Natural Kelp (Laminaria japonica) Hydrogel with Anisotropic Mechanical Properties, Low Friction and Self-Cleaning for Triboelectric Nanogenerator
Abstract
1. Introduction
2. Results and Discussion
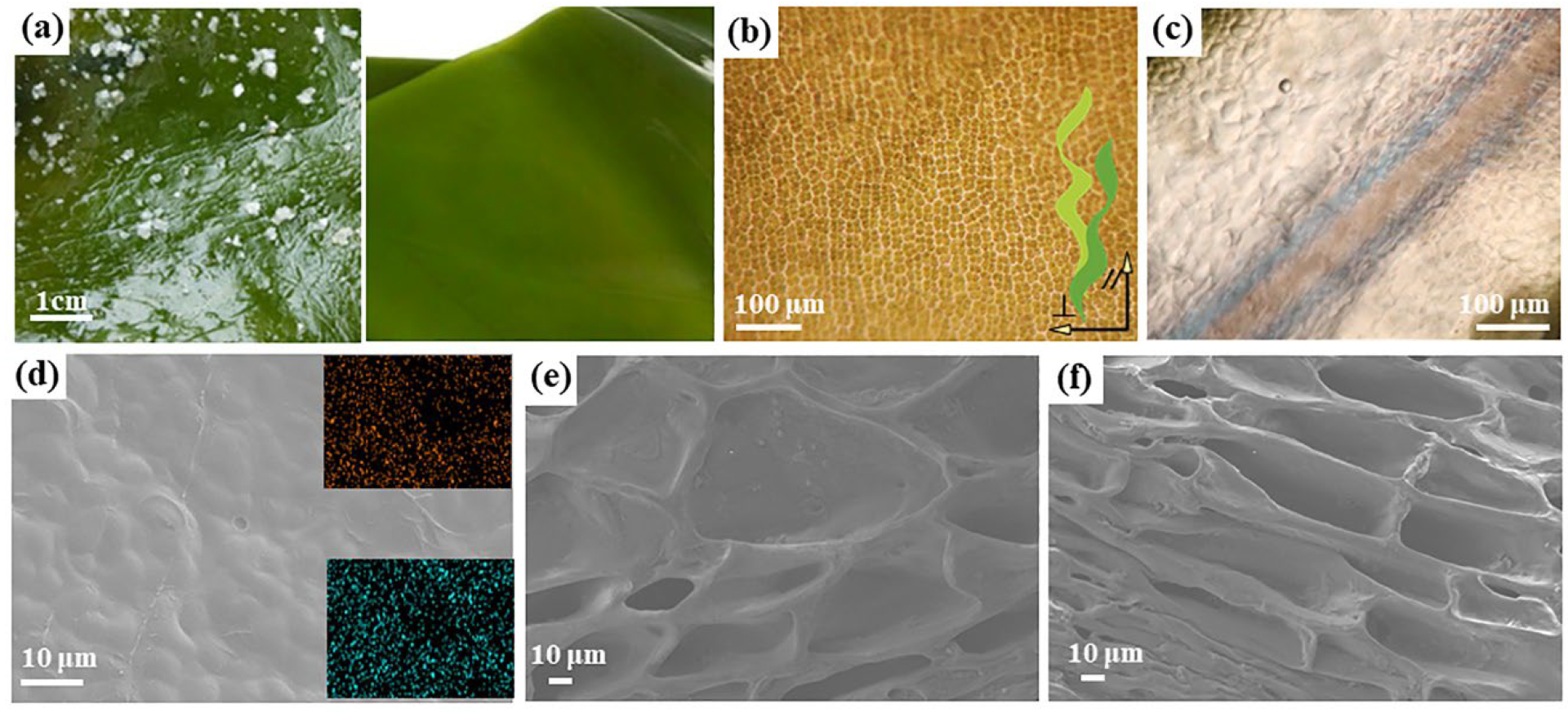
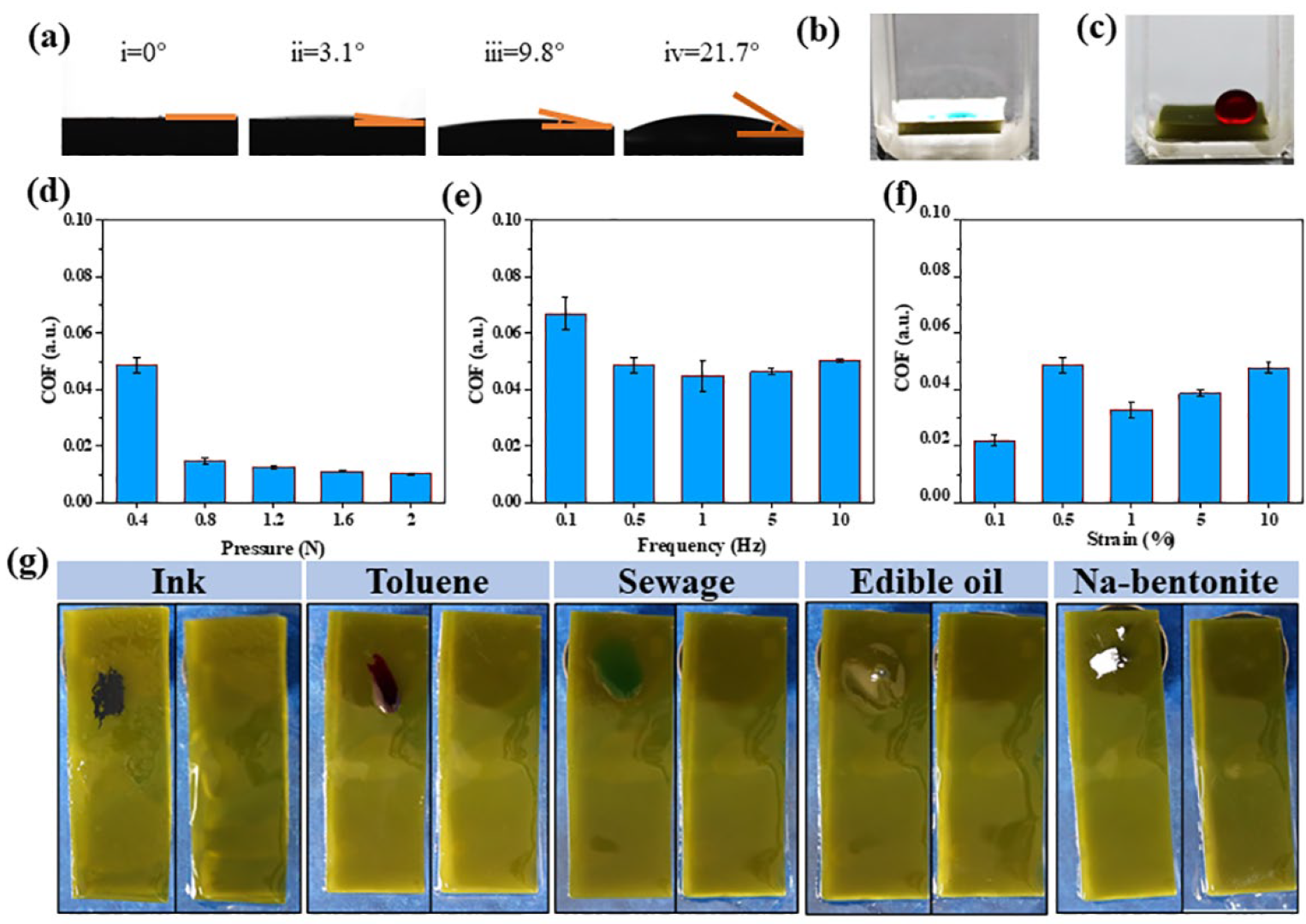
2.1. Surface Properties
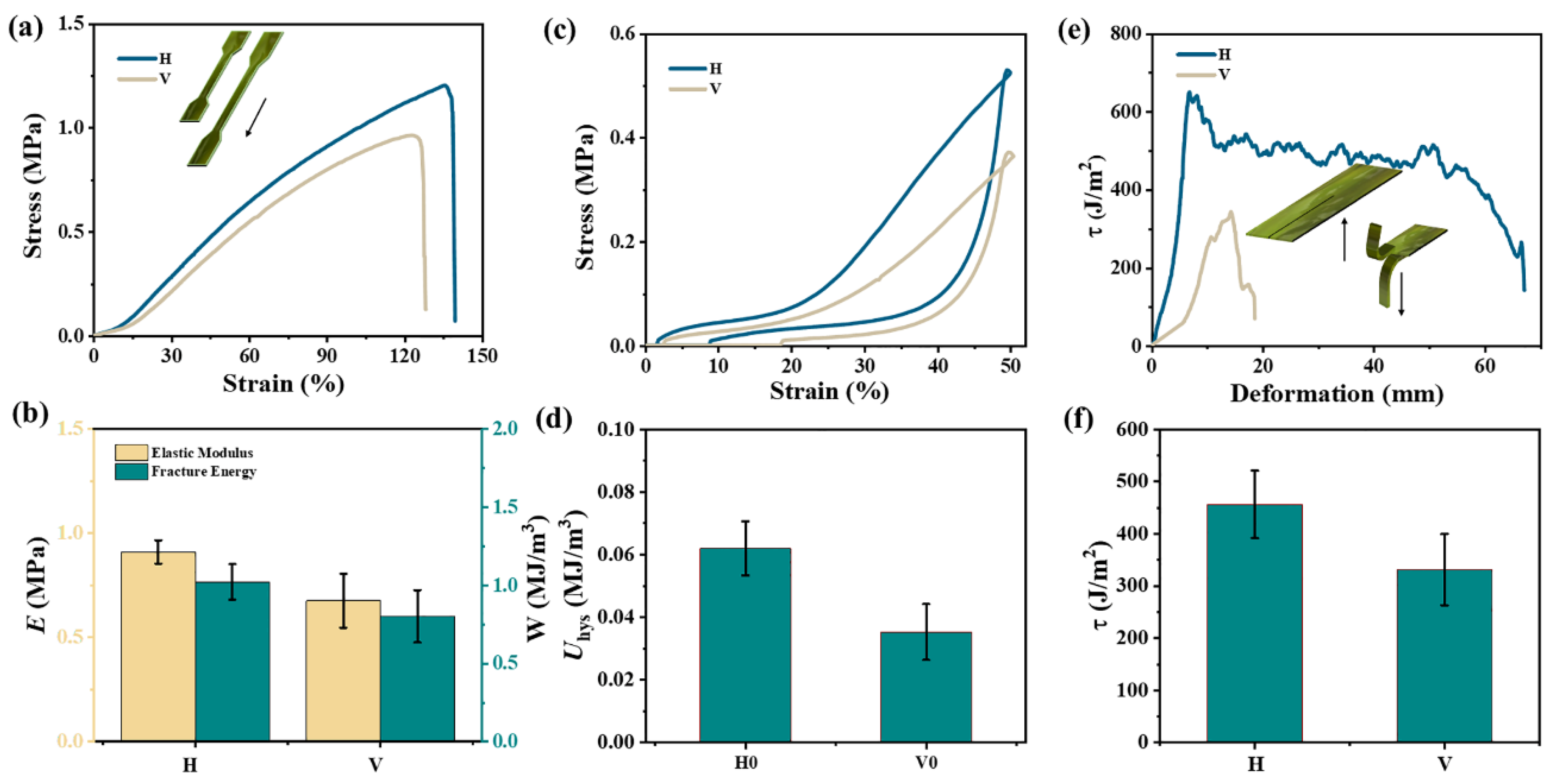
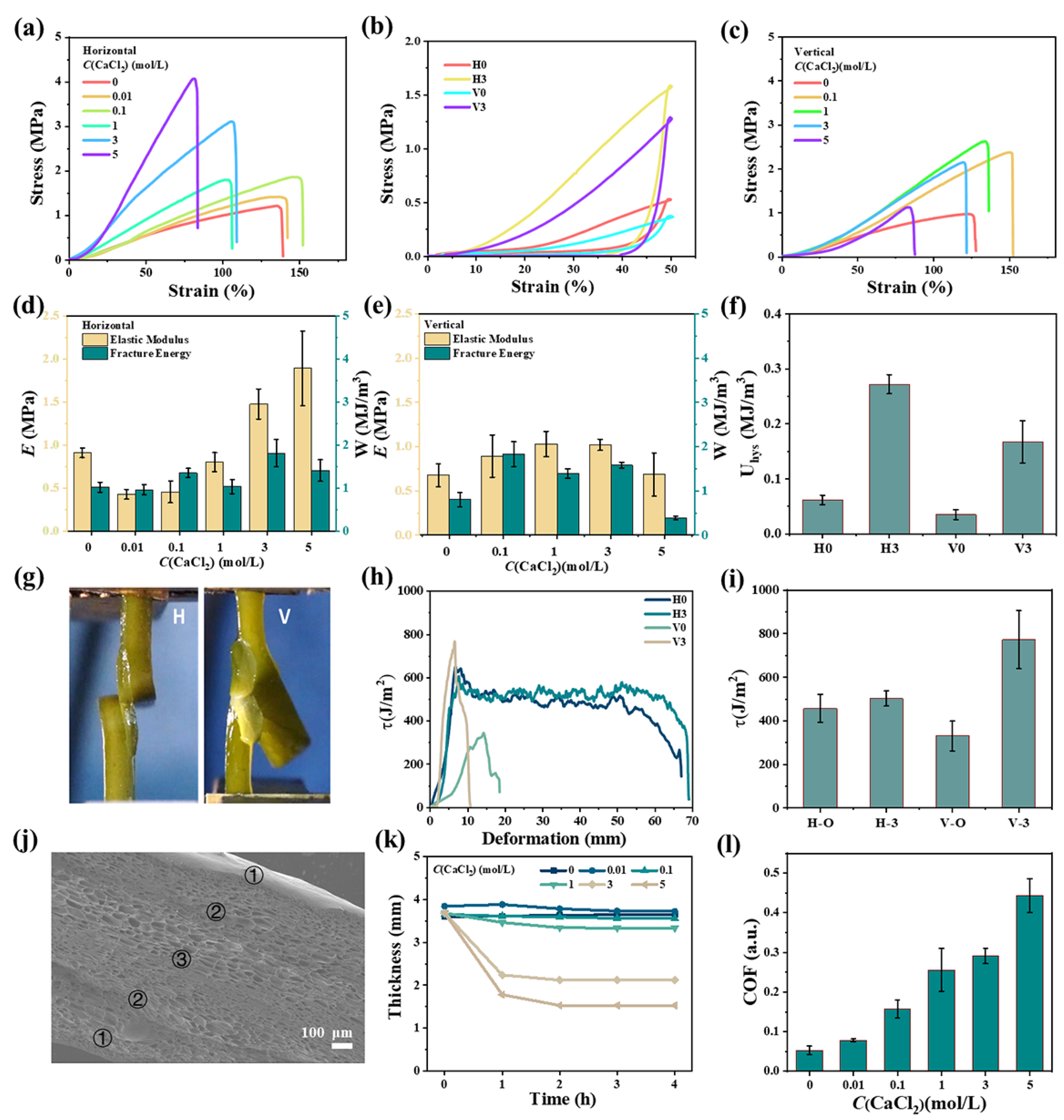
2.2. Mechanical Properties
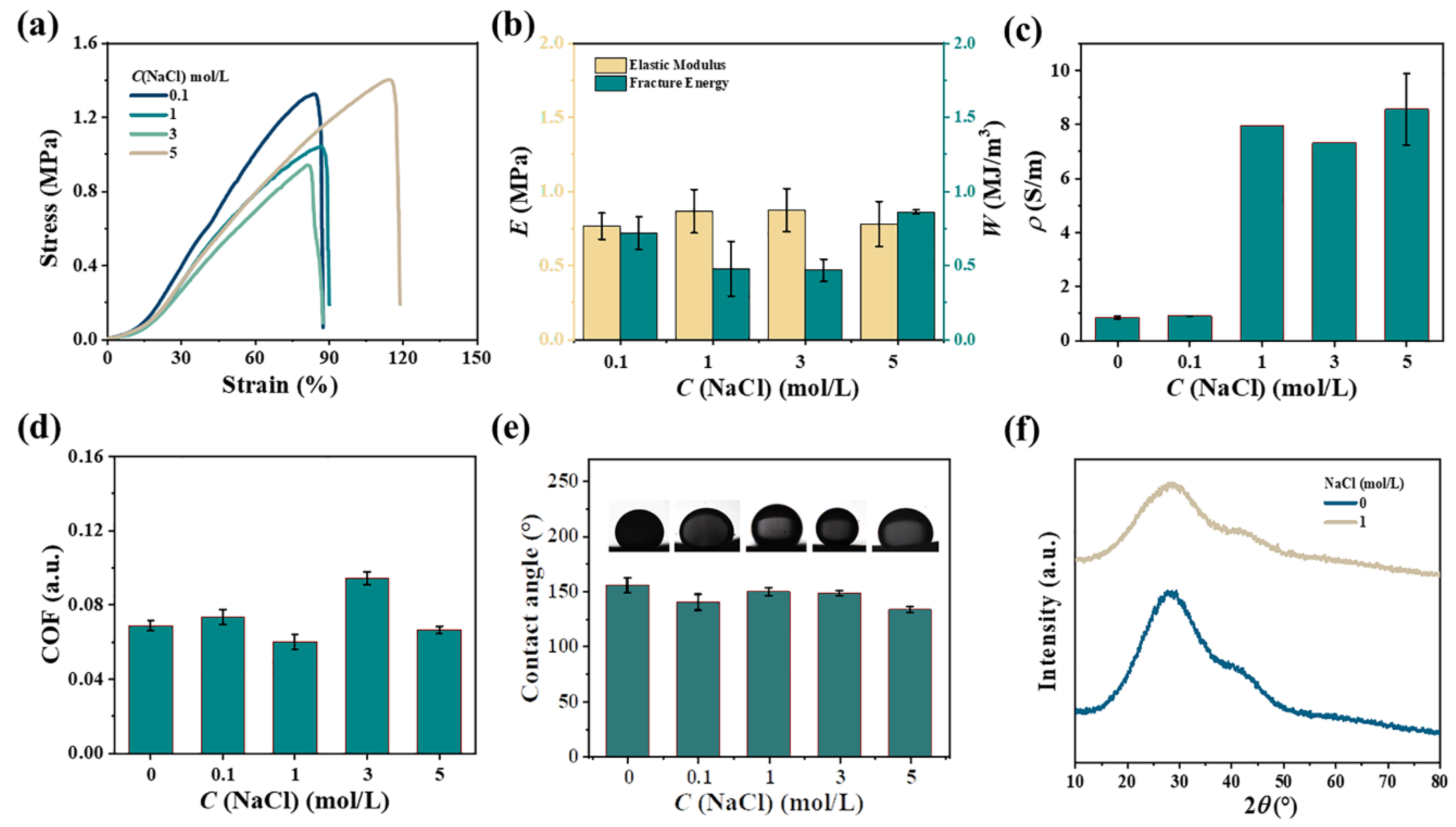
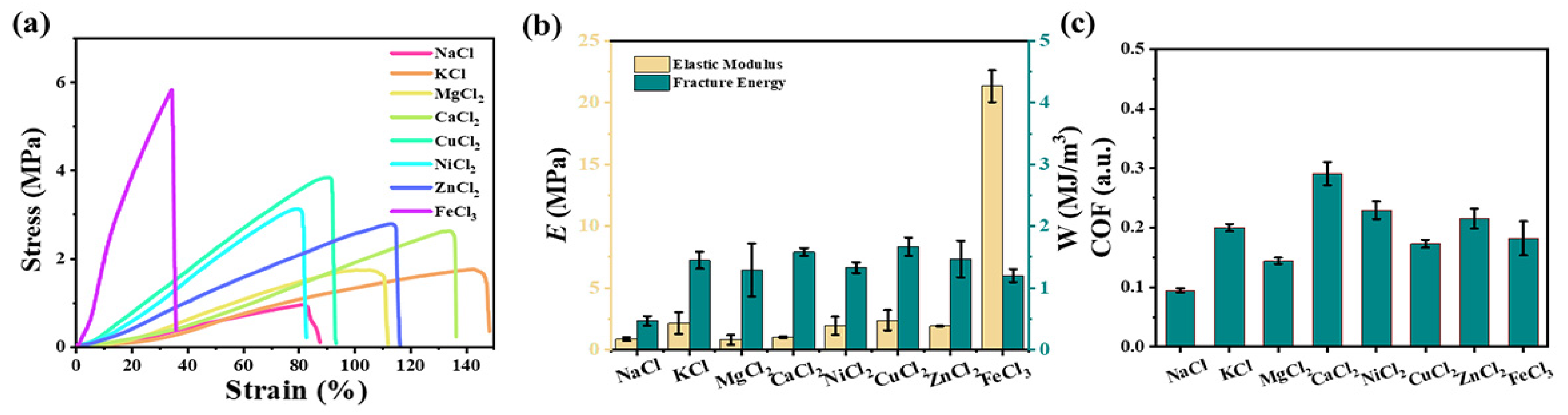
2.3. Enhancement with Various Metal Ions
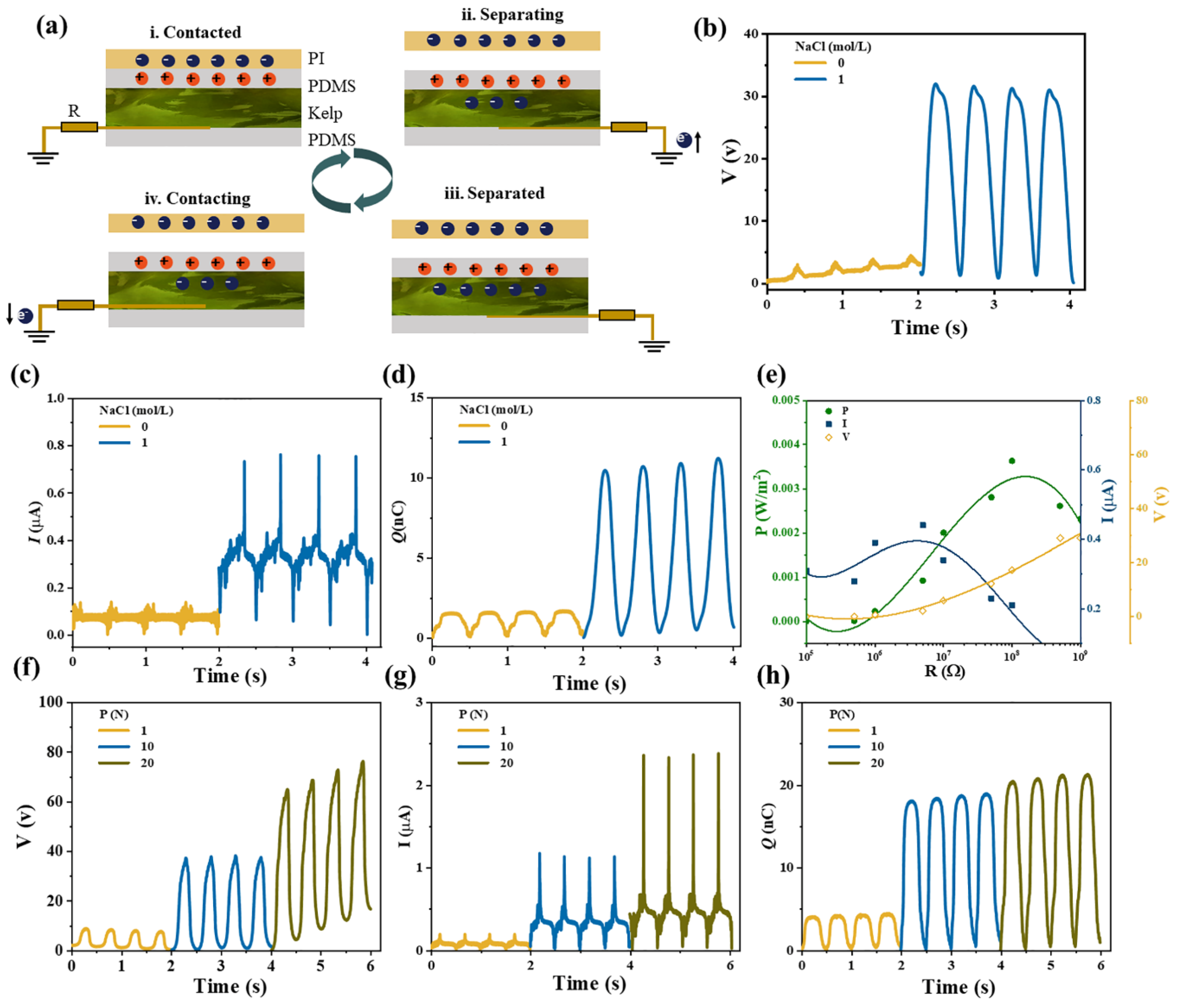
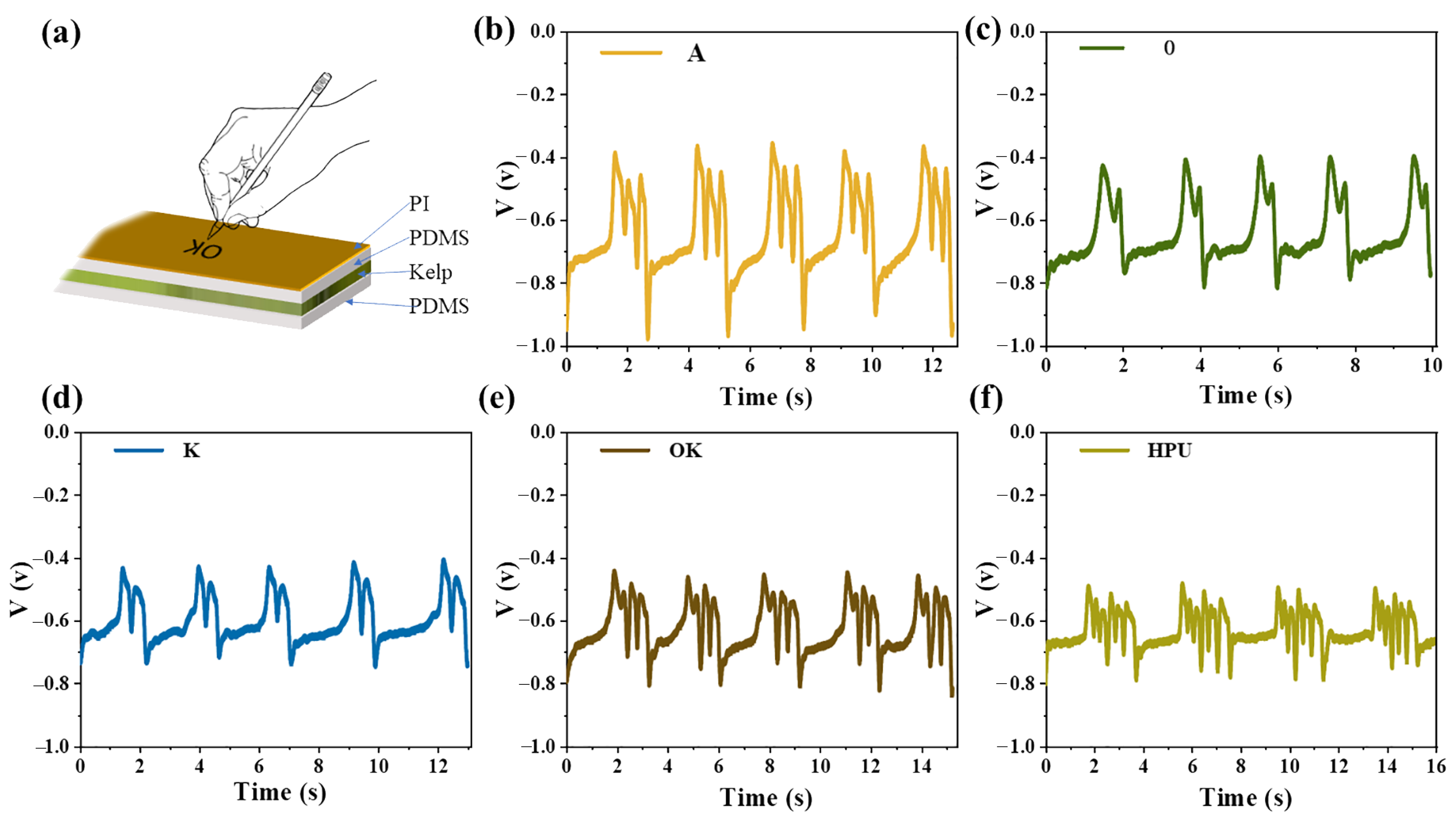
2.4. Properties of TENG
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Kelp Pretreatment
4.2.1. Removal of Cl− in Salted Kelp
4.2.2. Introducing Metal Ion Mn+ into Kelp
4.3. Testing and Characterization
4.3.1. Optical Microstructure Characterization
4.3.2. Scanning Electron Microscopy (SEM) Structural Characterization
4.3.3. Coefficient of Friction (COF)
4.3.4. Characterization of Surface Antifouling
4.3.5. Uniaxial Tensile Test
4.3.6. Energy Dissipation Performance Test
4.3.7. Shear Test of Trouser Samples
4.3.8. Static Contact Angle Test
4.3.9. Conductivity Test
4.3.10. Nanotriboelectricity Test
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ash, C.; Smith, J.; Sugden, A.M. Organic-matter flow in kelp forest. Science 2018, 361, 1084–1085. [Google Scholar] [CrossRef]
- Eger, A.M.; Eddy, N.; McHugh, T.A.; Arafeh-Dalmau, N.; Wernberg, T.; Krumhansl, K.; Verbeek, J.; Branigan, S.; Kuwae, T.; Caselle, J.E.; et al. State of the world’s kelp forests. One Earth 2024, 7, 1927–1931. [Google Scholar] [CrossRef]
- Eger, A.M.; Marzinelli, E.M.; Beas-Luna, R.; Blain, C.O.; Blamey, L.K.; Byrnes, J.E.K.; Carnell, P.E.; Choi, C.G.; Hessing-Lewis, M.; Kim, K.Y.; et al. The value of ecosystem services in global marine kelp forests. Nat. Commun. 2023, 14, 1894. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Puebla, S.T.; Weigel, B.L.; Jack, L.; Schlundt, C.; Pfister, C.A.; Mark Welch, J.L. Spatial organization of the kelp microbiome at micron scales. Microbiome 2022, 10, 7958. [Google Scholar] [CrossRef]
- King, N.G.; McKeown, N.J.; Smale, D.A.; Bradbury, S.; Stamp, T.; Jüterbock, A.; Egilsdóttir, H.; Groves, E.A.; Moore, P.J.; Stewart Grant, W. Hierarchical genetic structuring in the cool boreal kelp, Laminaria digitata: Implications for conservation and management. ICES J. Mar. Sci. 2020, 77, 1906–1913. [Google Scholar] [CrossRef]
- Ribeiro, P.A.; Næss, T.; Dahle, G.; Asplin, L.; Meland, K.; Fredriksen, S.; Sjøtun, K. Going with the Flow–Population Genetics of the Kelp Saccharina latissima (Phaeophyceae, Laminariales). Front. Mar. Sci. 2022, 9, 876420. [Google Scholar] [CrossRef]
- Paine, E.R.; Boyd, P.W.; Strzepek, R.F.; Ellwood, M.; Brewer, E.A.; Diaz-Pulido, G.; Schmid, M.; Hurd, C.L. Iron limitation of kelp growth may prevent ocean afforestation. Commun. Biol. 2023, 6, 607. [Google Scholar] [CrossRef]
- Flórez, M.; Lopez-Sanchez, P.; Vázquez, M.; Cazón, P. Sugar kelp Saccharina latissima extract as an innovative ingredient for chitosan films: Case study as cheese slice separators. Food Hydrocoll. 2024, 149, 109571. [Google Scholar] [CrossRef]
- van der Merwe, R.D.T.; Goosen, N.J.; Pott, R.W.M. Macroalgal-Derived Alginate Soil Amendments for Water Retention, Nutrient Release Rate Reduction, and Soil pH Control. Gels 2022, 8, 548. [Google Scholar] [CrossRef]
- Ge, H.; Zang, Y.; Cao, Z.; Ye, X.; Chen, J. Rheological properties, textural and compound preservative of kelp recombination noodles. LWT 2020, 118, 108729. [Google Scholar] [CrossRef]
- Li, H.; Yi, Y.; Guo, S.; Zhang, F.; Yan, H.; Zhan, Z.; Zhu, Y.; Duan, J. Isolation, structural characterization and bioactivities of polysaccharides from Laminaria japonica: A review. Food Chem. 2022, 370, 131010. [Google Scholar] [CrossRef] [PubMed]
- Millar, R.V.; Houghton, J.D.R.; Elsäβer, B.; Mensink, P.J.; Kregting, L.; Wernberg, T. Influence of waves and currents on the growth rate of the kelp Laminaria digitata (Phaeophyceae). J. Phycol. 2019, 56, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Krause-Jensen, D.; Marbà, N.; Sanz-Martin, M.; Hendriks, I.E.; Thyrring, J.; Carstensen, J.; Sejr, M.K.; Duarte, C.M. Long photoperiods sustain high pH in Arctic kelp forests. Sci. Adv. 2016, 2, 1501938. [Google Scholar] [CrossRef]
- Kregting, L.; Blight, A.J.; Elsäßer, B.; Savidge, G. The influence of water motion on the growth rate of the kelp Laminaria digitata. J. Exp. Mar. Biol. Ecol. 2016, 478, 86–95. [Google Scholar] [CrossRef]
- Strong-Wright, J.; Taylor, J.R. Modeling the Growth Potential of the Kelp Saccharina Latissima in the North Atlantic. Front. Mar. Sci. 2022, 8, 793977. [Google Scholar] [CrossRef]
- Henry, P.-Y. Variability and similarities in the structural properties of two related Laminaria kelp species. Estuar. Coast. Shelf Sci. 2018, 200, 395–405. [Google Scholar] [CrossRef]
- Smidsrød, O. Molecular basis for some physical properties of alginates in the gel state. Faraday Discuss. Chem. Soc. 1974, 57, 263–274. [Google Scholar] [CrossRef]
- Lee, H.-H.; Kim, J.-S.; Jeong, J.-H.; Park, S.M.; Sathasivam, R.; Lee, S.Y.; Kim, C.S. Effect of Different Solvents on the Extraction of Compounds from Different Parts of Undaria pinnatifida (Harvey) Suringar. J. Mar. Sci. Eng. 2022, 10, 1193. [Google Scholar] [CrossRef]
- Semprebon, C.; McHale, G.; Kusumaatmaja, H. Apparent contact angle and contact angle hysteresis on liquid infused surfaces. Soft Matter 2017, 13, 101–110. [Google Scholar] [CrossRef]
- Lee, S.J.; Heo, S.; Yoon, G.Y.; Seo, E.; Choi, W. Effects of surface morphological structure of a brown alga miyeok (Undaria pinnatifida) on sustainable drag reduction. AIP Adv. 2020, 10, 125123. [Google Scholar] [CrossRef]
- Lubsch, A.; Timmermans, K. Texture analysis of Laminaria digitata (Phaeophyceae) thallus reveals trade-off between tissue tensile strength and toughness along lamina. Bot. Mar. 2017, 60, 229–237. [Google Scholar] [CrossRef]
- Wang, J.; Ma, X.; Su, L.; Zhang, C.; Dong, X.; Teng, C.; Jiang, L.; Yu, C. Eco-friendly perforated kelp membrane with high strength for efficient oil/water separation in a complex environment. Sep. Purif. Technol. 2022, 282, 120114. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Show, P.L.; Lau, B.F.; Chang, J.S.; Ling, T.C. New Prospects for Modified Algae in Heavy Metal Adsorption. Trends Biotechnol. 2019, 37, 1255–1268. [Google Scholar] [CrossRef]
- Lin, W.; Wei, X.; Liu, S.; Zhang, J.; Yang, T.; Chen, S. Recent Advances in Mechanical Reinforcement of Zwitterionic Hydrogels. Gels 2022, 8, 580. [Google Scholar] [CrossRef]
- Fan, F.-R.; Tian, Z.-Q.; Wang, Z.L. Flexible triboelectric generator. Nano Energy 2012, 1, 328–334. [Google Scholar] [CrossRef]
- Horn, R.G.; Smith, D.T. Contact Electrification and Adhesion Between Dissimilar Materials. Science 1992, 256, 362–364. [Google Scholar] [CrossRef]
- Wang, N.; Zou, J.; Yang, Y.; Li, X.; Guo, Y.; Jiang, C.; Jia, X.; Cao, X. Kelp-inspired biomimetic triboelectric nanogenerator boosts wave energy harvesting. Nano Energy 2019, 55, 541–547. [Google Scholar] [CrossRef]
- Ahmed, A. Self-powered wireless sensing platform for monitoring marine life based on harvesting hydrokinetic energy of water currents. J. Mater. Chem. A 2022, 10, 1992–1998. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Y.; Wang, D.; He, P. Solid–liquid contact TENG using a melting near-field direct writing PCL nano-fiber structure. Int. J. Smart Nano Mater. 2023, 14, 90–102. [Google Scholar] [CrossRef]
- Jiang, C.; Li, X.; Yao, Y.; Lan, L.; Shao, Y.; Zhao, F.; Ying, Y.; Ping, J. A multifunctional and highly flexible triboelectric nanogenerator based on MXene-enabled porous film integrated with laser-induced graphene electrode. Nano Energy 2019, 66, 104121. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, L.; Kuang, X.; Li, V.; Lei, M.; Kang, G.; Wang, Z.L.; Qi, H.J. Dynamic Photomask-assisted Direct Ink Writing Multimaterial for Multilevel Triboelectric Nanogenerator. Adv. Funct. Mater. 2019, 29, 1903568. [Google Scholar] [CrossRef]
- Lv, X.; Yang, Y.; Guo, J.; Gao, J.; Li, J.; Rao, W.-F. One-click fabrication of triboelectric nanogenerators through multiple fused filaments fabrication. Mater. Today Commun. 2024, 39, 109283. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Yu, H.; Hao, J.; Chen, Q.; Zhu, L. Natural Kelp (Laminaria japonica) Hydrogel with Anisotropic Mechanical Properties, Low Friction and Self-Cleaning for Triboelectric Nanogenerator. Gels 2025, 11, 597. https://doi.org/10.3390/gels11080597
Chen D, Yu H, Hao J, Chen Q, Zhu L. Natural Kelp (Laminaria japonica) Hydrogel with Anisotropic Mechanical Properties, Low Friction and Self-Cleaning for Triboelectric Nanogenerator. Gels. 2025; 11(8):597. https://doi.org/10.3390/gels11080597
Chicago/Turabian StyleChen, Dongnian, Hui Yu, Jiajia Hao, Qiang Chen, and Lin Zhu. 2025. "Natural Kelp (Laminaria japonica) Hydrogel with Anisotropic Mechanical Properties, Low Friction and Self-Cleaning for Triboelectric Nanogenerator" Gels 11, no. 8: 597. https://doi.org/10.3390/gels11080597
APA StyleChen, D., Yu, H., Hao, J., Chen, Q., & Zhu, L. (2025). Natural Kelp (Laminaria japonica) Hydrogel with Anisotropic Mechanical Properties, Low Friction and Self-Cleaning for Triboelectric Nanogenerator. Gels, 11(8), 597. https://doi.org/10.3390/gels11080597






