Effects of Melatonin-Loaded Poly(N-vinylcaprolactam) Transdermal Gel on Sleep Quality
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation and Characterisation of Melatonin-Loaded p(NVCL) Transdermal Gel
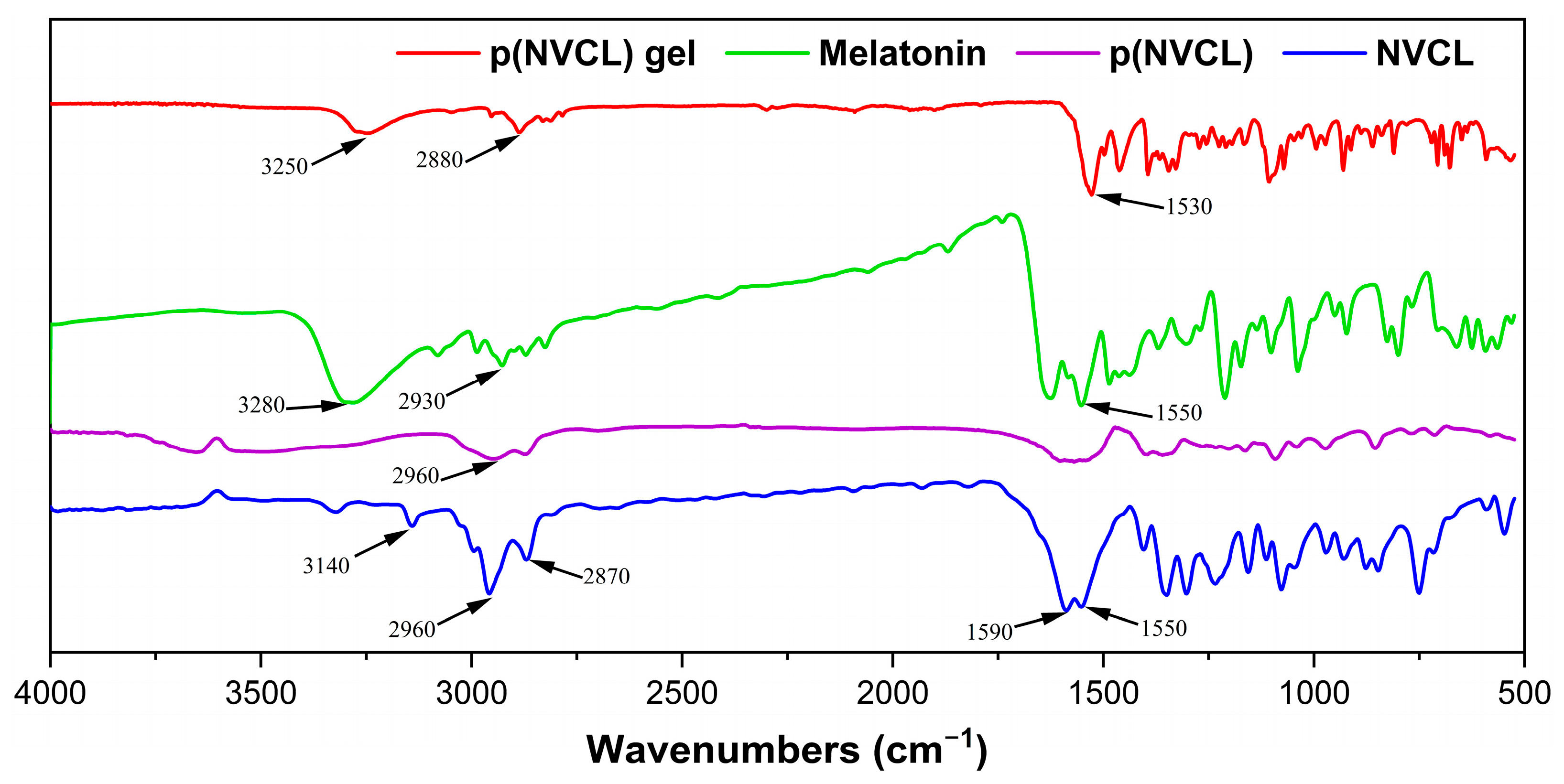
2.1.1. Polymer Synthesis Results and Structural Confirmation
2.1.2. Thermal Properties and Molecular Weight Distribution
2.1.3. Melatonin Loading and Encapsulation Characteristics
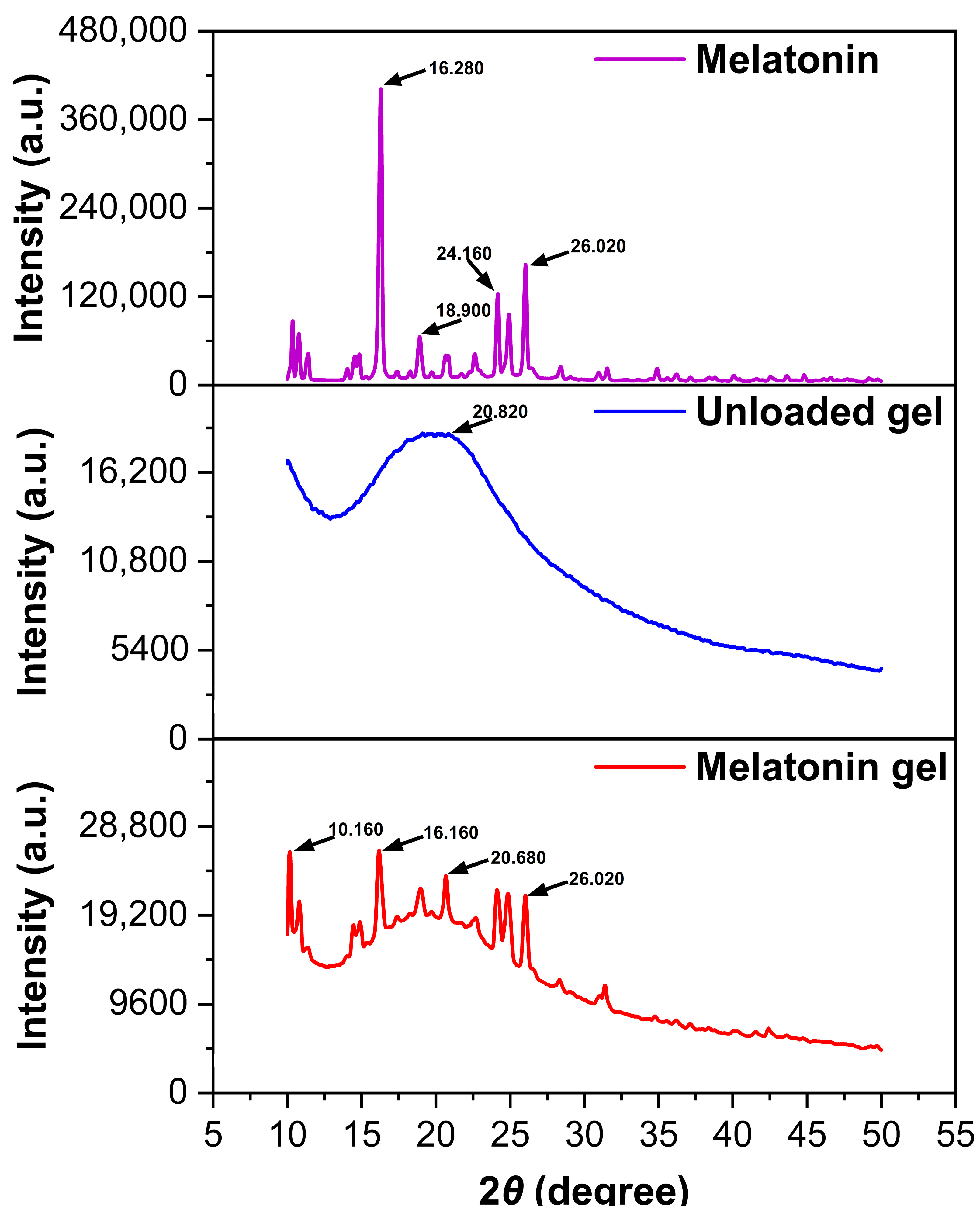
2.1.4. Powder X-Ray Diffraction Analysis and Crystalline State Characterization
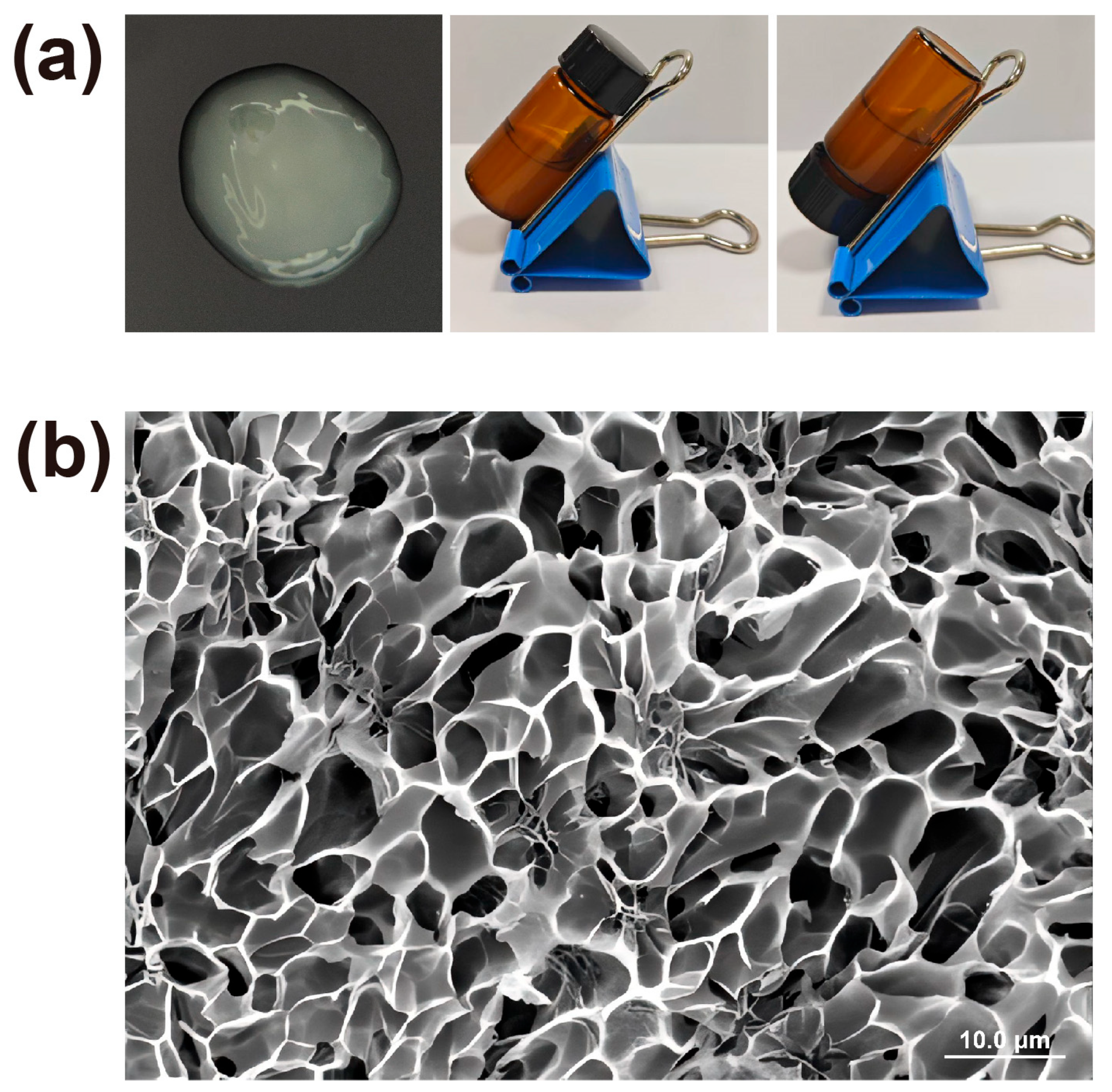
2.2. Physicochemical Properties of Melatonin-Loaded p(NVCL) Transdermal Gel
2.3. Optimisation of Transdermal Permeation Enhancers Transdermal Permeation Enhancement Performance
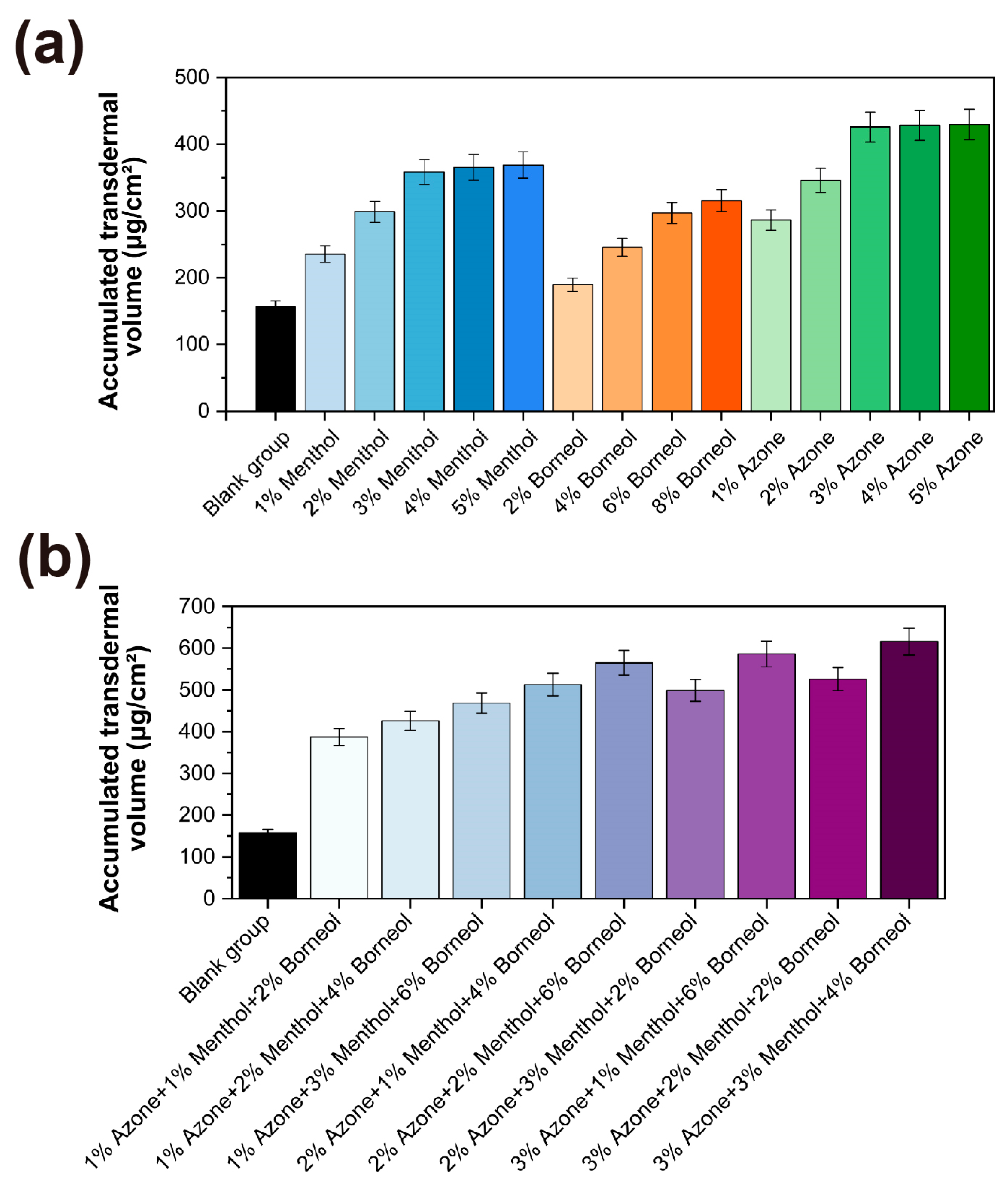
2.3.1. Screening of Single Permeation Enhancers Single Enhancer Efficacy Results
2.3.2. Composite Enhancer System Performance Evaluation
2.4. Physical–Mechanical Properties of Melatonin-Loaded p(NVCL) Transdermal Gel
2.4.1. Rheological Property Analysis
2.4.2. Thixotropic Property Evaluation
2.4.3. Stability Studies Formulation Stability Assessment Results
2.5. In Vivo Evaluation of Sleep Quality Improvement and Safety of Melatonin-Loaded p(NVCL) Transdermal Gel
2.5.1. Behavioral Assessment of Sleep Promotion Sleep Promotion Behavioral Outcomes
2.5.2. Electroencephalographic Sleep Structure Analysis EEG-Based Sleep Architecture Analysis Results
2.5.3. Neurochemical Mechanism Evaluation
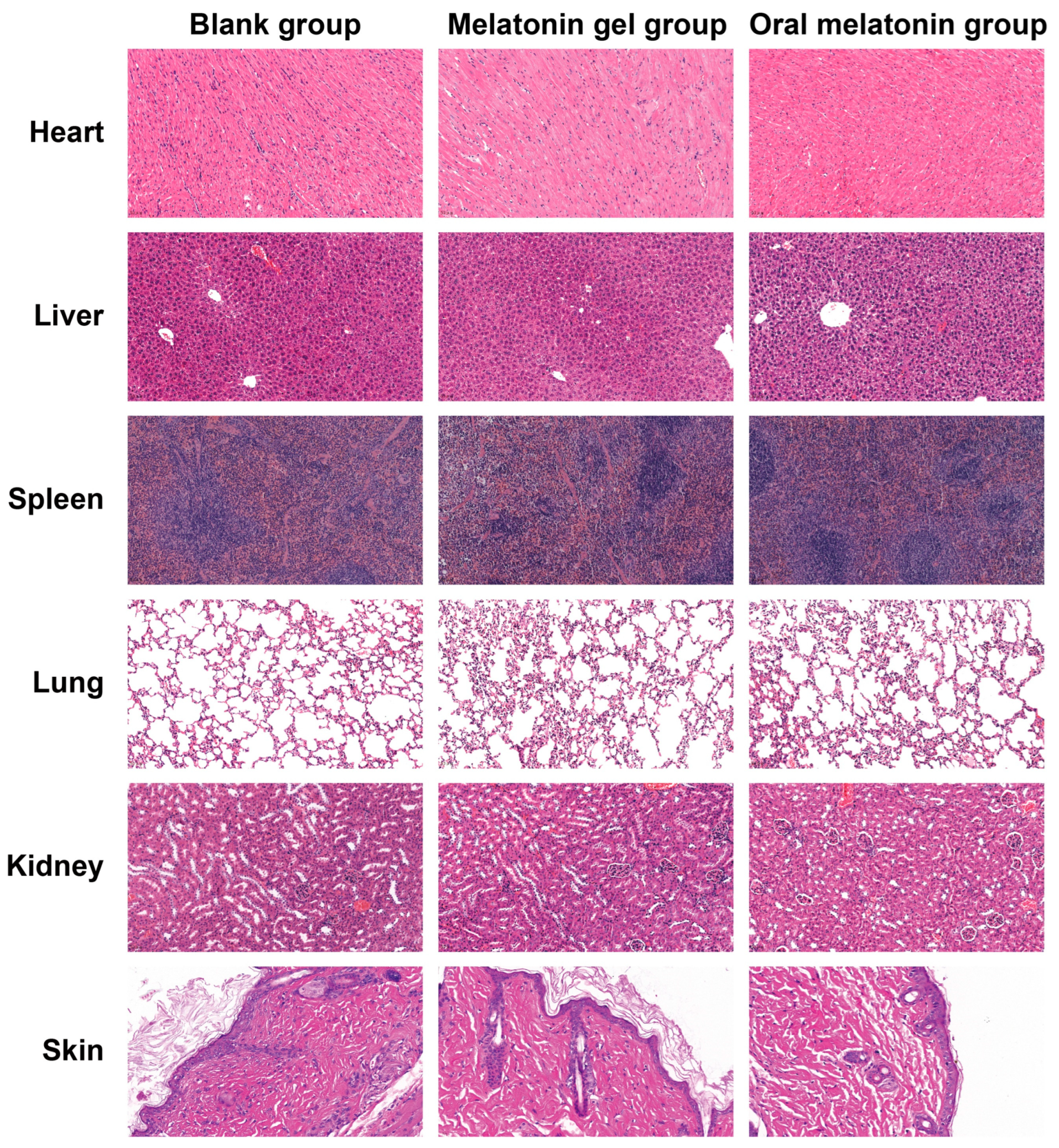
2.5.4. Safety and Tolerability Evaluation
2.6. In Vivo Biodistribution and Transdermal Absorption Visualization
2.6.1. Synthesis and Characterization of Cy5.5-Melatonin Fluorescent Probe
2.6.2. In Vivo Near-Infrared Fluorescence Imaging
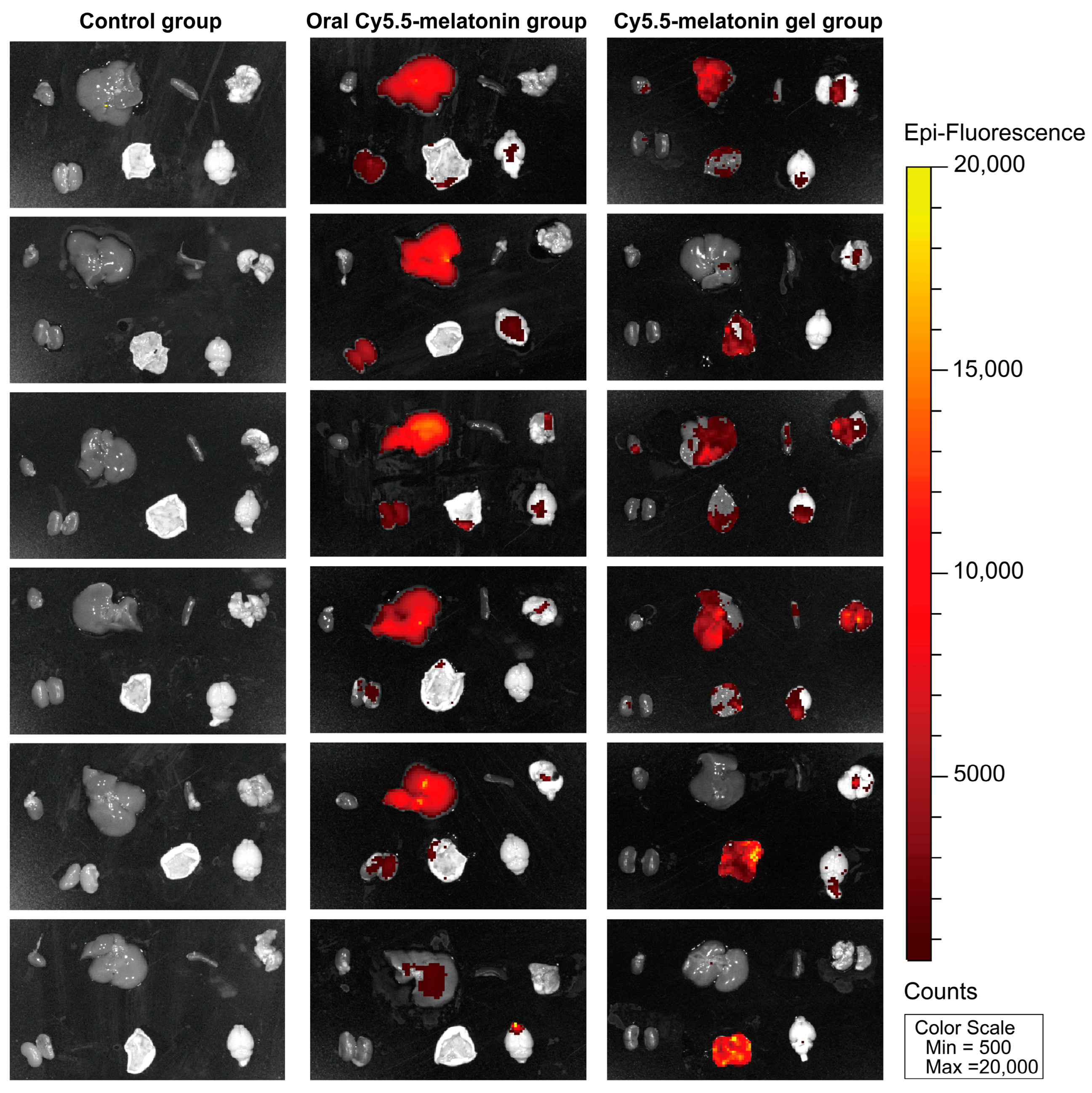
2.6.3. Biodistribution Analysis and Transdermal Absorption Confirmation
3. Conclusions
4. Materials and Methods
4.1. Materials and Reagents
4.2. Synthesis and Characterisation of p(NVCL)
4.2.1. Polymerization Procedure
4.2.2. FTIR Spectroscopic Analysis
4.2.3. LCST Determination
4.2.4. GPC Analysis
4.2.5. PXRD Analysis
4.3. Preparation of Melatonin-Loaded p(NVCL) Transdermal Gel
4.4. Determination of Melatonin Content
4.5. Optimisation of Transdermal Permeation Enhancers Transdermal Permeation Enhancer Optimization Methodology
4.5.1. Franz Diffusion Cell Setup
4.5.2. Screening of Single Permeation Enhancers Single Permeation Enhancer Screening Protocol
4.5.3. Composite Enhancer System Development Protocol
4.6. Characterisation of Melatonin-Loaded p(NVCL) Transdermal Gel
4.6.1. Physicochemical Property Evaluation
4.6.2. Rheological Characterisation
4.6.3. Thixotropic Property Assessment
4.6.4. Stability Studies Stability Testing Protocol
4.7. Evaluation of the Sleep Quality Effects and Safety of Melatonin-Loaded p(NVCL) Transdermal Gel
4.7.1. Animal Grouping and Administration Protocol
4.7.2. Behavioral Assessment of Sleep Promotion Behavioral Assessment Methodology
4.7.3. Electroencephalographic Sleep Structure Analysis Electroencephalographic Monitoring Protocol
4.7.4. Neurochemical Mechanism Investigation
4.7.5. Safety and Tolerability Assessment
4.8. Synthesis of Cy5.5-Melatonin Fluorescent Probe and In Vivo Imaging
4.8.1. Chemical Synthesis of Cy5.5-Melatonin
4.8.2. Analytical Characterization
4.8.3. In Vivo Near-Infrared Fluorescence Imaging Protocol
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gueye-Ndiaye, S.; Redline, S. Sleep Health Disparities. Annu. Rev. Med. 2025, 76, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Cavaillès, C.; Dintica, C.S.; Habes, M.; Leng, Y.; Carnethon, M.; Yaffe, K. Sleep Patterns in Midlife and Brain Age. Alzheimer’s Dement. 2024, 20, e085643. [Google Scholar] [CrossRef]
- Carnes, A.; Piñol-Ripoll, G.; Ariza, M.; Cano, N.; Segura, B.; Junqué, C.; Garoellda, M. Poor sleep quality as a potential trigger for cognitive deficits. Alzheimer’s Dement. 2024, 20, e087261. [Google Scholar] [CrossRef]
- Shan, W.; Peng, X.; Tan, W.; Zhou, Z.; Xie, H.; Wang, S. Prevalence of insomnia and associations with depression, anxiety among adults in guangdong, China: A large-scale cross-sectional study. Sleep Med. 2024, 115, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; Chai, Y.; Guo, B.; Quan, P.; Rao, H. Sleep Architecture and Sleep EEG Alterations are Associated with Impaired Cognition Under Sleep Restriction. Nat. Sci. Sleep 2023, 15, 823–838. [Google Scholar] [CrossRef]
- Zinchuk, A.V.; Chu, J.H.; Liang, J.; Celik, Y.; Op de Beeck, S.; Redeker, N.S.; Wellman, A.; Yaggi, H.K.; Peker, Y.; Sands, S.A. Physiological Traits and Adherence to Sleep Apnea Therapy in Individuals with Coronary Artery Disease. Am. J. Respir. Crit. Care Med. 2021, 204, 703–712. [Google Scholar] [CrossRef]
- Bonsignore, M.R.; Lombardi, C.; Lombardo, S.; Fanfulla, F. Epidemiology, Physiology and Clinical Approach to Sleepiness at the Wheel in OSA Patients: A Narrative Review. J. Clin. Med. 2022, 11, 3691. [Google Scholar] [CrossRef]
- Flaxer, J.M.; Heyer, A.; Francois, D. Evidenced-Based Review and Evaluation of Clinical Significance: Nonpharmacological and Pharmacological Treatment of Insomnia in the Elderly. Am. J. Geriatr. Psychiatry 2021, 29, 585–603. [Google Scholar] [CrossRef]
- Staines, A.C.; Broomfield, N.; Pass, L.; Orchard, F.; Bridges, J. Do non-pharmacological sleep interventions affect anxiety symptoms? A meta-analysis. J. Sleep Res. 2022, 31, e13451. [Google Scholar] [CrossRef]
- Soyka, M.; Wild, I.; Caulet, B.; Leontiou, C.; Lugoboni, F.; Hajak, G. Long-term use of benzodiazepines in chronic insomnia: A European perspective. Front. Psychiatry 2023, 14, 1212028. [Google Scholar] [CrossRef]
- Boutin, J.A.; Kennaway, D.J.; Jockers, R. Melatonin: Facts, Extrapolations and Clinical Trials. Biomolecules 2023, 13, 943. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.J.; Tang, H.; Pi, P.L.; Zhang, H.W.; Du, S.Y.; Ge, W.Y.; Dai, Q.; Zhao, Z.Y.; Li, J.; Sun, Z. Melatonin in cancer biology: Pathways, derivatives, and the promise of targeted delivery. Drug Metab. Rev. 2024, 56, 62–79. [Google Scholar] [CrossRef]
- Thanawala, S.; Abiraamasundari, R.; Shah, R. Comparative Pharmacokinetics of Sustained-Release versus Immediate-Release Melatonin Capsules in Fasting Healthy Adults: A Randomized, Open-Label, Cross-Over Study. Pharmaceutics 2024, 16, 1248. [Google Scholar] [CrossRef]
- Myburgh, J.; Liebenberg, W.; Willers, C.; Dube, A.; Gerber, M. Investigation and Evaluation of the Transdermal Delivery of Ibuprofen in Various Characterized Nano-Drug Delivery Systems. Pharmaceutics 2023, 15, 2413. [Google Scholar] [CrossRef]
- Karve, T.; Dandekar, A.; Agrahari, V.; Melissa Peet, M.; Banga, A.K.; Doncel, G.F. Long-acting transdermal drug delivery formulations: Current developments and innovative pharmaceutical approaches. Adv. Drug Deliv. Rev. 2024, 210, 115326. [Google Scholar] [CrossRef]
- Laohasiriwong, S.; Nukulkit, C.; Johns, N.P.; Soontornpas, C.; Priprem, A.; Yimtae, K. Sleep induction effect of daytime administration of melatonin niosome gel in healthy volunteers. Sleep Med. 2017, 40, e178. [Google Scholar] [CrossRef]
- Gola, A.; Pietrańczyk, R.; Musiał, W. Synthesis and Physicochemical Properties of Thermally Sensitive Polymeric Derivatives of N-vinylcaprolactam. Polymers 2024, 16, 1917. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Urias, A.; Licea-Claverie, A.; Sañudo-Barajas, J.A.; González-Ayón, M.A. NVCL-Based Hydrogels and Composites for Biomedical Applications: Progress in the Last Ten Years. Int. J. Mol. Sci. 2022, 23, 4722. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, J.G.; Prabhu, T.N.; Pal, K. Poly(N-isopropyl acrylamide)-co-poly(sodium acrylate) hydrogel for the adsorption of cationic dyes from aqueous solution. Eur. Phys. J. E 2023, 46, 11. [Google Scholar] [CrossRef]
- Wang, T.; Liu, C.; Li, Y.; Zhang, L.; Cheng, Z. Preparation of Temperature-Responsive Films Based on PNVCL Microgel with Varying Sizes and Cross-Linking Degrees for Cell Harvesting. Macromol. Rapid Commun. 2024, 46, e2400156. [Google Scholar] [CrossRef]
- Bahmani, M.; Akbarian, M.; Tayebi, L.; Farjadian, F. The inhibitory effect of curcumin loaded poly (vinyl caprolactam) nanohydrogel on insulin fibrillation. Process Biochem. 2022, 117, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Mohammad Gholiha, H.; Ehsani, M.; Saeidi, A.; Ghadami, A. Albumin-loaded thermo/pH dual-responsive nanogels based on sodium alginate and poly (N-vinyl caprolactam). Prog. Biomater. 2023, 12, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Kozlovskaya, V.; Kharlampieva, E.; Drachuk, I.; Cheng, D.; Tsukruk, V.V. Responsive microcapsule reactors based on hydrogen-bonded tannic acid layer-by-layer assemblies. Soft Matter 2010, 6, 3596–3608. [Google Scholar] [CrossRef]
- Tong, S.; Luo, S.; Yang, Q.; Song, B.; Chang, R.; Wu, J. Preparation and biological evaluation of a glucose-responsive block copolymer nanoparticle with the ability to ameliorate diabetic kidney damage. Eur. Polym. J. 2024, 220, 113472. [Google Scholar] [CrossRef]
- Tie, B.S.H.; Daly, M.; Zhuo, S.; Halligan, E.; Keane, G.; Geever, J.; Geever, L. The Exponential Shapeshifting Response of N-Vinylcaprolactam Hydrogel Bilayers Due to Temperature Change for Potential Minimally Invasive Surgery. J. Funct. Biomater. 2024, 15, 242. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.R.; Jiang, E.Y.; Duan, C.B.; Cheng, L.; Chen, Z.; Li, Y.; Wang, F.; Bian, Q.; Sukhbaatar, O.; Sun, Q.; et al. Design, Synthesis, and Antifungal Activity of Melatonin Derivatives Containing a (Thio)Semicarbazide Group. J. Pineal Res. 2025, 77, e70038. [Google Scholar] [CrossRef]
- Braga, G.P.A.; Caiaffa, K.S.; Pereira, J.A.; Santos, V.R.D.; Souza, A.C.A.; Ribeiro, L.D.S.; Camargo, E.R.; Prakki, A.; Duque, C. Microbiological Properties and Cytotoxicity of PNVCL Hydrogels Containing Flavonoids as Intracanal Medication for Endodontic Therapy. J. Funct. Biomater. 2022, 13, 305. [Google Scholar] [CrossRef]
- Briggs, F.; Browne, D.; Asuri, P. Role of Polymer Concentration and Crosslinking Density on Release Rates of Small Molecule Drugs. Int. J. Mol. Sci. 2022, 23, 4118. [Google Scholar] [CrossRef]
- Givler, D.; Givler, A.; Luther, P.M.; Wenger, D.M.; Ahmadzadeh, S.; Shekoohi, S.; Edinoff, A.N.; Dorius, B.K.; Jean Baptiste, C.; Cornett, E.M.; et al. Chronic Administration of Melatonin: Physiological and Clinical Considerations. Neurol. Int. 2023, 15, 518–533. [Google Scholar] [CrossRef]
- Sim, Y.S.; Wong, L.C.; Yeoh, S.C.; Almashhadani, A.; Alrimawi, B.H.; Goh, C.F. Skin penetration enhancers: Mechanistic understanding and their selection for formulation and design. Drug Deliv. Transl. Res. 2025. online ahead of print. [Google Scholar] [CrossRef]
- Oshizaka, T.; Kodera, S.; Kawakubo, R.; Takeuchi, I.; Mori, K.; Sugibayashi, K. Enhanced Drug Skin Permeation by Azone-Mimicking Ionic Liquids: Effects of Fatty Acids Forming Ionic Liquids. Pharmaceutics 2024, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Oshizaka, T.; Hayakawa, M.; Uesaka, M.; Yoshizawa, K.; Kamei, T.; Takeuchi, I.; Mori, K.; Itakura, S.; Todo, H.; Sugibayashi, K. Design of an Ante-enhancer with an Azone-Mimic Structure using Ionic Liquid. Pharm. Res. 2023, 40, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, Y.; Wang, S.; Liu, J.; Zhang, T.; Wei, Y.; Zhu, L.; Bai, W.; Ye, T.; Wang, S. Menthol nanoliposomes enhanced anti-tumor immunotherapy by increasing lymph node homing of dendritic cell vaccines. J. Clin. Immunol. 2022, 244, 109119. [Google Scholar] [CrossRef]
- Luo, M.; He, J.; Yin, L.; Zhan, P.; Zhao, Z.; Xiong, H.; Mei, Z. Borneol exerts its antipruritic effects by inhibiting TRPA1 and activating TRPM8. J. Ethnopharmacol. 2024, 322, 117581. [Google Scholar] [CrossRef]
- Menczel Schrire, Z.; Phillips, C.L.; Duffy, S.L.; Marshall, N.S.; Mowszowski, L.; La Monica, H.M.; Stranks, L.; Gordon, C.J.; Chapman, J.L.; Saini, B.; et al. 3-Month Melatonin Supplementation to Reduce Brain Oxidative Stress and Improve Sleep in Mild Cognitive Impairment: A Randomised Controlled Feasibility Trial. J. Pineal Res. 2024, 76, e70019. [Google Scholar] [CrossRef]
- Hannemann, J.; Laing, A.; Middleton, B.; Schwedhelm, E.; Marx, N.; Federici, M.; Kastner, M.; Skene, D.J.; Böger, R. Effect of oral melatonin treatment on insulin resistance and diurnal blood pressure variability in night shift workers. A double-blind, randomized, placebo-controlled study. Pharmacol. Res. 2024, 199, 107011. [Google Scholar] [CrossRef] [PubMed]
- Grosskopf, A.K.; Mann, J.L.; Baillet, J.; Lopez Hernandez, H.; Autzen, A.A.A.; Yu, A.C.; Appel, E.A. Extreme Extensibility in Physically Cross-Linked Nanocomposite Hydrogels Leveraging Dynamic Polymer-Nanoparticle Interactions. Macromolecules 2022, 55, 7498–7511. [Google Scholar] [CrossRef]
- Song, Y.; Yoon, M. Melatonin effects on animal behavior: Circadian rhythm, stress response, and modulation of behavioral patterns. J. Anim. Sci. Technol. 2025, 67, 1–16. [Google Scholar] [CrossRef]
- Nasini, S.; Tidei, S.; Shkodra, A.; De Gregorio, D.; Cambiaghi, M.; Comai, S. Age-Related Effects of Exogenous Melatonin on Anxiety-like Behavior in C57/B6J Mice. Biomedicines 2023, 11, 1705. [Google Scholar] [CrossRef]
- Sahin, K.; Gencoglu, H.; Korkusuz, A.K.; Orhan, C.; Aldatmaz, İ.E.; Erten, F.; Er, B.; Morde, A.; Padigaru, M.; Kilic, E. Impact of a Novel Valerian Extract on Sleep Quality, Relaxation, and GABA/Serotonin Receptor Activity in a Murine Model. Antioxidants 2024, 13, 657. [Google Scholar] [CrossRef]
- Hu, Y.; Lv, Y.; Long, X.; Yang, G.; Zhou, J. Melatonin attenuates chronic sleep deprivation-induced cognitive deficits and HDAC3-Bmal1/clock interruption. CNS Neurosci. Ther. 2024, 30, e14474. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, Z.; Han, Q.; Luo, F.; Jiang, C.; Zhang, Z.; Wang, N.; Zou, N.; Liu, G.; Long, M.; et al. Melatonin targets the paraventricular thalamus to promote non-rapid eye movement sleep in C3H/HeJ mice. Curr. Biol. 2024, 34, 3792–3803.e5. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, A.; Chéret, J.; Slominski, R.M.; Slominski, A.T.; Paus, R. Revisiting the role of melatonin in human melanocyte physiology: A skin context perspective. J. Pineal Res. 2022, 72, e12790. [Google Scholar] [CrossRef]
- Xia, T.J.; Jin, S.W.; Liu, Y.G.; Zhang, S.S.; Wang, Z.; Liu, X.M.; Pan, R.L.; Jiang, N.; Liao, Y.H.; Yan, M.Z.; et al. Shen Yuan extract exerts a hypnotic effect via the tryptophan/5-hydroxytryptamine/melatonin pathway in mice. J. Ethnopharmacol. 2024, 326, 117992. [Google Scholar] [CrossRef] [PubMed]
- Curado, D.F.; de Barros, V.V.; Noto, A.R.; Opaleye, E.S. Dependence on hypnotics: A comparative study between chronic users of benzodiazepines and Z-drugs. Braz. J. Psychiatry 2022, 44, 248–256. [Google Scholar] [CrossRef]
- Besag, F.M.C.; Vasey, M.J. Adverse events in long-term studies of exogenous melatonin. Expert Opin. Drug Saf. 2022, 21, 1469–1481. [Google Scholar] [CrossRef]
- Wu, J.Z.; Bremner, D.H.; Li, H.Y.; Sun, X.Z.; Zhu, L.M. Synthesis and evaluation of temperature- and glucose-sensitive nanoparticles based on phenylboronic acid and N-vinylcaprolactam for insulin delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 1026–1035. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Wang, F.; Huang, L.; Song, B.; Wu, J.; Zhang, Y.; Du, W.; Li, Y.; Tong, S. Effects of Melatonin-Loaded Poly(N-vinylcaprolactam) Transdermal Gel on Sleep Quality. Gels 2025, 11, 435. https://doi.org/10.3390/gels11060435
Zhao W, Wang F, Huang L, Song B, Wu J, Zhang Y, Du W, Li Y, Tong S. Effects of Melatonin-Loaded Poly(N-vinylcaprolactam) Transdermal Gel on Sleep Quality. Gels. 2025; 11(6):435. https://doi.org/10.3390/gels11060435
Chicago/Turabian StyleZhao, Wei, Fengyu Wang, Liying Huang, Bo Song, Junzi Wu, Yongbo Zhang, Wuyi Du, Yan Li, and Sen Tong. 2025. "Effects of Melatonin-Loaded Poly(N-vinylcaprolactam) Transdermal Gel on Sleep Quality" Gels 11, no. 6: 435. https://doi.org/10.3390/gels11060435
APA StyleZhao, W., Wang, F., Huang, L., Song, B., Wu, J., Zhang, Y., Du, W., Li, Y., & Tong, S. (2025). Effects of Melatonin-Loaded Poly(N-vinylcaprolactam) Transdermal Gel on Sleep Quality. Gels, 11(6), 435. https://doi.org/10.3390/gels11060435








