Exploring the Potential of Cleansing Hydrogel and Shampoo with Whey as a Contemporary Approach to Sustainability
Abstract
1. Introduction
2. Results and Discussion
2.1. Shampoo Development
2.1.1. The Effect of Surfactants’ Concentration
2.1.2. The Effect of Whey Concentration
2.2. Cleansing Hydrogel Development
2.2.1. The Effect of Xanthan Gum and Decyl Glucoside Concentration
2.2.2. The Effect of Whey Concentration
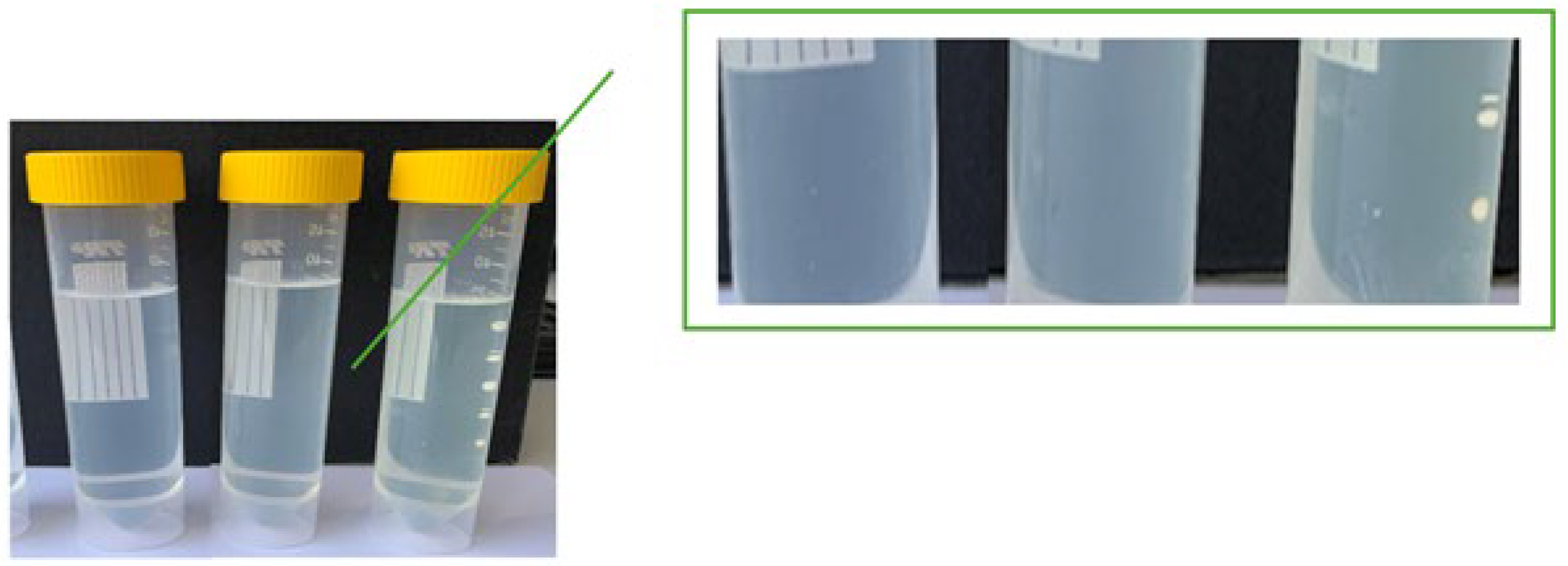
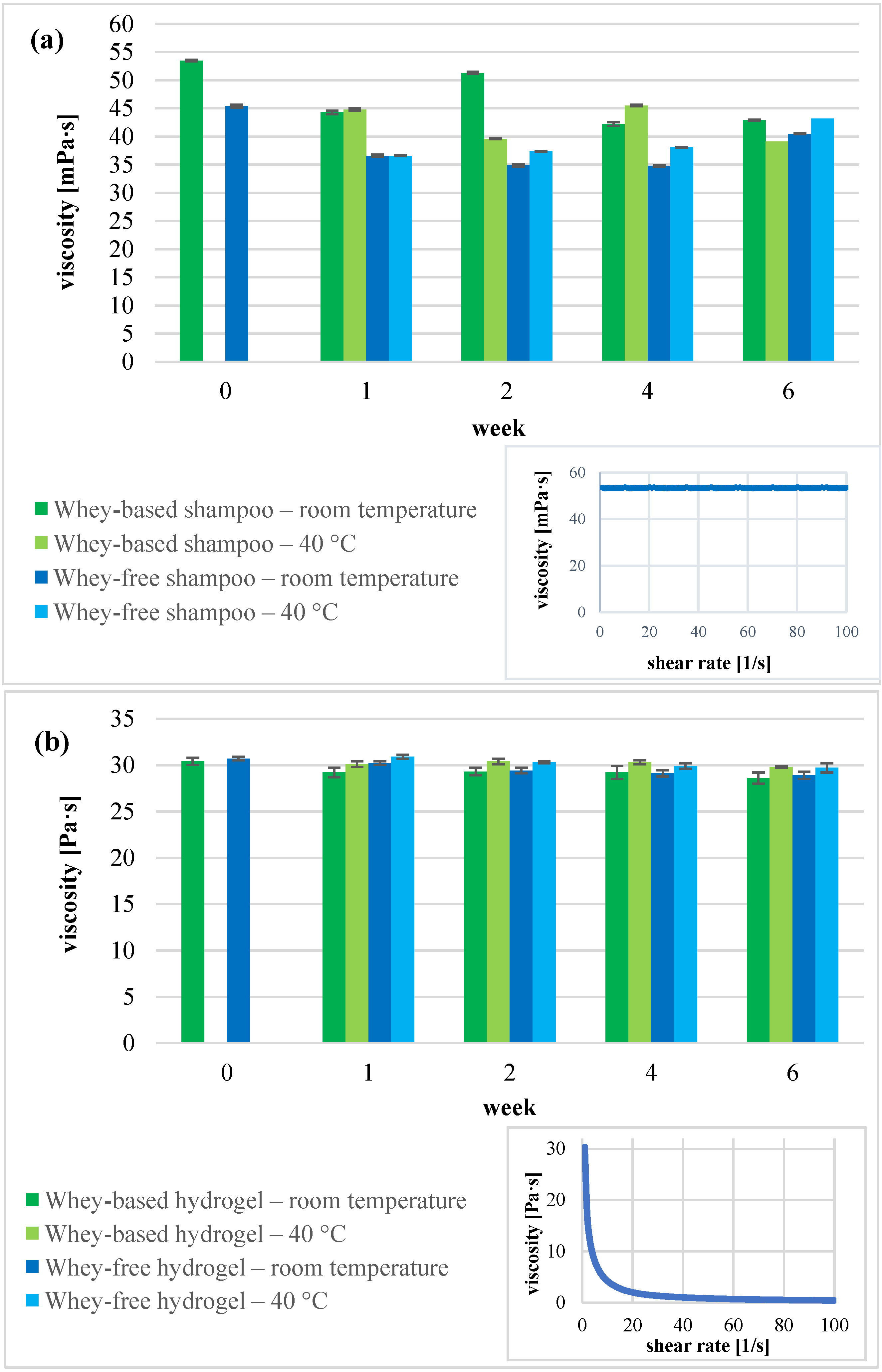
2.3. Stability Evaluation
2.4. In Vitro and In Vivo Evaluation
2.4.1. In Vitro Skin Irritation Evaluation
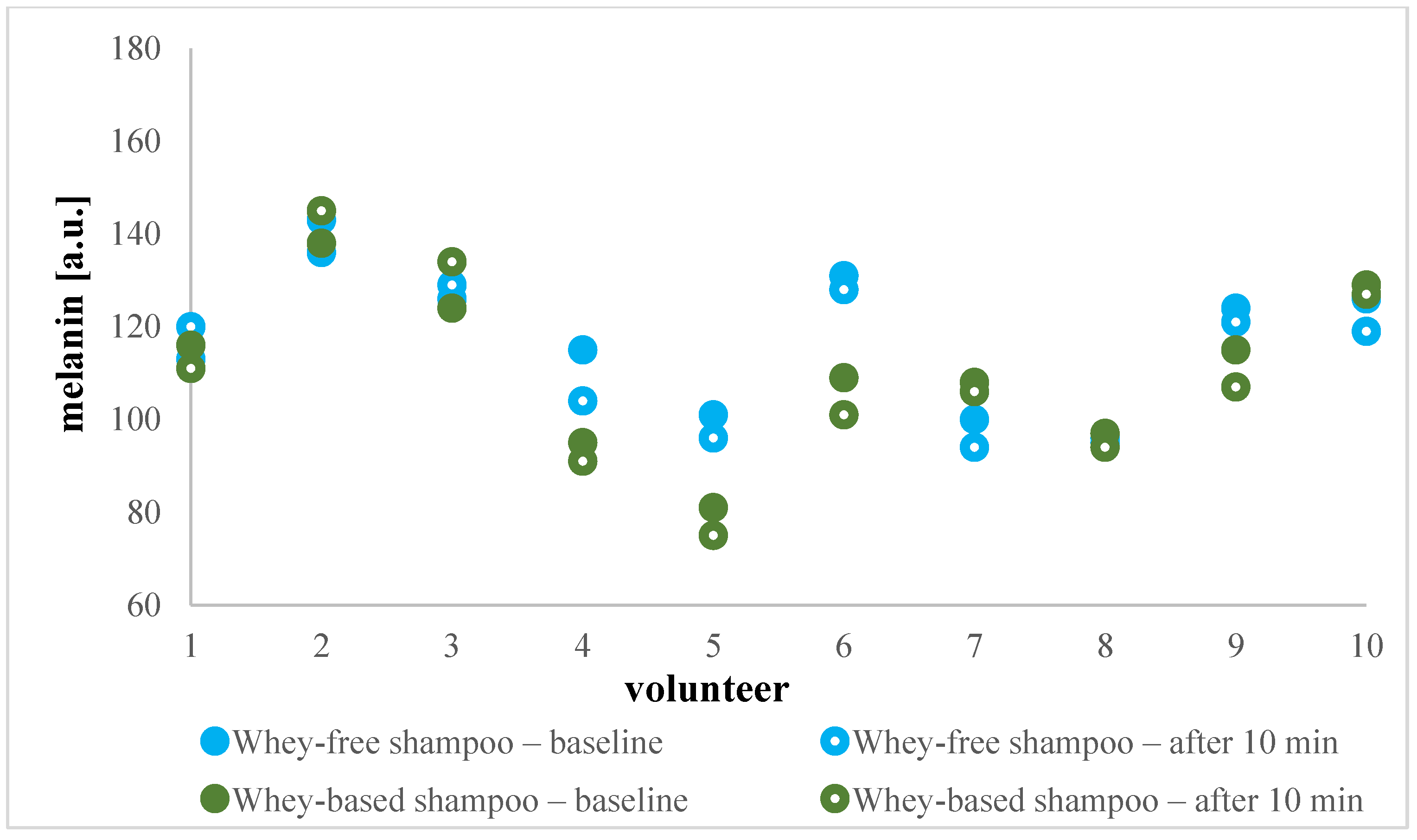
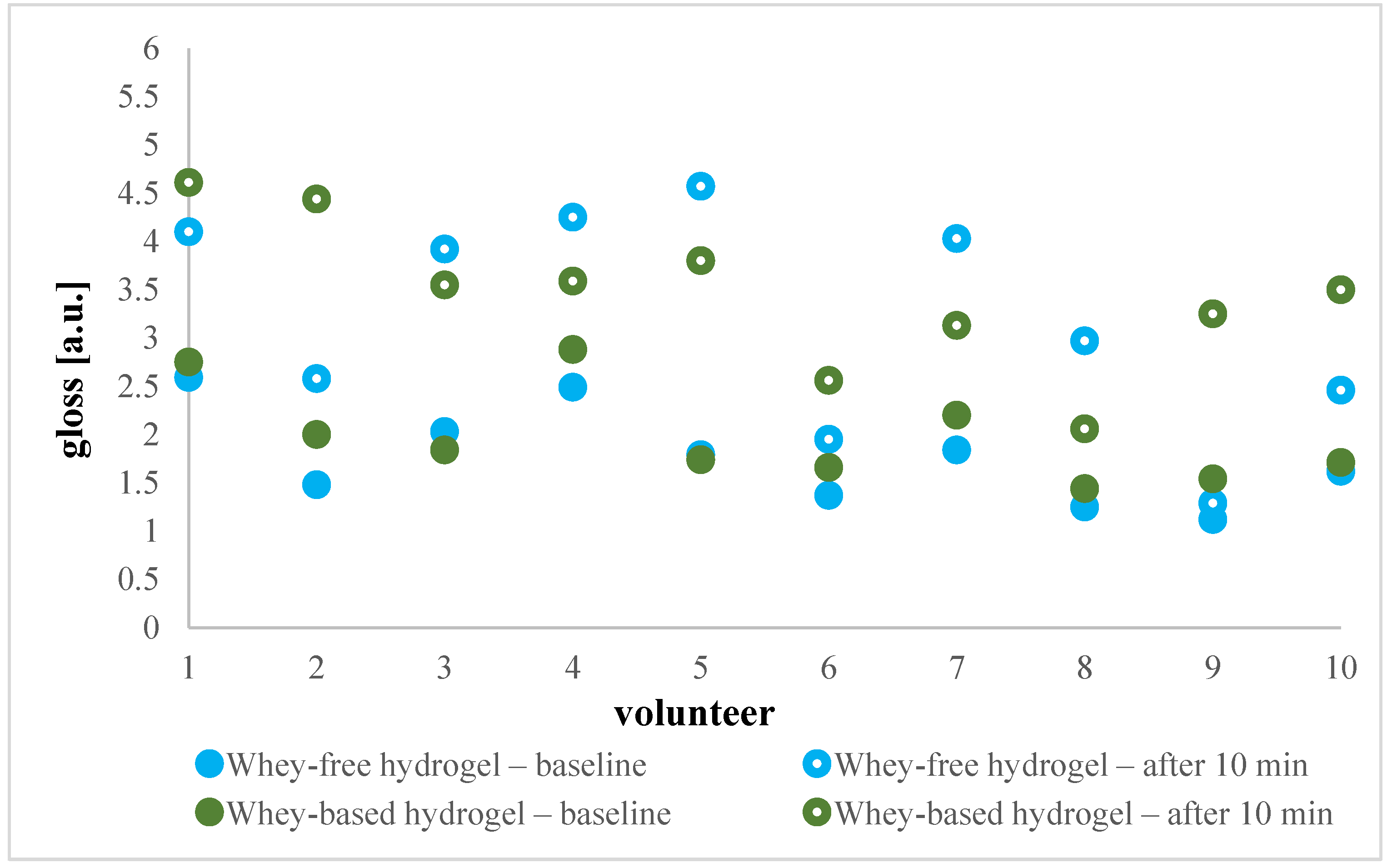
2.4.2. In Vivo Evaluation of Efficacy of Shampoo and Cleansing Hydrogel
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Cosmetic Product Development
Optimisation of Shampoo Formulation
Optimisation of Cleansing Hydrogel Formulation
Viscosity Measurements
pH Measurements
Organoleptic Evaluation
Stability Study
4.2.2. Evaluation of Irritancy Potential of Shampoo and Cleansing Hydrogel
4.2.3. In Vivo Study: Efficacy Evaluation of Shampoo and Cleansing Hydrogel
Initial Segment
Study Protocol
Transepidermal Water Loss
Stratum Corneum Hydration
Erythema and Melanin Index
Skin Gloss
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Secchi, G. Role of protein in cosmetics. Clin. Dermatol. 2008, 26, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Prokopowicz, M.; Różycki, K.M. Innovation in cosmetics. World Sci. News 2017, 72, 448–456. [Google Scholar]
- Zhang, L.; Falla, T.J. Cosmeceuticals and peptides. Clin. Dermatol. 2009, 27, 485–494. [Google Scholar] [CrossRef]
- Bjelošević, M.; Gašperlin, M.; Gosenca Matjaž, M. Peptidi v kozmetiki-smotrnost njihove uporabe. Farm. Vestn. 2019, 5, 361–369. [Google Scholar]
- Kumar, M.; Ghirnikar, R. Proteins and Peptides in Personal Care. In Biotechnology in Personal Care; Lad, R., Ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 57–79. [Google Scholar]
- Gorouhi, F.; Maibach, H.I. Role of topical peptides in preventing or treating aged skin. Int. J. Cosmet. Sci. 2009, 31, 327–345. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, T. Chapter 9—Pharmaceutical and Cosmetic Applications of Protein By-Products. In Protein Byproducts, 1st ed.; Singh Dhillon, G., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 147–160. [Google Scholar] [CrossRef]
- Speer, S.; Amin, S. Sustainable thermoresponsive whey protein- and chitosan-based oil-in-water emulsions for cosmetic applications. Int. J. Cosmet. Sci. 2021, 44, 30–41. [Google Scholar] [CrossRef]
- Amiri, S.; Rezazadeh-Bari, M.; Alizadeh-Khaledabad, M.; Rezaei-Mokarram, R.; Sowti-Khiabani, M. Fermentation optimization for co-production of postbiotics by Bifidobacterium lactis BB12 in cheese whey. Waste Biomass Valorization 2021, 12, 5869–5884. [Google Scholar] [CrossRef]
- Staples, L.C. Whey Protein Containing Cosmetic Formulations. C.A. Patent 1166576A, 1 May 1984. [Google Scholar]
- Wakabayashi, H.; Yamauchi, K.; Takase, M. Lactoferrin research, technology and applications. Int. Dairy J. 2006, 16, 1241–1251. [Google Scholar] [CrossRef]
- Hassoun, L.A.; Sivamani, R.K. A systematic review of lactoferrin use in dermatology. Crit. Rev. Food Sci. Nutr. 2017, 57, 3632–3639. [Google Scholar] [CrossRef]
- Song, W.; Liu, H.; Wang, C.; Wang, S. Cosmetics with Bovine Lactoferrin. C.N. Patent 103735424A, 26 January 2014. [Google Scholar]
- Pohl, S.; Varco, J.; Wallace, P.; Wolfram, L.J. Hair Preparations. In Chemical Technology of Cosmetics; Seidl, A., Ed.; A John Wiley & Sons, Inc., Publication: Hoboken, NJ, USA, 2013; pp. 85–122. [Google Scholar]
- Cornwell, P.A. A review of shampoo surfactant technology: Consumer benefits, raw materials and recent developments. Int. J. Cosmet. Sci. 2018, 40, 16–30. [Google Scholar] [CrossRef]
- Lochhead, R.Y. Shampoo and conditioner science. In Practical Modern Hair Science; Evans, T., Wickett, R.R., Eds.; Allured Pub. Corp.: Carol Stream, IL, USA, 2012; pp. 75–115. [Google Scholar]
- Anachkov, S.E.; Georgieva, G.S.; Abezgauz, L.; Danino, D.; Kralchevsky, P.A. Viscosity Peak due to Shape Transition from Wormlike to Disklike Micelles: Effect of Dodecanoic Acid. Langmuir 2018, 34, 4897–4907. [Google Scholar] [CrossRef] [PubMed]
- Mertens, S.; Gilissen, L.; Goossens, A. Allergic contact dermatitis caused by cocamide diethanolamine. Contact Dermat. 2016, 75, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Amin, S. Microrheological study of ternary surfactant-biosurfactant mixtures. Int. J. Cosmet. Sci. 2019, 41, 364–370. [Google Scholar] [CrossRef]
- Cai, W.; Gupta, R.B. Hydrogels. In Chemical Technology of Cosmetics; Seidl, A., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 597–632. [Google Scholar]
- Azizi Saadatlou, G.; Pircheraghi, G. Concentrated regimes of xanthan-based hydrogels crosslinked with multifunctional crosslinkers. Carbohydr. Polym. Technol. Appl. 2021, 2, 100047. [Google Scholar] [CrossRef]
- Fiume, M.M.; Heldreth, B.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; et al. Safety assessment of decyl glucoside and other alkyl glucosides as used in cosmetics. Int. J. Toxicol. 2013, 32, 22–48. [Google Scholar] [CrossRef]
- Vitek, M.; Gosenca Matjaž, M. Clinical application of hempseed or flaxseed oil-based lyotropic liquid crystals: Evaluation of their impact on skin barrier function. Acta Pharm. 2024, 74, 301–313. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; Nădăban, A.; Bras, W.; McCabe, C.; Bunge, A.; Gooris, G.S. The skin barrier: An extraordinary interface with an exceptional lipid organization. Prog. Lipid Res. 2023, 92, 101252. [Google Scholar] [CrossRef]
- Jansen van Rensburg, S.; Franken, A.; Du Plessis, J.L. Measurement of transepidermal water loss, stratum corneum hydration and skin surface pH in occupational settings: A review. Ski. Res. Technol. 2019, 25, 595–605. [Google Scholar] [CrossRef]
- Augustin, M.; Kirsten, N.; Körber, A.; Wilsmann-Theis, D.; Itschert, G.; Staubach-Renz, P.; Maul, J.T.; Zander, N. Prevalence, predictors and comorbidity of dry skin in the general population. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 147–150. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Darlenski, R.; Surber, C. Glycerol and the skin: Holistic approach to its origin and functions. Br. J. Dermatol. 2008, 159, 23–34. [Google Scholar] [CrossRef]
- Sobkowska, D.; Micek, I.; Urbańska, M.; Seraszek-Jaros, A.; Nowak, G.; Zaprutko, L.; Czajkowski, R.; Adamski, Z.; Gornowicz-Porowska, J. The effects of baths and wet wraps with a sweet whey solution on the level of hydration and barrier function of the epidermis. Postep. Dermatol. Alergol. 2021, 38, 798–803. [Google Scholar] [CrossRef]
- Abdlaty, R.; Haywar, J.; Farrell, T.; Fang, Q. Skin Erythema and Pigmentation Optical Assessment Techniques: Review Article. Photodiagnosis Photodyn. Ther. 2021, 33, 102127. [Google Scholar] [CrossRef] [PubMed]
- Frew, J.; Penzi, L.; Suarez-Farinas, M.; Garcet, S.; Brunner, P.M.; Czarnowicki, T.; Kim, J.; Bottomley, C.; Finney, R.; Cueto, I.; et al. The erythema Q-score, an imaging biomarker for redness in skin inflammation. Exp. Dermatol. 2021, 30, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xu, Z.; Qiu, Y.; Zheng, X. Comparative study of instrumental measurement and sensory evaluation methods for the repairing effect of mildly damaged hair bundles. Skin Res. Technol. 2023, 29, e13394. [Google Scholar] [CrossRef] [PubMed]
- National Health Surveillance Agency Brasilia. Cosmetic Products Stability Guide; National Health Surveillance Agency: Brasília, Brazil, 2005; Volume 1, pp. 1–50. [Google Scholar]
- Rozman, B.; Gosenca, M.; Gašperlin, M.; Padois, K.; Falson, F. Dual influence of colloidal silica on skin deposition of vitamins C and E simultaneously incorporated in topical microemulsions. Drug Dev. Ind. Pharm. 2010, 36, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Fentem, J.H.; Briggs, D.; Chesné, C.; Elliott, G.R.; Harbell, J.W.; Heylings, J.R.; Portes, P.; Roguet, R.; van de Sandt, J.J.; Botham, P.A. A prevalidation study on in vitro tests for acute skin irritation. results and evaluation by the Management Team. Toxicol. In Vitro 2001, 15, 57–93. [Google Scholar] [CrossRef]
- Courage + Khazaka Electronic GmbH. Skin-Glossymeter GL 200. Available online: https://www.courage-khazaka.de/en/scientific-products/efficacy-tests/skin?view=article&id=174&catid=16 (accessed on 2 April 2024).



| Component | % (m/m) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | |
| purified water | 88.5 | 83.5 | 78.5 | 73.5 | 78.5 | 78.5 | 78.5 | 68.5 | 58.5 | 48.5 |
| sodium laureth sulfate | 5 | 10 | 15 | 20 | 15 | 15 | 15 | 15 | 15 | 15 |
| sodium chloride | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| cocamidopropyl betaine | 1.5 | 1.5 | 1.5 | 1.5 | 0.5 | 2.5 | 3.0 | 2.5 | 2.5 | 2.5 |
| cocamide diethanolamine | 1.5 | 1.5 | 1.5 | 1.5 | 2.5 | 0.5 | - | 0.5 | 0.5 | 0.5 |
| propylene glycol | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| whey | / | / | / | / | / | / | / | 10 | 20 | 30 |
| Component | % (m/m) | |||||||
|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | |
| purified water | 80 | 79.5 | 79 | 74 | 69 | 69 | 59 | 49 |
| decyl glucoside | 10 | 10 | 10 | 15 | 20 | 10 | 10 | 10 |
| glycerol | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 |
| almond oil | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| xanthan gum | 0.5 | 1 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| whey | / | / | / | / | / | 10 | 20 | 30 |
| Formulation | Baseline | 95% Confidence Interval | Absolute Value After 10 min | 95% Confidence Interval | p-Value * | p-Value ** |
|---|---|---|---|---|---|---|
| shampoo without whey | 12.31 ± 3.54 | (9.78, 14.84) | 13.97 ± 2.85 | (11.93, 16.00) | 0.05 | 0.25 |
| shampoo with whey | 12.85 ± 2.28 | (11.21, 14.48) | 13.80 ± 3.88 | (11.02, 16.58) | 0.22 | |
| hydrogel without whey | 12.27 ± 2.89 | (10.07, 14.47) | 13.58 ± 2.59 | (11.72, 15.44) | 0.07 | 0.19 |
| hydrogel with whey | 13.62 ± 3.33 | (11.24, 16.01) | 13.93 ± 1.39 | (12.94, 14.92) | 0.37 |
| Baseline | Absolute Value After 10 min | p-Value * | p-Value ** | |
|---|---|---|---|---|
| shampoo without whey | 23.91 ± 6.47 | 27.18 ± 5.53 | 0.003 | 0.29 |
| shampoo with whey | 21.87 ± 3.54 | 25.69 ± 3.63 | 0.0005 | |
| hydrogel without whey | 25.81 ± 5.64 | 46.75 ± 8.04 | 5.24 × 10−7 | 0.42 |
| hydrogel with whey | 21.97 ± 5.66 | 43.59 ± 9.37 | 9.74 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bjelošević Žiberna, M.; Grilc, B.; Gašperlin, M.; Gosenca Matjaž, M. Exploring the Potential of Cleansing Hydrogel and Shampoo with Whey as a Contemporary Approach to Sustainability. Gels 2025, 11, 374. https://doi.org/10.3390/gels11050374
Bjelošević Žiberna M, Grilc B, Gašperlin M, Gosenca Matjaž M. Exploring the Potential of Cleansing Hydrogel and Shampoo with Whey as a Contemporary Approach to Sustainability. Gels. 2025; 11(5):374. https://doi.org/10.3390/gels11050374
Chicago/Turabian StyleBjelošević Žiberna, Maja, Blaž Grilc, Mirjana Gašperlin, and Mirjam Gosenca Matjaž. 2025. "Exploring the Potential of Cleansing Hydrogel and Shampoo with Whey as a Contemporary Approach to Sustainability" Gels 11, no. 5: 374. https://doi.org/10.3390/gels11050374
APA StyleBjelošević Žiberna, M., Grilc, B., Gašperlin, M., & Gosenca Matjaž, M. (2025). Exploring the Potential of Cleansing Hydrogel and Shampoo with Whey as a Contemporary Approach to Sustainability. Gels, 11(5), 374. https://doi.org/10.3390/gels11050374







