Sugar Alcohols as Crosslinking Delay Additives for Fracturing Fluids
Abstract
1. Introduction
2. Results and Discussion
2.1. Baseline Crosslinked Viscosity
2.2. Tests with Varying Crosslinker and Sugar Alcohol Concentrations
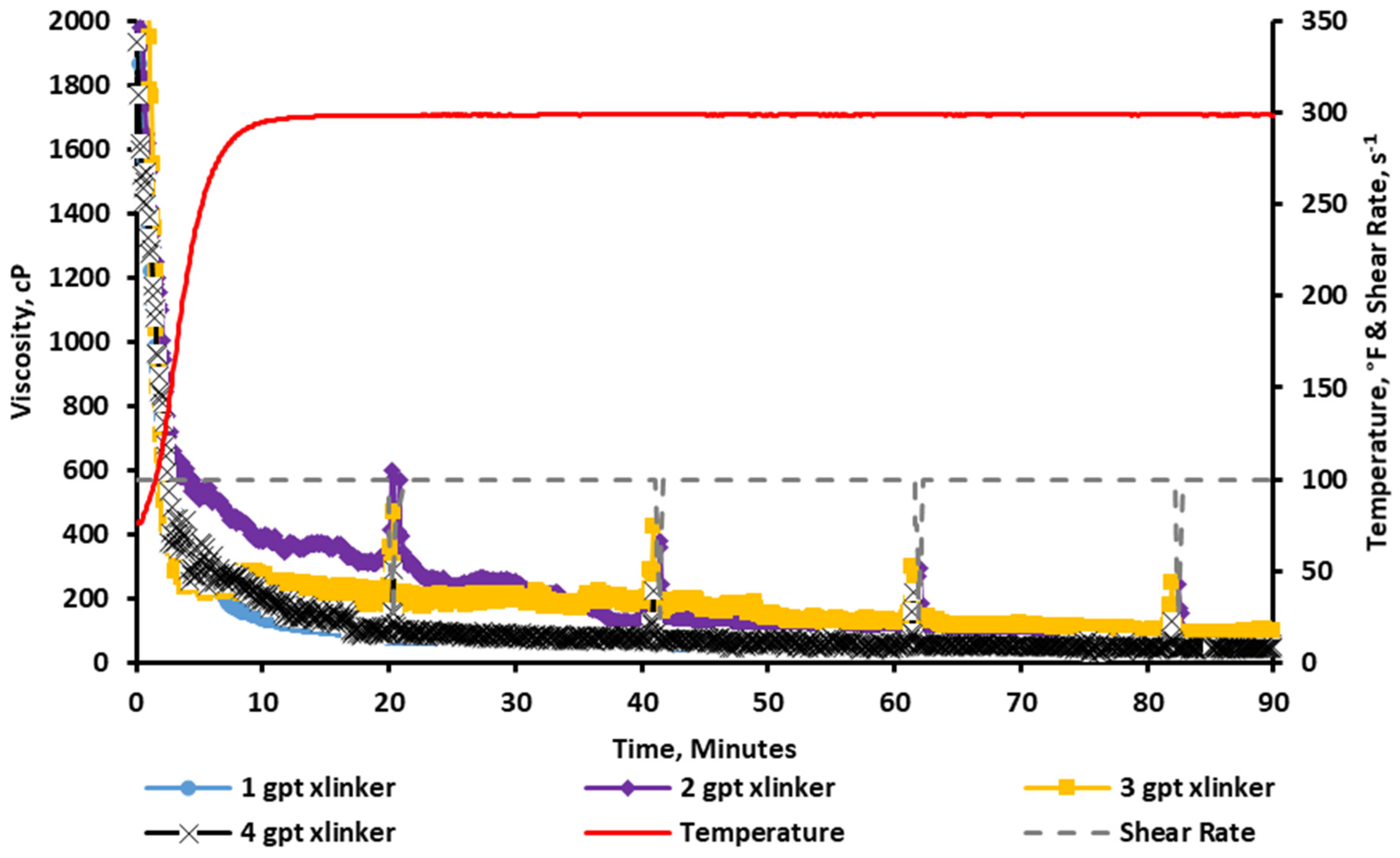
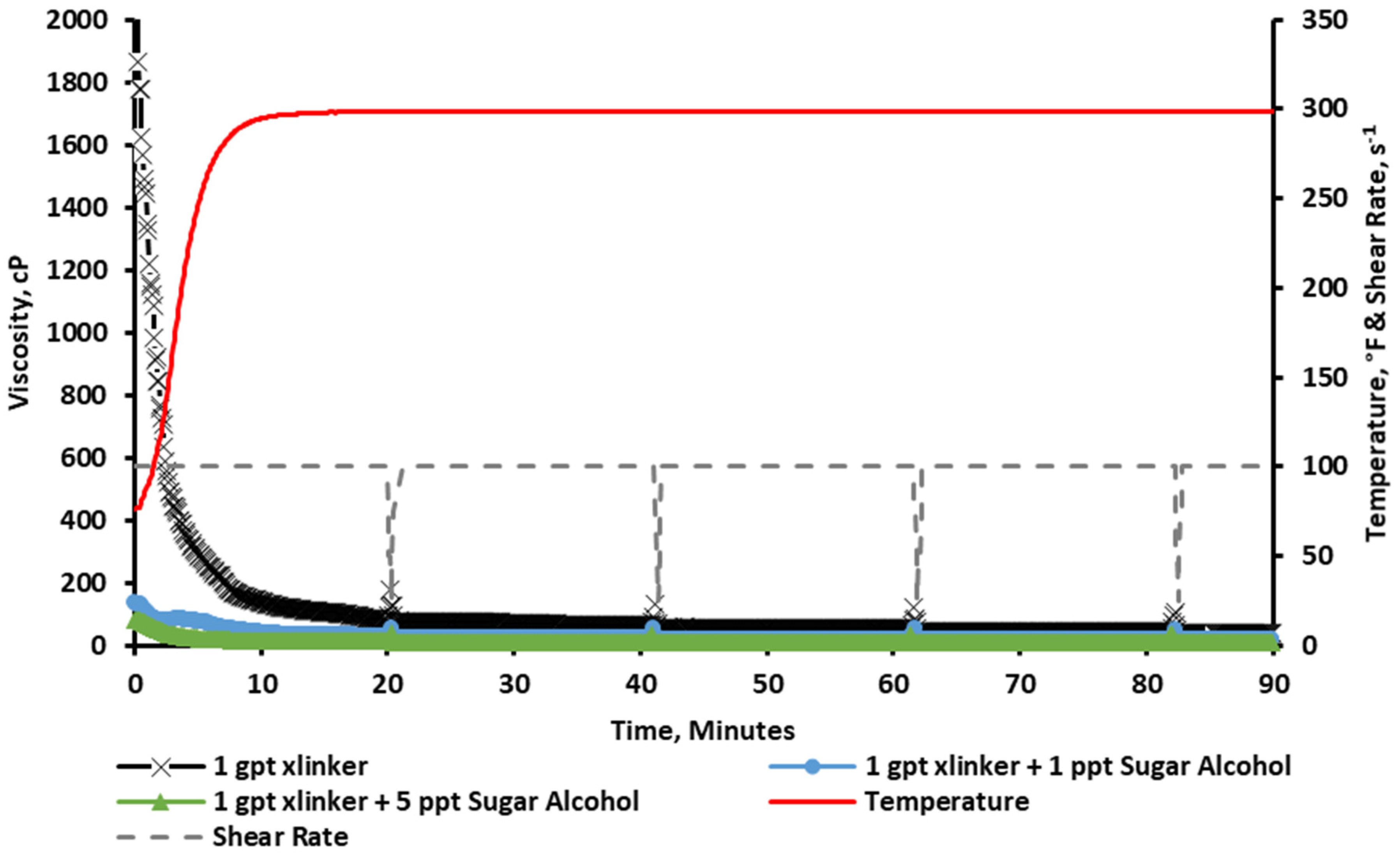
2.2.1. Rheology at a 1 gpt Crosslinker Concentration
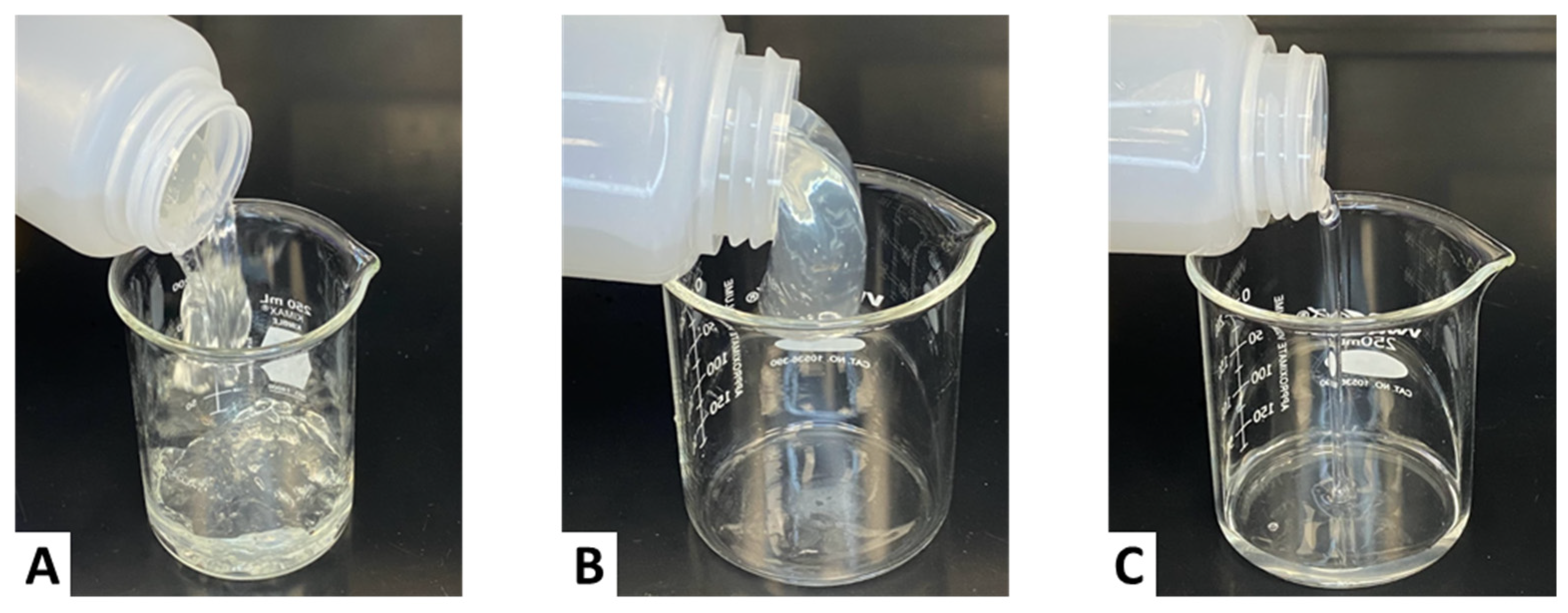
2.2.2. Visual Observation at a 1 gpt Crosslinker Concentration
2.2.3. Rheology at a 2 gpt Crosslinker Concentration
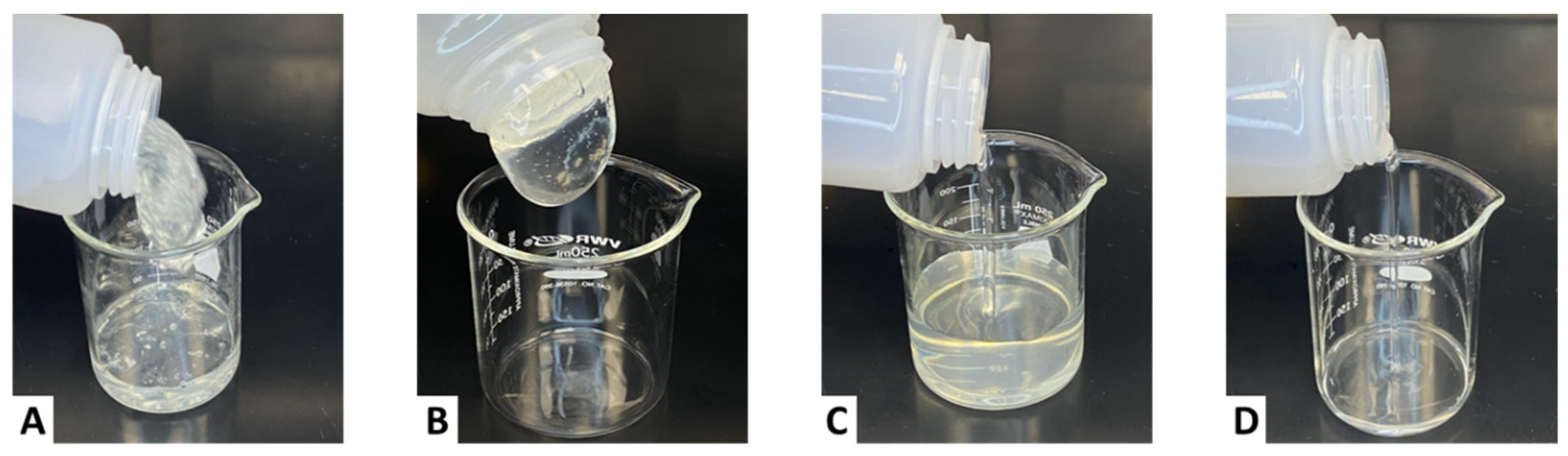
2.2.4. Visual Observation at a 2 gpt Crosslinker Concentration
2.2.5. Rheology at a 3 gpt Crosslinker Concentration
2.2.6. Visual Observation at a 3 gpt Crosslinker Concentration
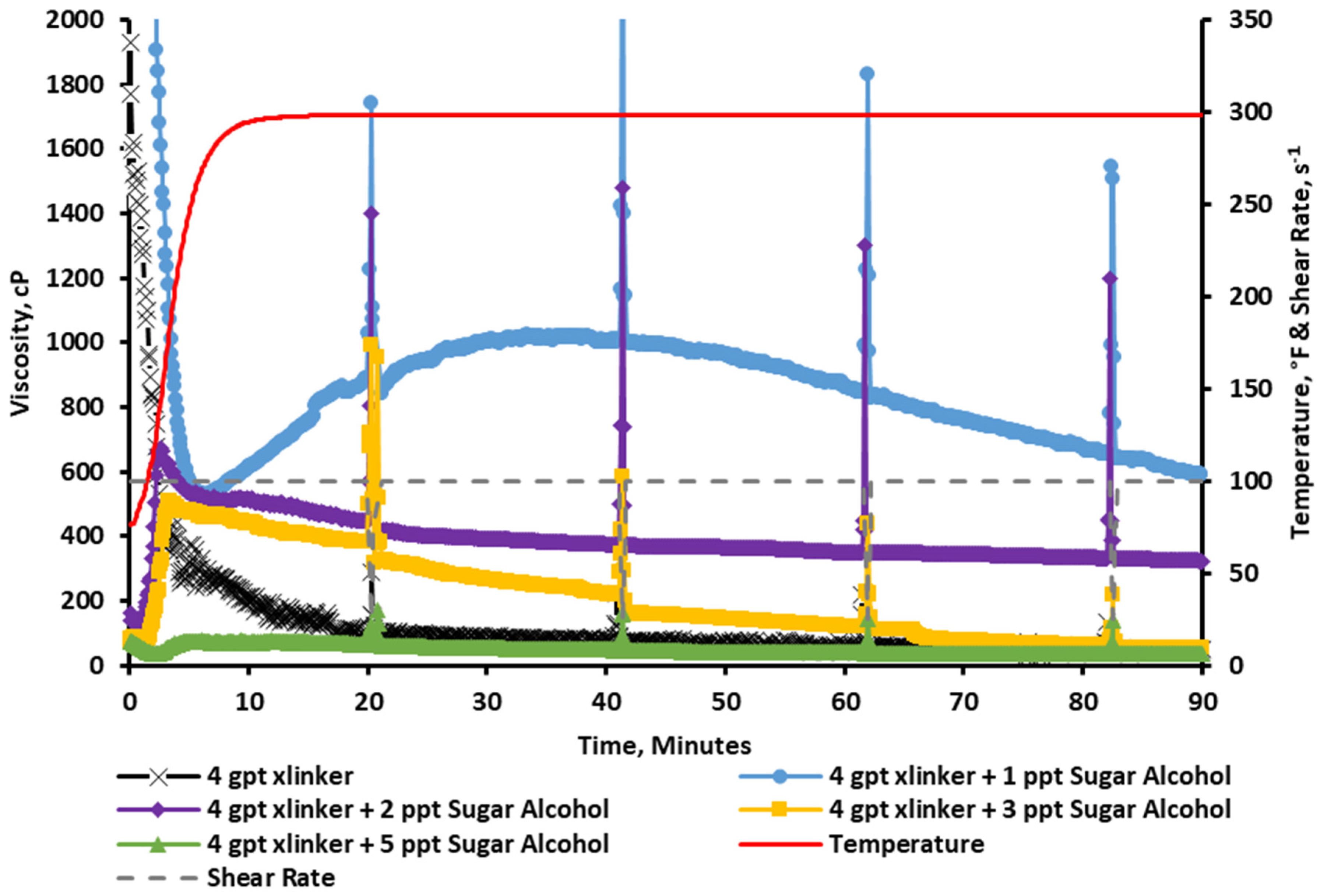
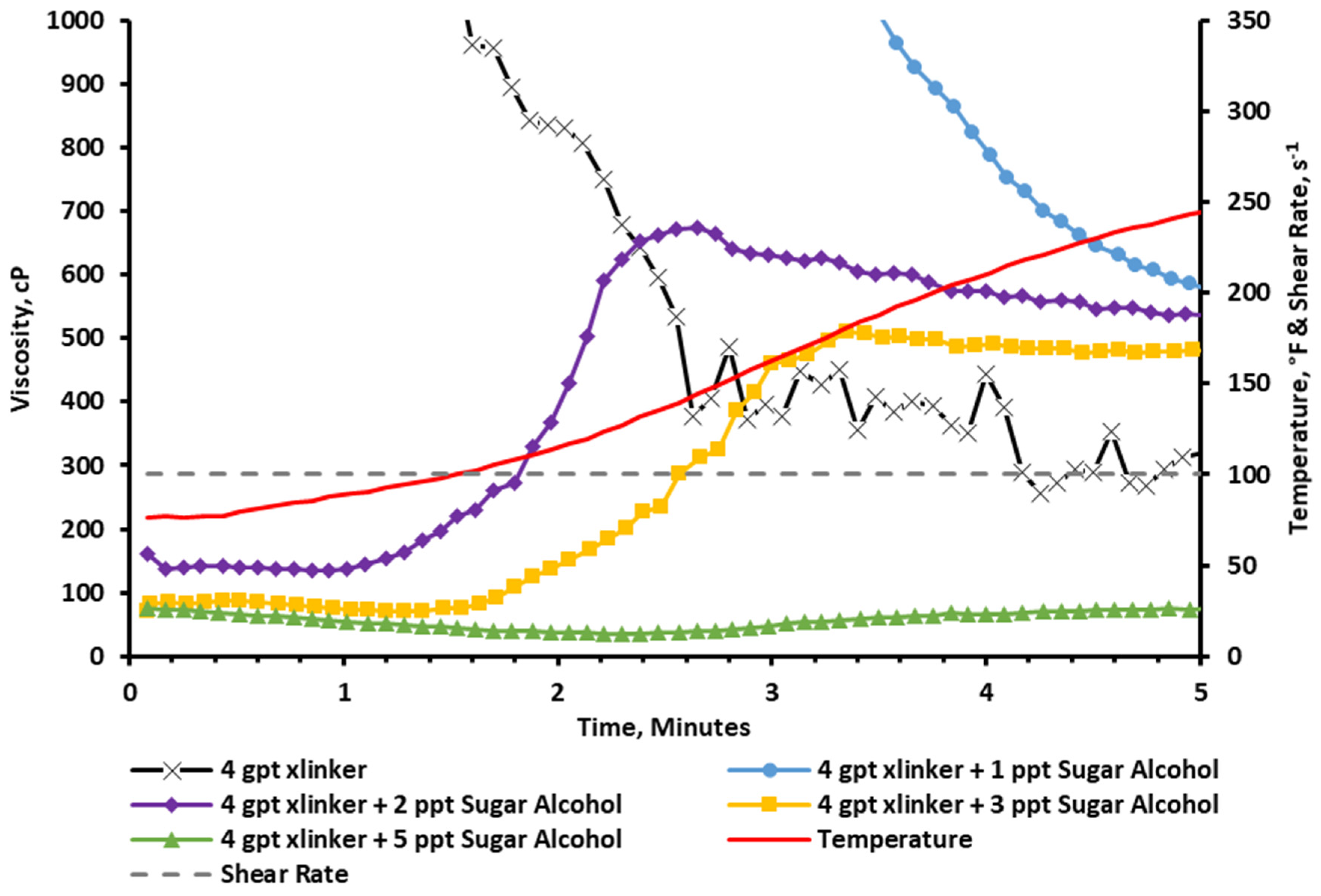
2.2.7. Rheology at a 4 gpt Crosslinker Concentration
2.2.8. Visual Observation at a 4 gpt Crosslinker Concentration
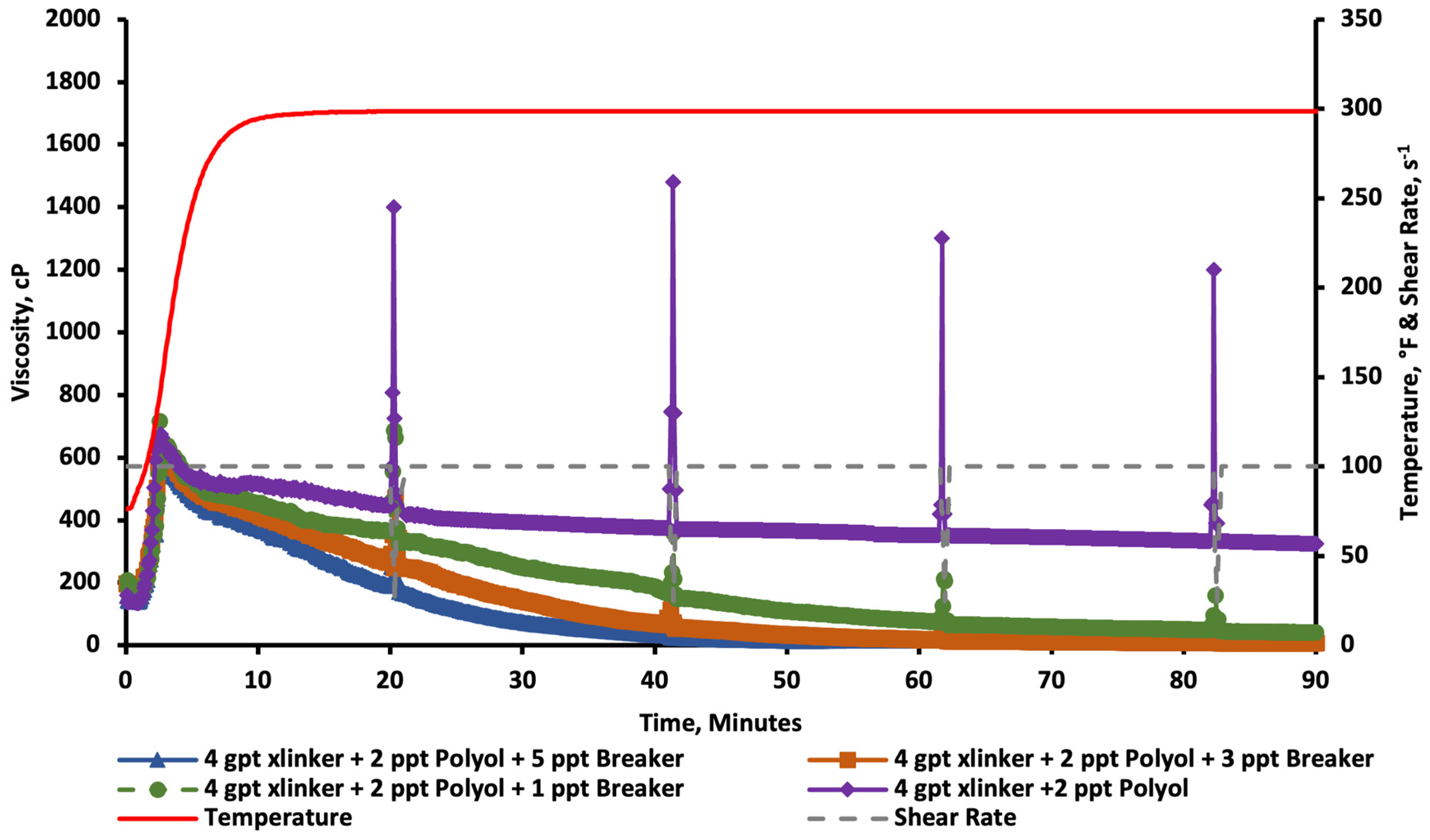
2.3. Breaker Tests
2.4. Proppant Suspension
3. Summary and Conclusions
- CMHPG fluids crosslinked at a pH of 10.7 exhibited rapid and unstable crosslinking behavior across 1–4 gpt of zirconium concentrations at 300 °F, leading to viscosity loss under shearing.
- Incorporating sugar alcohol successfully delayed crosslinking, with optimal results observed at 2 ppt of sugar alcohol combined with 4 gpt of zirconium crosslinker.
- The delayed crosslinking effect improved viscosity stability, enabling fluids to maintain viscosity above 300 cP for 1.5 h at 300 °F.
- Breaker tests using sodium bromate confirmed that sugar alcohols do not interfere with oxidative breakers, providing a controlled viscosity reduction profile.
- Proppant suspension was retained by over 95% of the proppant under both room temperature and 200 °F, demonstrating the additive’s suitability for field applications.
4. Recommendations
5. Materials and Fluid Preparation
6. Equipment and Laboratory Procedures
6.1. HPHT Viscometer
6.2. Lipping Behavior
6.3. Proppant Settling
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPG | Hydroxypropyl Guar |
| CMHPG | Carboxymethylhydroxypropyl Guar |
| cP | Centipoise |
| gpt | Gallons of chemical per thousand gallons of treatment |
| HPHT | High-pressure/high-temperature |
| lb | Pounds |
| ppg | Pounds of chemical per gallon of treatment |
| ppt | Pounds of chemical per thousand gallons of treatment |
| TEPA | Tetraethylenepentamine |
References
- Goel, N.; Shah, S. A Rheological Criterion for Fracturing Fluids to Transport Proppant during a Stimulation Treatment. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 30 September 2001; p. SPE-71663-MS. [Google Scholar] [CrossRef]
- Shah, S.N.; Patel, H.R. Proppant Transport. In Hydraulic Fracturing: Fundamentals and Advancements; Miskimins, J.L., Ed.; Society of Petroleum Engineers: Richardson, TX, USA, 2019; 784p, ISBN 978-1-61399-719-2. [Google Scholar]
- Harrington, L.J.; Hannah, R.R.; Williams, D. Dynamic Experiments On Proppant Settling In Crosslinked Fracturing Fluids. In Proceedings of the SPE Annual Technical Conference and Exhibition, Las Vegas, NV, USA, 23 September 1979; p. SPE-8342-MS. [Google Scholar] [CrossRef]
- Lei, C.; Clark, P.E. Crosslinking of guar and guar derivatives. SPE J. 2007, 12, 316–321. [Google Scholar] [CrossRef]
- Weaver, J.; Gdanski, R.; Karcher, A. Guar Gum Degradation: A Kinetic Study. In Proceedings of the International Symposium on Oilfield Chemistry, Houston, TX, USA, 5 February 2003; p. SPE-80226-MS. [Google Scholar] [CrossRef]
- Almubarak, T.; Li, L.; Ng, J.H.; Nasr-El-Din, H.; AlKhaldi, M. New Insights into Hydraulic Fracturing Fluids Used for High-Temperature Wells. Petroleum 2021, 7, 70–79. [Google Scholar] [CrossRef]
- Chetan, P.; Songire, S. A Sulfur-Free and Biodegradable Gel Stabilizer for High Temperature Fracturing Applications. In Proceedings of the SPE North Africa Technical Conference and Exhibition, Cairo, Egypt, 14 September 2015; p. SPE-175786-MS. [Google Scholar] [CrossRef]
- Ogunsanya, T.; Li, L. Safe Boundaries of High-Temperature Fracturing Fluids. In Proceedings of the SPE Western Regional Meeting, Garden Grove, CA, USA, 22 April 2018; p. SPE-190029-MS. [Google Scholar] [CrossRef]
- Brannon, H.D.; Hodge, R.M.; England, K.W. High Temperature Guar-Based Fracturing Fluid. U.S. Patent 4,801,389, 31 January 1989. [Google Scholar]
- Mirakyan, A.; Abad, C.; Parris, M.; Chen, Y.; Mueller, F. Rheological Characterization of Novel, Delayed-Transition Metal Crosslinked Fracturing Fluids: Correlation With First Field Applications. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 4 October 2009; p. SPE-121757-MS. [Google Scholar] [CrossRef]
- Sokhanvarian, K.; Nasr-El-Din, H.A.; Harper, T.L. Effect of Ligand Type Attached to Zirconium-Based Crosslinkers and the Effect of a New Dual Crosslinker on the Properties of Crosslinked Carboxymethylhydroxypropylguar. SPE J. 2019, 24, 1741–1756. [Google Scholar] [CrossRef]
- Knaak, J.B.; Leung, H.-W.; Stott, W.T.; Busch, J.; Bilsky, J. Toxicology of Mono-, Di-, and Triethanolamine. In Reviews of Environmental Contamination and Toxicology: Continuation of Residue Reviews; Ware, G.W., Ed.; Springer: New York, NY, USA, 1997; pp. 1–86. ISBN 978-1-4612-2272-9. [Google Scholar]
- National Toxicology Program; Public Health Service; National Institutes of Health; US Department of Health and Human Services. NTP Toxicology and Carcinogenesis Studies of Triethanolamine (Cas No. 102-71-6) in B6C3F1 Mice (Dermal Studies). Natl. Toxicol. Program Tech. Rep. Ser. 2004, 518, 5–163. [Google Scholar]
- Balwierz, R.; Biernat, P.; Jasińska-Balwierz, A.; Siodłak, D.; Kusakiewicz-Dawid, A.; Kurek-Górecka, A.; Olczyk, P.; Ochędzan-Siodłak, W. Potential Carcinogens in Makeup Cosmetics. Int. J. Environ. Res. Public Health 2023, 20, 4780. [Google Scholar] [CrossRef]
- Stringfellow, W.T.; Camarillo, M.K.; Domen, J.K.; Sandelin, W.L.; Varadharajan, C.; Jordan, P.D.; Reagan, M.T.; Cooley, H.; Heberger, M.G.; Birkholzer, J.T. Identifying Chemicals of Concern in Hydraulic Fracturing Fluids Used for Oil Production. Environ. Pollut. 2017, 220, 413–420. [Google Scholar] [CrossRef]
- Kahrilas, G.A.; Blotevogel, J.; Stewart, P.S.; Borch, T. Biocides in Hydraulic Fracturing Fluids: A Critical Review of Their Usage, Mobility, Degradation, and Toxicity. Environ. Sci. Technol. 2015, 49, 16–32. [Google Scholar] [CrossRef]
- Ng, J.H.; Almubarak, T.; Nasr-El-Din, H. Natural Alkaloid as a Non-Toxic, Environmentally Friendly Corrosion Inhibitor. Can. J. Chem. Eng. 2022, 100, 1214–1225. [Google Scholar] [CrossRef]
- Almubarak, T.; Ng, J.H.; Almubarak, M.; AlOtaibi, F. Fragrant Flower Extracts as Corrosion Inhibitors in the Oil and Gas Industry. In Proceedings of the International Petroleum Technology Conference, Bangkok, Thailand, 1 March 2023; p. IPTC-22877-MS. [Google Scholar] [CrossRef]
- Almubarak, T.; Almubarak, M.; Almoajil, A.; Alotaibi, F. Vitamin C: An Environmentally Friendly Multifunctional Additive for Hydraulic Fracturing Fluids. In Proceedings of the ADIPEC, Abu Dhabi, United Arab Emirates, 31 October 2022; p. SPE-211113-MS. [Google Scholar] [CrossRef]
- Li, L.; Al-Muntasheri, G.A.; Liang, F. A Review of Crosslinked Fracturing Fluids Prepared with Produced Water. Petroleum 2016, 2, 313–323. [Google Scholar] [CrossRef]
- Ng, J.H.; Almubarak, T.; Nasr-El-Din, H.A. Replacing the Use of Freshwater With Seawater: Problems, Solutions, and Applications. In Proceedings of the 6th Unconventional Resources Technology Conference, Houston, TX, USA, 23 July 2018; p. URTEC-2896321-MS. [Google Scholar] [CrossRef]
- Almubarak, M.; Almubarak, T.; Ng, J.H.; Hernandez, J.; Nasr-El-Din, H. Recent Advances in Waterless Fracturing Fluids: A Review. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 9 November 2020; p. SPE-202981-MS. [Google Scholar] [CrossRef]
- Almubarak, T.; Almubarak, M.; Rafie, M.; Almoajil, A. Turning the Most Abundant Form of Trash Worldwide into Effective Corrosion Inhibitors for Applications in the Oil and Gas Industry. In Proceedings of the ADIPEC, Abu Dhabi, United Arab Emirates, 31 October 2022; p. SPE-211161-MS. [Google Scholar] [CrossRef]
- Kalgaonkar, R.; Alkhowaildi, M.; Sahu, Q.; Alali, A. Novel Hydraulic Fracturing Fluid Supporting the Sustainability Initiatives for Freshwater Conservation. In Proceedings of the ADIPEC, Abu Dhabi, United Arab Emirates, 2 October 2023; p. SPE-216321-MS. [Google Scholar] [CrossRef]
- Budiman, O.; Alajmei, S. Seawater-Based Fracturing Fluid: A Review. ACS Omega 2023, 8, 41022–41038. [Google Scholar] [CrossRef]
- Manickvasagan, A.; Kumar, C.S.; Al-Attabi, Z.H. Effect of Sugar Replacement with Date Paste and Date Syrup on Texture and Sensory Quality of Kesari (Traditional Indian Dessert). J. Agric. Mar. Sci. 2017, 22, 67–74. [Google Scholar] [CrossRef]
- Arshad, S.; Rehman, T.; Saif, S.; Rajoka, M.S.R.; Ranjha, M.M.A.N.; Hassoun, A.; Cropotova, J.; Trif, M.; Younas, A.; Aadil, R.M. Replacement of Refined Sugar by Natural Sweeteners: Focus on Potential Health Benefits. Heliyon 2022, 8, e10711. [Google Scholar] [CrossRef] [PubMed]
- Kaartinen, N.E.; Similä, M.E.; Kanerva, N.; Valsta, L.M.; Harald, K.; Männistö, S. Naturally Occurring and Added Sugar in Relation to Macronutrient Intake and Food Consumption: Results from a Population-Based Study in Adults. J. Nutr. Sci. 2017, 6, e7. [Google Scholar] [CrossRef] [PubMed]
- Urbanus, B.L.; Cox, G.O.; Eklund, E.J.; Ickes, C.M.; Schmidt, S.J.; Lee, S.-Y. Sensory Differences Between Beet and Cane Sugar Sources. J. Food Sci. 2014, 79, S1763–S1768. [Google Scholar] [CrossRef]
- Clarke, M.A. SYRUPS. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 5711–5717. ISBN 978-0-12-227055-0. [Google Scholar]
- WebMD. Available online: https://www.webmd.com/diet/what-to-know-about-different-types-names-sugar (accessed on 7 January 2025).
- Godshall, M.A.; Eggleston, G.; Thompson, J.; Kochergin, V. Sugar. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: Hoboken, NJ, USA, 2001; pp. 1–84. ISBN 978-0-471-23896-6. [Google Scholar]
- Eggleston, G.; Legendre, B.; Godshall, M.A. Sugar and Other Sweeteners. In Handbook of Industrial Chemistry and Biotechnology; Kent, J.A., Bommaraju, T.V., Barnicki, S.D., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 933–978. ISBN 978-3-319-52287-6. [Google Scholar]
- East, C.P.; Fellows, C.M.; Doherty, W.O.S. Chapter 25—Scale in Sugar Juice Evaporators: Types, Cases, and Prevention. In Mineral Scales and Deposits; Amjad, Z., Demadis, K.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 619–637. ISBN 978-0-444-63228-9. [Google Scholar]
- Magistretti, P.J.; Allaman, I. A Cellular Perspective on Brain Energy Metabolism and Functional Imaging. Neuron 2015, 86, 883–901. [Google Scholar] [CrossRef]
- Pase, M.P.; Himali, J.J.; Jacques, P.F.; DeCarli, C.; Satizabal, C.L.; Aparicio, H.; Vasan, R.S.; Beiser, A.S.; Seshadri, S. Sugary Beverage Intake and Preclinical Alzheimer’s Disease in the Community. Alzheimer’s Dement. 2017, 13, 955–964. [Google Scholar] [CrossRef]
- Epner, M.; Yang, P.; Wagner, R.W.; Cohen, L. Understanding the Link between Sugar and Cancer: An Examination of the Preclinical and Clinical Evidence. Cancers 2022, 14, 6042. [Google Scholar] [CrossRef]
- Ahmad, A.; Isherwood, C.; Umpleby, M.; Griffin, B. Effects of High and Low Sugar Diets on Cardiovascular Disease Risk Factors. J. Nutr. Sci. Vitaminol. 2020, 66, S18–S24. [Google Scholar] [CrossRef]
- Jensen, T.; Abdelmalek, M.F.; Sullivan, S.; Nadeau, K.J.; Green, M.; Roncal, C.; Nakagawa, T.; Kuwabara, M.; Sato, Y.; Kang, D.-H.; et al. Fructose and Sugar: A Major Mediator of Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2018, 68, 1063–1075. [Google Scholar] [CrossRef]
- Harvard, T.H. Chan School of Public Health. Added Sugar. Available online: https://nutritionsource.hsph.harvard.edu/carbohydrates/added-sugar-in-the-diet (accessed on 7 January 2025).
- Ahmed, J.; Preissner, S.; Dunkel, M.; Worth, C.L.; Eckert, A.; Preissner, R. SuperSweet—A Re January 9 2025source on Natural and Artificial Sweetening Agents. Nucleic Acids Res. 2011, 39, D377–D382. [Google Scholar] [CrossRef]
- Yan, M.R.; Welch, R.; Rush, E.C.; Xiang, X.; Wang, X. A Sustainable Wholesome Foodstuff; Health Effects and Potential Dietotherapy Applications of Yacon. Nutrients 2019, 11, 2632. [Google Scholar] [CrossRef] [PubMed]
- Shivani; Thakur, B.K.; Mallikarjun, C.P.; Mahajan, M.; Kapoor, P.; Malhotra, J.; Dhiman, R.; Kumar, D.; Pal, P.K.; Kumar, S. Introduction, Adaptation and Characterization of Monk Fruit (Siraitia Grosvenorii): A Non-Caloric New Natural Sweetener. Sci. Rep. 2021, 11, 6205. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Amarnath, S.; Thulasimani, M.; Ramaswamy, S. Artificial Sweeteners as a Sugar Substitute: Are They Really Safe? Indian J. Pharmacol. 2016, 48, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Morales, P.; Ferreira, I.C. Sweeteners as Food Additives in the XXI Century: A Review of What Is Known, and What Is to Come. Food Chem. Toxicol. 2017, 107, 302–317. [Google Scholar] [CrossRef]
- Cummings, J.H.; Stephen, A.M. Carbohydrate Terminology and Classification. Eur. J. Clin. Nutr. 2007, 61, S5–S18. [Google Scholar] [CrossRef]
- Godswill, A.C. Sugar Alcohols: Chemistry, Production, Health Concerns and Nutritional Importance of Mannitol, Sorbitol, Xylitol, and Erythritol; IJAAR: Port Harcourt, Nigeria, 2017. [Google Scholar]
- Gauthier, E.; Milagro, F.I.; Navas-Carretero, S. Effect of Low-and Non-Calorie Sweeteners on the Gut Microbiota: A Review of Clinical Trials and Cross-Sectional Studies. Nutrition 2024, 117, 112237. [Google Scholar] [CrossRef]
- O’Brien-Nabors, L. Alternative Sweeteners, 4th ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-0-429-10908-9. [Google Scholar]
- Schiweck, H.; Ziesenitz, S. Physiological Properties of Polyols in Comparison with Easily Metabolisable Saccharides. In Advances in Sweeteners; Springer: New York, NY, USA, 1996; pp. 56–84. [Google Scholar]
- Kawanabe, J.; Hirasawa, M.; Takeuchi, T.; Oda, T.; Ikeda, T. Noncariogenicity of Erythritol as a Substrate. Caries Res. 1992, 26, 358–362. [Google Scholar] [CrossRef]
- Ortiz, M.E.; Bleckwedel, J.; Raya, R.R.; Mozzi, F. Biotechnological and in Situ Food Production of Polyols by Lactic Acid Bacteria. Appl. Microbiol. Biotechnol. 2013, 97, 4713–4726. [Google Scholar] [CrossRef]
- Al-Muntasheri, G.A.; Li, L.; Liang, F.; Gomaa, A.M. Concepts in Cleanup of Fracturing Fluids Used in Conventional Reservoirs: A Literature Review. SPE Prod. Oper. 2018, 33, 196–213. [Google Scholar] [CrossRef]
- ISO 13503-1; Petroleum and Natural Gas Industries-Completion Fluids and Materials—Part 1, Measurement of Viscous Properties of Completion Fluids, Second Edition. ISO: Geneva, Switzerland, 2011.








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almubarak, T.; Alabdrabalnabi, M.I.; Albaiz, A.; Yami, M. Sugar Alcohols as Crosslinking Delay Additives for Fracturing Fluids. Gels 2025, 11, 457. https://doi.org/10.3390/gels11060457
Almubarak T, Alabdrabalnabi MI, Albaiz A, Yami M. Sugar Alcohols as Crosslinking Delay Additives for Fracturing Fluids. Gels. 2025; 11(6):457. https://doi.org/10.3390/gels11060457
Chicago/Turabian StyleAlmubarak, Tariq, Mohammed I. Alabdrabalnabi, Abdualilah Albaiz, and Mohammed Yami. 2025. "Sugar Alcohols as Crosslinking Delay Additives for Fracturing Fluids" Gels 11, no. 6: 457. https://doi.org/10.3390/gels11060457
APA StyleAlmubarak, T., Alabdrabalnabi, M. I., Albaiz, A., & Yami, M. (2025). Sugar Alcohols as Crosslinking Delay Additives for Fracturing Fluids. Gels, 11(6), 457. https://doi.org/10.3390/gels11060457






