Bioactive Hydrogels for Spinal Cord Injury Repair: Emphasis on Gelatin and Its Derivatives
Abstract
1. Background
Materials and Methods
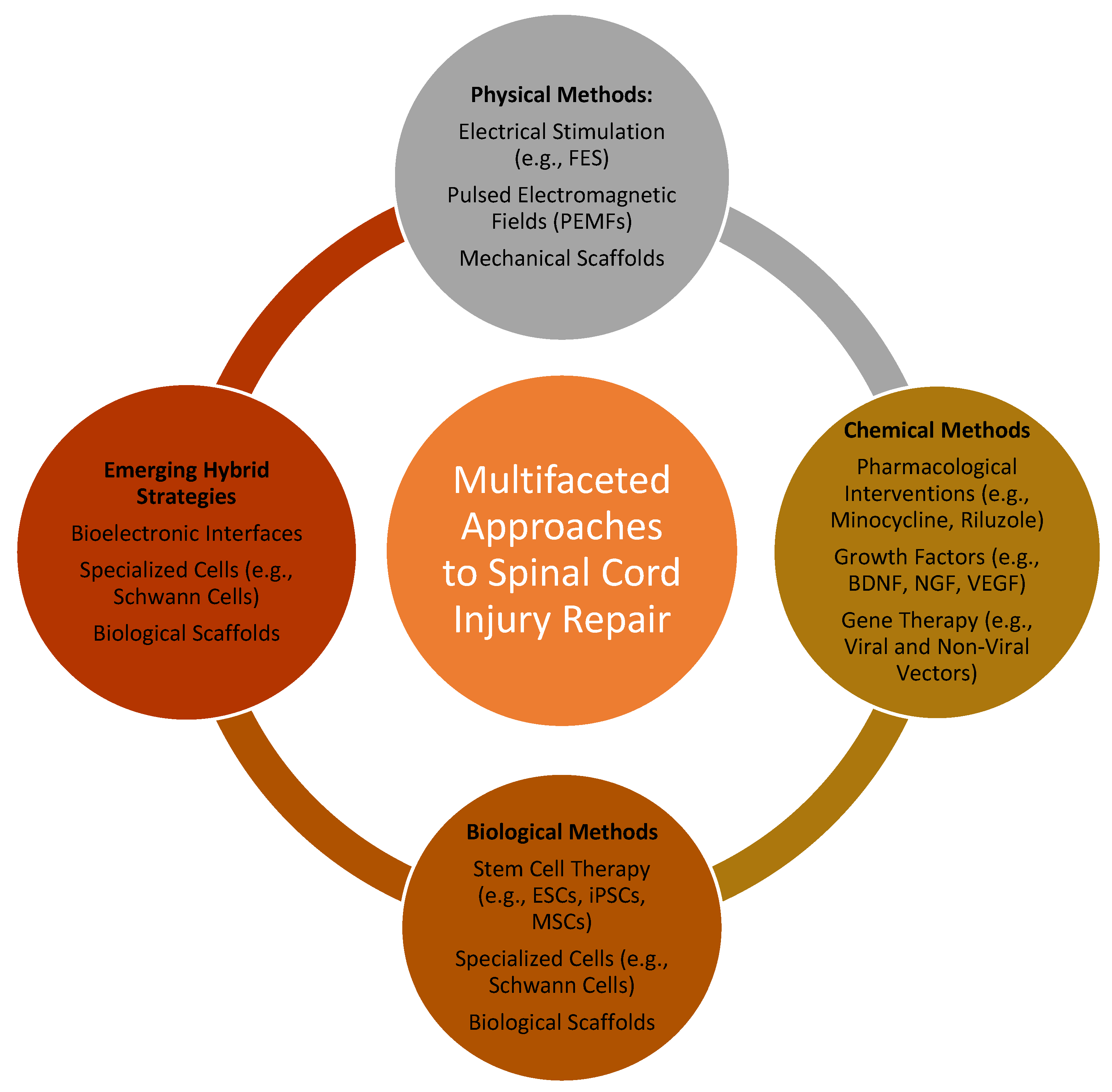
2. Multifaceted Approaches to Spinal Cord Injury Repair: Physical, Chemical, and Biological Strategies
2.1. Physical Methods
2.2. Chemical Methods
2.3. Biological Methods
2.4. Emerging Hybrid Strategies and Advanced Technologies
3. Expanding the Role of Biopolymer Gels in Particle Synthesis and Biomedical Applications
Gelatin-Based Hydrogels in SCI Repair
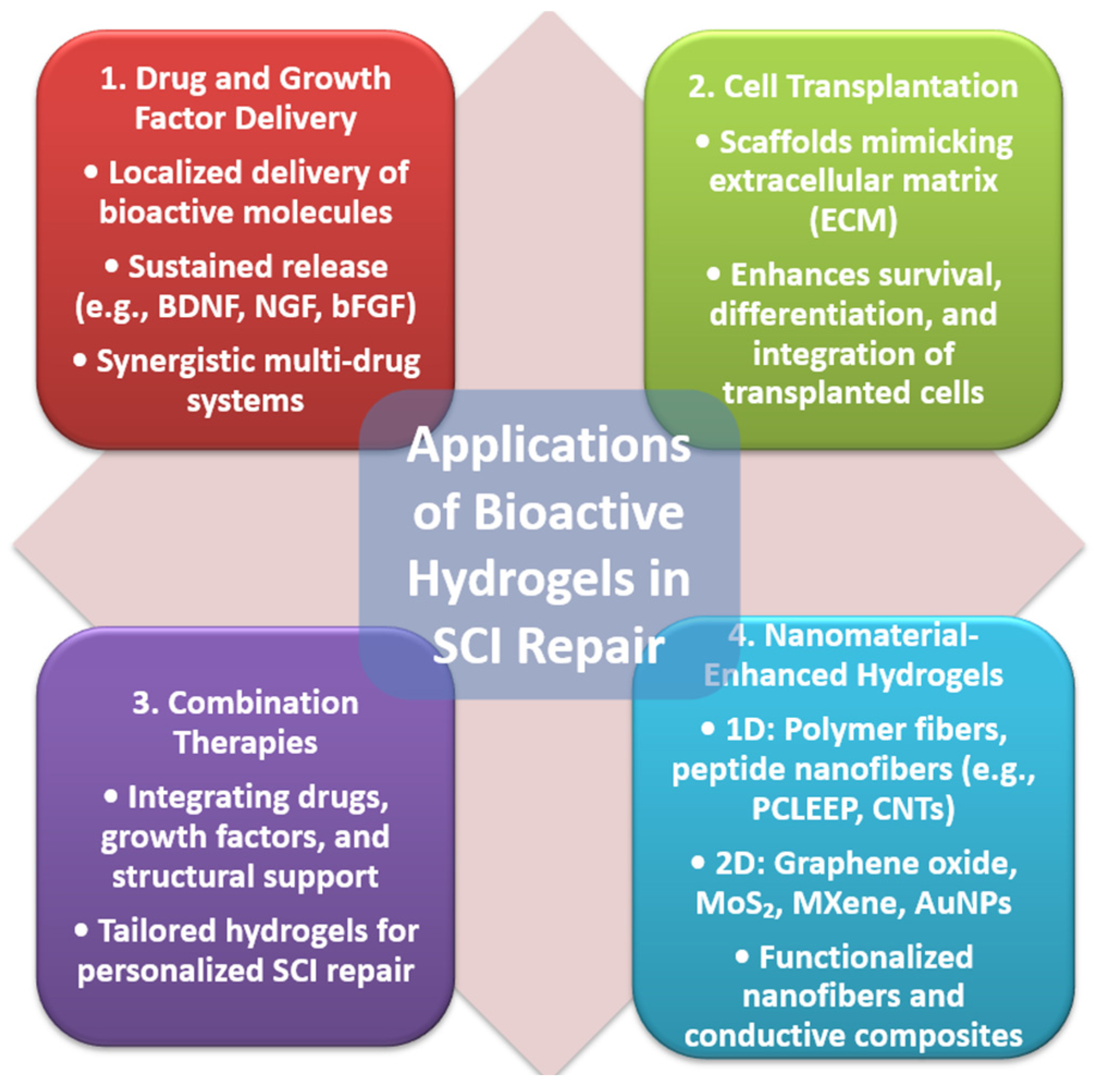
4. Applications of Bioactive Hydrogels in SCI Repair
4.1. Drug and Growth Factor Delivery
4.2. Cell Transplantation
4.3. Combination Therapies
5. Analysis of Publication Trends
6. Costs of Hydrogel Therapy in Spinal Cord Injury: Current Trends and Historical Perspective
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diop, M.; Epstein, D. A Systematic Review of the Impact of Spinal Cord Injury on Costs and Health-Related Quality of Life. PharmacoEcon. Open 2024, 8, 793–808. [Google Scholar] [CrossRef]
- Yari, D.; Saberi, A.; Salmasi, Z.; Ghoreishi, S.A.; Etemad, L.; Movaffagh, J.; Ganjeifar, B. Recent Advances in the Treatment of Spinal Cord Injury. Arch. Bone Jt. Surg. 2024, 12, 380–399. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zhang, S.; Yang, M. Recent progress and challenges in the treatment of spinal cord injury. Protein Cell 2023, 14, 635–652. [Google Scholar] [CrossRef]
- Anjum, A.; Yazid, M.D.; Fauzi Daud, M.; Idris, J.; Ng, A.M.H.; Selvi Naicker, A.; Ismail, O.H.R.; Athi Kumar, R.K.; Lokanathan, Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020, 21, 7533. [Google Scholar] [CrossRef] [PubMed]
- Revete, A.; Aparicio, A.; Cisterna, B.A.; Revete, J.; Luis, L.; Ibarra, E.; Segura González, E.A.; Molino, J.; Reginensi, D. Advancements in the Use of Hydrogels for Regenerative Medicine: Properties and Biomedical Applications. Int. J. Biomater. 2022, 2022, 3606765. [Google Scholar] [CrossRef]
- Omidian, H.; Chowdhury, S.D.; Cubeddu, L.X. Hydrogels for Neural Regeneration: Exploring New Horizons. Materials 2024, 17, 3472. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhu, D.; Zhao, H.; Liu, J.; He, P.; Luan, X.; Hu, H.; Zhang, X.; Wei, G.; Xi, Y. Recent advance in bioactive hydrogels for repairing spinal cord injury: Material design, biofunctional regulation, and applications. J. Nanobiotechnol. 2023, 21, 238. [Google Scholar] [CrossRef]
- Devi, V.K.A.; Shyam, R.; Palaniappan, A.; Jaiswal, A.K.; Oh, T.H.; Nathanael, A.J. Self-Healing Hydrogels: Preparation, Mechanism and Advancement in Biomedical Applications. Polymers 2021, 13, 3782. [Google Scholar] [CrossRef]
- Liu, S.; Schackel, T.; Weidner, N.; Puttagunta, R. Biomaterial-Supported Cell Transplantation Treatments for Spinal Cord Injury: Challenges and Perspectives. Front. Cell. Neurosci. 2017, 11, 430. [Google Scholar] [CrossRef]
- Peressotti, S.; Koehl, G.E.; Goding, J.A.; Green, R.A. Self-Assembling Hydrogel Structures for Neural Tissue Repair. ACS Biomater. Sci. Eng. 2021, 7, 4136–4163. [Google Scholar] [CrossRef]
- Kharbikar, B.N.; Mohindra, P.; Desai, T.A. Biomaterials to enhance stem cell transplantation. Cell Stem Cell 2022, 29, 692–721. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Lu, L.; Hu, L.; Cheng, P.; Hu, Y.; Xie, X.; Dai, G.; Mi, B.; Liu, X.; Liu, G. Recent advances in GelMA hydrogel transplantation for musculoskeletal disorders and related disease treatment. Theranostics 2023, 13, 2015–2039. [Google Scholar] [CrossRef]
- Chen, X.; Ren, L.; Zhang, H.; Hu, Y.; Liao, M.; Shen, Y.; Wang, K.; Cai, J.; Cheng, H.; Guo, J.; et al. Basic fibroblast growth factor-loaded methacrylate gelatin hydrogel microspheres for spinal nerve regeneration. Smart Med. 2023, 2, e20220038. [Google Scholar] [CrossRef] [PubMed]
- Dorrian, R.M.; Berryman, C.F.; Lauto, A.; Leonard, A.V. Electrical stimulation for the treatment of spinal cord injuries: A review of the cellular and molecular mechanisms that drive functional improvements. Front. Cell. Neurosci. 2023, 17, 1095259. [Google Scholar] [CrossRef]
- Khan, M.A.; Fares, H.; Ghayvat, H.; Brunner, I.C.; Puthusserypady, S.; Razavi, B.; Lansberg, M.; Poon, A.; Meador, K.J. A systematic review on functional electrical stimulation based rehabilitation systems for upper limb post-stroke recovery. Front. Neurol. 2023, 14, 1272992. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.L.; Zhou, Y.; McCall, C.E.; Soker, S.; Criswell, T.L. The Use of Pulsed Electromagnetic Field to Modulate Inflammation and Improve Tissue Regeneration: A Review. Bioelectricity 2019, 1, 247–259. [Google Scholar] [CrossRef]
- Hu, X.; Xu, W.; Ren, Y.; Wang, Z.; He, X.; Huang, R.; Ma, B.; Zhao, J.; Zhu, R.; Cheng, L. Spinal cord injury: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 245. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Li, J.; Li, X.; Xiao, J. Fibroblast growth factors in the management of spinal cord injury. J. Cell. Mol. Med. 2018, 22, 25–37. [Google Scholar] [CrossRef]
- Parambi, D.G.T.; Alharbi, K.S.; Kumar, R.; Harilal, S.; Batiha, G.E.; Cruz-Martins, N.; Magdy, O.; Musa, A.; Panda, D.S.; Mathew, B. Gene Therapy Approach with an Emphasis on Growth Factors: Theoretical and Clinical Outcomes in Neurodegenerative Diseases. Mol. Neurobiol. 2022, 59, 191–233. [Google Scholar] [CrossRef]
- Zeng, C.W. Advancing Spinal Cord Injury Treatment through Stem Cell Therapy: A Comprehensive Review of Cell Types, Challenges, and Emerging Technologies in Regenerative Medicine. Int. J. Mol. Sci. 2023, 24, 4349. [Google Scholar] [CrossRef]
- Tang, H.; Li, Y.; Liao, S.; Liu, H.; Qiao, Y.; Zhou, J. Multifunctional Conductive Hydrogel Interface for Bioelectronic Recording and Stimulation. Adv. Healthc. Mater. 2024, 13, e2400562. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Choi, W.S.; Jeong, J.O. A Review of Advanced Hydrogel Applications for Tissue Engineering and Drug Delivery Systems as Biomaterials. Gels 2024, 10, 693. [Google Scholar] [CrossRef]
- Fathi-Achachelouei, M.; Knopf-Marques, H.; Ribeiro da Silva, C.E.; Barthès, J.; Bat, E.; Tezcaner, A.; Vrana, N.E. Use of Nanoparticles in Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2019, 7, 113. [Google Scholar] [CrossRef]
- Kim, D.Y.; Liu, Y.; Kim, G.; An, S.B.; Han, I. Innovative Strategies in 3D Bioprinting for Spinal Cord Injury Repair. Int. J. Mol. Sci. 2024, 25, 9592. [Google Scholar] [CrossRef]
- Zöller, K.; To, D.; Bernkop-Schnürch, A. Biomedical applications of functional hydrogels: Innovative developments, relevant clinical trials and advanced products. Biomaterials 2025, 312, 122718. [Google Scholar] [CrossRef]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the potential of hydrogels for advanced therapeutic applications: Current achievements and future directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res. Pharm. Sci. 2015, 10, 1–16. [Google Scholar] [PubMed]
- Alven, S.; Aderibigbe, B.A. Chitosan-Based Scaffolds Incorporated with Silver Nanoparticles for the Treatment of Infected Wounds. Pharmaceutics 2024, 16, 327. [Google Scholar] [CrossRef]
- Li, Y.; Zou, Z.; An, J.; Liu, X.; Wu, Q.; Sun, J.; Du, J.; Xiong, Y.; Wu, C.; Mei, X.; et al. Folic acid-functionalized chitosan nanoparticles with bioenzyme activity for the treatment of spinal cord injury. Eur. J. Pharm. Sci. 2024, 192, 106667. [Google Scholar] [CrossRef]
- Wang, Z.; Duan, H.; Hao, F.; Hao, P.; Zhao, W.; Gao, Y.; Gu, Y.; Song, J.; Li, X.; Yang, Z. Circuit reconstruction of newborn neurons after spinal cord injury in adult rats via an NT3-chitosan scaffold. Prog. Neurobiol. 2023, 220, 102375. [Google Scholar] [CrossRef]
- Valachová, K.; Šoltés, L. Hyaluronan as a Prominent Biomolecule with Numerous Applications in Medicine. Int. J. Mol. Sci. 2021, 22, 7077. [Google Scholar] [CrossRef]
- Tamer, T.M.; Valachová, K.; Hassan, M.A.; Omer, A.M.; El-Shafeey, M.; Mohy Eldin, M.S.; Šoltés, L. Chitosan/hyaluronan/edaravone membranes for anti-inflammatory wound dressing: In vitro and in vivo evaluation studies. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 227–235. [Google Scholar] [CrossRef]
- Kumar, P.P.P.; Lim, D.K. Gold-Polymer Nanocomposites for Future Therapeutic and Tissue Engineering Applications. Pharmaceutics 2021, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Elkhenany, H.; Bonilla, P.; Giraldo, E.; Alastrue Agudo, A.; Edel, M.J.; Vicent, M.J.; Roca, F.G.; Ramos, C.M.; Doblado, L.R.; Pradas, M.M.; et al. A Hyaluronic Acid Demilune Scaffold and Polypyrrole-Coated Fibers Carrying Embedded Human Neural Precursor Cells and Curcumin for Surface Capping of Spinal Cord Injuries. Biomedicines 2021, 9, 1928. [Google Scholar] [CrossRef]
- Farshidfar, N.; Iravani, S.; Varma, R.S. Alginate-Based Biomaterials in Tissue Engineering and Regenerative Medicine. Mar. Drugs 2023, 21, 189. [Google Scholar] [CrossRef] [PubMed]
- Saadinam, F.; Azami, M.; Pedram, M.S.; Sadeghinezhad, J.; Jabbari Fakhr, M.; Salimi, A.; Aminianfar, H.; Molazem, M.; Mokhber Dezfouli, M.R.; Dehghan, M.M. Injectable alginate chitosan hydrogel as a promising bioengineered therapy for acute spinal cord injury. Sci. Rep. 2024, 14, 26747. [Google Scholar] [CrossRef]
- Manzari-Tavakoli, A.; Babajani, A.; Vousooghi, N.; Moghimi, A.; Tarasi, R.; Safaeinejad, F.; Norouzi, S.; Bahrami, S.; Niknejad, H. Therapeutic potential of placenta-derived stem cells cultivated on noggin-loaded nanochitosan/polypyrrole-alginate conductive scaffold to restore spinal cord injury. Stem Cell Res. Ther. 2024, 15, 497. [Google Scholar] [CrossRef]
- Sun, W.; Gregory, D.A.; Tomeh, M.A.; Zhao, X. Silk Fibroin as a Functional Biomaterial for Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 1499. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Ma, Y.; Dai, H.; Tan, S.; Han, B. Advancements and Applications in the Composites of Silk Fibroin and Graphene-Based Materials. Polymers 2022, 14, 3110. [Google Scholar] [CrossRef]
- Feng, F.; Song, X.; Tan, Z.; Tu, Y.; Xiao, L.; Xie, P.; Ma, Y.; Sun, X.; Ma, J.; Rong, L.; et al. Cooperative assembly of a designer peptide and silk fibroin into hybrid nanofiber gels for neural regeneration after spinal cord injury. Sci. Adv. 2023, 9, eadg0234. [Google Scholar] [CrossRef]
- Deng, W.S.; Liu, X.Y.; Ma, K.; Liang, B.; Liu, Y.F.; Wang, R.J.; Chen, X.Y.; Zhang, S. Recovery of motor function in rats with complete spinal cord injury following implantation of collagen/silk fibroin scaffold combined with human umbilical cord-mesenchymal stem cells. Rev. Assoc. Med. Bras. 2021, 67, 1342–1348. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Tang, X.C.; Li, X.; Qin, J.Z.; Zhong, W.T.; Wu, P.; Zhang, F.; Shen, Y.X.; Dai, T.T. Implantation of nanofibrous silk scaffolds seeded with bone marrow stromal cells promotes spinal cord regeneration (6686 words). Artif. Cells Nanomed. Biotechnol. 2021, 49, 699–708. [Google Scholar] [CrossRef]
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Soucy, J.R.; Shirzaei Sani, E.; Portillo Lara, R.; Diaz, D.; Dias, F.; Weiss, A.S.; Koppes, A.N.; Koppes, R.A.; Annabi, N. Photocrosslinkable Gelatin/Tropoelastin Hydrogel Adhesives for Peripheral Nerve Repair. Tissue Eng. Part A 2018, 24, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lu, G.; Shi, B.; Ni, H.; Wang, J.; Qiu, Y.; Yang, L.; Zhu, Z.; Yi, X.; Du, X. ROS-Scavenging Hydrogels Synergize with Neural Stem Cells to Enhance Spinal Cord Injury Repair via Regulating Microenvironment and Facilitating Nerve Regeneration. Adv. Healthc. Mater. 2023, 12, e2300123. [Google Scholar] [CrossRef]
- Caron, I.; Rossi, F.; Papa, S.; Aloe, R.; Sculco, M.; Mauri, E.; Sacchetti, A.; Erba, E.; Panini, N.; Parazzi, V.; et al. A new three dimensional biomimetic hydrogel to deliver factors secreted by human mesenchymal stem cells in spinal cord injury. Biomaterials 2016, 75, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, H.; Amiri, S.; Amiri, F.; Moradi, S.; Zarrintaj, P. Antibacterial Biocomposite Based on Chitosan/Pluronic/Agarose Noncovalent Hydrogel: Controlled Drug Delivery by Alginate/Tetracycline Beads System. J. Funct. Biomater. 2024, 15, 286. [Google Scholar] [CrossRef]
- Han, S.; Lee, J.Y.; Heo, E.Y.; Kwon, I.K.; Yune, T.Y.; Youn, I. Implantation of a Matrigel-loaded agarose scaffold promotes functional regeneration of axons after spinal cord injury in rat. Biochem. Biophys. Res. Commun. 2018, 496, 785–791. [Google Scholar] [CrossRef]
- Jia, Z.; Zeng, H.; Ye, X.; Dai, M.; Tang, C.; Liu, L. Hydrogel-based treatments for spinal cord injuries. Heliyon 2023, 9, e19933. [Google Scholar] [CrossRef]
- Peng, H.; Liu, Y.; Xiao, F.; Zhang, L.; Li, W.; Wang, B.; Weng, Z.; Chen, G. Research progress of hydrogels as delivery systems and scaffolds in the treatment of secondary spinal cord injury. Front. Bioeng. Biotechnol. 2023, 11, 1111882. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Dong, C.; Zhang, T.; Zhang, S. Hydrogels in Spinal Cord Injury Repair: A Review. Front. Bioeng. Biotechnol. 2022, 10, 931800. [Google Scholar] [CrossRef] [PubMed]
- Hassannejad, Z.; Zadegan, S.A.; Vaccaro, A.R.; Rahimi-Movaghar, V.; Sabzevari, O. Biofunctionalized peptide-based hydrogel as an injectable scaffold for BDNF delivery can improve regeneration after spinal cord injury. Injury 2019, 50, 278–285. [Google Scholar] [CrossRef]
- Alizadeh, A.; Moradi, L.; Katebi, M.; Ai, J.; Azami, M.; Moradveisi, B.; Ostad, S.N. Delivery of injectable thermo-sensitive hydrogel releasing nerve growth factor for spinal cord regeneration in rat animal model. J. Tissue Viability 2020, 29, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Nazemi, Z.; Nourbakhsh, M.S.; Kiani, S.; Heydari, Y.; Ashtiani, M.K.; Daemi, H.; Baharvand, H. Co-delivery of minocycline and paclitaxel from injectable hydrogel for treatment of spinal cord injury. J. Control. Release 2020, 321, 145–158. [Google Scholar] [CrossRef]
- Qi, Z.; Kong, W.; Fu, C.; Chang, Y.; Li, H.; Yang, X.; Pan, S. A dual-drug enhanced injectable hydrogel incorporated with neural stem cells for combination therapy in spinal cord injury. Chem. Eng. J. 2021, 427, 130906. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, H.; Xu, H.; Zhao, Y.; Li, Z.; Li, J.; Wang, H.; Zhuge, D.; Guo, X.; Jones, S.; et al. Novel multi-drug delivery hydrogel using scar-homing liposomes improves spinal cord injury repair. Theranostics 2018, 8, 4429–4446. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, J.; Liu, C.; Zhang, S.; Gao, F.; Guo, W.; Sun, X.; Zhang, C.; Li, H.; Rao, Z.; et al. Understanding the role of tissue-specific decellularized spinal cord matrix hydrogel for neural stem/progenitor cell microenvironment reconstruction and spinal cord injury. Biomaterials 2021, 268, 120596. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.S.; Zhao, C.; Zhang, A.; Duan, H.; Hao, P.; Wei, R.H.; Shang, J.; Zhao, W.; Liu, Z.; Yu, J.; et al. NT3-chitosan enables de novo regeneration and functional recovery in monkeys after spinal cord injury. Proc. Natl. Acad. Sci. USA 2018, 115, E5595–E5604. [Google Scholar] [CrossRef]
- Wu, F.; Wang, P.; Wei, X.; Yang, Y.; Al Mamun, A.; Zhang, X.; Zhu, Y.; Mo, T.; Zhang, H.; Jiang, C.; et al. Barrier-penetrating liposome targeted delivery of basic fibroblast growth factor for spinal cord injury repair. Mater. Today Bio 2023, 18, 100546. [Google Scholar] [CrossRef]
- Ji, R.; Hao, Z.; Wang, H.; Li, X.; Duan, L.; Guan, F.; Ma, S. Application of Injectable Hydrogels as Delivery Systems in Spinal Cord Injury. Gels 2023, 9, 907. [Google Scholar] [CrossRef] [PubMed]
- Furuya, T.; Hashimoto, M.; Koda, M.; Murata, A.; Okawa, A.; Dezawa, M.; Matsuse, D.; Tabata, Y.; Takahashi, K.; Yamazaki, M. Treatment with basic fibroblast growth factor-incorporated gelatin hydrogel does not exacerbate mechanical allodynia after spinal cord contusion injury in rats. J. Spinal Cord. Med. 2013, 36, 134–139. [Google Scholar] [CrossRef][Green Version]
- Shen, M.; Wang, L.; Li, K.; Tan, J.; Tang, Z.; Wang, X.; Yang, H. Gelatin Methacrylic Acid Hydrogel-Based Nerve Growth Factors Enhances Neural Stem Cell Growth and Differentiation to Promote Repair of Spinal Cord Injury. Int. J. Nanomed. 2024, 19, 10589–10604. [Google Scholar] [CrossRef]
- Cai, M.; Chen, L.; Wang, T.; Liang, Y.; Zhao, J.; Zhang, X.; Li, Z.; Wu, H. Hydrogel scaffolds in the treatment of spinal cord injury: A review. Front. Neurosci. 2023, 17, 1211066. [Google Scholar] [CrossRef]
- Walsh, C.M.; Wychowaniec, J.K.; Brougham, D.F.; Dooley, D. Functional hydrogels as therapeutic tools for spinal cord injury: New perspectives on immunopharmacological interventions. Pharmacol. Ther. 2022, 234, 108043. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xiao, B.; Mu, J.; Zhang, Y.; Zhang, C.; Cao, H.; Chen, R.; Patra, H.K.; Yang, B.; Feng, S.; et al. A MnO2 nanoparticle-dotted hydrogel promotes spinal cord repair via regulating reactive oxygen species microenvironment and synergizing with mesenchymal stem cells. ACS Nano 2019, 13, 14283–14293. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, S.; Zhou, T.; Wu, K.; Qiao, Z.; Zhang, Y.; Xin, N.; Liu, X.; Wei, D.; Sun, J.; et al. Antioxidative and Conductive Nanoparticles-Embedded Cell Niche for Neural Differentiation and Spinal Cord Injury Repair. ACS Appl. Mater. Interfaces 2021, 13, 52346–52361. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Xiao, Z.; Liu, X.; Wu, C.; Wu, K.; Liu, A.; Wei, D.; Sun, J.; Zhou, L.; et al. Magnetoelectric Nanoparticles Incorporated Biomimetic Matrix for Wireless Electrical Stimulation and Nerve Regeneration. Adv. Healthc. Mater. 2021, 10, e2100695. [Google Scholar] [CrossRef]
- Ko, W.K.; Lee, S.J.; Kim, S.J.; Han, G.H.; Han, I.B.; Hong, J.B.; Sheen, S.H.; Sohn, S. Direct Injection of Hydrogels Embedding Gold Nanoparticles for Local Therapy after Spinal Cord Injury. Biomacromolecules 2021, 22, 2887–2901. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Gao, M.; Lin, J.; Wu, W.; Wang, J.; Chew, S.Y. Three-dimensional aligned nanofibers-hydrogel scaffold for controlled non-viral drug/gene delivery to direct axon regeneration in spinal cord injury treatment. Sci. Rep. 2017, 7, 42212. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Haggerty, A.E.; Yan, J.; Lan, M.; Seu, M.; Yang, M.; Marlow, M.M.; Maldonado-Lasunción, I.; Cho, B.; et al. The effect of a nanofiber-hydrogel composite on neural tissue repair and regeneration in the contused spinal cord. Biomaterials 2020, 245, 119978. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kim, J.C.; Miller, A.L.; Waletzki, B.E.; Lu, L. Electrically conductive nanocomposite hydrogels embedded with functionalized carbon nanotubes for spinal cord injury. New J. Chem. 2018, 42, 17671–17681. [Google Scholar] [CrossRef]
- Girão, A.F.; Serrano, M.C.; Completo, A.; Marques, P.A.A.P. Is Graphene Shortening the Path toward Spinal Cord Regeneration? ACS Nano 2022, 16, 13430–13467. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, W.; Lin, Z.; Lu, Y.; Chen, H.; Li, B.; Li, Z.; Xia, H.; Li, L.; Zhang, T. Conducting molybdenum sulfide/graphene oxide/polyvinyl alcohol nanocomposite hydrogel for repairing spinal cord injury. J. Nanobiotechnol. 2022, 20, 210. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Yang, X.; Chen, J.; Chen, Y.; Zhao, Z.; Chen, X.; Wang, F.; Fu, C. Combined treatment using novel multifunctional MAu-GelMA hydrogel loaded with neural stem cells and electrical stimulation promotes functional recovery from spinal cord injury. Ceram. Int. 2023, 49, 20623–20636. [Google Scholar] [CrossRef]
- Yang, K.; Yang, J.; Man, W.; Meng, Z.; Yang, C.-Y.; Cao, Z.; Liu, J.; Kim, K.; Liu, Y.; Yang, S.; et al. N-Cadherin-Functionalized Nanofiber Hydrogel Facilitates Spinal Cord Injury Repair by Building a Favorable Niche for Neural Stem Cells. Adv. Fiber Mater. 2023, 5, 1349–1366. [Google Scholar] [CrossRef]
- Gao, X.; Cheng, W.; Zhang, X.; Zhou, Z.; Ding, Z.; Zhou, X.; Lu, Q.; Kaplan, D.L. Nerve Growth Factor-Laden Anisotropic Silk Nanofiber Hydrogels to Regulate Neuronal/Astroglial Differentiation for Scarless Spinal Cord Repair. ACS Appl. Mater. Interfaces 2022, 14, 3701–3715. [Google Scholar] [CrossRef]
- Man, W.; Yang, S.; Cao, Z.; Lu, J.; Kong, X.; Sun, X.; Zhao, L.; Guo, Y.; Yao, S.; Wang, G.; et al. A multi-modal delivery strategy for spinal cord regeneration using a composite hydrogel presenting biophysical and biochemical cues synergistically. Biomaterials 2021, 276, 120971. [Google Scholar] [CrossRef]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Romero, A.; Pérez-Puyana, V. Novel Trends in Hydrogel Development for Biomedical Applications: A Review. Polymers 2022, 14, 3023. [Google Scholar] [CrossRef]
- Rotaru-Zăvăleanu, A.D.; Dinescu, V.C.; Aldea, M.; Gresita, A. Hydrogel-Based Therapies for Ischemic and Hemorrhagic Stroke: A Comprehensive Review. Gels 2024, 10, 476. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, M.; Chong, C.M.; Lin, D.; Chen, S.; Zhen, Y.; Ding, H.; Zhong, H.J. Recent Advances in the 3D Printing of Conductive Hydrogels for Sensor Applications: A Review. Polymers 2024, 16, 2131. [Google Scholar] [CrossRef] [PubMed]
- Visan, A.I.; Negut, I. Development and Applications of PLGA Hydrogels for Sustained Delivery of Therapeutic Agents. Gels 2024, 10, 497. [Google Scholar] [CrossRef]
- Mandal, A.; Clegg, J.R.; Anselmo, A.C.; Mitragotri, S. Hydrogels in the clinic. Bioeng. Transl. Med. 2020, 5, e10158. [Google Scholar] [CrossRef] [PubMed]
- Sekar, M.P.; Suresh, S.; Zennifer, A.; Sethuraman, S.; Sundaramurthi, D. Hyaluronic Acid as Bioink and Hydrogel Scaffolds for Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2023, 9, 3134–3159. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Pramanik, S.; Abdelgawad, M.A.; Abualsoud, B.M.; Kadi, A.; Ansari, M.J.; Deepak, A. Recent Advances of Chitosan Formulations in Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 975. [Google Scholar] [CrossRef]
- Beheshtizadeh, N.; Gharibshahian, M.; Pazhouhnia, Z.; Rostami, M.; Zangi, A.R.; Maleki, R.; Azar, H.K.; Zalouli, V.; Rajavand, H.; Farzin, A.; et al. Commercialization and regulation of regenerative medicine products: Promises, advances and challenges. Biomed. Pharmacother. 2022, 153, 113431. [Google Scholar] [CrossRef]
| Biopolymer | Key Properties | Applications | Challenges | Recent Innovations |
|---|---|---|---|---|
| Gelatin/GelMA | Tunable degradation rate, MMP-sensitive, versatile crosslinking (UV, enzymatic) | Injectable scaffolds, growth factor delivery, neural and spinal repair | Thermosensitivity, batch variability, requires chemical modification for stability | Three-dimensional bioprinting of structured GelMA networks; conductive GelMA for neural interfaces |
| Chitosan | Biocompatible, antimicrobial, forms gels under mild conditions, cationic nature | Encapsulation of nanoparticles, wound healing, drug delivery | Limited solubility at physiological pH, weak mechanical properties | Chitosan-based injectable nanogels; co-delivery systems with growth factors or EVs |
| Hyaluronic acid | Biocompatible, promotes cell adhesion and proliferation, modulates inflammation | Neural tissue engineering, cancer therapy, targeted delivery | Rapid degradation, limited mechanical strength | HA hydrogels with enzymatic resistance; HA-integrated stem cell scaffolds |
| Alginate | Biocompatible, easy gelation, porous structure for drug encapsulation | Sustained drug release, neuroprotection, anti-inflammatory use | Lacks intrinsic cell adhesion | Oxidized alginate for tunable degradation; RGD-functionalized alginate scaffolds |
| Silk fibroin | Strong mechanical properties, biocompatible, slow degradation | Neural tissue engineering, scaffolding | Complex processing, chemical modification required | Composite silk-based scaffolds; aligned silk fibers for axonal guidance |
| Agarose | Thermoreversible gelation, high mechanical stiffness, inert to cell signaling unless modified | Multichannel guidance conduits, axonal bridging matrices, structural supports in lesion cavities | Poor intrinsic bioactivity, limited cell adhesion, difficult to functionalize for specific cell types | Functionalized agarose with peptides or neurotrophins; multichannel agarose constructs |
| Biopolymer | Crosslinking Method | Cell Compatibility | Mechanical Stability | Key Benefits | Potential Risks |
|---|---|---|---|---|---|
| Gelatin | None or enzymatic | Moderate | Low | Biocompatible, inexpensive | Poor mechanical properties |
| GelMA | Photoinitiated (UV/visible) | High | Tunable | Tailored stiffness, bioactive | Light/initiator toxicity |
| Agarose | Thermal gelation | High | High | Biostable, immuno-inert | Limited bioactivity |
| Chitosan | Ionic/enzymatic | High | Moderate | Anti-inflammatory, antimicrobial | Solubility and variability |
| Hyaluronic acid | Enzymatic/chemical | High | Tunable | Neuroprotective, ECM mimic | Rapid degradation |
| PEG derivatives | Click chemistry/photocrosslink | High | Precisely tunable | Predictable, customizable | Non-bioactive, synthetic |
| Material Type | Representative Materials | Hydrogel Integration | Key Benefits in SCI Repair | Example Studies |
|---|---|---|---|---|
| One-dimensional nanomaterials | Aligned polymer nanofibers (e.g., PCLEEP), electrospun PCL fibers, CNTs, peptide nanofibers | Embedded in collagen, HA, or GelMA matrices | Enhanced mechanical strength, neurite guidance, drug/gene delivery, electrical conductivity | [70,71,72] |
| Two-dimensional nanomaterials | Graphene oxide (GO), reduced GO (rGO), MoS2, MXene, AuNPs | Mixed with GelMA, composite networks | Improved conductivity, biocompatibility, NSC differentiation, inflammation control | [73,74,75] |
| Functionalized nanofibers | Aligned fibrin nanofibers (AFGs), silk fibroin nanofibers (SFNs) | Blended into hydrogels with growth factors like NGF | Enhanced axonal regeneration, scar-free healing, CNS function restoration | [76], SFN-NGF models |
| Composite platforms | rGO + xanthan gum, MoS2/GO hybrids, MXene + AuNPs | Conductive composite hydrogels | Electroconductivity, glial scar suppression, myelin regeneration | [74,75], AFG-tailored systems |
| Material | Estimated Cost (USD/mL) | Notes | Regulatory/Reimbursement Context |
|---|---|---|---|
| GelMA | USD 36–38/mL | Advanced BioMatrix 20% solution: USD 180 for 5 mL → USD 36/mL. CELLINK GelMA bioink: USD ≈ 38/mL. | Synthetic gelatin-based biomaterials often undergo GMP track; products for bioinks need sterility certification; reimbursement still limited due to classification as “experimental”. |
| Chitosan-based hydrogel | USD ≈4/mL (thermosensitive gelatin–chitosan) | Reported at USD ~4/mL in PLoS One study. Prices may increase by 2–3× when functionalized with growth factors or in high-purity medical grades. Chitosan hydrogels occasionally gain medical device approval; some wound-care chitosan hydrogels already reimbursed under skin grafts or adhesives. | |
| Hyaluronic acid (HA)-based cHydrogel | USD 30–50/mL | Market bioinks like PhotoHA-INK cost USD 325 for 5 mL → USD 65/mL; actual therapeutic-grade hydrogels run USD ~30–50/mL. | HA is widely FDA-approved for fillers and joint injections; high-ni HT-grade therapeutic hydrogels still under OUS or clinical trials. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotaru-Zavaleanu, A.D.; Bica, M.; Dinescu, S.-N.; Ruscu, M.A.; Vasile, R.C.; Zavate, A.C.; Dinescu, V.C. Bioactive Hydrogels for Spinal Cord Injury Repair: Emphasis on Gelatin and Its Derivatives. Gels 2025, 11, 497. https://doi.org/10.3390/gels11070497
Rotaru-Zavaleanu AD, Bica M, Dinescu S-N, Ruscu MA, Vasile RC, Zavate AC, Dinescu VC. Bioactive Hydrogels for Spinal Cord Injury Repair: Emphasis on Gelatin and Its Derivatives. Gels. 2025; 11(7):497. https://doi.org/10.3390/gels11070497
Chicago/Turabian StyleRotaru-Zavaleanu, Alexandra Daniela, Marius Bica, Sorin-Nicolae Dinescu, Mihai Andrei Ruscu, Ramona Constantina Vasile, Andrei Calin Zavate, and Venera Cristina Dinescu. 2025. "Bioactive Hydrogels for Spinal Cord Injury Repair: Emphasis on Gelatin and Its Derivatives" Gels 11, no. 7: 497. https://doi.org/10.3390/gels11070497
APA StyleRotaru-Zavaleanu, A. D., Bica, M., Dinescu, S.-N., Ruscu, M. A., Vasile, R. C., Zavate, A. C., & Dinescu, V. C. (2025). Bioactive Hydrogels for Spinal Cord Injury Repair: Emphasis on Gelatin and Its Derivatives. Gels, 11(7), 497. https://doi.org/10.3390/gels11070497







