Varying Synthesis Parameters of Potato Starch Aerogel for Aerospace Applications
Abstract
1. Introduction
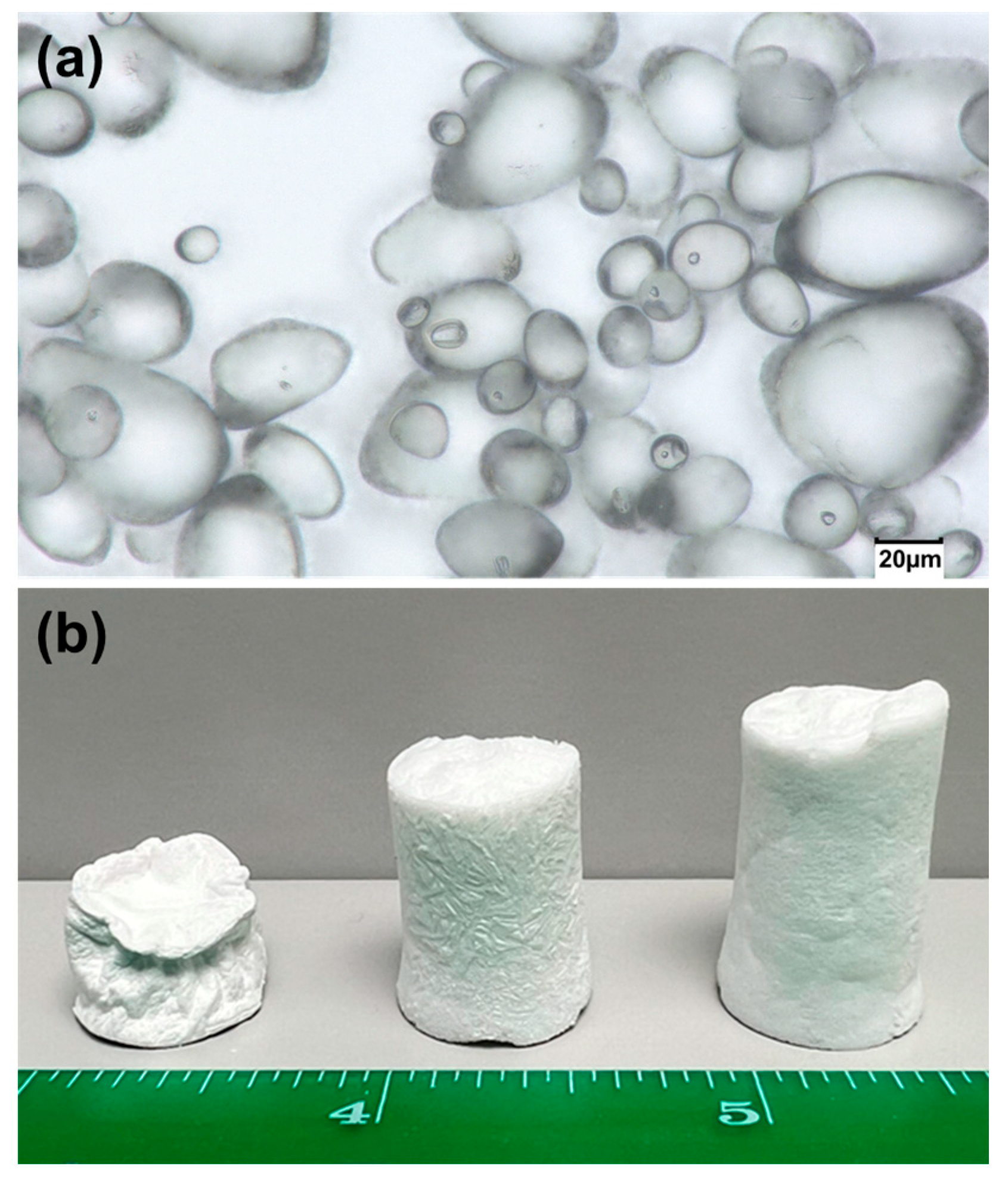
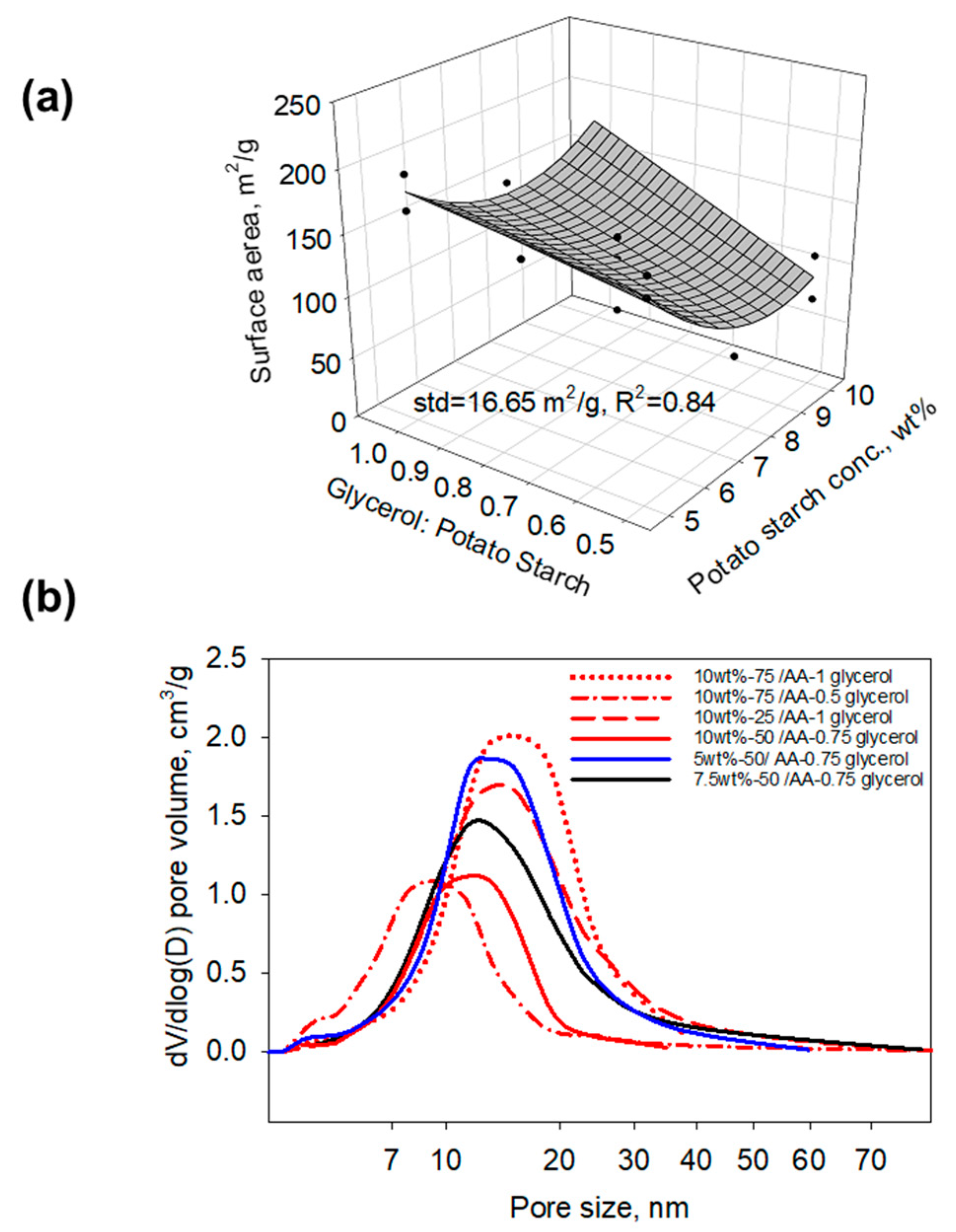
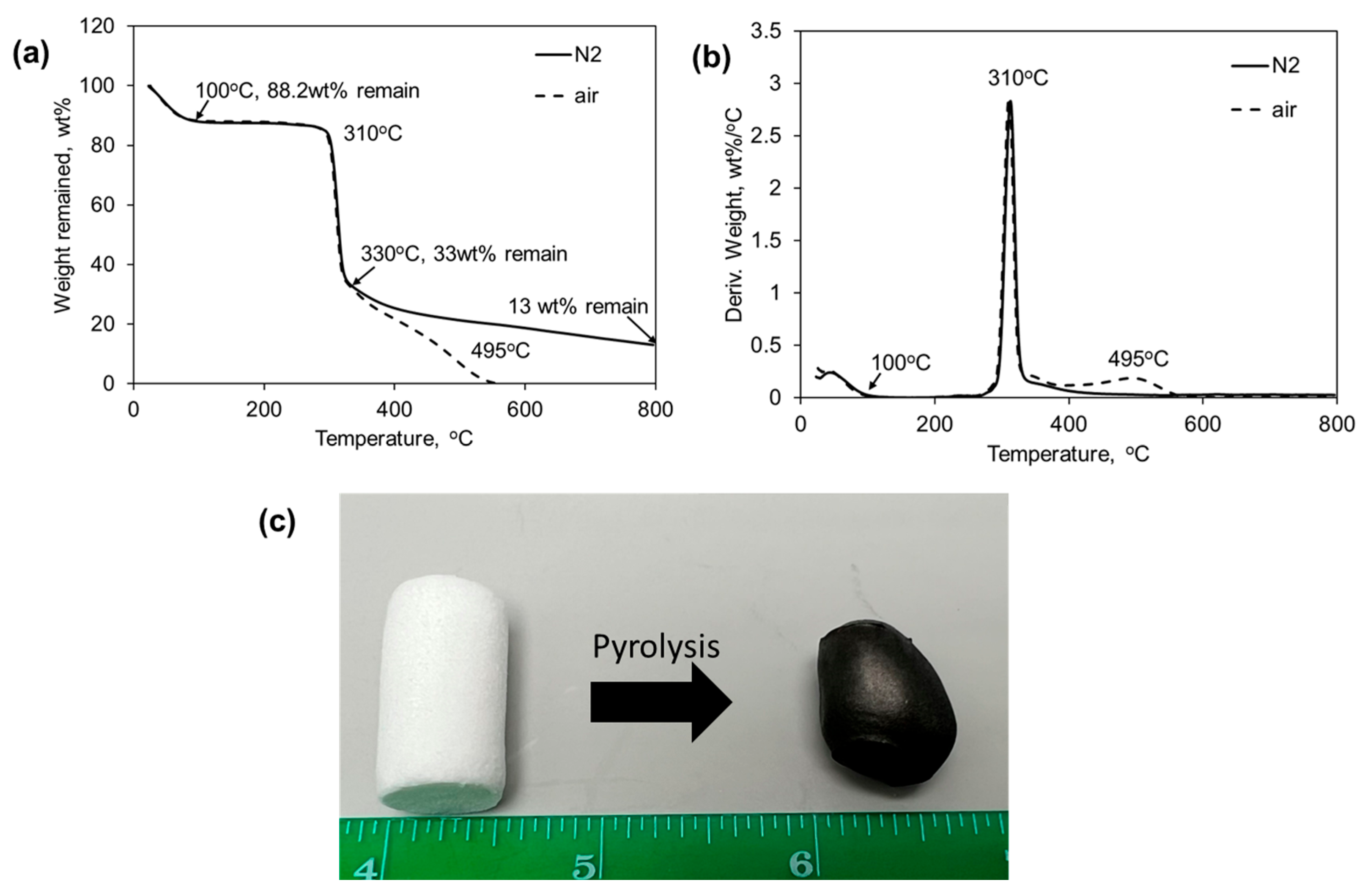
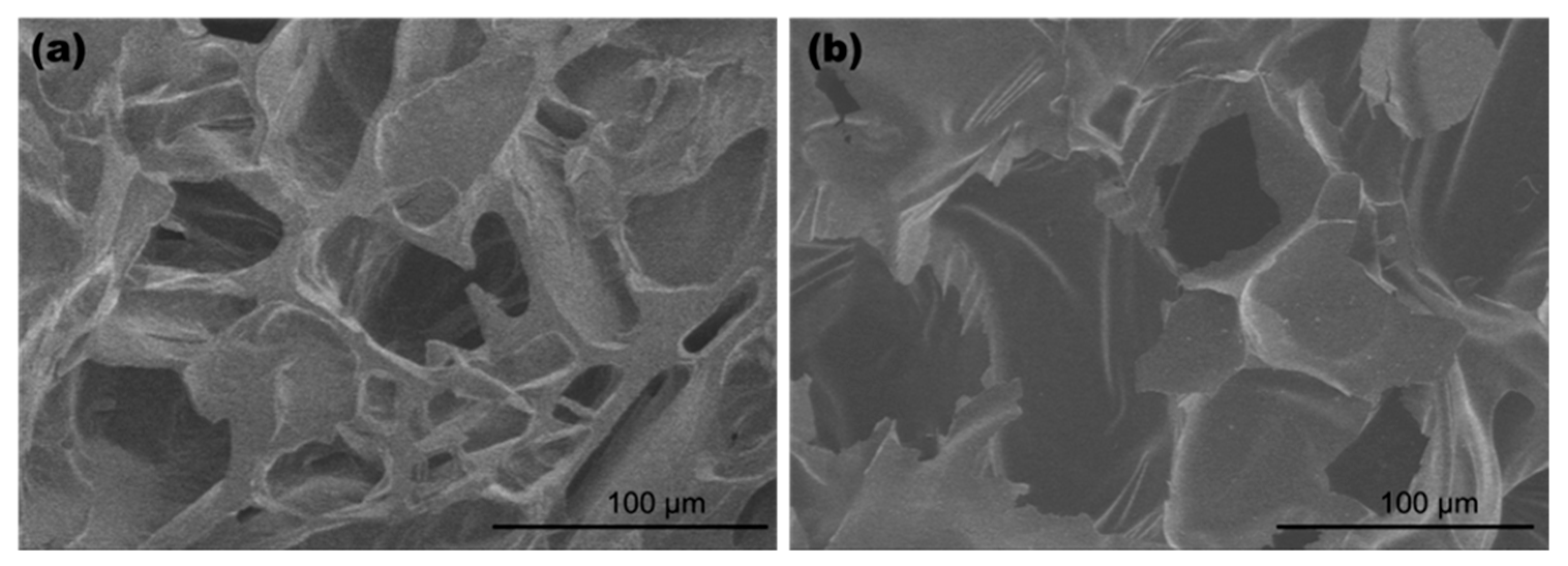
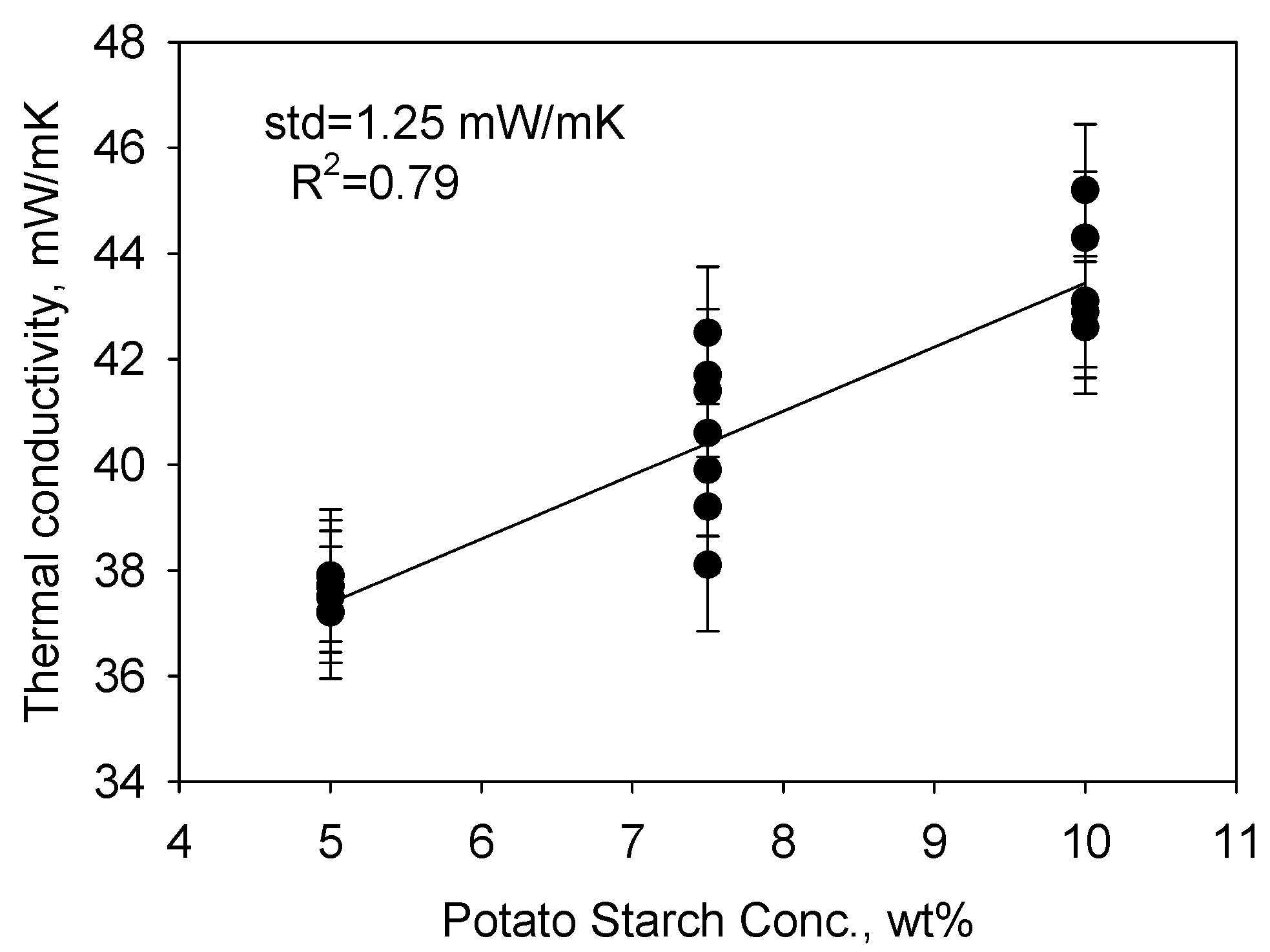
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials Used for Aerogel Formulations
4.2. Method for Fabricating Aerogel Including Supercritical Drying
4.3. Experimental Design and Analysis
4.4. Bulk Density, Shrinkage, and Porosity Determination
4.5. Instrument Characterization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bheekhun, N.; Talib, A.R.A.; Hassan, M.R. Aerogels in aerospace: An overview. Adv. Mater. Sci. Eng. 2013, 2013, 406065. [Google Scholar] [CrossRef]
- Dorchen, A.S.; Abbasi, M.H. Silica aerogel; synthesis, properties and characterization. J. Mater. Proc. Technol. 2008, 199, 10–26. [Google Scholar]
- Baptista, J.M.; Sagu, J.S.; KG, U.W.; Lobato, K. State-of-the-art materials for high power and high energy supercapacitors: Performance metrics and obstacles for the transition from lab to industrial scale—A critical approach. Chem. Eng. J. 2019, 374, 1153–1179. [Google Scholar] [CrossRef]
- Guo, H.; Meador, M.A.B.; McCorkle, L.; Quade, D.J.; Guo, J.; Hamilton, B.; Cakmak, M.; Sprow, G. Polyimide Aerogels Cross-Linked through Amine Functionalized Polyoligomeric Silsesquioxane. ACS Appl. Mater. Interfaces 2011, 3, 546–552. [Google Scholar] [CrossRef]
- Scheiman, D.A.; Guo, H.; Oosterbaan, K.J.; McCorkle, L.; Nguyen, B.N. Synthesis of Polyamide Aerogels Cross-Linked with a Tri-isocyanate. Gels 2024, 10, 519. [Google Scholar] [CrossRef] [PubMed]
- Merillas, B.; León, J.M.; Villafañe, F.; Rodríguez-Pérez, M.A. Transparent Polyisocyanurate-Polyurethane-Based Aerogels: KeyAspects on the Synthesis and Their Porous Structures. ACS Appl. Polym. Mater. 2021, 3, 4607–4615. [Google Scholar] [CrossRef]
- Bruce, W.E., III; Mesick, N.J.; Ferlemann, P.G.; Siemers, P.M., III; Del Corso, J.A.; Hughes, S.J.; Tobin, S.A.; Kardell, M. Aerothermal Ground Testing of Flexible Thermal Protection Systems for Hypersonic Inflatable Aerodynamic Decelerators. In Proceedings of the 9th International Planetary Probe Workshop, Toulouse, France, 16–22 June 2012. [Google Scholar]
- Mi, H.-Y.; Jing, X.; Meador, M.A.B.; Guo, H.; Turng, L.-S.; Gong, S. Triboelectric Nanogenerators Made of Porous Polyamide Nanofiber Mats and Polyimide Aerogel Film: Output Optimization and Performance in Circuits. ACS Appl. Mater. Interfaces 2018, 10, 30596–30606. [Google Scholar] [CrossRef]
- Cashman, J.L.; Nguyen, B.N.; Dosa, B.; Meador, M.A.B. Flexible Polyimide Aerogels Derived from the Use of a Neopentyl Spacer in the Backbone. ACS Appl. Polym. Mater. 2020, 2, 2179–2189. [Google Scholar] [CrossRef]
- Barlis, A.; Guo, H.; Helson, K.; Bennett, C.; Chan, C.Y.Y.; Marriage, T.; Quijada, M.; Tokarz, A.; Vivod, S.; Wollack, E.; et al. Fabrication and characterization of optical filters from polymeric aerogels loaded with diamond scattering particles. Appl. Opt. 2024, 63, 6036–6045. [Google Scholar] [CrossRef]
- Xu, Z.; Croft, Z.; Guo, D.; Cao, K.; Li, G. Recent development of polyimides: Synthesis, processing, and application in gas separation. J. Poly. Sci. 2021, 59, 943–962. [Google Scholar] [CrossRef]
- Environmental Protection Agency. n-Methylpyrrolidone (NMP); Revision to Toxic Substances Control Act (TSCA) Risk Determination; Notice of Availability; 87 FR 77596, 2022-27438; Environmental Protection Agency: Washington, DC, USA, 2022; pp. 77596–77602.
- Wang, Y. Airborne hydrophilic microplastics in cloud water at high altitudes and their role in cloud formation. Environ. Chem. Lett. 2023, 21, 3055–3062. [Google Scholar] [CrossRef]
- Kumar, K.; Saxena, D.C. A Review on Starch Aerogel: Application and Future Trends. In Proceedings of the 8th International Conference on Advancements in Engineering and Technology, (ICAET-2020) BGIET, Sangrur, India, 20–21 March 2020; ISBN 978-81-924893-5-3. [Google Scholar]
- Huang, S.; Chao, C.; Yu, J.; Coperland, L.; Wang, S. New insight into starch retrogradation: The effect of short-range molecular order in gelatinized starch. Food Hydrocoll. 2021, 120, 106921. [Google Scholar] [CrossRef]
- Jiamjariyatam, R.; Kongpensook, V.; Pradipasena, P. Effects of amylose content, cooling rate and aging time on properties and characteristics of rice starch gels and puffed products. J. Cereal Sci. 2014, 61, 16–25. [Google Scholar] [CrossRef]
- Zou, F.; Budtova, T. Tailoring the morphology and properties of starch aerogels and cryogels via starch source and process parameter. Carbohydr. Polym. 2021, 255, 117344. [Google Scholar] [CrossRef] [PubMed]
- Druel, L.; Bardl, R.; Vorwerg, W.; Budtova, T. Starch aerogels: A member of the family of thermal superinsulating materials. Biomacromolecules 2017, 18, 4232–4239. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. Supercritical CO2 adsorption of non-steroidal anti-inflammatory drugs into biopolymer aerogels. J. CO2 Util. 2020, 36, 40–53. [Google Scholar] [CrossRef]
- Aguirre, J.F.; Osella, C.A.; Carrara, C.R.; Sa´nchez, H.D.; del Buera, M.P. Effect of storage temperature on starch retrogradation of bread staling. Starch/Stärke 2011, 63, 587–593. [Google Scholar] [CrossRef]
- Staker, J.; White, G.M.; Pasilova, S.; Scheiman, D.A.; Guo, H.; Tovar, A.; Siegel, A.P. Mitigating Shrinkage and Enhancing Structure of Thermally Insulating Starch Aerogel via Solvent Exchange and Chitin Ad-dition. Macromol 2025, accepted. [Google Scholar]
- Hu, L.; He, R.; Lei, H.; Fang, D. Carbon Aerogel for Insulation Applications: A Review. Int. J. Thermophys. 2019, 40, 39. [Google Scholar] [CrossRef]
- Meador, M.A.B.; Malow, E.J.; Silva, R.; Wright, S.; Quade, D.; Vivod, S.L.; Guo, H.; Guo, J.; Cakmak, M. Mechanically Strong, Flexible Polyimide Aerogels Cross-Linked with Aromatic Triamine. ACS Appl. Mater. Interfaces 2012, 4, 536–544. [Google Scholar] [CrossRef]
- Namazi, H.; Fathi, F.; Dadkhah, A. Hydrophobically modified starch using long-chain fatty acids for preparation of nanosized starch particles. Sci. Iran. 2011, 18, 439–445. [Google Scholar] [CrossRef]
- Smith, A.M.; Zeeman, S.C.; Smith, S.M. Starch degradation. Annu. Rev. Plant Biol. 2005, 56, 73–98. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Corker, J.; Papathanasiou, T.; Wang, Y.; Zhou, Y.; Madyan, O.M.; Liao, F.; Fan, M. Critical review on the thermal conductivity modelling of silica aerogel composites. J. Build. Eng. 2022, 57, 104814–104840. [Google Scholar] [CrossRef]
- Feng, J.; Wang, X.; Jiang, Y.; Du, D.; Feng, J. Study on Thermal Conductivities of Aromatic Polyimide Aerogels. ACS Appl. Mater. Interfaces 2016, 8, 12992–12996. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lu, J.; Xi, S.; Wang, H.; Han, D.; Fan, C.; Zhang, Z.; Shen, J.; Zhou, B.; Du, A. Direct 3D print polyimide aerogels for synergy management of thermal insulation, gas permeability and light absorption. J. Mater. Chem. A 2023, 11, 21272–21284. [Google Scholar] [CrossRef]
- Anh, L.D.H.; Pάsztory, Z. An overview of factors influencing thermal conductivity of building insulation materials. J. Build. Eng. 2021, 44, 102604. [Google Scholar]
- ASTM D7984-21; Standard Test Method for Measurement of Thermal Effusivity of Fabrics Using a Modified Transient Plane Source (MTPS) Instrument. ASTM International: West Conshohocken, PA, USA, 2021.



| Run | Potato Starch, wt% | Potato Starch/Acetic Acid | Glycerol/Potato Starch | Density, g/cm3 | Shrinkage, % | Porosity, % | Surface Area, m2/g | Young’s Modulus, MPa | Thermal Conductivity, mW/mK |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | 75 | 1 | 0.235 | 26.8 | 84.4 | 172.0 | 12.4 | 42.6 |
| 2 | 10 | 50 | 0.75 | 0.232 | 26.7 | 84.5 | 151.6 | 37.4 | 44.3 |
| 3 | 7.5 | 50 | 0.5 | 0.277 | 36.0 | 77.6 | 70.5 | 13.7 | 38.1 |
| 4 | 7.5 | 75 | 0.75 | 0.216 | 32.2 | 86.0 | 78.3 | 23.9 | 42.5 |
| 5 | 10 | 75 | 0.5 | 0.278 | 33.2 | 78.4 | 109.7 | 37.8 | 45.2 |
| 6 | 7.5 | 50 | 1 | 0.170 | 25.9 | 88.3 | 157.3 | 9.9 | 41.7 |
| 7 | 7.5 | 50 | 0.75 | 0.178 | 28.2 | 86.0 | 109.9 | 15.8 | 41.4 |
| 8 | 7.5 | 50 | 0.75 | 0.174 | 25.4 | 87.9 | 137.6 | 8.1 | 39.9 |
| 9 | 5 | 50 | 0.75 | 0.106 | 25.3 | 91.1 | 160.1 | 5.6 | 37.2 |
| 10 | 5 | 25 | 1 | 0.104 | 26.4 | 92.8 | 170.7 | 2.7 | 37.7 |
| 11 | 10 | 25 | 1 | 0.222 | 24.7 | 84.6 | 163.3 | 30.4 | 43.1 |
| 12 | 7.5 | 50 | 0.75 | 0.163 | 25.3 | 89.7 | 112.4 | 25.1 | 38.1 |
| 13 | 5 | 75 | 1 | 0.118 | 28.1 | 89.5 | 198.6 | 14.5 | 37.5 |
| 14 | 5 | 25 | 0.5 | 0.123 | 28.6 | 91.5 | 159.1 | 5.4 | 37.9 |
| 15 | 7.5 | 50 | 0.75 | 0.200 | 27.6 | 87.3 | 73.4 | 21.2 | 39.2 |
| 16 | 10 | 25 | 0.5 | 0.295 | 32.7 | 80.5 | 175.3 | 19.3 | 42.9 |
| 17 | 5 | 75 | 0.5 | 0.114 | 27.0 | 92.2 | 175.3 | 7.8 | 37.5 |
| 18 | 7.5 | 25 | 0.75 | 0.164 | 24.6 | 89.5 | 138.1 | 24.2 | 40.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staker, J.; Scheiman, D.A.; Mather, J.; Stokes, J.L.; Guo, H. Varying Synthesis Parameters of Potato Starch Aerogel for Aerospace Applications. Gels 2025, 11, 467. https://doi.org/10.3390/gels11060467
Staker J, Scheiman DA, Mather J, Stokes JL, Guo H. Varying Synthesis Parameters of Potato Starch Aerogel for Aerospace Applications. Gels. 2025; 11(6):467. https://doi.org/10.3390/gels11060467
Chicago/Turabian StyleStaker, Jacob, Daniel A. Scheiman, Janice Mather, Jamesa L. Stokes, and Haiquan Guo. 2025. "Varying Synthesis Parameters of Potato Starch Aerogel for Aerospace Applications" Gels 11, no. 6: 467. https://doi.org/10.3390/gels11060467
APA StyleStaker, J., Scheiman, D. A., Mather, J., Stokes, J. L., & Guo, H. (2025). Varying Synthesis Parameters of Potato Starch Aerogel for Aerospace Applications. Gels, 11(6), 467. https://doi.org/10.3390/gels11060467








