Effect of Potassium-Ion-Triggered Double Helix Aggregation on Shakedown Behavior of κ-Carrageenan/Polyacrylamide Hydrogel
Abstract
1. Introduction
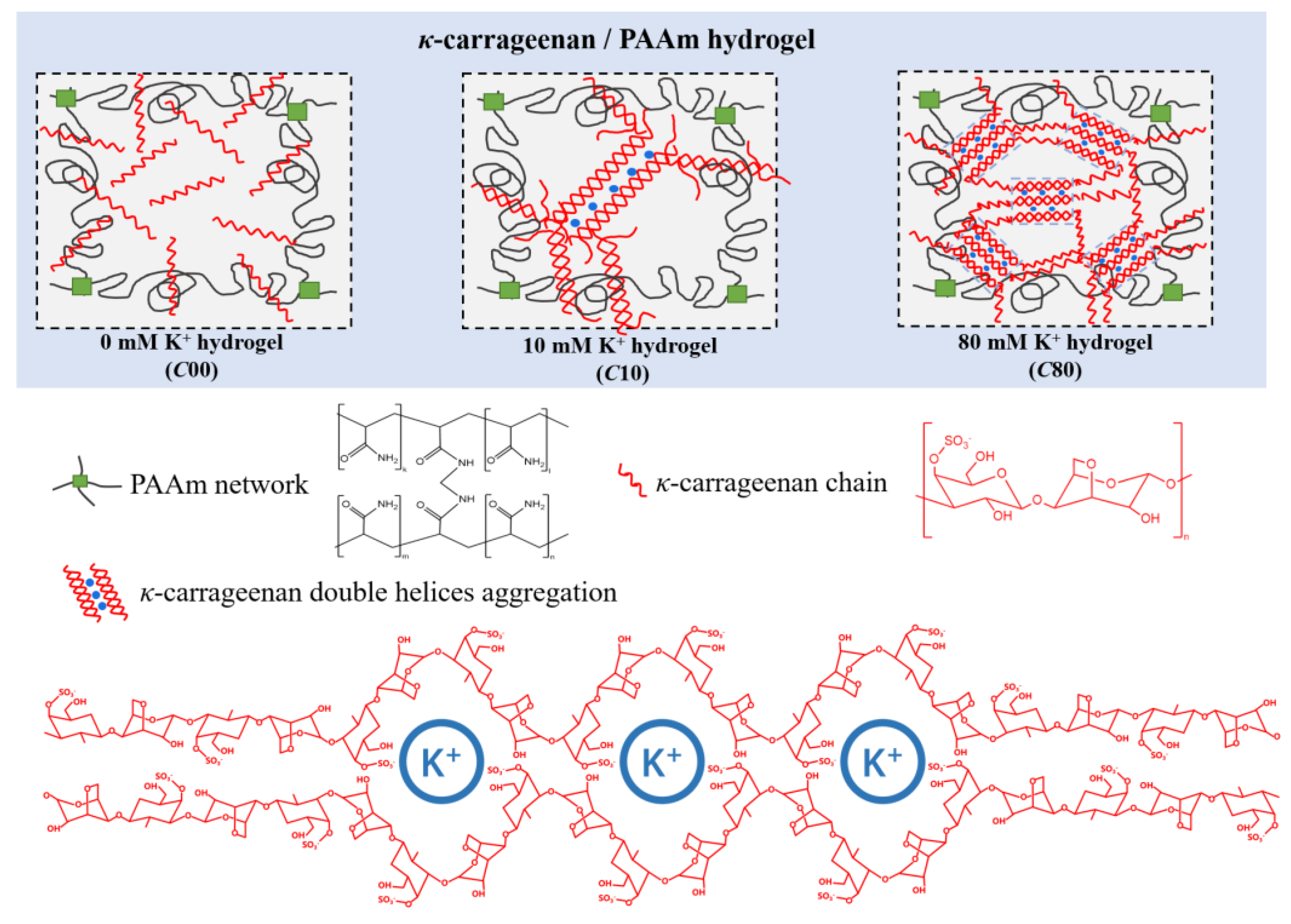
2. Results and Discussion
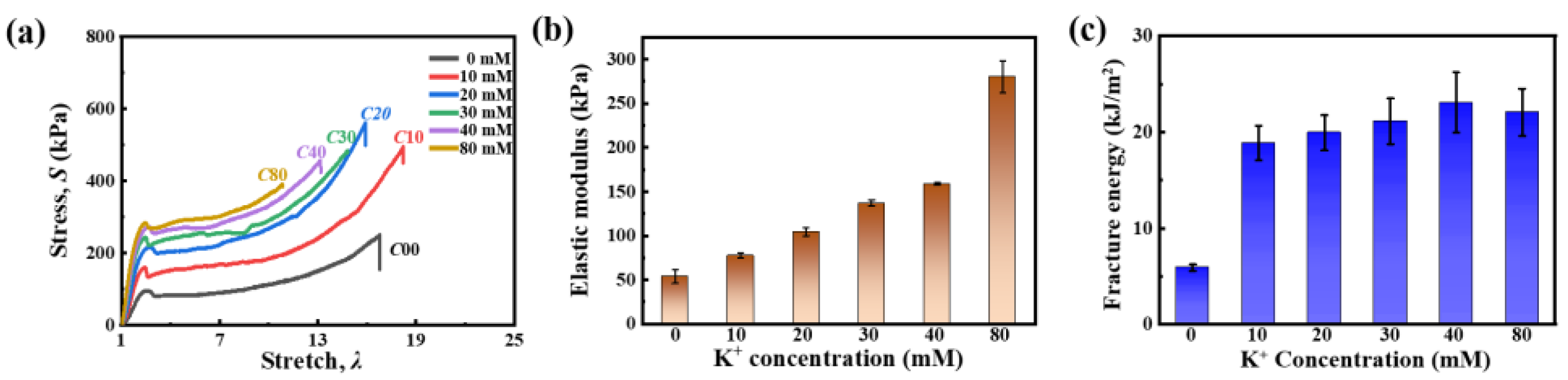
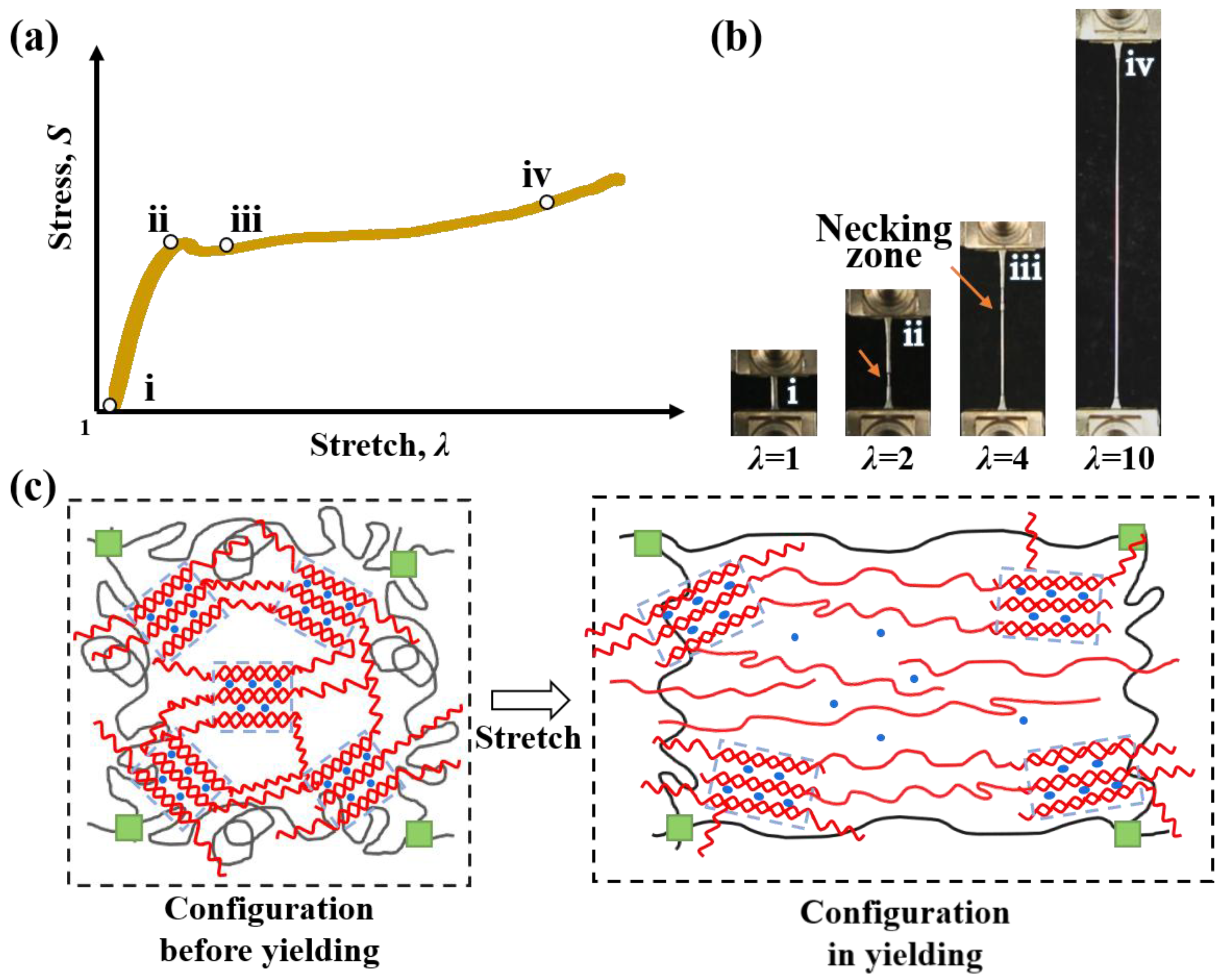
2.1. Tensile Mechanical Properties
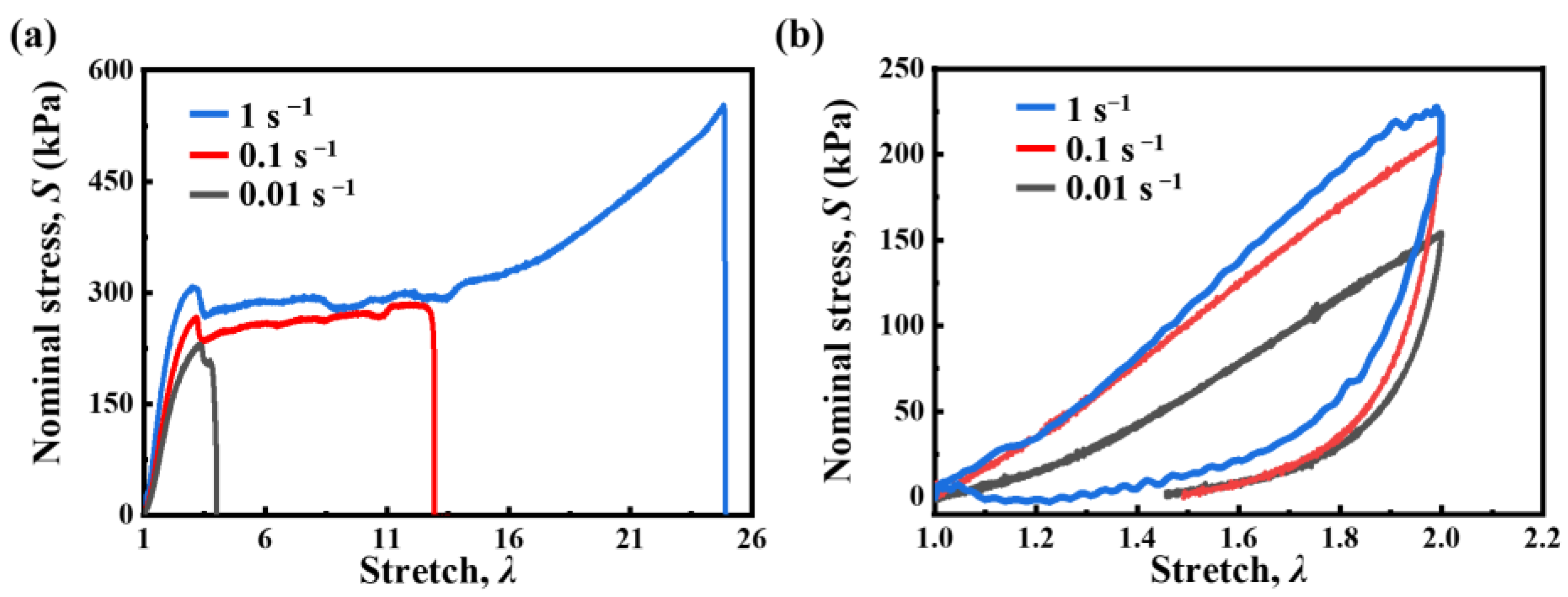
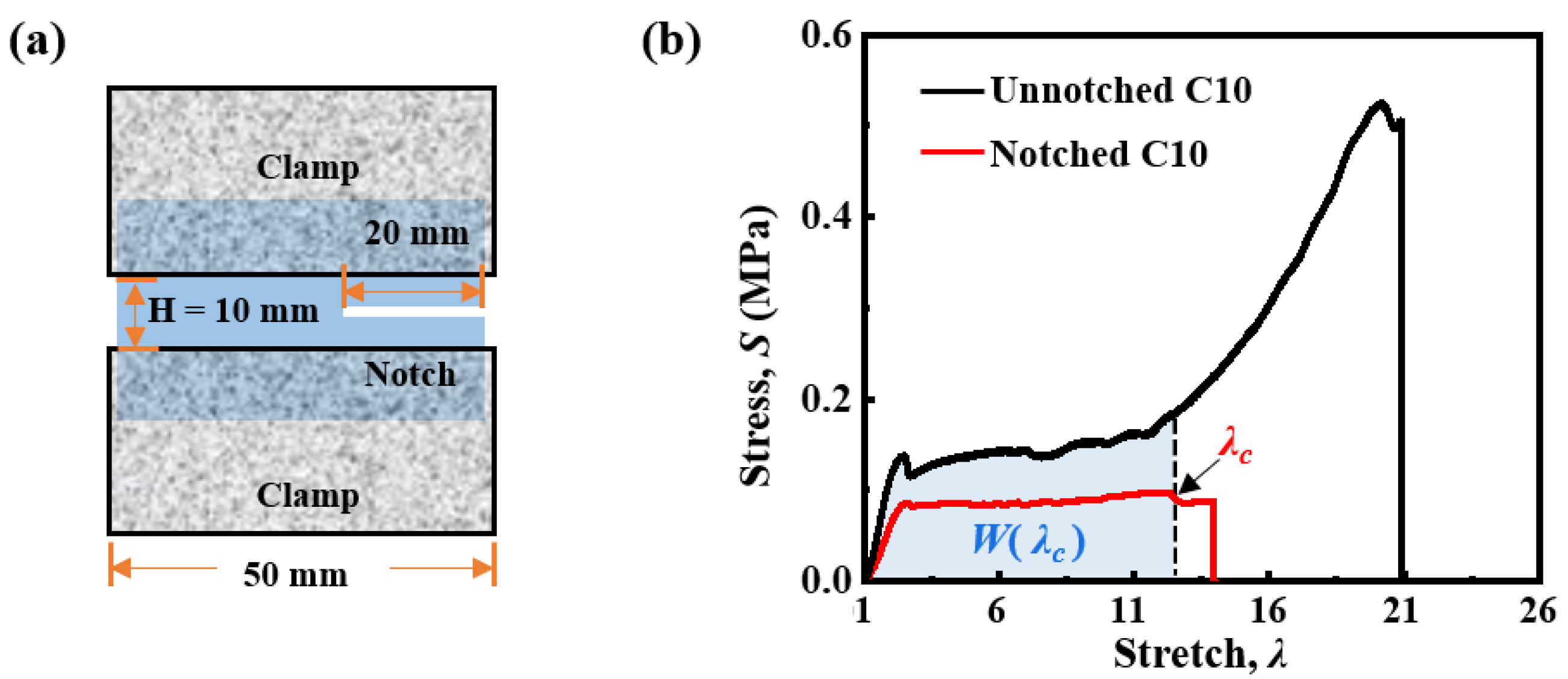
2.2. Viscoelastic Behavior
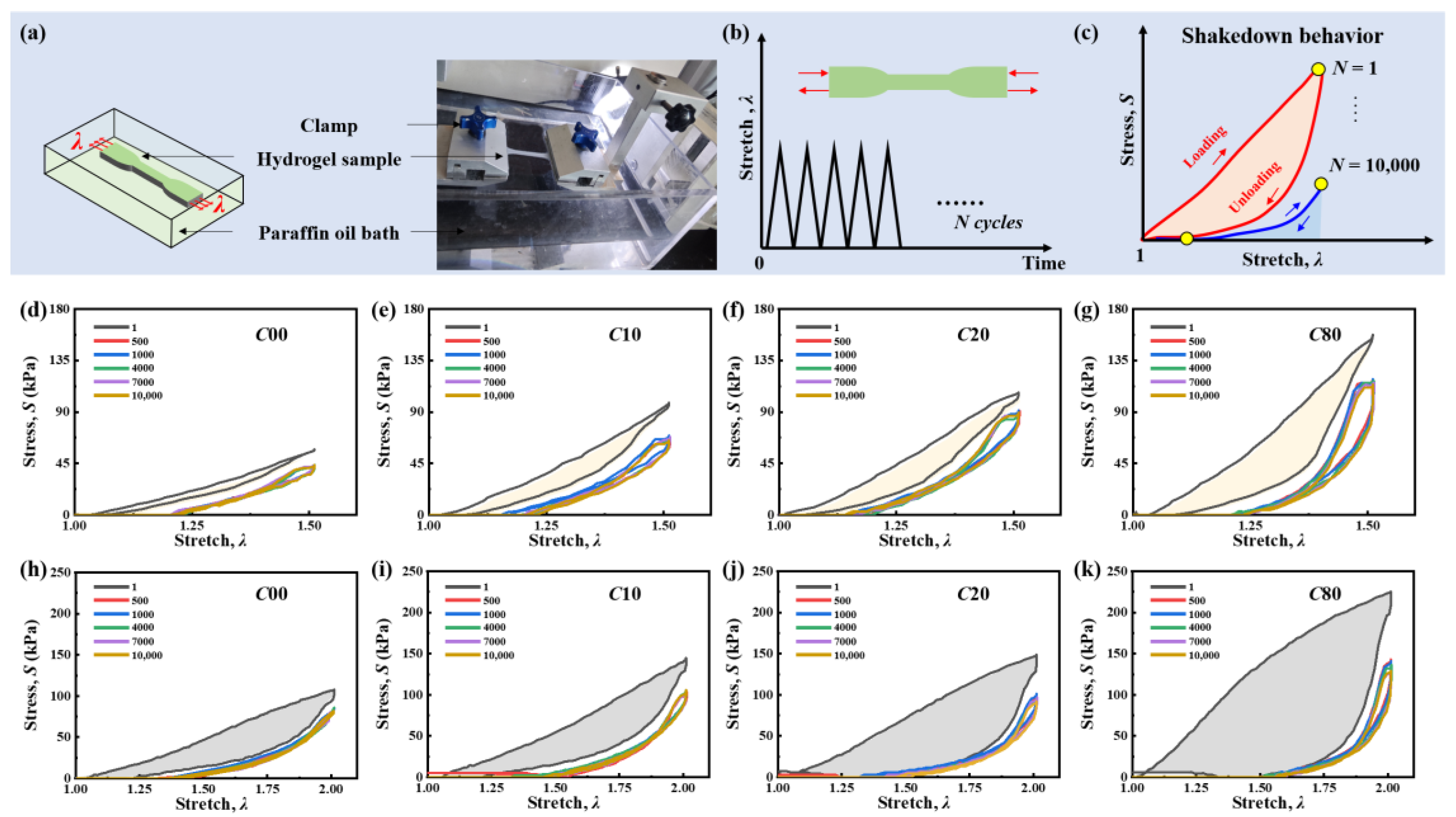
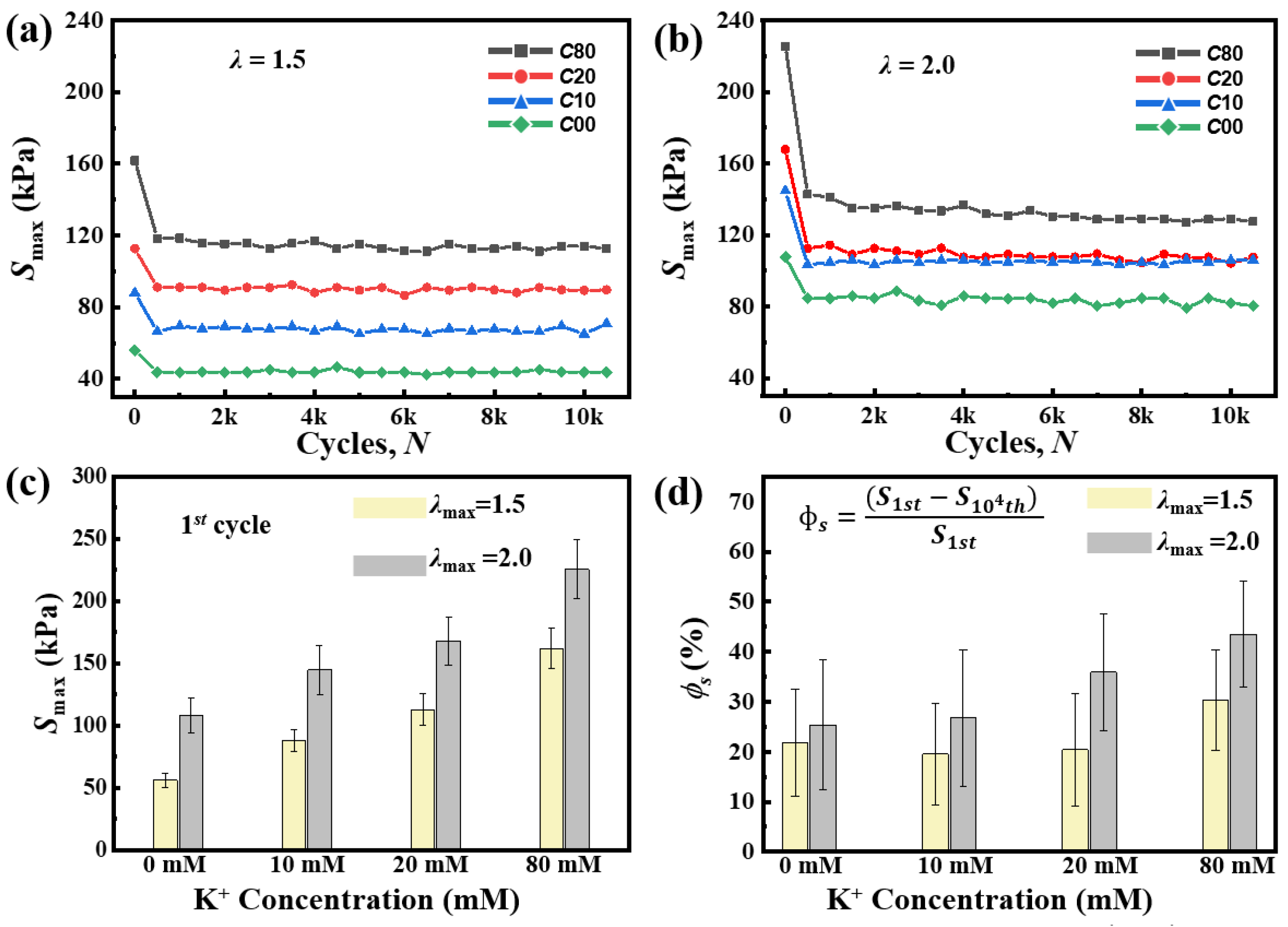
2.3. Cyclic Fatigue Damage and Shakedown Behavior
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Sample Preparation
4.3. Measurement
4.3.1. Tensile Test
4.3.2. Pure Shear Test
4.3.3. Fatigue Test
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Liu, F.; Yu, Q.; Yang, M.; Suo, Z.; Tang, J. A Soft and Fatigue-Resistant Material that Mimics Heart Valves. Matter 2025, 8, 101926. [Google Scholar] [CrossRef]
- Zhao, X. A Bioadhesive Robot to Activate Muscles. Nat. Mater. 2023, 22, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tan, S.; Zhang, X.; Li, Z.; Cai, J.; Liu, Y. Design Strategies and Emerging Applications of Conductive Hydrogels in Wearable Sensing. Gels 2025, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- Protsak, I.S.; Morozov, Y.M. Fundamentals and Advances in Stimuli-Responsive Hydrogels and Their Applications: A Review. Gels 2025, 11, 30. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, J.; Tang, J.; Wang, Z.; Wang, J.; Lu, T.; Suo, Z. Fracture Toughness and Fatigue Threshold of Tough Hydrogels. ACS Macro. Lett. 2019, 8, 17–23. [Google Scholar] [CrossRef]
- Han, Z.; Lu, Y.; Qu, S. Design of Fatigue-Resistant Hydrogels. Adv. Funct. Mater. 2024, 34, 2313498. [Google Scholar] [CrossRef]
- Bai, R.; Yang, Q.; Tang, J.; Morelle, X.P.; Vlassak, J.; Suo, Z. Fatigue Fracture of Tough Hydrogels. Extrem. Mech. Lett. 2017, 15, 91–96. [Google Scholar] [CrossRef]
- Bai, R.; Yang, J.; Morelle, X.; Yang, C.; Suo, Z. Fatigue Fracture of Self-Recovery Hydrogels. ACS Macro. Lett. 2018, 7, 312–317. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly Stretchable and Tough Hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef]
- Bai, R.; Yang, J.; Suo, Z. Fatigue of Hydrogels. Eur. J. Mech. A-Solids 2019, 74, 337–370. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, J.; Zhou, Y.; Pan, Y.; Lu, T.; Song, X.; Hu, J. Fatigue Behaviors of Physical Hydrogels Based on Hydrogen Bonds. Extrem. Mech. Lett. 2021, 46, 101320. [Google Scholar] [CrossRef]
- Li, X.; Gong, J. Design Principles for Strong and Tough Hydrogels. Nat. Rev. Mater. 2024, 9, 380–398. [Google Scholar] [CrossRef]
- Wang, Z.; Long, J.; Zhang, C.; Hua, Y.; Li, X. Effect of Polysaccharide on Structures and Gel Properties of Microgel Particle Reconstructed Soybean Protein Isolate/Polysaccharide Complex Emulsion Gels as Solid Fat Mimetic. Carbohydr. Polym. 2025, 347, 122759. [Google Scholar] [CrossRef] [PubMed]
- Zabik, M.E.; Aldrich, P.J. The Effect of Cations on the Viscosity of Lambda-Carrageenan. J. Food Sci. 1967, 32, 91–97. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Yu, W. Simultaneously Improved Strength and Toughness in κ-Carrageenan/Polyacrylamide Double Network Hydrogel via Synergistic Interaction. Carbohydr. Polym. 2020, 230, 115596. [Google Scholar] [CrossRef]
- Miao, Y.; Xu, M.D.; Zhang, L.D. Electrochemistry-Induced Improvements of Mechanical Strength, Self-Healing, and Interfacial Adhesion of Hydrogels. Adv. Mater. 2021, 33, 11. [Google Scholar] [CrossRef]
- Núñez-Santiago, M.; Pérez-López, A.; Espinosa-Solares, T.; Nicolás-Vázquez, M.; Laureano-López, B. Sol-gel transition diagram and theoretical study of κ-carrageenan in the presence of calcium ions. LWT-Food Sci. Technol. 2023, 182, 114867. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, M.; Sun, D.; Hou, Y.; Li, Y.B.; Dong, T.S.; Wang, X.H.; Zhang, L.; Yang, W.Z. Dual Physically Cross-Linked κ-Carrageenan-Based Double Network Hydrogels with Superior Self-Healing Performance for Biomedical Application. ACS Appl. Mater. Interfaces 2018, 10, 37544–37554. [Google Scholar] [CrossRef]
- Gonçalves, G.; Faria, B.; Moraes, I.; Hilliou, L. Role of the Molecular Mass on the Elastic Properties of Hybrid Carrageenan Hydrogels. Gels 2025, 11, 77. [Google Scholar] [CrossRef]
- Das, I.J.; Bal, T. Exploring Carrageenan: From Seaweed to Biomedicine—A Comprehensive Review. Int. J. Biol. Macromol. 2024, 268, 131822. [Google Scholar] [CrossRef]
- Batet, D.; Navarro-Segarra, M.; Gonçalves, R.; Costa, C.M.; Lanceros-Mendez, S.; Pablo Esquivel, J. The Versatility of Iota-Carrageenan Biopolymer for The Fabrication of Non-Toxic bio-Based Energy Storage Devices. Chem. Eng. J. 2024, 499, 156548. [Google Scholar] [CrossRef]
- Fan, Z.; Cheng, P.; Zhang, P.; Gao, Y.; Zhao, Y.; Liu, M.; Gu, J.; Wang, Z.; Han, J. A Novel Multifunctional Salecan/κ-Carrageenan Composite Hydrogel with Anti-Freezing Properties: Advanced Rheology, Thermal Analysis and Model Fitting. Int. J. Biol. Macromol. 2022, 208, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sabu Mathew, S.; Jaiswal, A.K.; Jaiswal, S. Carrageenan-Based Sustainable Biomaterials for Intelligent Food Packaging: A Review. Carbohydr. Polym. 2024, 342, 122267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, J.; Zhang, J.; Ren, T.; Wei, J.; Liang, Y.; Feng, E.; Han, X.; Ma, X. An Antifreeze, Self-Healing Dual-Network Organogel for Supercapacitor and Anticounterfeiting Equipment. ACS Appl. Energy Mater. 2024, 7, 4040–4049. [Google Scholar] [CrossRef]
- Cao, L.; Li, X.; Hu, X. An Antibacterial, Highly Sensitive Strain Sensor Based on an Anionic Copolymer Interpenetrating with κ-Carrageenan. ACS Biomater. Sci. Eng. 2024, 10, 5641–5652. [Google Scholar] [CrossRef]
- Mangione, M.R.; Giacomazza, D.; Bulone, D.; Martorana, V.; Cavallaro, G.; San Biagio, P.L. K+ and Na+ Effects on The Gelation Properties of κ-Carrageenan. Biophys. Chem. 2005, 113, 129–135. [Google Scholar] [CrossRef]
- Choudhury, A.R. Self-Assembled pH-Stable Gellan/κ-Carrageenan Bigel: Rheological Studies and Viscosity Prediction by Neural Network. Int. J. Biol. Macromol. 2023, 237, 124057. [Google Scholar] [CrossRef]
- Liu, S.; Li, L. Recoverable and Self-Healing Double Network Hydrogel Based on κ-Carrageenan. ACS Appl. Mater. Interfaces 2016, 8, 29749–29758. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, L.; Zhou, J. Recent Developments of Polysaccharide-Based Double-Network Hydrogels. J. Polym. Sci. 2023, 61, 7–43. [Google Scholar] [CrossRef]
- Fan, Z.; Duan, L.; Gao, G. Self-Healing Carrageenan-Driven Polyacrylamide Hydrogels for Strain Sensing. Sci. China-Technol. Sci. 2020, 63, 2677–2686. [Google Scholar] [CrossRef]
- Mirzaei, A.; Javanshir, S.; Servati, P. Thermal Insulation Properties of Lightweight, Self-Healing, and Mesoporous Carrageenan/PMMA Cryogels. RSC Adv. 2023, 13, 1094–1105. [Google Scholar] [CrossRef] [PubMed]
- Safarpour, F.; Kharaziha, M.; Mokhtari, H.; Emadi, R.; Bakhsheshi-Rad, H.R.; Ramakrishna, S. Kappa-Carrageenan Based Hybrid Hydrogel for Soft Tissue Engineering Applications. Biomed. Mater. 2023, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Horinaka, J.; Takagaki, H.; Tanaka, T.; Takigawa, T. Effects of Gelation Concentration on Cyclic Deformation Behavior of κ-Carrageenan Hydrogels. Int. J. Biol. Macromol. 2022, 218, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Alipour, S.; Pourjavadi, A.; Hosseini, S. Magnetite Embedded κ-Carrageenan-Based Double Network Nanocomposite Hydrogel with Two-Way Shape Memory Properties for Flexible Electronics and Magnetic Actuators. Carbohydr. Polym. 2023, 310, 120610. [Google Scholar] [CrossRef]
- Zeng, L.; Liu, B.; Duan, L.; Gao, G. Tough, Recyclable and Biocompatible Carrageenan-Modified Polyvinyl Alcohol Ionic Hydrogel with Physical Cross-Linked for Multimodal Sensing. Int. J. Biol. Macromol. 2023, 253, 126954. [Google Scholar] [CrossRef]
- Barbeyron, T.; Michel, G.; Potin, P.; Henrissat, B.; Kloareg, B. ι-Carrageenases Constitute a Novel Family of Glycoside Hydrolases, Unrelated to That of κ-Carrageenases. J. Biol. Chem. 2000, 275, 35499–35505. [Google Scholar] [CrossRef]
- Sun, X.Y.; Ye, L.N.; Liang, H.Y. Extremely Stretchable and Tough Hybrid Hydrogels Based on Gelatin, κ-Carrageenan and Polyacrylamide. Soft Matter 2021, 17, 9708–9715. [Google Scholar] [CrossRef]
- Li, D.; Yang, D.; Yang, X.; Wang, Y.; Guo, Z.; Xia, Y.; Sun, S.; Guo, S. Double-Helix Structure in Carrageenan-Metal Hydrogels: A General Approach to Porous Metal Sulfides/Carbon Aerogels with Excellent Sodium-Ion Storage. Angew. Chem. Int. Ed. Engl. 2016, 55, 15925–15928. [Google Scholar] [CrossRef]
- Kulkarni, R.V.; Boppana, R.; Krishna Mohan, G.; Mutalik, S.; Kalyane, N.V. pH-Responsive Interpenetrating Network Hydrogel Beads of Poly(acrylamide)-g-Carrageenan and Sodium Alginate for Intestinal Targeted Drug Delivery: Synthesis, in Vitro and In Vivo Evaluation. J. Colloid Interface Sci. 2012, 367, 509–517. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Pan, Y.; Zhou, Z.; Gao, Y.; Li, A.; Shi, B.; Hu, J.; Wang, L. Effect of Potassium-Ion-Triggered Double Helix Aggregation on Shakedown Behavior of κ-Carrageenan/Polyacrylamide Hydrogel. Gels 2025, 11, 412. https://doi.org/10.3390/gels11060412
Zhao X, Pan Y, Zhou Z, Gao Y, Li A, Shi B, Hu J, Wang L. Effect of Potassium-Ion-Triggered Double Helix Aggregation on Shakedown Behavior of κ-Carrageenan/Polyacrylamide Hydrogel. Gels. 2025; 11(6):412. https://doi.org/10.3390/gels11060412
Chicago/Turabian StyleZhao, Xueqi, Yudong Pan, Zhanrong Zhou, Yang Gao, Aijun Li, Binkai Shi, Jian Hu, and Liuying Wang. 2025. "Effect of Potassium-Ion-Triggered Double Helix Aggregation on Shakedown Behavior of κ-Carrageenan/Polyacrylamide Hydrogel" Gels 11, no. 6: 412. https://doi.org/10.3390/gels11060412
APA StyleZhao, X., Pan, Y., Zhou, Z., Gao, Y., Li, A., Shi, B., Hu, J., & Wang, L. (2025). Effect of Potassium-Ion-Triggered Double Helix Aggregation on Shakedown Behavior of κ-Carrageenan/Polyacrylamide Hydrogel. Gels, 11(6), 412. https://doi.org/10.3390/gels11060412





