.; El-Mohdy, H.L.A. Radiation Synthesis of Hydrogels to Enhance Sandy Soils Water Retention and Increase Plant Performance. J. Appl. Polym. Sci. 2004, 93, 1360–1371. [Google Scholar] [CrossRef]
- Elbarbary, A.M.; El-Rehim, H.A.A.; El-Sawy, N.M.; Hegazy, E.-S.A.; Soliman, E.-S.A. Radiation Induced Crosslinking of Polyacrylamide Incorporated Low Molecular Weights Natural Polymers for Possible Use in the Agricultural Applications. Carbohydr. Polym. 2017, 176, 19–28. [Google Scholar] [CrossRef]
- Seeponkai, N.; Khammuang, K.; Fuggate, P.; Seephonkai, P. Physical Properties and Ion Permeability of Crosslinking Hydrogel Membrane Based on Poly(Vinyl Alcohol) for Soilless Cultivation. J. Appl. Polym. Sci. 2023, 140, e53311. [Google Scholar] [CrossRef]
- Ma, Z.; Shi, W.; Yan, K.; Pan, L.; Yu, G. Doping Engineering of Conductive Polymer Hydrogels and Their Application in Advanced Sensor Technologies. Chem. Sci. 2019, 10, 6232–6244. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Choi, J.H.; Kim, S.; Jang, I.; Jeong, S.; Lee, J.Y. Micropatterned Conductive Hydrogels as Multifunctional Muscle-Mimicking Biomaterials: Graphene-Incorporated Hydrogels Directly Patterned with Femtosecond Laser Ablation. Acta Biomater. 2019, 97, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Dong, C.; Chen, B.; Li, W.; Hu, H.; Zhou, J.; Li, C.; Huang, Z. Strong, Tough, and Anti-Swelling Supramolecular Conductive Hydrogels for Amphibious Motion Sensors. Small 2023, 19, e2303612. [Google Scholar] [CrossRef] [PubMed]
- Ball, V. Specific Ion Effects in Hydrogels. Molecules 2024, 29, 5990. [Google Scholar] [CrossRef]
- Park, K.C.; Tsukahara, T. Expansion of Ion Effects on Water Induced by a High Hydrophilic Surface of a Polymer Network. Langmuir 2020, 36, 159–168. [Google Scholar] [CrossRef]
- Abdel-Raouf, M.E.; El-Saeed, S.M.; Zaki, E.G.; Al-Sabagh, A.M. Green Chemistry Approach for Preparation of Hydrogels for Agriculture Applications through Modification of Natural Polymers and Investigating Their Swelling Properties. Egypt. J. Pet. 2018, 27, 1345–1355. [Google Scholar] [CrossRef]
- Han, B.; Benner, S.G.; Flores, A.N. Evaluating Impacts of Climate Change on Future Water Scarcity in an Intensively Managed Semi-Arid Region Using a Coupled Model of Biophysical Processes and Water Rights. Hydrol. Earth Syst. Sci. Discuss. 2018, 140, 1–53. [Google Scholar]
- Udenni Gunathilake, T.; Ching, Y.; Ching, K.; Chuah, C.; Abdullah, L. Biomedical and Microbiological Applications of Bio-Based Porous Materials: A Review. Polymers 2017, 9, 160. [Google Scholar] [CrossRef]
- Peters, J.T.; Wechsler, M.E.; Peppas, N.A. Advanced Biomedical Hydrogels: Molecular Architecture and Its Impact on Medical Applications. Regen. Biomater. 2021, 8, rbab060. [Google Scholar] [CrossRef]
- Omidian, H.; Rocca, J.G.; Park, K. Elastic, Superporous Hydrogel Hybrids of Polyacrylamide and Sodium Alginate. Macromol. Biosci. 2006, 6, 703–710. [Google Scholar] [CrossRef]
- Liu, X.; He, X.; Yang, B.; Lai, L.; Chen, N.; Hu, J.; Lu, Q. Dual Physically Cross-Linked Hydrogels Incorporating Hydrophobic Interactions with Promising Repairability and Ultrahigh Elongation. Adv. Funct. Mater. 2021, 31, m2008187. [Google Scholar] [CrossRef]
- Islam, M.R.; Tanveer, S.; Chen, C.-C. Modeling Swelling Behavior of Hydrogels in Aqueous Organic Solvents. Chem. Eng. Sci. 2021, 242, 116744. [Google Scholar] [CrossRef]
- Yang, X.; Dargaville, B.; Hutmacher, D. Elucidating the Molecular Mechanisms for the Interaction of Water with Polyethylene Glycol-Based Hydrogels: Influence of Ionic Strength and Gel Network Structure. Polymers 2021, 13, 845. [Google Scholar] [CrossRef]
- Trivedi, J.; Chourasia, A. Sodium Salt of Partially Carboxymethylated Sodium Alginate-Graft-Poly(Acrylonitrile): II Superabsorbency, Salt Sensitivity and Swelling Kinetics of Hydrogel, H-Na-PCMSA-g-PAN. Gels 2023, 9, 407. [Google Scholar] [CrossRef]
- Wu, Y.; Li, S.; Chen, G. Hydrogels as Water and Nutrient Reservoirs in Agricultural Soil: A Comprehensive Review of Classification, Performance, and Economic Advantages. Environ. Dev. Sustain. 2023, 26, 24653–24685. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Q.; Lin, S.; Li, J. Water: The Soul of Hydrogels. Prog. Mater. Sci. 2025, 148, 101378. [Google Scholar] [CrossRef]
- Cavallo, A.; Madaghiele, M.; Masullo, U.; Lionetto, M.G.; Sannino, A. Photo-Crosslinked Poly(Ethylene Glycol) Diacrylate (PEGDA) Hydrogels from Low Molecular Weight Prepolymer: Swelling and Permeation Studies. J. Appl. Polym. Sci. 2017, 134, 44380. [Google Scholar] [CrossRef]
- Manaila, E.; Demeter, M.; Calina, I.C.; Craciun, G. NaAlg-g-AA Hydrogels: Candidates in Sustainable Agriculture Applications. Gels 2023, 9, 316. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, A. Synthesis and Swelling Properties of PH-Sensitive Semi-IPN Superabsorbent Hydrogels Based on Sodium Alginate-g-Poly(Sodium Acrylate) and Polyvinylpyrrolidone. Carbohydr. Polym. 2010, 80, 1028–1036. [Google Scholar] [CrossRef]
- Lee, C.-J.; Wu, H.; Hu, Y.; Young, M.; Wang, H.; Lynch, D.; Xu, F.; Cong, H.; Cheng, G. Ionic Conductivity of Polyelectrolyte Hydrogels. ACS Appl. Mater. Interfaces 2018, 10, 5845–5852. [Google Scholar] [CrossRef]
- Craciun, G.; Calina, I.C.; Demeter, M.; Scarisoreanu, A.; Dumitru, M.; Manaila, E. Poly(Acrylic Acid)-Sodium Alginate Superabsorbent Hydrogels Synthesized by Electron Beam Irradiation Part I: Impact of Initiator Concentration and Irradiation Dose on Structure, Network Parameters and Swelling Properties. Materials 2023, 16, 4552. [Google Scholar] [CrossRef]
- Manaila, E.; Craciun, G. Poly(Acrylic Acid)-Sodium Alginate Superabsorbent Hydrogels Synthesized by Electron-Beam Irradiation—Part II: Swelling Kinetics and Absorption Behavior in Various Swelling Media. Gels 2024, 10, 609. [Google Scholar] [CrossRef]
- Nagasawa, N.; Yagi, T.; Kume, T.; Yoshii, F. Radiation Crosslinking of Carboxymethyl Starch. Carbohydr. Polym. 2004, 58, 109–113. [Google Scholar] [CrossRef]
- Călina, I.; Demeter, M.; Crăciun, G.; Scărișoreanu, A.; Mănăilă, E. The Influence of the Structural Architecture on the Swelling Kinetics and the Network Behavior of Sodium-Alginate-Based Hydrogels Cross-Linked with Ionizing Radiation. Gels 2024, 10, 588. [Google Scholar] [CrossRef]
- Şen, M.; Hayrabolulu, H. Radiation Synthesis and Characterisation of the Network Structure of Natural/Synthetic Double-Network Superabsorbent Polymers. Radiat. Phys. Chem. 2012, 81, 1378–1382. [Google Scholar] [CrossRef]
- Ding, Z.Y.; Aklonis, J.J.; Salovey, R. Model Filled Polymers. VI. Determination of the Crosslink Density of Polymeric Beads by Swelling. J. Polym. Sci. B Polym. Phys. 1991, 29, 1035–1038. [Google Scholar] [CrossRef]
- Chen, J.; Park, H.; Park, K. Synthesis of Superporous Hydrogels: Hydrogels with Fast Swelling and Superabsorbent Properties. J. Biomed. Mater. Res. 1999, 44, 53–62. Available online: https://onlinelibrary.wiley.com/doi/10.1002/(SICI)1097-4636(199901)44:1%3C53::AID-JBM6%3E3.0.CO;2-W (accessed on 31 July 2025). [CrossRef]
- Karadag, E.; Saraydin, D.; Sahiner, N.; Güven, O. RADIATION INDUCED ACRYLAMIDE/CITRIC ACID HYDROGELS AND THEIR SWELLING BEHAVIORS. J. Macromol. Sci. Part. A 2001, 38, 1105–1121. [Google Scholar] [CrossRef]
- Jabbari, E.; Nozari, S. Swelling Behavior of Acrylic Acid Hydrogels Prepared by γ-Radiation Crosslinking of Polyacrylic Acid in Aqueous Solution. Eur. Polym. J. 2000, 36, 2685–2692. [Google Scholar] [CrossRef]
- Peppas, N.A.; Franson, N.M. The Swelling Interface Number as a Criterion for Prediction of Diffusional Solute Release Mechanisms in Swellable Polymers. J. Polym. Sci. Polym. Phys. Ed. 1983, 21, 983–997. [Google Scholar] [CrossRef]
- Espert, A.; Vilaplana, F.; Karlsson, S. Comparison of Water Absorption in Natural Cellulosic Fibres from Wood and One-Year Crops in Polypropylene Composites and Its Influence on Their Mechanical Properties. Compos. Part. A Appl. Sci. Manuf. 2004, 35, 1267–1276. [Google Scholar] [CrossRef]
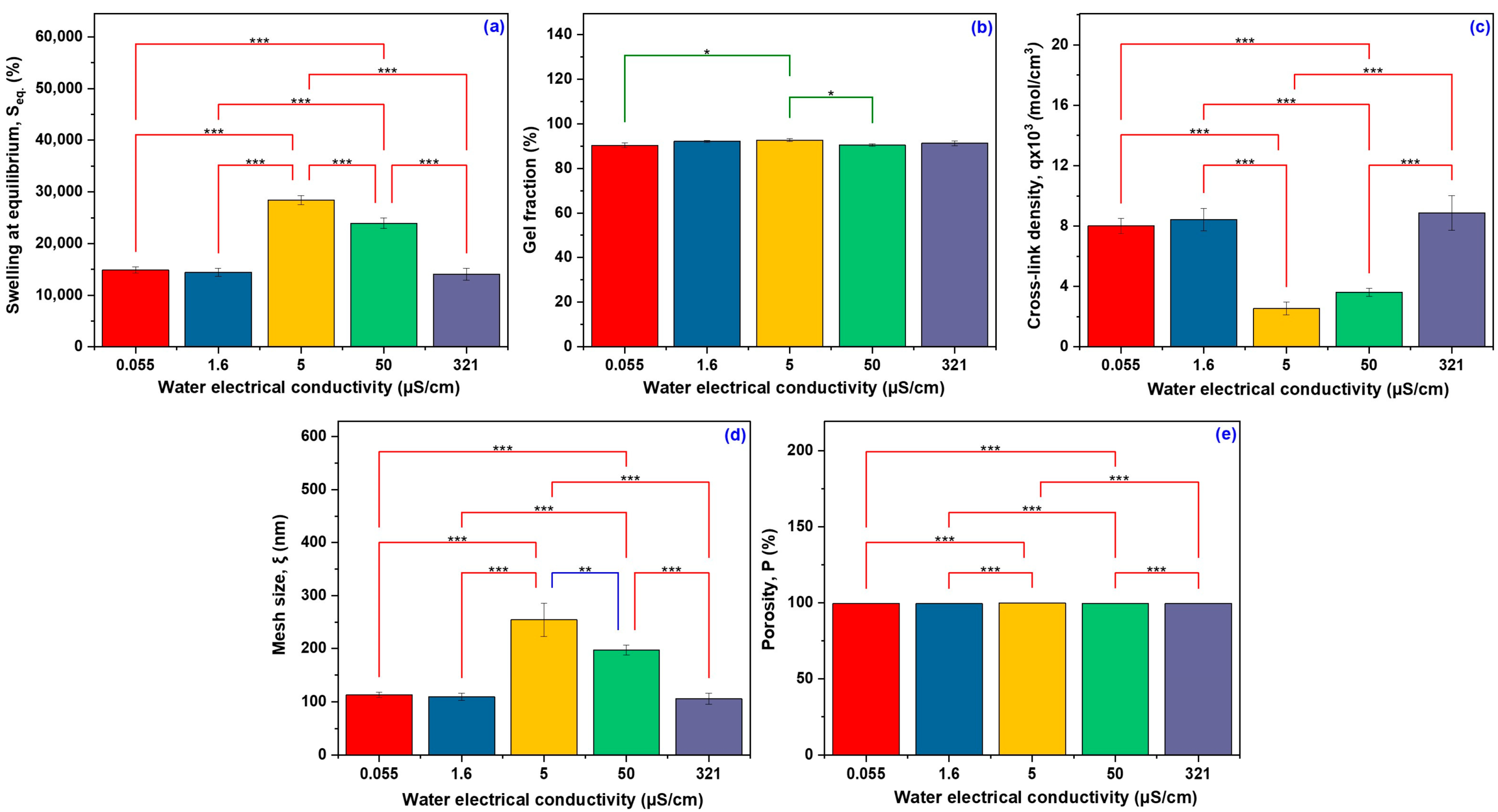
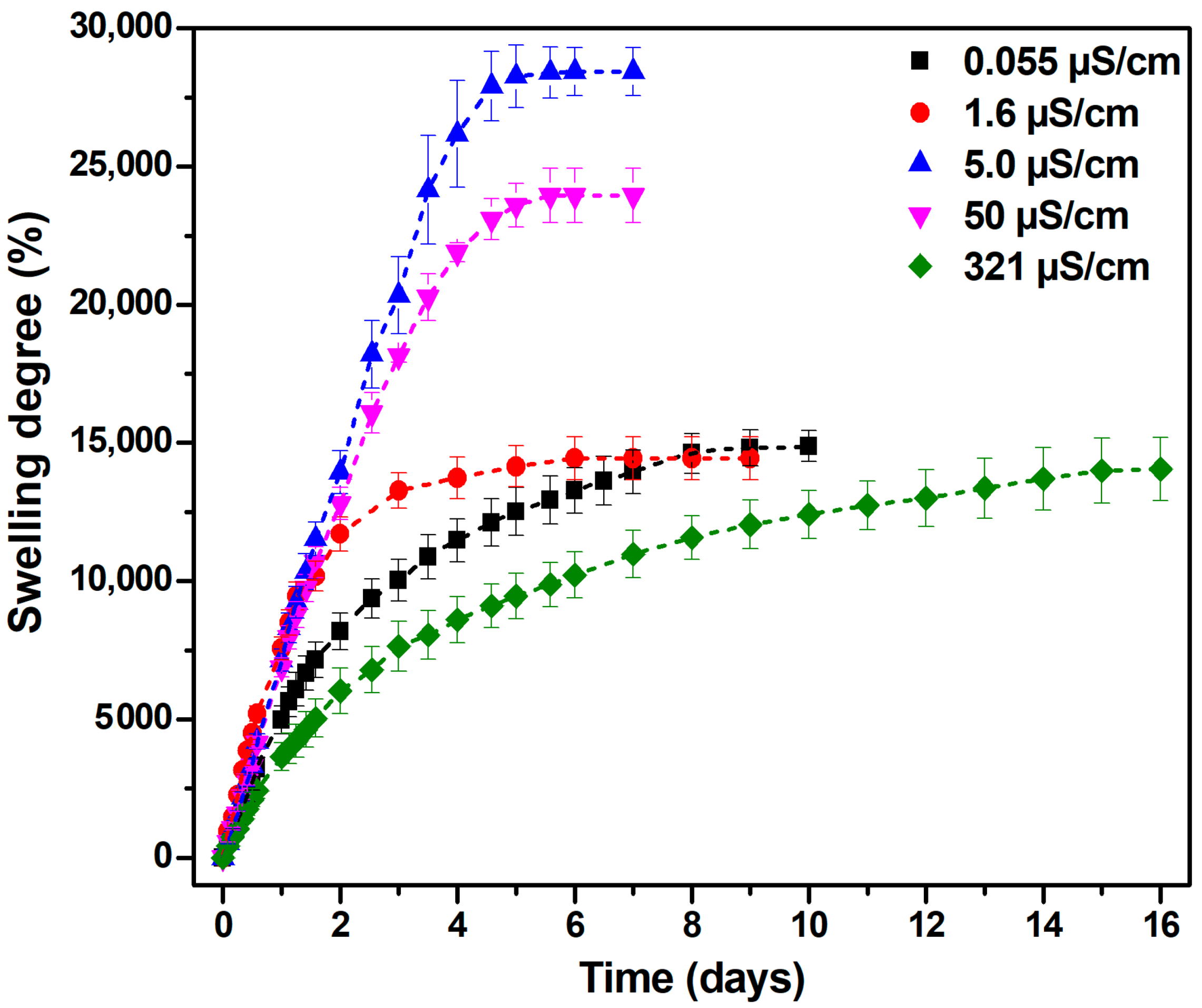
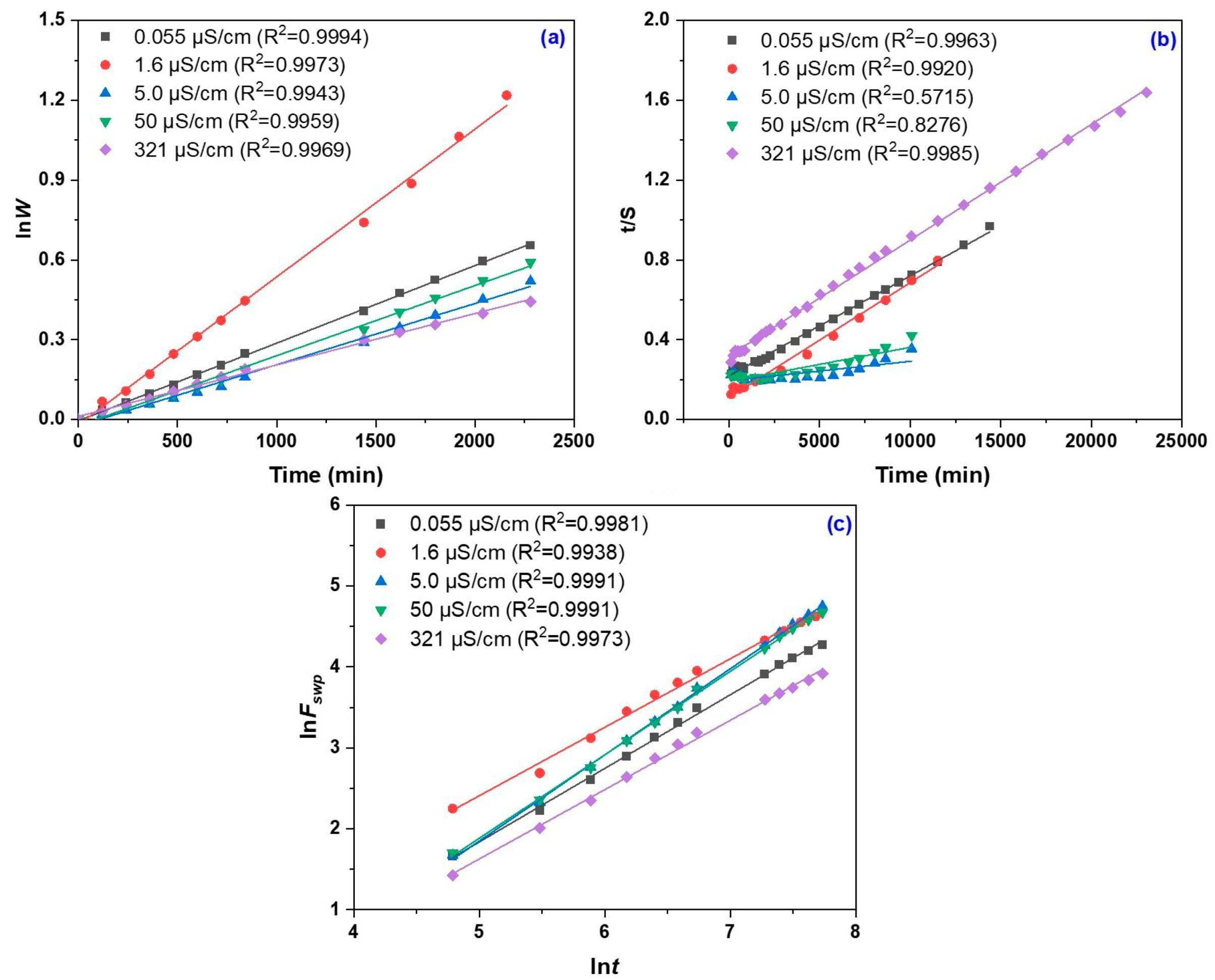

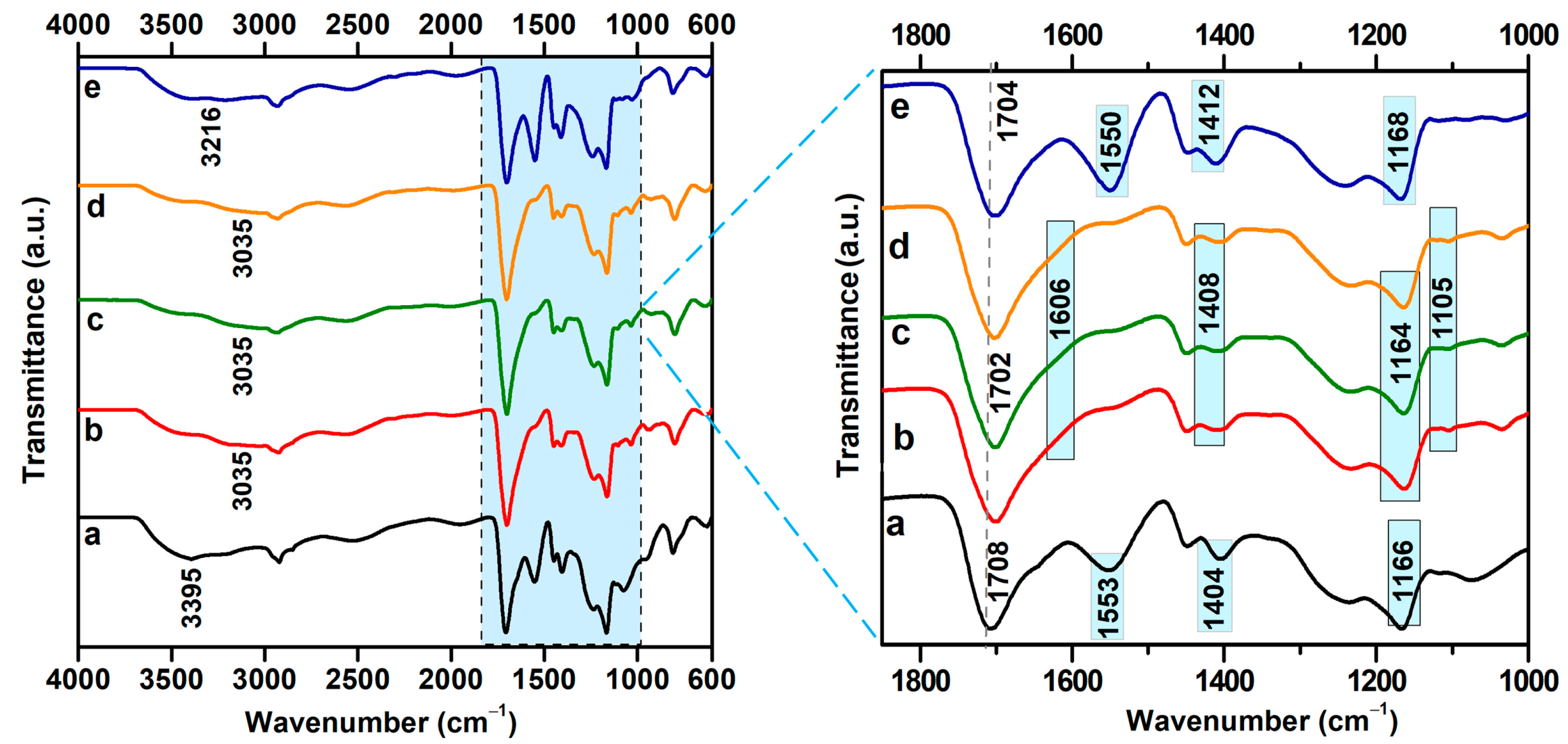
| Water Electrical Conductivity (µS/cm) | Seq (%) | G (%) | q ×103 (mol/cm3) | ξ (nm) | P (%) |
|---|---|---|---|---|---|
| 0.055 | 14,878 ± 3.77 c | 90.27 ± 1.06 b | 8.01 ± 0.51 a | 112.88 ± 4.97 c | 99.33 ± 0.03 b |
| 1.6 | 14,440 ± 5.33 c | 92.11 ± 0.42 ab | 8.43 ± 0.75 a | 109.01 ± 6.77 c | 99.31 ± 0.04 b |
| 5 | 28,428 ± 3.07 a | 92.66 ± 0.63 a | 2.54 ± 0.42 b | 254.26 ± 3.50 a | 99.66 ± 0.03 a |
| 50 | 23,945 ± 4.12 b | 90.40 ± 0.54 b | 3.61 ± 0.26 b | 196.89 ± 9.47 b | 99.58 ± 0.02 a |
| 321 | 14,053 ± 8.16 c | 91.26 ± 1.04 ab | 8.87 ± 1.15 a | 105.63 ± 10.11 c | 99.28 ± 0.06 b |
| F value | 166.1844 | 5.3225 | 55.8578 | 53.5606 | 69.4507 |
| p value | <0.0001 | 0.01468 | <0.0001 | <0.0001 | <0.0001 |
| Water Electrical Conductivity (µS/cm) | k1,S ×104/min−1 | k2,S ×109/g gel | Seq (Theoretical)/g Water |
|---|---|---|---|
| 0.055 | 0.292 ± 0.023 b | 11.37 ± 1.07 b | 20,004 ± 639 c |
| 1.6 | 0.556 ± 0.001 a | 30.03 ± 1.59 a | 17,370 ± 919 c |
| 5 | 0.230 ± 0.011 cd | 0.49 ± 0.15 c | 104,087 ± 12,330 a |
| 50 | 0.263 ± 0.010 bc | 1.53 ± 0.26 c | 58,860 ± 4759 b |
| 321 | 0.193 ± 0.010 d | 10.54 ± 1.19 b | 17,259 ± 1182 c |
| F value | 285.5630 | 406.6245 | 123.2881 |
| p value | <0.0001 | <0.0001 | <0.0001 |
| Water Electrical Conductivity (µS/cm) | k × 10 | n |
|---|---|---|
| 0.055 | 0.666 ± 0.051 b | 0.909 ± 0.010 b |
| 1.6 | 1.612 ± 0.091 a | 0.847 ± 0.003 c |
| 5 | 0.309 ± 0.033 c | 1.065 ± 0.014 a |
| 50 | 0.379 ± 0.027 c | 1.031 ± 0.015 a |
| 321 | 0.706 ± 0.023 b | 0.855 ± 0.023 c |
| F value | 305.5099 | 145.50916 |
| p value | <0.0001 | <0.0001 |
| Bands (cm−1) | Hydr. 12.5 kGy | Conductivity (µS/cm) | Δν (cm−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.055 | 1.6 | 5 | 50 | 321 | 0.055 | 1.6 | 5 | 50 | 321 | ||
| ν(C–H) | 2947 | 2921 | 2924 | 2933 | 2929 | 2931 | 26 | 23 | 14 | 18 | 16 |
| ν(C–O–C) | 1164 | 1075 | 1105 | 1105 | 1105 | 1118 | 85 | 59 | 59 | 59 | 46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mănăilă, E.; Călina, I.; Scărișoreanu, A.; Demeter, M.; Crăciun, G.; Dumitru, M. Impact of Water Conductivity on the Structure and Swelling Dynamics of E-Beam Cross-Linked Hydrogels. Gels 2025, 11, 611. https://doi.org/10.3390/gels11080611
Mănăilă E, Călina I, Scărișoreanu A, Demeter M, Crăciun G, Dumitru M. Impact of Water Conductivity on the Structure and Swelling Dynamics of E-Beam Cross-Linked Hydrogels. Gels. 2025; 11(8):611. https://doi.org/10.3390/gels11080611
Chicago/Turabian StyleMănăilă, Elena, Ion Călina, Anca Scărișoreanu, Maria Demeter, Gabriela Crăciun, and Marius Dumitru. 2025. "Impact of Water Conductivity on the Structure and Swelling Dynamics of E-Beam Cross-Linked Hydrogels" Gels 11, no. 8: 611. https://doi.org/10.3390/gels11080611
APA StyleMănăilă, E., Călina, I., Scărișoreanu, A., Demeter, M., Crăciun, G., & Dumitru, M. (2025). Impact of Water Conductivity on the Structure and Swelling Dynamics of E-Beam Cross-Linked Hydrogels. Gels, 11(8), 611. https://doi.org/10.3390/gels11080611









