Ultra-High Dose Rate Electron Beam Dosimetry Using Ag Nanoparticle-Enhanced nPAG and NIBMAGAT Gels
Abstract
1. Introduction
2. Results and Discussion
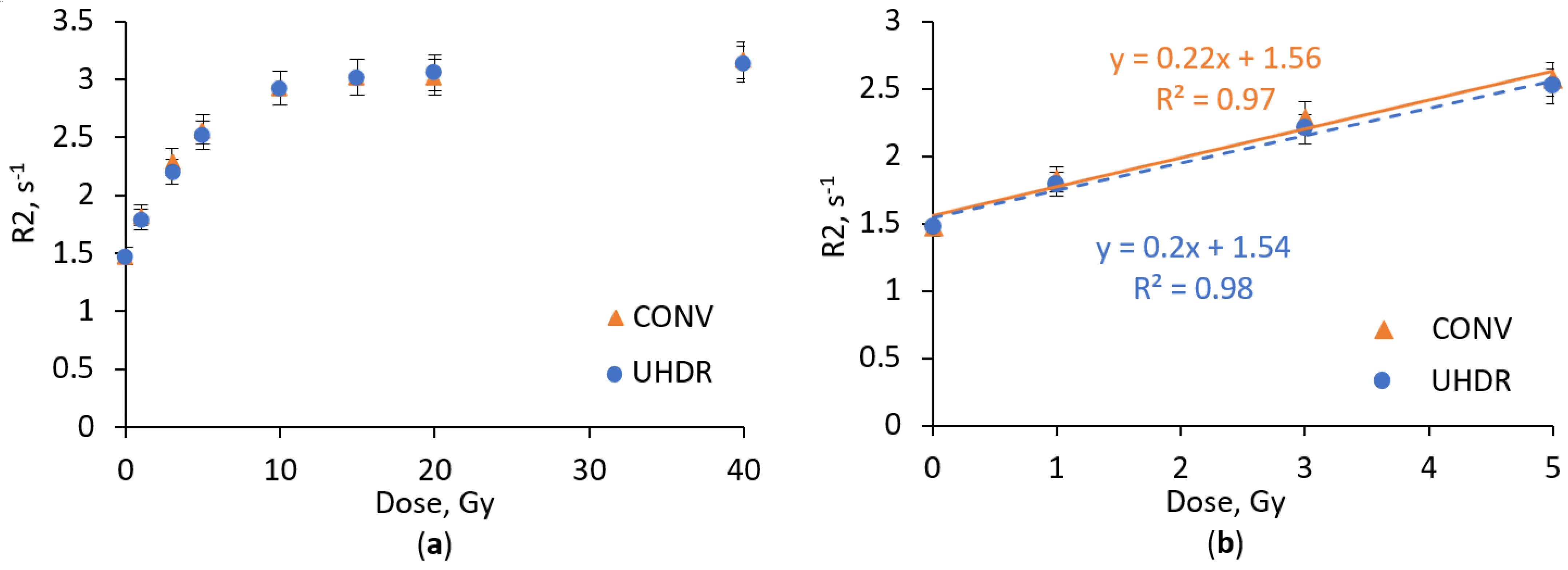
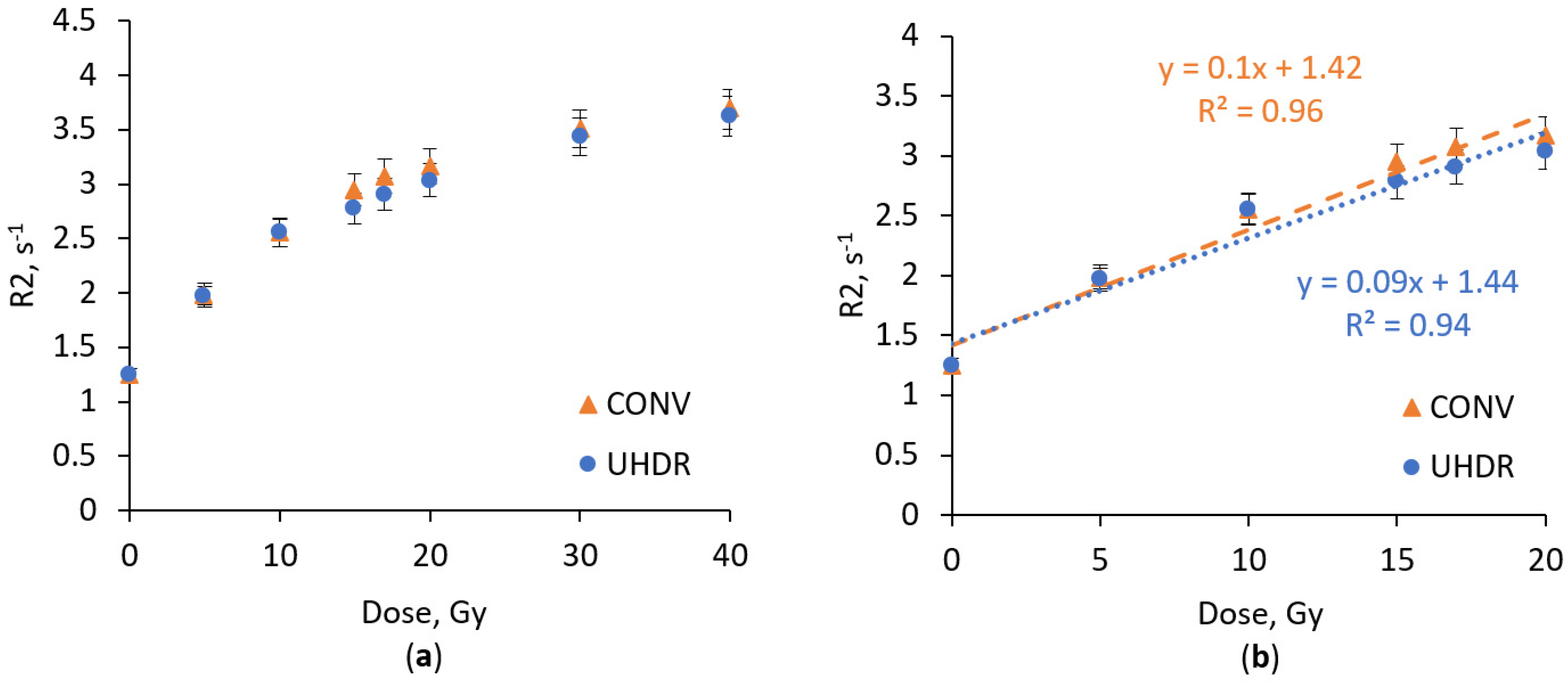
2.1. Dose Response Analysis
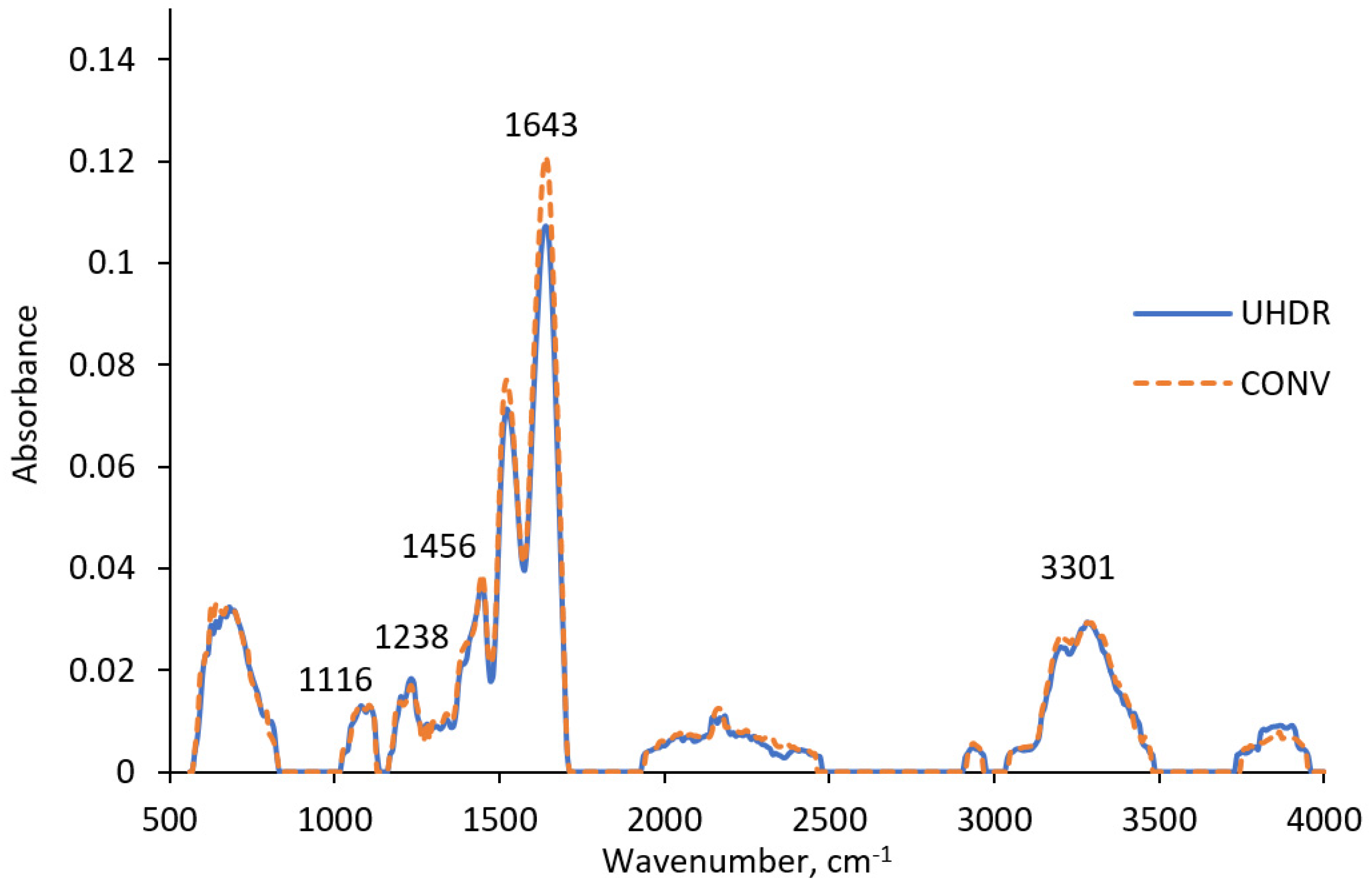
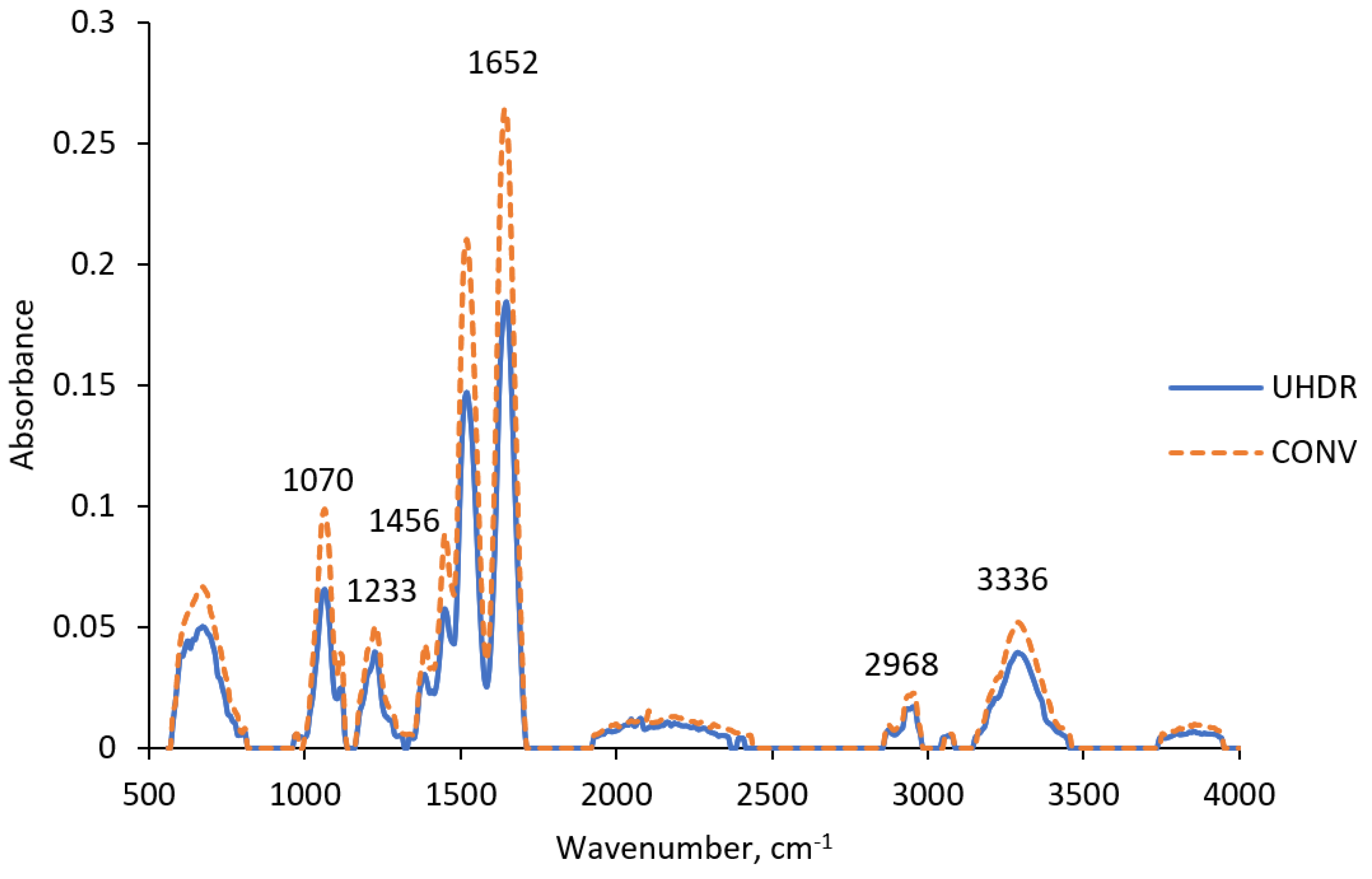
2.2. FTIR Analysis
2.3. Uncertainty Budget
3. Conclusions
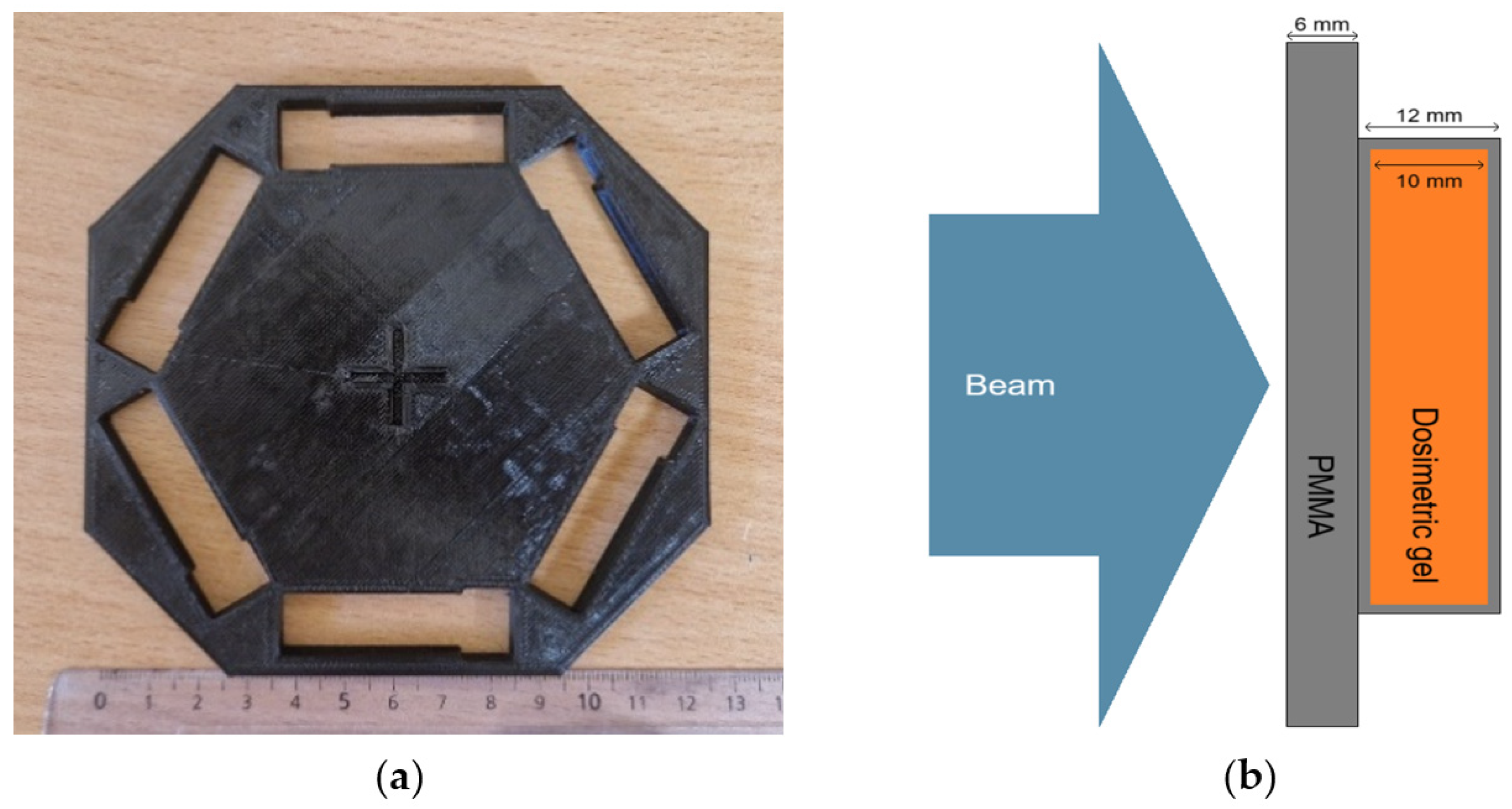
4. Materials and Methods
4.1. Fabrication of Dosimetric Gel
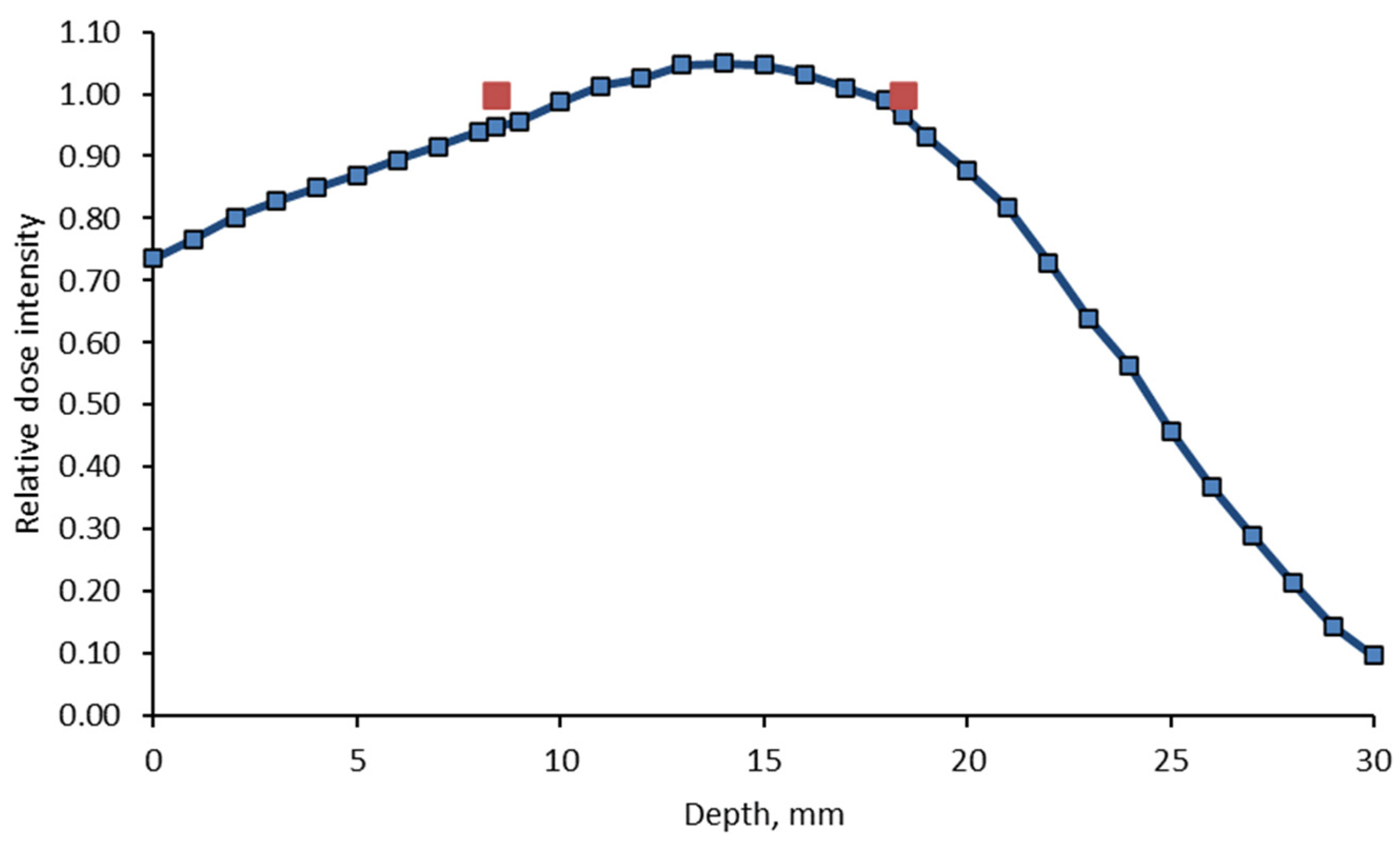
4.2. Irradiation of Dosimetric Gels
4.3. Analysis of Dosimetric Gel Samples
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.F.; Brito, I.; Hupé, P.; Bourhis, J.; et al. Ultrahigh Dose-Rate FLASH Irradiation Increases the Differential Response between Normal and Tumor Tissue in Mice. Sci. Transl. Med. 2014, 6, 245ra93. [Google Scholar] [CrossRef] [PubMed]
- Vozenin, M.C.; Hendry, J.H.; Limoli, C.L. Biological Benefits of Ultra-High Dose Rate FLASH Radiotherapy: Sleeping Beauty Awoken. Clin. Oncol. 2019, 31, 407–415. [Google Scholar] [CrossRef]
- Bourhis, J.; Montay-Gruel, P.; Gonçalves Jorge, P.; Bailat, C.; Petit, B.; Ollivier, J.; Jeanneret-Sozzi, W.; Ozsahin, M.; Bochud, F.; Moeckli, R.; et al. Clinical Translation of FLASH Radiotherapy: Why and How? Radiother. Oncol. 2019, 139, 11–17. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, H.J. FLASH Radiotherapy: Bridging Revolutionary Mechanisms and Clinical Frontiers in Cancer Treatment—A Narrative Review. Ewha Med. J. 2024, 47, e54. [Google Scholar] [CrossRef]
- Wilson, J.D.; Hammond, E.M.; Higgins, G.S.; Petersson, K. Ultra-High Dose Rate (FLASH) Radiotherapy: Silver Bullet or Fool’s Gold? Front. Oncol. 2020, 9, 1563. [Google Scholar] [CrossRef] [PubMed]
- Baldock, C.; De Deene, Y.; Doran, S.; Ibbott, G.; Jirasek, A.; Lepage, M.; McAuley, K.B.; Oldham, M.; Schreiner, L.J. Topical Review: Polymer Gel Dosimetry. Phys. Med. Biol. 2010, 55, R1–R63. [Google Scholar] [CrossRef]
- Ceberg, S.; Mannerberg, A.; Konradsson, E.; Blomstedt, M.; Kügele, M.; Kadhim, M.; Edvardsson, A.; Bäck, S.Å.J.; Petersson, K.; Jamtheim Gustafsson, C.; et al. FLASH Radiotherapy and the Associated Dosimetric Challenges. J. Phys. Conf. Ser. 2023, 2630, 012010. [Google Scholar] [CrossRef]
- McJury, M.; Oldham, M.; Cosgrove, V.P.; Murphy, P.S.; Doran, S.; Leach, M.O.; Webb, S. Radiation Dosimetry Using Polymer Gels: Methods and Applications. Br. J. Radiol. 2000, 73, 919–929. [Google Scholar] [CrossRef]
- Macchione, M.A.; Lechón Páez, S.; Strumia, M.C.; Valente, M.; Mattea, F. Chemical Overview of Gel Dosimetry Systems: A Comprehensive Review. Gels 2022, 8, 663. [Google Scholar] [CrossRef]
- McAuley, K.B. Fundamentals of Polymer Gel Gosimeters. J. Phys. Conf. Ser. 2006, 56, 35. [Google Scholar] [CrossRef]
- Rabaeh, K.A.; Al-Ajaleen, M.S.; Abuzayed, M.H.; Aldweri, F.M.; Eyadeh, M.M. High Dose Sensitivity of N-(Isobutoxymethyl)Acrylamide Polymer Gel Dosimeters with Improved Monomer Solubility Using Acetone Co-Solvent. Nucl. Instrum. Methods Phys. Res. Sect. B 2019, 442, 67–72. [Google Scholar] [CrossRef]
- Cheng, K.Y.; Hsieh, L.L.; Shih, C.T. A Comprehensive Evaluation of NIPAM Polymer Gel Dosimeters on Three Orthogonal Planes and Temporal Stability Analysis. PLoS ONE 2016, 11, e0155797. [Google Scholar] [CrossRef]
- Shih, T.Y.; Hsieh, B.T.; Yen, T.H.; Lin, F.Y.; Wu, J. Sensitivity Enhancement of Methacrylic Acid Gel Dosimeters by Incorporating Iodine for Computed Tomography Scans. Phys. Medica 2019, 63, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Aliasgharzadeh, A.; Mohammadi, A.; Farhood, B.; Anaraki, V.; Mohseni, M.; Moradi, H. Improvement of the Sensitivity of PASSAG Polymer Gel Dosimeter by Urea. Radiat. Phys. Chem. 2020, 166, 108470. [Google Scholar] [CrossRef]
- Koeva, V.I.; Csaszar, E.S.; Senden, R.J.; McAuley, K.B.; Schreiner, L.J. Polymer Gel Dosimeters with Increased Solubility: A Preliminary Investigation of the NMR and Optical Dose-Response Using Different Crosslinkers and Co-Solvents. Macromol. Symp. 2008, 261, 157–166. [Google Scholar] [CrossRef]
- Zhu, X.; Reese, T.G.; Crowley, E.M.; El Fakhri, G. Improved MAGIC Gel for Higher Sensitivity and Elemental Tissue Equivalent 3D Dosimetry. Med. Phys. 2010, 37, 183–188. [Google Scholar] [CrossRef]
- Hayashi, S.I.; Fujiwara, F.; Usui, S.; Tominaga, T. Effect of Inorganic Salt on the Dose Sensitivity of Polymer Gel Dosimeter. Radiat. Phys. Chem. 2012, 81, 884–888. [Google Scholar] [CrossRef]
- Cho, S.H. Estimation of Tumour Dose Enhancement Due to Gold Nanoparticles during Typical Radiation Treatments: A Preliminary Monte Carlo Study. Phys. Med. Biol. 2005, 50, N163–N173. [Google Scholar] [CrossRef]
- Vedelago, J.; Mattea, F.; Valente, M. Integration of Fricke Gel Dosimetry with Ag Nanoparticles for Experimental Dose Enhancement Determination in Theranostics. Appl. Radiat. Isot. 2018, 141, 182–186. [Google Scholar] [CrossRef]
- Farahani, S.; Riyahi Alam, N.; Haghgoo, S.; Khoobi, M.; Geraily, G.H.; Gorji, E. Dosimetry and Radioenhancement Comparison of Gold Nanoparticles in Kilovoltage and Megavoltage Radiotherapy Using MAGAT Polymer Gel Dosimeter. J. Biomed. Phys. Eng. 2019, 9, 199. [Google Scholar] [CrossRef]
- Mahdavi, M.; KhademAbolfazli, M.; Mahdavi, S.-R.-M.; Ataei, G. Effect of Gold Nanoparticle on Percentage Depth Dose Enhancement on Megavoltage Energy in MAGICA Polymer Gel Dosimeter. J. Biomed. Phys. Eng. 2013, 3, 37–44. [Google Scholar] [PubMed]
- Sabbaghizadeh, R.; Shamsudin, R.; Deyhimihaghighi, N.; Sedghi, A. Enhancement of Dose Response and Nuclear Magnetic Resonance Image of PAGAT Polymer Gel Dosimeter by Adding Silver Nanoparticles. PLoS ONE 2017, 12, e0168737. [Google Scholar] [CrossRef]
- Kudrevicius, L.; Jaselske, E.; Adliene, D.; Rudzianskas, V.; Radziunas, A.; Tamasauskas, A. Application of 3D Gel Dosimetry as a Quality Assurance Tool in Functional Leksell Gamma Knife Radiosurgery. Gels 2022, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Vandecasteele, J.; Vercauteren, T.; De Pasquale, S.; De Deene, Y. Reproducibility Study of Normoxic Polyacrylamide Gel (NPAG) Dosimeters. J. Phys. Conf. Ser. 2009, 164, 012010. [Google Scholar] [CrossRef]
- Nordström, F.; Ceberg, S.; Wetterstedt, S.A.; Nilsson, P.; Ceberg, C.; Bäck, S.Å.J. 3D Geometric Gel Dosimetry Verification of Intraprostatic Fiducial Guided Hypofractionated Radiotherapy of Prostate Cancer. J. Phys. Conf. Ser. 2010, 250, 012059. [Google Scholar] [CrossRef]
- Boudou, C.; Troprès, I.; Lamalle, L.; Gastaldo, J.; Rousseau, J.; Charvet, A.M.; Estève, F.; Elleaume, H. Utilization of NPAG Dosimeter for Synchrotron Radiotherapy: First Results. J. Phys. Conf. Ser. 2006, 56, 289. [Google Scholar] [CrossRef]
- Marques, T.; Schwarcke, M.; Garrido, C.; Zucolot, V.; Baffa, O.; Nicolucci, P. Gel Dosimetry Analysis of Gold Nanoparticle Application in Kilovoltage Radiation Therapy. J. Phys. Conf. Ser. 2010, 250, 012084. [Google Scholar] [CrossRef]
- Sellakumar, P.; Samuel, E.J.J.; Kumar, D.S. Dose-Rate Dependence of PAGAT Polymer Gel Dosimeter Evaluated Using X-Ray CT Scanner. J. Phys. Conf. Ser. 2010, 250, 012074. [Google Scholar] [CrossRef]
- Zehtabian, M.; Faghihi, R.; Zahmatkesh, M.H.; Meigooni, A.S.; Mosleh-Shirazi, M.A.; Mehdizadeh, S.; Sina, S.; Bagheri, S. Investigation of the Dose Rate Dependency of the PAGAT Gel Dosimeter at Low Dose Rates. Radiat. Meas. 2012, 47, 139–144. [Google Scholar] [CrossRef]
- Jirasek, A. Alternative Imaging Modalities for Polymer Gel Dosimetry. J. Phys. Conf. Ser. 2010, 250, 012070. [Google Scholar] [CrossRef]
- Guidelli, E.J.; Ramos, A.P.; Zaniquelli, M.E.D.; Nicolucci, P.; Baffa, O. Synthesis and Characterization of Silver/Alanine Nanocomposites for Radiation Detection in Medical Applications: The Influence of Particle Size on the Detection Properties. Nanoscale 2012, 4, 2884–2893. [Google Scholar] [CrossRef] [PubMed]
- Rahman, W.N.; Wong, C.J.; Ackerly, T.; Yagi, N.; Geso, M. Polymer Gels Impregnated with Gold Nanoparticles Implemented for Measurements of Radiation Dose Enhancement in Synchrotron and Conventional Radiotherapy Type Beams. Australas. Phys. Eng. Sci. Med. 2012, 35, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Basfar, A.A.; Moftah, B.; Lotfy, S.; Al-Moussa, A.A.; Soliman, Y.S. Evaluations of N-(Isobutoxymethyl) Acrylamide Gel Dosimeter by NMR Technique for Radiotherapy and Uncertainty in Dose Measurements. Appl. Radiat. Isot. 2019, 148, 240–245. [Google Scholar] [CrossRef]
- Lotfy, S.; Basfar, A.A.; Moftah, B.; Al-Moussa, A.A. Comparative Study of Nuclear Magnetic Resonance and UV–Visible Spectroscopy Dose-Response of Polymer Gel Based on N-(Isobutoxymethyl) Acrylamide. Nucl. Instrum. Methods Phys. Res. B 2017, 413, 42–50. [Google Scholar] [CrossRef]
- Smith, B.C. The Infrared Spectroscopy of Alkenes. Spectroscopy 2016, 31, 28–34. [Google Scholar]
- Lehmann, A.; Nijakowski, K.; Drożdżyńska, A.; Przybylak, M.; Woś, P.; Surdacka, A. Influence of the Polymerization Modes on the Methacrylic Acid Release from Dental Light-Cured Materials—In Vitro Study. Materials 2022, 15, 8976. [Google Scholar] [CrossRef]
- Lepage, M.; Jordan, K. 3D Dosimetry Fundamentals: Gels and Plastics. J. Phys. Conf. Ser. 2010, 250, 012055. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret Ftir Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Kurečič, M.; Majda, S.S.; Karin, S.K. Uv Polymerization of Poly (N-Isopropylacrylamide) Hydrogel. Mater. Tehnol. 2012, 46, 87–91. [Google Scholar]
- Soliman, Y.S.; Tadros, S.M.; Beshir, W.B.; Saad, G.R.; Gallo, S.; Ali, L.I.; Naoum, M.M. Study of Ag Nanoparticles in a Polyacrylamide Hydrogel Dosimeters by Optical Technique. Gels 2022, 8, 222. [Google Scholar] [CrossRef]
- Soliman, Y.S.; Beshir, W.B.; Abdelgawad, M.H.; Bräuer-Krisch, E.; Abdel-Fattah, A.A. Pergascript Orange-Based Polymeric Solution as a Dosimeter for Radiotherapy Dosimetric Validation. Phys. Medica 2019, 57, 169–176. [Google Scholar] [CrossRef] [PubMed]
- El Gohary, M.I.; Soliman, Y.S.; Amin, E.A.; Gawad, M.H.A.; Desouky, O.S. Effect of Perchloric Acid on the Performance of the Fricke Xylenol Gel Dosimeter. Appl. Radiat. Isot. 2016, 113, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, P.; Miller, A. Guidelines for the Calibration of Routine Dosimetry Systems for Use in Radiation Processing. Npl Rep. CIRM 2009, 29, 13–17. [Google Scholar]
- Guan, F.; Chen, H.; Draeger, E.; Li, Y.; Aydin, R.; Tien, C.J.; Chen, Z. Characterization of GafchromicTM EBT4 Film with Clinical KV/MV Photons and MeV Electrons. Precis. Radiat. Oncol. 2023, 7, 84–91. [Google Scholar] [CrossRef]
- Castro, P.; García-Vicente, F.; Mínguez, C.; Floriano, A.; Sevillano, D.; Pérez, L.; Torres, J.J. Study of the Uncertainty in the Determination of the Absorbed Dose to Water during External Beam Radiotherapy Calibration. J. Appl. Clin. Med. Phys. 2008, 9, 70–86. [Google Scholar] [CrossRef]
- Venning, A.J.; Brindha, S.; Hill, B.; Baldock, C. Preliminary Study of a Normoxic PAG Gel Dosimeter with Tetrakis (Hydroxymethyl) Phosphonium Chloride as an Anti-Oxidant. J. Phys. Conf. Ser. 2004, 3, 155. [Google Scholar] [CrossRef]
- Šileikaitčė, A.; Tamulevičius, T.; Tamulevičius, S.; Andrulevičius, M.; Puišo, J.; Guobienčė, A.; Prosyčevas, I.; Madsen, M.; Maibohm, C.; Rubahn, H.-G. Periodic Structures Modified with Silver Nanoparticles for Novel Plasmonic Application. In Proceedings of the Nanophotonics II, Strasbourg, France, 7–9 April 2008; Volume 6988. [Google Scholar]
- Paramelle, D.; Sadovoy, A.; Gorelik, S.; Free, P.; Hobley, J.; Fernig, D.G. A Rapid Method to Estimate the Concentration of Citrate Capped Silver Nanoparticles from UV-Visible Light Spectra. Analyst 2014, 139, 4855–4861. [Google Scholar] [CrossRef]
- Šlėktaitė-Kišonė, A.; Burkanas, M.; Cicinas, A.; Čeponis, T.; Džiugelis, M.; Merkis, M.; Adlienė, D.; Venius, J. First Experience On Varian Truebeam Generated Ultra-High Dose Rate Electron Beam Dosimetry Using Conventional Dosimetry Methods. In Proceedings of the International Conference “Medical Physics in the Baltic States 2023”, Kaunas, Lithuania, 9–11 November 2023; Adlienė, D., Ed.; Kaunas University of Technology: Kaunas, Lithuania, 2023; pp. 92–95. [Google Scholar]
- Ashraf, M.R.; Rahman, M.; Zhang, R.; Williams, B.B.; Gladstone, D.J.; Pogue, B.W.; Bruza, P. Dosimetry for FLASH Radiotherapy: A Review of Tools and the Role of Radioluminescence and Cherenkov Emission. Front. Phys. 2020, 8, 328. [Google Scholar] [CrossRef]
- Merkis, M.; Urbonavicius, B.G.; Adliene, D.; Laurikaitiene, J.; Puiso, J. Pilot Study of Polymerization Dynamics in NMAG Dose Gel. Gels 2022, 8, 288. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Huang, S.-W.; Chen, H.-Y.; Lan, H.-T.; Su, F.-Y.; Peng, S.-L.; Yao, C.-H. Investigating the Sensitivity of NIPAM Gel Dosimetry in CyberKnife Systems Using MRI-Based R2 Mapping. J. Med. Biol. Eng. 2025, 1–7. [Google Scholar] [CrossRef]




| Uncertainty Source | nPAG | nPAG + Ag | NIBMAGAT | NIBMAGAT + Ag | Uncertainty Type |
|---|---|---|---|---|---|
| Irradiation output | 2% | 2% | 2% | 2% | B |
| Irradiation dose evaluation | 2% | 2% | 2% | 2% | B |
| Sensitivity variation of imaging | 3.9% | 3.9% | 3.9% | 3.9% | A |
| Dose rate dependency | 0.9% | 2.5% | 1.3% | 1.8% | A |
| Calibration curve fit | 1% | 1.5% | 0.4% | 0.2% | A |
| Reproducibility | 0.5% | 0.4% | 0.9% | 0.6% | A |
| Combined uncertainty | 5% | 5.6% | 5.1% | 5.2% | |
| Total uncertainty (2σ) | 10% | 11.2% | 10.2% | 10.4% |
| Purpose | Material | Dosimetric Gel | |
|---|---|---|---|
| nPAG | NIBMAGAT | ||
| Monomer | N-(isobutoxymethyl)acrylamide | - | 2 wt% |
| Acrylamide | 3 wt% | - | |
| Crosslinker | N,N′-methylenebis(acrylamide) | 3 wt% | 3 wt% |
| Scaffold | Gelatin | 5 wt% | 4 wt% |
| Oxygen scavenger | Tetrakis(hydroxymethyl)phosphonium chloride | 5 mM | 5 mM |
| Cosolvent | Acetone | - | 30 wt% |
| Solvent | Water | 59–89 wt% | 41–71 wt% |
| Additive (optional) | Ag nanoparticle solution (1 mM) | 30 wt% | 30 wt% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merkis, M.; Slektaite-Kisone, A.; Burkanas, M.; Cicinas, A.; Dziugelis, M.; Klimkevicius, V.; Adliene, D.; Venius, J. Ultra-High Dose Rate Electron Beam Dosimetry Using Ag Nanoparticle-Enhanced nPAG and NIBMAGAT Gels. Gels 2025, 11, 336. https://doi.org/10.3390/gels11050336
Merkis M, Slektaite-Kisone A, Burkanas M, Cicinas A, Dziugelis M, Klimkevicius V, Adliene D, Venius J. Ultra-High Dose Rate Electron Beam Dosimetry Using Ag Nanoparticle-Enhanced nPAG and NIBMAGAT Gels. Gels. 2025; 11(5):336. https://doi.org/10.3390/gels11050336
Chicago/Turabian StyleMerkis, Mantvydas, Akvile Slektaite-Kisone, Marius Burkanas, Aleksandras Cicinas, Mindaugas Dziugelis, Vaidas Klimkevicius, Diana Adliene, and Jonas Venius. 2025. "Ultra-High Dose Rate Electron Beam Dosimetry Using Ag Nanoparticle-Enhanced nPAG and NIBMAGAT Gels" Gels 11, no. 5: 336. https://doi.org/10.3390/gels11050336
APA StyleMerkis, M., Slektaite-Kisone, A., Burkanas, M., Cicinas, A., Dziugelis, M., Klimkevicius, V., Adliene, D., & Venius, J. (2025). Ultra-High Dose Rate Electron Beam Dosimetry Using Ag Nanoparticle-Enhanced nPAG and NIBMAGAT Gels. Gels, 11(5), 336. https://doi.org/10.3390/gels11050336







