Multifunctional Hydrogels for Advanced Cancer Treatment: Diagnostic Imaging and Therapeutic Modalities
Abstract
1. Introduction
2. Fundamentals and Characteristics of Multifunctional Hydrogels
2.1. Definition and Classification
2.2. Physicochemical Properties
3. Preparation of Multifunctional Hydrogels
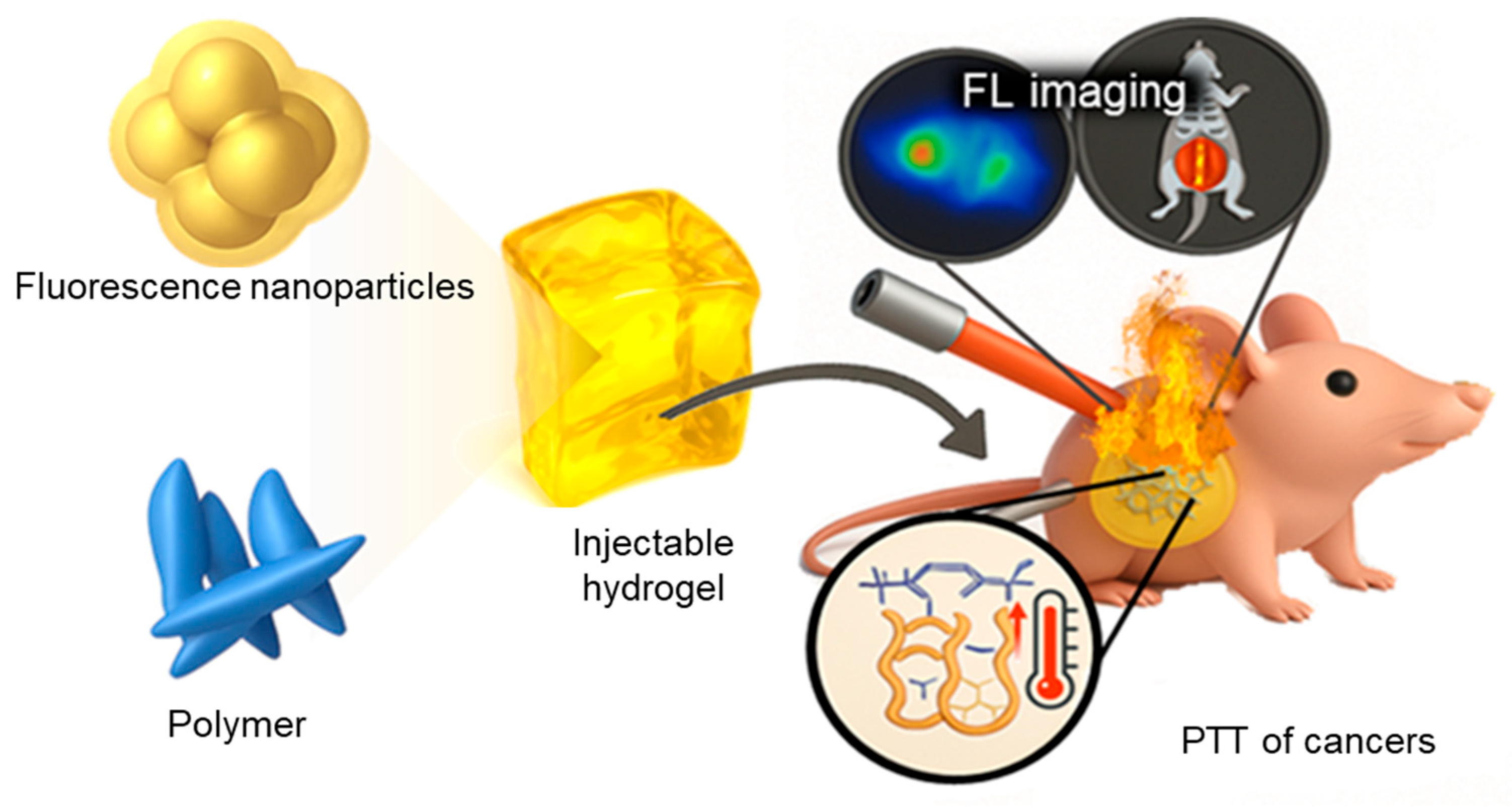
4. Design Strategies for Diagnostic Imaging Hydrogels
4.1. MRI
4.2. FL and Optical Imaging
4.3. CT Imaging
4.4. US and PA Imaging
4.5. Others Imaging Techniques
| Imaging Modality | Hydrogel Types | Key Features | Materials | References |
|---|---|---|---|---|
| MRI (T1-weighted, CEST) imaging | Gd3+-DTPA/DOTA hydrogels, CEST-active hydrogel | High spatial resolution, real-time imaging, label-free options, artifact minimization | Gadolinium compounds, hydroxyl/amine-functional hydrogels | [100,101,102,103,104,105,106,107] |
| FL and Optical imaging | Quantum dot or dye-loaded injectable hydrogels; NIR dye-integrated systems | High sensitivity, real-time visualization, FL-guided surgery compatibility | Quantum dots, carbon dots, indocyanine green | [108,109,110,111,112,113,114,115,116] |
| CT imaging | Radiopaque hydrogels with metallic nanoparticles or iodine-based agents | High resolution, accurate tumor localization, spectral CT compatibility | Gold, ytterbium, iodinated compounds | [117,118,119] |
| US and PA imaging | Bubble-encapsulated hydrogels, photo-absorber embedded systems | Non-ionizing, dual diagnostic and therapeutic capabilities, deep-tissue imaging | Microbubbles, carbon nanotubes, organic dyes | [120,121,122,123,124,125,126] |
| Raman imaging | Raman-active hydrogel systems with embedded nanoparticles | Molecular specificity, label-free detection | Gold nanorods | [127,128,129] |
5. Therapeutic Modalities Using Hydrogels
5.1. Chemotherapy
5.2. PTT
5.3. PDT
5.4. Immunotherapy
5.5. Combination Therapy
6. Applications of Multifunctional Hydrogels in Cancer Theranostics
6.1. In Situ Injectable Hydrogels for Localized Cancer Therapy
6.2. Hydrogels for the Prevention of Tumor Recurrence and Metastasis
6.3. Hydrogels for Minimally Invasive Localized Treatment
| Application Area | Cancers | Key Features | Advantages | References |
|---|---|---|---|---|
| In Situ Injectable Hydrogels for Localized Cancer Therapy | Breast, Liver, Prostate | Thermo-responsive, pH-responsive hydrogels (e.g., chitosan, hyaluronic acid-based) | Minimally invasive, reduced systemic toxicity, targeted therapy | [158,159,160,161,162] |
| Prevention of Tumor Recurrence and Metastasis | Colorectal, Breast, Lung | Biodegradable hydrogels (e.g., PLGA, alginate-based) | Effective prevention of recurrence and metastasis, reduced side effects | [163,164,165,166,167] |
| Minimally Invasive Localized Treatment | Skin, Pancreatic, Head and Neck | Photo-responsive, Magnetic-responsive hydrogels (e.g., gold nanoparticles, iron oxide nanoparticles embedded) | Enhanced therapeutic accuracy, reduced invasiveness, real-time monitoring | [168,169] |
7. Challenges and Future Perspectives
7.1. Current Limitations in Multifunctional Hydrogel Systems
7.2. Future Directions
7.2.1. Material Science Innovations and Advanced Hydrogel Designs
7.2.2. Integration with Advanced Technologies (AI-Driven Drug Delivery, Nanotechnology, and 3D Printing)
7.2.3. Personalized and Precision Medicine Approaches
7.2.4. Ethical and Socioeconomic Considerations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer treatment and survivorship statistics. CA Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef]
- Zahedifard, Z.; Mahmoodi, S.; Ghasemian, A. Genetically Engineered Bacteria as a Promising Therapeutic Strategy Against Cancer: A Comprehensive Review. Biotechnol. Appl. Biochem. 2025. [Google Scholar] [CrossRef]
- Khan, S.U.; Fatima, K.; Aisha, S.; Malik, F. Unveiling the mechanisms and challenges of cancer drug resistance. Cell Commun. Signal. 2024, 22, 109. [Google Scholar] [CrossRef]
- Eslami, M.; Memarsadeghi, O.; Davarpanah, A.; Arti, A.; Nayernia, K.; Behnam, B. Overcoming Chemotherapy Resistance in Metastatic Cancer: A Comprehensive Review. Biomedicines 2024, 12, 183. [Google Scholar] [CrossRef]
- Wang, K.; Tepper, J.E. Radiation therapy-associated toxicity: Etiology, management, and prevention. CA Cancer J. Clin. 2021, 71, 437–454. [Google Scholar] [CrossRef]
- Mahvi, D.A.; Liu, R.; Grinstaff, M.W.; Colson, Y.L.; Raut, C.P. Local Cancer Recurrence: The Realities, Challenges, and Opportunities for New Therapies. CA Cancer J. Clin. 2018, 68, 488–505. [Google Scholar] [CrossRef] [PubMed]
- Chargari, C.; Rassy, E.; Helissey, C.; Achkar, S.; Francois, S.; Deutsch, E. Impact of radiation therapy on healthy tissues. Int. Rev. Cell Mol. Biol. 2023, 376, 69–98. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Asea, A. Radiation-induced effects and the immune system in cancer. Front. Oncol. 2012, 2, 191. [Google Scholar] [CrossRef]
- Tohme, S.; Simmons, R.L.; Tsung, A. Surgery for Cancer: A Trigger for Metastases. Cancer Res. 2017, 77, 1548–1552. [Google Scholar] [CrossRef]
- Van Meir, H.; Nout, R.A.; Welters, M.J.; Loof, N.M.; de Kam, M.L.; van Ham, J.J.; Samuels, S.; Kenter, G.G.; Cohen, A.F.; Melief, C.J.; et al. Impact of (chemo)radiotherapy on immune cell composition and function in cervical cancer patients. Oncoimmunology 2017, 6, e1267095. [Google Scholar] [CrossRef] [PubMed]
- Mohan, G.; Ayisha Hamna, T.P.; Jijo, A.J.; Saradha Devi, K.M.; Narayanasamy, A.; Vellingiri, B. Recent advances in radiotherapy and its associated side effects in cancer—A review. J. Basic. Appl. Zool. 2019, 80, 14. [Google Scholar] [CrossRef]
- Brook, I. Late side effects of radiation treatment for head and neck cancer. Radiat. Oncol. J. 2020, 38, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.K.; Lee, J.H.; Lee, S.C.; Lee, C.S. MnCO3-mineralized polydopamine nanoparticles as an activatable theranostic agent for dual-modality imaging-guided photothermal therapy of cancers. Theranostics 2022, 12, 6762–6778. [Google Scholar] [CrossRef]
- Ryu, J.H.; Koo, H.; Sun, I.C.; Yuk, S.H.; Choi, K.; Kim, K.; Kwon, I.C. Tumor-targeting multi-functional nanoparticles for theragnosis: New paradigm for cancer therapy. Adv. Drug Deliv. Rev. 2012, 64, 1447–1458. [Google Scholar] [CrossRef]
- Dessale, M.; Mengistu, G.; Mengist, H.M. Nanotechnology: A Promising Approach for Cancer Diagnosis, Therapeutics and Theragnosis. Int. J. Nanomed. 2022, 17, 3735–3749. [Google Scholar] [CrossRef]
- Annigeri, N.C.; Mohan, R.; Vijayakumar, D.B.; Lingaraj, A.J. Theragnosis using fluorescence: A review. J. Adv. Dent. Pract. Res. 2024, 2, 59–61. [Google Scholar] [CrossRef]
- Ma, Q.; Li, Q.; Cai, X.; Zhou, P.; Wu, Z.; Wang, B.; Ma, W.; Fu, S. Injectable hydrogels as drug delivery platform for in-situ treatment of malignant tumor. J. Drug Deliv. Sci. Technol. 2022, 76, 103817. [Google Scholar] [CrossRef]
- Mohaghegh, N.; Ahari, A.; Zehtabi, F.; Buttles, C.; Davani, S.; Hoang, H.; Tseng, K.; Zamanian, B.; Khosravi, S.; Daniali, A.; et al. Injectable hydrogels for personalized cancer immunotherapies. Acta Biomater. 2023, 172, 67–91. [Google Scholar] [CrossRef]
- Zang, C.; Tian, Y.; Tang, Y.; Tang, M.; Yang, D.; Chen, F.; Ghaffarlou, M.; Tu, Y.; Ashrafizadeh, M.; Li, Y. Hydrogel-based platforms for site-specific doxorubicin release in cancer therapy. J. Transl. Med. 2024, 22, 879. [Google Scholar] [CrossRef]
- Zhong, Z.; Gan, L.; Feng, Z.; Wang, W.; Pan, X.; Wu, C.; Huang, Y. Hydrogel local drug delivery systems for postsurgical management of tumors: Status Quo and perspectives. Mater. Today Bio 2024, 29, 101308. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, X.; Xu, M.; Geng, Z.; Ji, P.; Liu, Y. Hydrogel systems for targeted cancer therapy. Front. Bioeng. Biotechnol. 2023, 11, 1140436. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.; Wu, Y.; Zhang, X.; Zheng, Z.; Zhang, M.; Long, L.; Liao, J.; Chen, W. Recent Advances in Hydrogel-Based Phototherapy for Tumor Treatment. Gels 2023, 9, 286. [Google Scholar] [CrossRef]
- Liu, C.; Liao, Y.; Liu, L.; Xie, L.; Liu, J.; Zhang, Y.; Li, Y. Application of injectable hydrogels in cancer immunotherapy. Front. Bioeng. Biotechnol. 2023, 11, 1121887. [Google Scholar] [CrossRef]
- Zhu, H.; Sun, H.; Dai, J.; Hao, J.; Zhou, B. Chitosan-based hydrogels in cancer therapy: Drug and gene delivery, stimuli-responsive carriers, phototherapy and immunotherapy. Int. J. Biol. Macromol. 2024, 282 Pt 2, 137047. [Google Scholar] [CrossRef]
- Lima-Sousa, R.; Alves, C.G.; Melo, B.L.; Costa, F.J.P.; Nave, M.; Moreira, A.F.; Mendonca, A.G.; Correia, I.J.; de Melo-Diogo, D. Injectable hydrogels for the delivery of nanomaterials for cancer combinatorial photothermal therapy. Biomater. Sci. 2023, 11, 6082–6108. [Google Scholar] [CrossRef]
- Dong, Y.C.; Bouche, M.; Uman, S.; Burdick, J.A.; Cormode, D.P. Detecting and Monitoring Hydrogels with Medical Imaging. ACS Biomater. Sci. Eng. 2021, 7, 4027–4047. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Ke, H.T. Nanomaterials incorporated ultrasound contrast agents for cancer theranostics. Cancer Biol. Med. 2016, 13, 313–324. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mieszawska, A.J.; Mulder, W.J.; Fayad, Z.A.; Cormode, D.P. Multifunctional gold nanoparticles for diagnosis and therapy of disease. Mol. Pharm. 2013, 10, 831–847. [Google Scholar] [CrossRef]
- Priester, M.I.; Ten Hagen, T.L.M. Image-guided drug delivery in nanosystem-based cancer therapies. Adv. Drug Deliv. Rev. 2023, 192, 114621. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Y.; Liu, Y.; Yu, Y.; Yang, M.; Lu, L.; Chan, L.; Liu, B. Functional hydrogels for hepatocellular carcinoma: Therapy, imaging, and in vitro model. J. Nanobiotechnol. 2024, 22, 381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tian, S.; Huang, L.; Li, Y.; Lu, Y.; Li, H.; Chen, G.; Meng, F.; Liu, G.L.; Yang, X.; et al. Reactive oxygen species-responsive and Raman-traceable hydrogel combining photodynamic and immune therapy for postsurgical cancer treatment. Nat. Commun. 2022, 13, 4553. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.; Linders, D.G.J.; Abenojar, E.; Wang, X.; Hazelbag, H.M.; Straver, M.E.; Bijlstra, O.D.; March, T.L.; Vahrmeijer, A.L.; Exner, A.; et al. Formulation of a Thermosensitive Imaging Hydrogel for Topical Application and Rapid Visualization of Tumor Margins in the Surgical Cavity. Cancers 2022, 14, 3459. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mao, D.; Wang, Y.; Wang, K.; Yi, X.; Kong, D.; Yang, Z.; Liu, Q.; Ding, D. Biocompatible fluorescent supramolecular nanofibrous hydrogel for long-term cell tracking and tumor imaging applications. Sci. Rep. 2015, 5, 16680. [Google Scholar] [CrossRef]
- Nicolson, F.; Andreiuk, B.; Lee, E.; O’Donnell, B.; Whitley, A.; Riepl, N.; Burkhart, D.L.; Cameron, A.; Protti, A.; Rudder, S.; et al. In vivo imaging using surface enhanced spatially offset raman spectroscopy (SESORS): Balancing sampling frequency to improve overall image acquisition. Npj Imaging 2024, 2, 7. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, B.M. Current Advances in Stimuli-Responsive Hydrogels as Smart Drug Delivery Carriers. Gels 2023, 9, 838. [Google Scholar] [CrossRef]
- Wells, C.M.; Harris, M.; Choi, L.; Murali, V.P.; Guerra, F.D.; Jennings, J.A. Stimuli-Responsive Drug Release from Smart Polymers. J. Funct. Biomater. 2019, 10, 34. [Google Scholar] [CrossRef]
- Liu, Y.; Ran, Y.; Ge, Y.; Raza, F.; Li, S.; Zafar, H.; Wu, Y.; Paiva-Santos, A.C.; Yu, C.; Sun, M.; et al. pH-Sensitive Peptide Hydrogels as a Combination Drug Delivery System for Cancer Treatment. Pharmaceutics 2022, 14, 652. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, G.; Su, W.K.; Shuai, Q. Enzyme-Responsive Nanoparticles for Anti-tumor Drug Delivery. Front. Chem. 2020, 8, 647. [Google Scholar] [CrossRef]
- Feng, H.; Chu, D.; Yang, F.; Li, Z.; Fan, B.; Jin, L.; Li, J. Hypoxia-Responsive Polymeric Micelles for Enhancing Cancer Treatment. Front. Chem. 2020, 8, 742. [Google Scholar] [CrossRef]
- Abed, H.F.; Abuwatfa, W.H.; Husseini, G.A. Redox-Responsive Drug Delivery Systems: A Chemical Perspective. Nanomaterials 2022, 12, 3183. [Google Scholar] [CrossRef] [PubMed]
- Tanga, S.; Aucamp, M.; Ramburrun, P. Injectable Thermoresponsive Hydrogels for Cancer Therapy: Challenges and Prospects. Gels 2023, 9, 418. [Google Scholar] [CrossRef]
- Londhe, P.V.; Londhe, M.V.; Salunkhe, A.B.; Laha, S.S.; Mefford, O.T.; Thorat, N.D.; Khot, V.M. Magnetic hydrogel (MagGel): An evolutionary pedestal for anticancer therapy. Coord. Chem. Rev. 2025, 522, 216228. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, L.G.; Fan, X.M.; Pang, J.L. Ultrasound Responsive Smart Implantable Hydrogels for Targeted Delivery of Drugs: Reviewing Current Practices. Int. J. Nanomed. 2022, 17, 5001–5026. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Pan, B.; Wang, T.; Yang, H.; Vance, D.; Li, X.; Zhao, H.; Hu, X.; Yang, T.; Chen, Z.; et al. Advances in NIR-Responsive Natural Macromolecular Hydrogel Assembly Drugs for Cancer Treatment. Pharmaceutics 2023, 15, 2729. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Y.; Fu, W.; Yao, M.; Ding, Z.; Xuan, J.; Li, D.; Wang, S.; Xia, Y.; Cao, M. Poly(N-isopropylacrylamide)-Based Thermoresponsive Composite Hydrogels for Biomedical Applications. Polymers 2020, 12, 580. [Google Scholar] [CrossRef]
- Gu, J.; Zhao, G.; Yu, J.; Xu, P.; Yan, J.; Jin, Z.; Chen, S.; Wang, Y.; Zhang, L.W.; Wang, Y. Injectable pH-responsive hydrogel for combinatorial chemoimmunotherapy tailored to the tumor microenvironment. J. Nanobiotechnol. 2022, 20, 372. [Google Scholar] [CrossRef]
- Bossmann, S.H.; Payne, M.M.; Kalita, M.; Bristow, R.M.D.; Afshar, A.; Perera, A.S. Iron-Based Magnetic Nanosystems for Diagnostic Imaging and Drug Delivery: Towards Transformative Biomedical Applications. Pharmaceutics 2022, 14, 2093. [Google Scholar] [CrossRef]
- Wang, Z.; Zhai, B.; Sun, J.; Zhang, X.; Zou, J.; Shi, Y.; Guo, D. Recent advances of injectable in situ-forming hydrogels for preventing postoperative tumor recurrence. Drug Deliv. 2024, 31, 2400476. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Z.; Tang, W.; Dai, Y. Gel/hydrogel-based in situ biomaterial platforms for cancer postoperative treatment and recovery. Exploration 2023, 3, 20220173. [Google Scholar] [CrossRef]
- Go, K.; Kim, D.-M.; Lee, K.J. 3D printable hydrogel filament with functionalizable moiety for in-situ flow-based sensor. Macromol. Res. 2024, 32, 467–473. [Google Scholar] [CrossRef]
- Bhuskute, H.; Shende, P.; Prabhakar, B. 3D Printed Personalized Medicine for Cancer: Applications for Betterment of Diagnosis, Prognosis and Treatment. AAPS PharmSciTech 2021, 23, 8. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Deng, B.; He, R.; Huang, P. Advancements of 3D bioprinting in regenerative medicine: Exploring cell sources for organ fabrication. Heliyon 2024, 10, e24593. [Google Scholar] [CrossRef]
- Fahad, A.; Bagher, F. Recent advances on chitosan/hyaluronic acid-based stimuli-responsive hydrogels and composites for cancer treatment: A comprehensive review. Int. J. Biol. Macromol. 2024, 280, 135893. [Google Scholar] [CrossRef]
- Pratikshya, P.; Tarun, K.U.; Nawaf, A.; Mohd, S.; Kavindra, K.K. Alginate-Chitosan Biodegradable and Biocompatible Based Hydrogel for Breast Cancer Immunotherapy and Diagnosis: A Comprehensive Review. ACS Appl. Bio Mater. 2024, 7, 3515–3534. [Google Scholar] [CrossRef]
- Ferreira, N.N.; Ferreira, L.M.B.; Miranda-Goncalves, V.; Reis, R.M.; Seraphim, T.V.; Borges, J.C.; Baltazar, F.; Gremiao, M.P.D. Alginate hydrogel improves anti-angiogenic bevacizumab activity in cancer therapy. Eur. J. Pharm. Biopharm. 2017, 119, 271–282. [Google Scholar] [CrossRef]
- Rezk, A.I.; Obiweluozor, F.O.; Choukrani, G.; Park, C.H.; Kim, C.S. Drug release and kinetic models of anticancer drug (BTZ) from a pH-responsive alginate polydopamine hydrogel: Towards cancer chemotherapy. Int. J. Biol. Macromol. 2019, 141, 388–400. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, T.; Zhuang, Y.; He, T.; Wu, X.; Su, L.; Kang, J.; Chang, J.; Wang, H. Sodium Alginate Hydrogel-Mediated Cancer Immunotherapy for Postoperative In Situ Recurrence and Metastasis. ACS Biomater. Sci. Eng. 2021, 7, 5717–5726. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Su, X.; Gregory, D.A.; Li, W.; Cai, Z.; Zhao, X. Magnetic Alginate/Chitosan Nanoparticles for Targeted Delivery of Curcumin into Human Breast Cancer Cells. Nanomaterials 2018, 8, 907. [Google Scholar] [CrossRef]
- Shanmugapriya, K.; Kang, H.W. Synthesis of nanohydroxyapatite/collagen-loaded fucoidan-based composite hydrogel for drug delivery to gastrointestinal cancer cells. Colloids Surf. B Biointerfaces 2021, 203, 111769. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, S.; Lin, R.; Cui, S.; Jing, X.; Coseri, S. Injectable multifunctional carboxymethyl chitosan/hyaluronic acid hydrogel for drug delivery systems. Int. J. Biol. Macromol. 2023, 249, 125801. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhao, X.; Liang, X.; Ma, P.X.; Guo, B. Injectable hydrogel based on quaternized chitosan, gelatin and dopamine as localized drug delivery system to treat Parkinson’s disease. Int. J. Biol. Macromol. 2017, 105 Pt 1, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, C.; Wang, X.; Wang, Y.; Zhang, Q.; Cheng, Y. A Polydopamine Nanoparticle-Knotted Poly(ethylene glycol) Hydrogel for On-Demand Drug Delivery and Chemo-photothermal Therapy. Chem. Mater. 2017, 29, 1370–1376. [Google Scholar] [CrossRef]
- Liu, C.; Guo, X.; Ruan, C.; Hu, H.; Jiang, B.P.; Liang, H.; Shen, X.C. An injectable thermosensitive photothermal-network hydrogel for near-infrared-triggered drug delivery and synergistic photothermal-chemotherapy. Acta Biomater. 2019, 96, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Rong, X.; Ji, Y.; Zhu, X.; Yang, J.; Qian, D.; Mo, X.; Lu, Y. Neuroprotective effect of insulin-loaded chitosan nanoparticles/PLGA-PEG-PLGA hydrogel on diabetic retinopathy in rats. Int. J. Nanomed. 2019, 14, 45–55. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, S.; Li, D.; Gong, Y.; Zang, J.; Liu, J.; Chen, X. The effect of PLGA-based hydrogel scaffold for improving the drug maximum-tolerated dose for in situ osteosarcoma treatment. Colloids Surf. B Biointerfaces 2018, 172, 387–394. [Google Scholar] [CrossRef]
- Zhai, L.; Shi, Y.; Yan, Y.; Lu, A.; Liu, X.; Lei, L.; Sun, Y.; Jiang, L.; Wang, X.; Qian, H.; et al. Local sustained release of PD-1 monoclonal antibody and lenvatinib by thermo-sensitive hydrogel for improving tumor immunotherapy. Chin. Chem. Lett. 2023, 34, 108104. [Google Scholar] [CrossRef]
- Dhamecha, D.; Le, D.; Chakravarty, T.; Perera, K.; Dutta, A.; Menon, J.U. Fabrication of PNIPAm-based thermoresponsive hydrogel microwell arrays for tumor spheroid formation. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 125, 112100. [Google Scholar] [CrossRef]
- Fang, Y.; Tan, J.; Lim, S.; Soh, S. Rupturing cancer cells by the expansion of functionalized stimuli-responsive hydrogels. NPG Asia Mater. 2018, 10, e465. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Qu, Q.; Pan, S.; Yu, K.; Liu, Y. Graphene oxide modified sodium alginate/polyethylene glycol phase change material hydrogel scaffold composite with photothermal temperature control for potential bone tissue regeneration. J. Mater. Res. Technol. 2024, 30, 2446–2457. [Google Scholar] [CrossRef]
- Baraian, A.I.; Raduly, L.; Zanoaga, O.; Iacob, B.C.; Barbu-Tudoran, L.; Dinte, E.; Berindan-Neagoe, I.; Bodoki, E. Targeting JAK/STAT3 in glioblastoma cells using an alginate-PNIPAm molecularly imprinted hydrogel for the sustained release of ruxolitinib. Int. J. Biol. Macromol. 2025, 298, 140025. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Stowers, R.; Lou, J.; Xia, Y.; Chaudhuri, O. Varying PEG density to control stress relaxation in alginate-PEG hydrogels for 3D cell culture studies. Biomaterials 2019, 200, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Sargeant, T.D.; Desai, A.P.; Banerjee, S.; Agawu, A.; Stopek, J.B. An in situ forming collagen-PEG hydrogel for tissue regeneration. Acta Biomater. 2012, 8, 124–132. [Google Scholar] [CrossRef]
- Jin, X.; Fu, Q.; Gu, Z.; Zhang, Z.; Lv, H. Injectable corilagin/low molecular weight chitosan/PLGA-PEG-PLGA thermosensitive hydrogels for localized cancer therapy and promoting drug infiltration by modulation of tumor microenvironment. Int. J. Pharm. 2020, 589, 119772. [Google Scholar] [CrossRef]
- Kim, T.T.; Malu, D.; He, D.; Hu, Y.; Kim, J. Development of Bioorthogonally Degradable Tough Hydrogels Using Enamine N-Oxide Based Crosslinkers. Adv. Mater. 2025, 37, 2414692. [Google Scholar] [CrossRef]
- Liu, Z.; Koseki, Y.; Suzuki, R.; Dao, A.T.N.; Kasai, H. Sustained Drug Release from Dual-Responsive Hydrogels for Local Cancer Chemo–Photothermal Therapy. Macromol. Biosci. 2025, 25, 2400413. [Google Scholar] [CrossRef]
- Tang, M.; Song, J.; Zhang, S.; Shu, X.; Liu, S.; Ashrafizadeh, M.; Ertas, Y.N.; Zhou, Y.; Lei, M. Innovative theranostic hydrogels for targeted gastrointestinal cancer treatment. J. Transl. Med. 2024, 22, 970. [Google Scholar] [CrossRef]
- Bilalis, P.; Skoulas, D.; Karatzas, A.; Marakis, J.; Stamogiannos, A.; Tsimblouli, C.; Sereti, E.; Stratikos, E.; Dimas, K.; Vlassopoulos, D.; et al. Self-Healing pH- and Enzyme Stimuli-Responsive Hydrogels for Targeted Delivery of Gemcitabine To Treat Pancreatic Cancer. Biomacromolecules 2018, 19, 3840–3852. [Google Scholar] [CrossRef] [PubMed]
- Kozlovskaya, V.; Chen, J.; Tedjo, C.; Liang, X.; Campos-Gomez, J.; Oh, J.; Saeed, M.; Lungu, C.T.; Kharlampieva, E. pH-responsive hydrogel cubes for release of doxorubicin in cancer cells. J. Mater. Chem. B 2014, 2, 2494–2507. [Google Scholar] [CrossRef]
- Raza, F.; Zhu, Y.; Chen, L.; You, X.; Zhang, J.; Khan, A.; Khan, M.W.; Hasnat, M.; Zafar, H.; Wu, J.; et al. Paclitaxel-loaded pH responsive hydrogel based on self-assembled peptides for tumor targeting. Biomater. Sci. 2019, 7, 2023–2036. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Z.; Zhu, J.; Wen, Y.; Zhao, F.; Lei, L.; Phan-Thien, N.; Khoo, B.C.; Li, J. Thermoresponsive Hydrogel Induced by Dual Supramolecular Assemblies and Its Controlled Release Property for Enhanced Anticancer Drug Delivery. Biomacromolecules 2020, 21, 1516–1527. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, J.; Hu, Y.; Gao, F.; Pak-Heng Leung, G.; Geng, F.; Fu, C.; Zhang, J. Injectable thermo-responsive nano-hydrogel loading triptolide for the anti-breast cancer enhancement via localized treatment based on “two strikes” effects. Acta Pharm. Sin. B 2020, 10, 2227–2245. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, G.; Liu, G.; Hu, J.; Liu, S. Photo- and thermo-responsive multicompartment hydrogels for synergistic delivery of gemcitabine and doxorubicin. J. Control. Release 2017, 259, 149–159. [Google Scholar] [CrossRef]
- Jaiswal, M.K.; De, M.; Chou, S.S.; Vasavada, S.; Bleher, R.; Prasad, P.V.; Bahadur, D.; Dravid, V.P. Thermoresponsive magnetic hydrogels as theranostic nanoconstructs. ACS Appl. Mater. Interfaces 2014, 6, 6237–6247. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; He, L.; Jiang, L.; Shan, S.; Su, H. Synthesis of magnetic/pH dual responsive dextran hydrogels as stimuli-sensitive drug carriers. Carbohydr. Res. 2022, 520, 108632. [Google Scholar] [CrossRef]
- Jo, Y.J.; Gulfam, M.; Jo, S.H.; Gal, Y.S.; Oh, C.W.; Park, S.H.; Lim, K.T. Multi-stimuli responsive hydrogels derived from hyaluronic acid for cancer therapy application. Carbohydr. Polym. 2022, 286, 119303. [Google Scholar] [CrossRef] [PubMed]
- Arjama, M.; Mehnath, S.; Jeyaraj, M. Self-assembled hydrogel nanocube for stimuli responsive drug delivery and tumor ablation by phototherapy against breast cancer. Int. J. Biol. Macromol. 2022, 213, 435–446. [Google Scholar] [CrossRef]
- He, G.; Chen, S.; Xu, Y.; Miao, Z.; Ma, Y.; Qian, H.; Lu, Y.; Zha, Z. Charge reversal induced colloidal hydrogel acts as a multi-stimuli responsive drug delivery platform for synergistic cancer therapy. Mater. Horiz. 2019, 6, 711–716. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, F.; Huang, N.; Li, J.; Wu, C.; Tan, B.; Liu, Y.; Li, L.; Yang, C.; Shao, D.; et al. Near-infrared light-responsive hybrid hydrogels for the synergistic chemo-photothermal therapy of oral cancer. Nanoscale 2021, 13, 17168–17182. [Google Scholar] [CrossRef]
- Lv, S.W.; Liu, Y.; Xie, M.; Wang, J.; Yan, X.W.; Li, Z.; Dong, W.G.; Huang, W.H. Near-Infrared Light-Responsive Hydrogel for Specific Recognition and Photothermal Site-Release of Circulating Tumor Cells. ACS Nano 2016, 10, 6201–6210. [Google Scholar] [CrossRef]
- Hao, Y.; Dong, Z.; Chen, M.; Chao, Y.; Liu, Z.; Feng, L.; Hao, Y.; Dong, Z.L.; Chen, M.C.; Chao, Y.; et al. Near-infrared light and glucose dual-responsive cascading hydroxyl radical generation for in situ gelation and effective breast cancer treatment. Biomaterials 2020, 228, 119568. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, A.; Harada, A.; Ueno, S.; Miyata, T. Weakly Acidic pH and Reduction Dual Stimuli-Responsive Gel Particles. Langmuir 2021, 37, 11484–11492. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Rajendran, R.R.; Singh, A.; Pramanik, S.; Shrivastav, P.; Ansari, J.; Manne, R.; Amaral, L.S.; Deepak, A. Alginate as a Promising Biopolymer in Drug Delivery and Wound Healing: A Review of the State-of-the-Art. Int. J. Mol. Sci. 2022, 23, 9035. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in Crosslinking Strategies of Biomedical Hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in Hydrogels—A Review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar] [CrossRef]
- Rebers, L.; Reichsöllner, R.; Regett, S.; Tovar, G.E.M.; Borchers, K.; Baudis, S.; Southan, A. Differentiation of Physical and Chemical Cross-Linking in Gelatin Methacryloyl Hydrogels. Sci. Rep. 2021, 11, 3256. [Google Scholar] [CrossRef] [PubMed]
- Nieto, D.; Marchal Corrales, J.A.; de Mora, A.J.; Moroni, L. Fundamentals of Light-Cell–Polymer Interactions in Photo-Cross-Linking Based Bioprinting. APL Bioeng. 2020, 4, 041502. [Google Scholar] [CrossRef]
- Gaudet, I.D.; Shreiber, D.I. Characterization of Methacrylated Type-I Collagen as a Dynamic, Photoactive Hydrogel. Biointerphases 2012, 7, 25. [Google Scholar] [CrossRef]
- Augustine, R.; Kalva, S.N.; Ahmad, R.; Zahid, A.A.; Hasan, S.; Nayeem, A.; McClements, L.; Hasan, A. 3D Bioprinted Cancer Models: Revolutionizing Personalized Cancer Therapy. Transl. Oncol. 2021, 14, 101015. [Google Scholar] [CrossRef]
- Shuhendler, A.J.; Staruch, R.; Oakden, W.; Gordijo, C.R.; Rauth, A.M.; Stanisz, G.J.; Chopra, R.; Wu, X.Y. Thermally-triggered ‘off-on-off’ response of gadolinium-hydrogel-lipid hybrid nanoparticles defines a customizable temperature window for non-invasive magnetic resonance imaging thermometry. J. Control. Release 2012, 157, 478–484. [Google Scholar] [CrossRef]
- Courant, T.; Roullin, V.G.; Cadiou, C.; Callewaert, M.; Andry, M.C.; Portefaix, C.; Hoeffel, C.; de Goltstein, M.C.; Port, M.; Laurent, S.; et al. Hydrogels incorporating GdDOTA: Towards highly efficient dual T1/T2 MRI contrast agents. Angew. Chem. Int. Ed. Engl. 2012, 51, 9119–9122. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, K.; Luan, J.; Wen, Z.; Wang, L.; Liu, Z.; Wu, G.; Zhuo, R. Visualization of in situ hydrogels by MRI in vivo. J. Mater. Chem. B 2016, 4, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Shazeeb, M.S.; Corazzini, R.; Konowicz, P.A.; Fogle, R.; Bangari, D.S.; Johnson, J.; Ying, X.; Dhal, P.K. Assessment of in vivo degradation profiles of hyaluronic acid hydrogels using temporal evolution of chemical exchange saturation transfer (CEST) MRI. Biomaterials 2018, 178, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, S.M.; Haris, M.; Singh, A.; Witschey, W.R.T.; Rodell, C.B.; Kogan, F.; Reddy, R.; Burdick, J.A. Visualization of Injectable Hydrogels Using Chemical Exchange Saturation Transfer MRI. ACS Biomater. Sci. Eng. 2015, 1, 227–237. [Google Scholar] [CrossRef]
- Zhu, W.; Chu, C.; Kuddannaya, S.; Yuan, Y.; Walczak, P.; Singh, A.; Song, X.; Bulte, J.W.M. In Vivo Imaging of Composite Hydrogel Scaffold Degradation Using CEST MRI and Two-Color NIR Imaging. Adv. Funct. Mater. 2019, 29, 1903753. [Google Scholar] [CrossRef]
- Kim, S.D.; Park, K.; Lee, S.; Kum, J.; Kim, Y.; An, S.; Kim, H.; Shin, M.; Son, D. Injectable and tissue-conformable conductive hydrogel for MRI-compatible brain-interfacing electrodes. Soft Sci. 2023, 3, 18. [Google Scholar] [CrossRef]
- Bermejo-Velasco, D.; Dou, W.; Heerschap, A.; Ossipov, D.; Hilborn, J. Injectable hyaluronic acid hydrogels with the capacity for magnetic resonance imaging. Carbohydr. Polym. 2018, 197, 641–648. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, J.; Wu, K.; Wu, X.; Tang, J.; Cui, S.; Cao, D.; Liu, R.; Peng, C.; Yu, L.; et al. Visualizing the In Vivo Evolution of an Injectable and Thermosensitive Hydrogel Using Tri-Modal Bioimaging. Small Methods 2020, 4, 2000310. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, J.; Wan, J.; Jiang, S.; Wu, Y.; Wang, W.; Gong, X.; Wang, H. Quantum dots-based hydrogels for sensing applications. Chem. Eng. J. 2021, 408, 127351. [Google Scholar] [CrossRef]
- Park, G.K.; Kim, S.H.; Kim, K.; Das, P.; Kim, B.G.; Kashiwagi, S.; Choi, H.S.; Hwang, N.S. Dual-Channel Fluorescence Imaging of Hydrogel Degradation and Tissue Regeneration in the Brain. Theranostics 2019, 9, 4255–4264. [Google Scholar] [CrossRef]
- Wang, L.; Li, B.; Xu, F.; Li, Y.; Xu, Z.; Wei, D.; Feng, Y.; Wang, Y.; Jia, D.; Zhou, Y. Visual in vivo degradation of injectable hydrogel by real-time and non-invasive tracking using carbon nanodots as fluorescent indicator. Biomaterials 2017, 145, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Kim, H.; Sohn, D.K.; Eom, J.B.; Seo, Y.S.; Yoon, H.M.; Choi, Y. Indocyanine green-loaded injectable alginate hydrogel as a marker for precision cancer surgery. Quant. Imaging Med. Surg. 2020, 10, 779–788. [Google Scholar] [CrossRef]
- Sachdev, A.; Matai, I.; Gopinath, P. Carbon dots incorporated polymeric hydrogels as multifunctional platform for imaging and induction of apoptosis in lung cancer cells. Colloids Surf. B Biointerfaces 2016, 141, 242–252. [Google Scholar] [CrossRef]
- Mohammadi, S.; Mohammadi, S.; Salimi, A. A 3D hydrogel based on chitosan and carbon dots for sensitive fluorescence detection of microRNA-21 in breast cancer cells. Talanta 2021, 224, 121895. [Google Scholar] [CrossRef]
- Dong, X.; Liang, J.; Yang, A.; Qian, Z.; Kong, D.; Lv, F. Fluorescence imaging guided CpG nanoparticles-loaded IR820-hydrogel for synergistic photothermal immunotherapy. Biomaterials 2019, 209, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Back, W.; Rho, J.; Kim, K.; Yong, H.S.; Jeon, O.H.; Choi, B.H.; Kim, H.K.; Park, J.H. An injectable fluorescent and iodinated hydrogel for preoperative localization and dual image-guided surgery of pulmonary nodules. Biomater. Sci. 2024, 12, 2943–2950. [Google Scholar] [CrossRef] [PubMed]
- Patrick, P.S.; Bear, J.C.; Fitzke, H.E.; Zaw-Thin, M.; Parkin, I.P.; Lythgoe, M.F.; Kalber, T.L.; Stuckey, D.J. Radio-metal cross-linking of alginate hydrogels for non-invasive in vivo imaging. Biomaterials 2020, 243, 119930. [Google Scholar] [CrossRef]
- Gu, X.; Shu, Z.; Zheng, X.; Wei, S.; Ma, M.; He, H.; Shi, Y.; Gong, X.; Chen, S.; Wang, X. A novel CT-responsive hydrogel for the construction of an organ simulation phantom for the repeatability and stability study of radiomic features. J. Mater. Chem. B 2023, 11, 11073–11081. [Google Scholar] [CrossRef]
- Dong, Y.C.; Kumar, A.; Rosario-Berrios, D.N.; Si-Mohamed, S.; Hsu, J.C.; Nieves, L.M.; Douek, P.; Noel, P.B.; Cormode, D.P. Ytterbium Nanoparticle Contrast Agents for Conventional and Spectral Photon-Counting CT and Their Applications for Hydrogel Imaging. ACS Appl. Mater. Interfaces 2022, 14, 39274–39284. [Google Scholar] [CrossRef]
- Exner, A.A.; Kolios, M.C. Bursting Microbubbles: How Nanobubble Contrast Agents Can Enable the Future of Medical Ultrasound Molecular Imaging and Image-Guided Therapy. Curr. Opin. Colloid Interface Sci. 2021, 54, 101463. [Google Scholar] [CrossRef]
- Helfield, B.; Zou, Y.; Matsuura, N. Acoustically-Stimulated Nanobubbles: Opportunities in Medical Ultrasound Imaging and Therapy. Front. Phys. 2021, 9, 654374. [Google Scholar] [CrossRef]
- Zhu, W.; Zhou, Z.; Huang, Y.; Liu, H.; He, N.; Zhu, X.; Han, X.; Zhou, D.; Duan, X.; Chen, X.; et al. A versatile 3D-printable hydrogel for antichondrosarcoma, antibacterial, and tissue repair. J. Mater. Sci. Technol. 2023, 136, 200–211. [Google Scholar] [CrossRef]
- Zheng, N.; Fitzpatrick, V.; Cheng, R.; Shi, L.; Kaplan, D.L.; Yang, C. Photoacoustic Carbon Nanotubes Embedded Silk Scaffolds for Neural Stimulation and Regeneration. ACS Nano 2022, 16, 2292–2305. [Google Scholar] [CrossRef]
- Jin, R.; Yang, X.; Zhao, D.; Hou, X.; Li, C.; Song, X.; Chen, W.; Wang, Q.; Zhao, Y.; Liu, B. An injectable hybrid hydrogel based on a genetically engineered polypeptide for second near-infrared fluorescence/photoacoustic imaging-monitored sustained chemo-photothermal therapy. Nanoscale 2019, 11, 16080–16091. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Pandey, K.; Nicolas-Boluda, A.; Onidas, D.; Nizard, P.; Carn, F.; Lucas, T.; Gateau, J.; Martin-Molina, A.; Quesada-Perez, M.; et al. Synergic Thermo- and pH-Sensitive Hybrid Microgels Loaded with Fluorescent Dyes and Ultrasmall Gold Nanoparticles for Photoacoustic Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2022, 14, 54439–54457. [Google Scholar] [CrossRef]
- Jin, R.-M.; Yao, M.-H.; Yang, J.; Zhao, D.-H.; Zhao, Y.-D.; Liu, B. One-Step in Situ Synthesis of Polypeptide–Gold Nanoparticles Hybrid Nanogels and Their Application in Targeted Photoacoustic Imaging. ACS Sustain. Chem. Eng. 2017, 5, 9841–9847. [Google Scholar] [CrossRef]
- Kotturi, D.; Paterson, S.; McShane, M. Comparison of SERS pH probe responses after microencapsulation within hydrogel matrices. J. Biomed. Opt. 2021, 26, 097001. [Google Scholar] [CrossRef]
- Wang, W.; Vikesland, P.J. SERS-Active Printable Hydrogel for 3D Cell Culture and Imaging. Anal. Chem. 2023, 95, 18055–18064. [Google Scholar] [CrossRef]
- Kim, D.; Gwon, G.; Lee, G.; Jeon, Y.; Kim, U.J.; Alothman, Z.A.; You, J. Surface-enhanced Raman scattering-active AuNR array cellulose films for multi-hazard detection. J. Hazard. Mater. 2021, 402, 123505. [Google Scholar] [CrossRef]
- Gallo, E.; Diaferia, C.; Rosa, E.; Smaldone, G.; Morelli, G.; Accardo, A. Peptide-Based Hydrogels and Nanogels for Delivery of Doxorubicin. Int. J. Nanomed. 2021, 16, 1617–1630. [Google Scholar] [CrossRef]
- Hyun, H.; Yoo, Y.B.; Kim, S.Y.; Ko, H.S.; Chun, H.J.; Yang, D.H. Hydrogel-Mediated DOX⋅HCl/PTX Delivery System for Breast Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 4671. [Google Scholar] [CrossRef]
- Sheu, M.T.; Jhan, H.J.; Su, C.Y.; Chen, L.C.; Chang, C.E.; Liu, D.Z.; Ho, H.O. Codelivery of doxorubicin-containing thermosensitive hydrogels incorporated with docetaxel-loaded mixed micelles enhances local cancer therapy. Colloids Surf. B Biointerfaces 2016, 143, 260–270. [Google Scholar] [CrossRef]
- Wu, Z.; Zou, X.; Yang, L.; Lin, S.; Fan, J.; Yang, B.; Sun, X.; Wan, Q.; Chen, Y.; Fu, S. Thermosensitive hydrogel used in dual drug delivery system with paclitaxel-loaded micelles for in situ treatment of lung cancer. Colloids Surf. B Biointerfaces 2014, 122, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Chen, X.; Luan, J.; Wang, D.; Yu, L.; Ding, J. Sustained Codelivery of Cisplatin and Paclitaxel via an Injectable Prodrug Hydrogel for Ovarian Cancer Treatment. ACS Appl. Mater. Interfaces 2017, 9, 40031–40046. [Google Scholar] [CrossRef] [PubMed]
- Nieto, C.; Vega, M.A.; Rodriguez, V.; Perez-Esteban, P.; Martin Del Valle, E.M. Biodegradable gellan gum hydrogels loaded with paclitaxel for HER2+ breast cancer local therapy. Carbohydr. Polym. 2022, 294, 119732. [Google Scholar] [CrossRef]
- Xu, X.; Huang, Z.; Huang, Z.; Zhang, X.; He, S.; Sun, X.; Shen, Y.; Yan, M.; Zhao, C. Injectable, NIR/pH-Responsive Nanocomposite Hydrogel as Long-Acting Implant for Chemophotothermal Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 20361–20375. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Gao, Q.; Guo, Z.; Wang, D.; Gao, F.; Wang, X.; Wei, Y.; Zhao, L. Injectable and Self-Healing Thermosensitive Magnetic Hydrogel for Asynchronous Control Release of Doxorubicin and Docetaxel to Treat Triple-Negative Breast Cancer. ACS Appl. Mater. Interfaces 2017, 9, 33660–33673. [Google Scholar] [CrossRef]
- Fong, Y.T.; Chen, C.H.; Chen, J.P. Intratumoral Delivery of Doxorubicin on Folate-Conjugated Graphene Oxide by In-Situ Forming Thermo-Sensitive Hydrogel for Breast Cancer Therapy. Nanomaterials 2017, 7, 388. [Google Scholar] [CrossRef]
- Liu, M.; Huang, P.; Wang, W.; Feng, Z.; Zhang, J.; Deng, L.; Dong, A. An injectable nanocomposite hydrogel co-constructed with gold nanorods and paclitaxel-loaded nanoparticles for local chemo-photothermal synergetic cancer therapy. J. Mater. Chem. B 2019, 7, 2667–2677. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, X.; Ji, Y.; Jiao, X.; Chen, Q.; Hou, L.; Zhang, H.; Zhang, Z. Near-infrared-triggered in situ hybrid hydrogel system for synergistic cancer therapy. J. Mater. Chem. B 2015, 3, 6310–6326. [Google Scholar] [CrossRef]
- Xia, L.Y.; Zhang, X.; Cao, M.; Chen, Z.; Wu, F.G. Enhanced Fluorescence Emission and Singlet Oxygen Generation of Photosensitizers Embedded in Injectable Hydrogels for Imaging-Guided Photodynamic Cancer Therapy. Biomacromolecules 2017, 18, 3073–3081. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Miao, P.; Li, L.; Yan, R.; Dong, W.F.; Mei, Q. Injectable Carbon Dots-Based Hydrogel for Combined Photothermal Therapy and Photodynamic Therapy of Cancer. ACS Appl. Mater. Interfaces 2022, 14, 49582–49591. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yao, T.; Peng, H.; Whittaker, A.K.; Li, Y.; Zhu, S.; Wang, Z. An Injectable Hydrogel for Simultaneous Photothermal Therapy and Photodynamic Therapy with Ultrahigh Efficiency Based on Carbon Dots and Modified Cellulose Nanocrystals. Adv. Funct. Mater. 2021, 31, 2106079. [Google Scholar] [CrossRef]
- Leung, B.; Dharmaratne, P.; Yan, W.; Chan, B.C.L.; Lau, C.B.S.; Fung, K.P.; Ip, M.; Leung, S.S.Y. Development of thermosensitive hydrogel containing methylene blue for topical antimicrobial photodynamic therapy. J. Photochem. Photobiol. B 2020, 203, 111776. [Google Scholar] [CrossRef]
- Karuppusamy, S.; Hyejin, K.; Kang, H.W. Nanoengineered chlorin e6 conjugated with hydrogel for photodynamic therapy on cancer. Colloids Surf. B Biointerfaces 2019, 181, 778–788. [Google Scholar] [CrossRef]
- Song, H.; Yang, P.; Huang, P.; Zhang, C.; Kong, D.; Wang, W. Injectable polypeptide hydrogel-based co-delivery of vaccine and immune checkpoint inhibitors improves tumor immunotherapy. Theranostics 2019, 9, 2299–2314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.; Liu, Y.; Jiang, Y.; Li, Z. Molecular Targeted Agent and Immune Checkpoint Inhibitor Co-Loaded Thermosensitive Hydrogel for Synergistic Therapy of Rectal Cancer. Front. Pharmacol. 2021, 12, 671611. [Google Scholar] [CrossRef]
- Wang, F.; Su, H.; Xu, D.; Monroe, M.K.; Anderson, C.F.; Zhang, W.; Oh, R.; Wang, Z.; Sun, X.; Wang, H.; et al. Therapeutic supramolecular tubustecan hydrogel combined with checkpoint inhibitor elicits immunity to combat cancer. Biomaterials 2021, 279, 121182. [Google Scholar] [CrossRef]
- Chen, Z.; Rong, Y.; Ding, J.; Cheng, X.; Chen, X.; He, C. Injectable Polypeptide Hydrogel Depots Containing Dual Immune Checkpoint Inhibitors and Doxorubicin for Improved Tumor Immunotherapy and Post-Surgical Tumor Treatment. Pharmaceutics 2023, 15, 428. [Google Scholar] [CrossRef]
- Hao, Y.; Chung, C.K.; Gu, Z.; Schomann, T.; Dong, X.; Veld, R.; Camps, M.G.M.; Ten Dijke, P.; Ossendorp, F.A.; Cruz, L.J. Combinatorial therapeutic approaches of photodynamic therapy and immune checkpoint blockade for colon cancer treatment. Mol. Biomed. 2022, 3, 26. [Google Scholar] [CrossRef]
- Du, Y.N.; Wei, Q.; Zhao, L.J.; Fan, C.Q.; Guo, L.R.; Ye, J.F.; Li, Y. Hydrogel-based co-delivery of CIK cells and oncolytic adenovirus armed with IL12 and IL15 for cancer immunotherapy. Biomed. Pharmacother. 2022, 151, 113110. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Sun, L.; Yang, H.; Xiao, Z.; Deng, Z.; Li, Q.; Wang, C.; Shen, F.; Liu, Z. Ni-Alginate Hydrogel Microspheres with Sustained Interleukin 2 Release to Boost Cytokine-Based Cancer Immunotherapy. Adv. Funct. Mater. 2022, 33, 2211423. [Google Scholar] [CrossRef]
- Lv, Q.; He, C.; Quan, F.; Yu, S.; Chen, X. DOX/IL-2/IFN-gamma co-loaded thermo-sensitive polypeptide hydrogel for efficient melanoma treatment. Bioact. Mater. 2018, 3, 118–128. [Google Scholar] [CrossRef]
- Sun, L.; Shen, F.; Tian, L.; Tao, H.; Xiong, Z.; Xu, J.; Liu, Z. ATP-Responsive Smart Hydrogel Releasing Immune Adjuvant Synchronized with Repeated Chemotherapy or Radiotherapy to Boost Antitumor Immunity. Adv. Mater. 2021, 33, e2007910. [Google Scholar] [CrossRef]
- Fathi, M.; Majidi, S.; Zangabad, P.S.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Chitosan-based multifunctional nanomedicines and theranostics for targeted therapy of cancer. Med. Res. Rev. 2018, 38, 2110–2136. [Google Scholar] [CrossRef]
- Wang, F.; Chen, J.; Liu, J.; Zeng, H. Cancer theranostic platforms based on injectable polymer hydrogels. Biomater. Sci. 2021, 9, 3543–3575. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, Y.; Kim, D.H.; Yoon, H.Y.; Kim, K. Injectable Hydrogel-Based Combination Cancer Immunotherapy for Overcoming Localized Therapeutic Efficacy. Pharmaceutics 2022, 14, 1908. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, L.; Liu, F.; Liu, C.; Shan, D.; Wang, Q.; Zhang, C.; Li, J.; Liu, J.; Qu, X.; et al. pH triggered injectable amphiphilic hydrogel containing doxorubicin and paclitaxel. Int. J. Pharm. 2011, 410, 83–91. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Q.; Qin, X.; Zhang, M.; Du, Q.; Luan, Y. An Injectable Hydrogel Reshaping Adenosinergic Axis for Cancer Therapy. Adv. Funct. Mater. 2022, 32, 2200801. [Google Scholar] [CrossRef]
- Kumar, S.; Bajaj, A. Advances in self-assembled injectable hydrogels for cancer therapy. Biomater. Sci. 2020, 8, 2055–2073. [Google Scholar] [CrossRef]
- Gil, M.S.; Thambi, T.; Phan, V.H.G.; Kim, S.H.; Lee, D.S. Injectable hydrogel-incorporated cancer cell-specific cisplatin releasing nanogels for targeted drug delivery. J. Mater. Chem. B 2017, 5, 7140–7152. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.K.; Lee, S.C.; Kim, H.; Lee, C.-S. Polydopamine Nanoparticle-Incorporated Fluorescent Hydrogel for Fluorescence Imaging-Guided Photothermal Therapy of Cancers. BioChip J. 2022, 17, 85–92. [Google Scholar] [CrossRef]
- Jia, Y.P.; Shi, K.; Yang, F.; Liao, J.F.; Han, R.X.; Yuan, L.P.; Hao, Y.; Pan, M.; Xiao, Y.; Qian, Z.Y.; et al. Multifunctional Nanoparticle Loaded Injectable Thermoresponsive Hydrogel as NIR Controlled Release Platform for Local Photothermal Immunotherapy to Prevent Breast Cancer Postoperative Recurrence and Metastases. Adv. Funct. Mater. 2020, 30, 2001059. [Google Scholar] [CrossRef]
- Mi, D.; Li, J.; Wang, R.; Li, Y.; Zou, L.; Sun, C.; Yan, S.; Yang, H.; Zhao, M.; Shi, S. Postsurgical wound management and prevention of triple-negative breast cancer recurrence with a pryoptosis-inducing, photopolymerizable hydrogel. J. Control. Release 2023, 356, 205–218. [Google Scholar] [CrossRef]
- Wang, H.; Jin, Y.; Tan, Y.; Zhu, H.; Huo, W.; Niu, P.; Li, Z.; Zhang, J.; Liang, X.J.; Yang, X. Photo-responsive hydrogel facilitates nutrition deprivation by an ambidextrous approach for preventing cancer recurrence and metastasis. Biomaterials 2021, 275, 120992. [Google Scholar] [CrossRef]
- Li, J.; Chen, Q.; Li, S.; Zeng, X.; Qin, J.; Li, X.; Chen, Z.; Zheng, W.; Zhao, Y.; Huang, Z.; et al. An adhesive hydrogel implant combining chemotherapy and tumor microenvironment remodeling for preventing postoperative recurrence and metastasis of breast cancer. Chem. Eng. J. 2023, 473, 145212. [Google Scholar] [CrossRef]
- Zhuang, B.; Chen, T.; Xiao, Z.; Jin, Y. Drug-loaded implantable surgical cavity-adaptive hydrogels for prevention of local tumor recurrence. Int. J. Pharm. 2020, 577, 119048. [Google Scholar] [CrossRef]
- Piantanida, E.; Alonci, G.; Bertucci, A.; De Cola, L. Design of Nanocomposite Injectable Hydrogels for Minimally Invasive Surgery. Acc. Chem. Res. 2019, 52, 2101–2112. [Google Scholar] [CrossRef]
- Tan, B.; Huang, L.; Wu, Y.; Liao, J. Advances and trends of hydrogel therapy platform in localized tumor treatment: A review. J. Biomed. Mater. Res. A 2021, 109, 404–425. [Google Scholar] [CrossRef]
- Zhao, F.; Yao, D.; Guo, R.; Deng, L.; Dong, A.; Zhang, J. Composites of Polymer Hydrogels and Nanoparticulate Systems for Biomedical and Pharmaceutical Applications. Nanomaterials 2015, 5, 2054–2130. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, X.; Liu, J.; Zhao, J.; Dong, H.; Deng, L.; Liu, J.; Dong, A. Sequential thermo-induced self-gelation and acid-triggered self-release process of drug-conjugated nanoparticles: A strategy for the sustained and controlled drug delivery to tumors. J. Mater. Chem. B 2013, 1, 4667–4677. [Google Scholar] [CrossRef]
- Laquerbe, S.; Es Sayed, J.; Lorthioir, C.; Meyer, C.; Narita, T.; Ducouret, G.; Perrin, P.; Sanson, N. Supramolecular Crosslinked Hydrogels: Similarities and Differences with Chemically Crosslinked Hydrogels. Macromolecules 2023, 56, 7406–7418. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, T.; Liu, Y.; Shang, F.; Chen, B.; Hu, Y.; Wang, S.; Guo, Z. Supramolecular hydrogel of poly(vinyl alcohol)/chitosan: A dual cross-link design. Adv. Polym. Technol. 2017, 37, 2186–2194. [Google Scholar] [CrossRef]
- Shigemitsu, H.; Hamachi, I. Design Strategies of Stimuli-Responsive Supramolecular Hydrogels Relying on Structural Analyses and Cell-Mimicking Approaches. Acc. Chem. Res. 2017, 50, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Yu, X.; Wu, H.; Li, J.; Lv, H.; Yang, X. Nonswellable and Tough Supramolecular Hydrogel Based on Strong Micelle Cross-Linkings. Biomacromolecules 2019, 20, 3399–3407. [Google Scholar] [CrossRef]
- Yu, B.; Zhan, A.; Liu, Q.; Ye, H.; Huang, X.; Shu, Y.; Yang, Y.; Liu, H. A designed supramolecular cross-linking hydrogel for the direct, convenient, and efficient administration of hydrophobic drugs. Int. J. Pharm. 2020, 578, 119075. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.; Gaitzsch, J.; Liubimtsev, N.; Zschoche, S.; Bauer, T.; Appelhans, D.; Voit, B. Double cross-linked supramolecular hydrogels with tunable properties based on host-guest interactions. Soft Matter 2020, 16, 6733–6742. [Google Scholar] [CrossRef]
- Le, X.; Lu, W.; Zhang, J.; Chen, T. Recent Progress in Biomimetic Anisotropic Hydrogel Actuators. Adv. Sci. 2019, 6, 1801584. [Google Scholar] [CrossRef]
- Xie, R.; Liang, Z.; Ai, Y.; Zheng, W.; Xiong, J.; Xu, P.; Liu, Y.; Ding, M.; Gao, J.; Wang, J.; et al. Composable microfluidic spinning platforms for facile production of biomimetic perfusable hydrogel microtubes. Nat. Protoc. 2021, 16, 937–964. [Google Scholar] [CrossRef]
- Adorinni, S.; Rozhin, P.; Marchesan, S. Smart Hydrogels Meet Carbon Nanomaterials for New Frontiers in Medicine. Biomedicines 2021, 9, 570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Chandra Sekhar, P.D.; Singh, M.; Tong, Y.; Kucukdeger, E.; Yoon, H.Y.; Haring, A.P.; Roman, M.; Kong, Z.; et al. Rapid, autonomous high-throughput characterization of hydrogel rheological properties via automated sensing and physics-guided machine learning. Appl. Mater. Today 2023, 30, 101720. [Google Scholar] [CrossRef]
- Owh, C.; Ho, D.; Loh, X.J.; Xue, K. Towards machine learning for hydrogel drug delivery systems. Trends Biotechnol. 2023, 41, 476–479. [Google Scholar] [CrossRef]
- Li, F.; Han, J.; Cao, T.; Lam, W.; Fan, B.; Tang, W.; Chen, S.; Fok, K.L.; Li, L. Design of self-assembly dipeptide hydrogels and machine learning via their chemical features. Proc. Natl. Acad. Sci. USA 2019, 116, 11259–11264. [Google Scholar] [CrossRef]
- Wang, Y.; Wallmersperger, T.; Ehrenhofer, A. Prediction of hydrogel swelling states using machine learning methods. Eng. Rep. 2024, 6, e12893. [Google Scholar] [CrossRef]
- Neufeld, L.; Yeini, E.; Reisman, N.; Shtilerman, Y.; Ben-Shushan, D.; Pozzi, S.; Madi, A.; Tiram, G.; Eldar-Boock, A.; Ferber, S.; et al. Microengineered perfusable 3D-bioprinted glioblastoma model for in vivo mimicry of tumor microenvironment. Sci. Adv. 2021, 7, eabi9119. [Google Scholar] [CrossRef]
- Moghimi, N.; Hosseini, S.A.; Dalan, A.B.; Mohammadrezaei, D.; Goldman, A.; Kohandel, M. Controlled tumor heterogeneity in a co-culture system by 3D bio-printed tumor-on-chip model. Sci. Rep. 2023, 13, 13648. [Google Scholar] [CrossRef] [PubMed]
- Datta, P.; Dey, M.; Ataie, Z.; Unutmaz, D.; Ozbolat, I.T. 3D bioprinting for reconstituting the cancer microenvironment. NPJ Precis. Oncol. 2020, 4, 18. [Google Scholar] [CrossRef]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef]
- Luo, Y.; Wei, X.; Wan, Y.; Lin, X.; Wang, Z.; Huang, P. 3D printing of hydrogel scaffolds for future application in photothermal therapy of breast cancer and tissue repair. Acta Biomater. 2019, 92, 37–47. [Google Scholar] [CrossRef]
- Kim, D.; Jo, S.; Lee, D.; Kim, S.M.; Seok, J.M.; Yeo, S.J.; Lee, J.H.; Lee, J.J.; Lee, K.; Kim, T.D.; et al. NK cells encapsulated in micro/macropore-forming hydrogels via 3D bioprinting for tumor immunotherapy. Biomater. Res. 2023, 27, 60. [Google Scholar] [CrossRef]
- Li, J.; Ji, C.; Lu, B.; Rodin, M.; Paradies, J.; Yin, M.; Kuckling, D. Dually Crosslinked Supramolecular Hydrogel for Cancer Biomarker Sensing. ACS Appl. Mater. Interfaces 2020, 12, 36873–36881. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Han, R.; Chen, M.; Luo, X. Antifouling Peptide Hydrogel Based Electrochemical Biosensors for Highly Sensitive Detection of Cancer Biomarker HER2 in Human Serum. Anal. Chem. 2021, 93, 7355–7361. [Google Scholar] [CrossRef] [PubMed]
- Mateti, T.; Likhith, K.; Laha, A.; Thakur, G. A critical analysis of the recent developments in multi-stimuli responsive smart hydrogels for cancer treatment. Curr. Opin. Biomed. Eng. 2023, 25, 100424. [Google Scholar] [CrossRef]
- Solanki, R.; Bhatia, D. Stimulus-Responsive Hydrogels for Targeted Cancer Therapy. Gels 2024, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.; Roca-Melendres, M.M.; Duran-Lara, E.F.; Rafael, D.; Schwartz, S., Jr. Stimuli-Responsive Hydrogels for Cancer Treatment: The Role of pH, Light, Ionic Strength and Magnetic Field. Cancers 2021, 13, 1164. [Google Scholar] [CrossRef]
- Sepantafar, M.; Maheronnaghsh, R.; Mohammadi, H.; Radmanesh, F.; Hasani-Sadrabadi, M.M.; Ebrahimi, M.; Baharvand, H. Engineered Hydrogels in Cancer Therapy and Diagnosis. Trends Biotechnol. 2017, 35, 1074–1087. [Google Scholar] [CrossRef]


| Type | Materials | Key Features | Applications | References |
|---|---|---|---|---|
| Natural hydrogel | Alginate, Hyaluronic Acid, Collagen, Chitosan | Biocompatibility, biodegradability, bioactivity | Drug delivery, tumor targeting | [54,55,56,57,58,59,60] |
| Synthetic hydrogel | PEG, PLGA, PVA, PNIPAm | Controlled mechanical strength, stimuli-responsiveness, customizable degradation rate | Controlled chemotherapy, localized immunotherapy, photothermal therapy | [61,62,63,64,65,66,67,68,69] |
| Hybrid hydrogel | PEG-Collagen, Chitosan-PLGA, Alginate-PEG | Optimized mechanical and biological characteristics, enhanced stimuli responsiveness | Advanced targeted delivery, precision oncology, prevention of metastasis | [70,71,72] |
| Type | Materials | Key Features | Applications | References |
|---|---|---|---|---|
| Stimuli-responsive hydrogel | pH-sensitive, Thermoresponsive, Magnetic-responsive, NIR-responsive hydrogels | Dynamic response to tumor microenvironment, controlled drug release, enhanced precision targeting | Localized chemotherapy, photothermal/photodynamic therapy, imaging-guided therapy | [78,79,80,81,82,83,84,85,86,87,88,89,90,91,92] |
| Therapuetic Modality | Cancers | Hydrogel Types | Activations | References |
|---|---|---|---|---|
| Chemotherapy | Breast, Lung, Ovarian | Doxorubicin, Paclitaxel-loaded hydrogels | Passive diffusion, biodegradation | [130,131,132,133,134,135] |
| PTT | Skin, Breast, Liver | Gold nanoparticles, Polydopamine-based hydrogels | NIR irradiation | [136,137,138,139,140] |
| PDT | Skin, Oral cavity | Photosensitizers (Chlorin e6, porphyrins) | Light irradiation | [141,142,143,144,145] |
| Immunotherapy | Melanoma, Lung | Hydrogels with checkpoint inhibitors, cytokines | Biological interaction, controlled release | [146,147,148,149,150,151,152,153,154] |
| Synergistic Chemo-Photothermal Therapy | Breast, Liver, Pancreatic | Hydrogels with chemotherapeutics + photothermal agents | NIR irradiation, biodegradation | [136,137] |
| Combined Immunotherapy and Phototherapy | Melanoma, Lung cancers | Immunotherapeutic agents + photosensitizers or photothermal agents | NIR irradiation, biodegradation, immune activation | [144,150] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.K.; Go, K.; Lee, E.; Kim, H.; Kim, S.; Kim, J.-H.; Chae, M.S.; Jeong, J.-O. Multifunctional Hydrogels for Advanced Cancer Treatment: Diagnostic Imaging and Therapeutic Modalities. Gels 2025, 11, 426. https://doi.org/10.3390/gels11060426
Lee KK, Go K, Lee E, Kim H, Kim S, Kim J-H, Chae MS, Jeong J-O. Multifunctional Hydrogels for Advanced Cancer Treatment: Diagnostic Imaging and Therapeutic Modalities. Gels. 2025; 11(6):426. https://doi.org/10.3390/gels11060426
Chicago/Turabian StyleLee, Kyung Kwan, Kwangmo Go, Eonjin Lee, Hongki Kim, Seonwook Kim, Ji-Hyun Kim, Min Suk Chae, and Jin-Oh Jeong. 2025. "Multifunctional Hydrogels for Advanced Cancer Treatment: Diagnostic Imaging and Therapeutic Modalities" Gels 11, no. 6: 426. https://doi.org/10.3390/gels11060426
APA StyleLee, K. K., Go, K., Lee, E., Kim, H., Kim, S., Kim, J.-H., Chae, M. S., & Jeong, J.-O. (2025). Multifunctional Hydrogels for Advanced Cancer Treatment: Diagnostic Imaging and Therapeutic Modalities. Gels, 11(6), 426. https://doi.org/10.3390/gels11060426







