Ionizing Radiation Crosslinked Chitosan-Based Hydrogels for Environmental Remediation
Abstract
1. Introduction
2. Structure of Chitosan and Its Properties
3. Crosslinking Strategies for Chitosan Hydrogel
3.1. Physical Crosslinking
3.2. Chemical Crosslinking
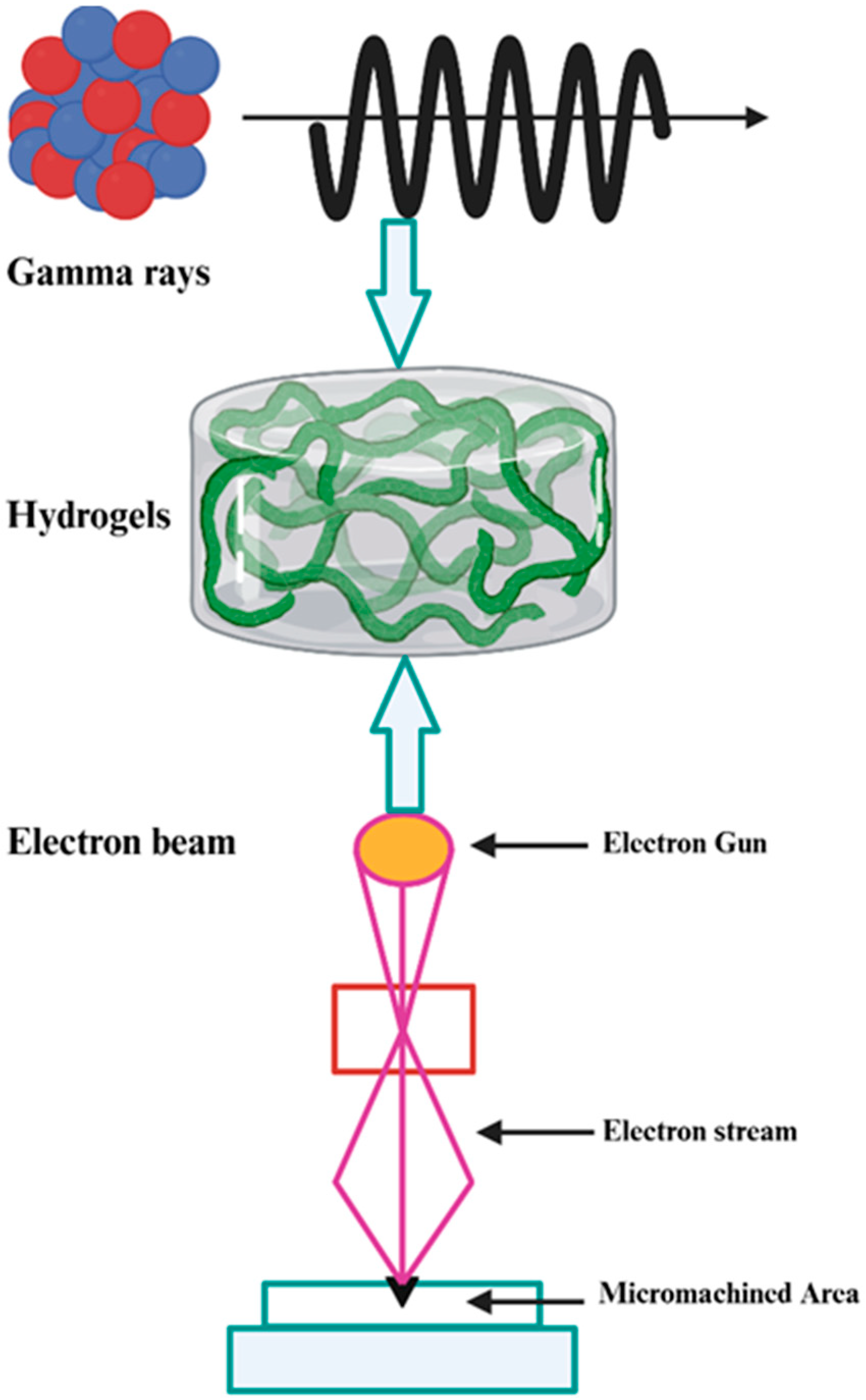
3.3. Irradiation Crosslinking
4. Fabrication of Chitosan Hydrogels Using Ionizing Radiation
Nanostructure Incorporated Chitosan-Based Nanocomposite Hydrogels
5. Environmental Remediation
5.1. Removal of Toxic Heavy Metals
5.2. Removal of Dyes
5.3. Extraction of Organic Contaminants
5.4. Removal of Radionuclides
5.5. Removal of Humic Substances
| Materials | Ionizing Radiation (Parameters) | Adsorption Kinetics, Isotherms, and Examined Number of Cycles | Application (Potential for Adsorbate Removal) | References |
|---|---|---|---|---|
| Poly(vinyl alcohol); Sodium carboxymethyl cellulose; Chitosan | γ radiation radiation doses: 2.5, 5, 10, and 20 kGy; (dose rate: 2.5 kGy/h) | MB adsorption: pseudo-first-order, DB1 adsorption: pseudo-second-order kinetic model; Langmuir model; 5 cycles | Removal of ionic dyes and their biodegradation (8.09 mg/g of methylene blue and 17.23 mg/g of direct blue) | [95] |
| Poly(acrylamide); Chitosan; Multi-walled carbon nanotubes | γ radiation radiation dose: 30 kGy; (dose rate: 0.6 kGy/h) | Pseudo-second-order model; 5 cycles | Potential for effectively removing oil and organic solvent contaminants from water (93.15% of oil and 80.68% of methanol) | [100] |
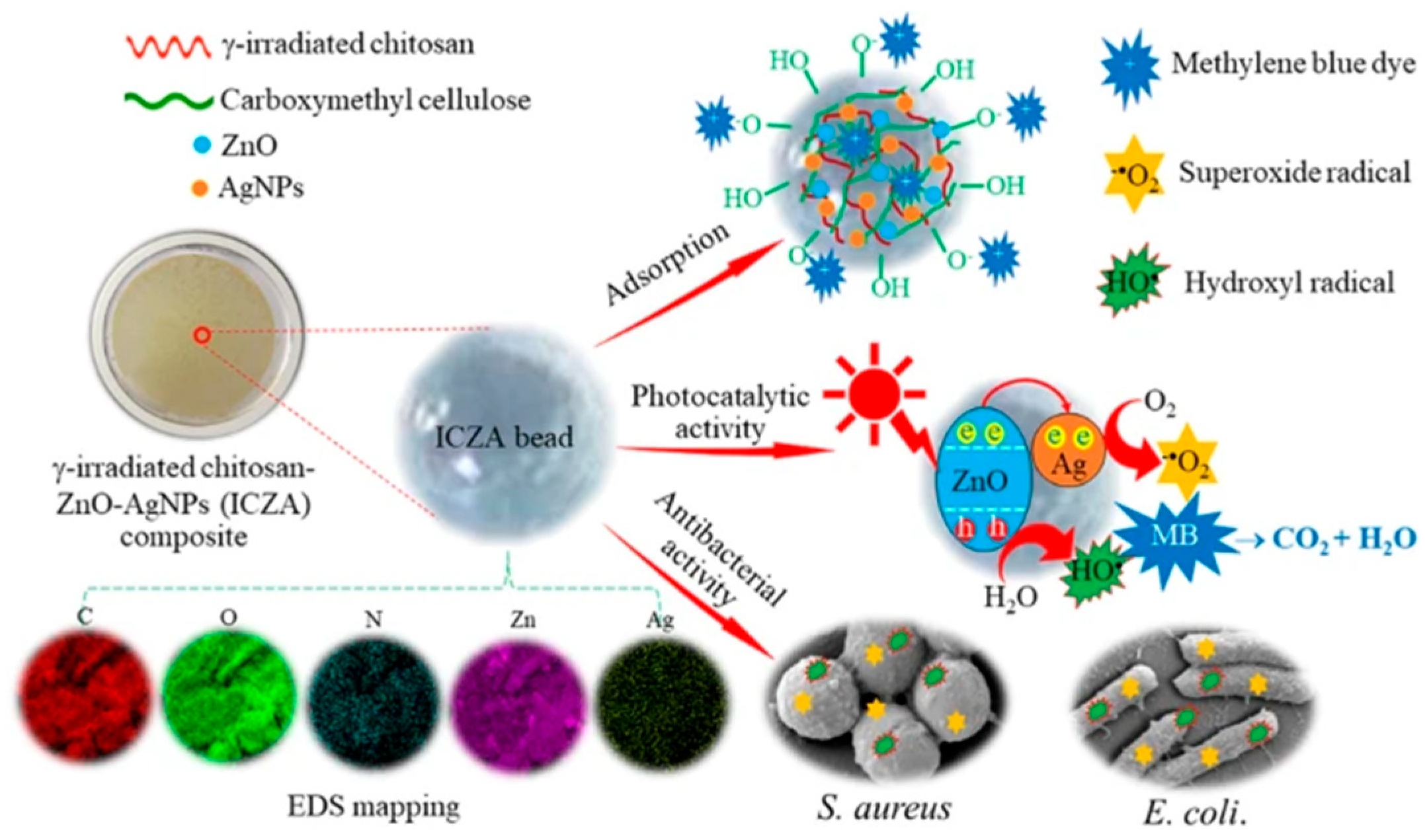
| Chitosan; Zinc oxide; Silver nanoparticles | γ radiation radiation dose: 40 kGy | Pseudo-first-order kinetic model; Langmuir isotherm model; 5 cycles | Organic dye removal (92.59 mg/g of methylene blue) | [75] |
| Graphitic carbon nitride; Chitosan; Poly(vinyl alcohol) | E. beam radiation dose: 30 kGy; (dose rate: 5 kGy/pass) | Quasi-second-order fitting | Potential for environmental wastewater treatment (65.92% of rhodamine B) | [77] |
| Chitosan; Maleic acid | γ radiation radiation dose: 1–5 kGy; (dose rate: 31.98 Gy/min) | Pseudo-second-order kinetic model; Temkin isotherm model | Potential for adsorption of radioisotopes (2.78 mg/g of cobalt-60) | [102] |
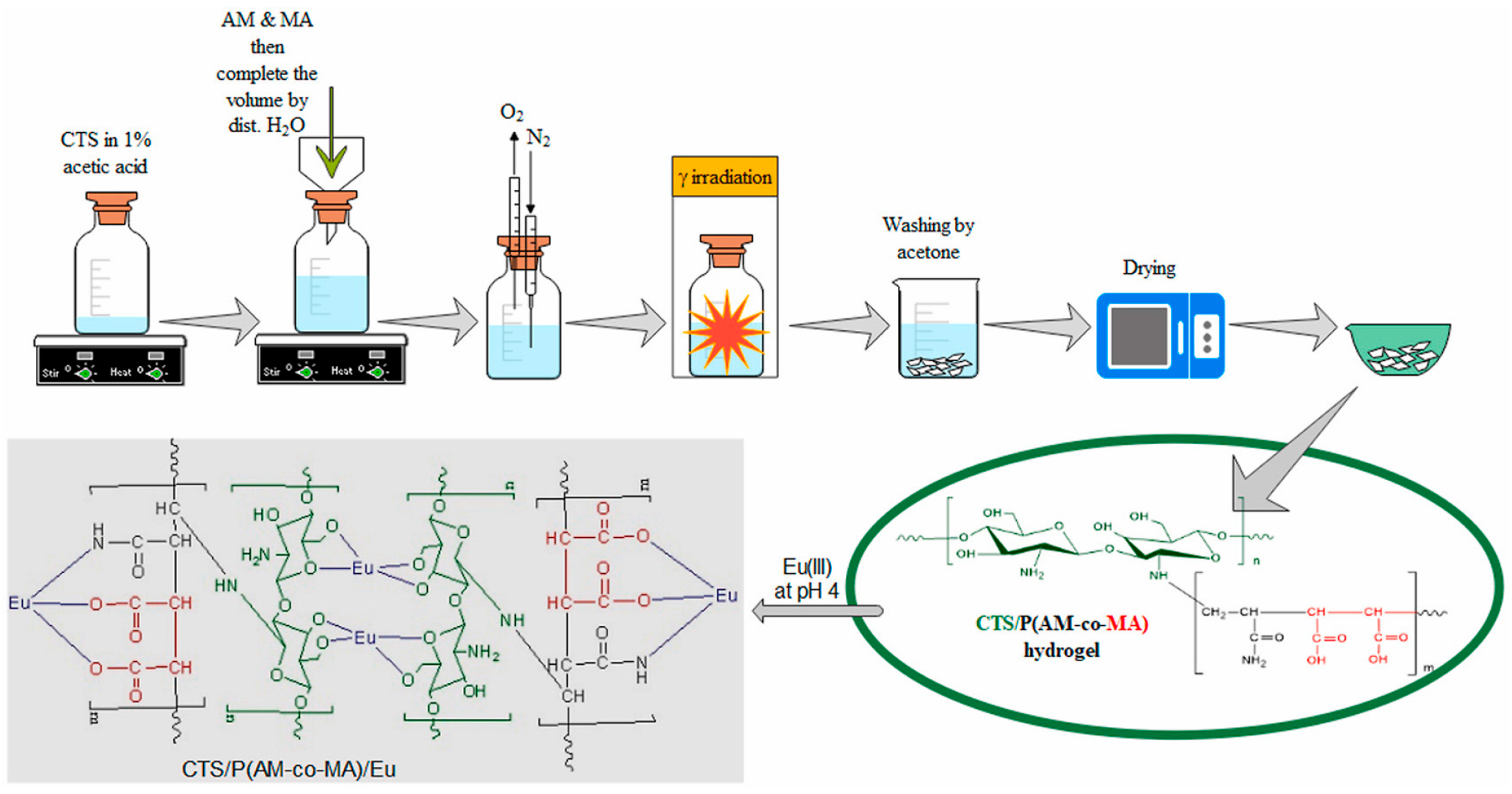
| Chitosan; Poly(acrylamide-co-maleic acid) | γ radiation radiation dose: 20 kGy | Pseudo-second-order model Langmuir isotherm | Potential for adsorption of trivalent lanthanide ions (144.96 mg/g of 152+154Eu (III)) | [103] |
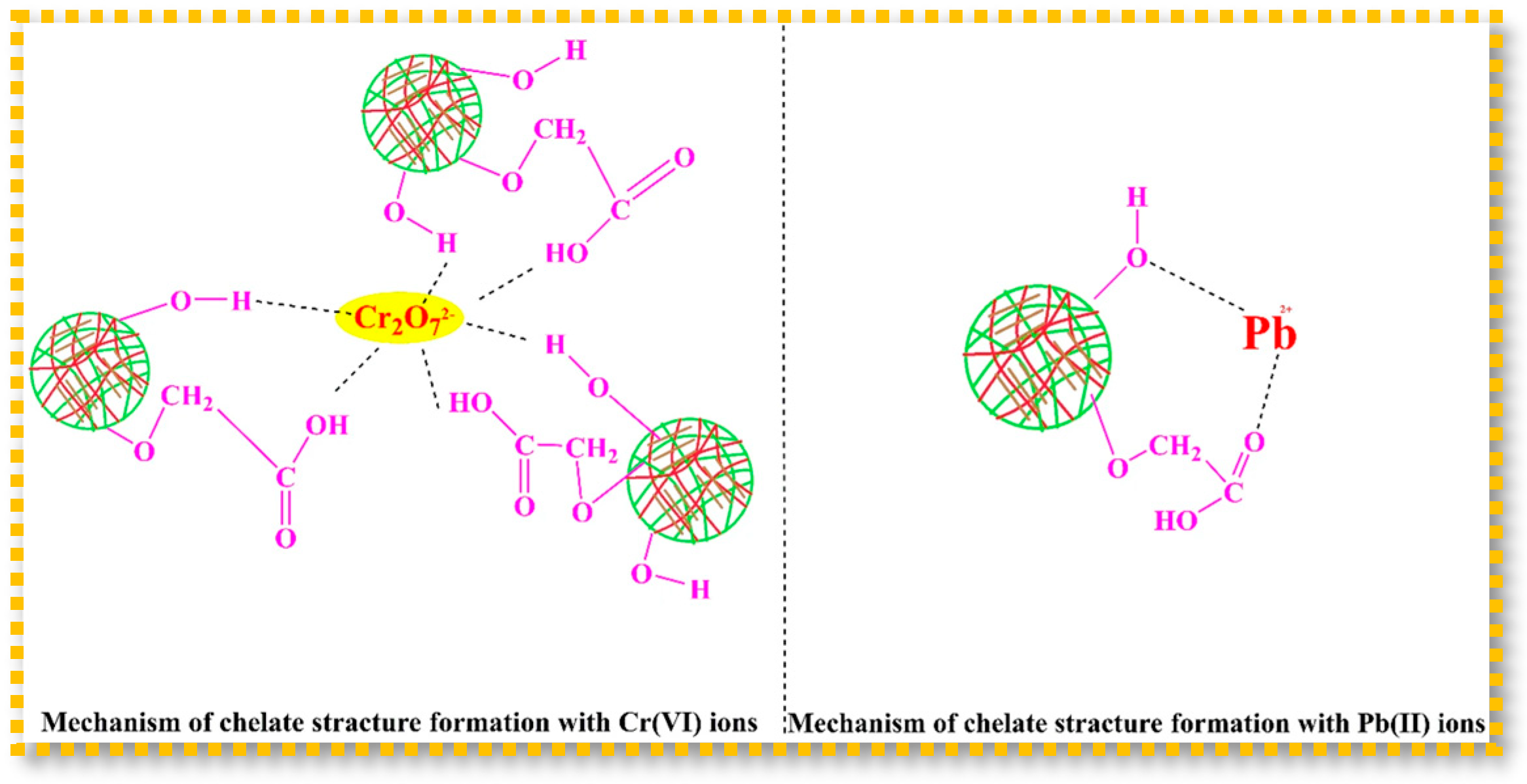
| N,O carboxymethyl Chitosan; Nanoclay; Acrylic acid | γ radiation radiation dose: 25 kGy | Pseudo-first-order model; Langmuir isotherm model; 3 cycles | Removal of Cr(VI) and Pb(II) from aqueous media (205 mg/g of Cr(VI) and 125 mg/g of Pb(II)) | [89] |
| Chitosan; Poly(propenoic acid); Ethylenediamine; Magnetite | γ radiation radiation dose: 30 kGy; (dose rate: 0.309 Gy/s) | Pseudo-second-order model; Langmuir isotherm model; 3 cycles | Potential for wastewater contamination remediation applications (193.21 mg/g of Astrazon blue and 51.9 mg/g of Lerui Acid Brilliant Blue dye) | [76] |
| Carboxymethyl chitosan; Carboxymethyl cellulose; Sodium sulfonate styrene | γ radiation radiation dose: 60 kGy | Pseudo-second-order models; Langmuir isotherm model; 4 cycles | Potential for wastewater treatment processes containing silver metal (451.74 × 10−3 mg/g of silver) | [88] |
| Chitosan; 2-Hydroxyethyl methacrylate | γ radiation radiation dose: 10, 20, and 30 kGy; (dose rate: 1.95 kGy/h) | Pseudo-second-order model; Langmuir and the Freundlich isotherm models; 5 cycles | Metal ions removal 66.3 mg/g of Cu(II), 48.7 mg/g of Ca(II), and 57.6 mg/g Zn (II) | [87] |
| Chitosan; Acrylamide | γ radiation radiation dose: 10–40 kGy | Langmuir and the Freundlich isotherm models | Metal ions removal (296.68 mg/g of Zn2+, 242.77 mg/g of Cr6+, 187.64 mg/g of Pb2+, 151.93 mg/g of Cu2+, 127.86 mg/g of Co2+ and 59.29 mg/g of Ni2+) | [86] |
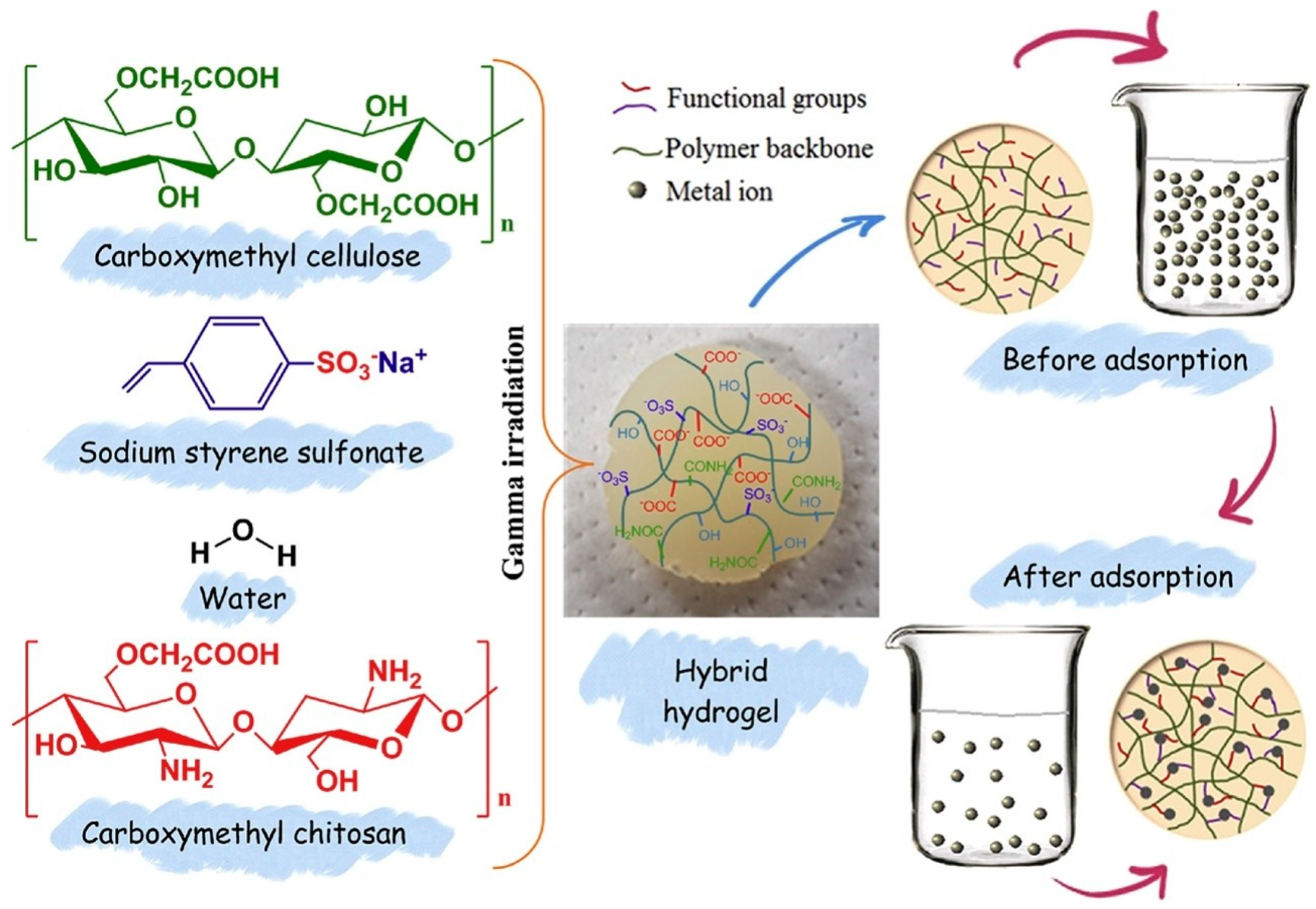
| Carboxymethyl cellulose; Carboxymethyl chitosan | γ radiation radiation dose: 10–200 kGy; (dose rate: 10 kGy/h) | – | Metal ions removal Distribution coefficient (2.5 × 103–1.1 × 104 of Pb and 2.7 × 102–1.8 × 104 of Au) | [67] |
| N,O-carboxymethyl chitosan | γ radiation radiation dose: 0–100 kGy | Langmuir isotherm model | Fe(III) ion adsorption (18.5 mg/g of Fe(III)) | [84] |
| Carboxymethyl chitosan | E. beam radiation dose: 50–200 kGy; (dose rate: 1 kGy/pass) | Langmuir model | Potential in water treatment for the removal of humic acid and electrically charged or other polarized species (57.14 mg/g of humic acid) | [105] |
| Chitosan; CM-cellulose | E. beam radiation dose: 10–100 kGy; (dose rate: 5 kGy/pass) | Langmuir model | Potential for applications in water treatment for the removal of heavy metal ions (169.49 mg/g of Cu(II)) | [85] |
| Carboxymethyl chitin; Carboxymethyl chitosan | E. beam radiation dose: 10 up to 200 kGy | Langmuir equation | Adsorption of metal ions (37.59 mg/g of Au for CM-CHT, 11.86 mg/g of Au for CM-chitin) | [66] |
6. Conclusion, Challenges, and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swindle-Reilly, K.E.; Reilly, M.A.; Ravi, N. Current Concepts in the Design of Hydrogels as Vitreous Substitutes. In Biomaterials and Regenerative Medicine in Ophthalmology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 101–130. [Google Scholar]
- Chatterjee, S.; Lee, M.W.; Woo, S.H. Adsorption of Congo Red by Chitosan Hydrogel Beads Impregnated with Carbon Nanotubes. Bioresour. Technol. 2010, 101, 1800–1806. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Ansari, M.; Ranjha, N.M.; Khan, I.U.; Shahzad, Y. Chemically Cross-Linked Poly(Acrylic-Co-vinylsulfonic) Acid Hydrogel for the Delivery of Isosorbide Mononitrate. Sci. World J. 2013, 2013, 340737. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, G.; Chu, L.; Liu, Y.; Liu, C.; Luo, S.; Wei, Y. Efficient Removal of Heavy Metal Ions with An EDTA Functionalized Chitosan/Polyacrylamide Double Network Hydrogel. ACS Sustain. Chem. Eng. 2017, 5, 843–851. [Google Scholar] [CrossRef]
- Sennakesavan, G.; Mostakhdemin, M.; Dkhar, L.K.; Seyfoddin, A.; Fatihhi, S.J. Acrylic Acid/Acrylamide Based Hydrogels and Its Properties—A Review. Polym. Degrad. Stab. 2020, 180, 109308. [Google Scholar] [CrossRef]
- Galal Ibrahim, A. Synthesis of Poly(Acrylamide-Graft-Chitosan) Hydrogel: Optimization of The Grafting Parameters and Swelling Studies. Am. J. Polym. Sci. Technol. 2019, 5, 55. [Google Scholar] [CrossRef]
- Abo-Zahra, S.F.; Abdelmonem, I.M.; Siyam, T.E.; El-Masry, A.M.; Abdel-Aziz, H.M. Radiation Synthesis of Polyacrylamide/Functionalized Multiwalled Carbon Nanotubes Composites for the Adsorption of Cu(II) Metal Ions from Aqueous Solution. Polym. Bull. 2022, 79, 4395–4415. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L. A Sustainable Solution for Environmental Purification: A Review of High-Performance Hydrogels Based on Chitosan. Int. J. Biol. Macromol. 2025, 309, 142834. [Google Scholar] [CrossRef] [PubMed]
- Alakhras, F.A.; Dari, K.A.; Mubarak, M.S. Synthesis and Chelating Properties of Some Poly(Amidoxime-hydroxamic Acid) Resins toward Some Trivalent Lanthanide Metal Ions. J. Appl. Polym. Sci. 2005, 97, 691–696. [Google Scholar] [CrossRef]
- Farinelli, G.; Di Luca, A.; Kaila, V.R.I.; MacLachlan, M.J.; Tiraferri, A. Fe-Chitosan Complexes for Oxidative Degradation of Emerging Contaminants in Water: Structure, Activity, and Reaction Mechanism. J. Hazard. Mater. 2021, 408, 124662. [Google Scholar] [CrossRef]
- Al-Remawi, M. Application of N-Hexoyl Chitosan Derivatives with High Degree of Substitution in the Preparation of Super-Disintegrating Pharmaceutical Matrices. J. Drug Deliv. Sci. Technol. 2015, 29, 31–41. [Google Scholar] [CrossRef]
- Li, C.; Marin, L.; Cheng, X. Chitosan Based Macromolecular Probes for the Selective Detection and Removal of Fe3+ Ion. Int. J. Biol. Macromol. 2021, 186, 303–313. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Fu, Z.; Lu, L.; Cheng, J.; Fei, Y. A ‘Top Modification’ Strategy for Enhancing the Ability of a Chitosan Aerogel to Efficiently Capture Heavy Metal Ions. J. Colloid Interface Sci. 2021, 594, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Kumar, I.A.; Jeyaprabha, C.; Meenakshi, S.; Viswanathan, N. Hydrothermal Encapsulation of Lanthanum Oxide Derived Aegle Marmelos Admixed Chitosan Bead System for Nitrate and Phosphate Retention. Int. J. Biol. Macromol. 2019, 130, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Rasool, A.; Ata, S.; Islam, A. Stimuli Responsive Biopolymer (Chitosan) Based Blend Hydrogels for Wound Healing Application. Carbohydr. Polym. 2019, 203, 423–429. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Chitin and Chitosan: Sources, Production and Medical Applications. In Renewable Resources for Functional Polymers and Biomaterials; The Royal Society of Chemistry: London, UK, 2011; pp. 292–318. [Google Scholar]
- Ahmed, S.; Ikram, S. Chitosan: Derivatives, Composites and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2017; ISBN 1119364817. [Google Scholar]
- Xu, Y.; Han, J.; Lin, H. Fabrication and Characterization of a Self-Crosslinking Chitosan Hydrogel under Mild Conditions without the Use of Strong Bases. Carbohydr. Polym. 2017, 156, 372–379. [Google Scholar] [CrossRef]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-Based Functional Materials for Skin Wound Repair: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Hussain, M.R.; Iman, M.; Maji, T.K. Determination of Degree of Deacetylation of Chitosan and Their Effect on the Release Behavior of Essential Oil from Chitosan and Chitosan-Gelatin Complex Microcapsules. Int. J. Adv. Eng. Appl. 2013, 2, 4–12. [Google Scholar]
- Patil, J.S.; Marapur, S.C.; Gurav, P.B.; Banagar, A.V. Ionotropic Gelation and Polyelectrolyte Complexation Technique: Novel Approach to Drug Encapsulation. In Handbook of Encapsulation and Controlled Release; CRC Press: Boca Raton, FL, USA, 2015; pp. 273–296. [Google Scholar]
- Shuai, H.-H.; Yang, C.-Y.; Hans, I.; Harn, C.; York, R.L.; Liao, T.-C.; Chen, W.-S.; Yeh, J.A.; Cheng, C.-M. Using Surfaces to Modulate the Morphology and Structure of Attached Cells—A Case of Cancer Cells on Chitosan Membranes. Chem. Sci. 2013, 4, 3058–3067. [Google Scholar] [CrossRef]
- Saberianpour, S.; Heidarzadeh, M.; Geranmayeh, M.H.; Hosseinkhani, H.; Rahbarghazi, R.; Nouri, M. Tissue Engineering Strategies for the Induction of Angiogenesis Using Biomaterials. J. Biol. Eng. 2018, 12, 36. [Google Scholar] [CrossRef]
- Geng, Y.; Xue, H.; Zhang, Z.; Panayi, A.C.; Knoedler, S.; Zhou, W.; Mi, B.; Liu, G. Recent Advances in Carboxymethyl Chitosan-Based Materials for Biomedical Applications. Carbohydr. Polym. 2023, 305, 120555. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z. Carboxymethyl Chitosan: Properties and Biomedical Applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef]
- Kłosiński, K.K.; Wach, R.A.; Girek-Bąk, M.K.; Rokita, B.; Kołat, D.; Kałuzińska-Kołat, Ż.; Kłosińska, B.; Duda, Ł.; Pasieka, Z.W. Biocompatibility and Mechanical Properties of Carboxymethyl Chitosan Hydrogels. Polymers 2023, 15, 144. [Google Scholar] [CrossRef]
- Chaiwarit, T.; Sommano, S.R.; Rachtanapun, P.; Kantrong, N.; Ruksiriwanich, W.; Kumpugdee-Vollrath, M.; Jantrawut, P. Development of Carboxymethyl Chitosan Nanoparticles Prepared by Ultrasound-Assisted Technique for a Clindamycin HCl Carrier. Polymers 2022, 14, 1736. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.C.; Signini, R.; Rosa, L.M.; Dias, Y.S.P.; Vinaud, M.C.; Lino Junior, R.d.S. Carboxymethyl Chitosan Hydrogel Formulations Enhance the Healing Process in Experimental Partial-Thickness (Second-Degree) Burn Wound Healing. Acta Cir. Bras. 2021, 36, e360303. [Google Scholar] [CrossRef]
- Hao, Y.; Zhao, W.; Zhang, H.; Zheng, W.; Zhou, Q. Carboxymethyl Chitosan-Based Hydrogels Containing Fibroblast Growth Factors for Triggering Diabetic Wound Healing. Carbohydr. Polym. 2022, 287, 119336. [Google Scholar] [CrossRef] [PubMed]
- Maitra, J.; Shukla, V. Cross-Linking in Hydrogels—A Review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar]
- Wang, Y.; Yu, W.; Liu, S. Physically Cross-Linked Gellan Gum/Hydrophobically Associated Polyacrylamide Double Network Hydrogel for Cartilage Repair. Eur. Polym. J. 2022, 167, 111074. [Google Scholar] [CrossRef]
- Hong, F.; Qiu, P.; Wang, Y.; Ren, P.; Liu, J.; Zhao, J.; Gou, D. Chitosan-Based Hydrogels: From Preparation to Applications, a Review. Food Chem. X 2024, 21, 101095. [Google Scholar] [CrossRef]
- Yang, J.; Liu, D.; Song, X.; Zhao, Y.; Wang, Y.; Rao, L.; Fu, L.; Wang, Z.; Yang, X.; Li, Y.; et al. Recent Progress of Cellulose-Based Hydrogel Photocatalysts and Their Applications. Gels 2022, 8, 270. [Google Scholar] [CrossRef]
- Chen, J.; An, R.; Han, L.; Wang, X.; Zhang, Y.; Shi, L.; Ran, R. Tough Hydrophobic Association Hydrogels with Self-Healing and Reforming Capabilities Achieved by Polymeric Core-Shell Nanoparticles. Mater. Sci. Eng. C 2019, 99, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, K.; Dhamodharan, R. Advances in Chitosan-Based Hydrogels: Evolution from Covalently Crosslinked Systems to Ionotropically Crosslinked Superabsorbents. React. Funct. Polym. 2020, 149, 104517. [Google Scholar] [CrossRef]
- Liu, T.; Peng, X.; Chen, Y.N.; Bai, Q.W.; Shang, C.; Zhang, L.; Wang, H. Hydrogen-Bonded Polymer–Small Molecule Complexes with Tunable Mechanical Properties. Macromol. Rapid Commun. 2018, 39, 1800050. [Google Scholar] [CrossRef]
- Chang, X.; Geng, Y.; Cao, H.; Zhou, J.; Tian, Y.; Shan, G.; Bao, Y.; Liang Wu, Z.; Pan, P.; Chang, X.; et al. Dual-Crosslink Physical Hydrogels with High Toughness Based on Synergistic Hydrogen Bonding and Hydrophobic Interactions. Macromol. Rapid Commun. 2018, 39, 1700806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chang, L.; Sun, P.; Cao, Z.; Chen, Y.; Liu, H. High-Performance Double-Network Ionogels Enabled by Electrostatic Interaction. RSC Adv. 2020, 10, 7424–7431. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Y.; Qin, D.; Sun, M.; Wang, T.; Chen, X. Research Status of Self-Healing Hydrogel for Wound Management: A Review. Int. J. Biol. Macromol. 2020, 164, 2108–2123. [Google Scholar] [CrossRef]
- Tian, B.; Hua, S.; Tian, Y.; Liu, J. Chemical and Physical Chitosan Hydrogels as Prospective Carriers for Drug Delivery: A Review. J. Mater. Chem. B 2020, 8, 10050–10064. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zou, L.; Zhou, X.; Huang, D.; Zhang, Y. High Strength Chitosan Hydrogels Prepared from NaOH/Urea Aqueous Solutions: The Role of Thermal Gelling. Carbohydr. Polym. 2022, 297, 120054. [Google Scholar] [CrossRef]
- Medellín-Castillo, N.A.; Isaacs-Páez, E.D.; Rodríguez-Méndez, I.; González-García, R.; Labrada-Delgado, G.J.; Aragón-Piña, A.; García-Arreola, M.E. Formaldehyde and Tripolyphosphate Crosslinked Chitosan Hydrogels: Synthesis, Characterization and Modeling. Int. J. Biol. Macromol. 2021, 183, 2293–2304. [Google Scholar] [CrossRef]
- Sang, Y.; Cao, Y.; Wang, L.; Yan, W.; Chen, T.; Huang, J.; Liu, Y.N. N-Rich Porous Organic Polymers Based on Schiff Base Reaction for CO2 Capture and Mercury(II) Adsorption. J. Colloid Interface Sci. 2021, 587, 121–130. [Google Scholar] [CrossRef]
- Huang, G.; Li, X. Applications of Michael Addition Reaction in Organic Synthesis. Curr. Org. Synth. 2017, 14, 568–571. [Google Scholar] [CrossRef]
- Summonte, S.; Racaniello, G.F.; Lopedota, A.; Denora, N.; Bernkop-Schnürch, A. Thiolated Polymeric Hydrogels for Biomedical Application: Cross-Linking Mechanisms. J. Control. Release 2021, 330, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Naredla, R.R.; Thompson, S.K.; Hoye, T.R. The Pentadehydro-Diels-Alder Reaction. Nature 2016, 532, 484–488. [Google Scholar] [CrossRef]
- Kaur, J.; Saxena, M.; Rishi, N. An Overview of Recent Advances in Biomedical Applications of Click Chemistry. Bioconjug Chem. 2021, 32, 1455–1471. [Google Scholar] [CrossRef]
- Makuuchi, K. Critical Review of Radiation Processing of Hydrogel and Polysaccharide. Radiat. Phys. Chem. 2010, 79, 267–271. [Google Scholar] [CrossRef]
- He, D.; Susanto, H.; Ulbricht, M. Photo-Irradiation for Preparation, Modification and Stimulation of Polymeric Membranes. Prog. Polym. Sci. 2009, 34, 62–98. [Google Scholar] [CrossRef]
- Nasef, S.M.; Khozemy, E.E.; Mahmoud, G.A. Characterization and in Vitro Drug Release Properties of Chitosan/Acrylamide/Gold Nanocomposite Prepared by Gamma Irradiation. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 723–732. [Google Scholar] [CrossRef]
- Elshahawy, M.F.; Ahmed, N.A.; Gad, Y.H.; Ali, A.E.H. Efficient Photocatalytic Remediation of Lerui Acid Brilliant Blue Dye Using Radiation- Prepared Carboxymethyl Cellulose/Acrylic Acid Hydrogel Supported by ZnO@Ag. Int. J. Biol. Macromol. 2024, 262, 129946. [Google Scholar] [CrossRef]
- Hayrabolulu, H.; Demeter, M.; Cutrubinis, M.; Şen, M. Radiation Synthesis and Characterization of Xanthan Gum Hydrogels. Radiat. Phys. Chem. 2021, 188, 109613. [Google Scholar] [CrossRef]
- Călina, I.; Demeter, M.; Scărișoreanu, A.; Abbas, A.; Raza, M.A. Role of Ionizing Radiation Techniques in Polymeric Hydrogel Synthesis for Tissue Engineering Applications. Gels 2025, 11, 47. [Google Scholar] [CrossRef]
- More, A.P.; Chapekar, S. Irradiation Assisted Synthesis of Hydrogel: A Review. Polym. Bull. 2024, 81, 5839–5908. [Google Scholar]
- Bucio, E.; Burillo, G.; Diaz, A. Characterization of Thermo and PH Sensitivity of Binary Graft Copolymers onto Polytetrafluoroethylene. Rev. Soc. Química México 2006, 50, 1–4. [Google Scholar]
- Melendez-Ortiz, H.I.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Grafting of N -vinyl Caprolactam and Methacrylic Acid onto Silicone Rubber Films for Drug-eluting Products. J. Appl. Polym. Sci. 2015, 132, 41855. [Google Scholar] [CrossRef]
- Lei, C.; Xiao, Q.; Zhou, S.; Zu, W.; Li, J.; Zeng, J.; Yan, L.; Huang, Y.; Wang, B. Synthesis and Characterization of Magnetic Carboxymethyl Chitosan-poly(Acrylic Acid-itaconic Acid) Hydrogel for the Efficient Adsorption of Malachite Green. J. Appl. Polym. Sci. 2022, 139, 52347. [Google Scholar] [CrossRef]
- Glass, S.; Kühnert, M.; Abel, B.; Schulze, A. Controlled Electron-Beam Synthesis of Transparent Hydrogels for Drug Delivery Applications. Polymers 2019, 11, 501. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Abdelmonem, I.M.; Metwally, E.; Siyam, T.E.; Abou El-Nour, F.; Mousa, A.-R.M. Gamma Radiation-Induced Preparation of Chitosan-Acrylic Acid-1-Vinyl-2-Vinylpyrrolidone/Multiwalled Carbon Nanotubes Composite for Removal of 152+154Eu, 60Co and 134Cs Radionuclides. Int. J. Biol. Macromol. 2020, 164, 2258–2266. [Google Scholar] [CrossRef]
- Gizawy, M.A.; Shamsel-Din, H.A.; Abdelmonem, I.M.; Ibrahim, M.I.A.; Mohamed, L.A.; Metwally, E. Synthesis of Chitosan-Acrylic Acid/Multiwalled Carbon Nanotubes Composite for Theranostic 47Sc Separation from Neutron Irradiated Titanium Target. Int. J. Biol. Macromol. 2020, 163, 79–86. [Google Scholar] [CrossRef]
- Şenol, Z.M.; Şimşek, S. Equilibrium, Kinetics and Thermodynamics of Pb(II) Ions from Aqueous Solution by Adsorption onto Chitosan-Dolomite Composite Beads. Int. J. Environ. Anal. Chem. 2022, 102, 4926–4940. [Google Scholar] [CrossRef]
- Kim, M.K.; Shanmuga Sundaram, K.; Anantha Iyengar, G.; Lee, K.-P. A Novel Chitosan Functional Gel Included with Multiwall Carbon Nanotube and Substituted Polyaniline as Adsorbent for Efficient Removal of Chromium Ion. Chem. Eng. J. 2015, 267, 51–64. [Google Scholar] [CrossRef]
- Benamer, S.; Mahlous, M.; Tahtat, D.; Nacer-Khodja, A.; Arabi, M.; Lounici, H.; Mameri, N. Radiation Synthesis of Chitosan Beads Grafted with Acrylic Acid for Metal Ions Sorption. Radiat. Phys. Chem. 2011, 80, 1391–1397. [Google Scholar] [CrossRef]
- Wasikiewicz, J.M.; Nagasawa, N.; Tamada, M.; Mitomo, H.; Yoshii, F. Adsorption of Metal Ions by Carboxymethylchitin and Carboxymethylchitosan Hydrogels. Nucl. Instrum. Methods Phys. Res. B 2005, 236, 617–623. [Google Scholar] [CrossRef]
- Hiroki, A.; Tran, H.T.; Nagasawa, N.; Yagi, T.; Tamada, M. Metal Adsorption of Carboxymethyl Cellulose/Carboxymethyl Chitosan Blend Hydrogels Prepared by Gamma Irradiation. Radiat. Phys. Chem. 2009, 78, 1076–1080. [Google Scholar] [CrossRef]
- Wang, J.-P.; Chen, Y.-Z.; Ge, X.-W.; Yu, H.-Q. Gamma Radiation-Induced Grafting of a Cationic Monomer onto Chitosan as a Flocculant. Chemosphere 2007, 66, 1752–1757. [Google Scholar] [CrossRef] [PubMed]
- Elbarbary, A.M.; El-Rehim, H.A.A.; El-Sawy, N.M.; Hegazy, E.S.A.; Soliman, E.S.A. Radiation Induced Crosslinking of Polyacrylamide Incorporated Low Molecular Weights Natural Polymers for Possible Use in the Agricultural Applications. Carbohydr. Polym. 2017, 176, 19–28. [Google Scholar] [CrossRef]
- Rafieian, S.; Mirzadeh, H.; Mahdavi, H.; Masoumi, M.E. A Review on Nanocomposite Hydrogels and Their Biomedical Applications. Sci. Eng. Compos. Mater. 2019, 26, 154–174. [Google Scholar] [CrossRef]
- Merino, S.; Martín, C.; Kostarelos, K.; Prato, M.; Vázquez, E. Nanocomposite Hydrogels: 3D Polymer–Nanoparticle Synergies for On-Demand Drug Delivery. ACS Nano 2015, 9, 4686–4697. [Google Scholar] [CrossRef]
- Wallyn, J.; Anton, N.; Vandamme, T.F. Synthesis, Principles, and Properties of Magnetite Nanoparticles for In Vivo Imaging Applications—A Review. Pharmaceutics 2019, 11, 601. [Google Scholar] [CrossRef]
- Yang, L.; Wang, H.; Fang, S.; Li, M. Research Progress on Energy Storage Performance Enhancement Strategies for Polyvinylidene Fluoride-Based Composites. J. Alloys Compd. 2023, 960, 170831. [Google Scholar] [CrossRef]
- Pan, Z.; Wang, Z.-Y.; Wang, M.-H.; Yang, L.; Yu, S.-H. Adhesive Aero-Hydrogel Hybrid Conductor Assembled from Silver Nanowire Architectures. Sci. China Mater. 2021, 64, 2868–2876. [Google Scholar] [CrossRef]
- Chaisorn, W.; Nuengmatcha, P.; Noypha, A.; Pimsen, R.; Porrawatkul, P.; Kuyyogsuy, A.; Thepchuay, Y.; Sricharoen, P.; Limchoowong, N.; Chanthai, S.; et al. Adsorption-Photocatalytic Degradation Abilities of γ-Irradiated Chitosan-ZnO-AgNP Composite for Organic Dye Removal and Antibacterial Activity. Environ. Sci. Pollut. Res. 2023, 30, 96840–96859. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.E.; Nasef, S.M.; Gad, Y.H. Remediation of Astrazon Blue and Lerui Acid Brilliant Blue Dyes from Waste Solutions Using Amphoteric Superparamagnetic Nanocomposite Hydrogels Based on Chitosan Prepared by Gamma Rays. Carbohydr. Polym. 2022, 283, 119149. [Google Scholar] [CrossRef] [PubMed]
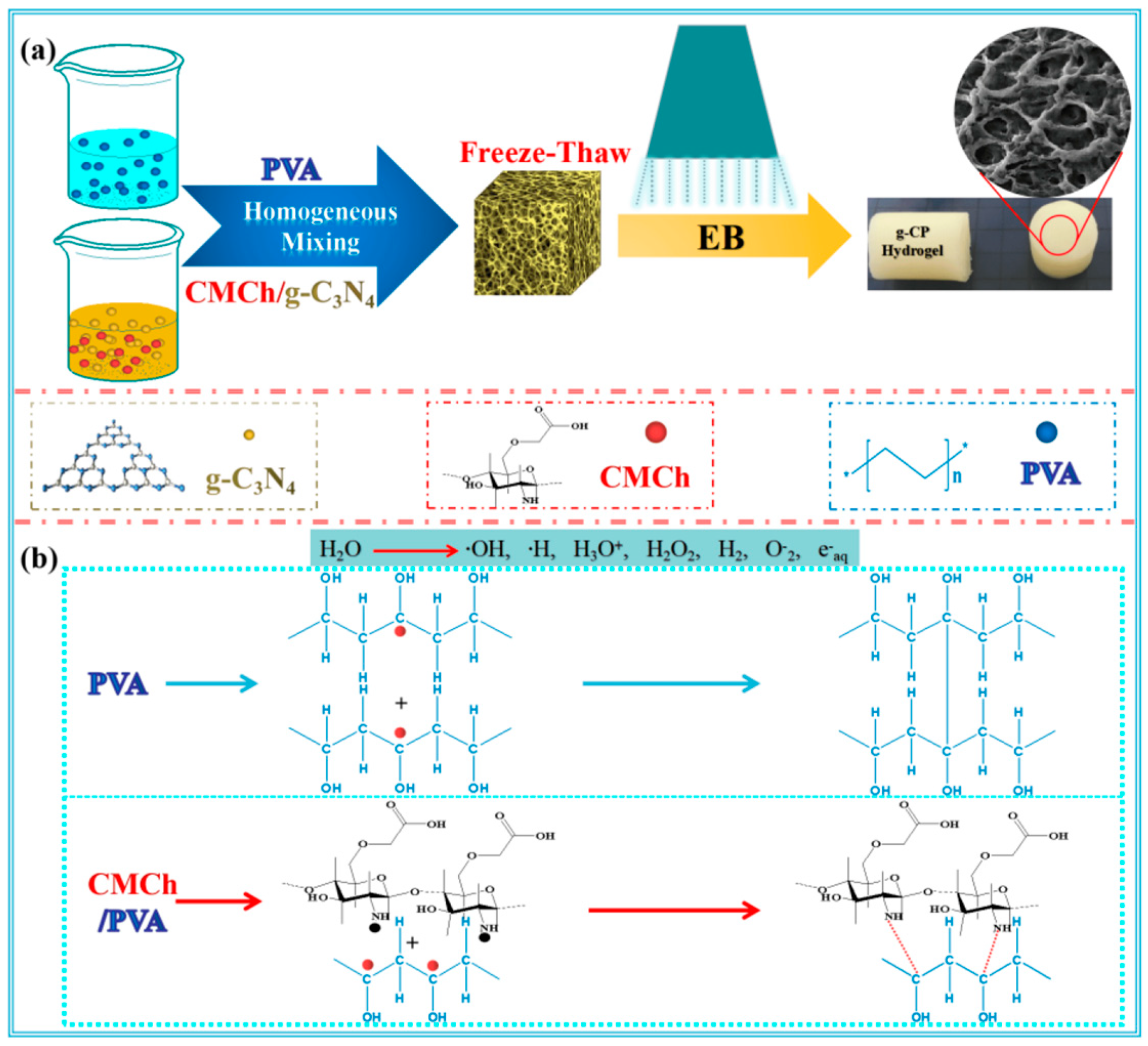
- Yang, J.Y.; Tang, D.X.; Liu, D.L.; Liu, K.; Yang, X.J.; Li, Y.S.; Liu, Y. Excellent Dark/Light Dual-Mode Photoresponsive Activities Based on g-C3N4/CMCh/PVA Nanocomposite Hydrogel Using Electron Beam Radiation Method. Molecules 2023, 28, 7544. [Google Scholar] [CrossRef] [PubMed]
- Amoatey, P.; Bani, R. Wastewater Management. In Waste Water—Evaluation and Management; InTech: London, UK, 2011. [Google Scholar]
- Tchobanoglous, G.; Burton, F.L.; Stensel, D.H. Metcalf &Eddy: Wastewater Engineering: Treatment and Reuse; McGraw Hill: New York, NY, USA, 2014; p. 421. [Google Scholar]
- Chakraborty, S.K.; Sanyal, P.; Ray, R. Pollution, Environmental Perturbation and Consequent Loss of Wetlands. In Wetlands Ecology; Springer International Publishing: Cham, Switzerland, 2023; pp. 521–582. [Google Scholar]
- Tchounwou, P.B.; Centeno, J.A. Toxicologic Pathology. In Preclinical Development Handbook: Toxicology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; Chapter 16; pp. 551–580. ISBN 978-0-470-24846-1. [Google Scholar]
- Beyersmann, D.; Hartwig, A. Carcinogenic Metal Compounds: Recent Insight into Molecular and Cellular Mechanisms. Arch. Toxicol. 2008, 82, 493–512. [Google Scholar] [CrossRef]
- Flora, S.J.S.; Flora, G.; Saxena, G. Environmental Occurrence, Health Effects and Management of Lead Poisoning. In Lead; Elsevier: Amsterdam, The Netherlands, 2006; pp. 158–228. [Google Scholar]
- Wang, M.; Xu, L.; Zhai, M.; Peng, J.; Li, J.; Wei, G. γ-Ray Radiation-Induced Synthesis and Fe(III) Ion Adsorption of Carboxymethylated Chitosan Hydrogels. Carbohydr. Polym. 2008, 74, 498–503. [Google Scholar] [CrossRef]
- Zhao, L.; Mitomo, H. Adsorption of Heavy Metal Ions from Aqueous Solution onto Chitosan Entrapped CM-Cellulose Hydrogels Synthesized by Irradiation. J. Appl. Polym. Sci. 2008, 110, 1388–1395. [Google Scholar] [CrossRef]
- Puspitasari, T.; Oktaviani; Pangerteni, D.S.; Nurfilah, E.; Darwis, D. Study of Metal Ions Removal from Aqueous Solution by Using Radiation Crosslinked Chitosan-Co-Poly(Acrylamide)-Based Adsorbent. Macromol. Symp. 2015, 353, 168–177. [Google Scholar] [CrossRef]
- Elbarbary, A.M.; Ghobashy, M.M. Phosphorylation of Chitosan/HEMA Interpenetrating Polymer Network Prepared by γ-Radiation for Metal Ions Removal from Aqueous Solutions. Carbohydr. Polym. 2017, 162, 16–27. [Google Scholar] [CrossRef]
- Tran, T.H.; Okabe, H.; Hidaka, Y.; Hara, K. Equilibrium and Kinetic Studies for Silver Removal from Aqueous Solution by Hybrid Hydrogels. J. Hazard. Mater. 2019, 365, 237–244. [Google Scholar] [CrossRef]
- El-Sayed Abdel-Raouf, M.; Kamal, R.S.; Hegazy, D.E.; Sayed, A. Gamma Irradiation Synthesis of Carboxymethyl Chitosan-Nanoclay Hydrogel for the Removal of Cr(VI) and Pb(II) from Aqueous Media. J. Inorg. Organomet. Polym. Mater. 2023, 33, 895–913. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Collins, M.N.; Naushad, M.; Shirazian, S.; Walker, G.; Mangwandi, C. Activated Lignin-Chitosan Extruded Blends for Efficient Adsorption of Methylene Blue. Chem. Eng. J. 2017, 307, 264–272. [Google Scholar] [CrossRef]
- Bulut, E.; Özacar, M.; Şengil, İ.A. Adsorption of Malachite Green onto Bentonite: Equilibrium and Kinetic Studies and Process Design. Microporous Mesoporous Mater. 2008, 115, 234–246. [Google Scholar] [CrossRef]
- Hao, Y.; Yan, L.; Yu, H.; Yang, K.; Yu, S.; Shan, R.; Du, B. Comparative Study on Adsorption of Basic and Acid Dyes by Hydroxy-Aluminum Pillared Bentonite. J. Mol. Liq. 2014, 199, 202–207. [Google Scholar] [CrossRef]
- Saleh, T.A.; Gupta, V.K. Photo-Catalyzed Degradation of Hazardous Dye Methyl Orange by Use of a Composite Catalyst Consisting of Multi-Walled Carbon Nanotubes and Titanium Dioxide. J. Colloid Interface Sci. 2012, 371, 101–106. [Google Scholar] [CrossRef]
- Nouri, N.; Khorram, P.; Duman, O.; Sibel, T.; Hassan, S. Overview of Nanosorbents Used in Solid Phase Extraction Techniques for the Monitoring of Emerging Organic Contaminants in Water and Wastewater Samples. Trends Environ. Anal. Chem. 2020, 25, e00081. [Google Scholar] [CrossRef]
- Helal, I.M.; Ahmed, S.B.; Abdulrahim, M.R. Synthesis and Characterization of Sodium Carboxymethyl Cellulose/Chitosan/Polyvinyl Alcohol Hydrogels to Removal Ionic Dyes and Their Biodegaration. Polym. Adv. Technol. 2025, 36, e70061. [Google Scholar] [CrossRef]
- Singh, H.; Bhardwaj, N.; Arya, S.K.; Khatri, M. Environmental Impacts of Oil Spills and Their Remediation by Magnetic Nanomaterials. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100305. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, N.; Shi, Y.; Ma, Y.; An, C.; Li, J.; Jiang, W.; Wang, C.; Shi, J. Construction of Antibacterial, Superwetting, Catalytic, and Porous Lignocellulosic Nanofibril Composites for Wastewater Purification. Adv. Mater. Interfaces 2022, 9, 2201388. [Google Scholar] [CrossRef]
- Aa, I.; Op, A.; Ujj, I.; Mt, B. A Critical Review of Oil Spills in the Niger Delta Aquatic Environment: Causes, Impacts, and Bioremediation Assessment. Env. Monit. Assess. 2022, 194, 816. [Google Scholar] [CrossRef]
- Xu, A.; Zhang, X.; Wu, S.; Xu, N.; Huang, Y.; Yan, X.; Zhou, J.; Cui, Z.; Dong, W. Pollutant Degrading Enzyme: Catalytic Mechanisms and Their Expanded Applications. Molecules 2021, 26, 4751. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; Aboulfotouh, M.E.; Gayed, H.M. Synthesis of Carbon Nanotube Aerogels from Cross-Linked Polyacrylamide/Chitosan Hydrogels via Gamma Irradiation for Efficient Oil Removal. J. Environ. Chem. Eng. 2024, 12, 113819. [Google Scholar] [CrossRef]
- El-Sweify, F.H.; Abdel Fattah, A.A.; El-Sheikh, R.; Aly, S.M.; Ghamry, M.A. Adsorption and Separation of 152+154Eu(III) and 60Co(II) Using Cerium(IV) Tungstate. Radiochemistry 2018, 60, 274–280. [Google Scholar] [CrossRef]
- Zhuang, S.; Yin, Y.; Wang, J. Removal of Cobalt Ions from Aqueous Solution Using Chitosan Grafted with Maleic Acid by Gamma Radiation. Nucl. Eng. Technol. 2018, 50, 211–215. [Google Scholar] [CrossRef]
- Abdelmonem, I.M.; Elsharma, E.M.; Emara, A.M. Radiation Synthesis of Chitosan/Poly(Acrylamide-Co-Maleic Acid) Hydrogel for the Removal of 152+154Eu (III) Ions. Appl. Radiat. Isot. 2023, 197, 110801. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lim, M.; Fabris, R.; Chow, C.; Chiang, K.; Drikas, M.; Amal, R. Removal of Humic Acid Using TiO2 Photocatalytic Process—Fractionation and Molecular Weight Characterisation Studies. Chemosphere 2008, 72, 263–271. [Google Scholar] [CrossRef]
- Zhao, L.; Luo, F.; Wasikiewicz, J.M.; Mitomo, H.; Nagasawa, N.; Yagi, T.; Tamada, M.; Yoshii, F. Adsorption of Humic Acid from Aqueous Solution onto Irradiation-Crosslinked Carboxymethylchitosan. Bioresour. Technol. 2008, 99, 1911–1917. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raza, M.A. Ionizing Radiation Crosslinked Chitosan-Based Hydrogels for Environmental Remediation. Gels 2025, 11, 492. https://doi.org/10.3390/gels11070492
Raza MA. Ionizing Radiation Crosslinked Chitosan-Based Hydrogels for Environmental Remediation. Gels. 2025; 11(7):492. https://doi.org/10.3390/gels11070492
Chicago/Turabian StyleRaza, Muhammad Asim. 2025. "Ionizing Radiation Crosslinked Chitosan-Based Hydrogels for Environmental Remediation" Gels 11, no. 7: 492. https://doi.org/10.3390/gels11070492
APA StyleRaza, M. A. (2025). Ionizing Radiation Crosslinked Chitosan-Based Hydrogels for Environmental Remediation. Gels, 11(7), 492. https://doi.org/10.3390/gels11070492








