Dual-Drug Delivery Systems Using Hydrogel–Nanoparticle Composites: Recent Advances and Key Applications
Abstract
1. Introduction
1.1. Importance of Dual-Drug Delivery
1.2. Need for Hydrogel–Nanoparticle Composites
2. Design and Fabrication Strategies of Hydrogel–Nanoparticle Dual-Drug Delivery Systems
2.1. Composite Formation Methods
2.1.1. Physical Embedding
2.1.2. Covalent Integration
2.1.3. Layer-by-Layer Assembly
- Physical compartmentalization: Each drug is confined to separate layers or zones, preventing interference and allowing distinct microenvironments optimal for each (e.g., a hydrophobic drug in a nanoparticle layer versus a hydrophilic drug in a hydrogel layer) [114].
- Tailored release kinetics: By adjusting layer composition and thickness, the release rate of each drug can be independently tuned—even enabling sequential or staged release for one drug before the other [115].
- Multifunctional versatility: LbL films can integrate many material types (polymers, inorganic nanoparticles, biomolecules, etc.), making it feasible to co-deliver drugs with very different properties or activation triggers within one composite [116].
| Formulation Strategy | Key Features | Advantages | Drawbacks |
|---|---|---|---|
| Physical Embedding | Pre-formed nanoparticles are physically dispersed within the hydrogel matrix via non-covalent interactions | - Simple fabrication - Benefits of modular design and rapid prototyping - Preserving hydrogel and nanoparticle functionality | - Non-uniformity of nanoparticle distribution - Release control limitations |
| Covalent Integration | Nanoparticles are chemically bonded to the hydrogel network (e.g., via EDC/NHS or click chemistry) | - Positional stability - Multimodal and hierarchical drug release design | - Requires precise reaction control and purification - Risk of activity loss or denaturation for sensitive therapeutics |
| Layer-by-Layer Assembly | Multilayer structures are sequentially deposited on hydrogel or nanoparticle surfaces | Spatially separated dual-drug release control | - Limited formulation flexibility in multilayer architectures - Restricted drug loading per layer |
2.2. Types of Hydrogels
2.2.1. Natural Polymers
2.2.2. Synthetic Polymers
2.3. Types of Nanoparticles
2.3.1. Polymeric Nanoparticles
2.3.2. Inorganic Nanoparticles (e.g., Gold, Iron Oxide, Silica)
2.3.3. Liposomes
3. Drug Release Mechanisms in Dual-Drug Delivery Systems
3.1. Simultaneous vs. Sequential Release
3.2. Spatial Separation in Composite Structures
3.3. Stimuli-Responsive Release Control
3.3.1. pH-Responsive Release
3.3.2. Temperature-Responsive Release
3.3.3. Enzyme-Responsive Release
3.3.4. Redox or ROS-Responsive Release
3.4. Mathematical Models for Dual-Drug Release
4. Key Biomedical Applications: Case Studies
4.1. Wound Healing
4.1.1. Metformin–Curcumin Compartmentalized Release via Injectable Self-Healing Hydrogel for Diabetic Wounds
4.1.2. Curcumin and Ciprofloxacin Dual Loading in Clay-Reinforced Chitosan–Hyaluronic Acid Hydrogel
4.1.3. Sequential Release of Ciprofloxacin and Insulin from Injectable Hydrogel for Infected Wounds
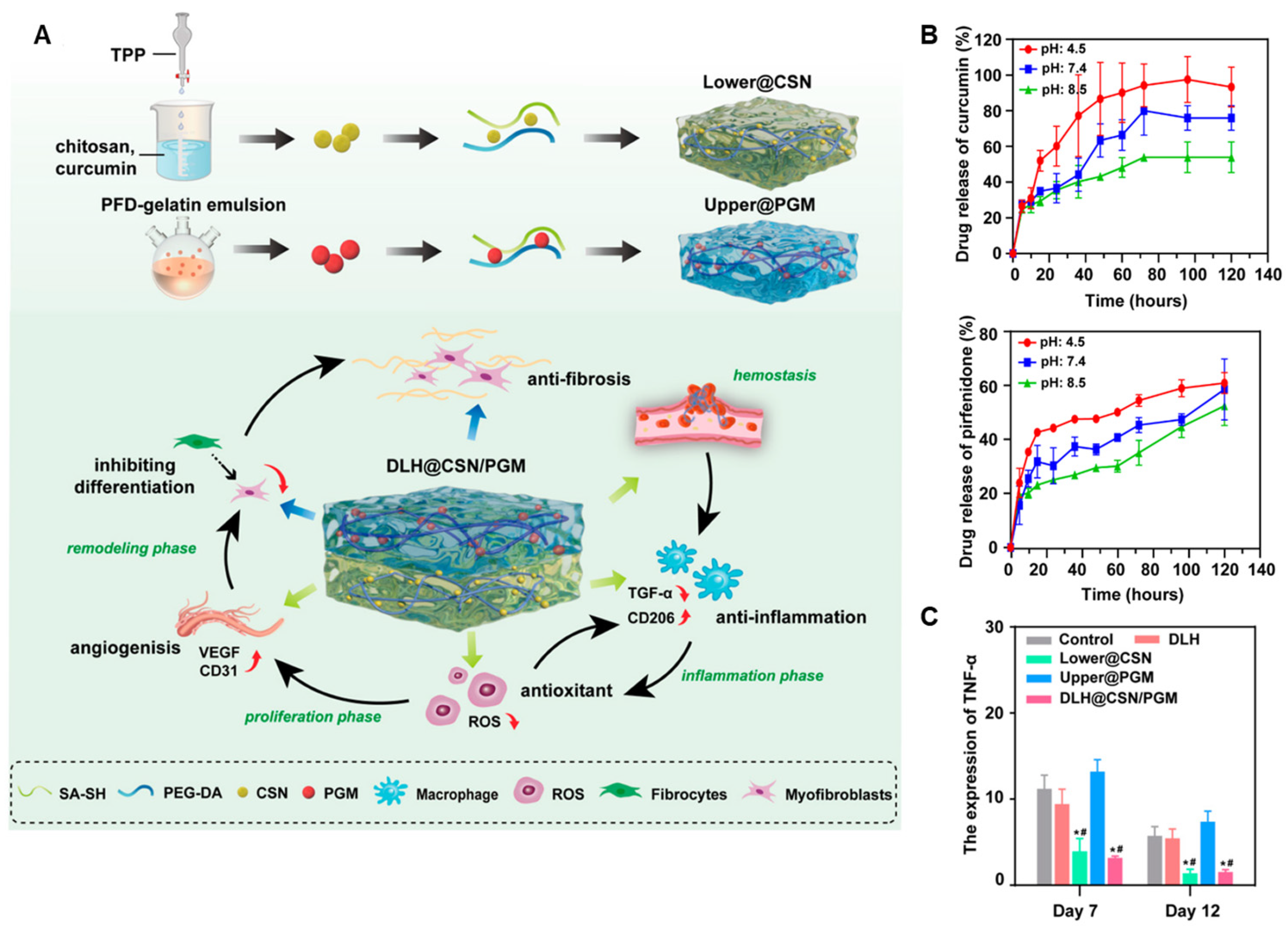
4.1.4. Layered Hydrogel System for Phase-Specific Release of Curcumin and Pirfenidone
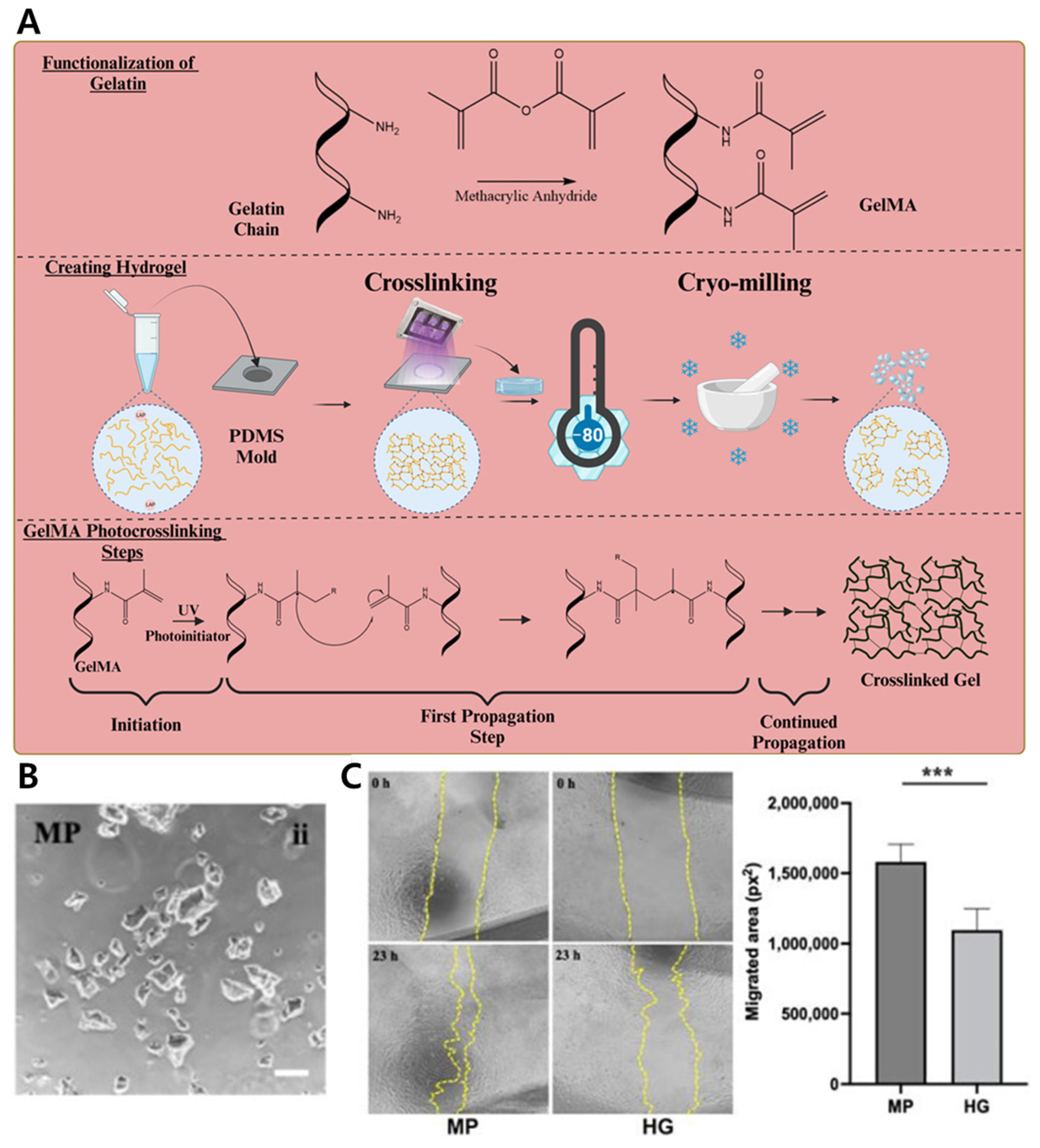
4.1.5. Co-Delivery of hMSCs and Antibiotic via GelMA Microparticle–Nanoparticle Hybrid for Enhanced Wound Repair
4.2. Cancer Therapy
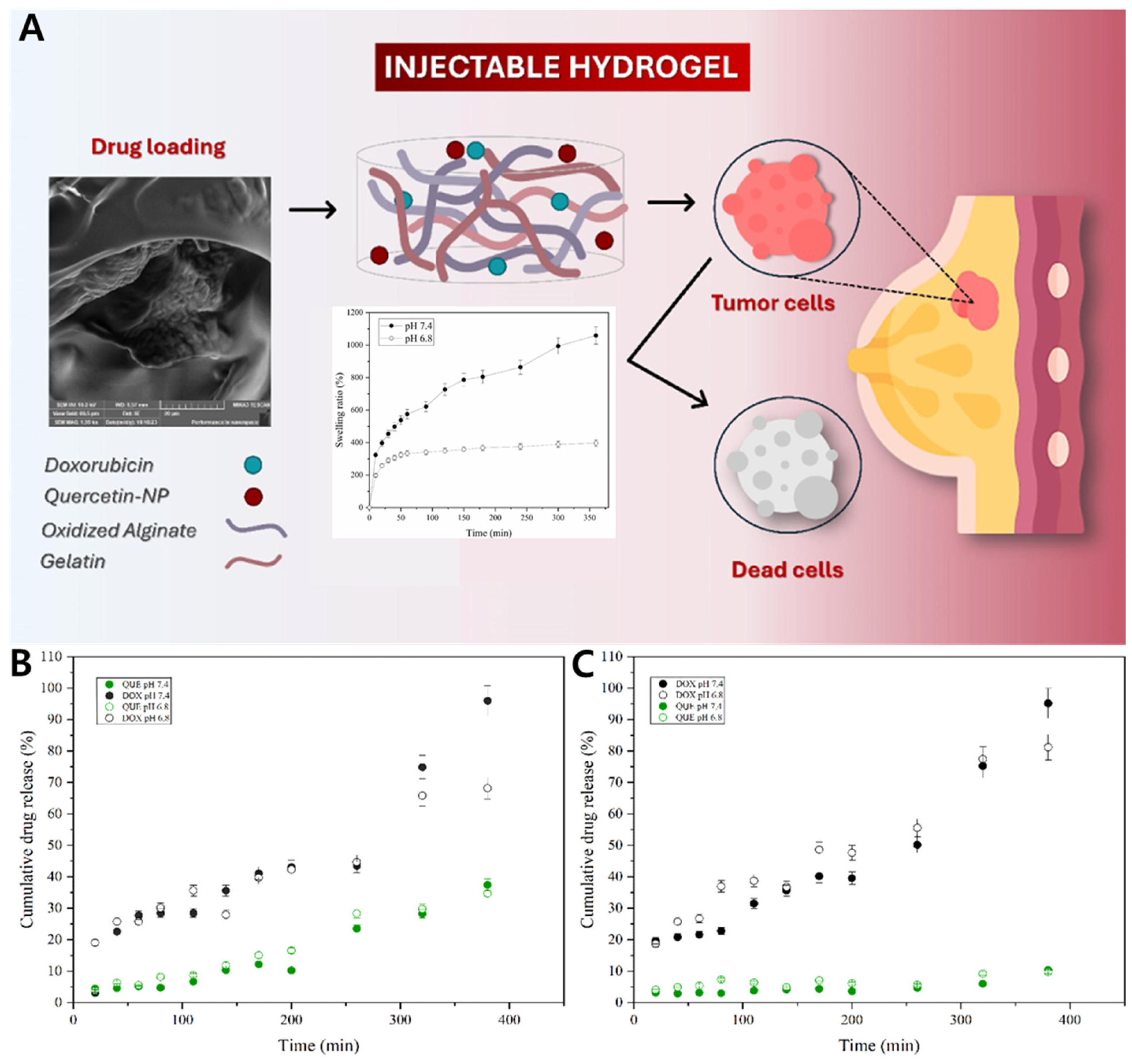
4.2.1. Gel–Zein Nanoparticle Composite for Co-Delivery of Doxorubicin and Quercetin in Breast Cancer Cells
4.2.2. Dual-Responsive Injectable Hydrogel with PDA Nanoparticles for Spatiotemporal Release of SN-38
4.2.3. Sequential Local Release of Doxorubicin and Docetaxel Using Thermoresponsive Hydrogel–Micelle Hybrid
4.2.4. Injectable Alginate Complex Hydrogel Loaded with Dual-Drug Nanovectors for Photochemotherapy of Triple-Negative Breast Cancer
4.2.5. pH-Responsive Chitosan Hydrogel for Co-Delivery of 5-Fluorouracil and Everolimus in Breast Cancer Therapy
4.3. Infection Control
4.3.1. Efflux Pump Inhibition Combined with Antibiotic Delivery for Resistant Skin Infections
4.3.2. Layered 3D-Printed Hydrogel for Dual Antibiotic Delivery Against Implant-Associated Infections
4.3.3. Sequential Triple Antibiotic Release from Hierarchical 3D Bioceramic–Polymer Scaffolds for Biofilm Eradication
4.3.4. Dual-Responsive Hydrogel Patch for Tailored Antibiotic Release in Chronic Wound Infections
4.4. Transplant Immunosuppression
4.4.1. Localized Dual Delivery of Immunosuppressant and Antibiotic via Micelle-Loaded Peptide Hydrogel for Corneal Graft Rejection
4.4.2. Vesicle-Crosslinked Hydrogel for Localized Immune Modulation in Allogeneic Transplantation
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| LBL | Layer-by-layer |
| DDS | Drug delivery system |
| PEG | Polyethylene glycol |
| PVA | Polyvinyl alcohol |
| PNIPAM | Poly(N-isopropylacrylamide) |
| PEGDA | Polyethylene glycol(PEG)-diacrylate |
| LCST | Lower critical solution temperature |
| PLGA | Polylactic-co-glycolic acid |
| PLA | Polylactic acid |
| AuNPs | Gold nanoparticles |
| MSNs | Mesoporous silica nanoparticles |
| NIR | Near-infrared |
| MMP | Matrix metalloproteinase |
| GSH | Glutathione |
| MPDA | Mesoporous polydopamine |
| MMT | Montmorillonite |
| CS | Chitosan |
| CSNPs | Chitosan nanoparticles |
| PGMs | Pirfenidone-loaded gelatin microspheres |
| Cur | Curcumin |
| hMSCs | Human mesenchymal stem cells |
| GelMA | Gelatin methacrylate |
| MPs | Microparticles |
| PS | Penicillin–streptomycin |
| OA | Oxidized alginate |
| QNP | Nanoparticles loaded with quercetin |
| PDA | Polydopamine |
| TPGS | D-α-tocopheryl polyethylene glycol 1000 succinate |
| TNBC | Triple-negative breast cancer |
| ICG | In-docyanine green |
| IPNEs | In-docyanine green-loaded perfluorocarbon nanoemulsions |
| CPT | Camptothecin |
| CCNPs | Camptothecin-loaded chitosan nanoparticles |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| CSH | Chitosan hydrogel |
| β-GP | Beta-glycerophosphate |
| CIP | Ciprofloxacin |
| 5-NPPP | 5-nitrophenylpiperazine |
| Rif | Rifampicin |
| Van | Vancomycin |
| Gel-Glu | Gelatin–glutaraldehyde |
| RSF | Regenerated silk fibroin |
| MoO2 | Molybdenum dioxide |
| RAPA | Rapamycin |
| Lev | Levofloxacin hydrochloride |
| NapFFKK | Cationic peptide-based hydrogel |
| MMVs | Membrane-derived vesicles |
References
- Lei, Z.-N.; Tian, Q.; Teng, Q.-X.; Wurpel, J.N.D.; Zeng, L.; Pan, Y.; Chen, Z.-S. Understanding and targeting resistance mechanisms in cancer. MedComm 2023, 4, e265. [Google Scholar] [CrossRef] [PubMed]
- Paton, N.I.; Stöhr, W.; Arenas-Pinto, A.; Clarke, A.; Williams, I.; Johnson, M.; Orkin, C.; Chen, F.; Lee, V.; Winston, A. Long-term efficacy and safety of a treatment strategy for HIV infection using protease inhibitor monotherapy: 8-year routine clinical care follow-up from a randomised, controlled, open-label pragmatic trial (PIVOT). EClinicalMedicine 2024, 69, 102457. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zuo, M.; Zhou, Q.; Wang, Y. Oncolytic virotherapy in cancer treatment: Challenges and optimization prospects. Front. Immunol. 2023, 14, 1308890. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Gong, J.; Shi, T.; Liu, J.; Pei, Z.; Liu, J.; Ren, X.; Li, F.; Qiu, F. Dual-drug codelivery nanosystems: An emerging approach for overcoming cancer multidrug resistance. Biomed. Pharmacother. 2023, 161, 114505. [Google Scholar] [CrossRef]
- Anupama Devi, V.K.; Ray, S.; Arora, U.; Mitra, S.; Sionkowska, A.; Jaiswal, A.K. Dual drug delivery platforms for bone tissue engineering. Front. Bioeng. Biotechnol. 2022, 10, 969843. [Google Scholar]
- Carvalho, A.M.; Greene, M.K.; Smyth, P.; Mutch, A.; McLaughlin, K.M.; Cairns, L.V.; Mills, K.I.; McCloskey, K.D.; Scott, C.J. Development of CD33-targeted dual drug-loaded nanoparticles for the treatment of pediatric acute myeloid leukemia. Biomacromolecules 2024, 25, 6503–6514. [Google Scholar] [CrossRef]
- Haley, R.M.; Qian, V.R.; Learn, G.D.; von Recum, H.A. Use of affinity allows anti-inflammatory and anti-microbial dual release that matches suture wound resolution. J. Biomed. Mater. Res. Part A 2019, 107, 1434–1442. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, L. Drug-induced self-assembled nanovesicles for doxorubicin resistance reversal via autophagy inhibition and delivery synchronism. Theranostics 2022, 12, 3977–3994. [Google Scholar] [CrossRef]
- Cai, J.; Yang, Y.; Zhang, J.; Bai, Z.; Zhang, X.; Li, K.; Shi, M.; Liu, Z.; Gao, L.; Wang, J. Multilayer nanodrug delivery system with spatiotemporal drug release improves tumor microenvironment for synergistic anticancer therapy. Biofabrication 2024, 16, 025012. [Google Scholar] [CrossRef]
- Anggelia, M.R.; Huang, R.-W.; Cheng, H.-Y.; Lin, C.-H.; Lin, C.-H. Implantable immunosuppressant delivery to prevent rejection in transplantation. Int. J. Mol. Sci. 2022, 23, 1592. [Google Scholar] [CrossRef] [PubMed]
- Ilinskaya, A.; Dobrovolskaia, M. Immunosuppressive and anti-inflammatory properties of engineered nanomaterials. Br. J. Pharmacol. 2014, 171, 3988–4000. [Google Scholar] [CrossRef] [PubMed]
- Feller, G.; Khammissa, R.A.G.; Ballyram, R.; Beetge, M.-M.; Lemmer, J.; Feller, L. Tumour genetic heterogeneity in relation to oral squamous cell carcinoma and anti-cancer treatment. Int. J. Environ. Res. Public Health 2023, 20, 2392. [Google Scholar] [CrossRef] [PubMed]
- Hata, A.N.; Niederst, M.J.; Archibald, H.L.; Gomez-Caraballo, M.; Siddiqui, F.M.; Mulvey, H.E.; Maruvka, Y.E.; Ji, F.; Bhang, H.-e.C.; Krishnamurthy Radhakrishna, V. Tumor cells can follow distinct evolutionary paths to become resistant to epidermal growth factor receptor inhibition. Nat. Med. 2016, 22, 262–269. [Google Scholar] [CrossRef]
- Sousa, C.; Videira, M. Dual Approaches in Oncology: The Promise of siRNA and Chemotherapy Combinations in Cancer Therapies. Onco 2025, 5, 2. [Google Scholar] [CrossRef]
- Wei, X.; Song, M.; Li, W.; Huang, J.; Yang, G.; Wang, Y. Multifunctional nanoplatforms co-delivering combinatorial dual-drug for eliminating cancer multidrug resistance. Theranostics 2021, 11, 6334–6354. [Google Scholar] [CrossRef]
- Liu, H.; Yao, X.; Zhu, W.; Zhang, J.; Ma, S.; Lu, D.; Yang, W. A Functionalized Metal–Organic Framework-Based Controlled Dual-Drug Delivery System with Short Hairpin RNA and Simvastatin for Ferroptosis in Colorectal Cancer. Adv. Ther. 2023, 6, 2200356. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, J.; Fan, Y.; Yu, C.; Yu, L.; Shao, F. A pH-Responsive Dendritic-DNA-Based Nanohydrogel for Dual Drug Delivery. Biomolecules 2025, 15, 537. [Google Scholar] [CrossRef]
- Christfort, J.F.; Milián-Guimerá, C.; Kamguyan, K.; Hansen, M.B.; Hagner Nielsen, L.; Thamdrup, L.H.E.; Zór, K.; Boisen, A. Sequential Drug Release Achieved with Dual-Compartment Microcontainers: Toward Combination Therapy. Adv. Ther. 2022, 5, 2200106. [Google Scholar] [CrossRef]
- Luo, S.; Zhao, C.; Wang, R.; Wu, D. Sequential Drug Release Nanocomposites for Diseases Synergistic Therapy. J. Mater. Chem. B 2025, 13, 4313–4329. [Google Scholar] [CrossRef]
- Yoon, M.S.; Lee, Y.J.; Shin, H.J.; Park, C.-W.; Han, S.-B.; Jung, J.-K.; Kim, J.-S.; Shin, D.H. Recent advances and challenges in controlling the spatiotemporal release of combinatorial anticancer drugs from nanoparticles. Pharmaceutics 2020, 12, 1156. [Google Scholar] [CrossRef] [PubMed]
- Kilic Boz, R.; Aydin, D.; Kocak, S.; Golba, B.; Sanyal, R.; Sanyal, A. Redox-responsive hydrogels for tunable and “on-demand” release of biomacromolecules. Bioconjug. Chem. 2022, 33, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, K.; Phan, T.T.V.; Santhamoorthy, M.; Ramkumar, V.; Kim, S.-C. pH and Thermoresponsive PNIPAm-co-Polyacrylamide Hydrogel for Dual Stimuli-Responsive Controlled Drug Delivery. Polymers 2023, 15, 167. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, D.; Hui, Q.; Bi, J.; Yu, B.; Huang, Z.; Hu, S.; Wang, Z.; Caranasos, T.; Rossi, J. Injection of ROS-responsive hydrogel loaded with basic fibroblast growth factor into the pericardial cavity for heart repair. Adv. Funct. Mater. 2021, 31, 2004377. [Google Scholar] [CrossRef]
- Mo, C.; Luo, R.; Chen, Y. Advances in the stimuli-responsive injectable hydrogel for controlled release of drugs. Macromol. Rapid Commun. 2022, 43, 2200007. [Google Scholar] [CrossRef]
- Kasiński, A.; Zielińska-Pisklak, M.; Oledzka, E.; Sobczak, M. Smart hydrogels–synthetic stimuli-responsive antitumor drug release systems. Int. J. Nanomed. 2020, 15, 4541–4572. [Google Scholar] [CrossRef]
- Deng, Z.; Yu, R.; Guo, B. Stimuli-responsive conductive hydrogels: Design, properties, and applications. Mater. Chem. Front. 2021, 5, 2092–2123. [Google Scholar] [CrossRef]
- Mohsin, M.E.A.; Siddiqa, A.J.; Mousa, S.; Shrivastava, N.K. Design, Characterization, and Release Kinetics of a Hybrid Hydrogel Drug Delivery System for Sustained Hormone Therapy. Polymers 2025, 17, 999. [Google Scholar] [CrossRef]
- Thakur, S.; Singh, H.; Singh, A.; Kaur, S.; Sharma, A.; Singh, S.K.; Kaur, G.; Jain, S.K. Thermosensitive injectable hydrogel containing carboplatin loaded nanoparticles: A dual approach for sustained and localized delivery with improved safety and therapeutic efficacy. J. Drug Deliv. Sci. Technol. 2020, 58, 101817. [Google Scholar] [CrossRef]
- Han, Q.; Du, L.; Zhu, L.; Yu, D. Review of the application of dual drug delivery nanotheranostic agents in the diagnosis and treatment of liver cancer. Molecules 2023, 28, 7004. [Google Scholar] [CrossRef]
- Thamilselvan, G.; David, H.; Sajeevan, A.; Rajaramon, S.; Solomon, A.P.; Durai, R.D.; Narayanan, V.H.B. Polymer based dual drug delivery system for targeted treatment of fluoroquinolone resistant Staphylococcus aureus mediated infections. Sci. Rep. 2023, 13, 11373. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Ma, J.; Luo, M.; Wang, Y.; Lei, B. Bioactive poly (salicylic acid)-poly (citric acid) scaffolds improve diabetic wound repair via regulating HIF-1α, Nrf2 and macrophage. J. Biomed. Mater. Res. Part A 2024, 112, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- van Straten, D.; Bimbo, J.F.; Hennink, W.E.; Vermonden, T.; Schiffelers, R.M. Nanoparticle-in-Hydrogel Delivery System for the Sequential Release of Two Drugs. Pharmaceutics 2025, 17, 127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Huang, Y.; Pan, W.; Tong, X.; Zeng, Q.; Su, T.; Qi, X.; Shen, J. Polydopamine-incorporated dextran hydrogel drug carrier with tailorable structure for wound healing. Carbohydr. Polym. 2021, 253, 117213. [Google Scholar] [CrossRef]
- Alshangiti, D.M.; El-Damhougy, T.K.; Zaher, A.; Madani, M. Revolutionizing biomedicine: Advancements, applications, and prospects of nanocomposite macromolecular carbohydrate-based hydrogel biomaterials: A review. RSC Adv. 2023, 13, 35251–35291. [Google Scholar] [CrossRef]
- Yin, B.; Gosecka, M.; Bodaghi, M.; Crespy, D.; Youssef, G.; Dodda, J.M.; Wong, S.H.D.; Imran, A.B.; Gosecki, M.; Jobdeedamrong, A. Engineering multifunctional dynamic hydrogel for biomedical and tissue regenerative applications. Chem. Eng. J. 2024, 487, 150403. [Google Scholar] [CrossRef]
- Kaur, H.; Gogoi, B.; Sharma, I.; Das, D.K.; Azad, M.A.; Pramanik, D.D.; Pramanik, A. Hydrogels as a potential biomaterial for multimodal therapeutic applications. Mol. Pharm. 2024, 21, 4827–4848. [Google Scholar] [CrossRef]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Yu, H.; Gao, R.; Liu, Y.; Fu, L.; Zhou, J.; Li, L. Stimulus-Responsive Hydrogels as Drug Delivery Systems for Inflammation Targeted Therapy. Adv. Sci. 2024, 11, 2306152. [Google Scholar] [CrossRef]
- Wahid, F.; Zhao, X.-J.; Jia, S.-R.; Bai, H.; Zhong, C. Nanocomposite hydrogels as multifunctional systems for biomedical applications: Current state and perspectives. Compos. Part B Eng. 2020, 200, 108208. [Google Scholar] [CrossRef]
- Chen, Y.; Song, G.; Yu, J.; Wang, Y.; Zhu, J.; Hu, Z. Mechanically strong dual responsive nanocomposite double network hydrogel for controlled drug release of asprin. J. Mech. Behav. Biomed. Mater. 2018, 82, 61–69. [Google Scholar] [CrossRef]
- Howard, E.; Li, M.; Kozma, M.; Zhao, J.; Bae, J. Self-strengthening stimuli-responsive nanocomposite hydrogels. Nanoscale 2022, 14, 17887–17894. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, D.; Chen, Z. Biomedical applications of stimuli-responsive nanomaterials. MedComm 2024, 5, e643. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Joo, S.W.; Mandal, T.K. Biodegradable and Stimuli-Responsive Nanomaterials for Targeted Drug Delivery in Autoimmune Diseases. J. Funct. Biomater. 2025, 16, 24. [Google Scholar] [CrossRef]
- Li, M.; Zhao, G.; Su, W.-K.; Shuai, Q. Enzyme-responsive nanoparticles for anti-tumor drug delivery. Front. Chem. 2020, 8, 647. [Google Scholar] [CrossRef]
- Abuwatfa, W.H.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. Thermosensitive polymers and thermo-responsive liposomal drug delivery systems. Polymers 2022, 14, 925. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Long, L.; Hu, C.; Kong, Q.; Wang, Y. A spatiotemporal release platform based on pH/ROS stimuli-responsive hydrogel in wound repairing. J. Control. Release 2022, 341, 147–165. [Google Scholar] [CrossRef]
- Attia, L.; Chen, L.H.; Doyle, P.S. Orthogonal Gelations to Synthesize Core–Shell Hydrogels Loaded with Nanoemulsion-Templated Drug Nanoparticles for Versatile Oral Drug Delivery. Adv. Healthc. Mater. 2023, 12, 2301667. [Google Scholar] [CrossRef]
- Chen, N.; Wang, H.; Ling, C.; Vermerris, W.; Wang, B.; Tong, Z. Cellulose-based injectable hydrogel composite for pH-responsive and controllable drug delivery. Carbohydr. Polym. 2019, 225, 115207. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiu, Z.; Jiang, X.; Zhang, H.; Li, X.; Feng, Y.; Li, B.; Cai, R.; Li, C.; Tao, G. Injectable hydrogels with ROS-triggered drug release enable the co-delivery of antibacterial agent and anti-inflammatory nanoparticle for periodontitis treatment. J. Nanobiotechnol. 2025, 23, 205. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.; Pan, C.; Ran, X.; Wen, Y.; Lang, R.; Peng, M.; Cao, J.; Yang, J. Dual-delivery temperature-sensitive hydrogel with antimicrobial and anti-inflammatory brevilin A and nitric oxide for wound healing in bacterial infection. Gels 2024, 10, 219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, Y.; Guo, J.; Yan, M.; Liu, C.; Du, B. Core–Shell Nanoparticles with Sequential Drug Release Depleting Cholesterol for Reverse Tumor Multidrug Resistance. ACS Appl. Mater. Interfaces 2025, 17, 6689–6702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zu, Q.; Deng, C.; Gao, X.; Liu, H.; Jin, Y.; Yang, X.; Wang, E. Biodegradable Double-Layer Hydrogels with Sequential Drug Release for Multi-Phase Collaborative Regulation in Scar-Free Wound Healing. J. Funct. Biomater. 2025, 16, 164. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jo, M.J.; Yoon, M.S.; Jin, C.E.; Shin, Y.B.; Lee, J.M.; Shin, H.J.; Oh, J.G.; Cho, J.M.; Kim, H. Gemcitabine and rapamycin-loaded mixed polymeric thermogel for metastatic pancreatic cancer therapy. J. Control. Release 2023, 360, 796–809. [Google Scholar] [CrossRef]
- Eom, S.; Park, S.G.; Koo, Y.; Noh, Y.; Choi, C.; Kim, Y.; Jun, H.; Cha, C.; Joo, J.; Kang, S. In situ forming and self-crosslinkable protein hydrogels for localized cancer therapy and topical wound healing. J. Control. Release 2025, 378, 460–475. [Google Scholar] [CrossRef]
- Liu, R.; Liang, Q.; Luo, J.Q.; Li, Y.X.; Zhang, X.; Fan, K.; Du, J.Z. Ferritin-Based Nanocomposite Hydrogel Promotes Tumor Penetration and Enhances Cancer Chemoimmunotherapy. Adv. Sci. 2024, 11, 2305217. [Google Scholar] [CrossRef]
- Liu, M.; Sun, X.; Liao, Z.; Li, Y.; Qi, X.; Qian, Y.; Fenniri, H.; Zhao, P.; Shen, J. Zinc oxide end-capped Fe3O4@mSiO2 core-shell nanocarriers as targeted and responsive drug delivery system for chemo-/ions synergistic therapeutics. Drug Deliv. 2019, 26, 732–743. [Google Scholar] [CrossRef]
- Tavares, M.; Santos, S.; Custódio, C.; Farinha, J.; Baleizão, C.; Mano, J. Platelet lysates-based hydrogels incorporating bioactive mesoporous silica nanoparticles for stem cell osteogenic differentiation. Mater. Today Bio 2021, 9, 100096. [Google Scholar] [CrossRef]
- Arvejeh, P.M.; Chermahini, F.A.; Marincola, F.; Taheri, F.; Mirzaei, S.A.; Alizadeh, A.; Deris, F.; Jafari, R.; Amiri, N.; Soltani, A. A novel approach for the co-delivery of 5-fluorouracil and everolimus for breast cancer combination therapy: Stimuli-responsive chitosan hydrogel embedded with mesoporous silica nanoparticles. J. Transl. Med. 2025, 23, 382. [Google Scholar] [CrossRef]
- Sun, J.; Shi, F.; Lu, Q.; Ye, W.; Liu, S.; Liu, J.; Zhang, C.; Zhao, J.; Ming, W. On-demand release of CO in dual-responsive nanocomposite hydrogels for wound dressing. Surf. Interfaces 2024, 54, 105133. [Google Scholar] [CrossRef]
- Chen, L.-H.; Liang, N.-W.; Huang, W.-Y.; Liu, Y.-C.; Ho, C.-Y.; Kuan, C.-H.; Huang, Y.-F.; Wang, T.-W. Supramolecular hydrogel for programmable delivery of therapeutics to cancer multidrug resistance. Biomater. Adv. 2023, 146, 213282. [Google Scholar] [CrossRef] [PubMed]
- Jalaladdiny, S.-s.; Badoei-dalfard, A.; Karami, Z.; Sargazi, G. Co-delivery of doxorubicin and curcumin to breast cancer cells by a targeted delivery system based on Ni/Ta core-shell metal-organic framework coated with folic acid-activated chitosan nanoparticles. J. Iran. Chem. Soc. 2022, 19, 4287–4298. [Google Scholar] [CrossRef]
- Liu, M.; Huang, P.; Wang, W.; Feng, Z.; Zhang, J.; Deng, L.; Dong, A. An injectable nanocomposite hydrogel co-constructed with gold nanorods and paclitaxel-loaded nanoparticles for local chemo-photothermal synergetic cancer therapy. J. Mater. Chem. B 2019, 7, 2667–2677. [Google Scholar] [CrossRef]
- Alamzadeh, Z.; Beik, J.; Mirrahimi, M.; Shakeri-Zadeh, A.; Ebrahimi, F.; Komeili, A.; Ghalandari, B.; Ghaznavi, H.; Kamrava, S.K.; Moustakis, C. Gold nanoparticles promote a multimodal synergistic cancer therapy strategy by co-delivery of thermo-chemo-radio therapy. Eur. J. Pharm. Sci. 2020, 145, 105235. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; Zhao, L.; Zhang, J.; Luo, H. Constructions and properties of physically cross-linked hydrogels based on natural polymers. Polym. Rev. 2023, 63, 574–612. [Google Scholar] [CrossRef]
- Pita-López, M.L.; Fletes-Vargas, G.; Espinosa-Andrews, H.; Rodríguez-Rodríguez, R. Physically cross-linked chitosan-based hydrogels for tissue engineering applications: A state-of-the-art review. Eur. Polym. J. 2021, 145, 110176. [Google Scholar] [CrossRef]
- Choi, W.; Kohane, D.S. Hybrid nanoparticle–hydrogel systems for drug delivery depots and other biomedical applications. ACS Nano 2024, 18, 22780–22792. [Google Scholar] [CrossRef]
- Jo, M.J.; Yoon, M.S.; Kim, S.Y.; Lee, J.M.; Kang, S.J.; Park, C.-W.; Kim, J.-S.; Yoon, J.-H.; Shin, D.H. A combination formulation of TPGS micelles loaded with paclitaxel and olaparib and a pH-thermosensitive hydrogel for treating peritoneal metastasis and drug-resistant ovarian cancer. J. Pharm. Investig. 2025, 55, 303–319. [Google Scholar] [CrossRef]
- Dannert, C.; Stokke, B.T.; Dias, R.S. Nanoparticle-Hydrogel Composites: From Molecular Interactions to Macroscopic Behavior. Polymers 2019, 11, 275. [Google Scholar] [CrossRef]
- Adibnia, V.; Mirbagheri, M.; Salimi, S.; De Crescenzo, G.; Banquy, X. Nonspecific interactions in biomedical applications. Curr. Opin. Colloid Interface Sci. 2020, 47, 70–83. [Google Scholar] [CrossRef]
- Sadeghi-Abandansari, H.; Pakian, S.; Nabid, M.-R.; Ebrahimi, M.; Rezalotfi, A. Local co-delivery of 5-fluorouracil and curcumin using Schiff’s base cross-linked injectable hydrogels for colorectal cancer combination therapy. Eur. Polym. J. 2021, 157, 110646. [Google Scholar] [CrossRef]
- Khan, Y.A.; Ozaltin, K.; Bernal-Ballen, A.; Di Martino, A. Chitosan-alginate hydrogels for simultaneous and sustained releases of ciprofloxacin, amoxicillin and vancomycin for combination therapy. J. Drug Deliv. Sci. Technol. 2021, 61, 102126. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, J.; Qiao, L.; Maleki, A.; Liang, Y.; Guo, B. ROS-responsive hydrogels with spatiotemporally sequential delivery of antibacterial and anti-inflammatory drugs for the repair of MRSA-infected wounds. Regen. Biomater. 2024, 11, rbad110. [Google Scholar] [CrossRef]
- Yang, H.; Lu, H.; Miao, Y.; Cong, Y.; Ke, Y.; Wang, J.; Yang, H.; Fu, J. Non-swelling, super-tough, self-healing, and multi-responsive hydrogels based on micellar crosslinking for smart switch and shape memory. Chem. Eng. J. 2022, 450, 138346. [Google Scholar] [CrossRef]
- Li, H.; Wen, H.; Zhang, H.; Li, J.; Cao, X.; Zhang, J.; Zheng, Y.; Huang, S.; Xue, W.; Cai, X. Polymeric micelle-hydrogel composites design for biomedical applications. Chin. Chem. Lett. 2024, 36, 110072. [Google Scholar] [CrossRef]
- Meis, C.M.; Grosskopf, A.K.; Correa, S.; Appel, E.A. Injectable supramolecular polymer-nanoparticle hydrogels for cell and drug delivery applications. J. Vis. Exp. JoVE 2021, 168, e62234. [Google Scholar] [CrossRef]
- Soni, S.S.; D’Elia, A.M.; Alsasa, A.; Cho, S.; Tylek, T.; O’Brien, E.M.; Whitaker, R.; Spiller, K.L.; Rodell, C.B. Sustained release of drug-loaded nanoparticles from injectable hydrogels enables long-term control of macrophage phenotype. Biomater. Sci. 2022, 10, 6951–6967. [Google Scholar] [CrossRef]
- Rojek, K.O.; Cwiklinska, M.; Kuczak, J.; Guzowski, J. Microfluidic formulation of topological hydrogels for microtissue engineering. Chem. Rev. 2022, 122, 16839–16909. [Google Scholar] [CrossRef]
- Moncure, P.J.; Simon, Z.C.; Millstone, J.E.; Laaser, J.E. Relationship between gel mesh and particle size in determining nanoparticle diffusion in hydrogel nanocomposites. J. Phys. Chem. B 2022, 126, 4132–4142. [Google Scholar] [CrossRef]
- Ray, P.; Maity, M.; Barik, H.; Sahoo, G.S.; Hasnain, M.S.; Hoda, M.N.; Nayak, A.K. Alginate-based hydrogels for drug delivery applications. In Alginates in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 41–70. [Google Scholar]
- Ávila-Salas, F.; Durán-Lara, E.F. An overview of injectable thermo-responsive hydrogels and advances in their biomedical applications. Curr. Med. Chem. 2020, 27, 5773–5789. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wu, Y.; Gu, R.; Zhang, Z.; Liu, X.; Hu, Y.; Li, X.; Lin, D.; Bao, Z. Nanoparticle-hydrogel composite as dual-drug delivery system for the potential application of corneal graft rejection. Eur. J. Pharm. Biopharm. 2024, 201, 114351. [Google Scholar] [CrossRef] [PubMed]
- Popescu, I.; Constantin, M.; Solcan, G.; Ichim, D.L.; Rata, D.M.; Horodincu, L.; Solcan, C. Composite hydrogels with embedded silver nanoparticles and ibuprofen as wound dressing. Gels 2023, 9, 654. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; Merrild, N.; Li, S.; Pinna, A.; Jones, J. Double-network hydrogels reinforced with covalently bonded silica nanoparticles via 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide chemistry. ACS Omega 2022, 7, 43904–43914. [Google Scholar] [CrossRef]
- Rahman Khan, M.M.; Rumon, M.M.H. Synthesis of PVA-Based Hydrogels for Biomedical Applications: Recent Trends and Advances. Gels 2025, 11, 88. [Google Scholar] [CrossRef]
- Min, Q.; Wang, C.; Zhang, Y.; Tian, D.; Wan, Y.; Wu, J. Strong and elastic hydrogels from dual-crosslinked composites composed of glycol chitosan and amino-functionalized bioactive glass nanoparticles. Nanomaterials 2022, 12, 1874. [Google Scholar] [CrossRef]
- Bailey, S.J.; Eckman, N.; Brunel, E.S.; Jons, C.K.; Sen, S.; Appel, E.A. A thiol–ene click-based strategy to customize injectable polymer–nanoparticle hydrogel properties for therapeutic delivery. Biomater. Sci. 2025, 13, 1323–1334. [Google Scholar] [CrossRef]
- Li, X.; Xiong, Y. Application of “click” chemistry in biomedical hydrogels. ACS Omega 2022, 7, 36918–36928. [Google Scholar] [CrossRef]
- Tarakci, E.C.; Gevrek, T.N. Isocyanate group containing reactive hydrogels: Facile synthesis and efficient biofunctionalization. Eur. Polym. J. 2022, 175, 111338. [Google Scholar] [CrossRef]
- Parvathy, P.; De, S.; Singh, M.; Manik, G.; Sahoo, S.K. Functional self-healing aldehyde-derived nanoparticle-crosslinked gelatin/PNIPAm-based adhesive gels. RSC Appl. Polym. 2025, 3, 662–674. [Google Scholar] [CrossRef]
- Devi, L.S.; Gigliobianco, M.R.; Gabrielli, S.; Agas, D.; Sabbieti, M.G.; Morelli, M.B.; Amantini, C.; Casadidio, C.; Di Martino, P.; Censi, R. Localized Cancer Treatment Using Thiol–Ene Hydrogels for Dual Drug Delivery. Biomacromolecules 2025, 26, 3234–3254. [Google Scholar] [CrossRef] [PubMed]
- Erfani, A.; Diaz, A.E.; Doyle, P.S. Hydrogel-enabled, local administration and combinatorial delivery of immunotherapies for cancer treatment. Mater. Today 2023, 65, 227–243. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, M.; Meng, M.; Wang, Q.; Wang, Y.; Lei, Y.; Zhang, Y.; Weng, L.; Chen, X. A dual-responsive hyaluronic acid nanocomposite hydrogel drug delivery system for overcoming multiple drug resistance. Chin. Chem. Lett. 2023, 34, 107583. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Che, X.; Guo, L.; Huang, L.; Li, X.; Gao, W. A novel pH/ROS dual responsive engineering hydrogels based on poly (tannic acid)-assisted surface deposition of nano-enzymes with efficient antibacterial and antioxidant activity for diabetic wound healing. Chem. Eng. J. 2024, 496, 153370. [Google Scholar] [CrossRef]
- Ma, X.; Li, S.-J.; Liu, Y.; Zhang, T.; Xue, P.; Kang, Y.; Sun, Z.-J.; Xu, Z. Bioengineered nanogels for cancer immunotherapy. Chem. Soc. Rev. 2022, 51, 5136–5174. [Google Scholar] [CrossRef]
- Abune, L.; Wang, Y. Affinity hydrogels for protein delivery. Trends Pharmacol. Sci. 2021, 42, 300–312. [Google Scholar] [CrossRef]
- Lin, S.H.; Huang, A.P.H.; Hsu, S.h. Injectable, Micellar Chitosan Self-Healing Hydrogel for Asynchronous Dual-Drug Delivery to Treat Stroke Rats. Adv. Funct. Mater. 2023, 33, 2303853. [Google Scholar] [CrossRef]
- Xian, S.; VandenBerg, M.A.; Xiang, Y.; Yu, S.; Webber, M.J. Glucose-responsive injectable thermogels via dynamic-covalent cross-linking of pluronic micelles. ACS Biomater. Sci. Eng. 2022, 8, 4873–4885. [Google Scholar] [CrossRef]
- Xiang, Y.; Su, B.; Liu, D.; Webber, M.J. Managing diabetes with hydrogel drug delivery. Adv. Ther. 2024, 7, 2300127. [Google Scholar] [CrossRef]
- Zhang, Z.; Zeng, J.; Groll, J.; Matsusaki, M. Layer-by-layer assembly methods and their biomedical applications. Biomater. Sci. 2022, 10, 4077–4094. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, T.; Ma, Q. Layer-by-Layer assembled nano-drug delivery systems for cancer treatment. Drug Deliv. 2021, 28, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Shende, P.; Patil, A.; Prabhakar, B. Layer-by-layer technique for enhancing physicochemical properties of actives. J. Drug Deliv. Sci. Technol. 2020, 56, 101519. [Google Scholar] [CrossRef]
- Srisang, S.; Nasongkla, N. Layer-by-layer dip coating of Foley urinary catheters by chlorhexidine-loaded micelles. J. Drug Deliv. Sci. Technol. 2019, 49, 235–242. [Google Scholar] [CrossRef]
- Hsu, B.B.; Hagerman, S.R.; Hammond, P.T. Rapid and efficient sprayed multilayer films for controlled drug delivery. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Kulikouskaya, V.; Chyshankou, I.; Pinchuk, S.; Vasilevich, I.; Volotovski, I.; Agabekov, V. Fabrication and characterization of ultrathin spin-coated poly (L-lactic acid) films suitable for cell attachment and curcumin loading. Biomed. Mater. 2020, 15, 065022. [Google Scholar] [CrossRef]
- Butt, M.A. Thin-film coating methods: A successful marriage of high-quality and cost-effectiveness—A brief exploration. Coatings 2022, 12, 1115. [Google Scholar] [CrossRef]
- Keeney, M.; Jiang, X.; Yamane, M.; Lee, M.; Goodman, S.; Yang, F. Nanocoating for biomolecule delivery using layer-by-layer self-assembly. J. Mater. Chem. B 2015, 3, 8757–8770. [Google Scholar] [CrossRef]
- Mensah, L.B.; Morton, S.W.; Li, J.; Xiao, H.; Quadir, M.A.; Elias, K.M.; Penn, E.; Richson, A.K.; Ghoroghchian, P.P.; Liu, J. Layer-by-layer nanoparticles for novel delivery of cisplatin and PARP inhibitors for platinum-based drug resistance therapy in ovarian cancer. Bioeng. Transl. Med. 2019, 4, e10131. [Google Scholar] [CrossRef]
- Skok, K.; Zidarič, T.; Orthaber, K.; Pristovnik, M.; Kostevšek, N.; Žužek Rožman, K.; Šturm, S.; Gradišnik, L.; Maver, U.; Maver, T. Novel Methacrylate-Based Multilayer Nanofilms with Incorporated FePt-Based Nanoparticles and the Anticancer Drug 5-Fluorouracil for Skin Cancer Treatment. Pharmaceutics 2022, 14, 689. [Google Scholar] [CrossRef]
- Sun, H.; Choi, D.; Heo, J.; Jung, S.Y.; Hong, J. Studies on the drug loading and release profiles of degradable chitosan-based multilayer films for anticancer treatment. Cancers 2020, 12, 593. [Google Scholar] [CrossRef]
- Park, S.; Han, U.; Choi, D.; Hong, J. Layer-by-layer assembled polymeric thin films as prospective drug delivery carriers: Design and applications. Biomater. Res. 2018, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Gundogdu, D.; Alemdar, C.; Turan, C.; Husnugil, H.H.; Banerjee, S.; Erel-Goktepe, I. Tuning stimuli-responsive properties of alginate hydrogels through layer-by-layer functionalization for dual-responsive dual drug release. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132213. [Google Scholar] [CrossRef]
- Martínez-Pérez, D.; Guarch-Pérez, C.; Purbayanto, M.A.K.; Choińska, E.; Riool, M.; Zaat, S.A.; Wojciech, Ś. 3D-printed dual drug delivery nanoparticle-loaded hydrogels to combat antibiotic-resistant bacteria. Int. J. Bioprint. 2023, 9, 683. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Li, B.; Wu, B.; Wang, J.; Müller-Buschbaum, P.; Zhong, Q. Controlled hydration, transition, and drug release realized by adjusting layer thickness in alginate-Ca2+/poly (N-isopropylacrylamide) interpenetrating polymeric network hydrogels on cotton fabrics. ACS Biomater. Sci. Eng. 2020, 6, 5051–5060. [Google Scholar] [CrossRef]
- Janardhanam, L.S.L.; Bandi, S.P.; Venuganti, V.V.K. Functionalized LbL film for localized delivery of STAT3 siRNA and oxaliplatin combination to treat colon cancer. ACS Appl. Mater. Interfaces 2022, 14, 10030–10046. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, Y.; Zhang, J.; Liang, H.; Chen, X.; Tan, H. Natural polymer-based hydrogels: From polymer to biomedical applications. Pharmaceutics 2023, 15, 2514. [Google Scholar] [CrossRef]
- Chellathurai, M.S.; Chung, L.Y.; Hilles, A.R.; Sofian, Z.M.; Singha, S.; Ghosal, K.; Mahmood, S. Pharmaceutical chitosan hydrogels: A review on its design and applications. Int. J. Biol. Macromol. 2024, 280, 135775. [Google Scholar] [CrossRef]
- Hong, F.; Qiu, P.; Wang, Y.; Ren, P.; Liu, J.; Zhao, J.; Gou, D. Chitosan-based hydrogels: From preparation to applications, a review. Food Chem. X 2024, 21, 101095. [Google Scholar] [CrossRef]
- Jaipan, P.; Nguyen, A.; Narayan, R.J. Gelatin-based hydrogels for biomedical applications. MRS Commun. 2017, 7, 416–426. [Google Scholar] [CrossRef]
- Bennacef, C.; Desobry-Banon, S.; Probst, L.; Desobry, S. Alginate Core-Shell Capsules Production through Coextrusion Methods: Principles and Technologies. Mar. Drugs 2023, 21, 235. [Google Scholar] [CrossRef]
- Rusu, A.G.; Nita, L.E.; Simionescu, N.; Ghilan, A.; Chiriac, A.P.; Mititelu-Tartau, L. Enzymatically-crosslinked gelatin hydrogels with nanostructured architecture and self-healing performance for potential use as wound dressings. Polymers 2023, 15, 780. [Google Scholar] [CrossRef] [PubMed]
- Ramdhan, T.; Ching, S.H.; Prakash, S.; Bhandari, B. Physical and mechanical properties of alginate based composite gels. Trends Food Sci. Technol. 2020, 106, 150–159. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Wilson, L.D. A review on the design and hydration properties of natural polymer-based hydrogels. Materials 2021, 14, 1095. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.T.; Navesit, K.; Wiwatkunupakarn, L.; Chomchalao, P.; Tiyaboonchai, W. Nanoparticles-hydrogel composites: A promising innovative system for local antimicrobial applications. J. Drug Deliv. Sci. Technol. 2023, 89, 105055. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Cowie, A.C. Recent advances in photo-crosslinkable hydrogels for biomedical applications. BioTechniques 2019, 66, 40–53. [Google Scholar] [CrossRef]
- Wang, Z.; Ye, Q.; Yu, S.; Akhavan, B. Poly ethylene glycol (PEG)-based hydrogels for drug delivery in cancer therapy: A comprehensive review. Adv. Healthc. Mater. 2023, 12, 2300105. [Google Scholar] [CrossRef]
- Wu, F.; Gao, J.; Xiang, Y.; Yang, J. Enhanced Mechanical Properties of PVA Hydrogel by Low-Temperature Segment Self-Assembly vs. Freeze–Thaw Cycles. Polymers 2023, 15, 3782. [Google Scholar] [CrossRef]
- Wu, S.; Lei, L.; Xia, Y.; Oliver, S.; Chen, X.; Boyer, C.; Nie, Z.; Shi, S. PNIPAM-immobilized gold-nanoparticles with colorimetric temperature-sensing and reusable temperature-switchable catalysis properties. Polym. Chem. 2021, 12, 6903–6913. [Google Scholar] [CrossRef]
- Ansari, M.J.; Rajendran, R.R.; Mohanto, S.; Agarwal, U.; Panda, K.; Dhotre, K.; Manne, R.; Deepak, A.; Zafar, A.; Yasir, M.; et al. Poly(N-isopropylacrylamide)-Based Hydrogels for Biomedical Applications: A Review of the State-of-the-Art. Gels 2022, 8, 454. [Google Scholar] [CrossRef]
- Castro, K.C.d.; Costa, J.M.; Campos, M.G.N. Drug-loaded polymeric nanoparticles: A review. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 1–13. [Google Scholar] [CrossRef]
- Hong, Y.; Che, S.; Hui, B.; Wang, X.; Zhang, X.; Ma, H. Combination therapy of lung cancer using layer-by-layer cisplatin prodrug and curcumin co-encapsulated nanomedicine. Drug Des. Dev. Ther. 2020, 14, 2263–2274. [Google Scholar] [CrossRef] [PubMed]
- Asad, M.I.; Khan, D.; Rehman, A.U.; Elaissari, A.; Ahmed, N. Development and in vitro/in vivo evaluation of pH-sensitive polymeric nanoparticles loaded hydrogel for the management of psoriasis. Nanomaterials 2021, 11, 3433. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yin, G.; Sun, S.; Xu, P. Medical applications and prospects of polylactic acid materials. iScience 2024, 27, 111512. [Google Scholar] [CrossRef]
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold nanoparticles for photothermal cancer therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef]
- Etemadi, H.; Buchanan, J.K.; Kandile, N.G.; Plieger, P.G. Iron oxide nanoparticles: Physicochemical characteristics and historical developments to commercialization for potential technological applications. ACS Biomater. Sci. Eng. 2021, 7, 5432–5450. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regí, M. Mesoporous silica nanoparticles for drug delivery. Adv. Funct. Mater. 2020, 30, 1902634. [Google Scholar] [CrossRef]
- Howaili, F.; Özliseli, E.; Küçüktürkmen, B.; Razavi, S.M.; Sadeghizadeh, M.; Rosenholm, J.M. Stimuli-responsive, plasmonic nanogel for dual delivery of curcumin and photothermal therapy for cancer treatment. Front. Chem. 2021, 8, 602941. [Google Scholar] [CrossRef]
- Zhang, N.; Xiong, G.; Liu, Z. Toxicity of metal-based nanoparticles: Challenges in the nano era. Front. Bioeng. Biotechnol. 2022, 10, 1001572. [Google Scholar] [CrossRef]
- Dymek, M.; Sikora, E. Liposomes as biocompatible and smart delivery systems–the current state. Adv. Colloid Interface Sci. 2022, 309, 102757. [Google Scholar] [CrossRef]
- Khan, A.A.; Allemailem, K.S.; Almatroodi, S.A.; Almatroudi, A.; Rahmani, A.H. Recent strategies towards the surface modification of liposomes: An innovative approach for different clinical applications. 3 Biotech 2020, 10, 163. [Google Scholar] [CrossRef]
- Binaymotlagh, R.; Hajareh Haghighi, F.; Chronopoulou, L.; Palocci, C. Liposome–Hydrogel Composites for Controlled Drug Delivery Applications. Gels 2024, 10, 284. [Google Scholar] [CrossRef]
- Pasarin, D.; Ghizdareanu, A.-I.; Enascuta, C.E.; Matei, C.B.; Bilbie, C.; Paraschiv-Palada, L.; Veres, P.-A. Coating materials to increase the stability of liposomes. Polymers 2023, 15, 782. [Google Scholar] [CrossRef] [PubMed]
- Sanati, M.; Yavari, S.A. Liposome-integrated hydrogel hybrids: Promising platforms for cancer therapy and tissue regeneration. J. Control. Release 2024, 368, 703–727. [Google Scholar] [CrossRef] [PubMed]
- Motevalli, S.M.; Eltahan, A.S.; Liu, L.; Magrini, A.; Rosato, N.; Guo, W.; Bottini, M.; Liang, X.-J. Co-encapsulation of curcumin and doxorubicin in albumin nanoparticles blocks the adaptive treatment tolerance of cancer cells. Biophys. Rep. 2019, 5, 19–30. [Google Scholar] [CrossRef]
- Shalmani, A.A.; Wang, A.; Ahmed, Z.; Sheybanifard, M.; Mihyar, R.; Buhl, E.M.; Pohl, M.; Hennink, W.E.; Kiessling, F.; Metselaar, J.M. Tunable polymeric micelles for taxane and corticosteroid co-delivery. Drug Deliv. Transl. Res. 2024, 14, 2642–2654. [Google Scholar] [CrossRef]
- Ahmadi, S.; Rabiee, N.; Bagherzadeh, M.; Elmi, F.; Fatahi, Y.; Farjadian, F.; Baheiraei, N.; Nasseri, B.; Rabiee, M.; Dastjerd, N.T. Stimulus-responsive sequential release systems for drug and gene delivery. Nano Today 2020, 34, 100914. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Long, L.; Yang, L.; Fu, D.; Hu, C.; Kong, Q.; Wang, Y. Inflammation-responsive drug-loaded hydrogels with sequential hemostasis, antibacterial, and anti-inflammatory behavior for chronically infected diabetic wound treatment. ACS Appl. Mater. Interfaces 2021, 13, 33584–33599. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Y.; Zhou, S.; Chen, D.; Zhou, M.; Chen, Q.; Lu, Y.; Cai, N.; Liu, C.; Guo, Y. Sequentially sustained release of anticarcinogens for postsurgical chemoimmunotherapy. J. Control. Release 2022, 350, 803–814. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, L.; Sun, S.; Zhou, Z.; Ma, Y.; Hong, H.; Yang, D. Sequential drug delivery by injectable macroporous hydrogels for combined photodynamic-chemotherapy. J. Nanobiotechnol. 2021, 19, 333. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, S.; Mi, F.; Yang, Y.; Song, Q.; Gao, Y.; Wu, C.; Wen, W. Nanoparticle-Reinforced Hydrogel with a Well-Defined Pore Structure for Sustainable Drug Release and Effective Wound Healing. ACS Appl. Bio Mater. 2025, 8, 1406–1417. [Google Scholar] [CrossRef]
- Briggs, F.; Browne, D.; Asuri, P. Role of Polymer Concentration and Crosslinking Density on Release Rates of Small Molecule Drugs. Int. J. Mol. Sci. 2022, 23, 4118. [Google Scholar] [CrossRef] [PubMed]
- Carrêlo, H.; Soares, P.I.P.; Borges, J.P.; Cidade, M.T. Injectable Composite Systems Based on Microparticles in Hydrogels for Bioactive Cargo Controlled Delivery. Gels 2021, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, H.; Zhang, N.; Zheng, Q. Micelle-Containing Hydrogels and Their Applications in Biomedical Research. Gels 2024, 10, 471. [Google Scholar] [CrossRef] [PubMed]
- Delechiave, G.; Naves, A.F.; Kolanthai, E.; da Silva, R.A.; Vlasman, R.C.; Petri, D.F.; Torresi, R.M.; Catalani, L.H. Tuning protein delivery from different architectures of layer-by-layer assemblies on polymer films. Mater. Adv. 2020, 1, 2043–2056. [Google Scholar] [CrossRef]
- Fischer, A.; Lilienthal, S.; Vázquez-González, M.; Fadeev, M.; Sohn, Y.S.; Nechushtai, R.; Willner, I. Triggered release of loads from microcapsule-in-microcapsule hydrogel microcarriers: En-route to an “artificial pancreas”. J. Am. Chem. Soc. 2020, 142, 4223–4234. [Google Scholar] [CrossRef]
- Meurs, S.; Aliabouzar, M. 3D Bioprintable GelMA Ultrasound Responsive Scaffolds for Precise Micropatterning and Spatiotemporal Drug Release. 2024. Available online: https://hdl.handle.net/2027.42/195338 (accessed on 20 June 2025).
- Wang, L.; Wei, Z.; Xue, C.; Yang, L. Co-delivery system based on multilayer structural nanoparticles for programmed sequential release of fucoxanthin and curcumin. Food Hydrocoll. 2023, 141, 108729. [Google Scholar] [CrossRef]
- Cui, H.; Zhao, Y.-Y.; Han, Y.-H.; Lan, Z.; Zou, K.-L.; Cheng, G.-W.; Chen, H.; Zhong, P.-L.; Chen, Y.; Ma, L.-M. Lymph node targeting strategy using a hydrogel sustained-release system to load effector memory T cells improves the anti-tumor efficacy of anti-PD-1. Acta Biomater. 2024, 180, 423–435. [Google Scholar] [CrossRef]
- Palanikumar, L.; Al-Hosani, S.; Kalmouni, M.; Nguyen, V.P.; Ali, L.; Pasricha, R.; Barrera, F.N.; Magzoub, M. pH-responsive high stability polymeric nanoparticles for targeted delivery of anticancer therapeutics. Commun. Biol. 2020, 3, 95. [Google Scholar] [CrossRef]
- Guo, H.; Tan, S.; Gao, J.; Wang, L. Sequential release of drugs form a dual-delivery system based on pH-responsive nanofibrous mats towards wound care. J. Mater. Chem. B 2020, 8, 1759–1770. [Google Scholar] [CrossRef]
- Rashedi, S.; Heydari, P.; Kharazi, A.Z.; Varshosaz, J.; Sheikholeslam, M. Chitosan/poly (β-amino ester) hydrogel by controlled release of Centella Asiatica promoted wound healing through improved collagen expression and antibacterial and anti-inflammatory properties. Polym. Eng. Sci. 2025, 65, 2418–2435. [Google Scholar] [CrossRef]
- Lv, L.; Cheng, W.; Wang, S.; Lin, S.; Dang, J.; Ran, Z.; Zhu, H.; Xu, W.; Huang, Z.; Xu, P. Poly (β-amino ester) dual-drug-loaded hydrogels with antibacterial and osteogenic properties for bone repair. ACS Biomater. Sci. Eng. 2023, 9, 1976–1990. [Google Scholar] [CrossRef]
- Chu, S.; Shi, X.; Tian, Y.; Gao, F. pH-responsive polymer nanomaterials for tumor therapy. Front. Oncol. 2022, 12, 855019. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lin, J.; Liu, C.; Zhang, Q.; Li, R.; Wang, C.; Zhao, C.; Lu, L.; Zhou, C.; Tian, J. Temperature-and pH-responsive injectable chitosan hydrogels loaded with doxorubicin and curcumin as long-lasting release platforms for the treatment of solid tumors. Front. Bioeng. Biotechnol. 2022, 10, 1043939. [Google Scholar] [CrossRef] [PubMed]
- Verkhovskii, R.A.; Ivanov, A.N.; Lengert, E.V.; Tulyakova, K.A.; Shilyagina, N.Y.; Ermakov, A.V. Current principles, challenges, and new metrics in pH-responsive drug delivery systems for systemic cancer therapy. Pharmaceutics 2023, 15, 1566. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, Z.; Zhu, J.; Wen, Y.; Zhao, F.; Lei, L.; Phan-Thien, N.; Khoo, B.C.; Li, J. Thermoresponsive hydrogel induced by dual supramolecular assemblies and its controlled release property for enhanced anticancer drug delivery. Biomacromolecules 2020, 21, 1516–1527. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Armelin, E. Poly (N-isopropylacrylamide) and copolymers: A review on recent progresses in biomedical applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef]
- Xing, Y.; Zeng, B.; Yang, W. Light responsive hydrogels for controlled drug delivery. Front. Bioeng. Biotechnol. 2022, 10, 1075670. [Google Scholar] [CrossRef]
- Mohan, A.; Santhamoorthy, M.; Phan, T.T.V.; Kim, S.-C. pNIPAm-Based pH and Thermoresponsive Copolymer Hydrogel for Hydrophobic and Hydrophilic Drug Delivery. Gels 2024, 10, 184. [Google Scholar] [CrossRef]
- Liu, L.; Sun, X.; Yan, Z.; Ye, B. NIR responsive AuNR/pNIPAM/PEGDA inverse opal hydrogel microcarriers for controllable drug delivery. New J. Chem. 2021, 45, 7893–7899. [Google Scholar] [CrossRef]
- Szwed, M.; Marczak, A. Application of Nanoparticles for Magnetic Hyperthermia for Cancer Treatment—The Current State of Knowledge. Cancers 2024, 16, 1156. [Google Scholar] [CrossRef]
- Wei, Y.; Lv, J.; Zhu, S.; Wang, S.; Su, J.; Xu, C. Enzyme-responsive liposomes for controlled drug release. Drug Discov. Today 2024, 29, 104014. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, F.; Chen, W.; Gong, T.; Zhou, Y.; Dai, X.; Leung, W.; Xu, C. Enzyme-responsive materials as carriers for improving photodynamic therapy. Front. Chem. 2021, 9, 763057. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhao, W.; Miao, Z.; Wang, J.; Ma, Y.; Wu, H.; Sun, T.; Qian, H.; Zha, Z. Folin–Ciocalteu assay inspired polyoxometalate nanoclusters as a renal clearable agent for non-inflammatory photothermal cancer therapy. ACS Nano 2020, 14, 2126–2136. [Google Scholar] [CrossRef]
- Shi, L.; Hu, Y.; Lin, A.; Ma, C.; Zhang, C.; Su, Y.; Zhou, L.; Niu, Y.; Zhu, X. Matrix metalloproteinase responsive nanoparticles for synergistic treatment of colorectal cancer via simultaneous anti-angiogenesis and chemotherapy. Bioconjug. Chem. 2016, 27, 2943–2953. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, M.; Li, D.; Li, C.; Luo, C.; Wang, Z.; Zhang, W.; Yang, Z.; Feng, Y.; Wang, S. Cytochrome P450 enzyme-mediated auto-enhanced photodynamic cancer therapy of co-nanoassembly between clopidogrel and photosensitizer. Theranostics 2020, 10, 5550–5564. [Google Scholar] [CrossRef]
- Zhang, R.X.; Dong, K.; Wang, Z.; Miao, R.; Lu, W.; Wu, X.Y. Nanoparticulate Drug Delivery Strategies to Address Intestinal Cytochrome P450 CYP3A4 Metabolism towards Personalized Medicine. Pharmaceutics 2021, 13, 1261. [Google Scholar] [CrossRef]
- Minehan, R.L.; Del Borgo, M.P. Controlled release of therapeutics from enzyme-responsive biomaterials. Front. Biomater. Sci. 2022, 1, 916985. [Google Scholar] [CrossRef]
- Wang, Z.; Li, R.; Zhang, J. On-demand drug delivery of triptolide and celastrol by poly (lactic-co-glycolic acid) nanoparticle/triglycerol monostearate-18 hydrogel composite for rheumatoid arthritis treatment. Adv. Compos. Hybrid Mater. 2022, 5, 2921–2935. [Google Scholar] [CrossRef]
- He, Y.; Lei, L.; Cao, J.; Yang, X.; Cai, S.; Tong, F.; Huang, D.; Mei, H.; Luo, K.; Gao, H. A combinational chemo-immune therapy using an enzyme-sensitive nanoplatform for dual-drug delivery to specific sites by cascade targeting. Sci. Adv. 2021, 7, eaba0776. [Google Scholar] [CrossRef]
- Zhou, W.; Duan, Z.; Zhao, J.; Fu, R.; Zhu, C.; Fan, D. Glucose and MMP-9 dual-responsive hydrogel with temperature sensitive self-adaptive shape and controlled drug release accelerates diabetic wound healing. Bioact. Mater. 2022, 17, 1–17. [Google Scholar] [CrossRef]
- Xu, C.; Yu, Y.; Sun, Y.; Kong, L.; Yang, C.; Hu, M.; Yang, T.; Zhang, J.; Hu, Q.; Zhang, Z. Transformable nanoparticle-enabled synergistic elicitation and promotion of immunogenic cell death for triple-negative breast cancer immunotherapy. Adv. Funct. Mater. 2019, 29, 1905213. [Google Scholar] [CrossRef]
- Shi, C.; Zhou, X.; Zhao, Q.; Zhang, Z.; Ma, H.; Lu, Y.; Huang, Z.; Sun, W.; Du, J.; Fan, J. CD44-specific targeting nanoreactors with glutathione depletion for magnifying photodynamic tumor eradication. CCS Chem. 2022, 4, 2662–2673. [Google Scholar] [CrossRef]
- Desideri, E.; Ciccarone, F.; Ciriolo, M.R. Targeting glutathione metabolism: Partner in crime in anticancer therapy. Nutrients 2019, 11, 1926. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Wang, X.; Zhang, D.; Mu, X. Research progress of disulfide bond based tumor microenvironment targeted drug delivery system. Int. J. Nanomed. 2024, 19, 7547–7566. [Google Scholar] [CrossRef]
- Fu, S.; Rempson, C.M.; Puche, V.; Zhao, B.; Zhang, F. Construction of disulfide containing redox-responsive polymeric nanomedicine. Methods 2022, 199, 67–79. [Google Scholar] [CrossRef]
- Parodi, A.; Rudzinska, M.; Leporatti, S.; Anissimov, Y.; Zamyatnin, A.A., Jr. Smart nanotheranostics responsive to pathological stimuli. Front. Bioeng. Biotechnol. 2020, 8, 503. [Google Scholar] [CrossRef]
- Mirhadi, E.; Mashreghi, M.; Maleki, M.F.; Alavizadeh, S.H.; Arabi, L.; Badiee, A.; Jaafari, M.R. Redox-sensitive nanoscale drug delivery systems for cancer treatment. Int. J. Pharm. 2020, 589, 119882. [Google Scholar] [CrossRef]
- Xu, X.; Xu, J.; Sun, Z.; Tetiana, D. Cyclodextrin-grafted redox-responsive hydrogel mediated by disulfide bridges for regulated drug delivery. Des. Monomers Polym. 2024, 27, 21–34. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Zhang, X.; Wei, X.; Xiong, X.; Zhou, S. Enzyme and redox dual-triggered intracellular release from actively targeted polymeric micelles. ACS Appl. Mater. Interfaces 2017, 9, 3388–3399. [Google Scholar] [CrossRef]
- Luo, Y.; Yin, X.; Yin, X.; Chen, A.; Zhao, L.; Zhang, G.; Liao, W.; Huang, X.; Li, J.; Zhang, C.Y. Dual pH/redox-responsive mixed polymeric micelles for anticancer drug delivery and controlled release. Pharmaceutics 2019, 11, 176. [Google Scholar] [CrossRef]
- Elmas, A.; Akyüz, G.; Bergal, A.; Andaç, M.; Andaç, Ö. Mathematical modelling of drug release. Res. Eng. Struct. Mater. 2020, 6, 327–350. [Google Scholar] [CrossRef]
- Sahai, N.; Gogoi, M.; Ahmad, N. Mathematical modeling and simulations for developing nanoparticle-based cancer drug delivery systems: A review. Curr. Pathobiol. Rep. 2021, 9, 1–8. [Google Scholar] [CrossRef]
- Mansha, S.; Sajjad, A.; Zarbab, A.; Afzal, T.; Kanwal, Z.; Iqbal, M.J.; Raza, M.A.; Ali, S. Development of pH-Responsive, thermosensitive, antibacterial, and anticancer CS/PVA/Graphene blended hydrogels for controlled drug delivery. Gels 2024, 10, 205. [Google Scholar] [CrossRef]
- Hsu, X.-L.; Wu, L.-C.; Hsieh, J.-Y.; Huang, Y.-Y. Nanoparticle-Hydrogel Composite Drug Delivery System for Potential Ocular Applications. Polymers 2021, 13, 642. [Google Scholar] [CrossRef]
- Pontrelli, G.; Toniolo, G.; McGinty, S.; Peri, D.; Succi, S.; Chatgilialoglu, C. Mathematical modelling of drug delivery from pH-responsive nanocontainers. Comput. Biol. Med. 2021, 131, 104238. [Google Scholar] [CrossRef]
- Marriott, R.; Spiridonova, T.; Tverdokhlebov, S.; Anissimov, Y. Using compartments to model drug delivery from biodegradable polymers. J. Pharm. Sci. 2022, 111, 3096–3107. [Google Scholar] [CrossRef]
- Morris, A.H.; Mahal, R.S.; Udell, J.; Wu, M.; Kyriakides, T.R. Multicompartment drug release system for dynamic modulation of tissue responses. Adv. Healthc. Mater. 2017, 6, 1700370. [Google Scholar] [CrossRef]
- Sungkhaphan, P.; Thavornyutikarn, B.; Muangsanit, P.; Kaewkong, P.; Kitpakornsanti, S.; Pornsuwan, S.; Singhatanadgit, W.; Janvikul, W. Dual-Functional Drug Delivery System for Bisphosphonate-Related Osteonecrosis Prevention and Its Bioinspired Releasing Model and In Vitro Assessment. ACS Omega 2023, 8, 26561–26576. [Google Scholar] [CrossRef]
- Sun, Y.; Qin, S.; Li, Y.; Hasan, N.; Li, Y.V.; Liu, J. Machine learning integrated with in vitro experiments for study of drug release from PLGA nanoparticles. Sci. Rep. 2025, 15, 4218. [Google Scholar] [CrossRef]
- Sheth, S.; Barnard, E.; Hyatt, B.; Rathinam, M.; Zustiak, S.P. Predicting drug release from degradable hydrogels using fluorescence correlation spectroscopy and mathematical modeling. Front. Bioeng. Biotechnol. 2019, 7, 410. [Google Scholar] [CrossRef]
- Ilgin, P.; Ozay, H.; Ozay, O. A new dual stimuli responsive hydrogel: Modeling approaches for the prediction of drug loading and release profile. Eur. Polym. J. 2019, 113, 244–253. [Google Scholar] [CrossRef]
- Tan, W.; Long, T.; Wan, Y.; Li, B.; Xu, Z.; Zhao, L.; Mu, C.; Ge, L.; Li, D. Dual-drug loaded polysaccharide-based self-healing hydrogels with multifunctionality for promoting diabetic wound healing. Carbohydr. Polym. 2023, 312, 120824. [Google Scholar] [CrossRef] [PubMed]
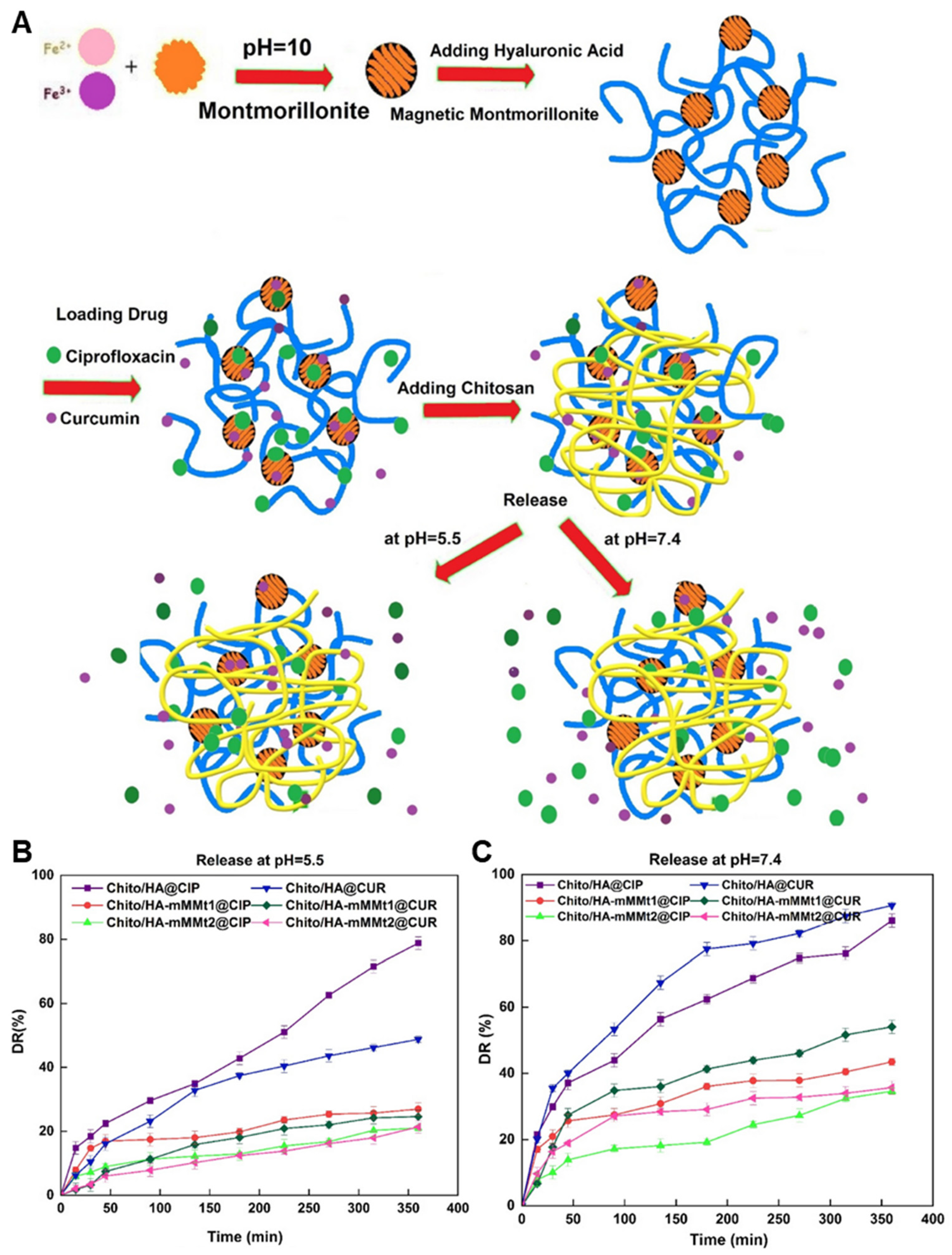
- Sayyar, Z.; Mahdavinia, G.R.; Khataee, A. Dual-drug (Curcumin/Ciprofloxacin) loading and release from chitosan-based hydrogels embedded with magnetic Montmorillonite/Hyaluronic acid for enhancing wound healing. J. Biol. Eng. 2023, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.J.; Heydari, P.; Javaherchi, P.; Kharazi, A.Z.; Zarrabi, A. Alginate-based nanocomposite incorporating chitosan nanoparticles: A dual-drug delivery system for infection control and wound regeneration. J. Drug Deliv. Sci. Technol. 2025, 107, 106755. [Google Scholar] [CrossRef]
- Winkler, P.; Mao, Y. Dual Delivery of Cells and Bioactive Molecules for Wound Healing Applications. Molecules 2025, 30, 1577. [Google Scholar] [CrossRef]
- Nascimento, S.d.P.D.; de Souza, R.R.M.; Sobral, M.V.; Xavier-Junior, F.H.; da Silva, M.V.S.; Viana, M.M.; da Silva, F.F.; Serpe, M.J.; de Souza, A.L. Gelatin-Oxidized Alginate and Chitosan-Coated Zein Nanoparticle Hydrogel Composite to Enhance Breast Cancer Cytotoxicity in Dual-Drug Delivery. ACS Omega 2024, 9, 45190–45202. [Google Scholar] [CrossRef]
- Liu, Z.; Koseki, Y.; Suzuki, R.; Dao, A.T.N.; Kasai, H. Sustained Drug Release from Dual-Responsive Hydrogels for Local Cancer Chemo–Photothermal Therapy. Macromol. Biosci. 2025, 25, 2400413. [Google Scholar] [CrossRef]
- Sheu, M.-T.; Jhan, H.-J.; Su, C.-Y.; Chen, L.-C.; Chang, C.-E.; Liu, D.-Z.; Ho, H.-O. Codelivery of doxorubicin-containing thermosensitive hydrogels incorporated with docetaxel-loaded mixed micelles enhances local cancer therapy. Colloids Surf. B Biointerfaces 2016, 143, 260–270. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Lin, C.-T. Injectable alginate complex hydrogel loaded with dual-drug nanovectors offers effective photochemotherapy against triple-negative breast cancer. Biomacromolecules 2024, 25, 2041–2051. [Google Scholar] [CrossRef]
- García-Alvarez, R.; Izquierdo-Barba, I.; Vallet-Regí, M. 3D scaffold with effective multidrug sequential release against bacteria biofilm. Acta Biomater. 2017, 49, 113–126. [Google Scholar] [CrossRef]
- Guo, J.; Yao, L.; Wang, X.; Song, R.; Yang, B.; Jin, D.; Guo, J.; Wu, G. Dual-Responsive Antibacterial Hydrogel Patch for Chronic-Infected Wound Healing. Biomacromolecules 2024, 25, 7283–7297. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, R.; Lu, Y.; Liu, M.; Mo, R. Immuno-protective vesicle-crosslinked hydrogel for allogenic transplantation. Nat. Commun. 2024, 15, 5176. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Krishnan, N.; Heo, J.; Fang, R.H.; Zhang, L. Nanoparticle–hydrogel superstructures for biomedical applications. J. Control. Release 2020, 324, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Kass, L.E.; Nguyen, J. Nanocarrier-hydrogel composite delivery systems for precision drug release. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1756. [Google Scholar] [CrossRef]
- Mei, E.; Chen, C.; Li, C.; Ding, X.; Chen, J.; Xi, Q.; Zhou, S.; Liu, J.; Li, Z. Injectable and Biodegradable Chitosan Hydrogel-Based Drug Depot Contributes to Synergistic Treatment of Tumors. Biomacromolecules 2021, 22, 5339–5348. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jeon, S.I.; Sim, S.B.; Byun, Y.; Ahn, C.-H. A supramolecular host-guest interaction-mediated injectable hydrogel system with enhanced stability and sustained protein release. Acta Biomater. 2021, 131, 286–301. [Google Scholar] [CrossRef]
- Cruz, L.J.; van Dijk, T.; Vepris, O.; Li, T.M.; Schomann, T.; Baldazzi, F.; Kurita, R.; Nakamura, Y.; Grosveld, F.; Philipsen, S. PLGA-nanoparticles for intracellular delivery of the CRISPR-complex to elevate fetal globin expression in erythroid cells. Biomaterials 2021, 268, 120580. [Google Scholar] [CrossRef]
- Tracey, S.R.; Smyth, P.; Herron, U.M.; Burrows, J.F.; Porter, A.J.; Barelle, C.J.; Scott, C.J. Development of a cationic polyethyleneimine-poly (lactic-co-glycolic acid) nanoparticle system for enhanced intracellular delivery of biologics. RSC Adv. 2023, 13, 33721–33735. [Google Scholar] [CrossRef]
- Xie, H.; Liu, C.; Gao, J.; Shi, J.; Ni, F.; Luo, X.; He, Y.; Ren, G.; Luo, Z. Fabrication of Zein-Lecithin-EGCG complex nanoparticles: Characterization, controlled release in simulated gastrointestinal digestion. Food Chem. 2021, 365, 130542. [Google Scholar] [CrossRef]
- She, W.; Li, H.; Wang, Z.; Liu, T.; Zhao, D.; Guo, Z.; Liu, Y.; Liu, Y. Site-specific controlled-release nanoparticles for immune reprogramming via dual metabolic inhibition against triple-negative breast cancer. J. Control. Release 2024, 366, 204–220. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan-alginate nanoparticles as effective oral carriers to improve the stability, bioavailability, and cytotoxicity of curcumin diethyl disuccinate. Carbohydr. Polym. 2021, 256, 117426. [Google Scholar] [CrossRef]
- Delgado-Pujol, E.J.; Martínez, G.; Casado-Jurado, D.; Vázquez, J.; León-Barberena, J.; Rodríguez-Lucena, D.; Torres, Y.; Alcudia, A.; Begines, B. Hydrogels and Nanogels: Pioneering the Future of Advanced Drug Delivery Systems. Pharmaceutics 2025, 17, 215. [Google Scholar] [CrossRef] [PubMed]
- Perrigue, P.M.; Murray, R.A.; Mielcarek, A.; Henschke, A.; Moya, S.E. Degradation of drug delivery nanocarriers and payload release: A review of physical methods for tracing nanocarrier biological fate. Pharmaceutics 2021, 13, 770. [Google Scholar] [CrossRef]
- Bhattacharjee, S. Craft of co-encapsulation in nanomedicine: A struggle to achieve synergy through reciprocity. ACS Pharmacol. Transl. Sci. 2022, 5, 278–298. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Liu, G.; Wang, L.; Yang, Q.; Liao, F.; Yang, X.; Xiao, B.; Duan, L. Synthesis and Properties of Injectable Hydrogel for tissue filling. Pharmaceutics 2024, 16, 430. [Google Scholar] [CrossRef]
- Pawelec, K.M.; Hix, J.M.; Shapiro, E.M. Biomaterial Degradation Products affect Regenerating Glia Independently of Surface Properties. bioRxiv 2024. [Google Scholar] [CrossRef]
- Mehta, M.; Bui, T.A.; Yang, X.; Aksoy, Y.; Goldys, E.M.; Deng, W. Lipid-based nanoparticles for drug/gene delivery: An overview of the production techniques and difficulties encountered in their industrial development. ACS Mater. Au 2023, 3, 600–619. [Google Scholar] [CrossRef]
- Vieira, C.C.; Peltonen, L.; Karttunen, A.; Ribeiro, A. Is it advantageous to use quality by design (QbD) to develop nanoparticle-based dosage forms for parenteral drug administration? Int. J. Pharm. 2024, 657, 124163. [Google Scholar] [CrossRef]
- Zheng, C.; Li, M.; Ding, J. Challenges and opportunities of nanomedicines in clinical translation. Bio Integr. 2021, 2, 57. [Google Scholar] [CrossRef]
- Bae, Y.H.; Park, K. Advanced drug delivery 2020 and beyond: Perspectives on the future. Adv. Drug Deliv. Rev. 2020, 158, 4–16. [Google Scholar] [CrossRef]
- Almawash, S.; Osman, S.K.; Mustafa, G.; El Hamd, M.A. Current and Future Prospective of Injectable Hydrogels—Design Challenges and Limitations. Pharmaceuticals 2022, 15, 371. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Bita, B. Exploring the potential of artificial intelligence for hydrogel development—A short review. Gels 2023, 9, 845. [Google Scholar] [CrossRef] [PubMed]
- Al-Rajabi, M.M.; Alzyod, S.; Patel, A.; Teow, Y.H. A hybrid machine learning framework for predicting drug-release profiles, kinetics, and mechanisms of temperature-responsive hydrogels. Polym. Bull. 2025, 82, 2911–2932. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.; Hui, Y.; Ranaweera, S.; Zhao, C.X. Microfluidic nanoparticles for drug delivery. Small 2022, 18, 2106580. [Google Scholar] [CrossRef]
- Chakraborty, A.; Roy, A.; Ravi, S.P.; Paul, A. Exploiting the role of nanoparticles for use in hydrogel-based bioprinting applications: Concept, design, and recent advances. Biomater. Sci. 2021, 9, 6337–6354. [Google Scholar] [CrossRef]
- Pandya, A.K.; Vora, L.K.; Umeyor, C.; Surve, D.; Patel, A.; Biswas, S.; Patel, K.; Patravale, V.B. Polymeric in situ forming depots for long-acting drug delivery systems. Adv. Drug Deliv. Rev. 2023, 200, 115003. [Google Scholar] [CrossRef]
- Liu, X.; Hu, Y.; Ju, Y.; Yang, P.; Shen, N.; Yang, A.; Wu, R.; Fang, B.; Liu, L. Immunomodulatory hydrogels for tissue repair and regeneration. APL Mater. 2024, 12, 080603. [Google Scholar] [CrossRef]
- Wang, C.-y.; Qin, Z.-x.; Wei, Y.; Hao, J.-x.; Zhu, Y.-f.; Zhao, F.; Jiao, K.; Ehrlich, H.; Tay, F.R.; Niu, L.-n. The immunomodulatory effects of RNA-based biomaterials on bone regeneration. Acta Biomater. 2023, 162, 32–43. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, F.; Zeng, Y.; Lin, L.; Yu, H.; Zhang, S.; Yang, W. Hydrogel systems for spatiotemporal controlled delivery of immunomodulators: Engineering the tumor immune microenvironment for enhanced cancer immunotherapy. Front. Cell Dev. Biol. 2024, 12, 1514595. [Google Scholar] [CrossRef]
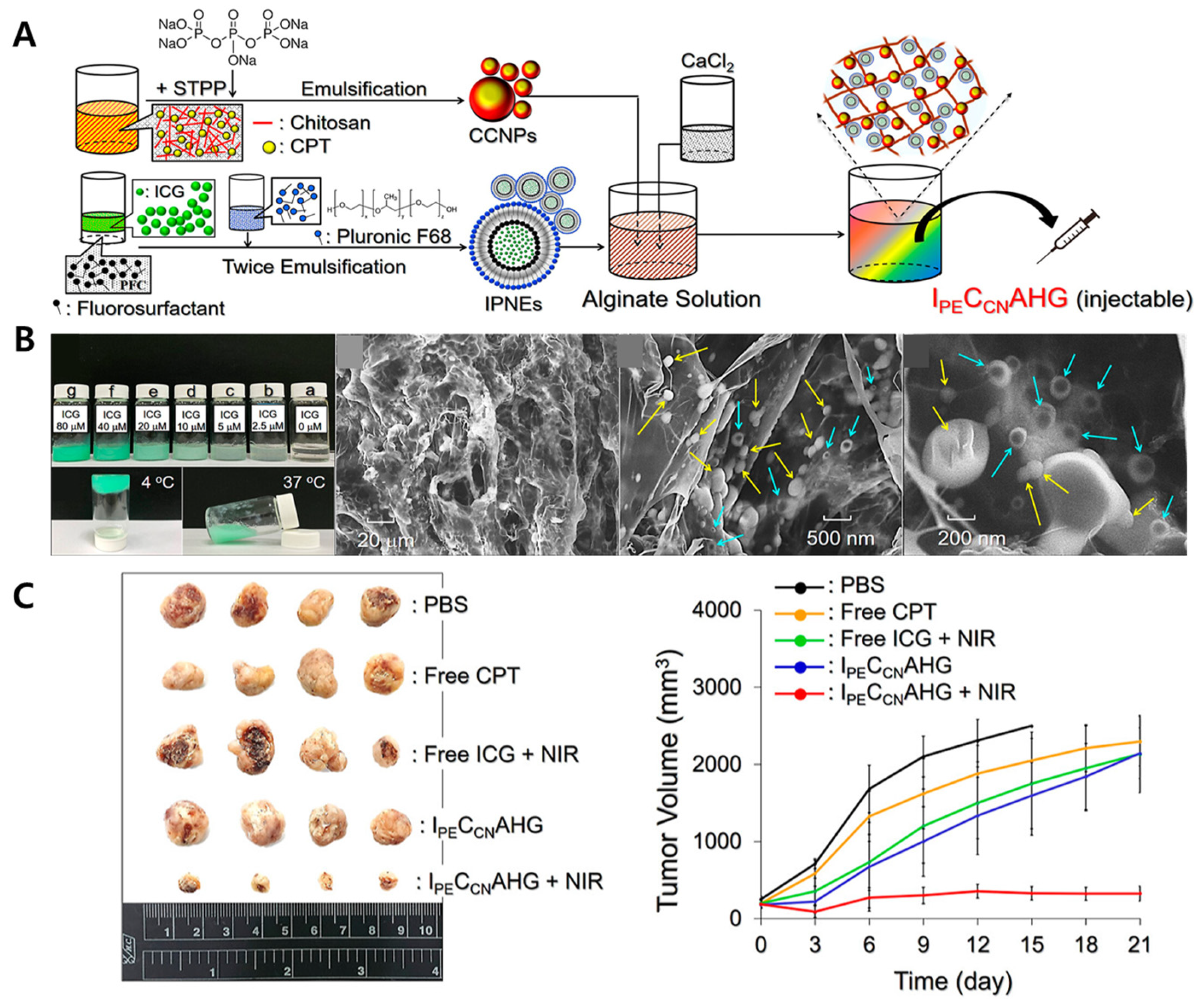
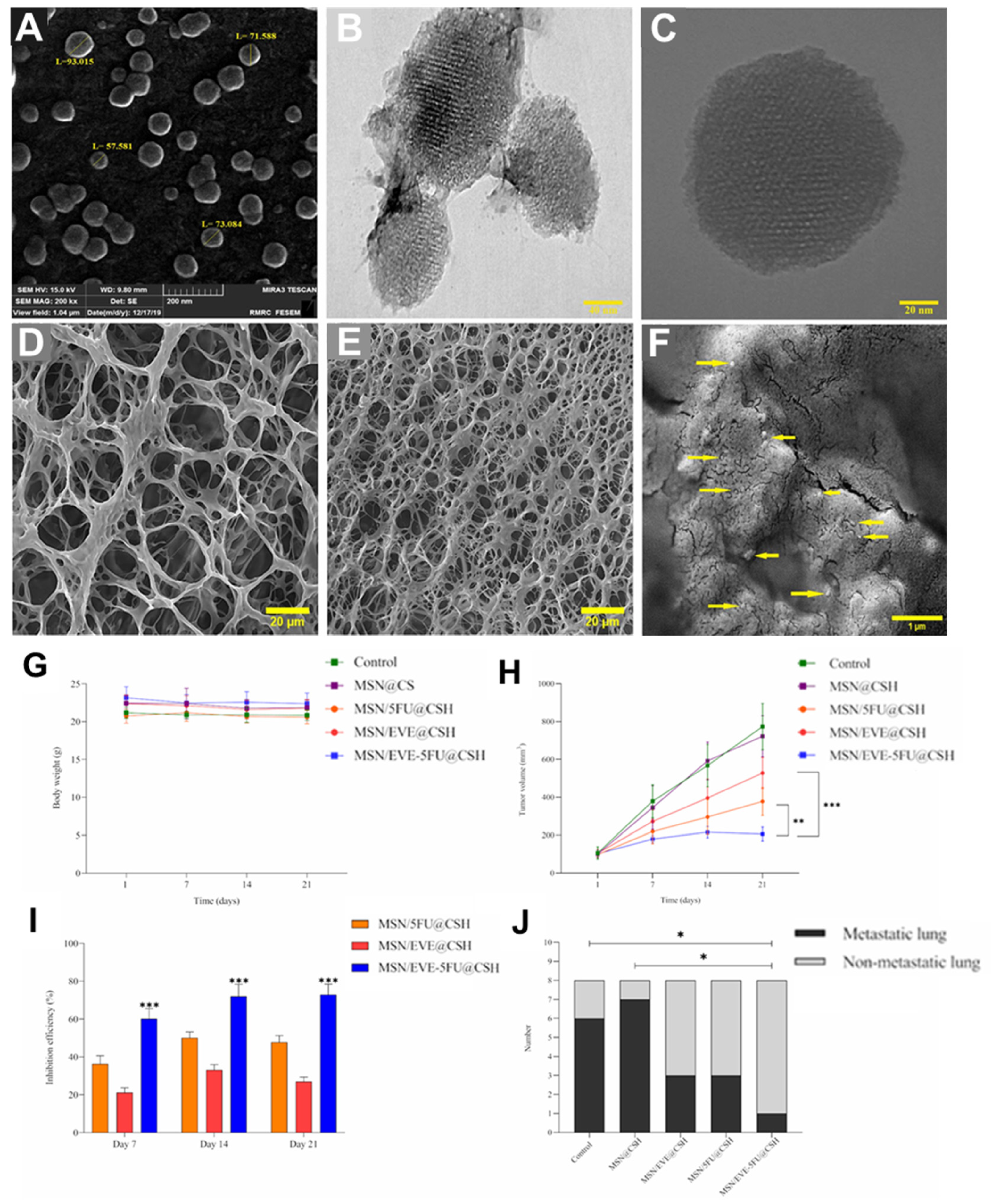
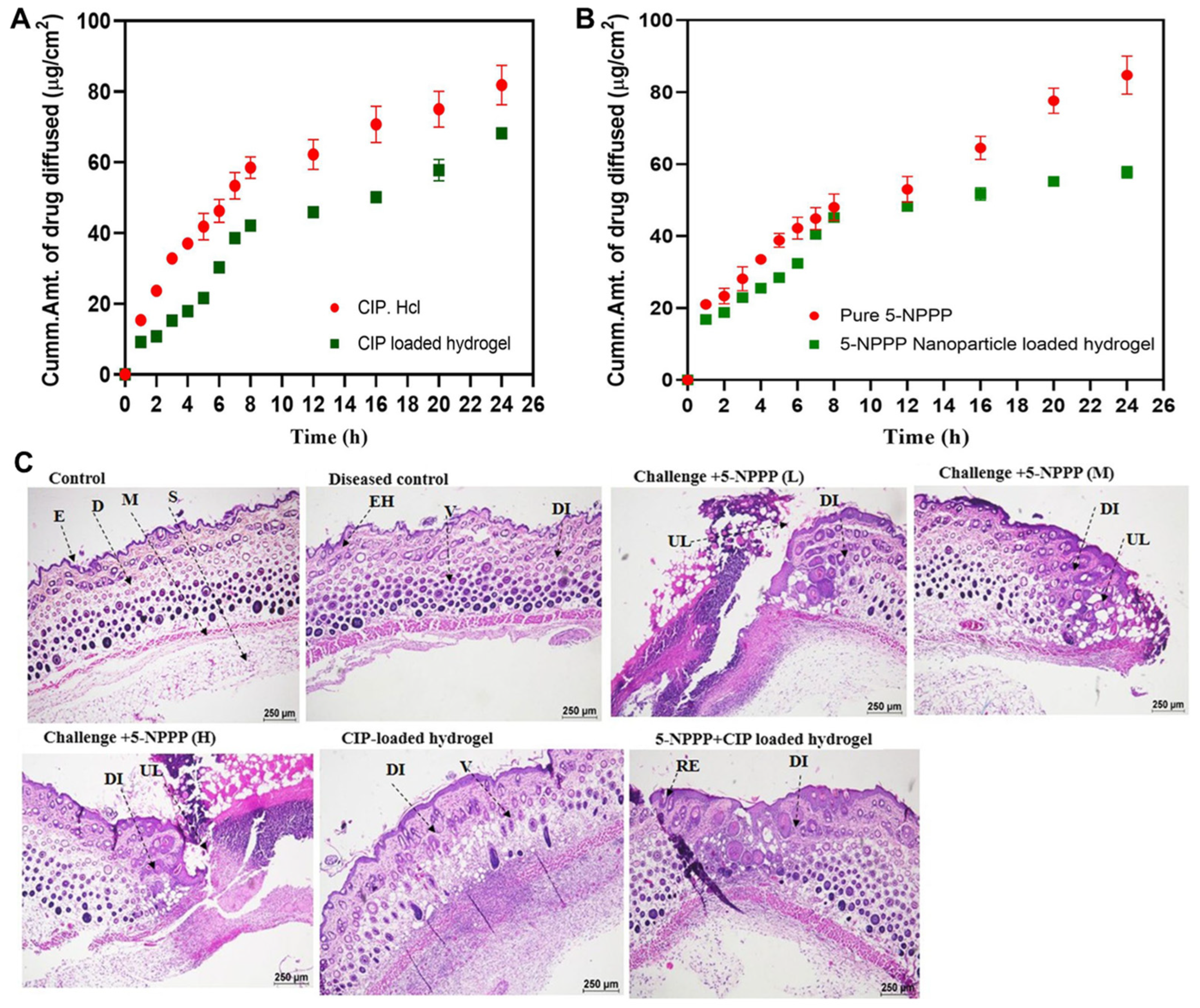
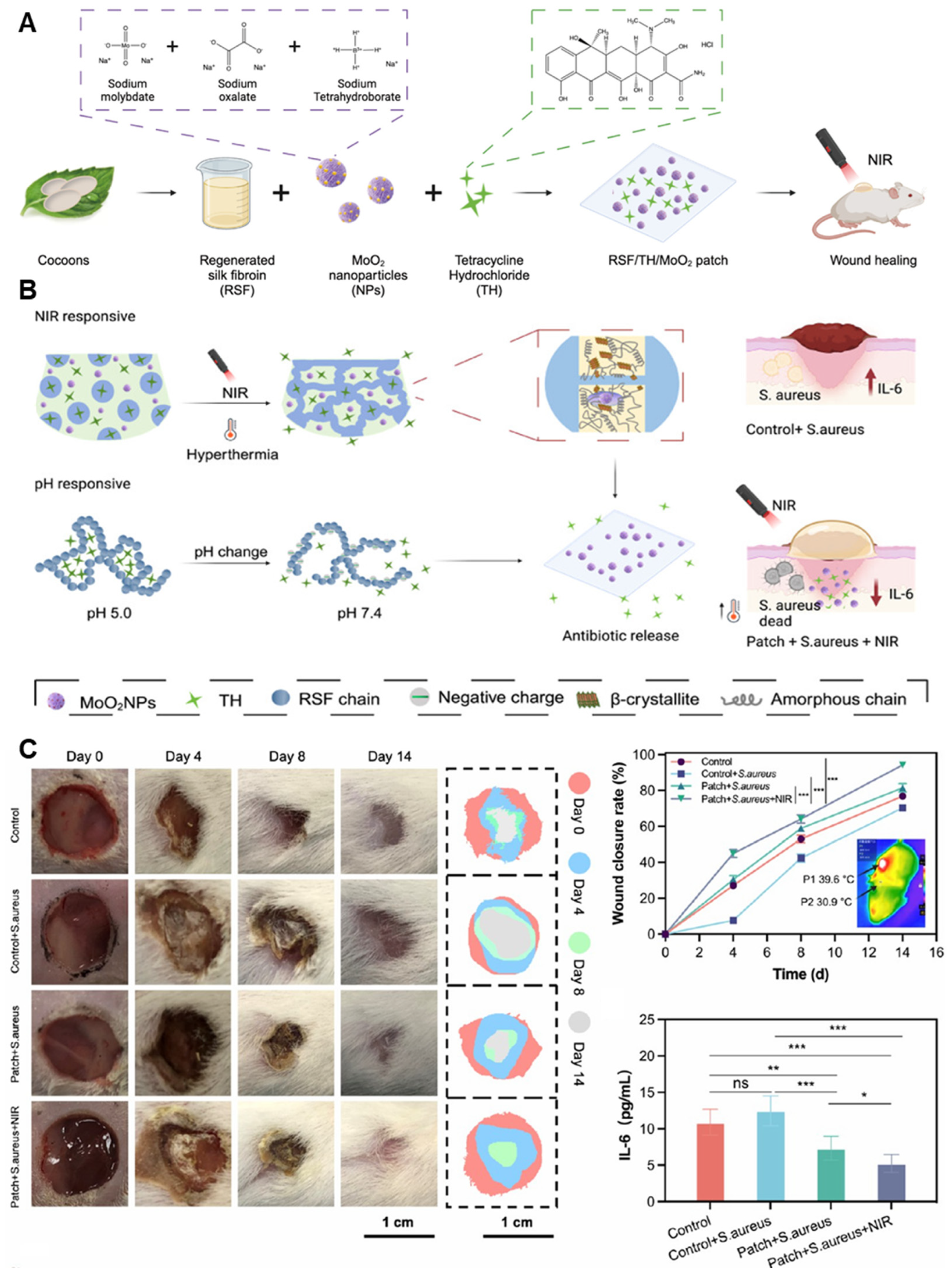

| Key Biomedical Applications | Used Hydrogel | Used NPs | Formulation Strategy | Authors |
|---|---|---|---|---|
| Wound Healing | N-carboxyethyl chitosan Oxidized sodium alginate | Mesoporous polydopamine | Physical Embedding | Tan et al. [204] |
| Chitosan | Magnetic montmorillonite | Physical Embedding | Sayyar et al. [205] | |
| Sodium alginate | Chitosan | Physical Embedding | Mousavi et al. [206] | |
| Polyethylene glycol diacrylate Thiolated alginate | Chitosan Gelatin | Layer-by-Layer Assembly | Zhang et al. [54] | |
| Gelatin methacrylate | Gelatin | Physical Embedding | Winkler et al. [207] | |
| Cancer Therapy | Oxidized alginate Gelatin | Chitosan | Physical Embedding | Nascimento et al. [208] |
| Poly(n-isopropylacrylamide) | Polydopamine Cholesterol | Covalent Integration Physical Embedding | Liu et al. [209] | |
| Pluronic F127 | Pluronic F127 Pluronic L121 | Physical Embedding | Sheu et al. [210] | |
| Sodium alginate | Pluronic F68 Chitosan | Physical Embedding | Lee et al. [211] | |
| Chitosan | Mesoporous silica | Physical Embedding | Arvejeh et al. [60] | |
| Infection Control | Polyvinyl alcohol | Eudragit RSPO | Physical Embedding | Thamilselvan et al. [31] |
| Gelatin methacrylate | PLGA | Layer-by-Layer Assembly | Martínez-Pérez et al. [114] | |
| Pluronic F127 Polyvinyl alcohol Gelatin–glutaraldehyde | Apatite | Layer-by-Layer Assembly | García-Alvarez et al. [212] | |
| Silk fibroin | Molybdenum dioxide | Physical Embedding | Guo. et al. [213] | |
| Transplant Immunosuppression | Cationic peptide | Methoxy poly(ethylene glycol)-poly(ε-caprolactone) | Physical Embedding | Xu et al. [83] |
| Thiol-terminated PEGylated phosphatidylethanolamine | Acylated hyaluronic acid | Covalent Integration | Wang et al. [214] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, M.S.; Lee, J.M.; Jo, M.J.; Kang, S.J.; Yoo, M.K.; Park, S.Y.; Bong, S.; Park, C.-S.; Park, C.-W.; Kim, J.-S.; et al. Dual-Drug Delivery Systems Using Hydrogel–Nanoparticle Composites: Recent Advances and Key Applications. Gels 2025, 11, 520. https://doi.org/10.3390/gels11070520
Yoon MS, Lee JM, Jo MJ, Kang SJ, Yoo MK, Park SY, Bong S, Park C-S, Park C-W, Kim J-S, et al. Dual-Drug Delivery Systems Using Hydrogel–Nanoparticle Composites: Recent Advances and Key Applications. Gels. 2025; 11(7):520. https://doi.org/10.3390/gels11070520
Chicago/Turabian StyleYoon, Moon Sup, Jae Min Lee, Min Jeong Jo, Su Jeong Kang, Myeong Kyun Yoo, So Yeon Park, Sunghyun Bong, Chan-Su Park, Chun-Woong Park, Jin-Seok Kim, and et al. 2025. "Dual-Drug Delivery Systems Using Hydrogel–Nanoparticle Composites: Recent Advances and Key Applications" Gels 11, no. 7: 520. https://doi.org/10.3390/gels11070520
APA StyleYoon, M. S., Lee, J. M., Jo, M. J., Kang, S. J., Yoo, M. K., Park, S. Y., Bong, S., Park, C.-S., Park, C.-W., Kim, J.-S., Han, S.-B., Lee, H. J., & Shin, D. H. (2025). Dual-Drug Delivery Systems Using Hydrogel–Nanoparticle Composites: Recent Advances and Key Applications. Gels, 11(7), 520. https://doi.org/10.3390/gels11070520










