Tissue Paper-Based Hydrogels for Soil Water Maintenance and Nitrogen Release
Abstract
1. Introduction
2. Results and Discussion
2.1. Hydrogel and Soil Properties
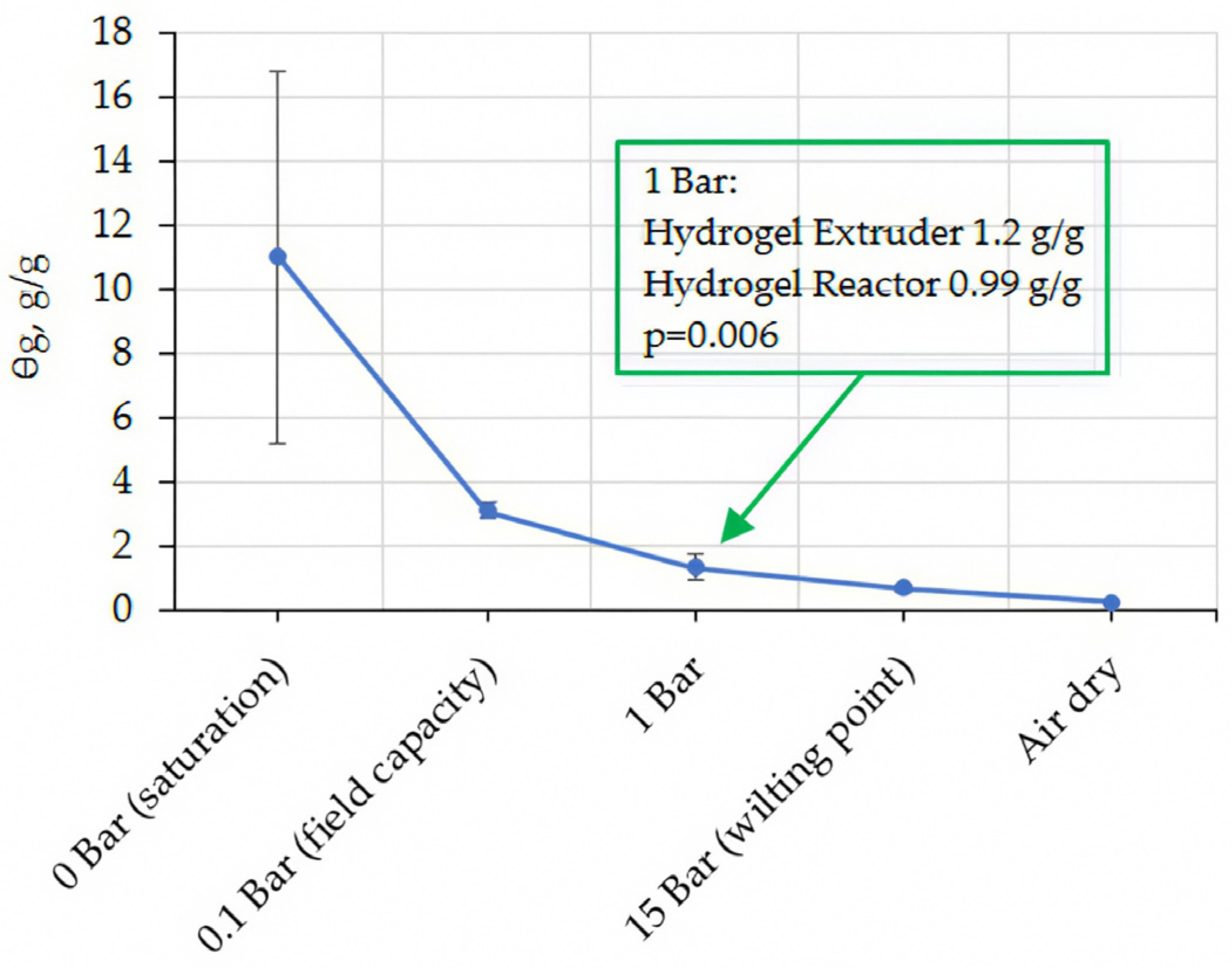
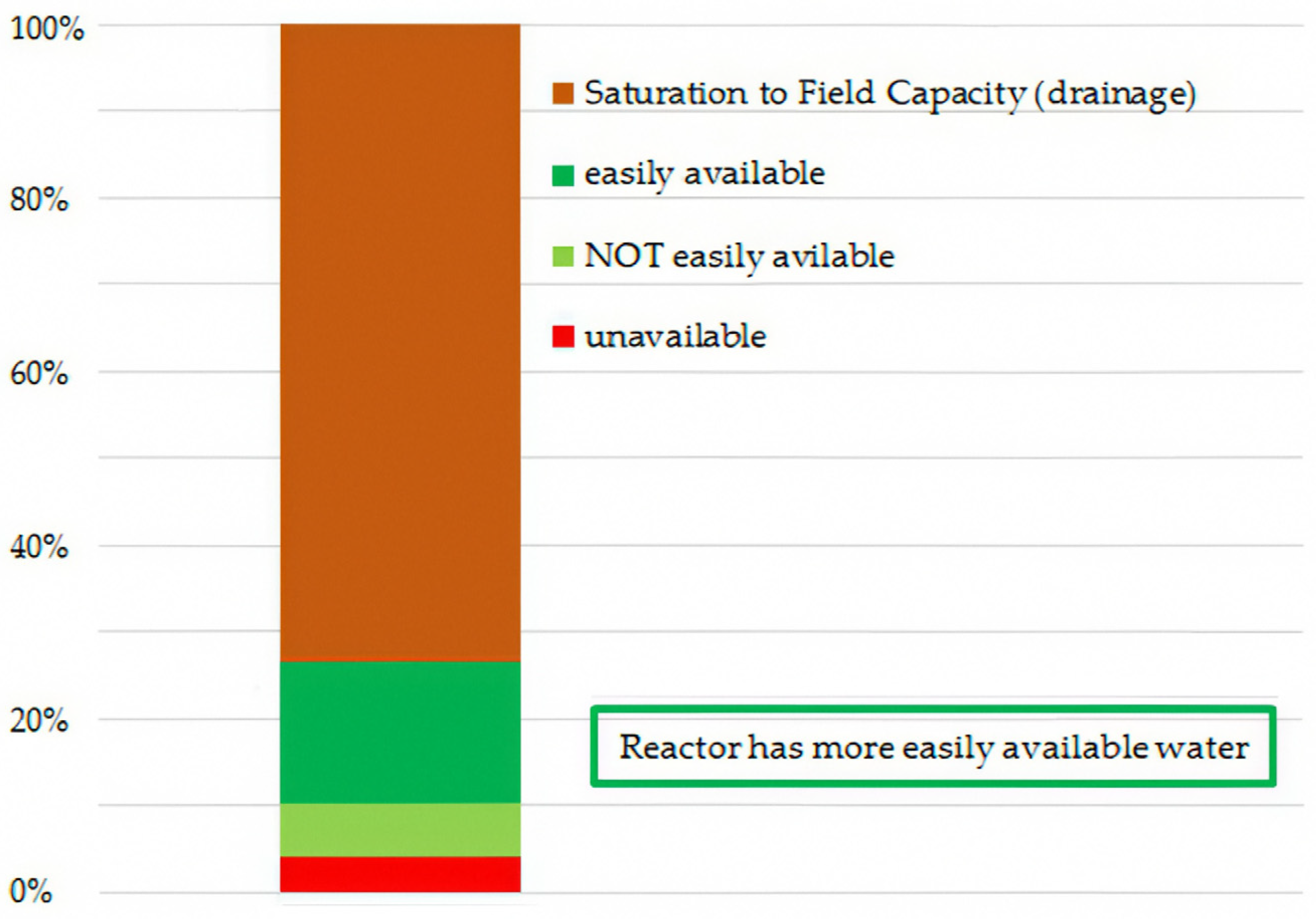
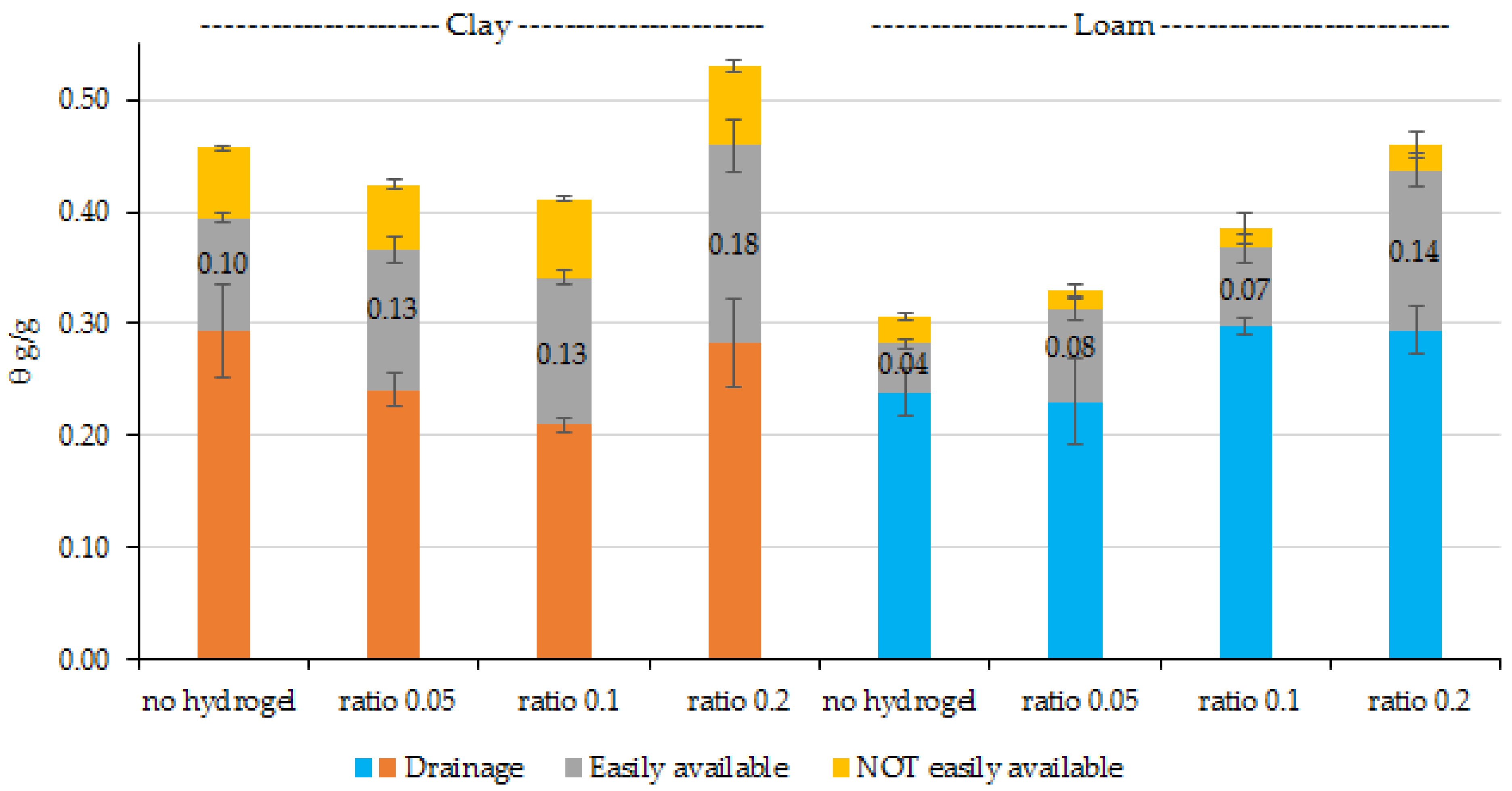
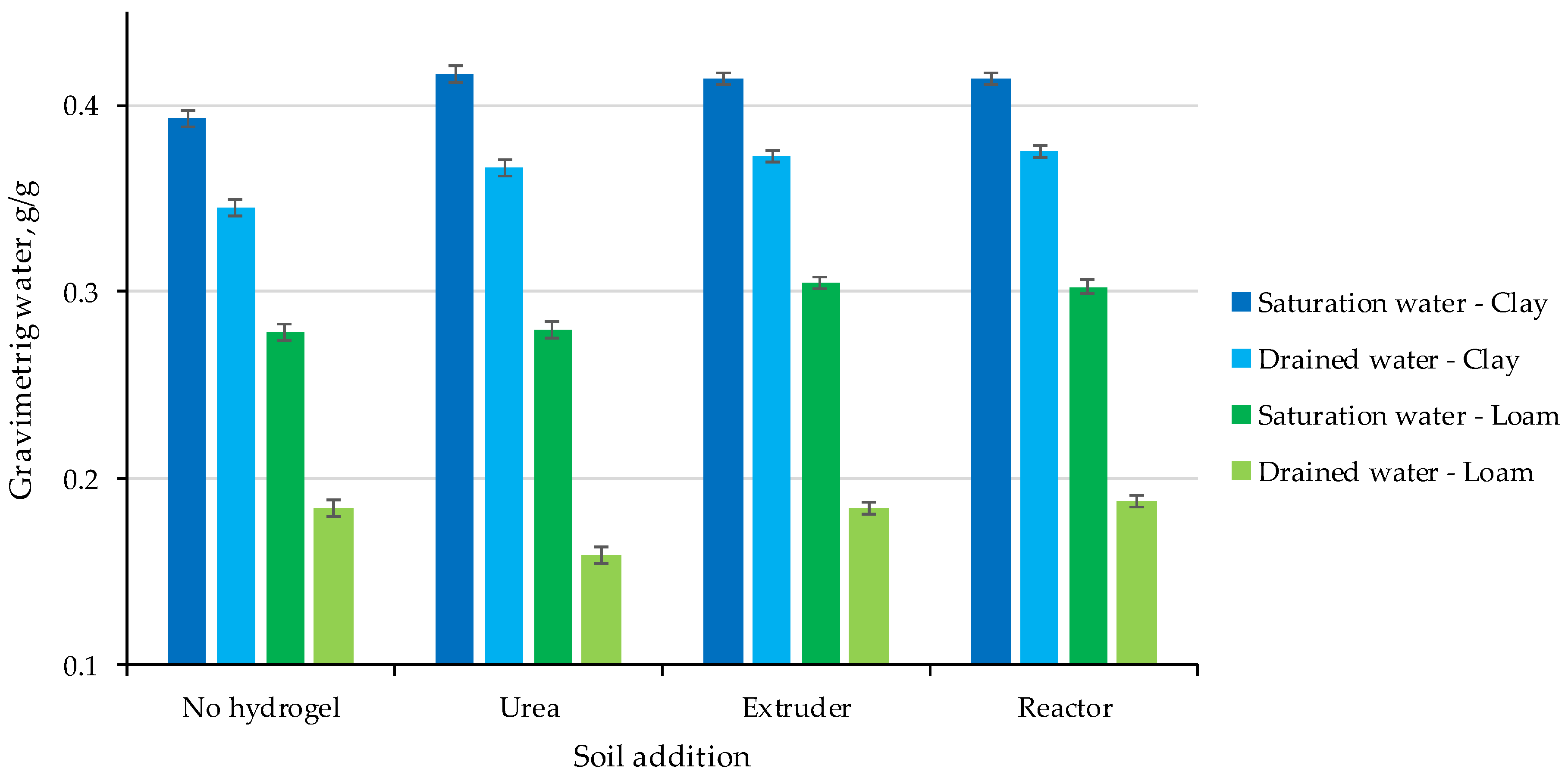
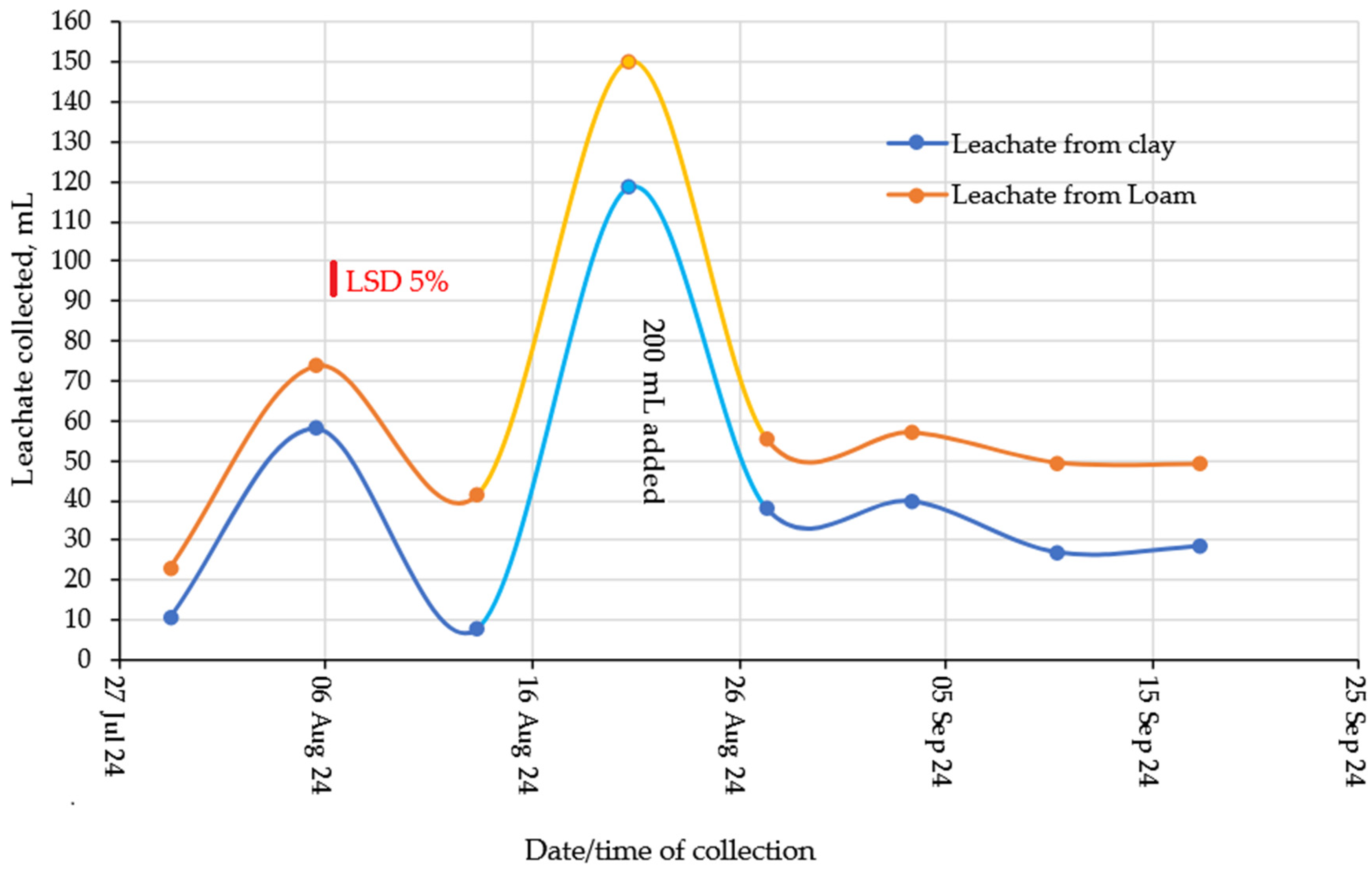
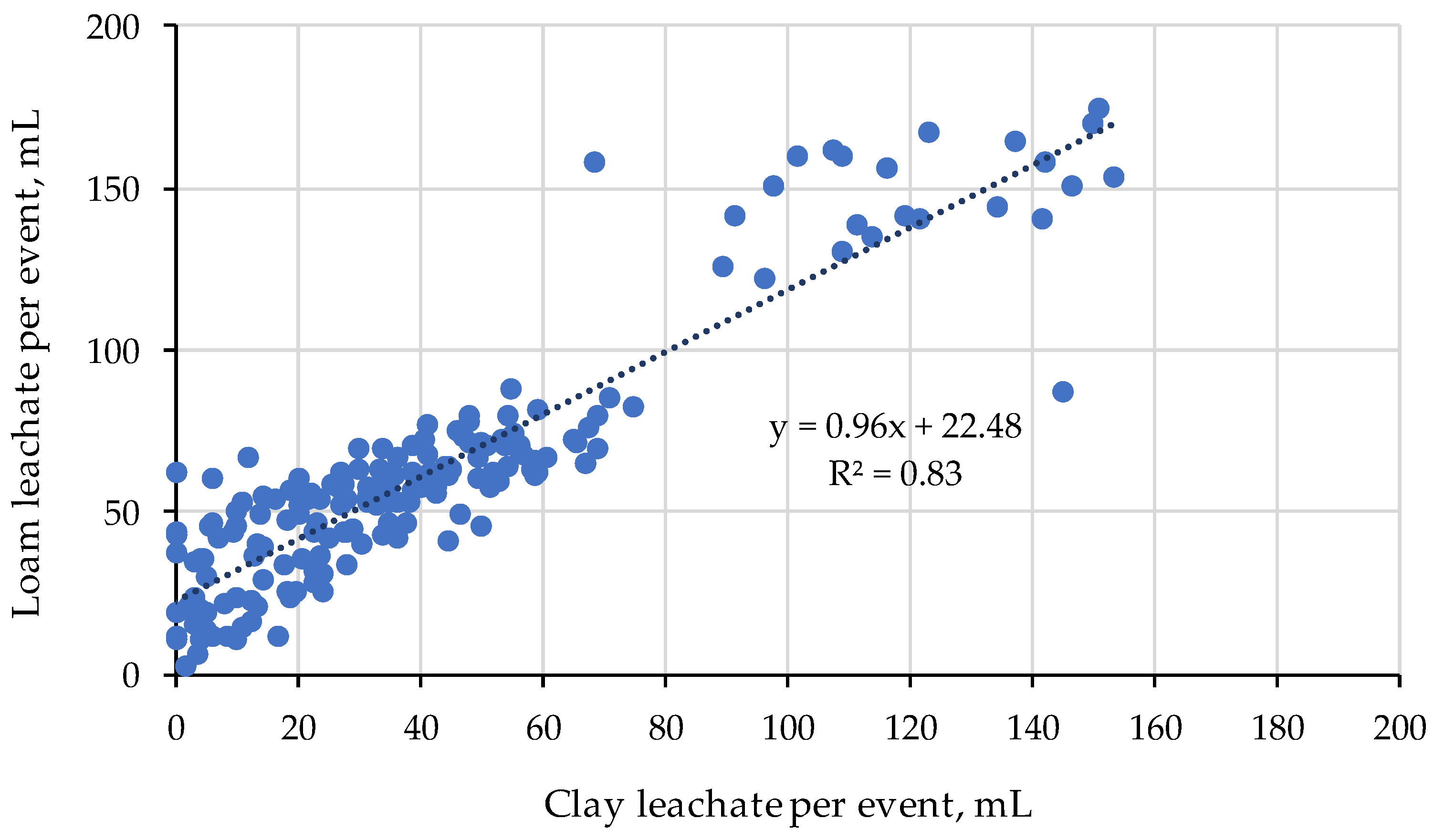
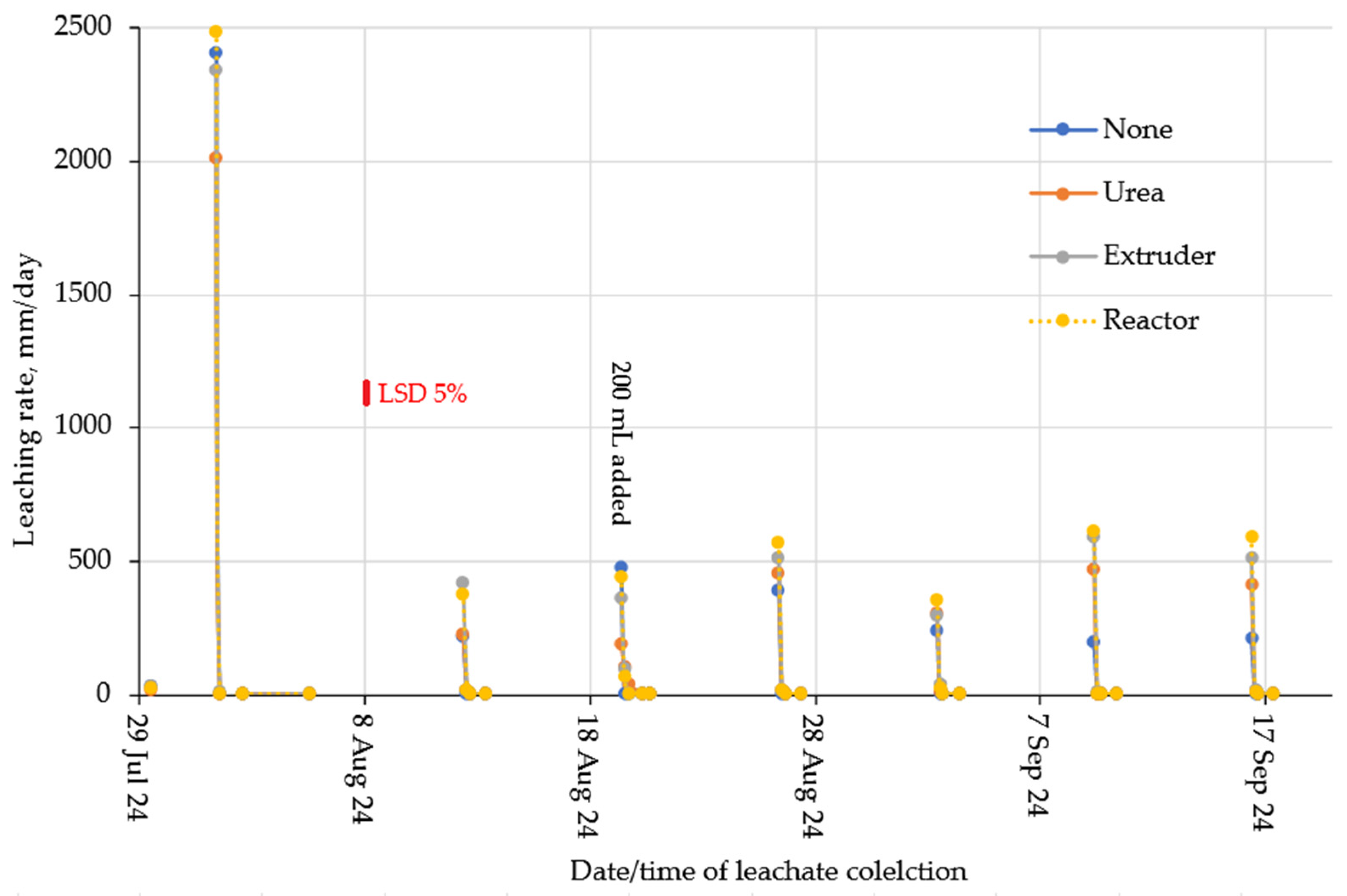
2.2. Water Holding Capacity of Soil Hydrogel Mixes
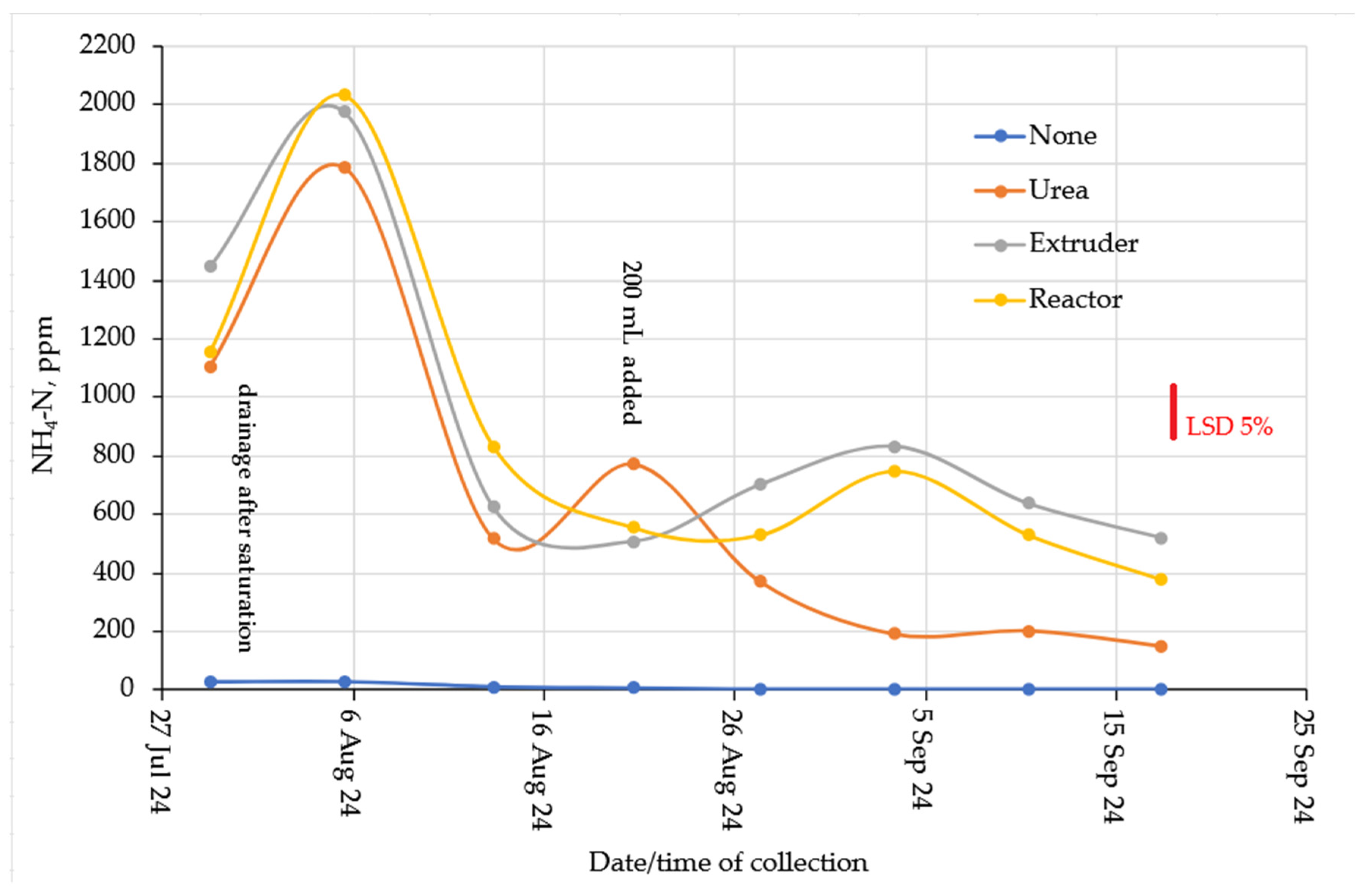
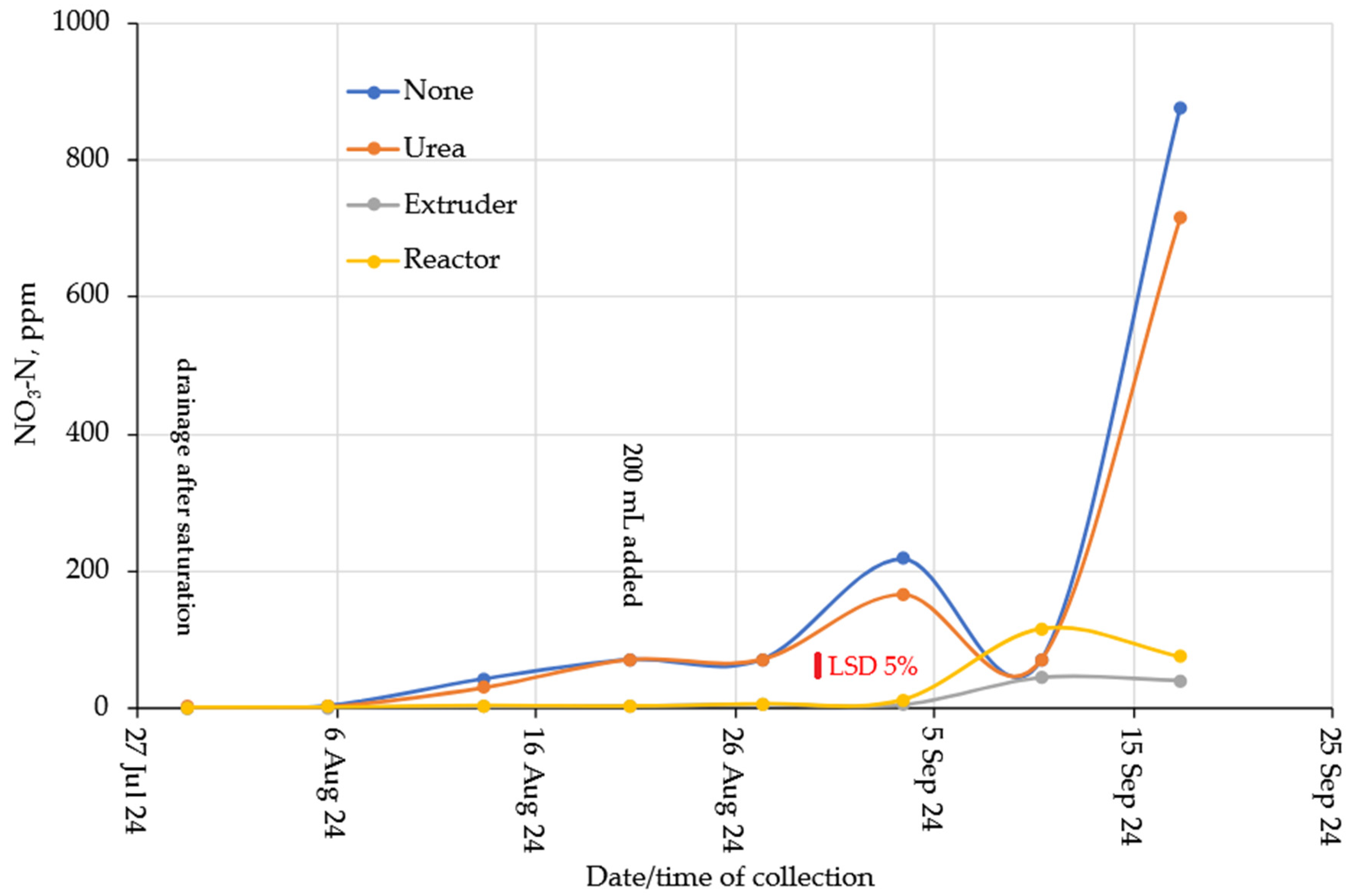
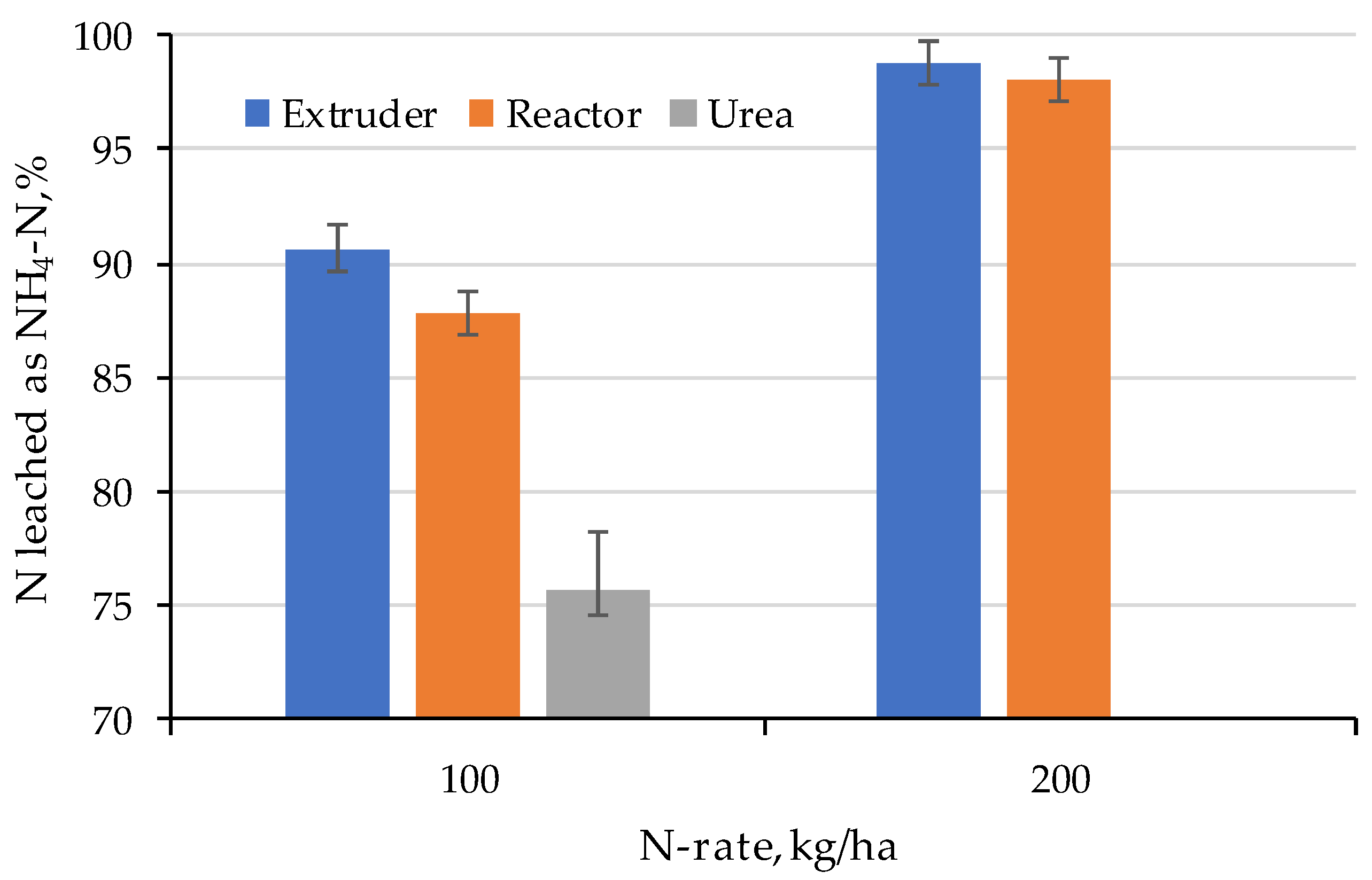
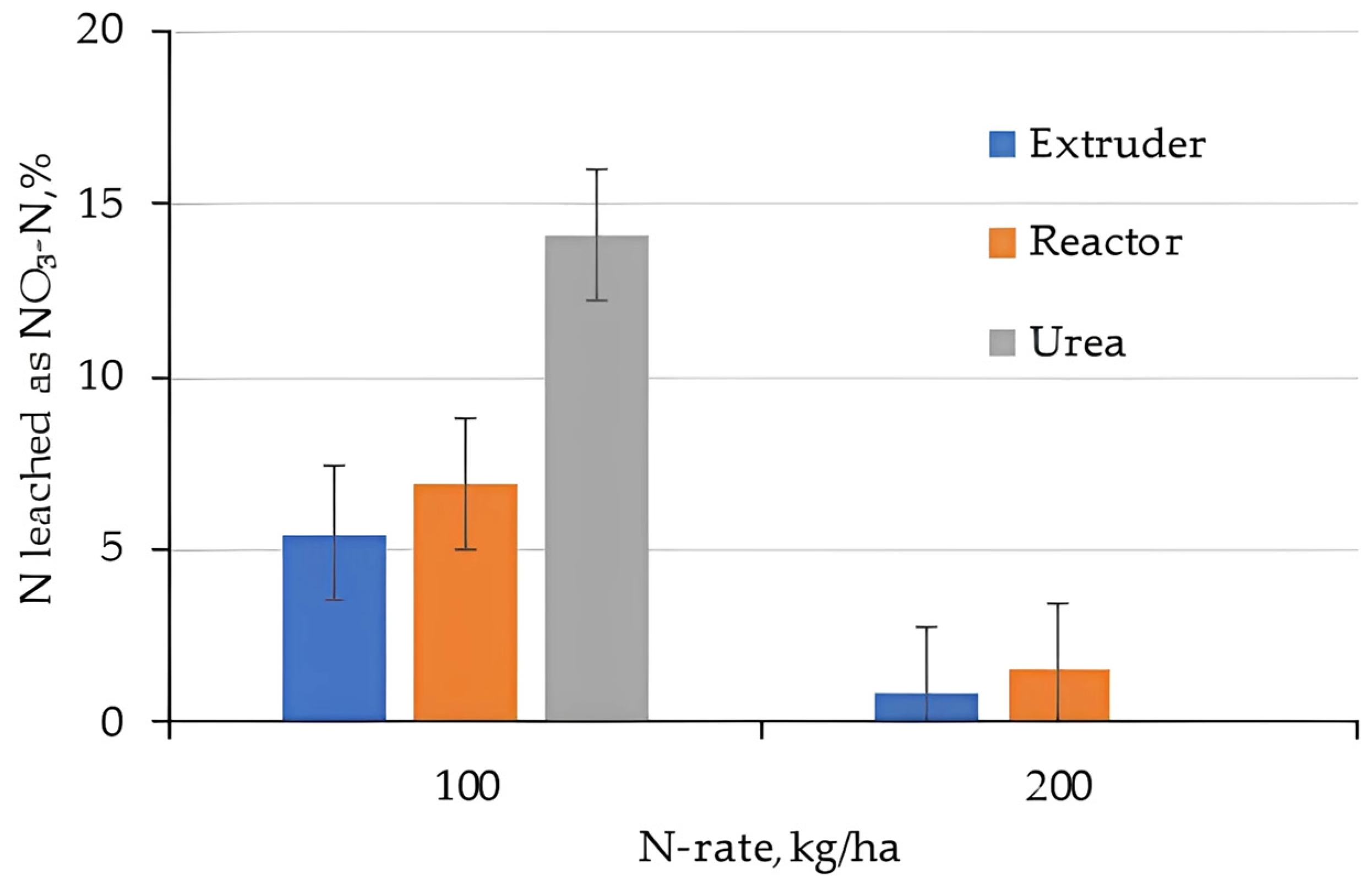
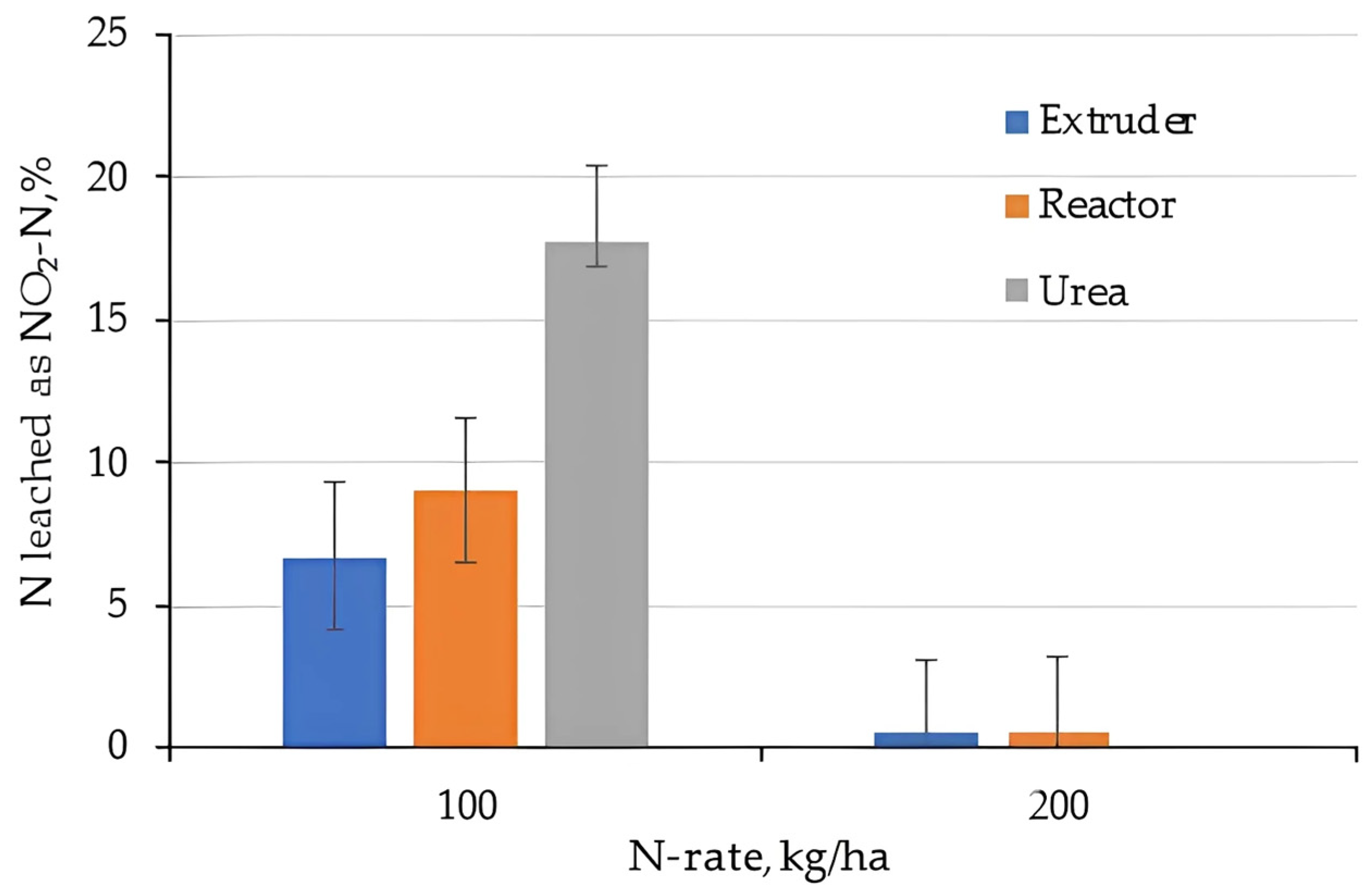
2.3. Nitrogen Leaching
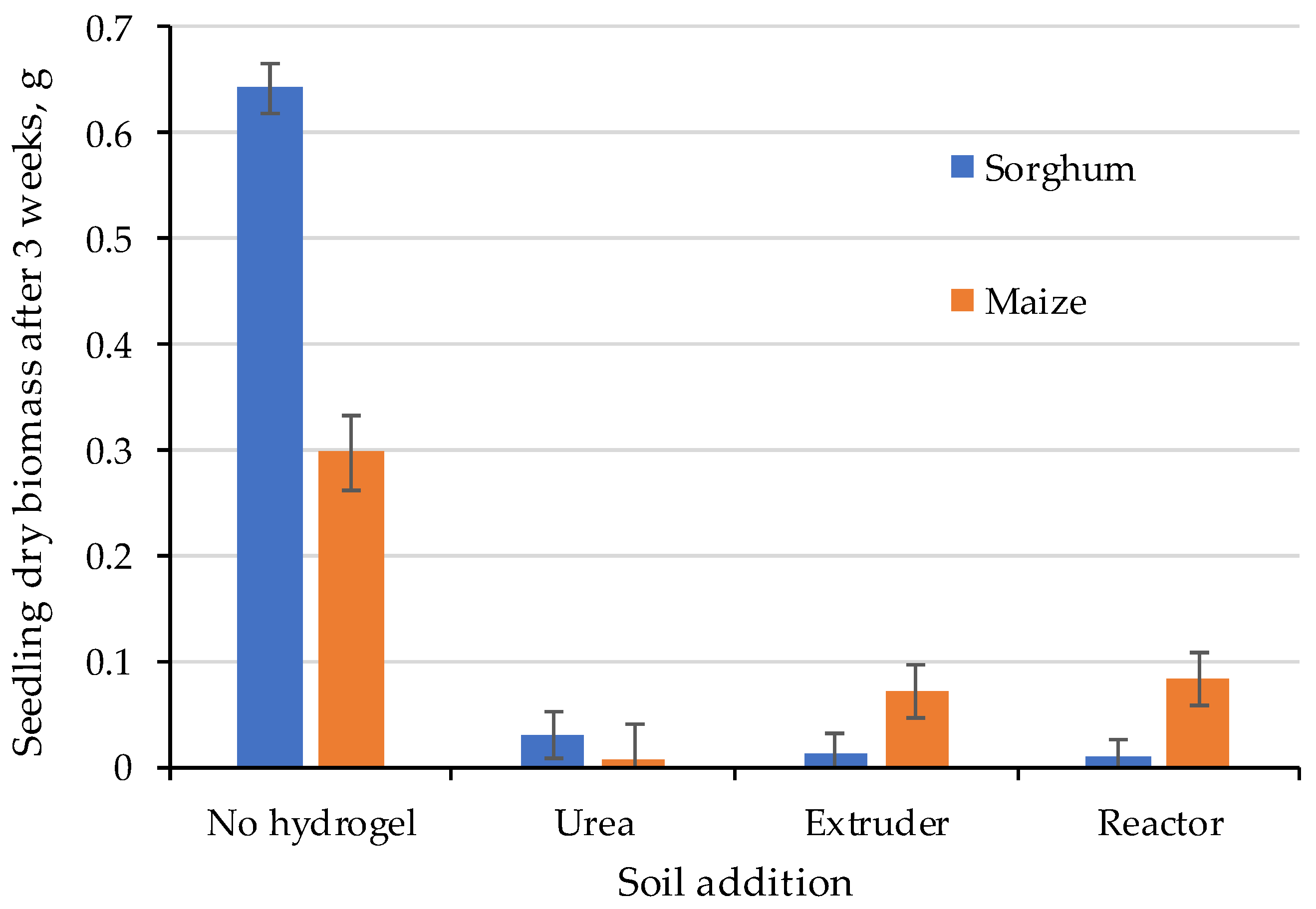
2.4. Seedling Growth
3. Conclusions
4. Materials and Methods
4.1. Soils
4.2. Hydrogels
4.3. Chemical Analysis of the Soil and Hydrogels
4.4. Soil Water Retention
4.5. Leaching Columns
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, H.; Cheng, S.; Zhen, J.; Lei, Z. Superabsorbent polymer with excellent water/salt absorbency and water retention, and fast swelling properties for preventing soil water evaporation. J. Polym. Environ. 2022, 31, 812–824. [Google Scholar] [CrossRef]
- Abdallah, A.M. The effect of hydrogel particle size on water retention properties and availability under water stress. Int. Soil Water Conse. 2019, 7, 275–285. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Omidian, H.; Akhzarmehr, A.; Chowdhury, S.D. Advancements in cellulose-based superabsorbent hydrogels: Sustainable solutions across industries. Gels 2024, 10, 174. [Google Scholar] [CrossRef]
- Zheng, H.; Mei, P.; Wang, W.; Yin, Y.; Li, H.; Zheng, M.; Ou, X.; Cui, Z. Effects of super absorbent polymer on crop yield, water productivity and soil properties: A global meta-analysis. Agric. Water Manag. 2023, 282, 108290. [Google Scholar] [CrossRef]
- Su, L.Q.; Li, J.G.; Xue, H.; Wang, X.F. Super absorbent polymer seed coatings promote seed germination and seedling growth of Caragana korshinskii in drought. J. Zhejiang Univ. Sci. B 2017, 18, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Sekharan, S.; Manna, U. Superabsorbent hydrogel (SAH) as a soil amendment for drought management: A review. Soil Till. Res. 2020, 204, 104736. [Google Scholar] [CrossRef]
- Albalasmeh, A.A.; Mohawesh, O.; Gharaibeh, M.A.; Alghamdi, A.G.; Alajlouni, M.A.; Alqudah, A.M. Effect of hydrogel on corn growth, water use efficiency, and soil properties in a semi-arid region. J. Saudi Soc. Agric. Sci. 2022, 21, 518–524. [Google Scholar] [CrossRef]
- Guan, Y.; Cui, H.; Ma, W.; Zheng, Y.; Tian, Y.; Hu, J. An enhanced drought-tolerant method using SA-loaded PAMPS polymer materials applied on tobacco pelleted seeds. Sci. World J. 2014, 2014, 752658. [Google Scholar] [CrossRef]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-based Hydrogels: Design and applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Taylor, K.C.; Halfacre, R.G. The effect of hydrophilic polymer on media water retention and nutrient availability to Ligustrum lucidum. Hortscience 1986, 21, 1159–1161. [Google Scholar] [CrossRef]
- Ye, X.; Peng, H.; Liu, X.; Xiong, H.; Wang, N.; Yang, F.; Kong, Y.; Yang, Z.; Lei, Z. Preparation and fertilizer retention/anti-leakage performances of superabsorbent composite based on hydroxypropyl methyl cellulose. Carbohydr. Polym. 2021, 274, 118636. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, A.; Dhamodharan, R. Super water-absorbing new material from chitosan, EDTA and urea. Carbohydr. Polym. 2015, 134, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chai, C.; Wu, W.; Qi, P.; Liu, X.; Hao, J. Hydrogels as the plant culture substrates: A review. Carbohydr. Polym. 2023, 305, 120544. [Google Scholar] [CrossRef]
- Wu, Y.; Li, S.; Chen, G. Hydrogels as water and nutrient reservoirs in agricultural soil: A comprehensive review of classification, performance, and economic advantages. Environ. Dev. Sustain. 2024, 26, 24653–24685. [Google Scholar] [CrossRef]
- Kaur, P.; Agrawal, R.; Pfeffer, F.M.; Williams, R.; Bohidar, H.B. Hydrogels in Agriculture: Prospects and challenges. J. Polym. Environ. 2023, 31, 3701–3718. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Muñoz, K.; Frör, O.; Schaumann, G.E. Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total Environ. 2016, 550, 690–705. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Jha, K.; Yahya, E.B.; Panchal, S.; Patel, N.; Garai, A.; Kumari, S.; Jameel, M. Insights into the potential of biopolymeric aerogels as an advanced soil-fertilizer delivery systems. Gels 2023, 9, 666. [Google Scholar] [CrossRef]
- Abedi-Koupai, J.; Sohrab, F.; Swarbrick, G. Evaluation of hydrogel application on soil water retention characteristics. J. Plant Nutr. 2008, 31, 317–331. [Google Scholar] [CrossRef]
- Narjary, B.; Aggarwal, P. Evaluation of soil physical quality under amendments and hydrogel applications in a soybean–wheat cropping system. Commun. Soil Sci. Plant Anal. 2014, 45, 1167–1180. [Google Scholar] [CrossRef]
- Guo, M.; Liu, M.; Hu, Z.; Zhan, F.; Wu, L. Preparation and properties of a slow release NP compound fertilizer with superabsorbent and moisture preservation. J. Appl. Polym. Sci. 2005, 96, 2132–2138. [Google Scholar] [CrossRef]
- Akhter, J.; Mahmood, K.; Malik, K.A.; Mardan, A.; Ahmad, M.; Iqbal, M.M. Effects of hydrogel amendment on water storage of sandy loam and loam soils and seedling growth of barley, wheat and chickpea. Plant Soil Environ. 2004, 50, 463–469. [Google Scholar] [CrossRef]
- Costa, M.C.G.; Freire, A.G.; Lourenço, D.V.; Sousa, R.R.; Feitosa, J.P.A.; Mota, J.C.A. Hydrogel composed of potassium acrylate, acrylamide, and mineral as soil conditioner under saline conditions. Sci. Agric. 2022, 79, e20200235. [Google Scholar] [CrossRef]
- Piccoli, I.; Camarotto, C.; Squartini, A.; Longo, M.; Gross, S.; Maggini, M.; Cabrera, M.L.; Morari, F. Hydrogels for agronomical application: From soil characteristics to crop growth. Agron. Sustain. Dev. 2024, 44, 22. [Google Scholar] [CrossRef]
- Neethu, T.M.; Dubey, P.K.; Kaswala, A.R. Prospects and applications of hydrogel technology in agriculture. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3155–3162. [Google Scholar] [CrossRef]
- Čechmánková, J.; Skála, J.; Sedlařík, V.; Duřpeková, S.; Drbohlav, J.; Šalaková, A.; Vácha, R. The synergic effect of whey-based hydrogel amendment on soil water holding capacity and availability of nutrients for more efficient valorization of dairy by-products. Sustainability 2021, 13, 10701. [Google Scholar] [CrossRef]
- Ni, B.; Liu, M.; Lü, S. Multifunctional slow-release urea fertilizer from ethylcellulose and superabsorbent coated formulations. J. Chem. Eng. 2009, 155, 892–898. [Google Scholar] [CrossRef]
- Lentz, R.D.; Ippolito, J.A. Cross-linked polymers increase nutrient sorption in degraded soils. Agron. J. 2021, 113, 1121–1135. [Google Scholar] [CrossRef]
- Koupai, J.A.; Eslamian, S.S.; Kazemi, J.A. Enhancing the available water content in unsaturated soil zone using hydrogel, to improve plant growth indices. Ecohydrol. Hydrobiol. 2008, 8, 67–75. [Google Scholar] [CrossRef]
- Hüttermann, A.; Zommorodi, M.; Reise, K. Addition of hydrogels to soil for prolonging the survival of Pinus halepensis seedlings subjected to drought. Soil Till. Res. 1999, 50, 295–304. [Google Scholar] [CrossRef]
- Adjuik, T.A.; Nokes, S.E.; Montross, M.D.; Wendroth, O. The impacts of bio-based and synthetic hydrogels on soil hydraulic properties: A review. Polymers 2022, 14, 4721. [Google Scholar] [CrossRef]
- Ahmad, D.F.B.A.; Wasli, M.E.; Tan, C.S.Y.; Musa, Z.; Chin, S. Eco-friendly cellulose-based hydrogels derived from wastepapers as a controlled-release fertilizer. Chem. Biol. Technol. Agric. 2023, 10, 36. [Google Scholar] [CrossRef]
- Rizwan, M.; Gilani, S.R.; Durani, A.I.; Naseem, S. Materials diversity of hydrogel: Synthesis, polymerization process and soil conditioning properties in agricultural field. J. Adv. Res. 2021, 33, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Cui, Y.J.; Dupla, J.C.; Canou, J. Soil-water retention behaviour of fine/coarse soil mixture with varying coarse grain contents and fine soil dry densities. Can. Geotech. J. 2022, 59, 291–299. [Google Scholar] [CrossRef]
- Maksimova, Y.G.; Shchetko, V.A.; Maksimov, A.Y. Polymer hydrogels in agriculture (Review). Sel’Skokhozyaistvennaya Biol. 2023, 58, 23–42. [Google Scholar] [CrossRef]
- Montesano, F.F.; Parente, A.; Santamaria, P.; Sannino, A.; Serio, F. Biodegradable superabsorbent hydrogel increases water retention properties of growing media and plant growth. Agric. Agric. Sci. Proc. 2015, 4, 451–458. [Google Scholar]
- Narjary, B.; Aggarwal, P.; Singh, A.; Chakraborty, D.; Singh, R. Water availability in different soils in relation to hydrogel application. Geoderma 2012, 187–188, 94–101. [Google Scholar] [CrossRef]
- Buchmann, C.; Steinmetz, Z.; Brax, M.; Peth, S.; Schaumann, G.E. Effect of matric potential and soil-water-hydrogel interactions on biohydrogel-induced soil microstructural stability. Geoderma 2020, 362, 114142. [Google Scholar] [CrossRef]
- Kuila, U.; Prasad, M. Specific surface area and pore size distribution in clays and shales. Geophys. Prospect. 2013, 61, 341–362. [Google Scholar] [CrossRef]
- Lu, Y.; Mccartney, J.S. Temperature effects on adsorption and capillarity water retention mechanisms in constrained unsaturated soils. Acta Geotech. 2024, 19, 6467–6482. [Google Scholar] [CrossRef]
- Leciejewski, P. The effect of hydrogel additives on the water retention curve of sandy soil from forest nursery in Julinek. J. Water Land Dev. 2009, 13, 239–247. [Google Scholar] [CrossRef]
- Senna, A.M.; Carmo, J.B.; Silva, J.M.S.; Botaro, V.R. Synthesis, characterization and application of hydrogel derived from cellulose acetate as a substrate for slow-release NPK fertilizer and water retention in soil. J. Environ. Chem. Eng. 2015, 3, 996–1002. [Google Scholar] [CrossRef]
- Benson, C.H.; Trast, J.M. Hydraulic conductivity of thirteen compacted clays. Clays Clay Miner. 1995, 43, 669–681. [Google Scholar] [CrossRef]
- Rousseau, M.; Pietro, L.D.; Angulo-Jaramillo, R.; Tessier, D.; Cabibel, B. Preferential transport of soil colloidal particles: Physicochemical effects on particle mobilization. Vadose Zone J. 2004, 3, 247–261. [Google Scholar] [CrossRef]
- Kumar, R.; Yadav, S.; Singh, V.; Kumar, M.; Kumar, M. Hydrogel and its effect on soil moisture status and plant growth: A review. J. Pharmacogn. Phytochem. 2020, 9, 1746–1753. [Google Scholar]
- Skrzypczak, D.; Mikula, K.; Kossińska, N.; Widera, B.; Warchoł, J.; Moustakas, K.; Chojnacka, K.; Witek-Krowiak, A. Biodegradable hydrogel materials for water storage in agriculture—Review of recent research. Desalin. Water Treat. 2020, 194, 324–332. [Google Scholar] [CrossRef]
- Tariq, Z.; Iqbal, D.N.; Rizwan, M.; Ahmad, M.; Faheem, M.; Ahmed, M. Significance of biopolymer-based hydrogels and their applications in agriculture: A review in perspective of synthesis and their degree of swelling for water holding. RSC Advances 2023, 13, 24731–24754. [Google Scholar] [CrossRef]
- Sarmah, D.; Karak, N. Biodegradable superabsorbent hydrogel for water holding in soil and controlled-release fertilizer. J. Appl. Polym. Sci. 2019, 137, 48495. [Google Scholar] [CrossRef]
- Hou, Y.; Deng, B.; Wang, S.; Ma, Y.; Long, X.; Wang, F.; Qin, C.; Liang, C.; Yao, S. High-strength, high-water-retention hemicellulose-based hydrogel and its application in urea slow release. Int. J. Mol. Sci. 2023, 24, 9208. [Google Scholar] [CrossRef]
- Tahir, S.; Marschner, P. Clay addition to sandy soil reduces nutrient leaching—Effect of clay concentration and ped size. Commun. Soil Sci. Plant Anal. 2017, 48, 1813–1821. [Google Scholar] [CrossRef]
- Zhang, G.; Ren, R.; Yan, X.; Zhang, H.; Zhu, Y. Effects of microplastics on dissipation of oxytetracycline and its relevant resistance genes in soil without and with Serratia marcescens: Comparison between biodegradable and conventional microplastics. Ecotoxicol. Environ. Saf. 2024, 287, 117235. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zou, G.; Li, Y.; Pan, B.; Wang, X.; Zhang, J.; Xu, L.; Li, C.; Chen, Y. Biodegradable microplastics coupled with biochar enhance Cd chelation and reduce Cd accumulation in Chinese cabbage. Biochar 2025, 7, 31. [Google Scholar] [CrossRef]
- Bortolin, A.; Aouada, F.A.; Moura, M.R.; Ribeiro, C.; Longo, E.; Mattoso, L.H.C. Application of polysaccharide hydrogels in adsorption and controlled-extended release of fertilizers processes. J. Appl. Polym. Sci. 2011, 123, 2291–2298. [Google Scholar] [CrossRef]
- Chen, R.; Zhu, Z. Characteristics of the fate and efficiency of nitrogen in supergranules of urea. Fert. Res. 1982, 3, 63–71. [Google Scholar] [CrossRef]
- Osman, M. Effect of polymer-coated urea and ammonium nitrate application on nitrogen behaviour in sandy soil and yield productivity. J. Soil Sci. Agric. Eng. 2017, 8, 101–113. [Google Scholar] [CrossRef]
- Stanley, N.; Mahanty, B. Preparation and characterization of biogenic CaCO3-reinforced polyvinyl alcohol–alginate hydrogel as controlled-release urea formulation. Polym. Bull. 2019, 77, 529–540. [Google Scholar] [CrossRef]
- Khan, E.; Ozaltin, K.; Bernal-Ballen, A.; Martino, A. Renewable mixed hydrogels based on polysaccharide and protein for release of agrochemicals and soil conditioning. Sustainability 2021, 13, 10439. [Google Scholar] [CrossRef]
- Osman, K.T. Management of Soil Problems, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 37–65. [Google Scholar]
- El-Asmar, J.; Jaafar, H.; Bashour, I.; Farran, M.T.; Saoud, I.P. Hydrogel banding improves plant growth, survival, and water use efficiency in two calcareous soils. Clean Soil Air Water 2017, 45, 1700251. [Google Scholar] [CrossRef]
- Dorraji, S.S.; Golchin, A.; Ahmadi, S. The effects of hydrophilic polymer and soil salinity on corn growth in sandy and loamy soils. Clean Soil Air Water 2010, 38, 584–591. [Google Scholar] [CrossRef]
- Qin, C.; Abdalkarim, S.Y.H.; Zhou, Y.; Yu, H.; He, X. Ultrahigh water-retention cellulose hydrogels as soil amendments for early seed germination under harsh conditions. J. Clean. Prod. 2022, 370, 133602. [Google Scholar] [CrossRef]
- Campo, A.D.D.; Hermoso, J.; Flors, J.; Lidon, A.; Navarro-Cerrillo, R.M. Nursery location and potassium enrichment in Aleppo pine stock 2. Performance under real and hydrogel-mediated drought conditions. Forestry 2011, 84, 235–245. [Google Scholar] [CrossRef]
- Wallace, A. Soil acidification from use of too much fertilizer. Commun. Soil Sci. Plant Anal. 1994, 25, 87–92. [Google Scholar] [CrossRef]
- Hu, S.; Zeng, R.J.; Haroon, M.F.; Keller, J.; Lant, P.A.; Tyson, G.W.; Yuan, Z. A laboratory investigation of interactions between denitrifying anaerobic methane oxidation (DAMO) and anammox processes in anoxic environments. Sci. Rep. 2015, 3, 8706. [Google Scholar] [CrossRef]
- Jermakka, J.; Brewster, E.T.; Ledezma, P.; Freguia, S. Electro-concentration for chemical-free nitrogen capture as solid ammonium bicarbonate. Sep. Purif. Technol. 2018, 203, 48–55. [Google Scholar] [CrossRef]







| Material | %C (se) | %N (se) | C:N Ratio |
|---|---|---|---|
| Hydrogel | 31.1 (1.6) | 19.6 (0.6) | 1.6 |
| Loam | 1.15 (0.09) | 0.09 (0.01) | 13 |
| Clay | 1.67 (0.09) | 0.07 (0.01) | 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuneski, A.C.; Haridevan, H.; Ninkovic, E.; McLeary, E.; Martin, D.; Kirchhof, G. Tissue Paper-Based Hydrogels for Soil Water Maintenance and Nitrogen Release. Gels 2025, 11, 599. https://doi.org/10.3390/gels11080599
Kuneski AC, Haridevan H, Ninkovic E, McLeary E, Martin D, Kirchhof G. Tissue Paper-Based Hydrogels for Soil Water Maintenance and Nitrogen Release. Gels. 2025; 11(8):599. https://doi.org/10.3390/gels11080599
Chicago/Turabian StyleKuneski, Ana Carla, Hima Haridevan, Elena Ninkovic, Ena McLeary, Darren Martin, and Gunnar Kirchhof. 2025. "Tissue Paper-Based Hydrogels for Soil Water Maintenance and Nitrogen Release" Gels 11, no. 8: 599. https://doi.org/10.3390/gels11080599
APA StyleKuneski, A. C., Haridevan, H., Ninkovic, E., McLeary, E., Martin, D., & Kirchhof, G. (2025). Tissue Paper-Based Hydrogels for Soil Water Maintenance and Nitrogen Release. Gels, 11(8), 599. https://doi.org/10.3390/gels11080599








