Hyaluronic Acid: Production Strategies, Gel-Forming Properties, and Advances in Drug Delivery Systems
Abstract
1. Introduction
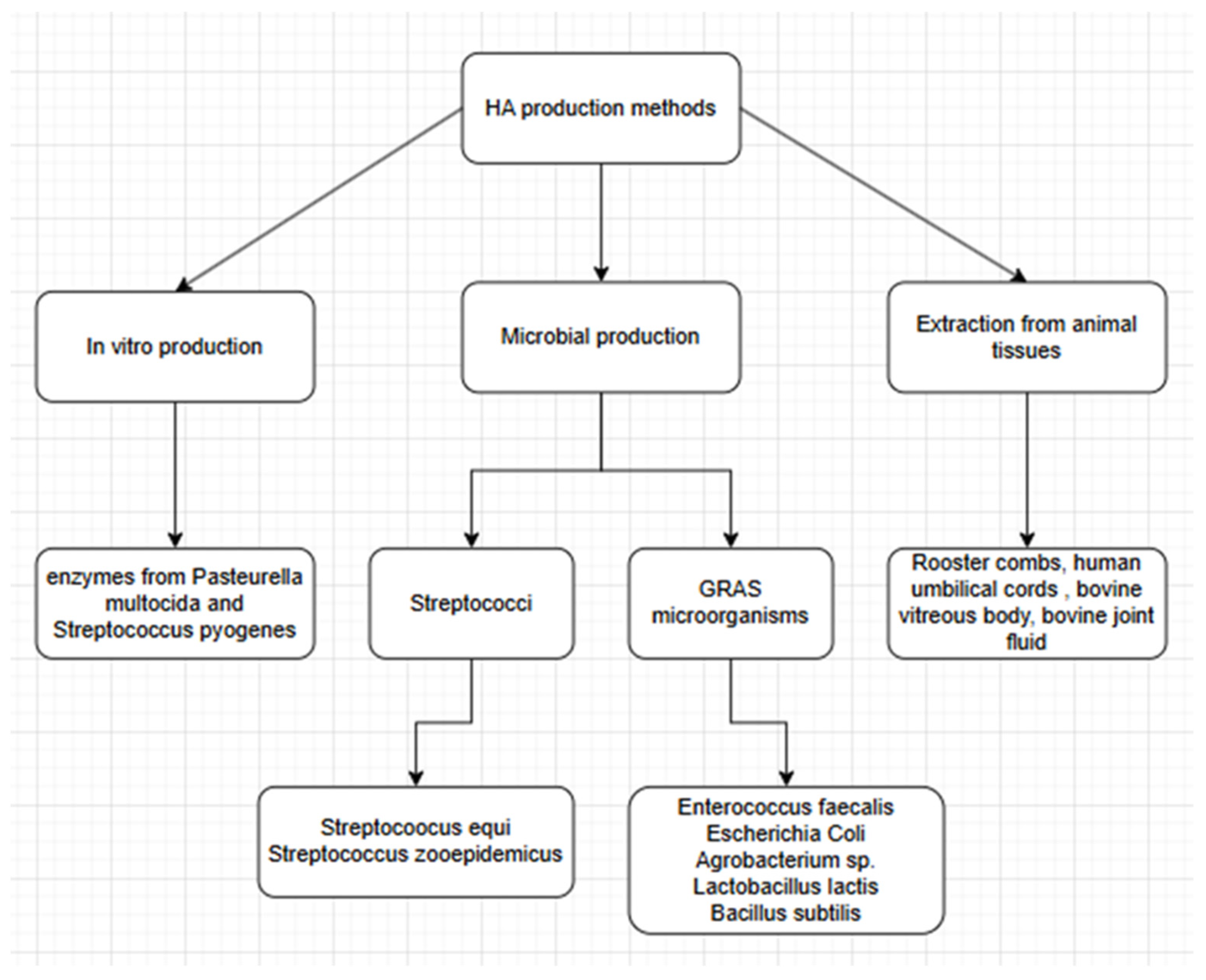
2. HA Production
2.1. Extraction from Animal Tissue
2.2. Enzymatic Synthesis
2.3. Microbial Production
2.3.1. Streptococcus sp.
2.3.2. Pasteurella multocida
2.3.3. “GRAS” Microorganisms
Bacillus subtillis
Lactococcus lactis
Escherichia coli
Streptococcus thermophilus
2.3.4. Other Microorganism
3. Bioreactors for HA Production
3.1. Increase in Viscosity
3.2. Mixing
3.3. Aeration
4. Process Modes
4.1. Batch
4.2. Continuous
4.3. Fed-Batch
5. HA Gel-Forming Strategies
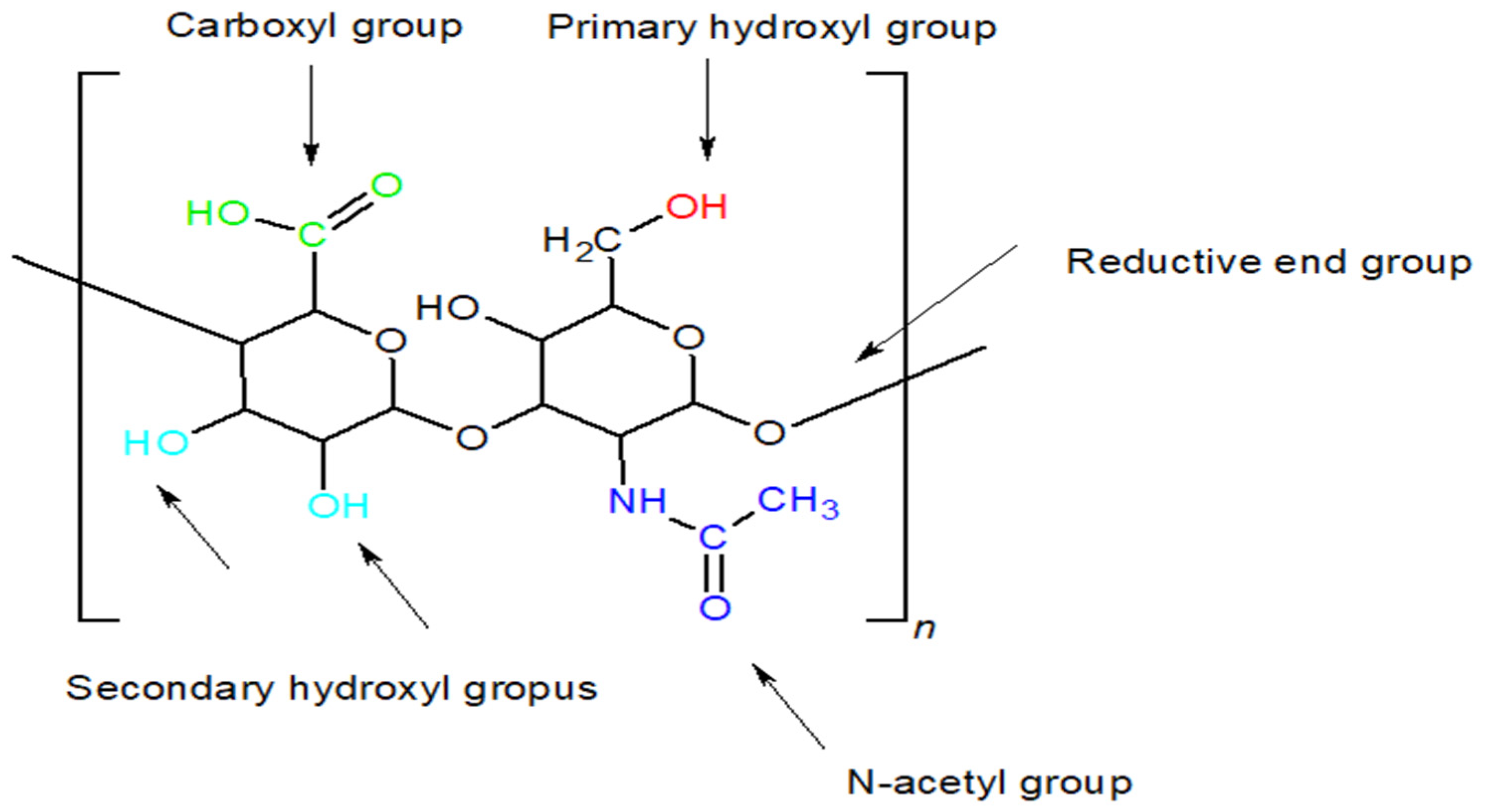
5.1. Chemical Modification of HA Functional Groups
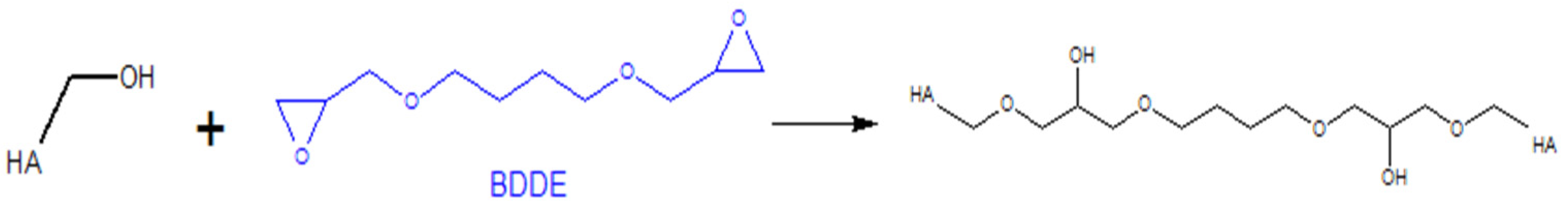
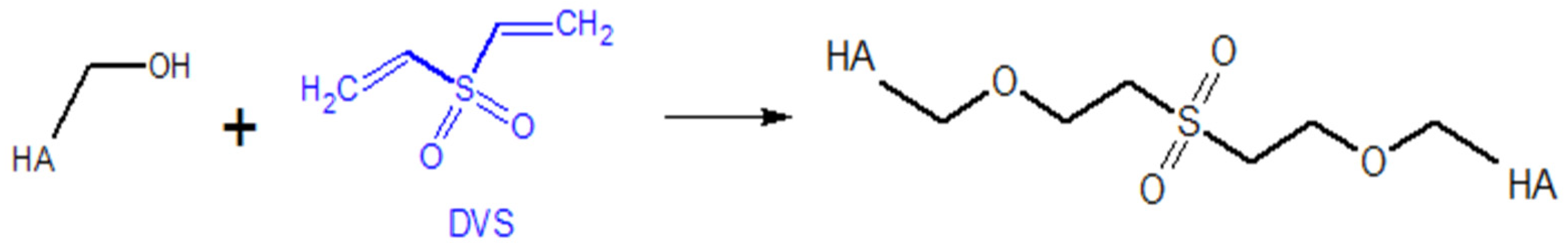
5.1.1. Modifications of the –OH Group
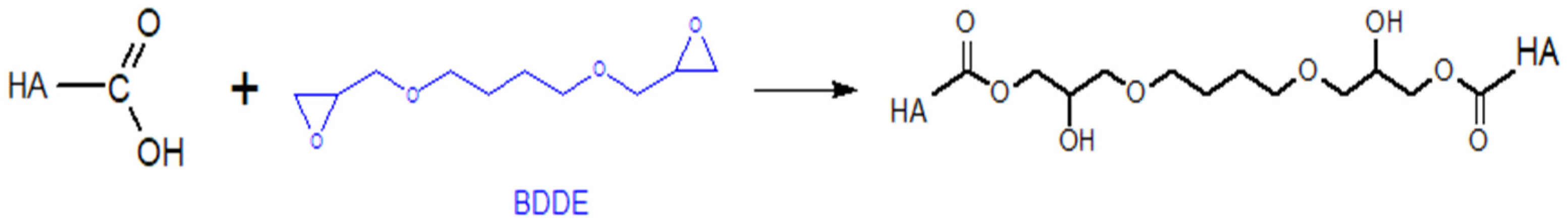
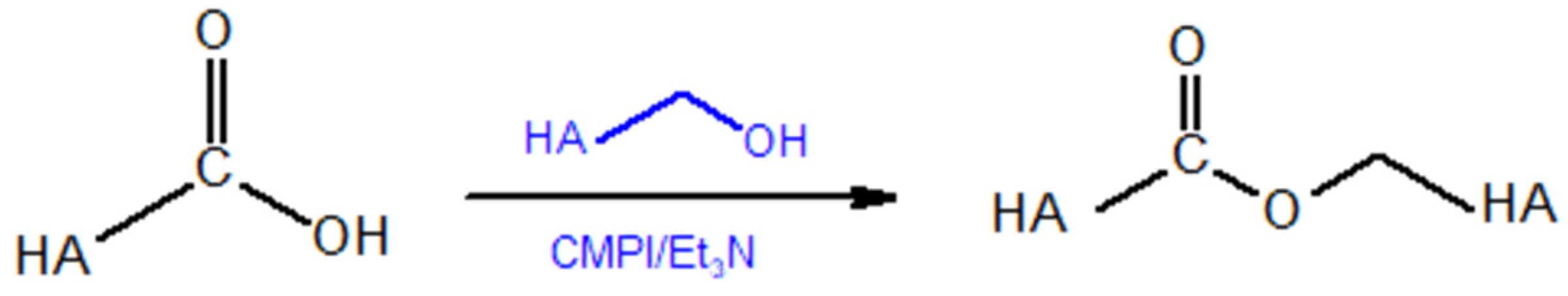
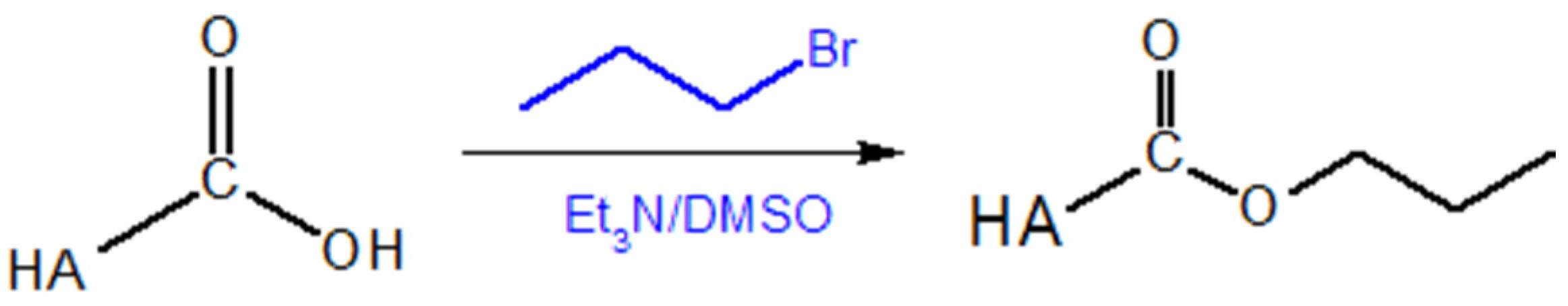
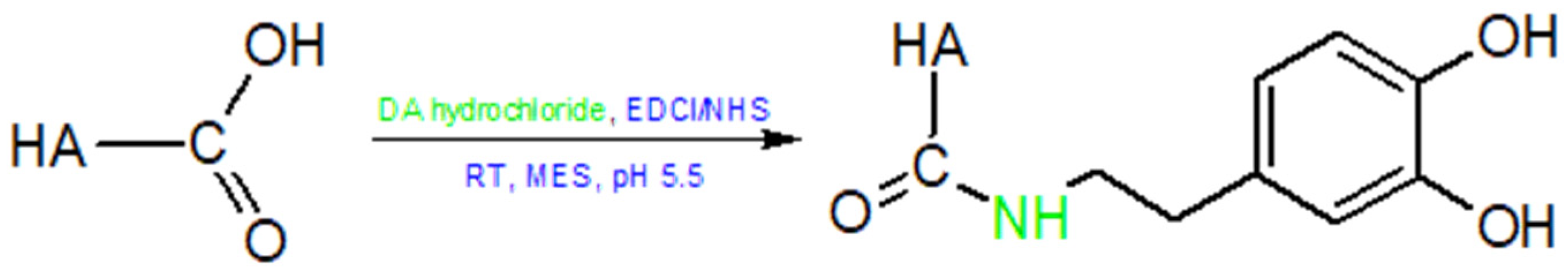
5.1.2. Modifications of the –COOH Group
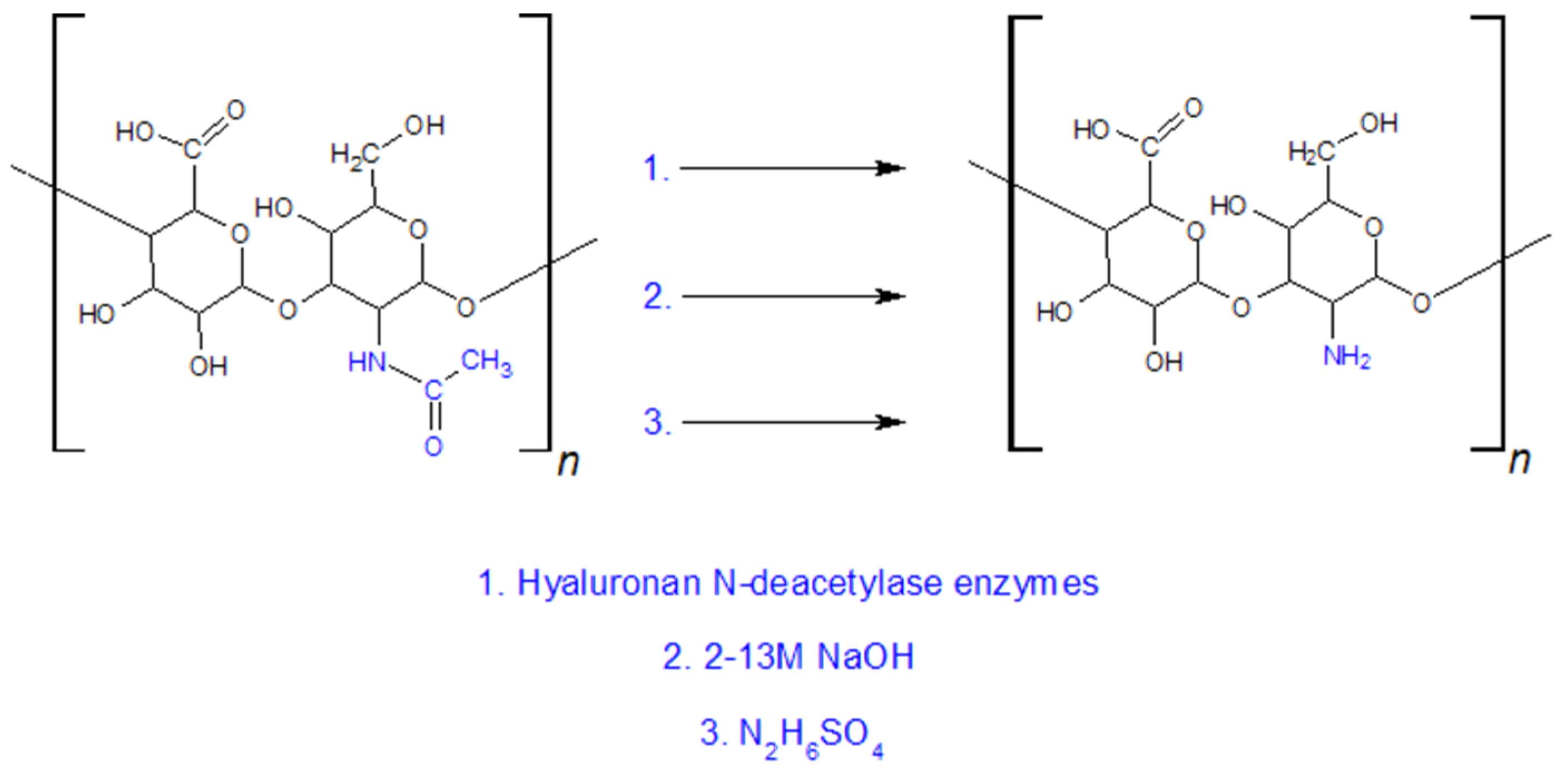
5.1.3. Modifications of the NHCOCH3 Group
5.2. Physical and Chemical Hydrogels Creation
5.2.1. Physical Crosslinking
5.2.2. Coordination Crosslinking
5.2.3. Chemical Crosslinking
Carbodiimide Crosslinking
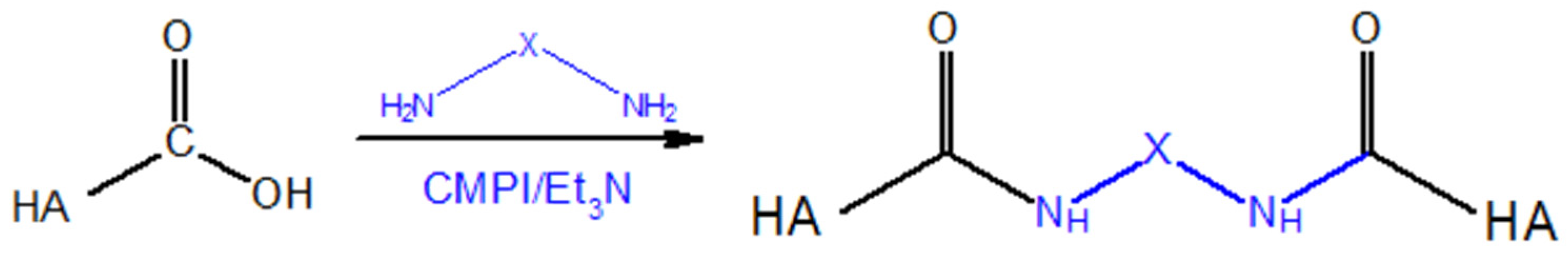
Diisocyanate Crosslinking
Michael Addition
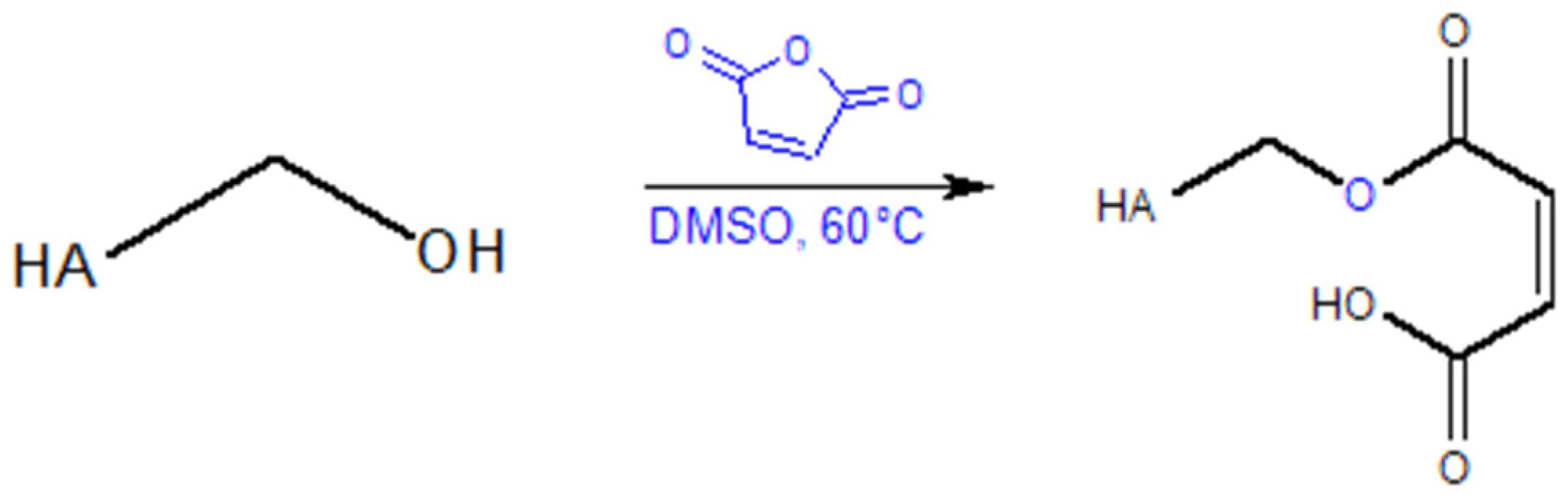
Diels–Alder Reaction
Host–Guest Complexes
Gelling Agents
Functionalisation of HA with Hydrophobic Molecules
Crosslinking via Condensation Reactions
Radical Polymerisation
Enzymatic Crosslinking
Thiol-Ene Photocoupling
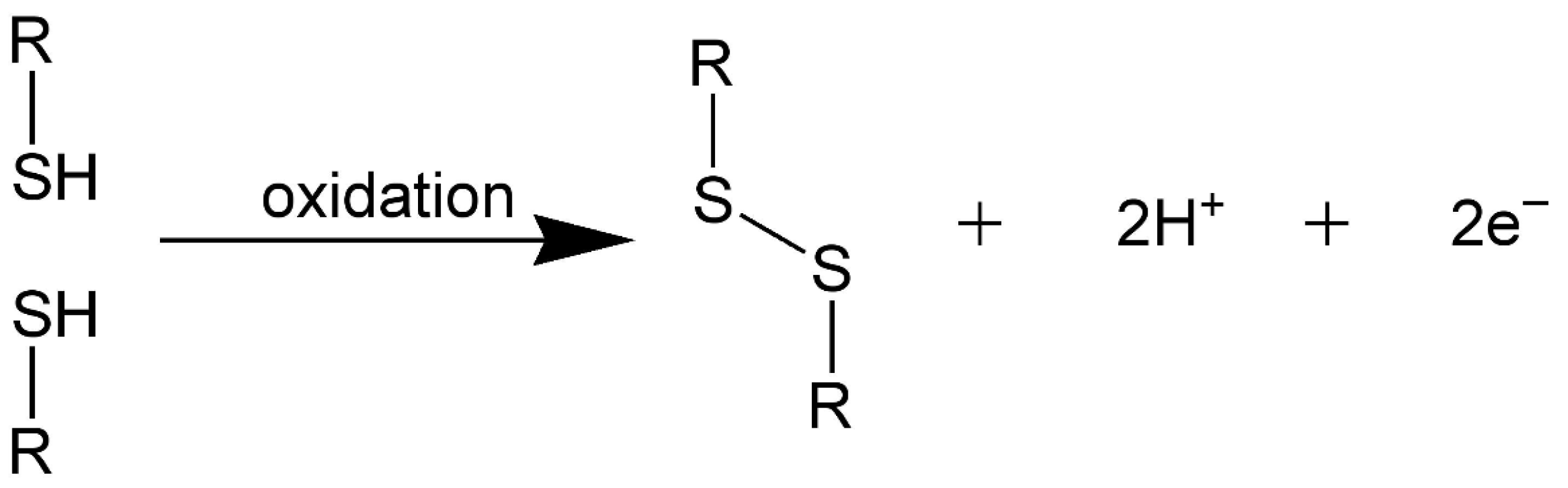
Disulfide Crosslinking
5.3. HA-Based Hydrogels
5.3.1. HA Hydrogels
5.3.2. Ha-Col Hydrogels
5.3.3. HA-Alg Hydrogels
5.3.4. HA-CS Hydrogels
5.3.5. HA-Gel Hydrogels
5.3.6. HA-PCL Combinations
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef]
- Hirschberg, J. Zentralblatt Für Praktische Augenheilkunde; Verlag von Veit & Comp.: Leibzig, Germany, 1880. [Google Scholar]
- Meyer, K.; Palmer, J.W. The Polysaccharide of the Vitreous Humor. J. Biol. Chem. 1934, 107, 629–634. [Google Scholar] [CrossRef]
- Balazs, E.A.; Laurent, T.C.; Jeanloz, R.W. Nomenclature of hyaluronic acid. Biochem. J. 1986, 235, 903. [Google Scholar] [CrossRef] [PubMed]
- Laurent, T.C.; Laurent, U.B.; Fraser, J.R.E. The structure and function of hyaluronan: An overview. Immunol. Cell Biol. 1996, 74, a1–a7. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Laurent, T.C.; Hascall, V.C. Hyaluronan: Structure and Physical Properties. Hyaluronan Today 1997, 1, A2. Available online: https://www.glycoforum.gr.jp/article/01A2.html (accessed on 15 April 2025).
- Scott, J.E.; Cummings, C.; Brass, A.; Chen, Y. Secondary and tertiary structures of hyaluronan in aqueous solution, investigated by rotary shadowing-electron microscopy and computer simulation. Hyaluronan is a very efficient network-forming polymer. Biochem. J. 1991, 274, 699–705. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Springer, J.; Kooy, F.K.; Van Den Broek, L.A.M.; Eggink, G. Production Methods for Hyaluronan. Int. J. Carbohydr. Chem. 2013, 2013, 624967. [Google Scholar] [CrossRef]
- Di Meo, C.; Stellavato, A.; d’Agostino, M.; D’Agostino, A.; Schiraldi, C.; La Gatta, A. Hyaluronan size and concentration: Effect on key biophysical and biochemical features. Int. J. Biol. Macromol. 2024, 282, 137125. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Okamoto, A.; Nishinari, K. Viscoelasticity of hyaluronic acid with different molecular weights. Biorheology 1994, 31, 235–244. [Google Scholar] [CrossRef]
- Scott, J.E. Supramolecular organization of extracellular matrix glycosaminoglycans, in vitro and in the tissues. FASEB J. 1992, 6, 2639–2645. [Google Scholar] [CrossRef] [PubMed]
- Lapčík, L.; De Smedt, S.; Demeester, J.; Chabreček, P. Hyaluronan: Preparation, Structure, Properties, and Applications. Chem. Rev. 1998, 98, 2663–2684. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; Kjøniksen, A.; Nyström, B. Effect of pH on the Behavior of Hyaluronic Acid in Dilute and Semidilute Aqueous Solutions. Macromol. Symp. 2008, 274, 131–140. [Google Scholar] [CrossRef]
- Cleland, R.L. Ionic polysaccharides. II. Comparison of polyelectrolyte behavior of hyaluronatc with that of carboxymethyl cellulose. Biopolymers 1968, 6, 1519–1529. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic Acid: The Influence of Molecular Weight on Structural, Physical, Physico-Chemical, and Degradable Properties of Biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef]
- Vigetti, D.; Karousou, E.; Viola, M.; Deleonibus, S.; De Luca, G.; Passi, A. Hyaluronan: Biosynthesis and signaling. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2014, 1840, 2452–2459. [Google Scholar] [CrossRef]
- Rwei, S.-P.; Chen, S.-W.; Mao, C.-F.; Fang, H.-W. Viscoelasticity and wearability of hyaluronate solutions. Biochem. Eng. J. 2008, 40, 211–217. [Google Scholar] [CrossRef]
- Nyström, B.; Kjøniksen, A.L.; Beheshti, N.; Maleki, A.; Zhu, K.; Knudsen, K.D.; De la Torre, J.G. Characterization of polyelectrolyte features in polysaccharide systems and mucin. Adv. Colloid Interface Sci. 2010, 158, 108–118. [Google Scholar] [CrossRef]
- Volpi, N.; Schiller, J.; Stern, R.; Soltes, L. Role, Metabolism, Chemical Modifications and Applications of Hyaluronan. Comput. Mater. Contin. 2009, 16, 1718–1745. [Google Scholar] [CrossRef]
- Cyphert, J.M.; Trempus, C.S.; Garantziotis, S. Size Matters: Molecular Weight Specificity of Hyaluronan Effects in Cell Biology. Int. J. Cell Biol. 2015, 2015, 563818. [Google Scholar] [CrossRef] [PubMed]
- Girish, K.S.; Kemparaju, K. The magic glue hyaluronan and its eraser hyaluronidase: A biological overview. Life Sci. 2007, 80, 1921–1943. [Google Scholar] [CrossRef] [PubMed]
- Toole, B.P. Hyaluronan: From extracellular glue to pericellular cue. Nat. Rev. Cancer 2004, 4, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Robert, L.; Robert, A.-M.; Renard, G. Biological effects of hyaluronan in connective tissues, eye, skin, venous wall. Role in aging. Pathol. Biol. 2010, 58, 187–198. [Google Scholar] [CrossRef]
- Fakhari, A.; Berkland, C. Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler and in osteoarthritis treatment. Acta Biomater. 2013, 9, 7081–7092. [Google Scholar] [CrossRef]
- Toole, B.P. Hyaluronan in morphogenesis. Semin. Cell Dev. Biol. 2001, 12, 79–87. [Google Scholar] [CrossRef]
- Christofori, G. NEW EMBO MEMBER’S REVIEW: Changing neighbours, changing behaviour: Cell adhesion molecule-mediated signalling during tumour progression. EMBO J. 2003, 22, 2318–2323. [Google Scholar] [CrossRef]
- Takeuchi, O.; Hoshino, K.; Kawai, T.; Sanjo, H.; Takada, H.; Ogawa, T.; Akira, S. Differential Roles of TLR2 and TLR4 in Recognition of Gram-Negative and Gram-Positive Bacterial Cell Wall Components. Immunity 1999, 11, 443–451. [Google Scholar] [CrossRef]
- Scheibner, K.A.; Lutz, M.A.; Boodoo, S.; Fenton, M.J.; Powell, J.D.; Horton, M.R. Hyaluronan Fragments Act as an Endogenous Danger Signal by Engaging TLR2. J. Immunol. 2006, 177, 1272–1281. [Google Scholar] [CrossRef]
- Chen, W.Y.J.; Abatangelo, G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999, 7, 79–89. [Google Scholar] [CrossRef]
- Ruppert, S.M.; Hawn, T.R.; Arrigoni, A.; Wight, T.N.; Bollyky, P.L. Tissue integrity signals communicated by high-molecular weight hyaluronan and the resolution of inflammation. Immunol. Res. 2014, 58, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Widner, B.; Behr, R.; Von Dollen, S.; Tang, M.; Heu, T.; Sloma, A.; Brown, S. Hyaluronic Acid Production in Bacillus subtilis. Appl. Environ. Microbiol. 2005, 71, 3747–3752. [Google Scholar] [CrossRef]
- Orlińska, K.; Komosińska-Vassev, K.; Olczyk, K.; Kowalczyk, A.; Olczyk, P. Glycosaminoglycans—Types, structure, functions, and the role in wound healing processes. Ann. Acad. Med. Siles. 2023, 77, 204–216. [Google Scholar] [CrossRef]
- Byrom, D. (Ed.) Biomaterials; Palgrave Macmillan: London, UK, 1991. [Google Scholar] [CrossRef]
- Fraser, J.R.E.; Laurent, T.C.; Laurent, U.B.G. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef]
- Graciela, C.-Q.; Juan, E.-C.J.; Gieraldin, C.-L.; Alejandra, P.-M.X.; Gabriel, A.-Á. Hyaluronic Acid. Extraction Methods, Sources and Applications. Polymers 2023, 15, 3473. [Google Scholar] [CrossRef]
- Itano, N. Hyaluronan Biosynthesis: A Multifaceted Process. Connect. Tissue 2018, 33, 221–226. [Google Scholar]
- Ignatova, E.Y.; Gurov, A.N. Principles of extraction and purification of hyaluronic acid (review). Pharm. Chem. J. 1990, 24, 211–216. [Google Scholar] [CrossRef]
- Balazs, E. Ultrapure Hyaluronic Acid and the Use Thereof. U.S. Patent 4141973A, 27 February 1989. [Google Scholar]
- O’Regan, M.; Martini, I.; Crescenzi, F.; De Luca, C.; Lansing, M. Molecular mechanisms and genetics of hyaluronan biosynthesis. Int. J. Biol. Macromol. 1994, 16, 283–286. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B-Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef]
- Sze, J.H.; Brownlie, J.C.; Love, C.A. Biotechnological production of hyaluronic acid: A mini review. 3 Biotech 2016, 6, 67. [Google Scholar] [CrossRef]
- Kang, D.Y.; Kim, W.; Heo, I.S.; Park, Y.H.; Lee, S. Extraction of hyaluronic acid (HA) from rooster comb and characterization using flow field-flow fractionation (FlFFF) coupled with multiangle light scattering (MALS). J. Sep. Sci. 2010, 33, 3530–3536. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Lansing, M.; Martini, I.; Crescenzi, F.; Shen, G.J.; O’Regan, M.; Wong, C.H. Enzymic Synthesis of Hyaluronic Acid with Regeneration of Sugar Nucleotides. J. Am. Chem. Soc. 1995, 117, 5869–5870. [Google Scholar] [CrossRef]
- DeAngelis, P. Monodisperse Hyaluronan Polymers: Synthesis and Potential Applications. Curr. Pharm. Biotechnol. 2008, 9, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Kooy, F.K.; Ma, M.; Beeftink, H.H.; Eggink, G.; Tramper, J.; Boeriu, C.G. Quantification and characterization of enzymatically produced hyaluronan with fluorophore-assisted carbohydrate electrophoresis. Anal. Biochem. 2009, 384, 329–336. [Google Scholar] [CrossRef]
- Ucm, R.; Aem, M.; Lhb, Z.; Kumar, V.; Taherzadeh, M.J.; Garlapati, V.K.; Chandel, A.K. Comprehensive review on biotechnological production of hyaluronic acid: Status, innovation, market and applications. Bioengineered 2022, 13, 9645–9661. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; DeAngelis, P.L. Synchronized Chemoenzymatic Synthesis of Monodisperse Hyaluronan Polymers. J. Biol. Chem. 2004, 279, 42345–42349. [Google Scholar] [CrossRef]
- Benedini, L.J.; Santana, M.H.A. Effects of soy peptone on the inoculum preparation of Streptococcus zooepidemicus for production of hyaluronic acid. Bioresour. Technol. 2013, 130, 798–800. [Google Scholar] [CrossRef]
- Chien, L.-J.; Lee, C.-K. Enhanced Hyaluronic Acid Production in Bacillus subtilis by Coexpressing Bacterial Hemoglobin. Biotechnol. Prog. 2007, 23, 1017–1022. [Google Scholar] [CrossRef]
- Chong, B.F.; Blank, L.M.; Mclaughlin, R.; Nielsen, L.K. Microbial hyaluronic acid production. Appl. Microbiol. Biotechnol. 2005, 66, 341–351. [Google Scholar] [CrossRef]
- Izawa, N.; Hanamizu, T.; Iizuka, R.; Sone, T.; Mizukoshi, H.; Kimura, K.; Chiba, K. Streptococcus thermophilus produces exopolysaccharides including hyaluronic acid. J. Biosci. Bioeng. 2009, 107, 119–123. [Google Scholar] [CrossRef]
- DeAngelis, P.L.; Jing, W.; Graves, M.V.; Burbank, D.E.; Van Etten, J.L. Hyaluronan Synthase of Chlorella Virus PBCV-1. Science 1997, 278, 1800–1803. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.D.; Carvalho, L.S.; Gomes, A.M.V.; Queiroz, L.R.; Magalhães, B.S.; Parachin, N.S. Genetic basis for hyper production of hyaluronic acid in natural and engineered microorganisms. Microb. Cell Factories 2016, 15, 119. [Google Scholar] [CrossRef]
- Pelkonen, S.; Lindahl, S.B.; Suomala, P.; Karhukorpi, J.; Vuorinen, S.; Koivula, I.; Tuuminen, T. Transmission of Streptococcus equi Subspecies zooepidemicus Infection from Horses to Humans. Emerg. Infect. Dis. 2013, 19, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- DeAngelis, P.L.; Weigel, P.H.; Papaconstantinou, J. Isolation of a Streptococcus pyogenes gene locus that directs hyaluronan biosynthesis in acapsular mutants and in heterologous bacteria. J. Biol. Chem. 1993, 268, 14568–14571. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Marcellin, E.; Hung, J.; Nielsen, L.K. Hyaluronan Molecular Weight Is Controlled by UDP-N-acetylglucosamine Concentration in Streptococcus zooepidemicus. J. Biol. Chem. 2009, 284, 18007–18014. [Google Scholar] [CrossRef] [PubMed]
- Streptococcus Pyogenes—Pathogen Safety Data Sheets; Government of Canada, Public Health Agency of Canada: Ottawa, ON, Canada, 2001.
- Blanchong, J.A.; Samuel, M.D.; Goldberg, D.R.; Shadduck, D.J.; Lehr, M.A. PERSISTENCE OF PASTEURELLA MULTOCIDA IN WETLANDS FOLLOWING AVIAN CHOLERA OUTBREAKS. J. Wildl. Dis. 2006, 42, 33–39. [Google Scholar] [CrossRef]
- Magyar, T.; Lax, A. Bacteria: Pasteurella Multocida. In Encyclopedia of Food Safety; Elsevier: Amsterdam, The Netherlands, 2014; pp. 476–479. [Google Scholar] [CrossRef]
- DeAngelis, P.L. Molecular Directionality of Polysaccharide Polymerization by the Pasteurella multocida Hyaluronan Synthase. J. Biol. Chem. 1999, 274, 26557–26562. [Google Scholar] [CrossRef]
- DeAngelis, P.L.; Jing, W.; Drake, R.R.; Achyuthan, A.M. Identification and Molecular Cloning of a Unique Hyaluronan Synthase from Pasteurella multocida. J. Biol. Chem. 1998, 273, 8454–8458. [Google Scholar] [CrossRef]
- Casolari, C.; Fabio, U. Isolation of Pasteurella multocida from human clinical specimens: First report in Italy. Eur. J. Epidemiol. 1988, 4, 389–390. [Google Scholar] [CrossRef]
- Mohan, N.; Balakrishnan, R.; Sivaprakasam, S. Optimization and effect of dairy industrial waste as media components in the production of hyaluronic acid by Streptococcus thermophilus. Prep. Biochem. Biotechnol. 2016, 46, 628–638. [Google Scholar] [CrossRef]
- Chien, L.-J.; Lee, C.-K. Hyaluronic acid production by recombinant Lactococcus lactis. Appl. Microbiol. Biotechnol. 2007, 77, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Chen, R.R. Recombinant Synthesis of Hyaluronan by Agrobacterium sp. Biotechnol. Prog. 2007, 23, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, O.P.; Beerthuyzen, M.M.; De Ruyter, P.G.G.A.; Luesink, E.J.; De Vos, W.M. Autoregulation of Nisin Biosynthesis in Lactococcus lactis by Signal Transduction. J. Biol. Chem. 1995, 270, 27299–27304. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, C.; Li, S.; Zhang, Y.; Yang, Z. Growth and exopolysaccharide production by Streptococcus thermophilus ST1 in skim milk. Braz. J. Microbiol. 2011, 42, 1470–1478. [Google Scholar] [CrossRef]
- Gunasekaran, V.; Gowdhaman, D.; Ponnusami, V. Role of membrane proteins in bacterial synthesis of hyaluronic acid and their potential in industrial production. Int. J. Biol. Macromol. 2020, 164, 1916–1926. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.B.; Ramachandran, K.B.; Jayaraman, G. Transcription analysis of hyaluronan biosynthesis genes in Streptococcus zooepidemicus and metabolically engineered Lactococcus lactis. Appl. Microbiol. Biotechnol. 2012, 94, 1593–1607. [Google Scholar] [CrossRef]
- Mao, Z.; Shin, H.-D.; Chen, R. A recombinant E. coli bioprocess for hyaluronan synthesis. Appl. Microbiol. Biotechnol. 2009, 84, 63–69. [Google Scholar] [CrossRef]
- Mercenier, A. Molecular genetics of Streptococcus thermophilus. FEMS Microbiol. Lett. 1990, 87, 61–78. [Google Scholar] [CrossRef]
- Izawa, N.; Hanamizu, T.; Sone, T.; Chiba, K. Effects of fermentation conditions and soybean peptide supplementation on hyaluronic acid production by Streptococcus thermophilus strain YIT 2084 in milk. J. Biosci. Bioeng. 2010, 109, 356–360. [Google Scholar] [CrossRef]
- Alburn, H.E.; Williams, E.C. Preparation of Hyaluronic Acid from Various Animal Sources. Ann. N. Y. Acad. Sci. 1950, 52, 971–976. [Google Scholar] [CrossRef]
- Follett, A.E. Preparation and Some Properties of Hyaluronic Acid from Umbilical Cord of the Pig. J. Biol. Chem. 1948, 176, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Swann, D.A. Studies on hyaluronic acid. Biochim. Biophys. Acta (BBA)—Gen. Subj. 1968, 156, 17–30. [Google Scholar] [CrossRef]
- Cowman, M.K.; Lee, H.-G.; Schwertfeger, K.L.; McCarthy, J.B.; Turley, E.A. The Content and Size of Hyaluronan in Biological Fluids and Tissues. Front. Immunol. 2015, 6, 261. [Google Scholar] [CrossRef]
- Serra, M.; Casas, A.; Toubarro, D.; Barros, A.N.; Teixeira, J.A. Microbial Hyaluronic Acid Production: A Review. Molecules 2023, 28, 2084. [Google Scholar] [CrossRef]
- Zanjani, F.S.A.; Afrasiabi, S.; Norouzian, D.; Ahmadian, G.; Hosseinzadeh, S.A.; Fayazi Barjin, A.; Keramati, M. Hyaluronic acid production and characterization by novel Bacillus subtilis harboring truncated Hyaluronan Synthase. AMB Express 2022, 12, 88. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Rai, A.K.; Tewari, R.P. Recent advancement in hyaluronic acid-based hydrogel for biomedical engineering application: A mini-review. Mater. Today Proc. 2023, 78, 138–144. [Google Scholar] [CrossRef]
- Bednarski, W.; Adamczak, M.; Fiedurek, J.; Gawroński, R.; Leman, J.; Szewczyk, K.W. (Eds.) Podstawy Biotechnologii Przemysłowej: Praca Zbiorowa, Wydanie I-1 Dodruk (PWN); Wydawnictwo WNT; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2017. [Google Scholar]
- Stirred-Tank Bioreactor Design. Available online: https://fermentorchina.com/zh/a-continuous-stirred-tank-bioreactor-cstr/ (accessed on 25 April 2025).
- Spier, M.; Vandenberghe, L.; Medeiros, A.; Soccol, C. Application of Different Types of Bioreactors in Bioprocesses. In Bioreactors: Design, Properties and Applications; NOVA/Nova Science Publishers: New York, NY, USA, 2011; pp. 53–87. ISBN 978-1-62100-164-5. [Google Scholar]
- Nienow, A.W. Mixing: Studies at the University of Birmingham on This Traditional Technology Critical in the Manufacture of New Biological Products. Food Bioprod. Process. 2000, 78, 145–151. [Google Scholar] [CrossRef]
- Yang, S.-T. (Ed.) Bioprocessing for Value-Added Products from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar] [CrossRef]
- Armstrong, D.C.; Johns, M.R. Culture Conditions Affect the Molecular Weight Properties of Hyaluronic Acid Produced by Streptococcus zooepidemicus. Appl. Environ. Microbiol. 1997, 63, 2759–2764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Duan, X.-J.; Tan, W.-S. Mechanism for the effect of agitation on the molecular weight of hyaluronic acid produced by Streptococcus zooepidemicus. Food Chem. 2010, 119, 1643–1646. [Google Scholar] [CrossRef]
- Mausolf, A.; Jungmann, J.; Robenek, H.; Prehm, P. Shedding of hyaluronate synthase from streptococci. Biochem. J. 1990, 267, 191–196. [Google Scholar] [CrossRef]
- Liu, L.; Du, G.; Chen, J.; Wang, M.; Sun, J. Comparative study on the influence of dissolved oxygen control approaches on the microbial hyaluronic acid production of Streptococcus zooepidemicus. Bioprocess Biosyst. Eng. 2009, 32, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.J.; Yang, L.; Zhang, X.; Tan, W.S. Effect of oxygen and shear stress on molecular weight of hyaluronic acid. J. Microbiol. Biotechnol. 2008, 18, 718–724. [Google Scholar] [PubMed]
- Chhabra, R.P.; Patel, S.A. Bubbles, Drops, and Particles in Non-Newtonian Fluids, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar] [CrossRef]
- Nickel, V.; Prehm, S.; Lansing, M.; Mausolf, A.; Podbielski, A.; Deutscher, J.; Prehm, P. An Ectoprotein Kinase of Group C Streptococci Binds Hyaluronan and Regulates Capsule Formation. J. Biol. Chem. 1998, 273, 23668–23673. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Chen, S.-J.; Chen, T.-L. The role of dissolved oxygen and function of agitation in hyaluronic acid fermentation. Biochem. Eng. J. 2006, 32, 239–243. [Google Scholar] [CrossRef]
- Richard, A.; Margaritis, A. Production and Mass Transfer Characteristics of Non-Newtonian Biopolymers for Biomedical Applications. Crit. Rev. Biotechnol. 2002, 22, 355–374. [Google Scholar] [CrossRef]
- Johns, M.R.; Goh, L.-T.; Oeggerli, A. Effect of pH, agitation and aeration on hyaluronic acid production by Streptococcus zooepidemicus. Biotechnol. Lett. 1994, 16, 507–512. [Google Scholar] [CrossRef]
- Nakayama, T.; Amachi, T.; Beta-Galactosidase, E. Encyclopedia of bioprocess technology: Fermentation, biocatalysis, and bioseparation. In Wiley Biotechnology Encyclopedias; Flickinger, M.C., Drew, S.W., Eds.; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Grau, M.D.; Nougués, J.M.; Puigjaner, L. Batch and semibatch reactor performance for an exothermic reaction. Chem. Eng. Process. Process Intensif. 2000, 39, 141–148. [Google Scholar] [CrossRef]
- Huang, W.-C.; Chen, S.-J.; Chen, T.-L. Production of hyaluronic acid by repeated batch fermentation. Biochem. Eng. J. 2008, 40, 460–464. [Google Scholar] [CrossRef]
- Don, M.M.; Shoparwe, N.F. Kinetics of hyaluronic acid production by Streptococcus zooepidemicus considering the effect of glucose. Biochem. Eng. J. 2010, 49, 95–103. [Google Scholar] [CrossRef]
- Wang, L.K.; Li, Y. Sequencing Batch Reactors. In Biological Treatment Processes; Wang, L.K., Pereira, N.C., Hung, Y.-T., Eds.; Humana Press: Totowa, NJ, USA, 2009; pp. 459–511. [Google Scholar] [CrossRef]
- Chen, S.-J.; Chen, J.-L.; Huang, W.-C.; Chen, H.-L. Fermentation process development for hyaluronic acid production by Streptococcus zooepidemicus ATCC 39920. Korean J. Chem. Eng. 2009, 26, 428–432. [Google Scholar] [CrossRef]
- Stephanopoulos, G.; Frederickson, A.G.; Aris, R. The growth of competing microbial populations in a CSTR with periodically varying inputs. AIChE J. 1979, 25, 863–872. [Google Scholar] [CrossRef]
- Liu, L.; Du, G.; Chen, J.; Wang, M.; Sun, J. Influence of culture modes on the microbial production of hyaluronic acid by Streptococcus zooepidemicus. Biotechnol. Bioprocess Eng. 2008, 13, 269–273. [Google Scholar] [CrossRef]
- Bokatyi, A.N.; Dubashynskaya, N.V.; Skorik, Y.A. Chemical modification of hyaluronic acid as a strategy for the development of advanced drug delivery systems. Carbohydr. Polym. 2024, 337, 122145. [Google Scholar] [CrossRef]
- Laffleur, F.; Netsomboon, K.; Erman, L.; Partenhauser, A. Evaluation of modified hyaluronic acid in terms of rheology, enzymatic degradation and mucoadhesion. Int. J. Biol. Macromol. 2019, 123, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Hintze, V.; Schnabelrauch, M.; Rother, S. Chemical Modification of Hyaluronan and Their Biomedical Applications. Front. Chem. 2022, 10, 830671. [Google Scholar] [CrossRef]
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Vasi, A.-M.; Popa, M.I.; Butnaru, M.; Dodi, G.; Verestiuc, L. Chemical functionalization of hyaluronic acid for drug delivery applications. Mater. Sci. Eng. C 2014, 38, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.J.; Park, K.; Kim, K.S.; Kim, J.; Yang, J.A.; Kong, J.H.; Hahn, S.K. Target specific and long-acting delivery of protein, peptide, and nucleotide therapeutics using hyaluronic acid derivatives. J. Control. Release 2010, 141, 2–12. [Google Scholar] [CrossRef]
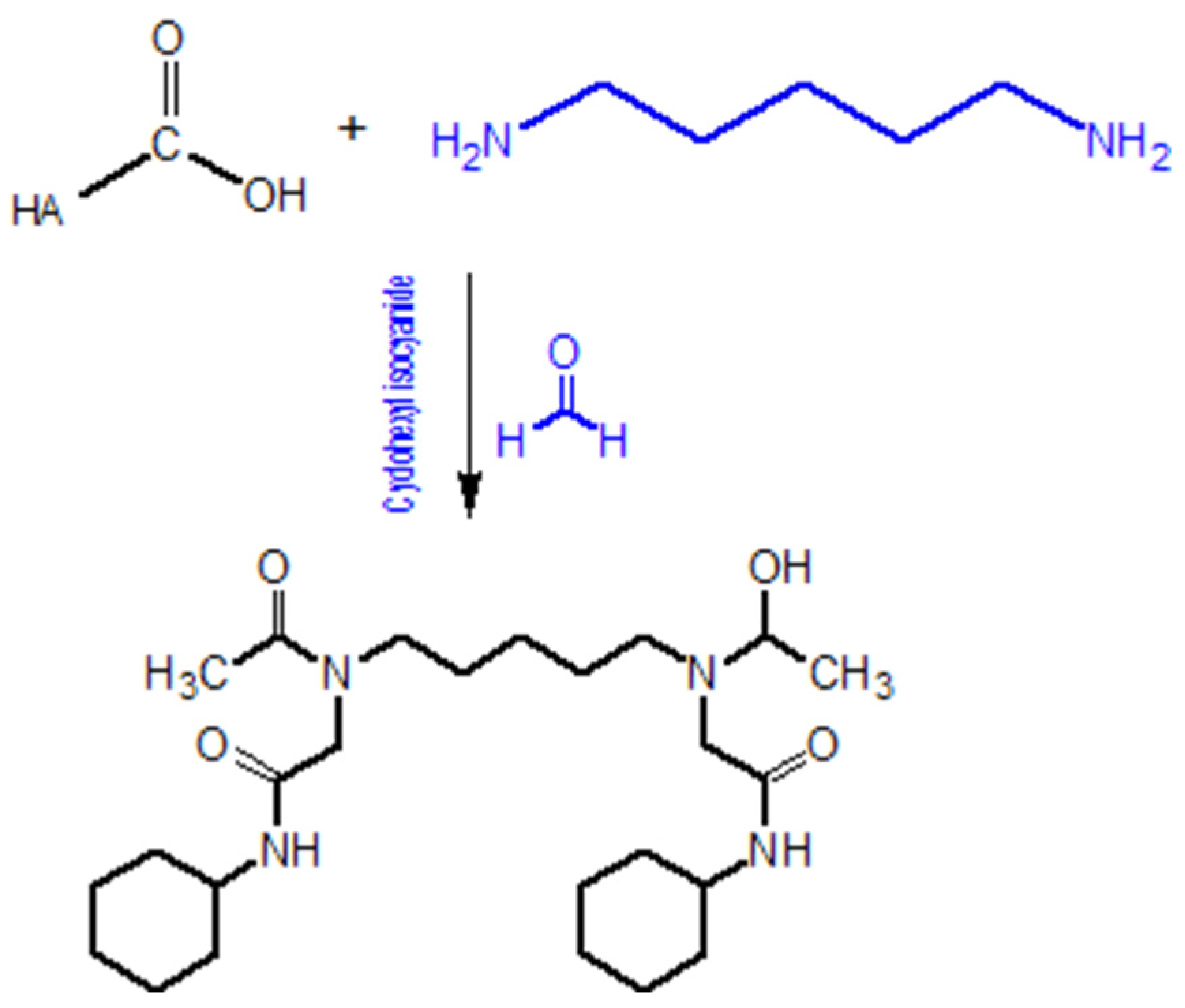
- De Nooy, A.E.J.; Capitani, D.; Masci, G.; Crescenzi, V. Ionic Polysaccharide Hydrogels via the Passerini and Ugi Multicomponent Condensations: Synthesis, Behavior and Solid-State NMR Characterization. Biomacromolecules 2000, 1, 259–267. [Google Scholar] [CrossRef]
- Zhang, G.; Song, D.; Ma, R.; Li, M.; Liu, B.; He, Z.; Fu, Q. Self-crosslinking hyaluronic acid hydrogel as an enteroprotective agent for the treatment of inflammatory bowel disease. Int. J. Biol. Macromol. 2024, 273, 132909. [Google Scholar] [CrossRef]
- Sedláček, J.; Hermannová, M.; Šatínský, D.; Velebný, V. Current analytical methods for the characterization of N-deacetylated hyaluronan: A critical review. Carbohydr. Polym. 2020, 249, 116720. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Chirachanchai, S.; Izawa, N.; Inaki, Y.; Takemoto, K. Synthesis and Properties of Hyaluronic Acid Conjugated Nucleic Acid Analogs—1: Synthesis of Deacetylhyaluronan and Introduction of Nucleic Acid Bases. J. Bioact. Compat. Polym. 1994, 9, 429–447. [Google Scholar] [CrossRef]
- Čožíková, D.; Šílová, T.; Moravcová, V.; Šmejkalová, D.; Pepeliaev, S.; Velebný, V.; Hermannová, M. Preparation and extensive characterization of hyaluronan with narrow molecular weight distribution. Carbohydr. Polym. 2017, 160, 134–142. [Google Scholar] [CrossRef]
- Koivusalo, L.; Kauppila, M.; Samanta, S.; Parihar, V.S.; Ilmarinen, T.; Miettinen, S.; Skottman, H. Tissue adhesive hyaluronic acid hydrogels for sutureless stem cell delivery and regeneration of corneal epithelium and stroma. Biomaterials 2019, 225, 119516. [Google Scholar] [CrossRef]
- Gholamali, I.; Vu, T.T.; Jo, S.-H.; Park, S.-H.; Lim, K.T. Exploring the Progress of Hyaluronic Acid Hydrogels: Synthesis, Characteristics, and Wide-Ranging Applications. Materials 2024, 17, 2439. [Google Scholar] [CrossRef]
- Prakash, G.; Clasky, A.J.; Gadani, K.; Nazeri, M.; Gu, F.X. Ion-Mediated Cross-Linking of Hyaluronic Acid into Hydrogels without Chemical Modification. Biomacromolecules 2024, 25, 7723–7735. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, F.; Ryan, E.; Culebras, M.; Collins, M.N. Labile crosslinked hyaluronic acid via urethane formation using bis(β-isocyanatoethyl) disulphide with tuneable physicochemical and immunomodulatory properties. Carbohydr. Polym. 2020, 245, 116501. [Google Scholar] [CrossRef]
- Kim, M.; Hwang, Y.; Tae, G. The enhanced anti-tissue adhesive effect of injectable pluronic-HA hydrogel by poly(γ-glutamic acid). Int. J. Biol. Macromol. 2016, 93, 1603–1611. [Google Scholar] [CrossRef]
- Petit, N.; Chang, Y.Y.J.; Lobianco, F.A.; Hodgkinson, T.; Browne, S. Hyaluronic acid as a versatile building block for the development of biofunctional hydrogels: In vitro models and preclinical innovations. Mater. Today Bio 2025, 31, 101596. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, Y.; Xu, Y.; Wang, J.; Yu, Y. Modification and crosslinking strategies for hyaluronic acid-based hydrogel biomaterials. Smart Med. 2023, 2, e20230029. [Google Scholar] [CrossRef]
- Jung, Y.; Park, W.; Park, H.; Lee, D.-K.; Na, K. Thermo-sensitive injectable hydrogel based on the physical mixing of hyaluronic acid and Pluronic F-127 for sustained NSAID delivery. Carbohydr. Polym. 2017, 156, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Choi, H.; Choi, E.S.; Park, M.-H.; Ryu, J.-H. Hyaluronic Acid-Coated Nanomedicine for Targeted Cancer Therapy. Pharmaceutics 2019, 11, 301. [Google Scholar] [CrossRef]
- Bermejo-Velasco, D.; Kadekar, S.; Tavares da Costa, M.V.; Oommen, O.P.; Gamstedt, K.; Hilborn, J.; Varghese, O.P. First Aldol Cross-Linked Hyaluronic Acid Hydrogel: Fast and Hydrolytically Stable Hydrogel with Tissue Adhesive Properties. ACS Appl. Mater. Interfaces 2019, 11, 38232–38239. [Google Scholar] [CrossRef] [PubMed]
- Pérez, L.A.; Hernández, R.; Alonso, J.M.; Pérez-González, R.; Sáez-Martínez, V. Hyaluronic Acid Hydrogels Crosslinked in Physiological Conditions: Synthesis and Biomedical Applications. Biomedicines 2021, 9, 1113. [Google Scholar] [CrossRef]
- Grover, G.N.; Braden, R.L.; Christman, K.L. Oxime Cross-Linked Injectable Hydrogels for Catheter Delivery. Adv. Mater. 2013, 25, 2937–2942. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Tan, J.; Zhou, Y.; Guo, Y.; Liao, X.; He, L.; Liu, Y. From crosslinking strategies to biomedical applications of hyaluronic acid-based hydrogels: A review. Int. J. Biol. Macromol. 2023, 231, 123308. [Google Scholar] [CrossRef]
- Steudter, T.; Lam, T.; Pirmahboub, H.; Stoppel, C.; Kloke, L.; Pearson, S.; Del Campo, A. A Comparative Study between Thiol-Ene and Acrylate Photocrosslinkable Hyaluronic Acid Hydrogel Inks for Digital Light Processing. Macromol. Biosci. 2025, 25, 2400535. [Google Scholar] [CrossRef]
- Zhang, M.; Ye, Q.; Zhu, Z.; Shi, S.; Xu, C.; Xie, R.; Li, Y. Hyaluronic Acid-Based Dynamic Hydrogels for Cartilage Repair and Regeneration. Gels 2024, 10, 703. [Google Scholar] [CrossRef]
- Yang, X.; Wang, B.; Peng, D.; Nie, X.; Wang, J.; Yu, C.Y.; Wei, H. Hyaluronic Acid-Based Injectable Hydrogels for Wound Dressing and Localized Tumor Therapy: A Review. Adv. NanoBiomed Res. 2022, 2, 2200124. [Google Scholar] [CrossRef]
- Disulfide Bond. Available online: https://www.wikidoc.org/index.php/Disulfide_bond (accessed on 27 April 2025).
- Yasin, A.; Ren, Y.; Li, J.; Sheng, Y.; Cao, C.; Zhang, K. Advances in Hyaluronic Acid for Biomedical Applications. Front. Bioeng. Biotechnol. 2022, 10, 910290. [Google Scholar] [CrossRef]
- Dulong, V.; Lack, S.; Le Cerf, D.; Picton, L.; Vannier, J.P.; Muller, G. Hyaluronan-based hydrogels particles prepared by crosslinking with trisodium trimetaphosphate. Synthesis and characterization. Carbohydr. Polym. 2004, 57, 1–6. [Google Scholar] [CrossRef]
- Thanh, T.N.; Laowattanatham, N.; Ratanavaraporn, J.; Sereemaspun, A.; Yodmuang, S. Hyaluronic acid crosslinked with alginate hydrogel: A versatile and biocompatible bioink platform for tissue engineering. Eur. Polym. J. 2022, 166, 111027. [Google Scholar] [CrossRef]
- Ying, H.; Zhou, J.; Wang, M.; Su, D.; Ma, Q.; Lv, G.; Chen, J. In situ formed collagen-hyaluronic acid hydrogel as biomimetic dressing for promoting spontaneous wound healing. Mater. Sci. Eng. C 2019, 101, 487–498. [Google Scholar] [CrossRef]
- Trusek, A.; Grabowski, M.; Ajayi, O.; Kijak, E. Hyaluronic Acid–Alginate Homogeneous Structures with Polylactide Coating Applied in Controlled Antibiotic Release. Gels 2023, 9, 526. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-D.; Zhai, P.; Schreyer, D.J.; Zheng, R.S.; Sun, X.D.; Cui, F.Z.; Chen, X.B. Novel crosslinked alginate/hyaluronic acid hydrogels for nerve tissue engineering. Front. Mater. Sci. 2013, 7, 269–284. [Google Scholar] [CrossRef]
- Hwang, H.S.; Lee, C.-S. Recent Progress in Hyaluronic-Acid-Based Hydrogels for Bone Tissue Engineering. Gels 2023, 9, 588. [Google Scholar] [CrossRef]
- Deng, Y.; Ren, J.; Chen, G.; Li, G.; Wu, X.; Wang, G.; Li, J. Injectable in situ cross-linking chitosan-hyaluronic acid based hydrogels for abdominal tissue regeneration. Sci. Rep. 2017, 7, 2699. [Google Scholar] [CrossRef]
- Ziadlou, R.; Rotman, S.; Teuschl, A.; Salzer, E.; Barbero, A.; Martin, I.; Grad, S. Optimization of hyaluronic acid-tyramine/silk-fibroin composite hydrogels for cartilage tissue engineering and delivery of anti-inflammatory and anabolic drugs. Mater. Sci. Eng. C 2021, 120, 111701. [Google Scholar] [CrossRef]
- Kiani-Dehkordi, B.; Vatanara, A.; Amini, M.; Hamidi, M.; Dibaei, M.; Norouzi, P.; Rouini, M.R. Preparation of polymersomes from synthesized hyaluronic acid-graft-poly(ε-caprolactone) copolymers for drug delivery to the brain. Mater. Today Chem. 2023, 30, 101504. [Google Scholar] [CrossRef]



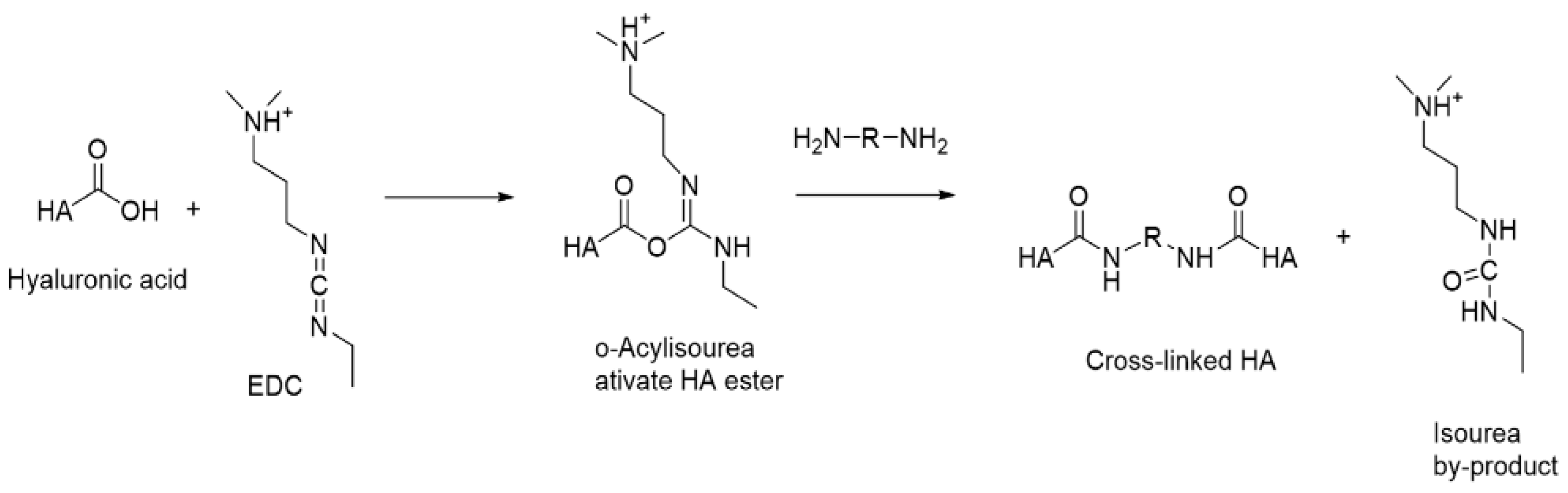
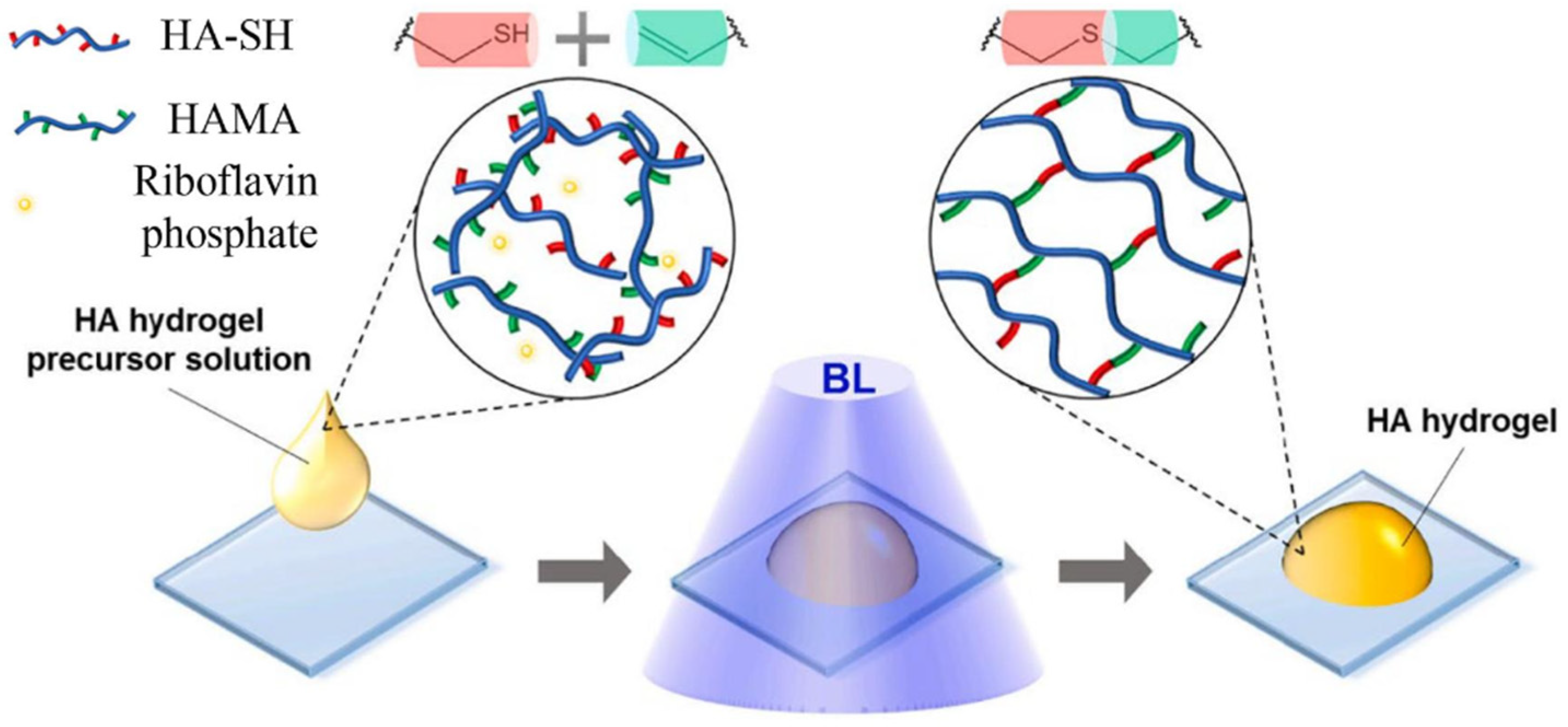
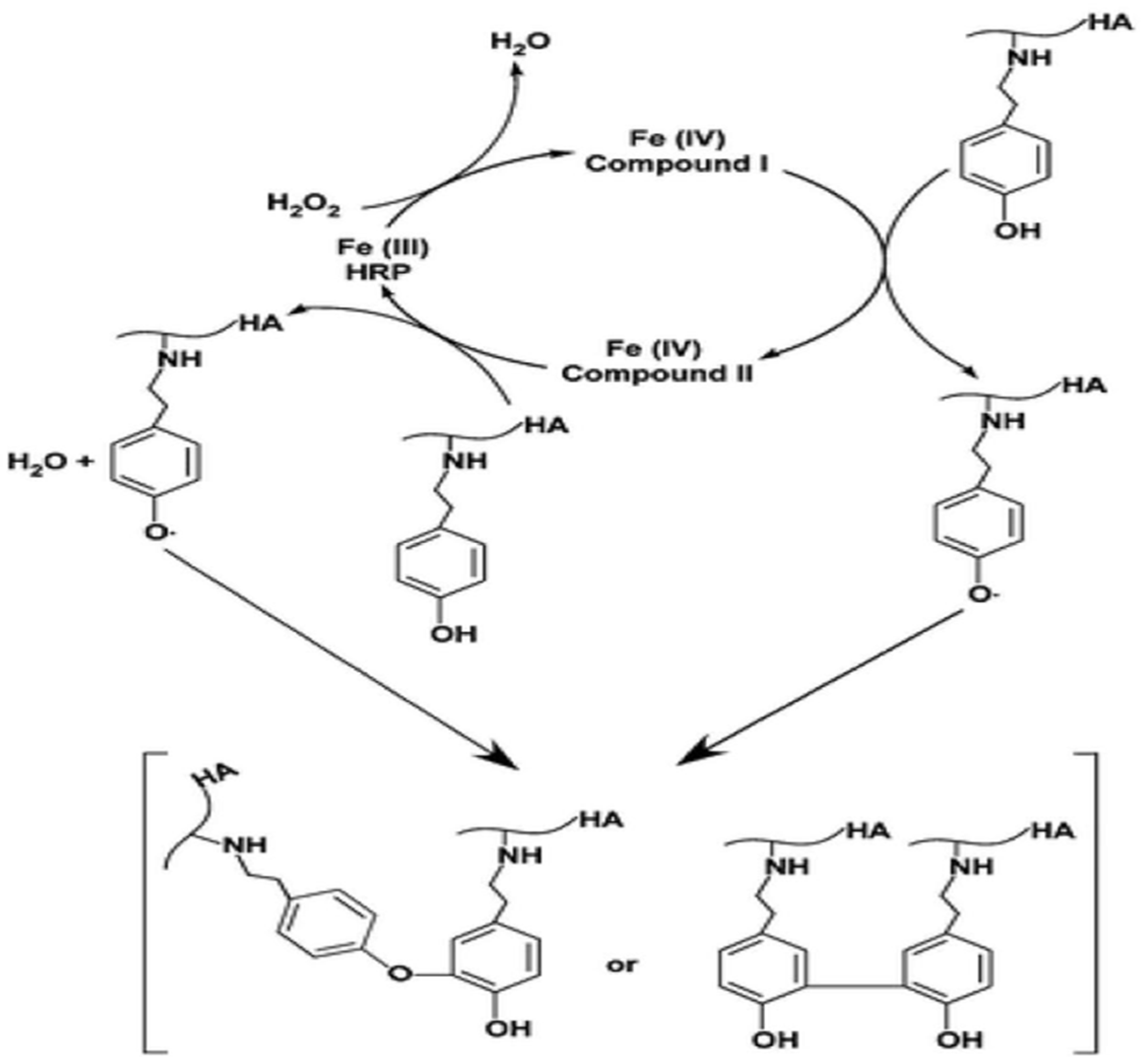
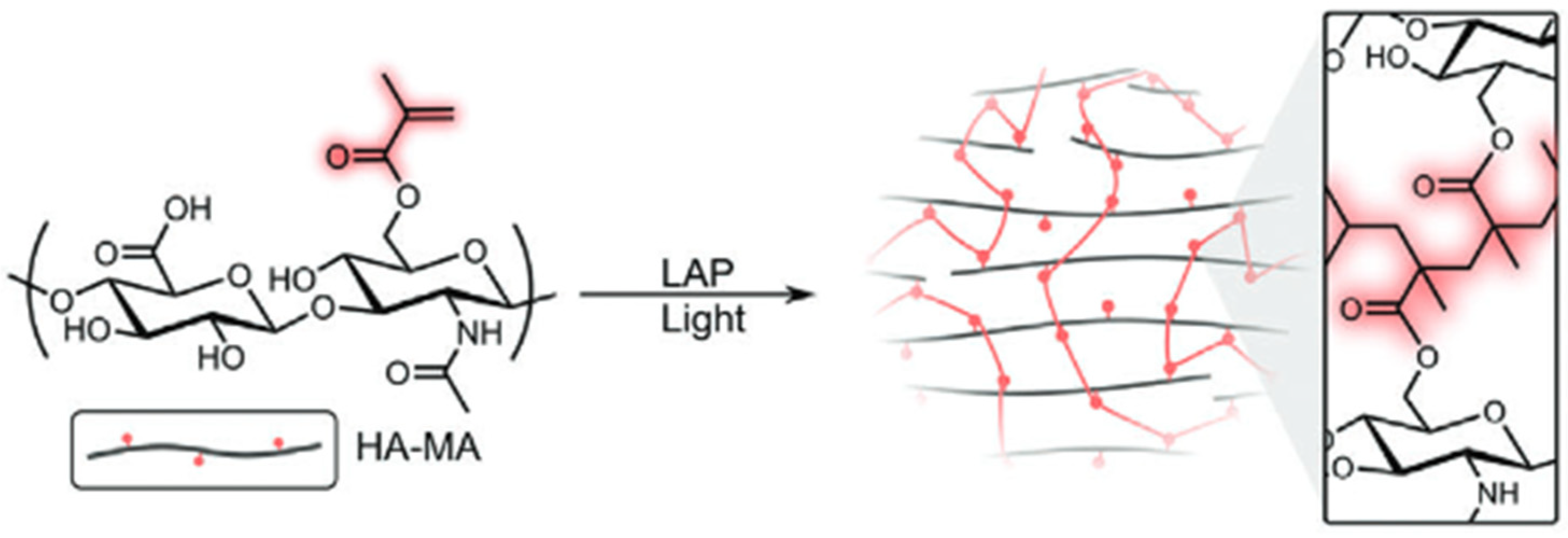
| MW Range | Network Formation | Viscosity Behavior | Stability |
|---|---|---|---|
| 60–90 kDa | None (rigid rods) | Newtonian (no shear-thinning) | High thermal and enzymatic stability |
| ~200 kDa | Intermediate | Shear-thinning at high concentration | Moderate stability |
| 400–2500 kDa | Strong Entanglement | Pseudoplastic (shear-thinning) | Lower stability, depolymerisation more likely |
| Production Method | Key Characteristics | Advantages | Limitations |
|---|---|---|---|
| Animal tissue Extraction | Extraction via chemical and enzymatic digestion (e.g., papain, trypsin) | High-molecular-weight HA; widely available tissues | Risk of pathogens; batch variability; ethical concerns |
| Bacterial Fermentation | Fermentation in bioreactors using glucose or other carbon sources; produces capsular HA | Scalable; “vegan” HA; cost-effective | May contain endotoxins; requires downstream purification |
| Cell-free In vitro systems | Uses isolated HAS enzymes in a cell-free system to synthesise HA | High specificity; potential for defined Mw products | Currently limited to research scale; complex enzyme stabilisation; low yield |
| Production Method | Source | Molecular Weight [Da] | Yield | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|
| From animal tissues | Rooster combs | 1.2 × 106 | 6.00% | Well-established technology | Product quality variability | [75] |
| Pig umbilical cord | 3.4 × 106 | 4.40% | Low-cost and accessible raw material | Risk of polymer degradation | [76] | |
| Bovine vitreous body | 7.7 × 104–1.7 × 106 | 0.12% | High molecular weight product | Risk of contamination (e.g., proteins) | [77] | |
| Bovine synovial fluid | 1.4 × 106 | 0.0037% | Natural product | Requires extensive purification, very low yield | [78] | |
| Fermentation | Streptococci | 2.1 × 106–7.4 × 107 | 0.44–6.94 g/L | High yield | Use of pathogens and GMOs | [79] |
| Streptococcus thermophilus | 2 × 106 | 1.2 g/L | Optimised technology | Risk of endotoxin contamination | [79] | |
| Bacillus subtilis | 1–1.2 × 106 | 5 g/L | High molecular weight product | Contamination risk (proteins, heavy metals) | [80] | |
| Lactococcus lactis | 0.4–4.1 × 106 | 0.65 g/L | Food-grade organism; potential for genetic engineering | Lower yield; requires metabolic pathway optimisation | [9,66] | |
| Escherichia coli | 1.5 × 106 | 3.8 g/L | Well-characterised genetics; rapid growth; scalable fermentation | Endotoxin contamination risk; requires extensive purification | [43] | |
| Pichia pastoris | 8 × 102–2.5 × 106 | 20–90% | High yield; scalable; suitable for pharmaceutical applications | Produces low molecular weight HA; emerging technology | [37] | |
| In vitro synthesis | — | 8 × 102–2.5 × 106 | 20–90% | Versatile technology; free from contamination; stable product quality; | Emerging and still developing technology; not economically viable | [81] |
| Physical Crosslinking | Chemical Croslinking | References | |
|---|---|---|---|
| Mechanism | Non-covalent interactions: hydrogen bonds, electrostatic interactions, metal coordination | Covalent bonds: carbodiimide crosslinking, diisocyanate crosslinking, Schiff base crosslinking | [107,119,121,122,129,130,131] |
| Crosslinking agents | Does not require the use of chemical crosslinking agents | Requires the use of chemical compounds that react with functional groups (e.g., EDC) | [119,121,122,124,129,130,131] |
| Method difficulty | Simple method, does not require complex chemical reactions; does not require the removal of byproducts | Requires precise selection and concentration of the crosslinking agent, control of reaction conditions; requires the removal of unbound crosslinking agent residues and potential byproducts | [107,119,121,129,130,131] |
| Biocompatibility | Preservation of natural biocompatibility | Biocompatibility depends on the type and amount of crosslinking agent used and the effectiveness of its removal after the crosslinking process | [107,119,121,129,130,131] |
| Durability | Gels are less durable, susceptible to environmental changes | Gels are more stable anddurable, resistant to rapid enzymatic degradation | [107,119,121,129,130,131] |
| Mechanical properties | May have lower mechanical strength and structural stability | Allows for obtaining structures with a wide range of mechanical properties, i.e., high viscosity, elasticity, ability to support tissues | [107,119,121,129,130,131] |
| Porosity | Difficult to control; structures with larger pores | Enables control; allows for the design of structures with specific properties | [107,119,121,129,130,131] |
| Rheological properties | Exhibit shear-thinning behavior; recovering viscosity after shear removal | It is possible to obtain gels with the desired viscosity and storage modulus | [107,119,121,129,130,131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabowski, M.; Gmyrek, D.; Żurawska, M.; Trusek, A. Hyaluronic Acid: Production Strategies, Gel-Forming Properties, and Advances in Drug Delivery Systems. Gels 2025, 11, 424. https://doi.org/10.3390/gels11060424
Grabowski M, Gmyrek D, Żurawska M, Trusek A. Hyaluronic Acid: Production Strategies, Gel-Forming Properties, and Advances in Drug Delivery Systems. Gels. 2025; 11(6):424. https://doi.org/10.3390/gels11060424
Chicago/Turabian StyleGrabowski, Maciej, Dominika Gmyrek, Maria Żurawska, and Anna Trusek. 2025. "Hyaluronic Acid: Production Strategies, Gel-Forming Properties, and Advances in Drug Delivery Systems" Gels 11, no. 6: 424. https://doi.org/10.3390/gels11060424
APA StyleGrabowski, M., Gmyrek, D., Żurawska, M., & Trusek, A. (2025). Hyaluronic Acid: Production Strategies, Gel-Forming Properties, and Advances in Drug Delivery Systems. Gels, 11(6), 424. https://doi.org/10.3390/gels11060424







