Biopolymers for Liver Tissue Engineering: A Systematic Review
Abstract
1. Introduction
2. Results and Discussion
2.1. Summative Results of the Systematic Searches
2.2. Biomaterials Used to Derive Liver Cells and Organoids from PSCs
2.3. Biomaterials Used to Derive Liver Cells and Organoids from LSCs
2.4. Biomaterials Used to Derive Liver Cells and Organoids from NLSCs
2.5. Discussion of Results
3. Conclusions
4. Materials and Methods
4.1. Data Sources and Searches
4.2. Study Selection
4.3. Data Extraction and Quality Assessment
4.4. Data Synthesis and Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2D | Two dimensional |
| 3D | Three dimensional |
| AFP | Alpha-fetoprotein |
| CYP | Cytochrome P450 |
| DED | Definitive Endoderm Differentiation |
| ECM | Extracellular Matrix |
| ESCs | Embryonic Stem Cells |
| GelMA | Gelatine Methacryloyl |
| GFR | Growth Factor Reduced |
| HA | Hyaluronic acid |
| HNF4 | Hepatocyte Nuclear Factor-4 |
| iPSCs | Induced Pluripotent Stem Cells |
| PIC-LEC | PIC-Laminin 111-Entactin-Complex |
| LSCs | Liver Stem Cells |
| LSECs | Liver Sinusoidal Endothelial Cells |
| MSCs | Mesenchymal Stem Cells |
| NLSCs | Non-Liver Stem Cells |
| PSCs | Pluripotent Stem Cells |
| PAA | Polyacrylamide |
| PEG | Polyethylene Glycol |
| PIC | Polyisocyanopeptides |
| PLLA/PCL | Poly-L-lactic acid/poly (ε-caprolactone) |
| PCL-Gel-HA | Poly ε-caprolactone-gelatine-hyaluronic acid |
References
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef]
- Karlsen, T.H.; Sheron, N.; Zelber-Sagi, S.; Carrieri, P.; Dusheiko, G.; Bugianesi, E.; Pryke, R.; Hutchinson, S.J.; Sangro, B.; Martin, N.K.; et al. The EASL-Lancet Liver Commission: Protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet 2022, 399, 61–116. [Google Scholar] [CrossRef]
- Organ Transplantation—NHS Blood and Transplant. How Long Is the Wait for a Liver? Available online: https://www.nhsbt.nhs.uk/organ-transplantation/liver/receiving-a-liver/how-long-is-the-wait-for-a-liver/ (accessed on 4 March 2025).
- Kim, W.R.; Therneau, T.M.; Benson, J.T.; Kremers, W.K.; Rosen, C.B.; Gores, G.J.; Dickson, E.R. Deaths on the liver transplant waiting list: An analysis of competing risks. Hepatology 2006, 43, 345–351. [Google Scholar] [CrossRef]
- Fink, M.A.; Berry, S.R.; Gow, P.J.; Angus, P.W.; Wang, B.-Z.; Muralidharan, V.; Christophi, C.; Jones, R.M. Risk factors for liver transplantation waiting list mortality. J. Gastroenterol. Hepatol. 2007, 22, 119–124. [Google Scholar] [CrossRef]
- The Government of Japan-JapanGov. ES Cells Give Small Lives a Chance for Tomorrow/The Government of Japan-JapanGov. Available online: https://www.japan.go.jp/tomodachi/2020/summer2020/es_cells.html (accessed on 4 March 2025).
- Bengrine, A.; Brochot, E.; Louchet, M.; Herpe, Y.E.; Duverlie, G. Modeling of HBV and HCV hepatitis with hepatocyte-like cells. Front. Biosci. (Sch. Ed.) 2016, 8, 97–105. [Google Scholar] [CrossRef][Green Version]
- Holmgren, G.; Sjögren, A.-K.; Barragan, I.; Sabirsh, A.; Sartipy, P.; Synnergren, J.; Björquist, P.; Ingelman-Sundberg, M.; Andersson, T.B.; Edsbagge, J. Long-term chronic toxicity testing using human pluripotent stem cell-derived hepatocytes. Drug Metab. Dispos. 2014, 42, 1401–1406. [Google Scholar] [CrossRef]
- Takayama, K.; Morisaki, Y.; Kuno, S.; Nagamoto, Y.; Harada, K.; Furukawa, N.; Ohtaka, M.; Nishimura, K.; Imagawa, K.; Sakurai, F.; et al. Prediction of interindividual differences in hepatic functions and drug sensitivity by using human iPS-derived hepatocytes. Proc. Natl. Acad. Sci. USA 2014, 111, 16772–16777. [Google Scholar] [CrossRef]
- Olson, H.; Betton, G.; Robinson, D.; Thomas, K.; Monro, A.; Kolaja, G.; Lilly, P.; Sanders, J.; Sipes, G.; Bracken, W.; et al. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 2000, 32, 56–67. [Google Scholar] [CrossRef]
- Underhill, G.H.; Khetani, S.R. Bioengineered Liver Models for Drug Testing and Cell Differentiation Studies. Cell. Mol. Gastroenterol. Hepatol. 2017, 5, 426–439.e1. [Google Scholar] [CrossRef]
- Messina, A.; Luce, E.; Benzoubir, N.; Pasqua, M.; Pereira, U.; Humbert, L.; Eguether, T.; Rainteau, D.; Duclos-Vallée, J.-C.; Legallais, C.; et al. Evidence of Adult Features and Functions of Hepatocytes Differentiated from Human Induced Pluripotent Stem Cells and Self-Organized as Organoids. Cells 2022, 11, 537. [Google Scholar] [CrossRef]
- Harrison, S.P.; Baumgarten, S.F.; Verma, R.; Lunov, O.; Dejneka, A.; Sullivan, G.J. Liver Organoids: Recent Developments, Limitations and Potential. Front. Med. 2021, 8, 574047. [Google Scholar] [CrossRef]
- Orford, K.W.; Scadden, D.T. Deconstructing stem cell self-renewal: Genetic insights into cell-cycle regulation. Nat. Rev. Genet. 2008, 9, 115–128. [Google Scholar] [CrossRef]
- Morrison, S.J.; Spradling, A.C. Stem cells and niches: Mechanisms that promote stem cell maintenance throughout life. Cell 2008, 132, 598–611. [Google Scholar] [CrossRef]
- Godoy, P.; Hewitt, N.J.; Albrecht, U.; Andersen, M.E.; Ansari, N.; Bhattacharya, S.; Bode, J.G.; Bolleyn, J.; Borner, C.; Böttger, J.; et al. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch. Toxicol. 2013, 87, 1315–1530. [Google Scholar] [CrossRef]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the potential of hydrogels for advanced therapeutic applications: Current achievements and future directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef]
- Barajaa, M.A.; Otsuka, T.; Ghosh, D.; Kan, H.-M.; Laurencin, C.T. Development of porcine skeletal muscle extracellular matrix-derived hydrogels with improved properties and low immunogenicity. Proc. Natl. Acad. Sci. USA 2024, 121, e2322822121. [Google Scholar] [CrossRef]
- Ong, J.; Gibbons, G.; Siang, L.Y.; Lei, Z.; Zhao, J.; Justin, A.W.; Cammarata, F.; Rajarethinam, R.; Limegrover, C.; Sinha, S.; et al. A clinically defined and xeno-free hydrogel system for regenerative medicine. bioRxiv 2025. [Google Scholar] [CrossRef]
- Talbot, N.C.; Caperna, T.J. Proteome array identification of bioactive soluble proteins/peptides in Matrigel: Relevance to stem cell responses. Cytotechnology 2015, 67, 873–883. [Google Scholar] [CrossRef]
- Vukicevic, S.; Kleinman, H.K.; Luyten, F.P.; Roberts, A.B.; Roche, N.S.; Reddi, A.H. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp. Cell Res. 1992, 202, 1–8. [Google Scholar] [CrossRef]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef]
- Soofi, S.S.; Last, J.A.; Liliensiek, S.J.; Nealey, P.F.; Murphy, C.J. The elastic modulus of Matrigel as determined by atomic force microscopy. J. Struct. Biol. 2009, 167, 216–219. [Google Scholar] [CrossRef]
- Kohen, N.T.; Little, L.E.; Healy, K.E. Characterization of Matrigel interfaces during defined human embryonic stem cell culture. Biointerphases 2009, 4, 69–79. [Google Scholar] [CrossRef]
- Serban, M.A.; Liu, Y.; Prestwich, G.D. Effects of extracellular matrix analogues on primary human fibroblast behavior. Acta Biomater. 2008, 4, 67–75. [Google Scholar] [CrossRef]
- Aisenbrey, E.A.; Murphy, W.L. Synthetic alternatives to Matrigel. Nat. Rev. Mater. 2020, 5, 539–551. [Google Scholar] [CrossRef]
- Liu, H.; Bockhorn, J.; Dalton, R.; Chang, Y.-F.; Qian, D.; Zitzow, L.A.; Clarke, M.F.; Greene, G.L. Removal of lactate dehydrogenase-elevating virus from human-in-mouse breast tumor xenografts by cell-sorting. J. Virol. Methods 2011, 173, 266–270. [Google Scholar] [CrossRef]
- Peterson, N.C. From bench to cageside: Risk assessment for rodent pathogen contamination of cells and biologics. ILAR J. 2008, 49, 310–315. [Google Scholar] [CrossRef]
- Segers, V.F.M.; Lee, R.T. Biomaterials to enhance stem cell function in the heart. Circ. Res. 2011, 109, 910–922. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Roudaut, M.; Caillaud, A.; Souguir, Z.; Bray, L.; Girardeau, A.; Rimbert, A.; Croyal, M.; Lambert, G.; Patitucci, M.; Delpouve, G.; et al. Human induced pluripotent stem cells-derived liver organoids grown on a Biomimesys® hyaluronic acid-based hydroscaffold as a new model for studying human lipoprotein metabolism. Bioeng. Transl. Med. 2024, 9, e10659. [Google Scholar] [CrossRef]
- Fan, H.; Shang, J.; Li, J.; Yang, B.; Zhou, D.; Jiang, S.; Fan, Y.; Zhou, Y.; Wang, Y.; Liu, P.; et al. High-Throughput Formation of Pre-Vascularized hiPSC-Derived Hepatobiliary Organoids on a Chip via Nonparenchymal Cell Grafting. Adv. Sci. 2025, 12, e2407945. [Google Scholar] [CrossRef]
- Kumar, M.; Toprakhisar, B.; Van Haele, M.; Antoranz, A.; Boon, R.; Chesnais, F.; De Smedt, J.; Tricot, T.; Idoype, T.I.; Canella, M.; et al. A fully defined matrix to support a pluripotent stem cell derived multi-cell-liver steatohepatitis and fibrosis model. Biomaterials 2021, 276, 121006. [Google Scholar] [CrossRef]
- Mobarra, N.; Raji, S.; Najafi, S.; Kafi, F.K.; Ferns, G.A.; Pakzad, R. Hypoxia-Induced miR-210 Overexpression Promotes the Differentiation of Human-Induced Pluripotent Stem Cells to Hepatocyte-Like Cells on Random Nanofiber Poly-L-Lactic Acid/Poly (ε-Caprolactone) Scaffolds. Oxid. Med. Cell. Longev. 2021, 2021, 4229721. [Google Scholar] [CrossRef]
- Septiana, W.L.; Ayudyasari, W.; Gunardi, H.; Pawitan, J.A.; Balachander, G.M.; Yu, H.; Antarianto, R.D. Liver organoids cocultured on decellularized native liver scaffolds as a bridging therapy improves survival from liver failure in rabbits. In Vitro Cell. Dev. Biol. Anim. 2023, 59, 747–763. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, F.; Jin, M.; Wang, Z.; Xu, X.; Zhou, Y.; Wang, J.; Gu, L.; Fan, H.; Fan, Y.; et al. Development of a high-throughput micropatterned agarose scaffold for consistent and reproducible hPSC-derived liver organoids. Biofabrication 2022, 15, 015006. [Google Scholar] [CrossRef]
- Duarte Campos, D.F.; Blaeser, A.; Korsten, A.; Neuss, S.; Jäkel, J.; Vogt, M.; Fischer, H. The stiffness and structure of three-dimensional printed hydrogels direct the differentiation of mesenchymal stromal cells toward adipogenic and osteogenic lineages. Tissue Eng. Part A 2015, 21, 740–756. [Google Scholar] [CrossRef]
- Ye, S.; Boeter, J.W.B.; Mihajlovic, M.; van Steenbeek, F.G.; van Wolferen, M.E.; Oosterhoff, L.A.; Marsee, A.; Caiazzo, M.; van der Laan, L.J.W.; Penning, L.C.; et al. A Chemically Defined Hydrogel for Human Liver Organoid Culture. Adv. Funct. Mater. 2020, 30, 2000893. [Google Scholar] [CrossRef]
- Willemse, J.; van Tienderen, G.; van Hengel, E.; Schurink, I.; van der Ven, D.; Kan, Y.; de Ruiter, P.; Rosmark, O.; Westergren-Thorsson, G.; Schneeberger, K.; et al. Hydrogels derived from decellularized liver tissue support the growth and differentiation of cholangiocyte organoids. Biomaterials 2022, 284, 121473. [Google Scholar] [CrossRef]
- Justin, A.W.; Cammarata, F.; Guy, A.A.; Estevez, S.R.; Burgess, S.; Davaapil, H.; Stavropoulou-Tatla, A.; Ong, J.; Jacob, A.G.; Saeb-Parsy, K.; et al. Densified collagen tubular grafts for human tissue replacement and disease modelling applications. Biomater. Adv. 2023, 145, 213245. [Google Scholar] [CrossRef]
- Di Matteo, S.; Di Meo, C.; Carpino, G.; Zoratto, N.; Cardinale, V.; Nevi, L.; Overi, D.; Costantini, D.; Pinto, C.; Montanari, E.; et al. Therapeutic effects of dexamethasone-loaded hyaluronan nanogels in the experimental cholestasis. Drug Deliv. Transl. Res. 2022, 12, 1959–1973. [Google Scholar] [CrossRef]
- Broguiere, N.; Isenmann, L.; Hirt, C.; Ringel, T.; Placzek, S.; Cavalli, E.; Ringnalda, F.; Villiger, L.; Züllig, R.; Lehmann, R.; et al. Growth of Epithelial Organoids in a Defined Hydrogel. Adv. Mater. 2018, 30, e1801621. [Google Scholar] [CrossRef]
- Zhang, C.J.; Meyer, S.R.; O’Meara, M.J.; Huang, S.; Capeling, M.M.; Ferrer-Torres, D.; Childs, C.J.; Spence, J.R.; Fontana, R.J.; Sexton, J.Z. A human liver organoid screening platform for DILI risk prediction. J. Hepatol. 2023, 78, 998–1006. [Google Scholar] [CrossRef]
- Gil-Recio, C.; Montori, S.; Al Demour, S.; Ababneh, M.A.; Ferrés-Padró, E.; Marti, C.; Ferrés-Amat, E.; Barajas, M.; Al Madhoun, A.; Atari, M. Chemically Defined Conditions Mediate an Efficient Induction of Dental Pulp Pluripotent-Like Stem Cells into Hepatocyte-Like Cells. Stem Cells Int. 2021, 2021, 5212852. [Google Scholar] [CrossRef]
- Yuniartha, R.; Yamaza, T.; Sonoda, S.; Yoshimaru, K.; Matsuura, T.; Yamaza, H.; Oda, Y.; Ohga, S.; Taguchi, T. Cholangiogenic potential of human deciduous pulp stem cell-converted hepatocyte-like cells. Stem Cell Res. Ther. 2021, 12, 57. [Google Scholar] [CrossRef]
- Mitani, S.; Onodera, Y.; Hosoda, C.; Takabayashi, Y.; Sakata, A.; Shima, M.; Tatsumi, K. Generation of functional liver sinusoidal endothelial-like cells from human bone marrow-derived mesenchymal stem cells. Regen. Ther. 2023, 24, 274–281. [Google Scholar] [CrossRef]
- Danoy, M.; Jellali, R.; Tauran, Y.; Bruce, J.; Leduc, M.; Gilard, F.; Gakière, B.; Scheidecker, B.; Kido, T.; Miyajima, A.; et al. Characterization of the proteome and metabolome of human liver sinusoidal endothelial-like cells derived from induced pluripotent stem cells. Differentiation 2021, 120, 28–35. [Google Scholar] [CrossRef]
- Son, J.S.; Park, C.-Y.; Lee, G.; Park, J.Y.; Kim, H.J.; Kim, G.; Chi, K.Y.; Woo, D.-H.; Han, C.; Kim, S.K.; et al. Therapeutic correction of hemophilia A using 2D endothelial cells and multicellular 3D organoids derived from CRISPR/Cas9-engineered patient iPSCs. Biomaterials 2022, 283, 121429. [Google Scholar] [CrossRef]
- Wu, R.; Li, H.; Yang, Y.; Zheng, Q.; Li, S.; Chen, Y. Bioactive Silk Fibroin-Based Hybrid Biomaterials for Musculoskeletal Engineering: Recent Progress and Perspectives. ACS Appl. Bio Mater. 2021, 4, 6630–6646. [Google Scholar] [CrossRef]
- Huang, T.-Y.; Wang, G.-S.; Ko, C.-S.; Chen, X.-W.; Su, W.-T. A study of the differentiation of stem cells from human exfoliated deciduous teeth on 3D silk fibroin scaffolds using static and dynamic culture paradigms. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110563. [Google Scholar] [CrossRef]
- Yeh, W.-C.; Li, P.-C.; Jeng, Y.-M.; Hsu, H.-C.; Kuo, P.-L.; Li, M.-L.; Yang, P.-M.; Lee, P.H. Elastic modulus measurements of human liver and correlation with pathology. Ultrasound Med. Biol. 2002, 28, 467–474. [Google Scholar] [CrossRef]
- Pook, M.; Teino, I.; Kallas, A.; Maimets, T.; Ingerpuu, S.; Jaks, V. Changes in Laminin Expression Pattern during Early Differentiation of Human Embryonic Stem Cells. PLoS ONE 2015, 10, e0138346. [Google Scholar] [CrossRef]
- Rodin, S.; Antonsson, L.; Niaudet, C.; Simonson, O.E.; Salmela, E.; Hansson, E.M.; Domogatskaya, A.; Xiao, Z.; Damdimopoulou, P.; Sheikhi, M.; et al. Clonal culturing of human embryonic stem cells on laminin-521/E-cadherin matrix in defined and xeno-free environment. Nat. Commun. 2014, 5, 3195. [Google Scholar] [CrossRef]
- Horejs, C.-M.; Serio, A.; Purvis, A.; Gormley, A.J.; Bertazzo, S.; Poliniewicz, A.; Wang, A.J.; DiMaggio, P.; Hohenester, E.; Stevens, M.M. Biologically-active laminin-111 fragment that modulates the epithelial-to-mesenchymal transition in embryonic stem cells. Proc. Natl. Acad. Sci. USA 2014, 111, 5908–5913. [Google Scholar] [CrossRef]
- Zeisberg, M.; Neilson, E.G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Investig. 2009, 119, 1429–1437. [Google Scholar] [CrossRef]
- Wang, Z.; Ye, S.; van der Laan, L.J.W.; Schneeberger, K.; Masereeuw, R.; Spee, B. Chemically Defined Organoid Culture System for Cholangiocyte Differentiation. Adv. Healthc. Mater. 2024, 13, e2401511. [Google Scholar] [CrossRef]
- Carpentier, N.; Ye, S.; Delemarre, M.D.; Van der Meeren, L.; Skirtach, A.G.; van der Laan, L.J.W.; Schneeberger, K.; Spee, B.; Dubruel, P.; Van Vlierberghe, S. Gelatin-Based Hybrid Hydrogels as Matrices for Organoid Culture. Biomacromolecules 2024, 25, 590–604. [Google Scholar] [CrossRef]
- Passaretta, F.; Bosco, D.; Centurione, L.; Centurione, M.A.; Marongiu, F.; Di Pietro, R. Differential response to hepatic differentiation stimuli of amniotic epithelial cells isolated from four regions of the amniotic membrane. J. Cell. Mol. Med. 2020, 24, 4350–4355. [Google Scholar] [CrossRef]
- Ong, J.; Serra, M.P.; Segal, J.; Cujba, A.-M.; Ng, S.S.; Butler, R.; Millar, V.; Hatch, S.; Zimri, S.; Koike, H.; et al. Imaging-Based Screen Identifies Laminin 411 as a Physiologically Relevant Niche Factor with Importance for i-Hep Applications. Stem Cell Rep. 2018, 10, 693–702. [Google Scholar] [CrossRef]
- Takayama, K.; Mitani, S.; Nagamoto, Y.; Sakurai, F.; Tachibana, M.; Taniguchi, Y.; Sekiguchi, K.; Mizuguchi, H. Laminin 411 and 511 promote the cholangiocyte differentiation of human induced pluripotent stem cells. Biochem. Biophys. Res. Commun. 2016, 474, 91–96. [Google Scholar] [CrossRef]
- Blackford, S.J.I.; Yu, T.T.L.; Norman, M.D.A.; Syanda, A.M.; Manolakakis, M.; Lachowski, D.; Yan, Z.; Guo, Y.; Garitta, E.; Riccio, F.; et al. RGD density along with substrate stiffness regulate hPSC hepatocyte functionality through YAP signalling. Biomaterials 2023, 293, 121982. [Google Scholar] [CrossRef]
- Van Norman, G.A. Drugs, Devices, and the FDA: Part 1: An Overview of Approval Processes for Drugs. JACC Basic Transl. Sci. 2016, 1, 170–179. [Google Scholar] [CrossRef]
- Federal Register. Intent To Consider the Appropriate Classification of Hyaluronic Acid Intra-Articular Products Intended for the Treatment of Pain in Osteoarthritis of the Knee Based on Scientific Evidence. 2018. Available online: https://www.federalregister.gov/documents/2018/12/18/2018-27351/intent-to-consider-the-appropriate-classification-of-hyaluronic-acid-intra-articular-products (accessed on 28 June 2025).
- Tajima, G.; Huh, S.; Schmidt, N.A.; Macdonald, J.C.; Fleischmann, T.; Wonnacott, K.M. Impact of genetically modified organism requirements on gene therapy development in the EU, Japan, and the US. Mol. Ther. Methods Clin. Dev. 2022, 26, 74–83. [Google Scholar] [CrossRef]
- Dhawan, A.; Chaijitraruch, N.; Fitzpatrick, E.; Bansal, S.; Filippi, C.; Lehec, S.C.; Heaton, N.D.; Kane, P.; Verma, A.; Hughes, R.D.; et al. Alginate microencapsulated human hepatocytes for the treatment of acute liver failure in children. J. Hepatol. 2020, 72, 877–884. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
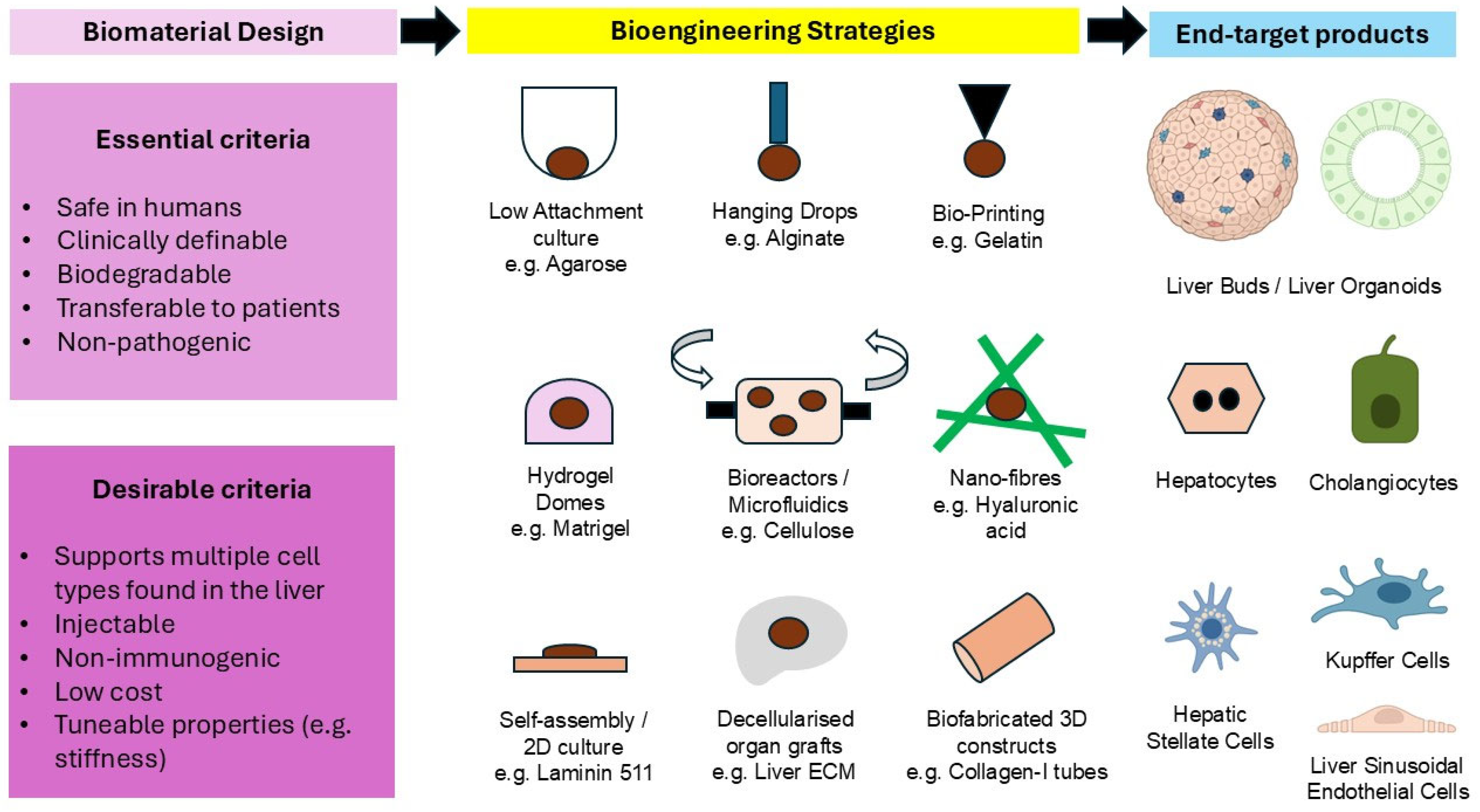

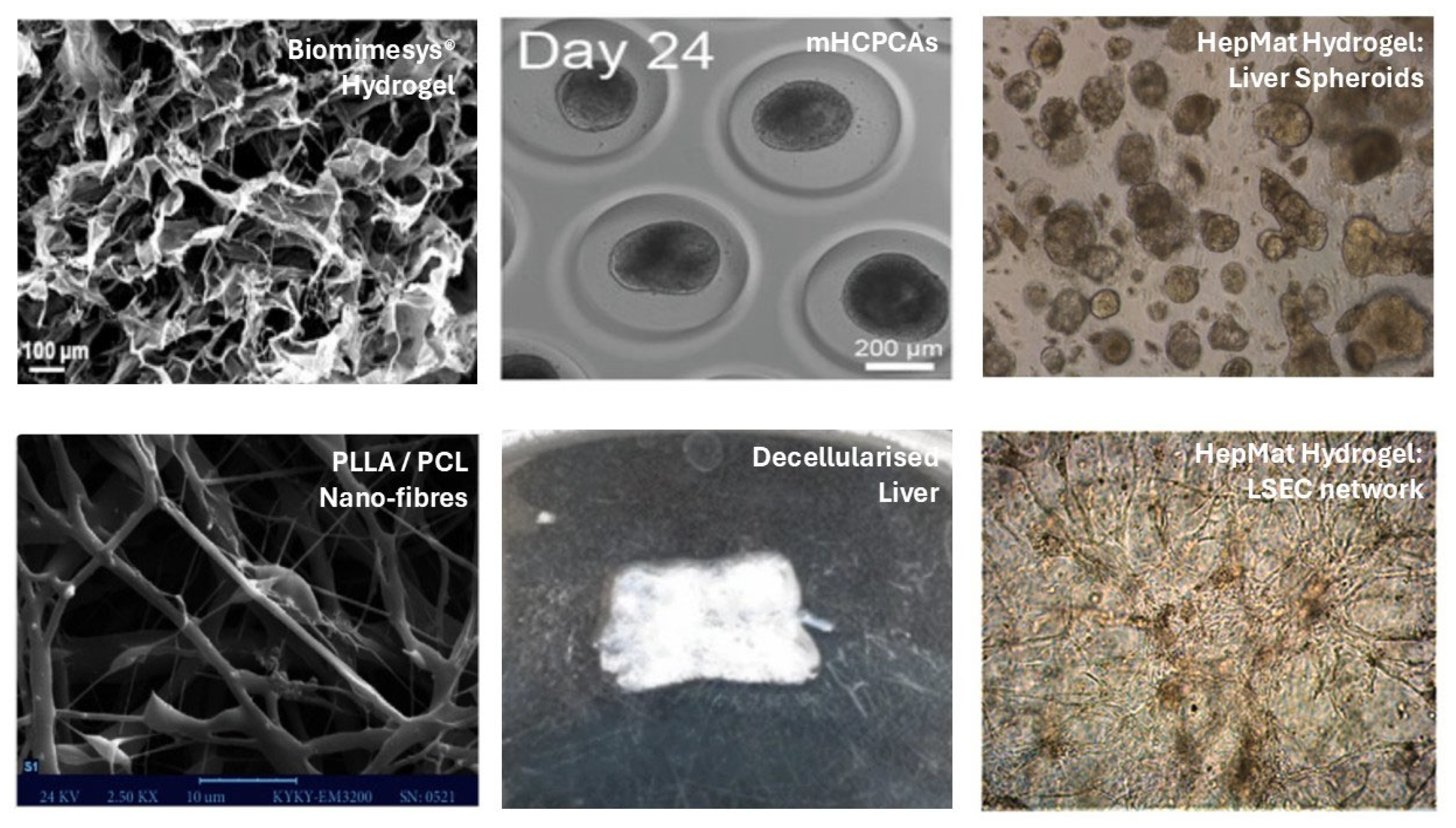
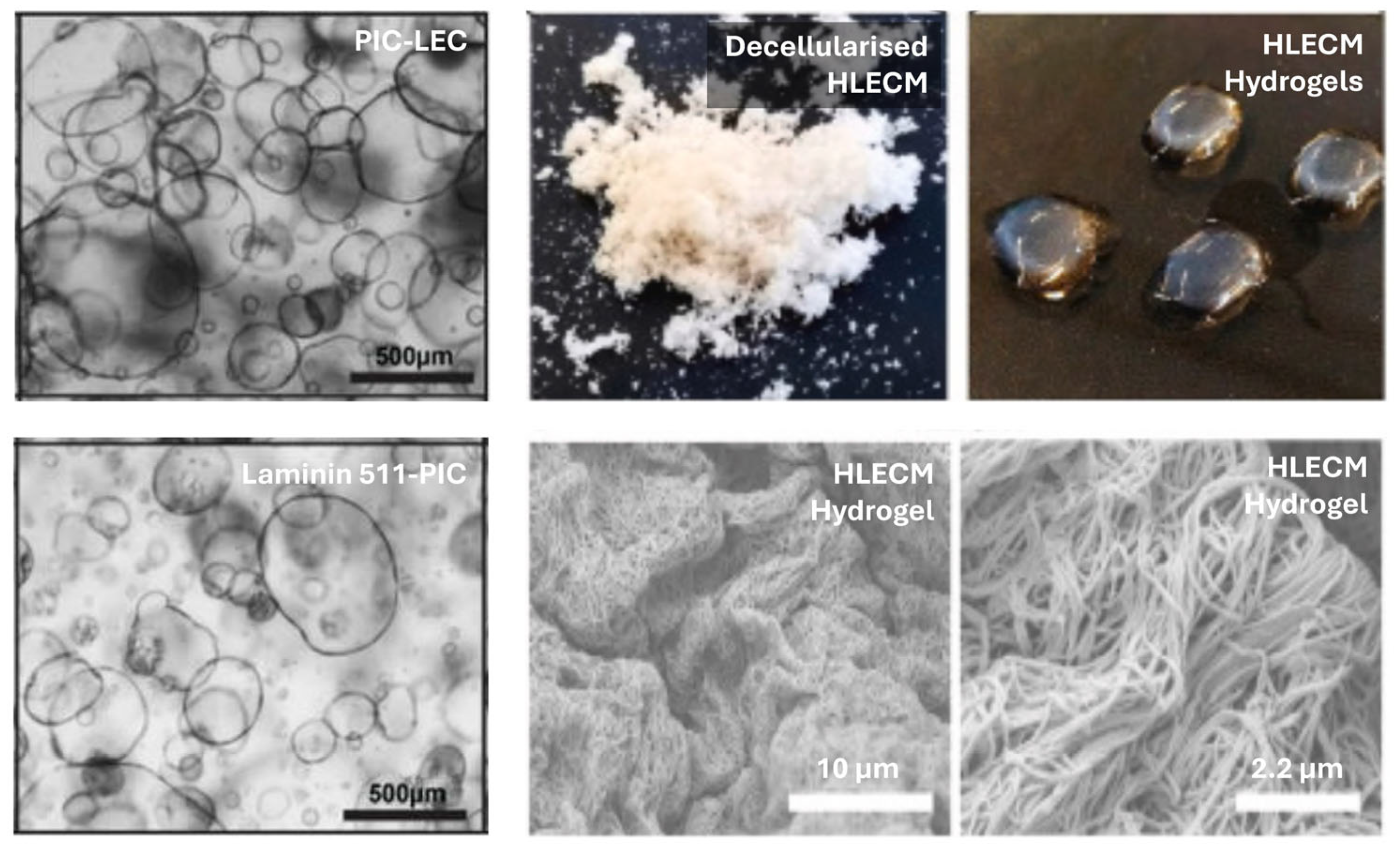
| Biopolymer/Substrate | Maintenance | Differentiation |
|---|---|---|
| Matrigel | 112 | 155 |
| Matrigel (GFR) 1 | 54 | 43 |
| Biomimesys | - | 1 |
| Collagen-I | - | 13 |
| Cellulose | - | 1 |
| Cellartis DEF COAT-1 | 9 | 1 |
| Cellartis DED coating 2 | - | 7 |
| Decellularised liver ECM | - | 3 |
| Feeder cells | 26 | 3 |
| Fibronectin | - | 2 |
| Gelatine | 5 | 13 |
| GelMA 3 | - | 1 |
| hE-cad-Fc | 1 | - |
| HepMat | - | 1 |
| HydroxTM coating | - | 1 |
| Laminin 111 | - | 1 |
| Laminin 511 | 20 | 9 |
| Laminin 511-Silk | 6 | 6 |
| Laminin 521 | 16 | 18 |
| Laminin 521-Silk (Biosilk) | 1 | - |
| PEG-coated constructs 4 | - | 1 |
| PEG-peptides | - | 1 |
| PLLA/PCL fibres 5 | - | 1 |
| PCL-Gel-HA 6 | - | 1 |
| Placenta ECM | 1 | 2 |
| PAA 7 | - | 1 |
| Suspension Culture | 1 | 11 |
| Synthemax II coating | 1 | 1 |
| Vitronectin | 35 | 2 |
| Biopolymer/Substrate | Maintenance | Differentiation |
|---|---|---|
| Matrigel | 90 | 96 |
| Matrigel (GFR) | 28 | 23 |
| Collagen-I | 14 | 11 |
| Fibronectin | 1 | - |
| Gelatine | 1 | 1 |
| Gelatine-Alginate | - | 1 |
| GelMA | - | 1 |
| Hyaluronic Acid | 1 | 1 |
| HydroxTM | - | 1 |
| Liver ECM | - | 1 |
| Laminin 332 | - | 1 |
| Laminin 511-PIC 1 | - | 1 |
| PIC-LEC 2 | - | 1 |
| PEG | - | 1 |
| PCL | - | 1 |
| Suspension Culture | 3 | 10 |
| No coating | 21 | 15 |
| Biopolymer/Substrate | Maintenance | Differentiation |
|---|---|---|
| Matrigel | 1 | - |
| Matrigel (GFR) | 1 | 1 |
| Agarose | - | 1 |
| Collagen-I | 5 | 11 |
| Fibronectin | 3 | 2 |
| Fibroin | - | 1 |
| Gelatine | 3 | 4 |
| Liver ECM (animal) | - | 4 |
| Lipidure coating 1 | - | 1 |
| Wharton’s Jelly | 1 | 1 |
| No coating | 45 | 33 |
| Biopolymers/ Synthetic Substrates | Bioactive | Fully Defined | Easily Transferable | Biodegradable In Vivo | Biosafety Studies |
|---|---|---|---|---|---|
| Collagen-I | ✔ | ✔ | ✔ | ✔ | ✔ |
| Hyaluronic Acid | ✔ | ✔ | ✔ | ✔ | ✔ |
| Laminin 111 | ✔ | ✔ | ✗ | ✔ | ✗ |
| Laminin 332 | ✔ | ✔ | ✗ | ✔ | ✗ |
| Laminin 511 | ✔ | ✔ | ✗ | ✔ | ✔ |
| Laminin 521 | ✔ | ✔ | ✗ | ✔ | ✔ |
| PIC-LEC | ✔ | ✔ | ✔ | ✗ | ✗ |
| Laminin 511-PIC | ✔ | ✔ | ✔ | ✗ | ✗ |
| Cellartis DEF COAT-1 | ✔ | ✔ * | ✗ | ✗ | ✗ |
| Cellartis DED Coating | ✔ | ✔ * | ✗ | ✗ | ✗ |
| Fibronectin | ✔ | ✔ | ✗ | ✗ | ✗ |
| Fibroin | ✔ | ✔ | ✗ | ✗ | ✗ |
| hE-cad-Fc | ✔ | ✔ | ✗ | ✗ | ✗ |
| HepMat | ✔ | ✔ | ✗ | ✗ | ✗ |
| PEG-peptides | ✔ | ✔ | ✗ | ✗ | ✗ |
| PCL-Gel-HA | ✔ | ✔ | ✗ | ✗ | ✗ |
| Vitronectin | ✔ | ✔ | ✗ | ✗ | ✗ |
| Laminin 511-Silk | ✔ | ✔ | ✗ | ✗ | ✗ |
| Laminin 521-Silk | ✔ | ✔ | ✗ | ✗ | ✗ |
| HydroxTM coating | ✗ | ✔ | ✗ | ✗ | ✗ |
| Lipidure® coating | ✗ | ✔ | ✗ | ✗ | ✗ |
| PCL | ✗ | ✔ | ✗ | ✗ | ✗ |
| PLLA/PCL fibres | ✗ | ✔ | ✗ | ✗ | ✗ |
| PEG-coated constructs | ✗ | ✔ | ✗ | ✗ | ✗ |
| PAA | ✗ | ✔ | ✗ | ✗ | ✗ |
| Synthemax II | ✗ | ✔ | ✗ | ✗ | ✗ |
| Cellulose | ✗ | ✔ | ✗ | ✗ | ✗ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, J.; Zhao, J.J.; Swift, C.; Markaki, A.E. Biopolymers for Liver Tissue Engineering: A Systematic Review. Gels 2025, 11, 525. https://doi.org/10.3390/gels11070525
Ong J, Zhao JJ, Swift C, Markaki AE. Biopolymers for Liver Tissue Engineering: A Systematic Review. Gels. 2025; 11(7):525. https://doi.org/10.3390/gels11070525
Chicago/Turabian StyleOng, John, Jacky Junzhe Zhao, Carla Swift, and Athina E. Markaki. 2025. "Biopolymers for Liver Tissue Engineering: A Systematic Review" Gels 11, no. 7: 525. https://doi.org/10.3390/gels11070525
APA StyleOng, J., Zhao, J. J., Swift, C., & Markaki, A. E. (2025). Biopolymers for Liver Tissue Engineering: A Systematic Review. Gels, 11(7), 525. https://doi.org/10.3390/gels11070525









