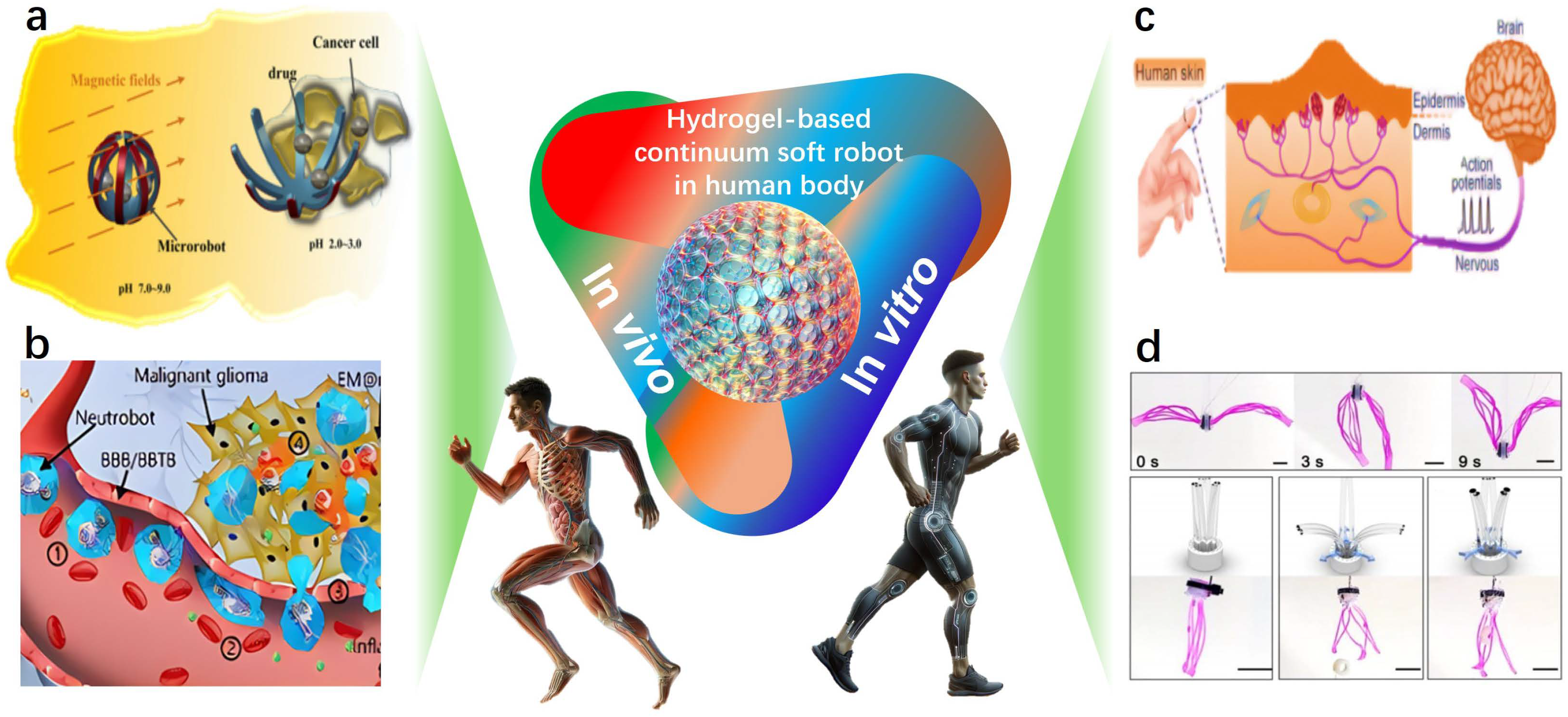
Hydrogel-Based Continuum Soft Robots
Abstract
1. Introduction
2. Applications of Hydrogel-Based Continuum Soft Robots
2.1. Industrial Applications
2.2. Medical Applications
2.3. Agriculture and Other Applications
3. Fabrication of Hydrogel-Based Continuum Soft Robots
3.1. Crosslinking Method
3.2. Additive Manufacturing
| Type | Application | Hydrogel | Ref. |
|---|---|---|---|
| 3D | Bioelectronics interface | Conductive polymer hydrogel | [176] |
| Bioelectronics and tissue engineering | PEDOT:PSS hydrogel | [177] | |
| Biomimetic soft robotics | DIW printed hydrogel | [161] | |
| Electroactive soft robotics | Electroactive hydrogel | [157] | |
| Stretchable electronics | Hydrogel–elastomer composite | [158] | |
| 4D | AI soft robotics | AI-based soft module | [178] |
| Actuator design | Diffusion-path hydrogel actuator | [170] | |
| Soft robotic actuator | Functional 4D-printed hydrogel | [172] | |
| Humidity-responsive robotics | Humidity-driven hydrogel robot | [173] | |
| Biomimetic shape transformation | Multi-responsive hydrogel | [174] | |
| Dual stimuli-responsive robotics | Dual stimuli soft carrier | [175] | |
| Self-healing robotics | Self-healing hydrogel | [179] | |
| Microfluidic | Soft robot actuation | Composite hydrogel fiber | [180] |
| Soft robot grasping | Microfluidic micro-gripper | [181] | |
| Intelligent agent integration | PAAm hydrogel array | [182] | |
| Integrated control | Light-responsive hydrogel | [183] |
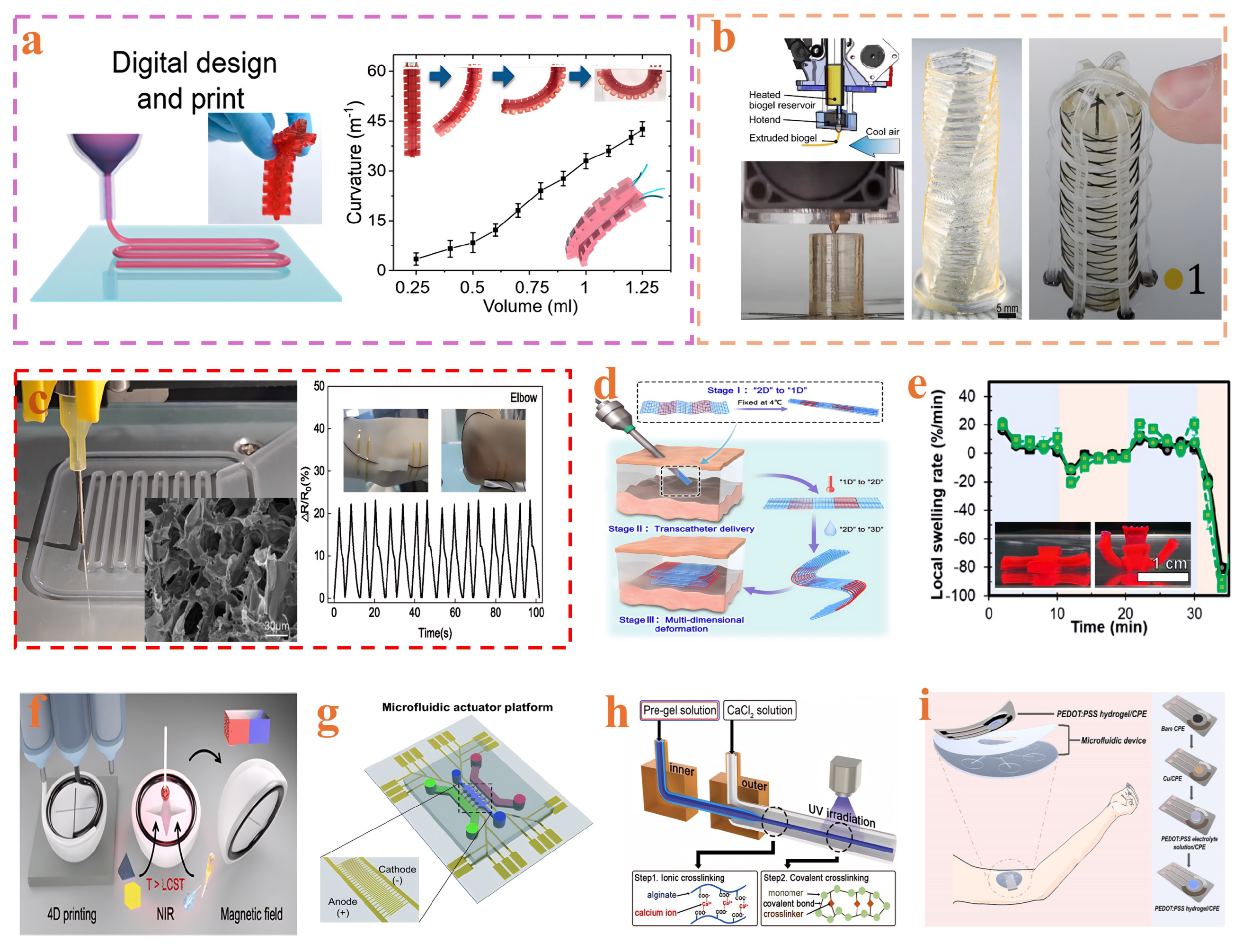
3.3. Microfluidic Fabrication
4. Hydrogel-Based Actuators
4.1. Solute or Solvent Responsive Actuators
4.2. pH Responsive Actuator
4.3. Chemical Reaction Actuator
4.4. Temperature Responsive Actuators
4.5. Light-Responsive Actuators
4.6. Magnetically Responsive Actuators
4.7. Electrically Responsive Actuators
4.8. Hydraulic or Electro-Osmotic Actuators
4.9. Humidity Responsive Actuators
4.10. Multifunctional Actuator
4.11. Perspectives
5. Hydrogel-Based Sensors
5.1. Strain Sensors
5.2. Pressure Sensors
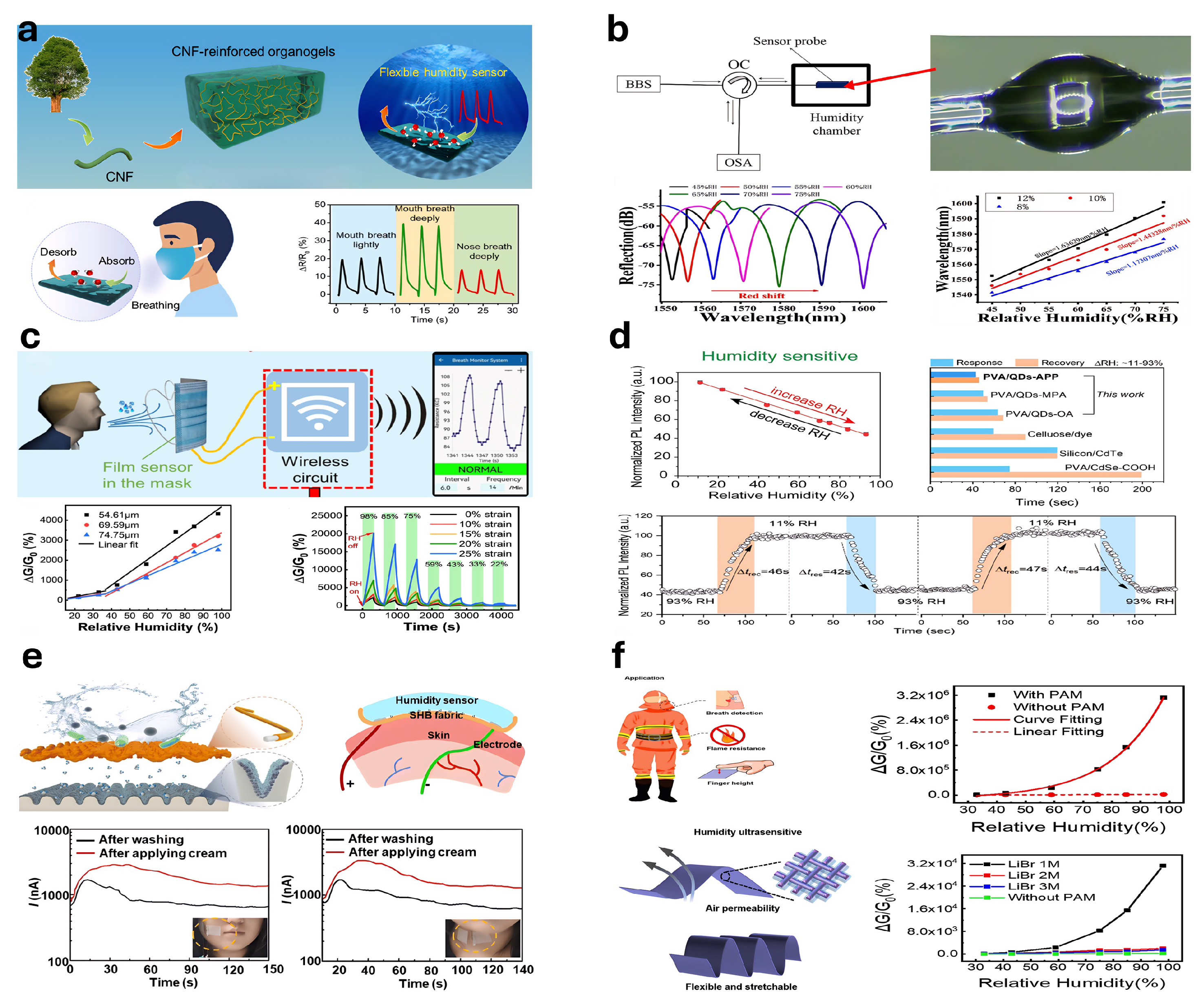
5.3. Humidity-Sensitive Hydrogel-Based Sensors
5.4. Conductive Hydrogel-Based Sensors
5.5. Magnetically Sensitive Hydrogel-Based Sensors
5.6. Thermosensitive Hydrogel-Based Sensors
5.7. Gas-Sensitive Hydrogel-Based Sensors
5.8. Photosensitive Hydrogel-Based Sensors
5.9. Multifunctional Hydrogel-Based Sensors
5.10. Perspectives
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, V.C.; Horn, R.C. Tensor Arm Manipulator. US Patent 3,497,083, 24 February 1970. [Google Scholar]
- Cerrillo, D.; Barrientos, A.; Del Cerro, J. Kinematic Modelling for Hyper-Redundant Robots—A Structured Guide. Mathematics 2022, 10, 2891. [Google Scholar] [CrossRef]
- Davies, J.B.C.; Lane, D.; Robinson, G.; O’Brien, D.; Pickett, M.; Sfakiotakis, M.; Deacon, B. Subsea applications of continuum robots. In Proceedings of the 1998 International Symposium on Underwater Technology, Tokyo, Japan, 15–17 April 1998; pp. 363–369. [Google Scholar] [CrossRef]
- Robinson, G.; Davies, J.B.C. Continuum robots-a state of the art. In Proceedings of the 1999 IEEE International Conference on Robotics and Automation (Cat. No. 99CH36288C), Detroit, Michigan, 10–15 May 1999; Volume 4, pp. 2849–2854. [Google Scholar] [CrossRef]
- Hirose, S.; Umetani, Y. The development of soft gripper for the versatile robot hand. Mech. Mach. Theory 1978, 13, 351–359. [Google Scholar] [CrossRef]
- Rus, D.; Tolley, M.T. Design, fabrication and control of soft robots. Nature 2015, 521, 467–475. [Google Scholar] [CrossRef]
- Walker, J.; Zidek, T.; Harbel, C.; Yoon, S.; Strickland, F.S.; Kumar, S.; Shin, M. Soft robotics: A review of recent developments of pneumatic soft actuators. Actuators 2020, 9, 3. [Google Scholar] [CrossRef]
- Pagoli, A.; Chapelle, F.; Corrales-Ramon, J.A.; Mezouar, Y.; Lapusta, Y. Review of soft fluidic actuators: Classification and materials modeling analysis. Smart Mater. Struct. 2021, 31, 013001. [Google Scholar] [CrossRef]
- O’Halloran, A.; O’Malley, F.; McHugh, P. A review on dielectric elastomer actuators, technology, applications, and challenges. J. Appl. Phys. 2008, 104, 071101. [Google Scholar] [CrossRef]
- Russo, M.; Sadati, S.M.H.; Dong, X.; Mohammad, A.; Walker, I.D.; Bergeles, C.; Xu, K.; Axinte, D.A. Continuum robots: An overview. Adv. Intell. Syst. 2023, 5, 2200367. [Google Scholar] [CrossRef]
- Wang, H.; Du, J.; Mao, Y. Cosserat Rod-Based Tendon Friction Modeling, Simulation, and Experiments for Tendon-Driven Continuum Robots. Micromachines 2025, 16, 346. [Google Scholar] [CrossRef]
- Kim, Y.; Zhao, X. Magnetic soft materials and robots. Chem. Rev. 2022, 122, 5317–5364. [Google Scholar] [CrossRef]
- Wang, H.; Mao, Y.; Du, J. Continuum robots and magnetic soft robots: From models to interdisciplinary challenges for medical applications. Micromachines 2024, 15, 313. [Google Scholar] [CrossRef]
- Campea, M.A.; Majcher, M.J.; Lofts, A.; Hoare, T. A review of design and fabrication methods for nanoparticle network hydrogels for biomedical, environmental, and industrial applications. Adv. Funct. Mater. 2021, 31, 2102355. [Google Scholar] [CrossRef]
- Yuk, H.; Wu, J.; Zhao, X. Hydrogel interfaces for merging humans and machines. Nat. Rev. Mater. 2022, 7, 935–952. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Wei, J.; Yang, Z.; Ren, C.; Li, B. Recent progress of conductive hydrogel fibers for flexible electronics: Fabrications, applications, and perspectives. Adv. Funct. Mater. 2023, 33, 2213485. [Google Scholar] [CrossRef]
- Kim, Y.; Parada, G.A.; Liu, S.; Zhao, X. Ferromagnetic soft continuum robots. Sci. Robot. 2019, 4, eaax7329. [Google Scholar] [CrossRef]
- Cianchetti, M.; Laschi, C.; Menciassi, A.; Dario, P. Biomedical applications of soft robotics. Nat. Rev. Mater. 2018, 3, 143–153. [Google Scholar] [CrossRef]
- Lee, Y.; Song, W.; Sun, J.Y. Hydrogel soft robotics. Mater. Today Phys. 2020, 15, 100258. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Lin, S.; Zhao, X. Hydrogel machines. Mater. Today 2020, 36, 102–124. [Google Scholar] [CrossRef]
- Jiang, J.; Xu, S.; Ma, H.; Li, C.; Huang, Z. Photoresponsive hydrogel-based soft robot: A review. Mater. Today Bio 2023, 20, 100657. [Google Scholar] [CrossRef]
- Wichterle, O.; Lim, D. Hydrophilic gels for biological use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Mehta, P.; Sharma, M.; Devi, M. Hydrogels: An overview of its classifications, properties, and applications. J. Mech. Behav. Biomed. Mater. 2023, 147, 106145. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Zhao, X. Bioadhesive Technology Platforms. Chem. Rev. 2023, 123, 14084–14118. [Google Scholar] [CrossRef]
- Han, L.; Wang, M.; Prieto-López, L.O.; Deng, X.; Cui, J. Self-Hydrophobization in a Dynamic Hydrogel for Creating Nonspecific Repeatable Underwater Adhesion. Adv. Funct. Mater. 2020, 30, 1907064. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef]
- Zhang, K.; Xue, K.; Loh, X.J. Thermo-responsive hydrogels: From recent progress to biomedical applications. Gels 2021, 7, 77. [Google Scholar] [CrossRef]
- Kocak, G.; Tuncer, C.; Bütün, V. pH-Responsive polymers. Polym. Chem. 2017, 8, 144–176. [Google Scholar] [CrossRef]
- Zhu, T.; Ni, Y.; Biesold, G.M.; Cheng, Y.; Ge, M.; Li, H.; Huang, J.; Lin, Z.; Lai, Y. Recent advances in conductive hydrogels: Classifications, properties, and applications. Chem. Soc. Rev. 2023, 52, 473–509. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Chen, C.; Cheng, Y. Magnetic-responsive hydrogels: From strategic design to biomedical applications. J. Control. Release 2021, 335, 541–556. [Google Scholar] [CrossRef]
- Chen, L.; Liu, F.; Abdiryim, T.; Liu, X. Stimuli-responsive hydrogels as promising platforms for soft actuators. Mater. Today Phys. 2023, 40, 101281. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging role of hydrogels in drug delivery systems, tissue engineering and wound management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Bril, M.; Saberi, A.; Jorba, I.; van Turnhout, M.C.; Sahlgren, C.M.; Bouten, C.V.; Schenning, A.P.; Kurniawan, N.A. Shape-Morphing Photoresponsive Hydrogels Reveal Dynamic Topographical Conditioning of Fibroblasts. Adv. Sci. 2023, 10, 2303136. [Google Scholar] [CrossRef]
- Jiang, Z.; Diggle, B.; Shackleford, I.C.; Connal, L.A. Tough, Self-Healing Hydrogels Capable of Ultrafast Shape Changing. Adv. Mater. 2019, 31, 1904956. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Roy, P.; Bonilla-Petriciolet, A.; Badawi, M.; Ganachari, S.V.; Shetti, N.P.; Aminabhavi, T.M. Polymeric hydrogels-based materials for wastewater treatment. Chemosphere 2023, 331, 138743. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.; Zhu, Y.J.; Huang, X.Y.; Xia, X.X.; Qian, Z.G. Programmable adhesion and morphing of protein hydrogels for underwater robots. Nat. Commun. 2024, 15, 195. [Google Scholar] [CrossRef]
- Heiden, A.; Preninger, D.; Lehner, L.; Baumgartner, M.; Drack, M.; Woritzka, E.; Schiller, D.; Gerstmayr, R.; Hartmann, F.; Kaltenbrunner, M. 3D printing of resilient biogels for omnidirectional and exteroceptive soft actuators. Sci. Robot. 2022, 7, eabk2119. [Google Scholar] [CrossRef]
- Morales, D.; Palleau, E.; Dickey, M.; Velev, O. Electro-actuated hydrogel walkers with dual responsive legs. Soft Matter 2014, 10, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wei, H.; Tang, J. Self-sensing magnetic actuators of bilayer hydrogels. Int. J. Smart Nano Mater. 2023, 14, 496–509. [Google Scholar] [CrossRef]
- Yang, J.; Huang, W.; Peng, K.; Cheng, Z.; Lin, L.; Yuan, J.; Sun, Y.; Cho, N.J.; Chen, Y. Versatile Agar-Zwitterion Hybrid Hydrogels for Temperature Self-Sensing and Electro-Responsive Actuation. Adv. Funct. Mater. 2024, 34, 2313725. [Google Scholar]
- Rana, H.; Rana, J.; Sareen, D.; Goswami, S. Value Addition to Agro-Industrial Waste Through Pectin Extraction: Chemometric Categorization, Density Functional Theory Analysis, Rheology Investigation, Optimization Using Response Surface Methodology and Prospective Applications Through Hydrogel Preparation. J. Polym. Environ. 2023, 32, 2965–2987. [Google Scholar] [CrossRef]
- Ghobril, C.; Rodriguez, E.K.; Nazarian, A.; Grinstaff, M.W. Recent advances in dendritic macromonomers for hydrogel formation and their medical applications. Biomacromolecules 2016, 17, 1235–1252. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Wang, S.; Dai, R.; Chen, H.; Shan, Z. Hydrogel beads derived from chrome leather scraps for the preparation of lightweight gypsum. Environ. Technol. Innov. 2022, 25, 102224. [Google Scholar] [CrossRef]
- Vijayaraghavan, R.; Unnikrishnan, G. Preparation of Hydrogel from Agriculture Waste For the Improvement of Soil Irrigation System. J. Solid Waste Technol. Manag. 2022, 48, 208–216. [Google Scholar] [CrossRef]
- Trombino, S.; Sole, R.; Gioia, M.L.D.; Procopio, D.; Curcio, F.; Cassano, R. Green Chemistry Principles for Nano- and Micro-Sized Hydrogel Synthesis. Molecules 2023, 28, 2107. [Google Scholar] [CrossRef]
- Chen, M.; Jiang, J.; Guan, W.; Zhang, Z.; Zhang, X.; Shi, W.; Lin, L.; Zhao, K.; Yu, G. Sustainable and Rapid Water Purification at the Confined Hydrogel Interface. Adv. Mater. 2024, 36, 2311416. [Google Scholar] [CrossRef]
- Yan, L.; Yang, X.; Li, Y.; Song, R.; Lin, Y.; Huang, Q.; Shao, L. Acid-resistant supramolecular nanofibrous hydrogel membrane with core-shell structure for highly efficient oil/water separation. Journal of Membrane Science 2023, 679, 121705. [Google Scholar] [CrossRef]
- Ko, B.; Jeon, N.; Kim, J.; Kang, H.; Seong, J.; Yun, S.; Badloe, T.; Rho, J. Hydrogels for active photonics. Microsyst. Nanoeng. 2024, 10, 1. [Google Scholar] [CrossRef]
- Flores-Valenzuela, L.E.; González-Fernández, J.V.; Carranza-Oropeza, M.V. Hydrogel applicability for the industrial effluent treatment: A systematic review and bibliometric analysis. Polymers 2023, 15, 2417. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y.; Xia, S.; Gao, G. An environment-stable hydrogel with skin-matchable performance for human-machine interface. Sci.-China-Mater. 2021, 64, 2313–2324. [Google Scholar]
- Guan, X.; Zheng, S.; Zhang, B.; Sun, X.; Meng, K.; Elafify, M.S.; Zhu, Y.; El-Gowily, A.H.; An, M.; Li, D.; et al. Masking Strategy Constructed Metal Coordination Hydrogels with Improved Mechanical Properties for Flexible Electronic Sensors. ACS Appl. Mater. Interfaces 2024, 16, 5168–5182. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Guillomaitre, N.; Christie, K.S.S.; Bay, R.; Bizmark, N.; Datta, S.; Ren, Z.; Priestley, R.D. Quick-Release Antifouling Hydrogels for Solar-Driven Water Purification. ACS Cent. Sci. 2023, 9, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, Y.; Qu, X.; Shi, B.; Zheng, Q.; Lin, X.; Chao, S.; Wang, C.; Zhou, J.; Sun, Y.; et al. Ultra-stretchable and fast self-healing ionic hydrogel in cryogenic environments for artificial nerve fiber. Adv. Mater. 2022, 34, 2105416. [Google Scholar] [CrossRef]
- Yu, F.; Yang, P.; Yang, Z.; Zhang, X.; Ma, J. Double-network hydrogel adsorbents for environmental applications. Chem. Eng. J. 2021, 426, 131900. [Google Scholar] [CrossRef]
- Liu, H.; Chu, H.; Yuan, H.; Li, D.; Deng, W.; Fu, Z.; Liu, R.; Liu, Y.; Han, Y.; Wang, Y.; et al. Bioinspired Multifunctional Self-Sensing Actuated Gradient Hydrogel for Soft-Hard Robot Remote Interaction. Nano-Micro Lett. 2024, 16, 69. [Google Scholar] [CrossRef]
- Hsiao, L.; Jing, L.; Li, K.; Yang, H.; Li, Y.; Chen, P. Carbon nanotube-integrated conductive hydrogels as multifunctional robotic skin. Carbon 2020, 161, 784–793. [Google Scholar] [CrossRef]
- Banerjee, H.; Sivaperuman Kalairaj, M.; Chang, T.H.; Fu, F.; Chen, P.Y.; Ren, H. Highly Stretchable Flame-Retardant Skin for Soft Robotics with Hydrogel–Montmorillonite-Based Translucent Matrix. Soft Robot. 2022, 9, 98–118. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Wang, B.; Zhu, H.; Wu, S.; Xu, X.; Li, X.; Cao, Y. Security-Enhanced 3D Data Encryption Using a Degradable pH-Responsive Hydrogel. Nanomaterials 2021, 11, 1744. [Google Scholar] [CrossRef]
- Wen, H.; Zeng, X.; Xu, X.; Li, W.; Xie, F.; Xiong, Z.; Song, S.; Wang, B.; Li, X.; Cao, Y. Reversible data encryption–decryption using a pH stimuli-responsive hydrogel. J. Mater. Chem. C 2021, 9, 2455–2463. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.; Zhu, H.; Qiu, H.; Li, S.; Yang, K.; Xu, J. Antibacterial self-fused supramolecular polymer hydrogel for infected wound healing. Mater. Res. Express 2022, 9, 035401. [Google Scholar] [CrossRef]
- Pham, D.T.; Thuy, N.T.N.; Thao, N.T.P.; Nhi, L.T.; Thuy, B.T.P. Naturally derived hydrogels for wound healing. Ther. Deliv. 2025, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sheikh-Oleslami, S.; Tao, B.; D’Souza, J.; Butt, F.; Suntharalingam, H.; Rempel, L.; Amiri, N. A Review of Metal Nanoparticles Embedded in Hydrogel Scaffolds for Wound Healing In Vivo. Gels 2023, 9, 591. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, J.; Li, D.; Cheng, F. The Recent Progress of the Cellulose-Based Antibacterial Hydrogel. Gels 2024, 10, 109. [Google Scholar] [CrossRef]
- Clegg, J.R.; Adebowale, K.; Zhao, Z.; Mitragotri, S. Hydrogels in the clinic: An update. Bioeng. Transl. Med. 2024, 9, e10680. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Lin, Y.K.; Lin, Y.T.; Lin, C.W.; Lan, G.Y.; Su, Y.C.; Hu, F.R.; Chang, K.H.; Chen, V.; Yeh, Y.C.; et al. Injectable, Antioxidative, and Tissue-Adhesive Nanocomposite Hydrogel as a Potential Treatment for Inner Retina Injuries. Adv. Sci. 2024, 11, 2308635. [Google Scholar] [CrossRef]
- Xu, R.; You, Y.; Zheng, W.; Ma, L.; Chang, Y.; Pan, S.; He, Y.; Zhou, M.; Xu, Z.; Chen, T.; et al. Selenoprotein-Regulated Hydrogel for Ultrasound-Controlled Microenvironment Remodeling to Promote Bone Defect Repair. Adv. Funct. Mater. 2024, 34, 2313122. [Google Scholar] [CrossRef]
- Li, H.; Go, G.; Ko, S.; Park, J.O.; Park, S. Magnetic actuated pH-responsive hydrogel-based soft micro-robot for targeted drug delivery. Smart Mater. Struct. 2016, 25, 027001. [Google Scholar] [CrossRef]
- Wei, T.; Zhao, R.; Fang, L.; Li, Z.; Yang, M.; Zhan, Z.; Cheang, U.K.; Hu, C. Encoded Magnetization for Programmable Soft Miniature Machines by Covalent Assembly of Modularly Coupled Microgels. Adv. Funct. Mater. 2023, 34, 2311908. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Gao, C.; Fan, X.; Pang, Y.; Li, T.; Wu, Z.; Xie, H.; He, Q. Dual-responsive biohybrid neutrobots for active target delivery. Sci. Robot. 2021, 6, eaaz9519. [Google Scholar] [CrossRef]
- Liu, D.; Cao, Y.; Jiang, P.; Wang, Y.; Lu, Y.; Ji, Z.; Wang, X.; Liu, W. Tough, transparent, and slippery PVA hydrogel led by syneresis. Small 2023, 19, 2206819. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, Z.; Cao, S.; Liu, D.; Zhang, Y.; Chen, C.; Jiang, Z.; Ma, J.; Wang, Y. Connective tissue inspired elastomer-based hydrogel for artificial skin via radiation-indued penetrating polymerization. Nat. Commun. 2024, 15, 636. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tian, C.; Zhang, H.; Xie, H. Biodegradable Magnetic Hydrogel Robot with Multimodal Locomotion for Targeted Cargo Delivery. ACS Appl. Mater. Interfaces 2023, 15, 28922–28932. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, X.; Zhang, W.; Wang, M.; Yan, L.; Wang, K.; Han, L.; Lu, X. Infant skin friendly adhesive hydrogel patch activated at body temperature for bioelectronics securing and diabetic wound healing. ACS Nano 2022, 16, 8662–8676. [Google Scholar] [CrossRef]
- Ying, B.; Nan, K.; Zhu, Q.; Khuu, T.; Ro, H.; Qin, S.; Wang, S.; Jiang, K.; Chen, Y.; Bao, G.; et al. Electroadhesive hydrogel interface for prolonged mucosal theranostics. bioRxiv 2023. [Google Scholar] [CrossRef]
- Michalicha, A.; Tomaszewska, A.; Vivcharenko, V.; Budzyńska, B.; Kulpa-Greszta, M.; Fila, D.; Pązik, R.; Belcarz, A. Poly(levodopa)-Functionalized Polysaccharide Hydrogel Enriched in Fe3O4 Particles for Multiple-Purpose Biomedical Applications. Int. J. Mol. Sci. 2023, 24, 8002. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, S.; Lu, H.; Ying, J.Y. Acid-resistant and physiological pH-responsive DNA hydrogel composed of A-motif and i-motif toward oral insulin delivery. J. Am. Chem. Soc. 2022, 144, 5461–5470. [Google Scholar] [CrossRef]
- Wu, J.; Pan, Z.; Zhao, Z.Y.; Wang, M.H.; Dong, L.; Gao, H.L.; Liu, C.Y.; Zhou, P.; Chen, L.; Shi, C.J.; et al. Anti-Swelling, Robust, and Adhesive Extracellular Matrix-Mimicking Hydrogel Used as Intraoral Dressing. Adv. Mater. 2022, 34, 2200115. [Google Scholar] [CrossRef]
- Bian, S.; Hao, L.; Qiu, X.; Wu, J.; Chang, H.; Kuang, G.M.; Zhang, S.; Hu, X.; Dai, Y.; Zhou, Z.; et al. An injectable rapid-adhesion and anti-swelling adhesive hydrogel for hemostasis and wound sealing. Adv. Funct. Mater. 2022, 32, 2207741. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, S.; Zhao, Y.; Zhong, Z.; Zuo, L. Discriminating Soft Actuators’ Thermal Stimuli and Mechanical Deformation by Hydrogel Sensors and Machine Learning. Adv. Intell. Syst. 2022, 4, 2200089. [Google Scholar] [CrossRef]
- Yan, H.; Wang, Y.; Shen, W.; Li, F.; Gao, G.; Zheng, T.; Xu, Z.; Qian, S.; Chen, C.Y.; Zhang, C.; et al. Cable-Driven Continuum Robot Perception Using Skin-Like Hydrogel Sensors. Adv. Funct. Mater. 2022, 32, 2203241. [Google Scholar] [CrossRef]
- Liu, W.; Gao, R.; Yang, C.; Feng, Z.; Ou-Yang, W.; Pan, X.; Huang, P.; Zhang, C.; Kong, D.; Wang, W. ECM-mimetic immunomodulatory hydrogel for methicillin-resistant Staphylococcus aureus–infected chronic skin wound healing. Sci. Adv. 2022, 8, eabn7006. [Google Scholar] [CrossRef]
- Alonci, G.; Mocchi, R.; Sommatis, S.; Capillo, M.C.; Liga, E.; Janowska, A.; Nachbaur, L.; Zerbinati, N. Physico-Chemical Characterization and In Vitro Biological Evaluation of a Bionic Hydrogel Based on Hyaluronic Acid and l-Lysine for Medical Applications. Pharmaceutics 2021, 13, 1194. [Google Scholar] [CrossRef]
- Baumgartner, M.; Hartmann, F.; Drack, M.; Preninger, D.; Wirthl, D.; Gerstmayr, R.; Lehner, L.; Mao, G.; Pruckner, R.; Demchyshyn, S.; et al. Resilient yet entirely degradable gelatin-based biogels for soft robots and electronics. Nat. Mater. 2020, 19, 1102–1109. [Google Scholar] [CrossRef]
- Yu, Y.; Feng, Y.; Liu, F.; Wang, H.; Yu, H.; Dai, K.; Zheng, G.; Feng, W. Carbon Dots-Based Ultrastretchable and Conductive Hydrogels for High-Performance Tactile Sensors and Self-Powered Electronic Skin. Small 2023, 19, 2204365. [Google Scholar] [CrossRef]
- Qu, J.; Yuan, Q.; Li, Z.; Wang, Z.; Xu, F.; Fan, Q.; Zhang, M.; Qian, X.; Wang, X.; Wang, X.; et al. All-in-one strain-triboelectric sensors based on environment-friendly ionic hydrogel for wearable sensing and underwater soft robotic grasping. Nano Energy 2023, 111, 108387. [Google Scholar] [CrossRef]
- Ying, B.; Chen, R.Z.; Zuo, R.; Li, J.; Liu, X. An anti-freezing, ambient-stable and highly stretchable ionic skin with strong surface adhesion for wearable sensing and soft robotics. Adv. Funct. Mater. 2021, 31, 2104665. [Google Scholar] [CrossRef]
- Jing, L.; Hsiao, L.Y.; Li, S.; Yang, H.; Ng, P.L.P.; Ding, M.; Van Truong, T.; Gao, S.P.; Li, K.; Guo, Y.X.; et al. 2D-Material-integrated hydrogels as multifunctional protective skins for soft robots. Mater. Horiz. 2021, 8, 2065–2078. [Google Scholar] [CrossRef]
- Duan, X.J.; Yu, J.; Zhu, Y.; Zheng, Z.; Liao, Q.; Xiao, Y.; Li, Y.; He, Z.; Zhao, Y.; Wang, H.; et al. Large-Scale Spinning Approach to Engineering Knittable Hydrogel Fiber for Soft Robots. ACS Nano 2020, 14, 14929–14938. [Google Scholar] [CrossRef]
- Anuar, W.A.N.W.; Ramli, R.A.; Mahmoud, M.M.E.S.; Warkar, S.G. Recent study on biodegradable hydrogels for agriculture application: A review. J. Environ. Chem. Eng. 2025, 13, 115679. [Google Scholar] [CrossRef]
- Liu, S.; Rahman, M.R.; Wu, H.; Qin, W.; Wang, Y.; Su, G. Development and application of hydrogels in pathogenic bacteria detection in foods. J. Mater. Chem. B 2025, 13, 1229–1251. [Google Scholar] [CrossRef]
- Zhao, M.; Han, P.; Mu, H.; Sun, S.; Dong, J.; Sun, J.; Lu, S.; Wang, Q.; Ji, H. Food packaging films from natural polysaccharides and protein hydrogels: A comprehensive review. Food Chem. X 2025, 25, 102174. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Chen, H.; Cheng, D. Environmentally friendly hydrogel: A review of classification, preparation and application in agriculture. Sci. Total Environ. 2022, 846, 157303. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Ma, Y.; Lu, Y.; Su, C.; Liu, X.; Li, H.; Lu, G.; Sun, P. Zeolitic Imidazolate-Framework-Engineered Heterointerface Catalysis for the Construction of Plant-Wearable Sensors. Adv. Mater. 2024, 36, 2311144. [Google Scholar] [CrossRef]
- Palanivelu, S.D.; Armir, N.A.Z.; Zulkifli, A.; Hair, A.H.A.; Salleh, K.M.; Lindsey, K.; Che-Othman, M.H.; Zakaria, S. Hydrogel application in urban farming: Potentials and limitations—A review. Polymers 2022, 14, 2590. [Google Scholar] [CrossRef]
- Li, S.; Hernandez, S.; Salazar, N.A. Biopolymer-Based Hydrogels for Harvesting Water from Humid Air: A Review. Sustainability 2023, 15, 848. [Google Scholar] [CrossRef]
- Kaur, P.; Agrawal, R.; Pfeffer, F.M.; Williams, R.; Bohidar, H.B. Hydrogels in Agriculture: Prospects and Challenges. J. Polym. Environ. 2023, 31, 3701–3718. [Google Scholar] [CrossRef]
- Xu, C.; Cao, L.; Cao, C.; Chen, H.; Zhang, H.; Li, Y.; Huang, Q. Fungicide itself as a trigger to facilely construct Hymexazol-Encapsulated polysaccharide supramolecular hydrogels with controllable rheological properties and reduced environmental risks. Chem. Eng. J. 2023, 452, 139195. [Google Scholar] [CrossRef]
- Adjuik, T.A.; Nokes, S.; Montross, M.; Wendroth, O.; Walton, R. Alkali Lignin-Based Hydrogel: Synthesis, Characterization, and Impact on Soil Water Retention From Near Saturation to Dryness. J. ASABE 2023, 66, 85–98. [Google Scholar] [CrossRef]
- Tamer, Y.B. A New Design of Poly(N-Isopropylacrylamide) Hydrogels Using Biodegradable Poly(Beta-Aminoester) Crosslinkers as Fertilizer Reservoirs for Agricultural Applications. Gels 2023, 9, 127. [Google Scholar] [CrossRef]
- Qu, B.; Luo, Y. Chitosan-based hydrogel beads: Preparations, modifications and applications in food and agriculture sectors–A review. Int. J. Biol. Macromol. 2020, 152, 437–448. [Google Scholar] [CrossRef]
- Vieira, A.L.S.; de Sousa, R.C.S.; da Veiga Correia, V.T.; Queiroz, V.A.V.; da Silva, W.A.; Garcia, M.A.V.T.; de Araújo, R.L.B. Starch-based hydrogels: Current status and applications in food. Contrib. Las Cienc. Soc. 2023, 16, 28707–28727. [Google Scholar] [CrossRef]
- Li, Y.; Yang, S.; Qu, C.; Ding, Z.; Liu, H. Hydrogel-based surface-enhanced Raman spectroscopy for food contaminant detection: A review on classification, strategies, and applications. Food Saf. Health 2023, 1, 110–125. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Qi, Y.; You, C. Recent Studies and Applications of Hydrogel-Based Biosensors in Food Safety. Foods 2023, 12, 4405. [Google Scholar] [CrossRef]
- Hadi, A.; Nawab, A.; Alam, F.; Naqvi, S. Development of sodium alginate–aloe vera hydrogel films enriched with organic fibers: Study of the physical, mechanical, and barrier properties for food-packaging applications. Sustain. Food Technol. 2023, 1, 863–873. [Google Scholar] [CrossRef]
- Acevedo-Puello, V.; Figueroa-López, K.J.; Ortega-Toro, R. Gelatin-based hydrogels containing microcrystalline and nanocrystalline cellulose as moisture absorbers for food packaging applications. J. Compos. Sci. 2023, 7, 337. [Google Scholar] [CrossRef]
- Zafar, A.; Khosa, M.K.; Noor, A.; Qayyum, S.; Saif, M.J. Carboxymethyl Cellulose/Gelatin hydrogel films loaded with zinc oxide nanoparticles for sustainable food packaging applications. Polymers 2022, 14, 5201. [Google Scholar] [CrossRef]
- Cui, D.; Huang, S.; Cheng, H.; Zheng, H.; Zhu, W.; Yu, J.; Zhong, Y.; Chen, Z. High-sensitivity humidity sensing of a U-shaped microfiber coated with porous methacryloxyethyl trimethyl ammonium chloride film. Appl. Opt. 2023, 62, 6106–6112. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Sobczak, M. Hydrogel-based active substance release systems for cosmetology and dermatology application: A review. Pharmaceutics 2020, 12, 396. [Google Scholar] [CrossRef]
- Ha, N.G.; Kim, S.L.; Lee, S.H.; Lee, W.J. A novel hydrogel-based moisturizing cream composed of hyaluronic acid for patients with xerosis: An intraindividual comparative analysis. Skin Res. Technol. 2023, 29, e13499. [Google Scholar] [CrossRef]
- Yu, H.M.; Yu, X.Y.; Chen, Y.; Liu, Y. Fullerene-polysaccharide supramolecular hydrogel displaying antioxidation/antiglycation behavior. Soft Matter 2023, 19, 3162–3166. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, M.; Cao, Y.; Yin, J.; Zhou, H.; Yu, W.; Liu, H.; Wang, J.; Huang, C.; Ma, P.; et al. Hydrogel sunscreen based on yeast/gelatin demonstrates excellent UV-shielding and skin protection performance. Colloids Surfaces B Biointerfaces 2021, 205, 111885. [Google Scholar] [CrossRef]
- Ishwarya S, P.; Nisha, P. Advances and prospects in the food applications of pectin hydrogels. Crit. Rev. Food Sci. Nutr. 2022, 62, 4393–4417. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, X.; Yin, L. Hydrogel: Diversity of structures and applications in food science. Food Rev. Int. 2021, 37, 313–372. [Google Scholar] [CrossRef]
- Yu, S.; Huang, Y.; Shen, B.; Zhang, W.; Xie, Y.; Gao, Q.; Zhao, D.; Wu, Z.; Liu, Y. Peptide hydrogels: Synthesis, properties, and applications in food science. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3053–3083. [Google Scholar] [CrossRef]
- Mitura, S.; Sionkowska, A.; Jaiswal, A. Biopolymers for hydrogels in cosmetics. J. Mater. Sci. Mater. Med. 2020, 31, 50. [Google Scholar] [CrossRef] [PubMed]
- Rashid, F.; Childs, S.; Dodou, K. Comparison of Analytical Methods for the Detection of Residual Crosslinker in Hyaluronic Acid Hydrogel Films. Cosmetics 2023, 10, 70. [Google Scholar] [CrossRef]
- Ioannou, E.; Labrou, N.E. Development of Enzyme-Based Cosmeceuticals: Studies on the Proteolytic Activity of Arthrospira Platensis and Its Efficient Incorporation in a Hydrogel Formulation. Cosmetics 2022, 9, 106. [Google Scholar] [CrossRef]
- Zahra, A.; Lim, S.K.; Shin, S.J.; Yeon, I.J. Properties of Green Tea Waste as Cosmetics Ingredients and Rheology Enhancers. Appl. Sci. 2022, 12, 12871. [Google Scholar] [CrossRef]
- Wang, B.X.; Xu, W.; Yang, Z.; Wu, Y.; Pi, F. An overview on recent progress of the hydrogels: From material resources, properties, to functional applications. Macromol. Rapid Commun. 2022, 43, 2100785. [Google Scholar] [CrossRef]
- Dodda, J.M.; Azar, M.G.; Sadiku, R. Crosslinking trends in multicomponent hydrogels for biomedical applications. Macromol. Biosci. 2021, 21, 2100232. [Google Scholar] [CrossRef]
- Huang, X.; Li, J.; Luo, J.; Gao, Q.; Mao, A.; Li, J. Research progress on double-network hydrogels. Mater. Today Commun. 2021, 29, 102757. [Google Scholar] [CrossRef]
- Nasution, H.; Harahap, H.; Dalimunthe, N.F.; Ginting, M.; Jaafar, M.; Tan, O.O.H.; Aruan, H.K.; Herfananda, A.L. Hydrogel and Effects of Crosslinking Agent on Cellulose-Based Hydrogels: A Review. Gels 2022, 8, 568. [Google Scholar] [CrossRef]
- Gao, Y.; Peng, K.; Mitragotri, S. Covalently Crosslinked hydrogels via step-growth reactions: Crosslinking chemistries, polymers, and clinical impact. Adv. Mater. 2021, 33, 2006362. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Peng, Y.; Zhang, S.; Zhang, Y.; Min, P. Effects and Progress of Photo-Crosslinking Hydrogels in Wound Healing Improvement. Gels 2022, 8, 609. [Google Scholar] [CrossRef] [PubMed]
- Stubbe, B.; Mignon, A.; Van Damme, L.; Claes, K.; Hoeksema, H.; Monstrey, S.; Van Vlierberghe, S.; Dubruel, P. Photo-crosslinked gelatin-based hydrogel films to support wound healing. Macromol. Biosci. 2021, 21, 2100246. [Google Scholar] [CrossRef]
- Haas, S.; Hubbuch, J. Mechanical Properties of Protein-Based Hydrogels Derived from Binary Protein Mixtures—A Feasibility Study. Polymers 2023, 15, 964. [Google Scholar] [CrossRef]
- Nelson, B.R.; Kirkpatrick, B.E.; Miksch, C.; Davidson, M.D.; Skillin, N.P.; Hach, G.K.; Khang, A.; Hummel, S.N.; Fairbanks, B.D.; Burdick, J.; et al. Photoinduced Dithiolane Crosslinking for Multiresponsive Dynamic Hydrogels. Adv. Mater. 2023, 36, e2211209. [Google Scholar] [CrossRef]
- Rusu, A.; Nita, L.; Simionescu, N.; Ghilan, A.; Chiriac, A.; Mititelu-Tarțău, L. Enzymatically-Crosslinked Gelatin Hydrogels with Nanostructured Architecture and Self-Healing Performance for Potential Use as Wound Dressings. Polymers 2023, 15, 780. [Google Scholar] [CrossRef]
- Singh, M.; Zhang, J.; Bethel, K.; Liu, Y.; Davis, E.M.; Zeng, H.; Kong, Z.; Johnson, B.N. Closed-loop controlled photopolymerization of hydrogels. ACS Appl. Mater. Interfaces 2021, 13, 40365–40378. [Google Scholar] [CrossRef]
- Tang, S.; Yang, J.; Lin, L.; Peng, K.; Chen, Y.; Jin, S.; Yao, W. Construction of physically crosslinked chitosan/sodium alginate/calcium ion double-network hydrogel and its application to heavy metal ions removal. Chem. Eng. J. 2020, 393, 124728. [Google Scholar] [CrossRef]
- Yu, H.; Xiao, Q.; Qi, G.; Chen, F.; Tu, B.; Zhang, S.; Li, Y.; Chen, Y.; Yu, H.; Duan, P. A Hydrogen Bonds-Crosslinked Hydrogels With Self-Healing and Adhesive Properties for Hemostatic. Front. Bioeng. Biotechnol. 2022, 10, 855013. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Gao, S.; Guo, Q.; Wang, C.; Qiao, Y.; Qiu, D. A solvent-exchange strategy to regulate noncovalent interactions for strong and antiswelling hydrogels. Adv. Mater. 2020, 32, 2004579. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zou, S.; Hang, C.; Li, J.; Di, X.; Li, X.; Wu, Q.; Wang, F.; Sun, P. Mechanically strong and tough hydrogels with pH-triggered self-healing and shape memory properties based on a dual physically crosslinked network. Polym. Chem. 2020, 11, 1906–1918. [Google Scholar] [CrossRef]
- Li, B.; Han, Y.; Zhang, Y.; Cao, X.; Luo, Z. Dual physically crosslinked nanocomposite hydrogels reinforced by poly(N-vinylpyrrolidone) grafted cellulose nanocrystal with high strength, toughness, and rapid self-recovery. Cellulose 2020, 27, 9913–9925. [Google Scholar] [CrossRef]
- Chen, C.; Duan, N.; Chen, S.; Guo, Z.; Hu, J.; Guo, J.; Chen, Z.; Yang, L. Synthesis mechanical properties and self-healing behavior of aliphatic polycarbonate hydrogels based on cooperation hydrogen bonds. J. Mol. Liq. 2020, 319, 114134. [Google Scholar] [CrossRef]
- Liu, S.; Xu, L.; Yuan, Z.; Huang, M.; Yang, T.; Chen, S. 3D Interlayer Slidable Multilayer Nano-Graphene Oxide Acrylate Crosslinked Tough Hydrogel. Langmuir ACS J. Surfaces Colloids 2022, 38, 8200–8210. [Google Scholar] [CrossRef]
- Wei, S.; Chen, T.H.; Kao, F.S.; Hsu, Y.; Chen, Y.C. Strategy for improving cell-mediated vascularized soft tissue formation in a hydrogen peroxide-triggered chemically-crosslinked hydrogel. J. Tissue Eng. 2022, 13, 20417314221084096. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Li, C.; Sun, J.; Zhang, X.; Zhou, M.; Deng, Y.; Xiao, A. GelMA/κ-carrageenan double-network hydrogels with superior mechanics and biocompatibility. RSC Adv. 2023, 13, 1558–1566. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, Q.; Li, Q.; Li, X. Fabrication of Silk Hydrogel Scaffolds with Aligned Porous Structures and Tunable Mechanical Properties. Gels 2023, 9, 181. [Google Scholar] [CrossRef]
- Chen, Q.; Tian, X.; Fan, J.; Tong, H.; Ao, Q.; Wang, X. An Interpenetrating Alginate/Gelatin Network for Three-Dimensional (3D) Cell Cultures and Organ Bioprinting. Molecules 2020, 25, 756. [Google Scholar] [CrossRef]
- Kong, W.; Gao, Y.; Liu, Q.; Dong, L.; Guo, L.; Fan, H.; Fan, Y.; Zhang, X. The effects of crosslinking manners on the physical properties and biocompatibility of collagen type I/hyaluronic acid composite hydrogels. Int. J. Biol. Macromol. 2020, 160, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dong, H.; Jing, X.; Wang, X.; Shi, Y.; He, C.; Ma, B.; Nie, J.; Zhang, J.; Ma, G. Injectable In Situ Photocrosslinked Hydrogel Dressing for Infected Wound Healing. ACS Appl. Bio Mater. 2023, 6, 1992–2002. [Google Scholar] [CrossRef]
- Gholami, M.; Tajabadi, M.; Khavandi, A.; Azarpira, N. Synthesis, optimization, and cell response investigations of natural-based, thermoresponsive, injectable hydrogel: An attitude for 3D hepatocyte encapsulation and cell therapy. Front. Bioeng. Biotechnol. 2023, 10, 1075166. [Google Scholar] [CrossRef]
- Rizwan, M.; Baker, A.E.; Shoichet, M.S. Designing hydrogels for 3D cell culture using dynamic covalent crosslinking. Adv. Healthc. Mater. 2021, 10, 2100234. [Google Scholar] [CrossRef]
- Jaramillo-Quiceno, N.; Rueda-Mira, S.; Marín, J.F.S.; Álvarez-López, C. Development of a novel silk sericin-based hydrogel film by mixture design. J. Polym. Res. 2023, 30, 120. [Google Scholar] [CrossRef]
- Salma-Ancane, K.; Sceglovs, A.; Tracuma, E.; Wychowaniec, J.K.; Aunina, K.; Ramata-Stunda, A.; Nikolajeva, V.; Loca, D. Effect of crosslinking strategy on the biological, antibacterial and physicochemical performance of hyaluronic acid and ε-polylysine based hydrogels. Int. J. Biol. Macromol. 2022, 208, 995–1008. [Google Scholar] [CrossRef]
- Erikci, S. Environementally Sensitive Hyaluronan Hydrogel Adjustable by Physical and Chemical Cross-Links for Biomedical Applications. Ph.D. Thesis, Heidelberg University, Heidelberg, Germany, 2020; pp. 1–151. [Google Scholar] [CrossRef]
- Xu, S.; Wencheng, L.; Xu, G.; Huang, C.; Zhang, J.; Lang, M. A fast and dual crosslinking hydrogel based on vinyl ether sodium alginate. Appl. Surf. Sci. 2020, 515, 145811. [Google Scholar] [CrossRef]
- Kędzierska, M.; Jamroży, M.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Bańkosz, M.; Gruca, M.; Potemski, P.; Tyliszczak, B. Analysis of the Influence of Both the Average Molecular Weight and the Content of Crosslinking Agent on Physicochemical Properties of PVP-Based Hydrogels Developed as Innovative Dressings. Int. J. Mol. Sci. 2022, 23, 11618. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Zeng, Q.; Li, K.; Wen, J.; Zhang, Y.; Zheng, Y.; Zhou, R.; Shi, C.; Chen, T.; Xiao, C.; et al. Rapid fabrication of physically robust hydrogels. Nat. Mater. 2023, 22, 1253–1260. [Google Scholar] [CrossRef]
- Debertrand, L.; Zhao, J.; Creton, C.; Narita, T. Swelling and Mechanical Properties of Polyacrylamide-Derivative Dual-Crosslink Hydrogels Having Metal–Ligand Coordination Bonds as Transient Crosslinks. Gels 2021, 7, 72. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Y.; Chen, X.; He, Q.; Wu, T.; Cao, X.; Liu, J.; You, X. Hydrogel-Crosslinked Microneedles Based on Microwave-Assisted Drying Method. Adv. Polym. Technol. 2022, 2022, 2220918. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Singhmar, R.; Garg, M.; Gupta, D.; Dhiman, A.; Han, S.S.; Agrawal, G. Designing Advanced Hydrogel Inks with Direct Ink Writing based 3D Printability for Engineered Biostructures. Eur. Polym. J. 2024, 205, 112736. [Google Scholar] [CrossRef]
- Han, D.; Farino, C.; Yang, C.; Scott, T.; Browe, D.; Choi, W.; Freeman, J.W.; Lee, H. Soft robotic manipulation and locomotion with a 3D printed electroactive hydrogel. ACS Appl. Mater. Interfaces 2018, 10, 17512–17518. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Hu, X.; Liu, B.; Chen, Z.; Qu, S. 3D printing of conductive hydrogel–elastomer hybrids for stretchable electronics. ACS Appl. Mater. Interfaces 2021, 13, 59243–59251. [Google Scholar] [CrossRef]
- Zhang, L.; Du, H.; Sun, X.; Cheng, F.; Lee, W.; Li, J.; Dai, G.; Fang, N.X.; Liu, Y. 3D Printing of Interpenetrating Network Flexible Hydrogels with Enhancement of Adhesiveness. ACS Appl. Mater. Interfaces 2023, 15, 41892–41905. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, M.; Roppolo, I.; Chiappone, A.; Larush, L.; Pirri, C.F.; Magdassi, S. 3D-printed self-healing hydrogels via Digital Light Processing. Nat. Commun. 2021, 12, 2462. [Google Scholar] [CrossRef]
- Cheng, Y.; Chan, K.H.; Wang, X.Q.; Ding, T.; Li, T.; Lu, X.; Ho, G.W. Direct-ink-write 3D printing of hydrogels into biomimetic soft robots. ACS Nano 2019, 13, 13176–13184. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, P.; Jannini, A.V.S.; Xiong, Z.; Ransbottom, M.J.; Perkins, J.S.; Henderson, J.H.; Hasenwinkel, J.M.; Soman, P. High-resolution 3D printing of stretchable hydrogel structures using optical projection lithography. ACS Appl. Mater. Interfaces 2019, 12, 1640–1649. [Google Scholar] [CrossRef]
- Champeau, M.; Heinze, D.A.; Viana, T.N.; de Souza, E.R.; Chinellato, A.C.; Titotto, S. 4D printing of hydrogels: A review. Adv. Funct. Mater. 2020, 30, 1910606. [Google Scholar] [CrossRef]
- Sood, S.K.; Lamba, Y.S.; Singh, A.K. Exploring the Shift from 3D Printing to 4D Printing: A Review. IEEE Sens. J. 2025, 25, 9224–9232. [Google Scholar] [CrossRef]
- Waidi, Y.O. Recent Advances in 4D-Printed Shape Memory Actuators. Macromol. Rapid Commun. 2025, 2401141. [Google Scholar] [CrossRef]
- Liu, B.; Li, H.; Meng, F.; Xu, Z.; Hao, L.; Yao, Y.; Zhu, H.; Wang, C.; Wu, J.; Bian, S.; et al. 4D printed hydrogel scaffold with swelling-stiffening properties and programmable deformation for minimally invasive implantation. Nat. Commun. 2024, 15, 1587. [Google Scholar] [CrossRef] [PubMed]
- Solis, D.M.; Czekanski, A. The effect of the printing temperature on 4D DLP printed pNIPAM hydrogels. Soft Matter 2022, 18, 3422–3429. [Google Scholar] [CrossRef] [PubMed]
- Sheikhi, M.; Vakili, S.; Karimi, N.; Rafiemanzelat, F.; Maleki, A.; Taheri, A.; Mohamadnia, Z.; Ramazani, A. 4D Printing of Self-Healing, Thermally, and Near-Infrared Light-Responsive Granular Hydrogels with Segmental Directed Movement for Soft Robotic. ACS Appl. Polym. Mater. 2025, 7, 1717–1728. [Google Scholar] [CrossRef]
- Uchida, T.; Onoe, H. 4D printing of multi-hydrogels using direct ink writing in a supporting viscous liquid. Micromachines 2019, 10, 433. [Google Scholar] [CrossRef]
- Pruksawan, S.; Lin, Z.; Lee, Y.L.; Chee, H.L.; Wang, F. 4D-Printed Hydrogel Actuators through Diffusion-Path Architecture Design. ACS Appl. Mater. Interfaces 2023, 15, 46388–46399. [Google Scholar] [CrossRef]
- Joshi, A.; Choudhury, S.; Baghel, V.S.; Ghosh, S.; Gupta, S.; Lahiri, D.; Ananthasuresh, G.; Chatterjee, K. 4D Printed Programmable Shape-morphing Hydrogels as Intraoperative Self-folding Nerve Conduits for Sutureless Neurorrhaphy. Adv. Healthc. Mater. 2023, 12, 2300701. [Google Scholar] [CrossRef]
- López-Valdeolivas, M.; Liu, D.; Broer, D.J.; Sánchez-Somolinos, C. 4D printed actuators with soft-robotic functions. Macromol. Rapid Commun. 2018, 39, 1700710. [Google Scholar] [CrossRef]
- Cecchini, L.; Mariani, S.; Ronzan, M.; Mondini, A.; Pugno, N.M.; Mazzolai, B. 4D Printing of Humidity-Driven Seed Inspired Soft Robots. Adv. Sci. 2023, 10, 2205146. [Google Scholar] [CrossRef]
- Tran, T.S.; Balu, R.; Mettu, S.; Roy Choudhury, N.; Dutta, N.K. 4D Printing of Hydrogels: Innovation in Material Design and Emerging Smart Systems for Drug Delivery. Pharmaceuticals 2022, 15, 1282. [Google Scholar] [CrossRef]
- Choi, I.; Jang, S.; Jung, S.; Woo, S.; Kim, J.; Bak, C.; Lee, Y.; Park, S. A dual stimuli-responsive smart soft carrier using multi-material 4D printing. Mater. Horiz. 2023, 10, 3668–3679. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yuk, H.; Hu, F.; Wu, J.; Tian, F.; Roh, H.; Shen, Z.; Gu, G.; Xu, J.; Lu, B.; et al. 3D printable high-performance conducting polymer hydrogel for all-hydrogel bioelectronic interfaces. Nat. Mater. 2023, 22, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Garcia, J.; Leahy, L.M.; Song, R.; Mullarkey, D.; Fei, B.; Dervan, A.; Shvets, I.V.; Stamenov, P.; Wang, W.; et al. 3D Printing of Multifunctional Conductive Polymer Composite Hydrogels. Adv. Funct. Mater. 2023, 33, 2214196. [Google Scholar] [CrossRef]
- Zolfagharian, A.; Khosravani, M.R.; Duong Vu, H.; Nguyen, M.K.; Kouzani, A.Z.; Bodaghi, M. AI-based soft module for safe human–robot interaction towards 4D printing. Polymers 2022, 14, 3302. [Google Scholar] [CrossRef]
- Taylor, D.L.; in het Panhuis, M. Self-healing hydrogels. Adv. Mater. 2016, 28, 9060–9093. [Google Scholar]
- Peng, L.; Liu, Y.; Gong, J.; Zhang, K.; Ma, J. Continuous fabrication of multi-stimuli responsive graphene oxide composite hydrogel fibres by microfluidics. RSC Adv. 2017, 7, 19243–19249. [Google Scholar] [CrossRef]
- Geng, Y.; Ge, X.; Zhang, S.; Zhou, Y.; Wang, Z.Q.; Chen, J.; Xu, J. Microfluidic preparation of flexible micro-grippers with precise delivery function. Lab Chip 2018, 18, 1838–1843. [Google Scholar] [CrossRef]
- Jiao, C.H.; Liubimtsev, N.; Zagradska-Paromova, Z.; Appelhans, D.; Gaitzsch, J.; Voit, B. Reversible Molecular Capture and Release in Microfluidics by Host-Guest Interactions in Hydrogel Microdots. Macromol. Rapid Commun. 2023, 44, 2200869. [Google Scholar] [CrossRef]
- Pan, J.; Chen, K.; Zhang, W.; Feng, L.; Zhang, D. Fabrication method and performance of a light-responsive hydrogel microvalve in a microfluidic chip. Soft Science 2022, 2, 4. [Google Scholar] [CrossRef]
- Ha, J.H.; Shin, H.; Choi, H.; Lim, J.; Mo, S.; Ahrberg, C.D.; Lee, J.M.; Chung, B. Electro-responsive hydrogel-based microfluidic actuator platform for photothermal therapy. Lab Chip 2020, 20, 3354–3364. [Google Scholar] [CrossRef]
- Kim, D.; Ahn, S.K.; Yoon, J. Highly stretchable strain sensors comprising double network hydrogels fabricated by microfluidic devices. Adv. Mater. Technol. 2019, 4, 1800739. [Google Scholar] [CrossRef]
- Xu, Z.; Song, J.; Liu, B.; Lv, S.; Gao, F.; Luo, X.; Wang, P. A conducting polymer PEDOT: PSS hydrogel based wearable sensor for accurate uric acid detection in human sweat. Sens. Actuators B Chem. 2021, 348, 130674. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Z.; Li, G.; Cai, Z.; Wu, J.; Wang, L.; Deng, L.; Cai, M.; Cui, W. Injectable Microfluidic Hydrogel Microspheres for Cell and Drug Delivery. Adv. Funct. Mater. 2021, 31, 202103339. [Google Scholar] [CrossRef]
- Chai, N.; Zhang, J.; Zhang, Q.; Du, H.; He, X.; Yang, J.; Zhou, X.; He, J.; He, C. Construction of 3D printed constructs based on microfluidic microgel for bone regeneration. Compos. Part B-Eng. 2021, 223, 109100. [Google Scholar] [CrossRef]
- Sood, A.; Kumar, A.; Dev, A.; Gupta, V.K.; Han, S.S. Advances in hydrogel-based microfluidic blood–brain-barrier models in oncology research. Pharmaceutics 2022, 14, 993. [Google Scholar] [CrossRef]
- Luo, Z.; Dong, Y.; Yu, M.; Fu, X.; Qiu, Y.; Sun, X.; Chu, X. A novel insulin delivery system by β cells encapsulated in microcapsules. Front. Chem. 2023, 10, 1104979. [Google Scholar] [CrossRef]
- Tolabi, H.; Davari, N.; Khajehmohammadi, M.; Malektaj, H.; Nazemi, K.; Vahedi, S.; Ghalandari, B.; Reis, R.; Ghorbani, F.; Oliveira, J. Progress of Microfluidic Hydrogel-based Scaffolds and Organ-on-Chips for the Cartilage Tissue Engineering. Adv. Mater. 2023, 35, e2208852. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, Y.; Xu, N.; Zhang, Z. Cell-loaded hydrogel microspheres based on droplet microfluidics: A review. Sheng Gong Cheng Xue Bao=Chin. J. Biotechnol. 2023, 39, 74–85. [Google Scholar] [CrossRef]
- Gao, R.Z.; Ren, C.L. Synergizing microfluidics with soft robotics: A perspective on miniaturization and future directions. Biomicrofluidics 2021, 15, 011302. [Google Scholar] [CrossRef]
- Chen, Z.; Lv, Z.; Zhang, Z.; Weitz, D.A.; Zhang, H.; Zhang, Y.; Cui, W. Advanced microfluidic devices for fabricating multi-structural hydrogel microsphere. Exploration 2021, 1, 20210036. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, S.; He, C.; Li, C.; Louh, R.F. Enhancing Bone Cement Efficacy with Hydrogel Beads Synthesized by Droplet Microfluidics. Nanomaterials 2024, 14, 302. [Google Scholar] [CrossRef] [PubMed]
- Ridha, I.; Gorenca, P.; Urie, R.; Shanbhag, S.; Rege, K. Repulsion of Polar Gels From Water: Hydration-Triggered Actuation, Self-Folding, and 3D Fabrication. Adv. Mater. Interfaces 2020, 7, 2000509. [Google Scholar] [CrossRef]
- Liang, Y.; Shen, Y.; Liang, H. Solvent-responsive strong hydrogel with programmable deformation and reversible shape memory for load-carrying soft robot. Mater. Today Commun. 2022, 30, 103067. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, H.; Zhou, J.; Zhao, Z.; Huang, J.; Chen, L.; Ru, Y.; Liu, M. High-Performance Organohydrogel Artificial Muscle with Compartmentalized Anisotropic Actuation Under Microdomain Confinement. Adv. Mater. 2023, 35, 2202193. [Google Scholar] [CrossRef]
- Li, G.; Gao, T.; Fan, G.; Liu, Z.; Liu, Z.; Jiang, J.; Zhao, Y. Photoresponsive shape memory hydrogels for complex deformation and solvent-driven actuation. ACS Appl. Mater. Interfaces 2019, 12, 6407–6418. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Z.; Liu, Z.; Jiang, J.; Li, G. Photo-dissociable Fe3+-carboxylate coordination: A general approach toward hydrogels with shape programming and active morphing functionalities. ACS Appl. Mater. Interfaces 2021, 13, 59310–59319. [Google Scholar] [CrossRef]
- Pinto, B.D.S.; Ronsin, O.; Baumberger, T. Syneresis of self-crowded calcium-alginate hydrogels as a self-driven athermal aging process. Soft Matter 2023, 19, 1720–1731. [Google Scholar] [CrossRef]
- Wang, J.; Tang, J.; Lin, Y.; He, H.; Zhao, C.; Ma, W.; Wang, X.; Zeng, M.; Li, S. Construction of organic–inorganic hybrid heterostructure towards solvent responsive hydrogel with high stiffness. Smart Mater. Struct. 2023, 32, 085018. [Google Scholar] [CrossRef]
- Debta, S.; Kumbhar, P.Y.; Ghosh, P.; Annabattula, R.K. Solvent-Responsive Functionally Graded Hydrogel Thin Films for Programmed Actuation. ACS Appl. Eng. Mater. 2024, 2, 1336–1347. [Google Scholar] [CrossRef]
- Parimita, S.; Kumar, A.; Krishnaswamy, H.; Ghosh, P. 4D printing of pH-responsive bilayer with programmable shape-shifting behaviour. Eur. Polym. J. 2025, 222, 113581. [Google Scholar] [CrossRef]
- Yang, L.; Yuan, Q.Y.; Li, T.T.; Lou, C.W.; Hung, C.Y.; Lin, J.H. Recent developments and applications of pH-responsive polymers. Text. Res. J. 2025, 00405175241305543. [Google Scholar] [CrossRef]
- Dai, L.; Ma, M.; Xu, J.; Si, C.; Wang, X.; Liu, Z.; Ni, Y. All-lignin-based hydrogel with fast pH-stimuli responsiveness for mechanical switching and actuation. Chem. Mater. 2020, 32, 4324–4330. [Google Scholar] [CrossRef]
- Han, Z.; Wang, P.; Mao, G.; Yin, T.; Zhong, D.; Yiming, B.; Hu, X.; Jia, Z.; Nian, G.; Qu, S.; et al. Dual pH-responsive hydrogel actuator for lipophilic drug delivery. ACS Appl. Mater. Interfaces 2020, 12, 12010–12017. [Google Scholar] [CrossRef] [PubMed]
- Houben, S.J.; Lugger, S.J.; van Raak, R.J.; Schenning, A.P. A pH-Responsive Liquid Crystal Hydrogel Actuator with Calcium-Induced Reprogrammable Shape Fixing. ACS Appl. Polym. Mater. 2022, 4, 1298–1304. [Google Scholar] [CrossRef]
- Yang, C.; Su, F.; Liang, Y.; Xu, W.; Li, S.; Liang, E.; Wang, G.; Zhou, N.; Wan, Q.; Ma, X. Fabrication of a biomimetic hydrogel actuator with rhythmic deformation driven by a pH oscillator. Soft Matter 2020, 16, 2928–2932. [Google Scholar] [CrossRef]
- Yang, C.; Su, F.; Xu, Y.; Ma, Y.; Tang, L.; Zhou, N.; Liang, E.; Wang, G.; Tang, J. pH oscillator-driven jellyfish-like hydrogel actuator with dissipative synergy between deformation and fluorescence color change. ACS Macro Lett. 2022, 11, 347–353. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Zhang, J.; Hou, Y.; Xu, X.; Liu, Q. A programmable bilayer hydrogel actuator based on the asymmetric distribution of crystalline regions. J. Mater. Chem. B 2022, 10, 120–130. [Google Scholar] [CrossRef]
- Xiang, Y.; Liu, C.; Ma, S.; Wang, X.; Zhu, L.; Bao, C. Stimuli-Responsive Peptide Self-Assembly to Construct Hydrogels with Actuation and Shape Memory Behaviors. Adv. Funct. Mater. 2023, 33, 2300416. [Google Scholar] [CrossRef]
- Lai, Y.P.; Li, Z.; Naguib, H.; Diller, E. Hybrid Hydrogel-Magnet Actuators with pH-Responsive Hydrogels for Gastrointestinal Microrobots. Adv. Eng. Mater. 2023, 25, 2301060. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y.; Gao, W.; Dong, R.; Wang, C. Emulsion Hydrogel Soft Motor Actuated by Thermal Stimulation. ACS Appl. Mater. Interfaces 2017, 9, 43211–43219. [Google Scholar] [CrossRef]
- Xu, H.; Bai, S.; Gu, G.; Gao, Y.; Sun, X.; Guo, X.; Xuan, F.; Wang, Y. Bioinspired Self-Resettable Hydrogel Actuators Powered by a Chemical Fuel. ACS Appl. Mater. Interfaces 2022, 14, 43825–43832. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Zhang, K.; Wang, X. Temporary Actuation of Bilayer Polymer Hydrogels Mediated by the Enzymatic Reaction. Langmuir ACS J. Surfaces Colloids 2022, 38, 15433–15441. [Google Scholar] [CrossRef]
- Fusi, G.; Giudice, D.; Skarsetz, O.; Stefano, S.; Walther, A. Autonomous Soft Robots Empowered by Chemical Reaction Networks. Adv. Mater. 2022, 35, 202209870. [Google Scholar] [CrossRef]
- Nan, M.; Guo, K.; Jia, T.; Wang, G.; Liu, S. Novel Acid-Driven Bioinspired Self-Resettable Bilayer Hydrogel Actuator Mimicking Natural Muscles. ACS Appl. Mater. Interfaces 2024, 16, 9224–9230. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Tan, Y.; Li, Y.; Wang, X. Ionic fuel-powered hydrogel actuators for soft robotics. J. Colloid Interface Sci. 2025, 677, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, Q.; Xu, Y.; Yang, M.; Wu, Q.; Wang, F.; Sun, P. Highly Bidirectional Bendable Actuator Engineered by LCST-UCST Bilayer Hydrogel with Enhanced Interface. ACS Appl. Mater. Interfaces 2020, 12, 55290–55298. [Google Scholar] [CrossRef]
- Liu, J.; Xu, W.; Kuang, Z.; Dong, P.; Yao, Y.; Wu, H.; Liu, A.; Ye, F. Gradient porous PNIPAM-based hydrogel actuators with rapid response and flexibly controllable deformation. J. Mater. Chem. C 2020, 8, 12092–12099. [Google Scholar] [CrossRef]
- Jiang, Z.; Seraji, S.M.; Tan, X.; Zhang, X.; Dinh, T.; Mollazade, M.; Rowan, A.; Whittaker, A.; Song, P.; Wang, H. Strong, Ultrafast, Reprogrammable Hydrogel Actuators with Muscle-Mimetic Aligned Fibrous Structures. Chem. Mater. 2021, 33, 7818–7828. [Google Scholar] [CrossRef]
- Asoh, T.; Takai, S.; Uyama, H. Actuation of Hydrogel Architectures Prepared by Electrophoretic Adhesion of Thermoresponsive Microgels. Langmuir ACS J. Surfaces Colloids 2021, 38, 5183–5187. [Google Scholar] [CrossRef]
- Zhao, J.; Bae, J. Microphase separation-driven sequential self-folding of nanocomposite hydrogel/elastomer actuators. Adv. Funct. Mater. 2022, 32, 2200157. [Google Scholar] [CrossRef]
- Hong, J.; Han, J.; Cha, C. Precision Control of Programmable Actuation of Thermoresponsive Nanocomposite Hydrogels with Multilateral Engineering. Int. J. Mol. Sci. 2022, 23, 5044. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L.; Xu, H.; Cheng, Z.; Yan, J.; Xie, X.M. Biomimetic gradient hydrogel actuators with ultrafast thermo-responsiveness and high strength. ACS Appl. Mater. Interfaces 2022, 14, 32541–32550. [Google Scholar] [CrossRef]
- Li, Y.; Coodley, S.N.; Chen, S.; Dong, P.; Li, S.; Yao, S. Thermally responsive spatially programmable soft actuators with multiple response states enabled by Grayscale UV light processing. Mater. Horiz. 2025, 12, 1568–1580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Aziz, S.; Zhu, Z. Tough and Fast Thermoresponsive Hydrogel Soft Actuators. Adv. Mater. Technol. 2025, 2401920. [Google Scholar] [CrossRef]
- Xiong, J.; Wu, R.; Hu, Y.; Guo, Z.; Wang, S.; Mao, J. A temperature-sensitive actuator based on AG/NIPAM delignification wood-based hydrogel. Compos. Struct. 2025, 351, 118580. [Google Scholar] [CrossRef]
- Wang, H.X.; Zhao, X.Y.; Jiang, J.Q.; Liu, Z.T.; Liu, Z.W.; Li, G. Thermal-Responsive Hydrogel Actuators with Photo-Programmable Shapes and Actuating Trajectories. ACS Appl. Mater. Interfaces 2022, 14, 51244–51252. [Google Scholar] [CrossRef]
- Spratte, T.; Arndt, C.; Wacker, I.; Hauck, M.; Adelung, R.; Schröder, R.; Schütt, F.; Selhuber-Unkel, C. Thermoresponsive Hydrogels with Improved Actuation Function by Interconnected Microchannels. Adv. Intell. Syst. 2022, 4, 202100081. [Google Scholar] [CrossRef]
- Ni, C.; Chen, D.; Wen, X.; Jin, B.; He, Y.; Xie, T.; Zhao, Q. High speed underwater hydrogel robots with programmable motions powered by light. Nat. Commun. 2023, 14, 7672. [Google Scholar] [CrossRef]
- Chen, Y.; Kuang, P.; Shen, X.; Lv, X.; Wang, Y.; Yin, W.; Zou, T.; Wang, B.; Liu, Y.; Fan, Q. Lignin precipitation-driven fabrication of gradient porous hydrogel actuator with temperature response. Smart Mater. Struct. 2023, 32, 035037. [Google Scholar] [CrossRef]
- Fan, Z.; Xu, W.; Wang, R.; Wu, H.; Liu, A. Fast-response thermo-sensitive actuator based on asymmetric structured PNIPAM hydrogel with inorganic particles embedding. Macromol. Res. 2023, 31, 625–633. [Google Scholar] [CrossRef]
- Huang, Y.C.; Cheng, Q.; Jeng, U.; Hsu, S. A Biomimetic Bilayer Hydrogel Actuator Based on Thermoresponsive Gelatin Methacryloyl-Poly(N-isopropylacrylamide) Hydrogel with Three-Dimensional Printability. ACS Appl. Mater. Interfaces 2023, 15, 5798–5810. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xiao, Y.; Wu, X.; Deng, J.; Wei, R.; Liu, A.; Chai, H.; Wang, R. Microgel-Crosslinked Thermo-Responsive Hydrogel Actuators with High Mechanical Properties and Rapid Response. Macromol. Rapid Commun. 2024, 45, 2300643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fan, G.; Jiang, J.; Liu, Z.; Liu, Z.; Li, G. Light-guided growth of gradient hydrogels with programmable geometries and thermally responsive actuations. ACS Appl. Mater. Interfaces 2022, 14, 29188–29196. [Google Scholar] [CrossRef]
- Zhao, Y.; Xuan, C.; Qian, X.; Alsaid, Y.; Hua, M.; Jin, L.; He, X. Soft phototactic swimmer based on self-sustained hydrogel oscillator. Sci. Robot. 2019, 4, eaax7112. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Jin, F.; Zhao, Y.; Zheng, M.; Liu, J.; Dong, X.; Xiong, Z.; Xia, Y.; Duan, X. Light-driven micron-scale 3D hydrogel actuator produced by two-photon polymerization microfabrication. Sens. Actuators B-Chem. 2020, 304, 127345. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Dong, B.; Sitti, M. In-air fast response and high speed jumping and rolling of a light-driven hydrogel actuator. Nat. Commun. 2020, 11, 3988. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Du, C.; Dai, Y.; Daab, M.; Matejdes, M.; Breu, J.; Hong, W.; Zheng, Q.; Wu, Z. Light-steered locomotion of muscle-like hydrogel by self-coordinated shape change and friction modulation. Nat. Commun. 2020, 11, 5166. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Liu, W.; Khoruzhenko, O.; Breu, J.; Hong, W.; Zheng, Q.; Wu, Z.L. Animating hydrogel knotbots with topology-invoked self-regulation. Nat. Commun. 2024, 15, 300. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Zhang, C.Y.; Liao, L. Robust Near-Infrared-Responsive Composite Hydrogel Actuator Using Fe3+/Tannic Acid as the Photothermal Transducer. ACS Appl. Mater. Interfaces 2021, 13, 18175–18183. [Google Scholar] [CrossRef]
- Xue, P.; Bisoyi, H.K.; Chen, Y.; Zeng, H.; Yang, J.; Yang, X.; Lv, P.; Zhang, X.; Priimagi, A.; Wang, L.; et al. Near-infrared light-driven shape-morphing of programmable anisotropic hydrogels enabled by MXene nanosheets. Angew. Chem. Int. Ed. 2021, 60, 3390–3396. [Google Scholar] [CrossRef]
- Chen, P.; Ruan, Q.; Nasseri, R.; Zhang, H.; Xi, X.; Xia, H.; Xu, G.; Xie, Q.; Yi, C.; Sun, Z.; et al. Light-Fueled Hydrogel Actuators with Controlled Deformation and Photocatalytic Activity. Adv. Sci. 2022, 9, 2204730. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Gu, Y.; Jing, Z.; Pan, L.; Ju, J.; Neogi, A.; Wang, Z. Photo-responsive superhydrophobic films for soft robotics: A new approach to remote actuation and multifunctional performance. Chem. Eng. J. 2025, 506, 160270. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Yang, W. A light-driven swimming micro-fish based on photo-responsive hydrogel. In Proceedings of the 2022 12th International Conference on CYBER Technology in Automation, Control, and Intelligent Systems (CYBER), Baishan, China, 27–31 July 2022; pp. 1066–1071. [Google Scholar] [CrossRef]
- Wu, Q.; Ma, C.; Chen, L.; Sun, Y.; Wei, X.; Ma, C.; Zhao, H.; Yang, X.; Ma, X.; Zhang, C.; et al. A Tissue Paper/Hydrogel Composite Light-Responsive Biomimetic Actuator Fabricated by In Situ Polymerization. Polymers 2022, 14, 5454. [Google Scholar] [CrossRef]
- Xiang, S.; Su, Y.; Yin, H.; Li, C.; Zhu, M. Visible-light-driven isotropic hydrogels as anisotropic underwater actuators. Nano Energy 2021, 85, 105965. [Google Scholar] [CrossRef]
- Zhang, X.; Xue, P.; Yang, X.; Valenzuela, C.; Chen, Y.; Lv, P.; Wang, Z.; Wang, L.; Xu, X. Near-Infrared Light-Driven Shape-Programmable Hydrogel Actuators Loaded with Metal-Organic Frameworks. ACS Appl. Mater. Interfaces 2022, 14, 11834–11841. [Google Scholar] [CrossRef]
- Yan, Q.; Ding, R.; Zheng, H.; Li, P.; Liu, Z.; Chen, Z.; Xiong, J.; Xue, F.; Zhao, X.; Peng, Q.; et al. Bio-Inspired Stimuli-Responsive Ti3C2Tx/PNIPAM Anisotropic Hydrogels for High-Performance Actuators. Adv. Funct. Mater. 2023, 33, 2301982. [Google Scholar] [CrossRef]
- Cao, Y.; Li, W.; Quan, F.; Xia, Y.; Xiong, Z. Green–light–driven poly (N-isopropylacrylamide-acrylamide)/Fe3O4 nanocomposite hydrogel actuators. Front. Mater. 2022, 9, 827608. [Google Scholar] [CrossRef]
- Yan, H.; Si, L.; Li, G.; Zhao, L.; Luo, W.; Ma, H.; Guan, J. Heterogeneous Thermochromic Hydrogel Film Based on Photonic Nanochains. Nanomaterials 2022, 12, 1867. [Google Scholar] [CrossRef]
- Cao, X.; Xuan, S.; Sun, S.; Xu, Z.; Li, J.; Gong, X. 3D printing magnetic actuators for biomimetic applications. ACS Appl. Mater. Interfaces 2021, 13, 30127–30136. [Google Scholar] [CrossRef]
- Hou, Y.; Dai, Y.; Zhang, W.; Wang, M.; Zhao, H.; Feng, L. Ultrasound-Based Real-Time Imaging of Hydrogel-Based Millirobots with Volume Change Capability. Micromachines 2023, 14, 422. [Google Scholar] [CrossRef]
- Tang, J.; Sun, B.; Yin, Q.; Yang, M.; Hu, J.; Wang, T. 3D printable, tough, magnetic hydrogels with programmed magnetization for fast actuation. J. Mater. Chem. B 2021, 9, 9183–9190. [Google Scholar] [CrossRef] [PubMed]
- Chand, D.; Srinivasan, S.M. Pattern architected soft magnetic actuation. Soft Matter 2025, 21, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Li, D.; Salim, T.; Li, Y.; Cheng, G.; Lam, Y.M.; Ding, J.N. Electrically Driven Hydrogel Actuators: Working Principle, Material design and Applications. J. Mater. Chem. C 2024, 12, 1565–1582. [Google Scholar] [CrossRef]
- Ying, Z.; Wang, Q.; Xie, J.; Li, B.; Lin, X.; Hui, S. Novel electrically-conductive electro-responsive hydrogels for smart actuators with a carbon-nanotube-enriched three-dimensional conductive network and a physical-phase-type three-dimensional interpenetrating network. J. Mater. Chem. C 2020, 8, 4192–4205. [Google Scholar] [CrossRef]
- Rotjanasuworapong, K.; Thummarungsan, N.; Lerdwijitjarud, W.; Sirivat, A. Facile formation of agarose hydrogel and electromechanical responses as electro-responsive hydrogel materials in actuator applications. Carbohydr. Polym. 2020, 247, 116709. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lin, Z.; Yang, Y.; Jiang, T.; Shang, J.; Luo, Z. Flexible Actuator Based on Conductive PAM Hydrogel Electrodes with Enhanced Water Retention Capacity and Conductivity. Micromachines 2022, 13, 1951. [Google Scholar] [CrossRef]
- Shin, Y.; Choi, M.; Choi, J.; Na, J.; Kim, S.Y. Design of an Electro-Stimulated Hydrogel Actuator System with Fast Flexible Folding Deformation under a Low Electric Field. ACS Appl. Mater. Interfaces 2021, 13, 15633–15646. [Google Scholar] [CrossRef]
- Lee, S.; Kim, M.; Choi, J.; Kim, S. Double-crosslinked reduced graphene oxide-based hydrogel actuator system with fast electro-responsive deformation and enhanced mechanical properties. Mater. Today Chem. 2023, 29, 101434. [Google Scholar] [CrossRef]
- Yuk, H.; Lin, S.; Ma, C.; Takaffoli, M.; Fang, N.X.; Zhao, X. Hydraulic hydrogel actuators and robots optically and sonically camouflaged in water. Nat. Commun. 2017, 8, 14230. [Google Scholar] [CrossRef]
- He, X.; Zhu, J.; Yang, C. Harnessing osmotic swelling stress for robust hydrogel actuators. Soft Matter 2022, 18, 5177–5184. [Google Scholar] [CrossRef]
- Na, H.R.; Kang, Y.W.; Park, C.S.; Jung, S.; Kim, H.Y.; Sun, J.Y. Hydrogel-based strong and fast actuators by electroosmotic turgor pressure. Science 2022, 376, 301–307. [Google Scholar] [PubMed]
- Zhang, M.; Li, G.; Yang, X.; Xiao, Y.; Yang, T.; Wong, T.; Li, T. Artificial muscle driven soft hydraulic robot: Electromechanical actuation and simplified modeling. Smart Mater. Struct. 2018, 27, 095016. [Google Scholar] [CrossRef]
- Levin, M.; Cohen, N. Swelling under Constraints: Exploiting 3D-Printing to Optimize the Performance of Gel-Based Devices. Adv. Mater. Technol. 2023, 8, 2202136. [Google Scholar] [CrossRef]
- Hu, X.; Jiang, J.; Wei, H.; Chen, J.; Wang, Z.; Mhenni, G.; Li, H.; Hu, H.; Dai, S.; Kim, S.H.; et al. Electroosmotic-driven coiled hydrogel-based yarn muscles. Chem. Eng. J. 2025, 507, 160398. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Chen, Z.D.; Han, D.D.; Mao, J.W.; Ma, J.N.; Zhang, Y.L.; Sun, H.B. Bioinspired soft robots based on the moisture-responsive graphene oxide. Adv. Sci. 2021, 8, 2002464. [Google Scholar] [CrossRef]
- Pu, W.; Wei, F.; Yao, L.; Xie, S. A review of humidity-driven actuator: Toward high response speed and practical applications. J. Mater. Sci. 2022, 57, 12202–12235. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Wang, R.; Tan, W.; Gu, Y.; Yu, X.; Zhu, L.; Liu, L. Development and challenges of smart actuators based on water-responsive materials. Soft Matter 2022, 18, 5725–5741. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Zhao, X.; Liu, S.; Mei, D.; Zhao, C.; Li, L.; Wang, Y. Moisture-Driven Actuators. Adv. Funct. Mater. 2025, 35, 2412254. [Google Scholar] [CrossRef]
- Tan, H.; Yu, X.; Tu, Y.; Zhang, L. Humidity-driven soft actuator built up layer-by-layer and theoretical insight into its mechanism of energy conversion. J. Phys. Chem. Lett. 2019, 10, 5542–5551. [Google Scholar] [CrossRef]
- Yang, L.; Cui, J.; Zhang, L.; Xu, X.; Chen, X.; Sun, D. A moisture-driven actuator based on polydopamine-modified MXene/bacterial cellulose nanofiber composite film. Adv. Funct. Mater. 2021, 31, 2101378. [Google Scholar] [CrossRef]
- Tang, X.Y.; Liu, Z.; Liu, W.Y.; Pu, X.Q.; Liu, L.Y.; Chen, S.K.; Xie, R.; Ju, X.J.; Wang, W.; Chu, L.Y. Moisture-Responsive Actuators Based on Hyaluronic Acid with Biocompatibility and Fast Response as Smart Breather Valves. ACS Appl. Polym. Mater. 2023, 5, 815–827. [Google Scholar] [CrossRef]
- Tang, X.Y.; Liu, Z.; Xie, R.; Ju, X.J.; Wang, W.; Chu, L.Y. Humidity-Responsive Actuators Based on Firm Heterojunction of Glycerol-Cross-linked Polyvinyl Alcohol and Porous Polyvinylidene Fluoride as Smart Gates for Anti-condensation. Ind. Eng. Chem. Res. 2022, 61, 8101–8111. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, Z.; Li, J.; Wei, J.; Guo, J.; Yin, M. Spontaneous and Continuous Actuators Driven by Fluctuations in Ambient Humidity for Energy-Harvesting Applications. ACS Appl. Mater. Interfaces 2022, 14, 38972–38980. [Google Scholar] [CrossRef]
- Hou, T.; Wang, S.; Li, J.; Wang, Y.; Zhang, Y.; Cao, L.; Xiong, S.; Fan, L.; Gu, F. Optimal humidity-responsive actuators of heterostructured MXene nanosheets/3D-MXene membrane. Smart Mater. Struct. 2023, 32, 065014. [Google Scholar] [CrossRef]
- Li, J.; Zhang, G.; Cui, Z.; Bao, L.; Xia, Z.; Liu, Z.; Zhou, X. High Performance and Multifunction Moisture-Driven Yin–Yang-Interface Actuators Derived from Polyacrylamide Hydrogel. Small 2023, 19, 2303228. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, H.; Cui, T.; Li, J.; Li, M.H.; Hu, J. Humidity-Responsive Actuator with Viewable and Programmable Deformation Enabled by Tannic Acid as Physical Cross-linker. Adv. Mater. Technol. 2023, 8, 2300603. [Google Scholar] [CrossRef]
- Mao, T.; Liu, Z.; Guo, X.; Wang, Z.; Liu, J.; Wang, T.; Geng, S.; Chen, Y.; Cheng, P.; Zhang, Z. Engineering Covalent Organic Frameworks with Polyethylene Glycol as Self-Sustained Humidity-Responsive Actuators. Angew. Chem. Int. Ed. 2023, 62, e202216318. [Google Scholar] [CrossRef]
- Chen, Z.; Peng, Q.; Hu, Y.; Liu, Z.; Zhao, X.; Li, P.; Xu, L.; Zheng, H.; Xue, F.; Ding, R.; et al. Dried bonito flakes-inspired moisture-responsive actuator with a gradient structure for smart devices. J. Mater. Sci. Technol. 2023, 167, 152–160. [Google Scholar] [CrossRef]
- Zeng, W.; Jiang, C.; Wu, D. Heterogeneity regulation of bilayer polysaccharide hydrogels for integrating pH-and humidity-responsive actuators and sensors. ACS Appl. Mater. Interfaces 2023, 15, 16097–16108. [Google Scholar] [CrossRef]
- Cheng, Z.; Hua, Y.; Zhang, D.; Wang, M.; Ge, X.; Shen, Y.; Zhao, B.; Qi, N.; Lin, H. Bilayer oriented nanofiber membrane for enhancing response deformation and stability of humidity-responsive actuator. Chem. Eng. J. 2025, 506, 160260. [Google Scholar] [CrossRef]
- Li, C.; Lau, G.C.; Yuan, H.; Aggarwal, A.; Domínguez, V.L.; Liu, S.; Sai, H.; Palmer, L.C.; Sather, N.A.; Pearson, T.J.; et al. Fast and programmable locomotion of hydrogel-metal hybrids under light and magnetic fields. Sci. Robot. 2020, 5, eabb9822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, X.; Chen, L.; Zhang, C.; Liao, L. Multi-responsive hydrogel actuator with photo-switchable color changing behaviors. Dye Pigment. 2020, 174, 108042. [Google Scholar] [CrossRef]
- Zhao, Y.; Lo, C.Y.; Ruan, L.; Pi, C.H.; Kim, C.; Alsaid, Y.; Frenkel, I.; Rico, R.; Tsao, T.; He, X. Somatosensory actuator based on stretchable conductive photothermally responsive hydrogel. Sci. Robot. 2021, 6, eabd5483. [Google Scholar] [CrossRef]
- Lo, C.Y.; Zhao, Y.; Kim, C.; Alsaid, Y.; Khodambashi, R.; Peet, M.; Fisher, R.; Marvi, H.; Berman, S.; Aukes, D.; et al. Highly stretchable self-sensing actuator based on conductive photothermally-responsive hydrogel. Mater. Today 2021, 50, 35–43. [Google Scholar] [CrossRef]
- Wang, L.; Liu, F.; Qian, J.; Wu, Z.; Xiao, R. Multi-responsive PNIPAM-PEGDA hydrogel composite. Soft Matter 2021, 17, 10421–10427. [Google Scholar] [CrossRef]
- Sun, Z.; Hu, Y.; Wei, C.; Hao, R.; Hao, C.; Liu, W.; Liu, H.; Huang, M.; He, S.; Yang, M. Transparent, photothermal and stretchable alginate-based hydrogels for remote actuation and human motion sensing. Carbohydr. Polym. 2022, 293, 119727. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, Y.; Zhang, Y.; Zeng, L.; Fang, J.; Chen, C.; Zhang, C.; Wu, D.; Lao, Z.; Xia, H. High-Load Shape Memory Microgripper with Embedded Resistive Heating and Magnetic Actuation. Adv. Funct. Mater. 2025, 2421798. [Google Scholar] [CrossRef]
- Li, M.; Mei, J.; Friend, J.; Bae, J. Acousto-Photolithography for Programmable Shape Deformation of Composite Hydrogel Sheets. Small 2022, 18, e2204288. [Google Scholar] [CrossRef]
- Zheng, Z.; Han, J.; Demir, S.O.; Wang, H.; Jiang, W.; Liu, H.; Sitti, M. Electrodeposited Superhydrophilic-Superhydrophobic Composites for Untethered Multi-Stimuli-Responsive Soft Millirobots. Adv. Sci. 2023, 10, 2302409. [Google Scholar] [CrossRef]
- Wei, X.; Wu, Q.; Chen, L.; Sun, Y.; Chen, L.; Zhang, C.; Li, S.; Ma, C.; Jiang, S. Remotely Controlled Light/Electric/Magnetic Multiresponsive Hydrogel for Fast Actuations. ACS Appl. Mater. Interfaces 2023, 15, 10030–10043. [Google Scholar] [CrossRef]
- Jiang, C.; Zeng, W.; Ding, X.; Wu, D. Regulating Boronic Ester Bonds in Bilayer Hydrogels toward Fabricating Multistimuli-Triggered Actuators. ACS Sustain. Chem. Eng. 2023, 11, 14094–14102. [Google Scholar] [CrossRef]
- Zhou, K.; Sun, R.; Wojciechowski, J.P.; Wang, R.; Yeow, J.; Zuo, Y.; Song, X.; Wang, C.; Shao, Y.; Stevens, M.M. 4D Multi-Material Printing of Soft Actuators with Spatial and Temporal Control. Adv. Mater. 2024, 36, 2312135. [Google Scholar] [CrossRef]
- Li, J.; Wang, M.; Cui, Z.; Liu, S.; Feng, D.; Mei, G.; Zhang, R.; An, B.; Qian, D.; Zhou, X.; et al. Dual-responsive jumping actuators by light and humidity. J. Mater. Chem. A 2022, 10, 25337–25346. [Google Scholar] [CrossRef]
- Saadli, M.; Braunmiller, D.L.; Mourran, A.; Crassous, J. Thermally and Magnetically Programmable Hydrogel Microactuators. Small 2023, 19, 2207035. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Li, Y.; Xu, W.; Liang, H.; Xue, Z.; Niu, Y.; Pang, M.; Ren, C. Drosera-Inspired Dual-Actuating Double-Layer Hydrogel Actuator. Macromol. Rapid Commun. 2021, 42, 2100416. [Google Scholar] [CrossRef]
- Wei, J.; Li, R.; Li, L.; Wang, W.; Chen, T. Touch-Responsive Hydrogel for Biomimetic Flytrap-Like Soft Actuator. Nano-Micro Lett. 2022, 14, 182. [Google Scholar] [CrossRef]
- He, F.; You, X.; Gong, H.; Yang, Y.; Bai, T.; Wang, W.; Guo, W.; Liu, X.; Ye, M. Stretchable, Biocompatible and Multifunctional Silk Fibroin-based Hydrogels towards Wearable Strain/Pressure Sensors and Triboelectric Nanogenerators. ACS Appl. Mater. Interfaces 2020, 12, 6442–6450. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Lv, X.; Sun, S. A highly sensitive strain sensor based on a silica@polyaniline core-shell particle reinforced hydrogel with excellent flexibility, stretchability, toughness and conductivity. Soft Matter 2021, 17, 2142–2150. [Google Scholar] [CrossRef]
- Sun, Z.; Song, C.; Zhou, J.; Hao, C.; Liu, W.; Liu, H.; Wang, J.; Huang, M.; He, S.; Yang, M. Rapid Photothermal Responsive Conductive MXene Nanocomposite Hydrogels for Soft Manipulators and Sensitive Strain Sensors. Macromol. Rapid Commun. 2021, 42, 2100499. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, G.; Ren, X. Graphene assisted ion-conductive hydrogel with super sensitivity for strain sensor. Polymer 2021, 215, 123340. [Google Scholar] [CrossRef]
- Zhao, L.; Ren, Z.; Liu, X.; Ling, Q.; Li, Z.; Gu, H. A Multifunctional, Self-Healing, Self-Adhesive, and Conductive Sodium Alginate/Poly(vinyl alcohol) Composite Hydrogel as a Flexible Strain Sensor. ACS Appl. Mater. Interfaces 2021, 13, 11344–11355. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, Y.; Cao, Y.; Wang, X.; Chen, Y.R.; Liu, H.; Gao, Y.; Wang, J.; Liu, C.; Wang, W.; et al. Anti-freezing, resilient and tough hydrogels for sensitive and large-range strain and pressure sensors. Chem. Eng. J. 2021, 403, 126431. [Google Scholar] [CrossRef]
- He, Z.; Yuan, W. Adhesive, Stretchable, and Transparent Organohydrogels for Antifreezing, Antidrying, and Sensitive Ionic Skins. ACS Appl. Mater. Interfaces 2021, 13, 1474–1485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Q.; Wang, G.; Zhang, Z.; Xia, S.; Gao, G. Ultrathin and Highly Tough Hydrogel Films for Multifunctional Strain Sensors. ACS Appl. Mater. Interfaces 2021, 13, 50411–50421. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Q.; Wei, J.; Zhu, C.; Wang, Y.; Yu, A.; Wang, W.; Zou, J.; Xie, J.; Fu, Z. Biocomposite silk fibroin hydrogel with stretchability, conductivity and biocompatibility for wireless strain sensor. J. Mater. Sci. Technol. 2025, 210, 195–203. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, Z.; Zhang, N.; Li, J.; Zhou, P.; Hu, F.; Rong, Y.; Lu, B.; Gu, G. High-stretchability, ultralow-hysteresis conductingpolymer hydrogel strain sensors for soft machines. Adv. Mater. 2022, 34, 2203650. [Google Scholar] [CrossRef]
- Guan, L.; Liu, H.; Ren, X.; Wang, T.; Zhu, W.; Zhao, Y.; Feng, Y.; Shen, C.; Zvyagin, A.; Fang, L.; et al. Balloon Inspired Conductive Hydrogel Strain Sensor for Reducing Radiation Damage in Peritumoral Organs During Brachytherapy. Adv. Funct. Mater. 2022, 32, 202112281. [Google Scholar] [CrossRef]
- Chen, C.; Zheng, N.; Wu, W.; Tang, M.; Feng, W.; Zhang, W.; Li, X.; Jiang, Y.; Pang, J.; Min, D.; et al. Self-Adhesive and Conductive Dual-Network Polyacrylamide Hydrogels Reinforced by Aminated Lignin, Dopamine, and Biomass Carbon Aerogel for Ultrasensitive Pressure Sensor. ACS Appl. Mater. Interfaces 2022, 14, 54127–54140. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Lv, X.; Li, Y.; Lv, A.; Sui, X.; Tian, S.; Jiang, L.; Li, R.; Sun, S. Carbon Nanotubes and Silica@polyaniline Core-Shell Particles Synergistically Enhance the Toughness and Electrical Conductivity in Hydrophobic Associated Hydrogels. Langmuir ACS J. Surfaces Colloids 2023, 39, 1299–1308. [Google Scholar] [CrossRef]
- Ryplida, B.; In, I.; Park, S.Y. Tunable pressure sensor of f-carbon dot-based conductive hydrogel with electrical, mechanical, and shape recovery for monitoring human motion. ACS Appl. Mater. Interfaces 2020, 12, 51766–51775. [Google Scholar] [CrossRef]
- Kang, K.; Jung, H.; An, S.; Baac, H.W.; Shin, M.; Son, D. Skin-like transparent polymer-hydrogel hybrid pressure sensor with pyramid microstructures. Polymers 2021, 13, 3272. [Google Scholar] [CrossRef]
- Han, X.; Lv, Z.; Ran, F.; Dai, L.; Li, C.; Si, C. Green and stable piezoresistive pressure sensor based on lignin-silver hybrid nanoparticles/polyvinyl alcohol hydrogel. Int. J. Biol. Macromol. 2021, 176, 78–86. [Google Scholar] [CrossRef]
- Liu, W.; Xiao, Y.; Wang, X.; Deng, F. Plantar Pressure Detection System Based on Flexible Hydrogel Sensor Array and WT-RF. Sensors 2021, 21, 5964. [Google Scholar] [CrossRef]
- Ding, H.; Wu, Z.; Wang, H.; Zhou, Z.; Wei, Y.; Tao, K.; Xie, X.; Wu, J. An ultrastretchable, high-performance, and crosstalk-free proximity and pressure bimodal sensor based on ionic hydrogel fibers for human-machine interfaces. Mater. Horiz. 2022, 9, 1935–1946. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zeng, J.; Zhang, X.; Guo, G.; Liu, R.; Yan, Z.; Yin, Y. Fatigue resistant aerogel/hydrogel nanostructured hybrid for highly sensitive and ultrabroad pressure sensing. Small 2022, 18, 2104706. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, G.; Yang, H.; Zhu, C.; Li, S.; Wang, W.; Ren, J.; Cong, Y.; Xu, X.; Wang, X.; et al. 3D printed microstructured ultra-sensitive pressure sensors based on microgel-reinforced double network hydrogels for biomechanical applications. Mater. Horiz. 2023, 10, 4232–4242. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, N.; Chu, X.; Sun, F.; Ali, M.U.; Zhang, Y.; Yang, B.; Cai, Y.; Liu, M.; Gasparini, N.; et al. Wide-Humidity Range Applicable, Anti-Freezing, and Healable Zwitterionic Hydrogels for Ion-Leakage-Free Iontronic Sensors. Adv. Mater. 2023, 35, 2211617. [Google Scholar] [CrossRef]
- Chen, M.; Wan, H.; Hu, Y.; Zhao, F.; An, X.; Lu, A. Rationally designed cellulose hydrogel for an ultrasensitive pressure sensor. Mater. Horiz. 2023, 10, 4510–4520. [Google Scholar] [CrossRef]
- Cai, Y.; Hardman, D.; Iida, F.; Thuruthel, T.G. Design and Development of a Hydrogel-based Soft Sensor for Multi-Axis Force Control. In Proceedings of the 2023 IEEE International Conference on Robotics and Automation (ICRA), London, UK, 29 May–2 June 2023; pp. 594–600. [Google Scholar] [CrossRef]
- Wang, J.; Shu, J.; Liu, H.; Ren, D.; Song, J.; Zhang, T.; Li, Z.; Huang, Y.; Huang, Y. Flexible capacitive pressure sensor with high sensitivity and wide detection range applying solvent-exchanged porous lignin-cellulose hydrogel as the dielectric layer. Int. J. Biol. Macromol. 2025, 303, 140702. [Google Scholar] [CrossRef]
- Luo, Y.; Li, J.; Ding, Q.; Wang, H.; Liu, C.; Wu, J. Functionalized Hydrogel-Based Wearable Gas and Humidity Sensors. Nano-Micro Lett. 2023, 15, 136. [Google Scholar] [CrossRef]
- Yu, J.; Feng, Y.; Sun, D.; Ren, W.; Shao, C.; Sun, R. Highly Conductive and Mechanically Robust Cellulose Nanocomposite Hydrogels with Antifreezing and Antidehydration Performances for Flexible Humidity Sensors. ACS Appl. Mater. Interfaces 2022, 14, 10886–10897. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.L.; Liu, J.; Shi, J.; He, X.D.; Yuan, J.; Pike, A.R.; Chu, L.; Wu, Q.; Liu, B. Compact fiber Fabry–Perot sensors filled with PNIPAM hydrogel for highly sensitive relative humidity measurement. Measurement 2022, 201, 111781. [Google Scholar] [CrossRef]
- Wu, Z.; Ding, Q.; Li, Z.; Zhou, Z.; Luo, L.; Tao, K.; Xie, X.; Wu, J. Ultrasensitive, stretchable, and transparent humidity sensor based on ion-conductive double-network hydrogel thin films. Sci. China Mater. 2022, 65, 2540–2552. [Google Scholar] [CrossRef]
- Liang, Y.; Ding, Q.; Wang, H.; Wu, Z.; Li, J.; Li, Z.; Tao, K.; Gui, X.; Wu, J. Humidity sensing of stretchable and transparent hydrogel films for wireless respiration monitoring. Nano-Micro Lett. 2022, 14, 183. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Yang, H.; Zhao, R.; Chen, X.; Nie, B.; Hu, L. A flexible organohydrogel-based humidity sensor for noncontact artificial sensation. Sci. China Technol. Sci. 2022, 65, 191–200. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, L.; Meredith, C.H.; Balaj, R.V.; Wang, D.; Pan, M.; Han, T.; Yang, J.; Wang, Q.; Dong, L.; et al. Photoluminescent Humidity Sensors Based on Droplet-Enabled Porous Composite Gels. ACS Mater. Lett. 2023, 5, 2074–2083. [Google Scholar] [CrossRef]
- Pan, B.; Su, P.; Jin, M.; Huang, X.; Wang, Z.; Zhang, R.; Xu, H.; Liu, W.; Ye, Y. Ultrathin hierarchical hydrogel–carbon nanocomposite for highly stretchable fast-response water-proof wearable humidity sensors. Mater. Horiz. 2023, 10, 5263–5276. [Google Scholar] [CrossRef]
- Cesnik, S.; Hernández Rodríguez, G.; Bergmann, A.; Coclite, A.M. Ultrafast Humidity Sensing Layers Made by Two-Photon Polymerization and Initiated Chemical Vapor Deposition. Adv. Sens. Res. 2023, 2, 2200100. [Google Scholar] [CrossRef]
- Yang, J.; Rong, L.; Huang, W.; Wu, Z.; Ding, Q.; Zhang, H.; Lin, Y.; Li, F.; Li, C.; Yang, B.R.; et al. Flame-retardant, flexible, and breathable smart humidity sensing fabrics based on hydrogels for respiratory monitoring and non-contact sensing. View 2023, 4, 20220060. [Google Scholar] [CrossRef]
- Ding, Q.; Wang, H.; Zhou, Z.; Wu, Z.; Tao, K.; Gui, X.; Liu, C.; Shi, W.; Wu, J. Stretchable, self-healable, and breathable biomimetic iontronics with superior humidity-sensing performance for wireless respiration monitoring. SmartMat 2023, 4, e1147. [Google Scholar] [CrossRef]
- Liu, R.; Liu, Y.; Fu, S.; Cheng, Y.; Jin, K.; Ma, J.; Wan, Y.; Tian, Y. Humidity Adaptive Antifreeze Hydrogel Sensor for Intelligent Control and Human-Computer Interaction. Small 2024, 20, 2308092. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Zhu, H.; Cheng, J.H. Constructing a novel humidity sensor using acrylic acid/bagasse cellulose porous hydrogel combining graphene oxide and citral for antibacterial and intelligent fruit preservation. Carbohydr. Polym. 2024, 326, 121639. [Google Scholar] [CrossRef]
- Song, L.; Chen, W.J.; Huang, J.; Hu, D.; Ji, X.; Hua, L.; Lu, Z. Conductive hydrogels with HPC additions for humidity sensing and temperature response. Chem. Eng. J. 2025, 506, 160000. [Google Scholar] [CrossRef]
- An, R.; Zhang, X.; Han, L.; Wang, X.; Zhang, Y.; Shi, L.; Ran, R. Healing, flexible, high thermal sensitive dual-network ionic conductive hydrogels for 3D linear temperature sensor. Mater. Sci. Eng. C 2020, 107, 110310. [Google Scholar] [CrossRef]
- Wang, T.; Ren, X.; Bai, Y.; Liu, L.; Wu, G. Adhesive and tough hydrogels promoted by quaternary chitosan for strain sensor. Carbohydr. Polym. 2021, 254, 117298. [Google Scholar] [CrossRef]
- Ding, H.; Liang, X.; Wang, Q.; Wang, M.; Li, Z.; Sun, G. A semi-interpenetrating network ionic composite hydrogel with low modulus, fast self-recoverability and high conductivity as flexible sensor. Carbohydr. Polym. 2020, 248, 116797. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Zhao, C.; Liu, L.Y.; Li, Y.; Xiang, D.; Wu, Y.; Li, H. Adhesive, transparent, stretchable, and strain-sensitive hydrogel as flexible strain sensor. Compos. Commun. 2021, 25, 100733. [Google Scholar] [CrossRef]
- Lu, Y.; Yue, Y.; Ding, Q.; Mei, C.; Xu, X.; Wu, Q.; Xiao, H.; Han, J. Self-Recovery, Fatigue-Resistant, and Multifunctional Sensor Assembled by a Nanocellulose/Carbon Nanotube Nanocomplex-Mediated Hydrogel. ACS Appl. Mater. Interfaces 2021, 13, 50281–50297. [Google Scholar] [CrossRef]
- Yang, J.; Sun, X.; Kang, Q.; Zhu, L.; Qin, G.; Chen, Q. Freezing-tolerant and robust gelatin-based supramolecular conductive hydrogels with double-network structure for wearable sensors. Polym. Test. 2021, 93, 106879. [Google Scholar] [CrossRef]
- Li, X.; He, L.; Li, Y.; Chao, M.; Li, M.; Wan, P.; Zhang, L. Healable, Degradable, and Conductive MXene Nanocomposite Hydrogel for Multifunctional Epidermal Sensors. ACS Nano 2021, 15, 7765–7773. [Google Scholar] [CrossRef]
- Kang, B.; Yan, X.; Zhao, Z.; Song, S. Dual-Sensing, Stretchable, Fatigue-Resistant, Adhesive, and Conductive Hydrogels Used as Flexible Sensors for Human Motion Monitoring. Langmuir ACS J. Surfaces Colloids 2022, 38, 7013–7023. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Tang, Y.; Liu, Z.; Zhang, Z.; Wu, H.; Shen, B.; Su, M.; Liu, M.; Li, F. Touchable Gustation via a Hoffmeister Gel Iontronic Sensor. ACS Nano 2023, 17, 5129–5139. [Google Scholar] [CrossRef]
- Liu, J.; Fan, X.; Astruc, D.; Gu, H. Robust conductive skin hydrogel e-skin constructed by top–down strategy for motion-monitoring. Collagen Leather 2023, 5, 17. [Google Scholar] [CrossRef]
- Zhou, L.; Li, Y.; Xiao, J.; Chen, S.W.; Tu, Q.; Yuan, M.S.; Wang, J. Liquid Metal-Doped Conductive Hydrogel for Construction of Multifunctional Sensors. Anal. Chem. 2023, 95, 3811–3820. [Google Scholar] [CrossRef] [PubMed]
- Ling, Q.; Fan, X.; Ling, M.; Liu, J.; Zhao, L.; Gu, H. Collagen-Based Organohydrogel Strain Sensor with Self-Healing and Adhesive Properties for Detecting Human Motion. ACS Appl. Mater. Interfaces 2023, 15, 12350–12362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liang, Y.; Deng, Z.; Xu, H.; Zhang, H.; Guo, B.; Zhang, J. Adhesive Ion-Conducting Hydrogel Strain Sensor with High Sensitivity, Long-Term Stability, and Extreme Temperature Tolerance. ACS Appl. Mater. Interfaces 2023, 15, 29902–29913. [Google Scholar] [CrossRef]
- Lee, D.; Song, J.; Kim, J.; Lee, J.; Son, D.; Shin, M. Soft and Conductive Polyethylene Glycol Hydrogel Electrodes for Electrocardiogram Monitoring. Gels 2023, 9, 957. [Google Scholar] [CrossRef]
- Liang, Q.; Shen, Z.; Sun, X.; Yu, D.; Liu, K.; Mugo, S.M.; Chen, W.; Wang, D.; Zhang, Q. Electron conductive and transparent hydrogels for recording brain neural signals and neuromodulation. Adv. Mater. 2023, 35, 2211159. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Inda, M.E.; Lin, S.; Wu, J.; Kim, Y.; Chen, X.; Ma, D.; Lu, T.; Zhao, X. Magnetic Living Hydrogels for Intestinal Localization, Retention, and Diagnosis. Adv. Funct. Mater. 2021, 31, 202010918. [Google Scholar] [CrossRef]
- Ning, Y.; Zhang, Y.; Guo, H.; Zhang, M.; Zhang, Y.; Li, S.; Liu, Z.; Zhang, J.; Yang, X.; Yuan, L. Optical Fiber Magnetic Field Sensor Based on Silk Fibroin Hydrogel. IEEE Sens. J. 2022, 22, 14878–14882. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, G.; Xue, L.; Dong, G.; Su, W.; Cui, M.; Wang, Z.; Liu, M.; Zhou, Z.; Zhang, X. Ultrasoft and Biocompatible Magnetic-Hydrogel-Based Strain Sensors for Wireless Passive Biomechanical Monitoring. ACS Nano 2022, 16, 21555–21564. [Google Scholar] [CrossRef]
- Keßler, C.; Gerlach, G. Magnetic Functionalization of Poly (N-isopropylacrylamide) Hydrogels for Sensor Applications. Phys. Status Solidi 2022, 259, 2200017. [Google Scholar] [CrossRef]
- Park, J.; Song, S.; Ochoa, M.; Jiang, H. Susceptibility of Stimuli-Responsive Hydrogels With Embedded Magnetic Microparticles for Inductively Wireless Chemical Sensing. IEEE Sens. J. 2022, 22, 1121–1127. [Google Scholar] [CrossRef]
- Yu, L.; Chen, L.; Liu, Y.; Zhu, J.; Wang, F.; Ma, L.; Yi, K.; Xiao, H.; Zhou, F.; Wang, F.; et al. Magnetically Actuated Hydrogel Stamping-Assisted Cellular Mechanical Analyzer for Stored Blood Quality Detection. ACS Sens. 2023, 8, 1183–1191. [Google Scholar] [CrossRef]
- Zhao, B.; Bai, Z.; Lv, H.; Yan, Z.; Du, Y.; Guo, X.; Zhang, J.; Wu, L.; Deng, J.; Zhang, D.W.; et al. Self-Healing Liquid Metal Magnetic Hydrogels for Smart Feedback Sensors and High-Performance Electromagnetic Shielding. Nano-Micro Lett. 2023, 15, 79. [Google Scholar] [CrossRef]
- Heidarian, P.; Kouzani, A.Z. Starch-g-Acrylic Acid/Magnetic Nanochitin Self-Healing Ferrogels as Flexible Soft Strain Sensors. Sensors 2023, 23, 1138. [Google Scholar] [CrossRef]
- Cao, Q.; Shu, Z.; Zhang, T.; Ji, W.; Chen, J.; Wei, Y. Magnetic nanocomposite hydrogel with ultra-stretchable as strain sensors for monitoring human motion and the change of magnetic field. J. Appl. Polym. Sci. 2024, 141, e54934. [Google Scholar] [CrossRef]
- Xiao, Y.; Gu, Y.; Qin, L.; Chen, L.; Chen, X.; Cui, W.; Li, F.; Xiang, N.; He, X. Injectable thermosensitive hydrogel-based drug delivery system for local cancer therapy. Colloids Surfaces B Biointerfaces 2021, 200, 111581. [Google Scholar] [CrossRef]
- Wu, Z.; Ding, H.; Tao, K.; Wei, Y.; Gui, X.; Shi, W.; Xie, X.; Wu, J. Ultrasensitive, Stretchable, and Fast-Response Temperature Sensors Based on Hydrogel Films for Wearable Applications. ACS Appl. Mater. Interfaces 2021, 13, 21854–21864. [Google Scholar] [CrossRef]
- Wu, Z.; Ding, H.; Wei, Y.; Tao, K.; Wu, J. Transparent, Anti-Freezing Hydrogels for Ultrasensitive Temperature and Strain Sensor Based on A Thin-Film Structure. In Proceedings of the 2021 IEEE 16th International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Xiamen, China, 25–29 April 2021; pp. 303–306. [Google Scholar] [CrossRef]
- Gao, T.; Gao, X.; Li, T.; Zhang, J. Microstructured biphasic hydrogels for highly sensitive and asymmetric sensors with temperature-dependent sensitivity. J. Polym. Sci. 2022, 60, 2701–2709. [Google Scholar] [CrossRef]
- Han, F.; Xie, X.; Wang, T.; Cao, C.; Li, J.; Sun, T.; Liu, H.; Geng, S.; Wei, Z.; Li, J.; et al. Wearable hydrogel-based epidermal sensor with thermal compatibility and long term stability for smart colorimetric multi-signals monitoring. Adv. Healthc. Mater. 2023, 12, 2201730. [Google Scholar] [CrossRef]
- Hao, S.; Dai, R.; Fu, Q.; Wang, Y.; Zhang, X.; Li, H.; Liu, X.; Yang, J. A Robust and Adhesive Hydrogel Enables Interfacial Coupling for Continuous Temperature Monitoring. Adv. Funct. Mater. 2023, 33, 2302840. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Wang, J.; Ye, T.; Li, Q.; Li, L.; Gao, R.; Wang, Y.; Ren, J.; Li, F.; et al. A Temperature-Sensing Hydrogel Coating on The Medical Catheter. Adv. Funct. Mater. 2023, 34, 2310260. [Google Scholar] [CrossRef]
- Wu, J.; Li, Y.; Duan, S.; Wang, Z.; Jing, X.; Lin, Y.; Zhu, D.; Lei, W.; Shi, Q.; Tao, L. Bioinspired Stretchable MXene Deformation-Insensitive Hydrogel Temperature Sensors for Plant and Skin Electronics. Research 2023, 6, 0106. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.W.; Song, Y.; Mustakim, N. Hydrogel micro-pillar array for temperature sensing in fluid. IEEE Sens. J. 2023, 23, 19021–19027. [Google Scholar] [CrossRef]
- Ryu, K.; Li, G.; Zhang, K.; Guan, J.; Long, Y.; Dong, Z. Thermoresponsive Hydrogels for the Construction of Smart Windows, Sensors, and Actuators. Acc. Mater. Res. 2025. [Google Scholar] [CrossRef]
- Puttasakul, T.; Pintavirooj, C.; Sangma, C.; Sukjee, W. Hydrogel based-electrochemical gas sensor for explosive material detection. IEEE Sens. J. 2019, 19, 8556–8562. [Google Scholar] [CrossRef]
- Zhi, H.; Gao, J.; Feng, L. Hydrogel-based gas sensors for NO2 and NH3. ACS Sens. 2020, 5, 772–780. [Google Scholar] [CrossRef]
- Wu, Z.; Rong, L.; Yang, J.; Wei, Y.; Tao, K.; Zhou, Y.; Yang, B.R.; Xie, X.; Wu, J. Ion-conductive hydrogel-based stretchable, self-healing, and transparent NO2 sensor with high sensitivity and selectivity at room temperature. Small 2021, 17, 2104997. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, H.; Ding, Q.; Tao, K.; Shi, W.; Liu, C.; Chen, J.; Wu, J. A Self-Powered, Rechargeable, and Wearable Hydrogel Patch for Wireless Gas Detection with Extraordinary Performance. Adv. Funct. Mater. 2023, 33, 2300046. [Google Scholar] [CrossRef]
- Wu, Z.; Ding, Q.; Wang, H.; Ye, J.; Luo, Y.; Yu, J.; Zhan, R.; Zhang, H.; Tao, K.; Liu, C.; et al. A Humidity-Resistant, Sensitive, and Stretchable Hydrogel-Based Oxygen Sensor for Wireless Health and Environmental Monitoring. Adv. Funct. Mater. 2023, 34, 2308280. [Google Scholar] [CrossRef]
- Zhou, B.; Guo, J.; Yang, C.; Kong, L. Upconversion-luminescent hydrogel optical probe for in situ dopamine monitoring. Photonics Res. 2020, 8, 1800–1807. [Google Scholar] [CrossRef]
- Xie, G.; Du, S.; Huang, Q.; Mo, M.; Gao, Y.; Li, M.; Tao, J.; Zhang, L.; Zhu, J. Photonic hydrogels for synergistic visual bacterial detection and on-site photothermal disinfection. ACS Appl. Mater. Interfaces 2022, 14, 5856–5866. [Google Scholar] [CrossRef]
- Hu, B.; Kang, X.; Xu, S.; Zhu, J.; Yang, L.; Jiang, C. Multiplex Chroma Response Wearable Hydrogel Patch: Visual Monitoring of Urea in Body Fluids for Health Prognosis. Anal. Chem. 2023, 95, 3587–3595. [Google Scholar] [CrossRef]
- Lu, D.; Qin, M.; Zhao, Y.; Li, H.; Luo, L.; Ding, C.; Cheng, P.; Su, M.; Li, H.; Song, Y.; et al. Supramolecular Photonic Hydrogel for High-Sensitivity Alkaline Phosphatase Detection via Synergistic Driving Force. Small 2023, 19, e2206461. [Google Scholar] [CrossRef]
- Davies, S.; Hu, Y.; Blyth, J.; Jiang, N.; Yetisen, A.K. Reusable Dual-Photopolymerized Holographic Glucose Sensors. Adv. Funct. Mater. 2023, 33, 2214197. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Y.; Lee, J.; Hoover, A.; King, S.A.; Chen, L.; Zhao, J.; Lin, Q.; Yu, C.; Zhu, L.; et al. Continuous Glucose Monitoring Enabled by Fluorescent Nanodiamond Boronic Hydrogel. Adv. Sci. 2023, 10, 2203943. [Google Scholar] [CrossRef]
- Li, Y.; Luo, S.; Gui, Y.; Wang, X.; Tian, Z.; Yu, H. Difunctional hydrogel optical fiber fluorescence sensor for continuous and simultaneous monitoring of glucose and pH. Biosensors 2023, 13, 287. [Google Scholar] [CrossRef]
- Liu, X.; Rao, S.; Chen, W.; Felix, K.; Ni, J.; Sahasrabudhe, A.; Lin, S.; Wang, Q.; Liu, Y.; He, Z.; et al. Fatigue-resistant hydrogel optical fibers enable peripheral nerve optogenetics during locomotion. Nat. Methods 2023, 20, 1802–1809. [Google Scholar] [CrossRef]
- Geng, S.; Guo, P.; Wang, J.; Zhang, Y.; Shi, Y.; Li, X.; Cao, M.; Song, Y.; Zhang, H.; Zhang, Z.; et al. Ultrasensitive Optical Detection and Elimination of Residual Microtumors with a Postoperative Implantable Hydrogel Sensor for Preventing Cancer Recurrence. Adv. Mater. 2024, 36, 2307923. [Google Scholar] [CrossRef]
- Li, B.; Zhang, F.; Yan, X.; Zhang, X.; Zhao, Y.; Cheng, T. Fluorescent Microspheres Based on Sodium Carboxymethyl Cellulose Hydrogel for Optical Temperature Measurement. IEEE Trans. Instrum. Meas. 2024, 73, 9500906. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Shi, Z.; Tan, D.; Yang, X.; Xiong, L.; Li, G.; Lei, Y.; Xue, L. Fast Self-Assembly of Photonic Crystal Hydrogel for Wearable Strain and Temperature Sensor. Small Methods 2022, 6, 2200461. [Google Scholar]
- Pang, Q.; Hu, H.; Zhang, H.; Qiao, B.; Ma, L. Temperature-responsive ionic conductive hydrogel for strain and temperature sensors. ACS Appl. Mater. Interfaces 2022, 14, 26536–26547. [Google Scholar] [CrossRef]
- Pang, Q.; Wu, K.; Jiang, Z.; Yang, F.; Shi, Z.; Gao, H.; Zhang, C.; Hou, R.; Zhu, Y. Nanostructured ionic hydrogel with integrated conductivity, stretchability and thermal responsiveness for a high-performance strain and temperature sensor. Biomater. Sci. 2023, 11, 3603–3615. [Google Scholar] [CrossRef]
- Zhao, R.; Zhao, Z.; Song, S.; Wang, Y. Multifunctional Conductive Double-Network Hydrogel Sensors for Multiscale Motion Detection and Temperature Monitoring. ACS Appl. Mater. Interfaces 2023, 15, 59854–59865. [Google Scholar] [CrossRef]
- Xu, Q.; Hou, M.; Wang, L.; Zhang, X.; Liu, L. Anti-bacterial, anti-freezing starch/ionic liquid/PVA ion-conductive hydrogel with high performance for multi-stimulation sensitive responsive sensors. Chem. Eng. J. 2023, 477, 147065. [Google Scholar] [CrossRef]
- Ge, S.J.; Liu, S.N.; Gu, Z.Z.; Xu, H. A Skin-Inspired Multifunctional Conductive Hydrogel with High Stretchable, Adhesive, Healable, and Decomposable Properties for Highly Sensitive Dual-Sensing of Temperature and Strain. Small Methods 2023, 7, 2300749. [Google Scholar] [CrossRef]
- Qu, X.; Liu, J.; Wang, S.; Shao, J.; Wang, Q.; Wang, W.; Gan, L.; Zhong, L.; Dong, X.; Zhao, Y. Photothermal regulated multi-perceptive poly (ionic liquids) hydrogel sensor for bioelectronics. Chem. Eng. J. 2023, 453, 139785. [Google Scholar] [CrossRef]
- Qu, X.; Sun, H.; Kan, X.; Lei, B.; Shao, J.; Wang, Q.; Wang, W.; Ni, Z.; Dong, X. Temperature-sensitive and solvent-resistance hydrogel sensor for ambulatory signal acquisition in “moist/hot environment”. Nano Res. 2023, 16, 10348–10357. [Google Scholar] [CrossRef]
- Yin, H.; Liu, F.; Abdiryim, T.; Chen, J.; Liu, X. Sodium carboxymethyl cellulose and MXene reinforced multifunctional conductive hydrogels for multimodal sensors and flexible supercapacitors. Carbohydr. Polym. 2024, 327, 121677. [Google Scholar] [CrossRef]
- Fu, D.; Xie, Y.; Zhou, L.; Zhang, L.; Zheng, T.; Shen, J. Triple physical cross-linking cellulose nanofibers-based poly(ionic liquid) hydrogel as wearable multifunctional sensors. Carbohydr. Polym. 2024, 325, 121572. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, Y.; Zhang, X.; Zheng, A.; Xia, Q. Ionic Conductive, Antidrying, and Flexible Organohydrogels Suitable for Pressure Sensors and Gas Sensors. ACS Appl. Electron. Mater. 2023, 5, 2758–2768. [Google Scholar] [CrossRef]
- Sun, S.; Xu, Y.; Maimaitiyiming, X. Tough polyvinyl alcohol-gelatin biological macromolecules ionic hydrogel temperature, humidity, stress and strain, sensors. Int. J. Biol. Macromol. 2023, 249, 125978. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Fang, Z.; Hu, Z.; Wei, X.; Cao, Y.; Han, J.; Li, Y. Temperature-Stress Bimodal Sensing Conductive Hydrogel-Liquid Metal by Facile Synthesis for Smart Wearable Sensor. Macromol. Rapid Commun. 2021, 43, 2100543. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, R.; Zhu, W.; Zhong, H.; Liu, S.; Yi, J.; Shao, L.; Wang, W.; Lam, J.; Wang, Z. A Multimodal Hydrogel Soft-Robotic Sensor for Multi-Functional Perception. Front. Robot. AI 2021, 8, 692754. [Google Scholar] [CrossRef]
- Feng, E.; Li, X.; Li, X.; Zhang, M.; Cao, L.; Wu, Z.; Ma, X. Toughened, self-healing and self-adhesive conductive gels with extraordinary temperature adaptability for dual-responsive sensors. J. Mater. Chem. A 2022, 10, 25527–25538. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Z.; Wu, C.; Cong, Y.; Zhang, R.; Fu, J. Highly Sensitive Pressure and Strain Sensors Based on Stretchable and Recoverable Ion-Conductive Physically Cross-Linked Double-Network Hydrogels. ACS Appl. Mater. Interfaces 2020, 12, 51969–51977. [Google Scholar] [CrossRef]
- Gao, J.; Li, X.; Xu, L.; Yan, M.; Bi, H.; Wang, Q. Transparent multifunctional cellulose-based conductive hydrogel for wearable strain sensors and arrays. Carbohydr. Polym. 2024, 329, 121784. [Google Scholar] [CrossRef]
- Zhu, Y.; Liang, B.; Zhu, J.; Gong, Z.; Gao, X.; Yao, D.; Chen, J.; Lu, C.; Pang, X. Hydrogel-based bimodal sensors for high-sensitivity independent detection of temperature and strain. J. Colloid Interface Sci. 2025, 680, 832–844. [Google Scholar] [CrossRef]
- He, X.; Ma, P.; Ma, S.; Cao, R.; Tian, X.; Liang, Y.; Li, J.; Lu, Y.; Wang, Z.; Lu, X. Self-healing, adhesive liquid metal hydrogels based on PNIPAM microgels for high-performance temperature and strain sensors. J. Mater. Chem. C 2025, 13, 2427–2439. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, Y.; Shen, S.; Du, Z.; Lin, Z.; Zhou, P.; Huang, H.; Lyu, X.; Zou, Z. Intrinsic Anti-Freezing, Tough, and Transparent Hydrogels for Smart Optical and Multi-Modal Sensing Applications. Adv. Mater. 2025, 37, 2413856. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, S.; Yu, W.; Wang, L.; Lv, F.; Yang, L.; Yu, H.; Shi, H.; Huang, Y. Hydrogel based flexible wearable sweat sensor for SERS-AI monitoring treatment effect of lung cancer. Sens. Actuators B Chem. 2025, 427, 137155. [Google Scholar] [CrossRef]
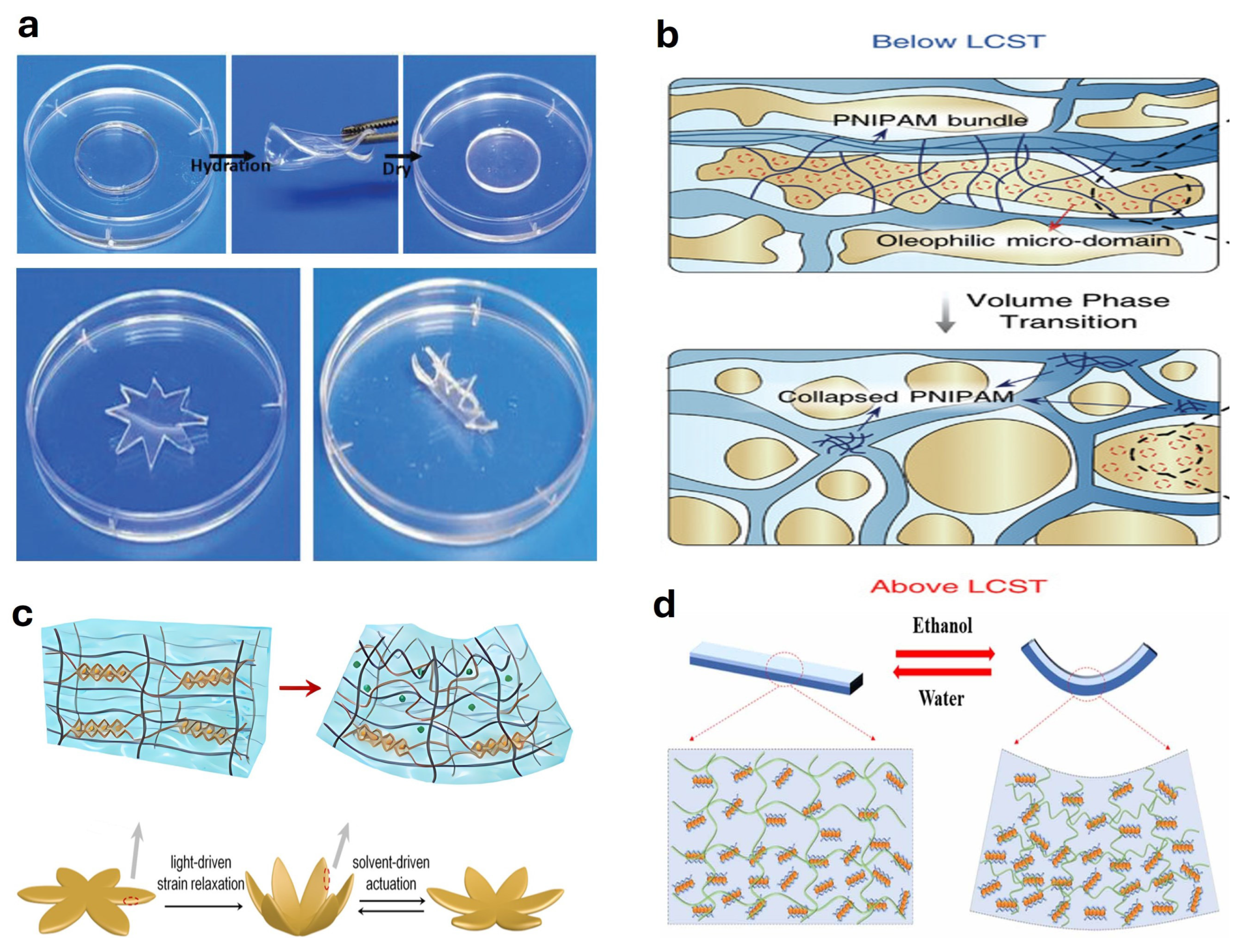
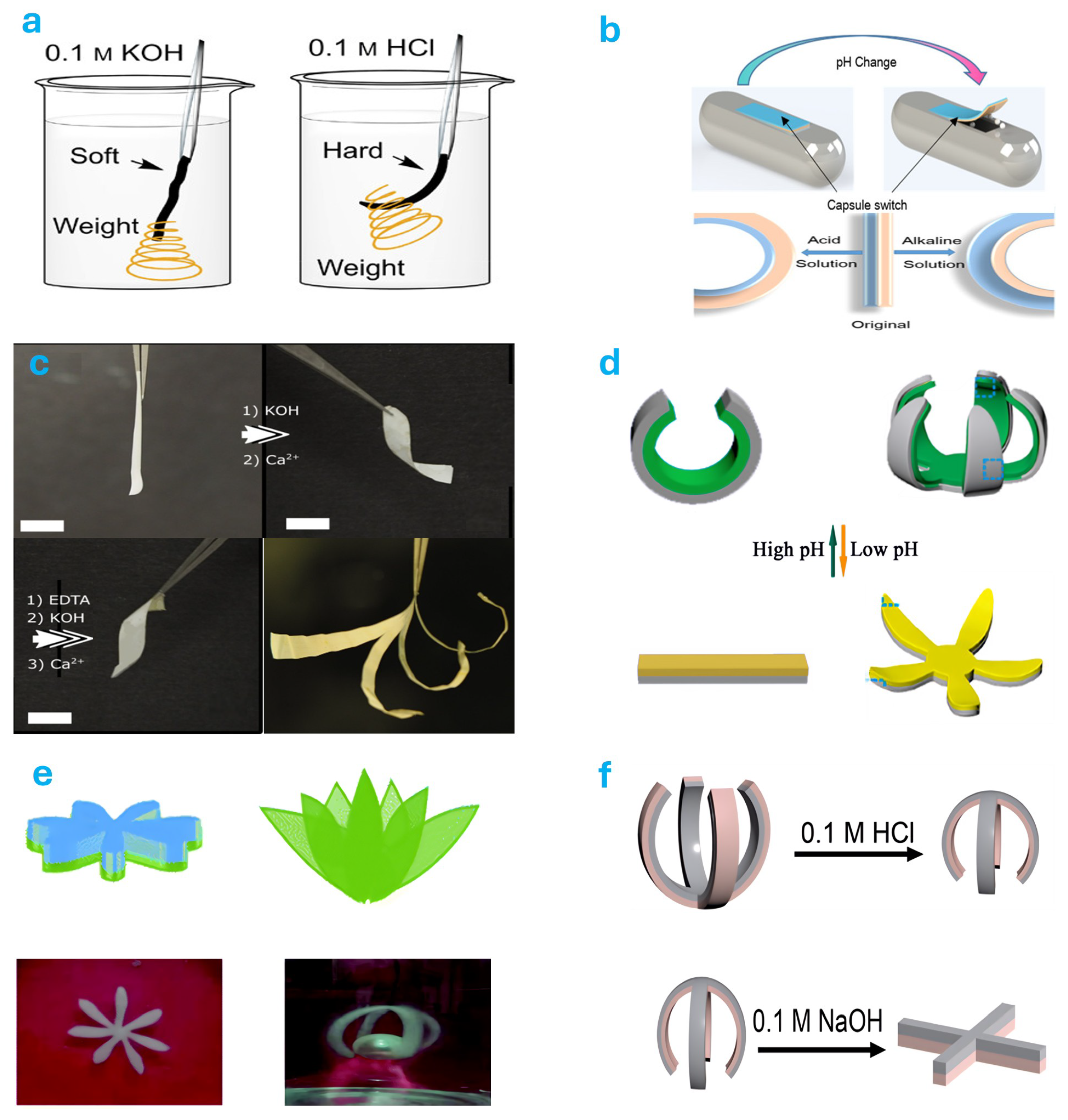
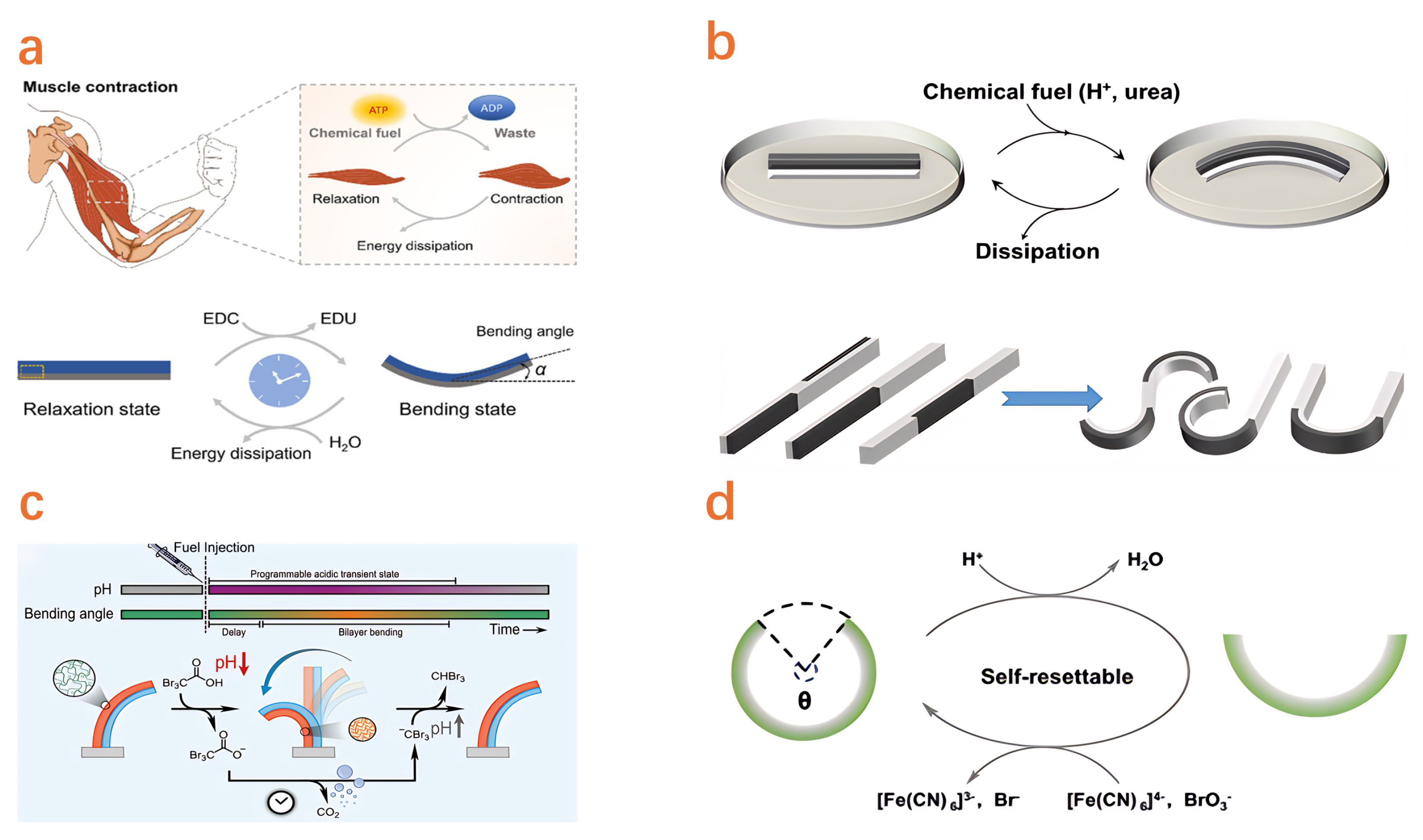
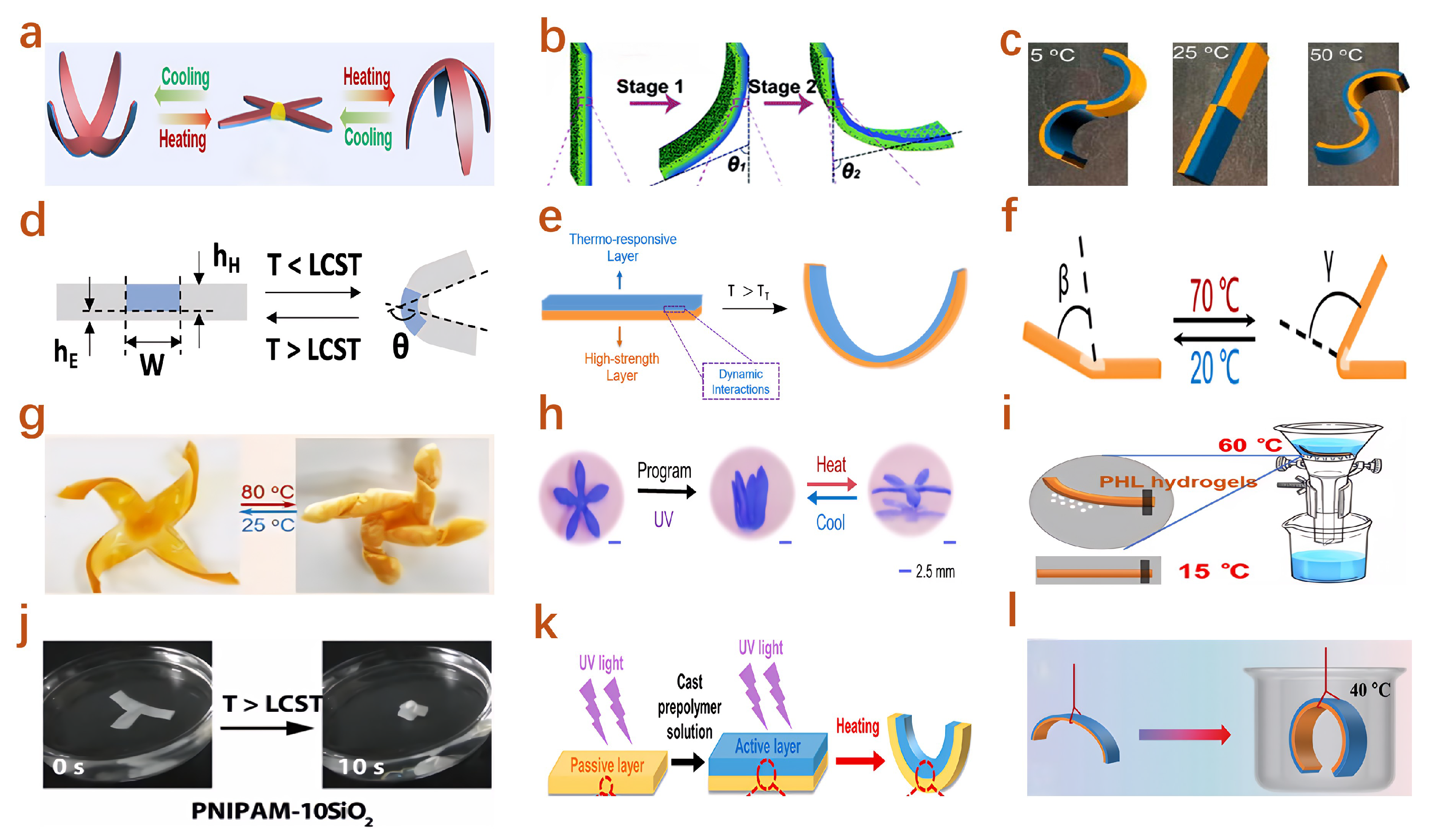
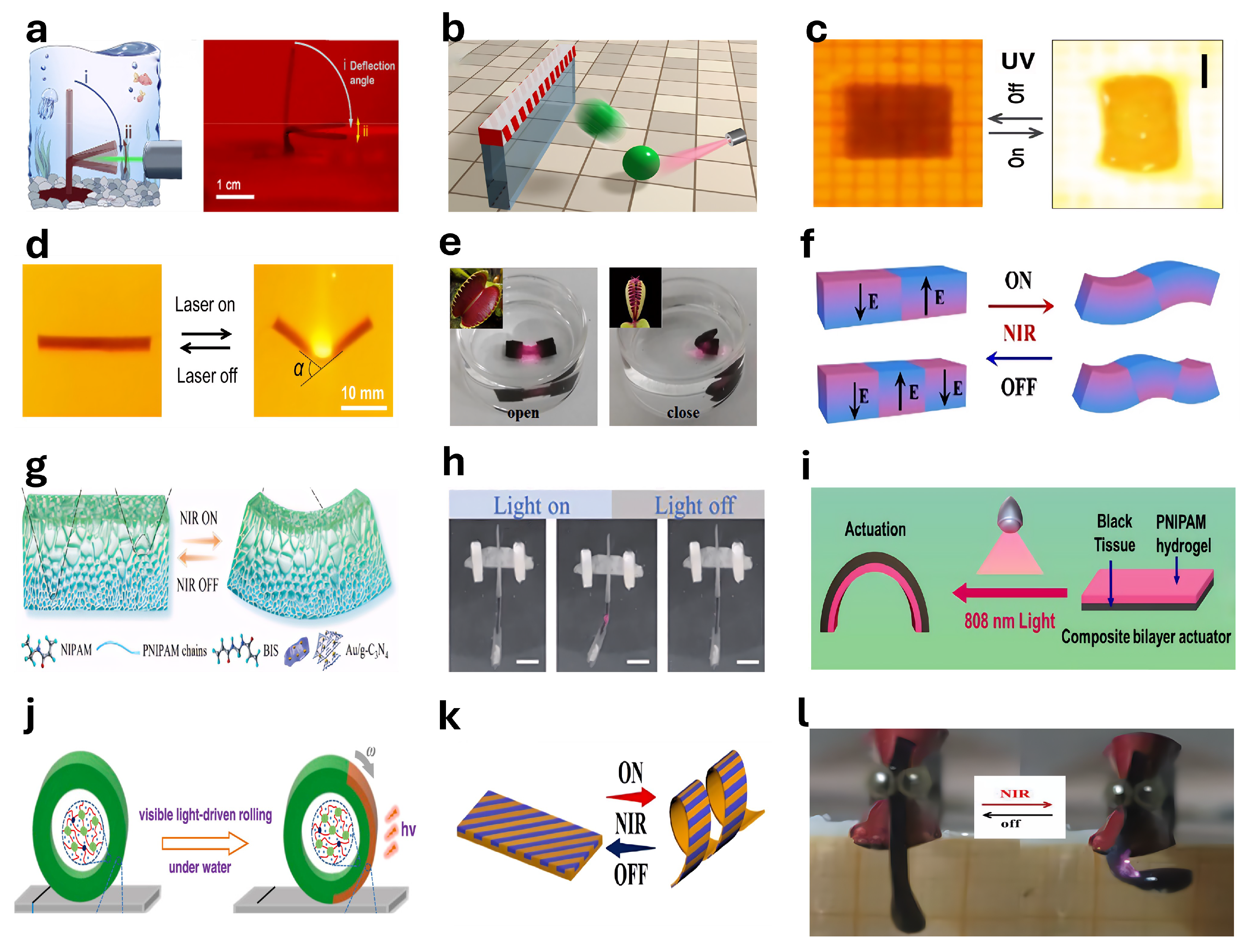
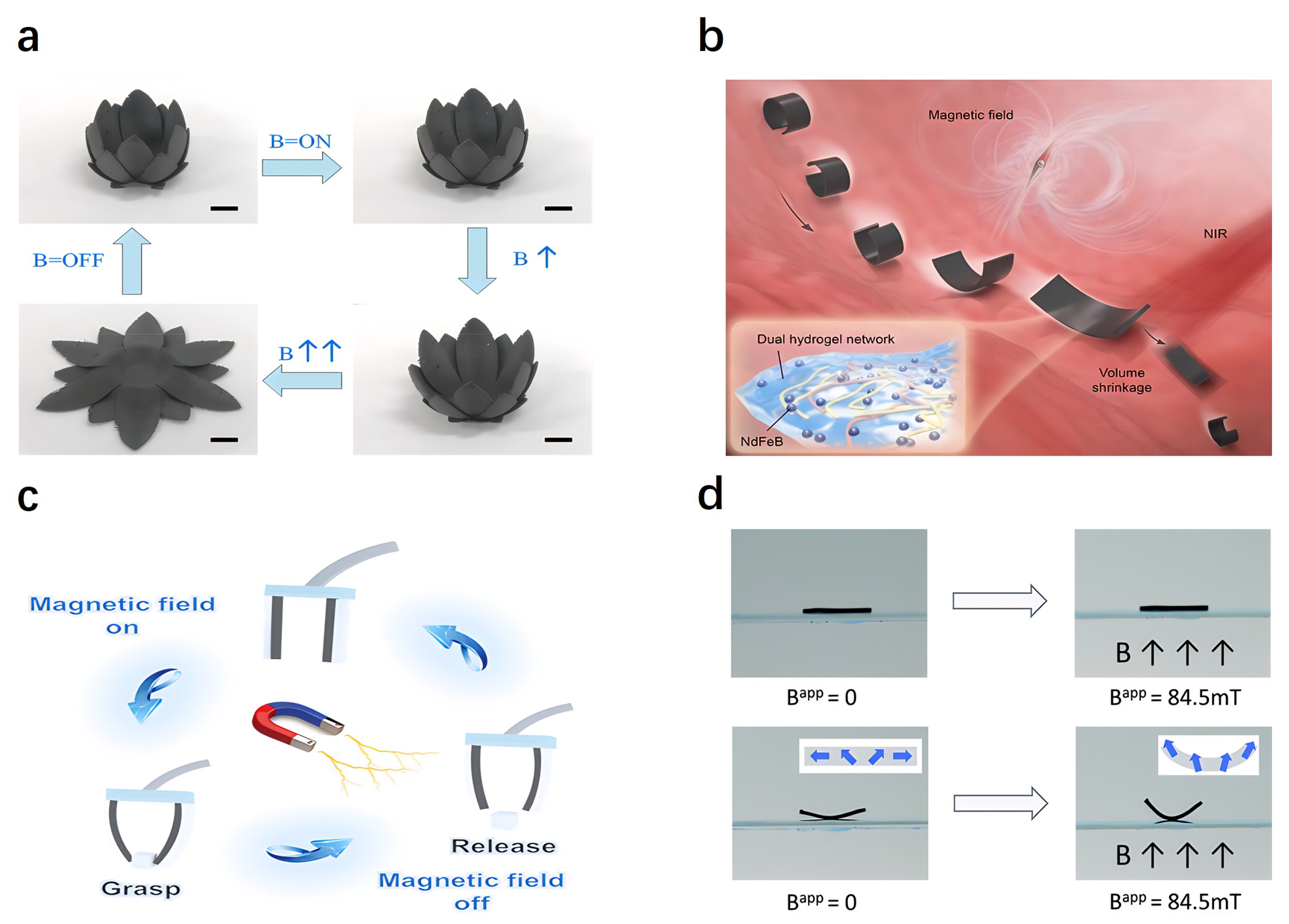
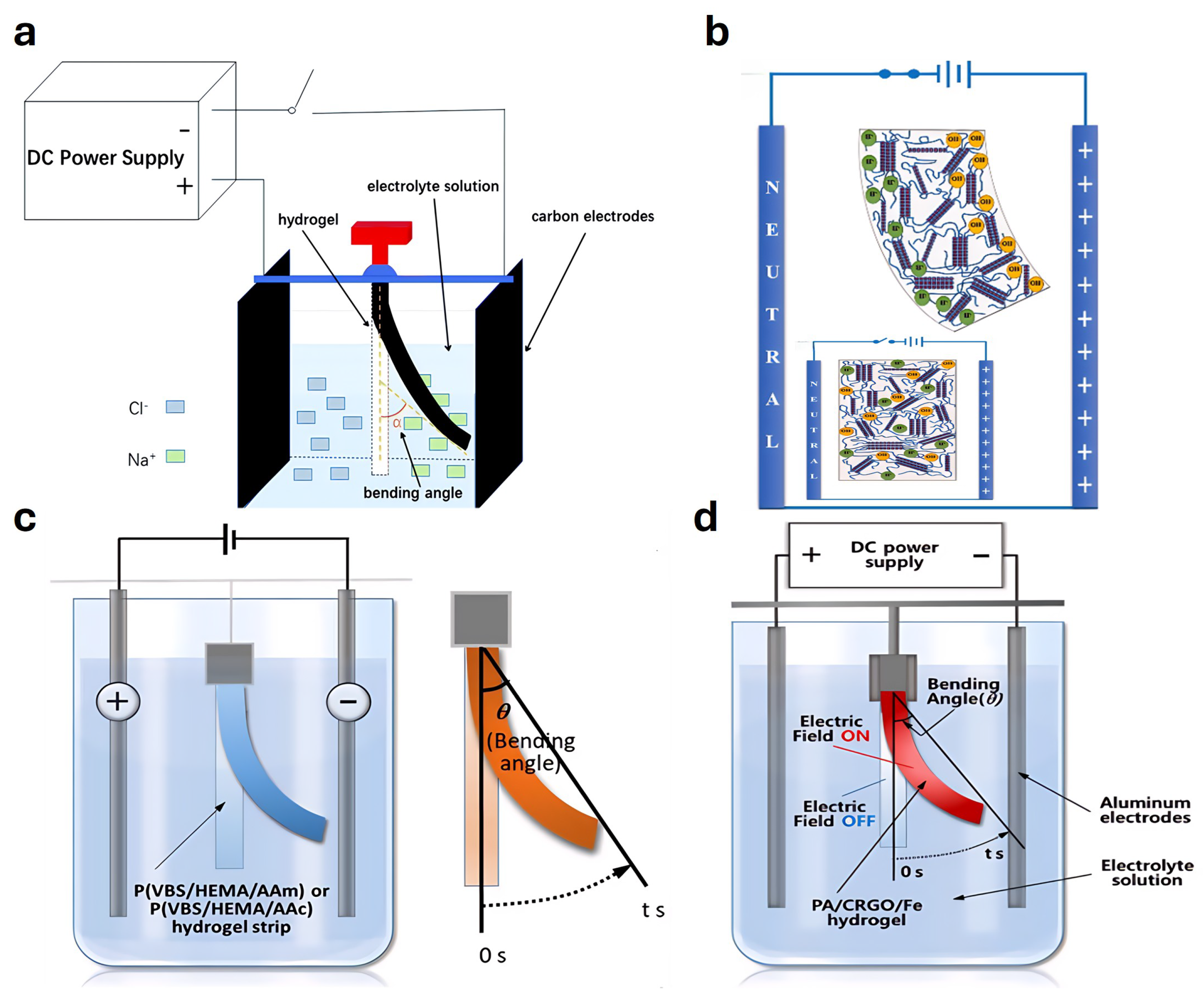
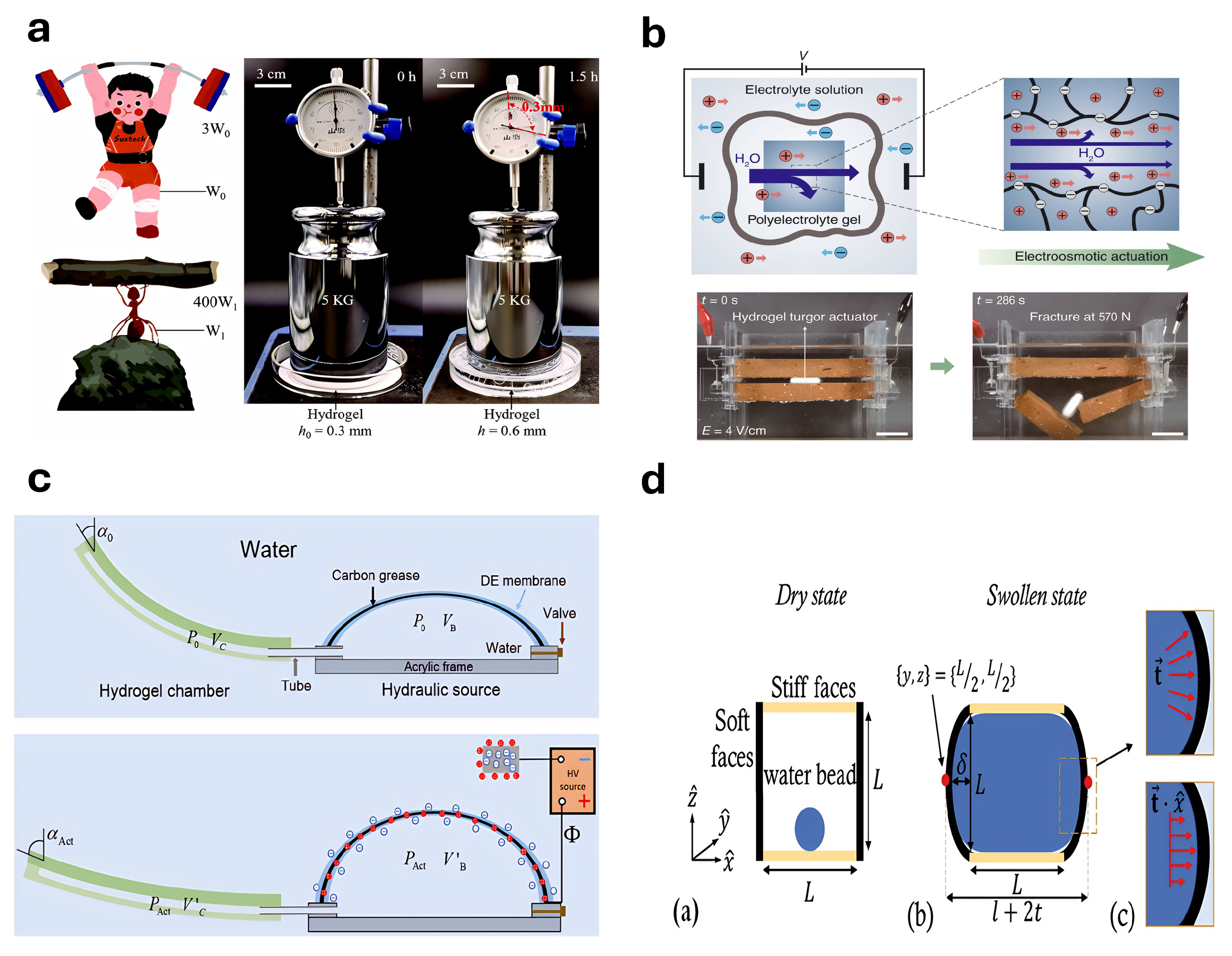
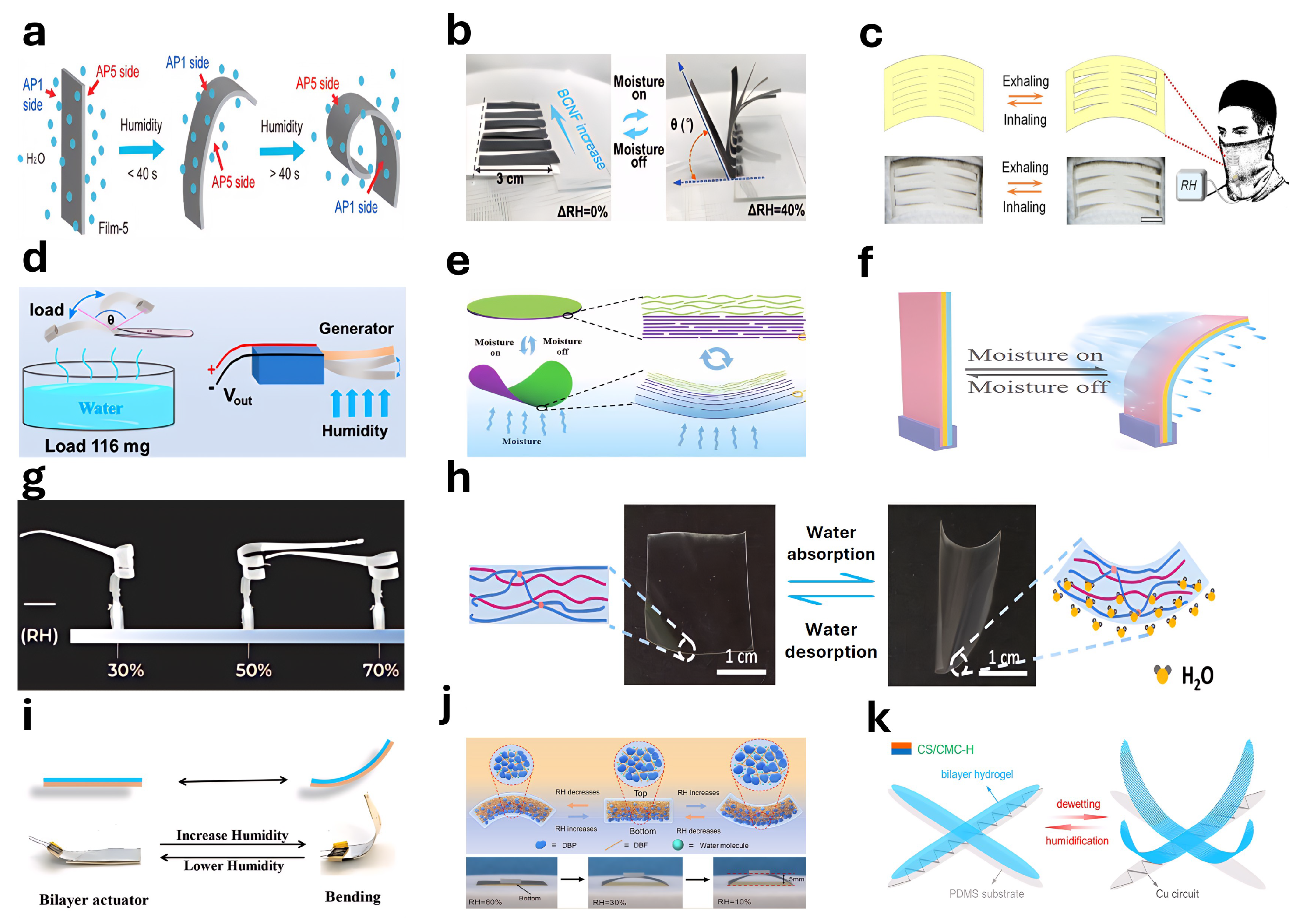
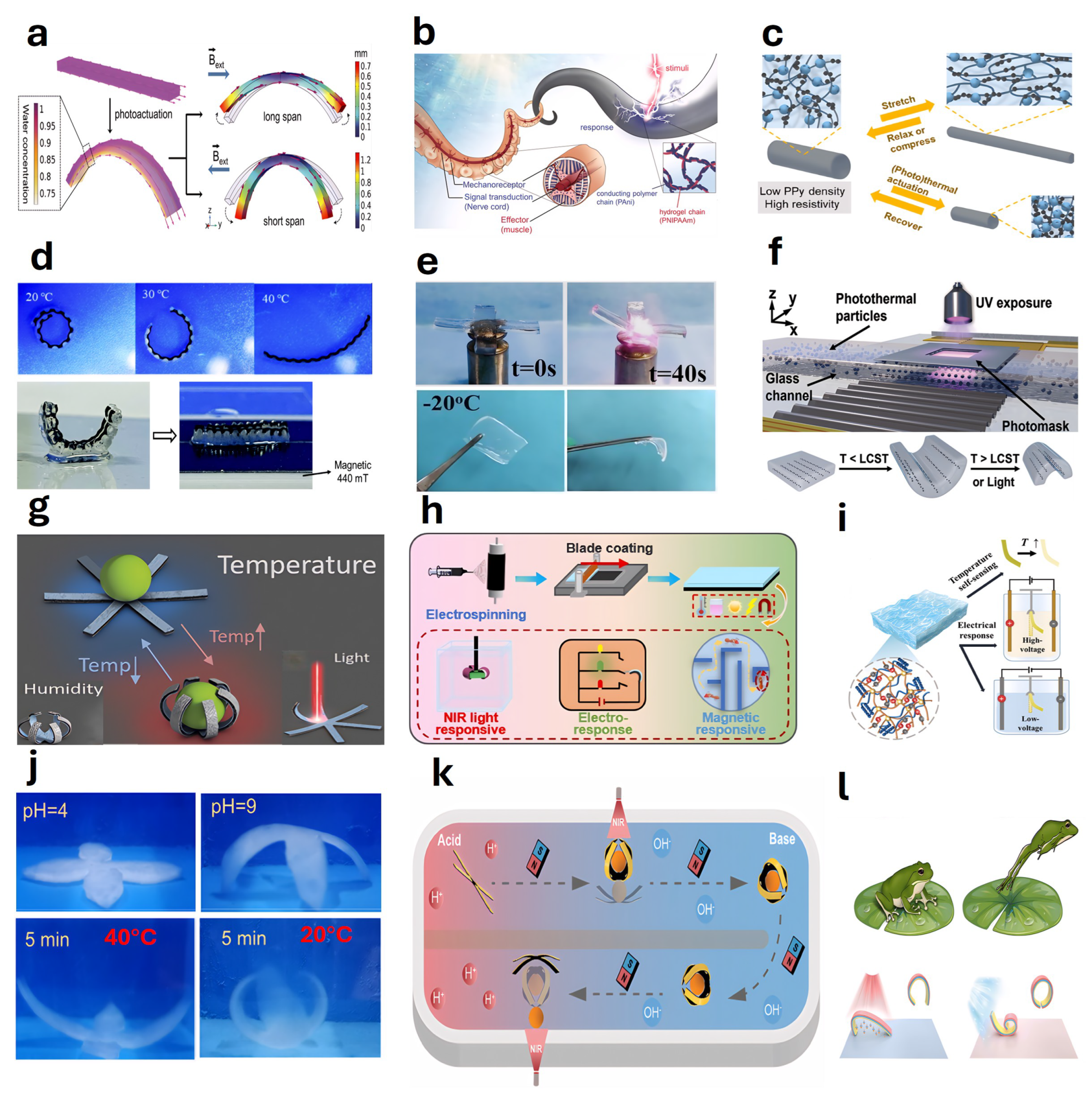
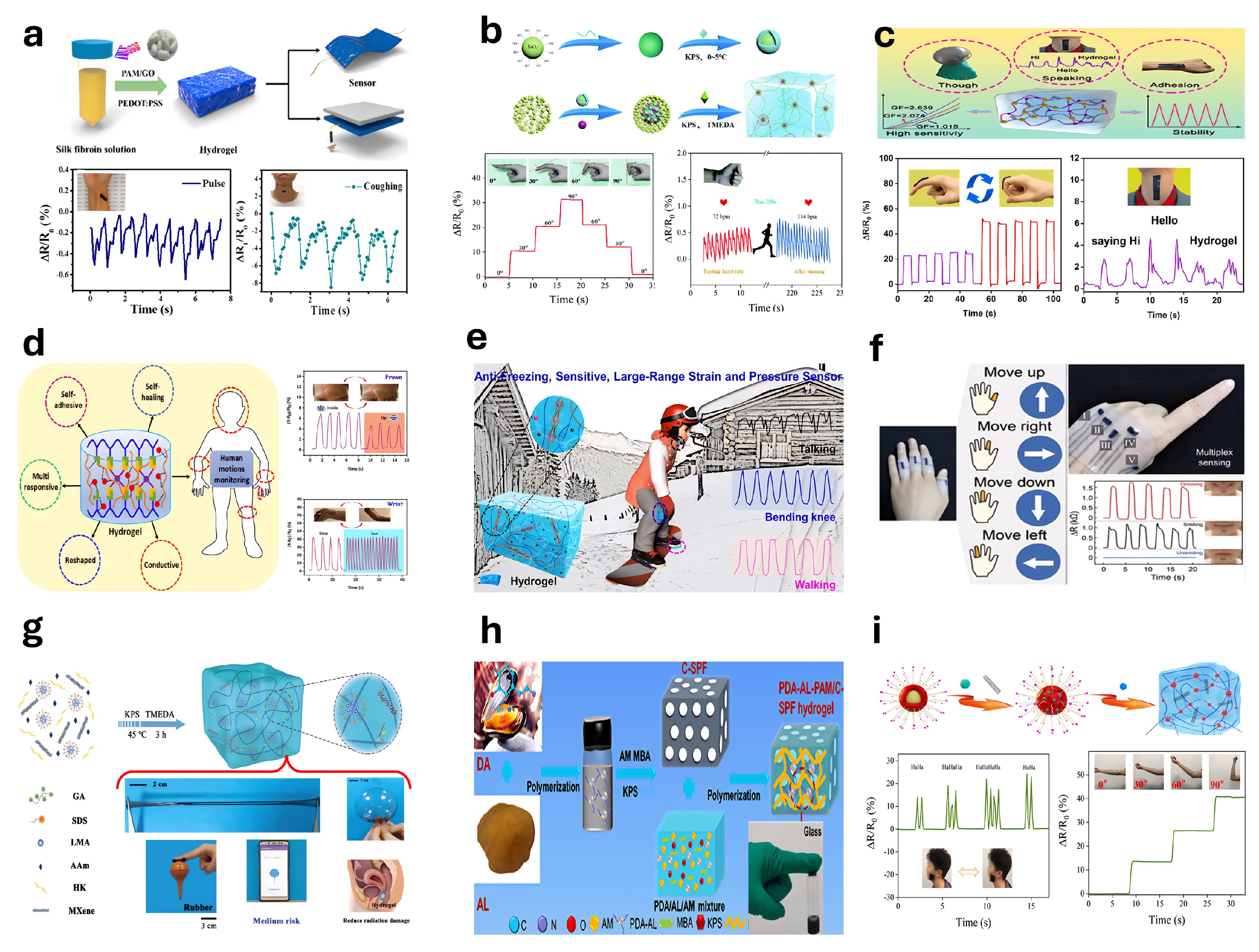

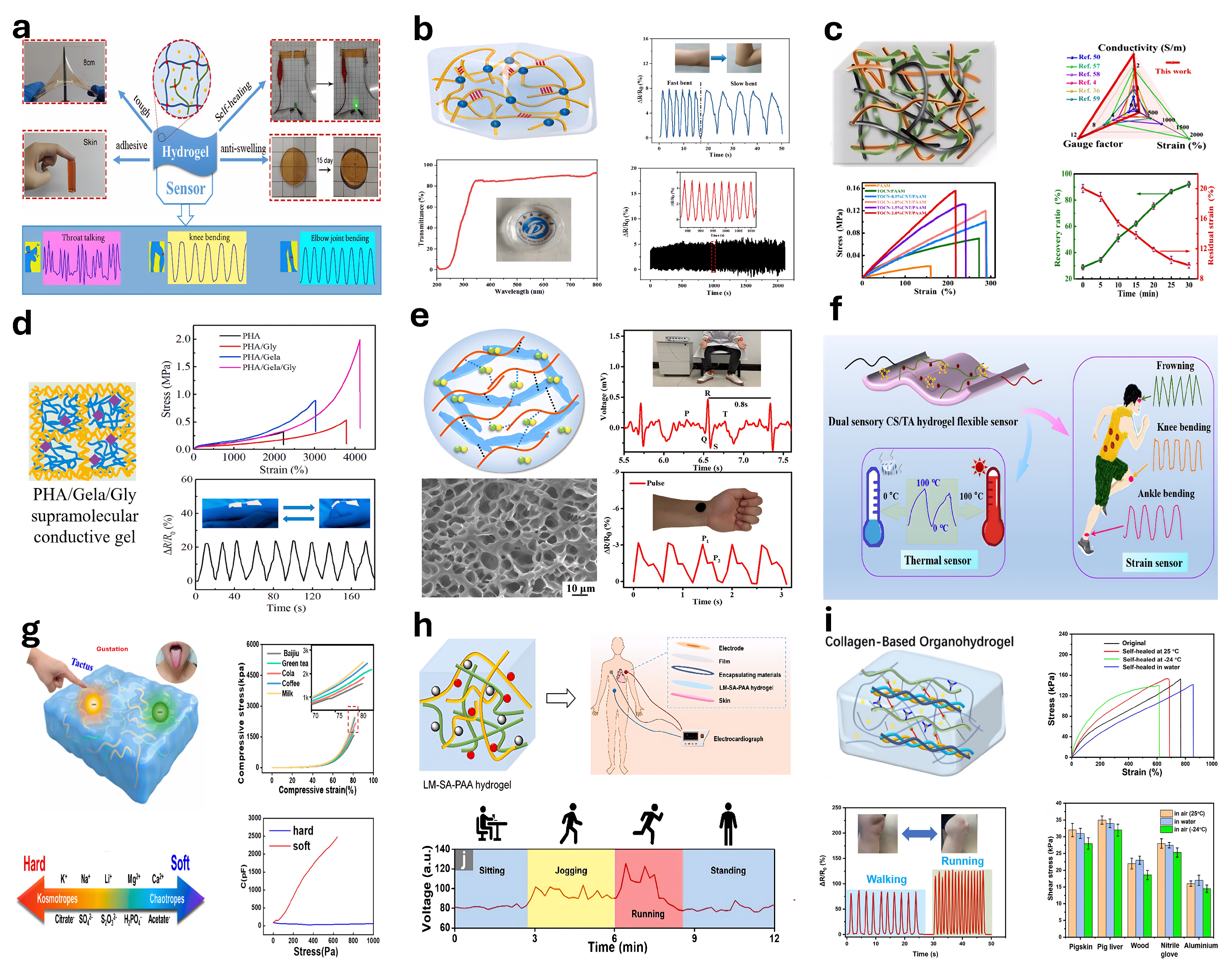
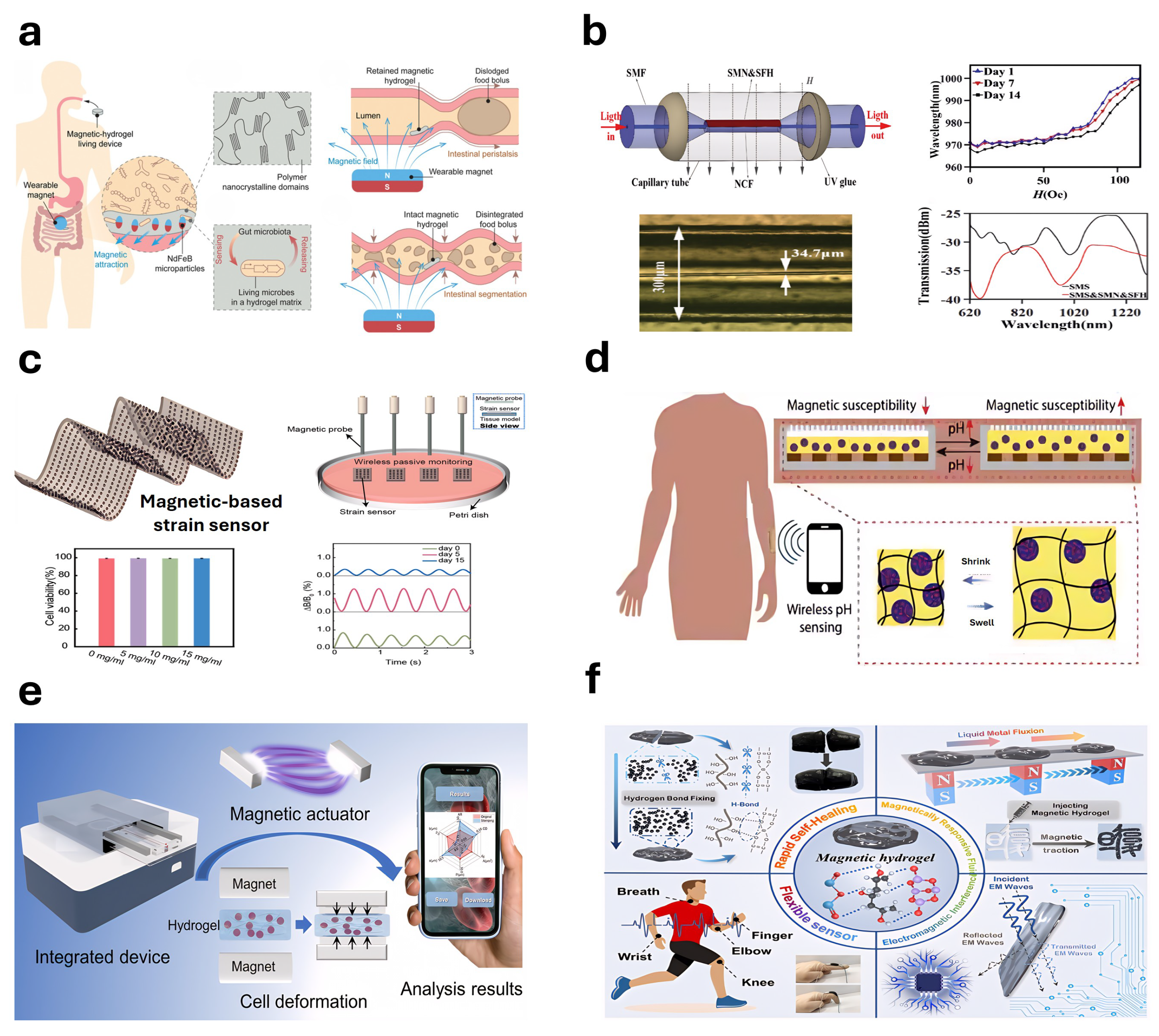
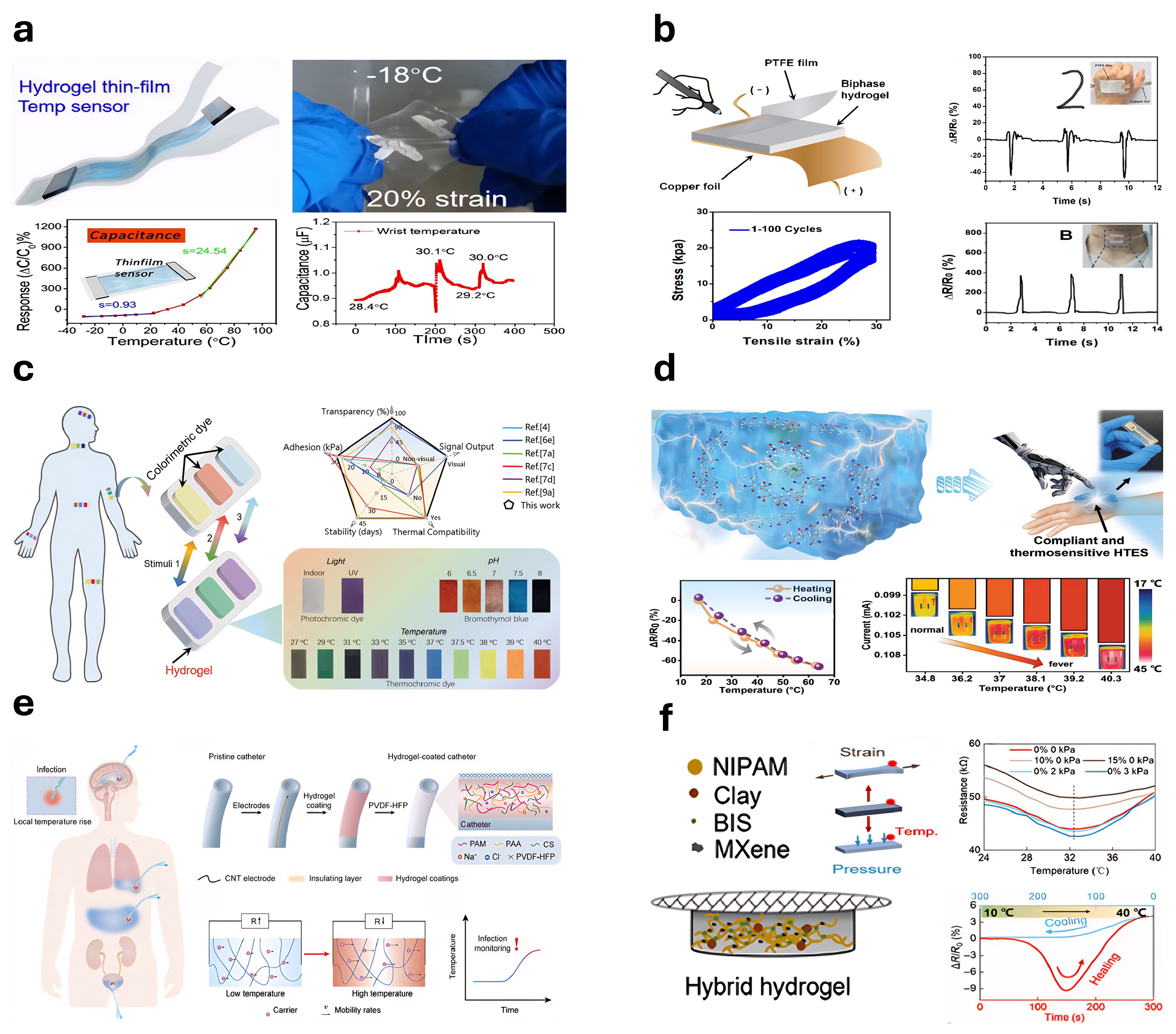
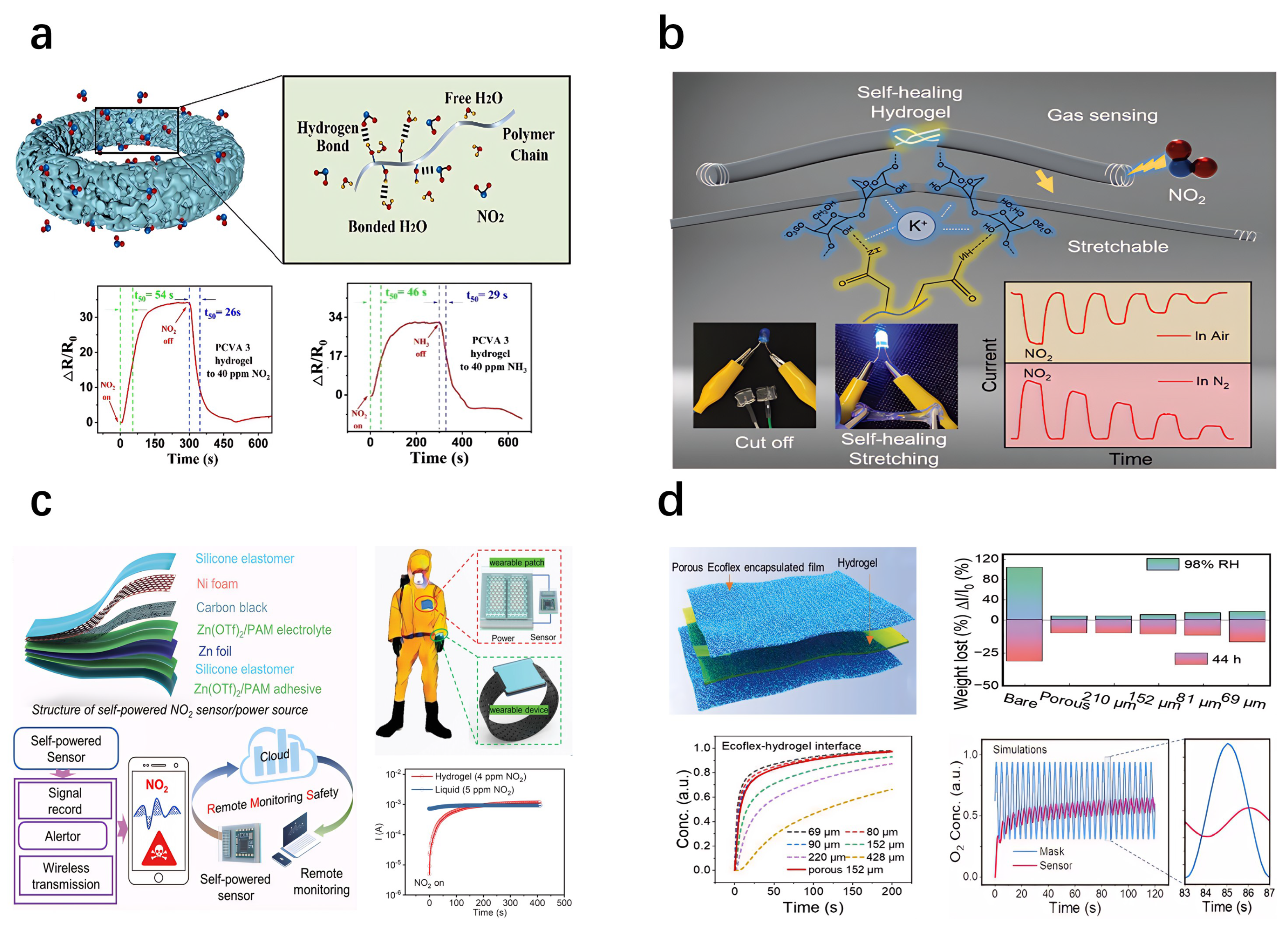
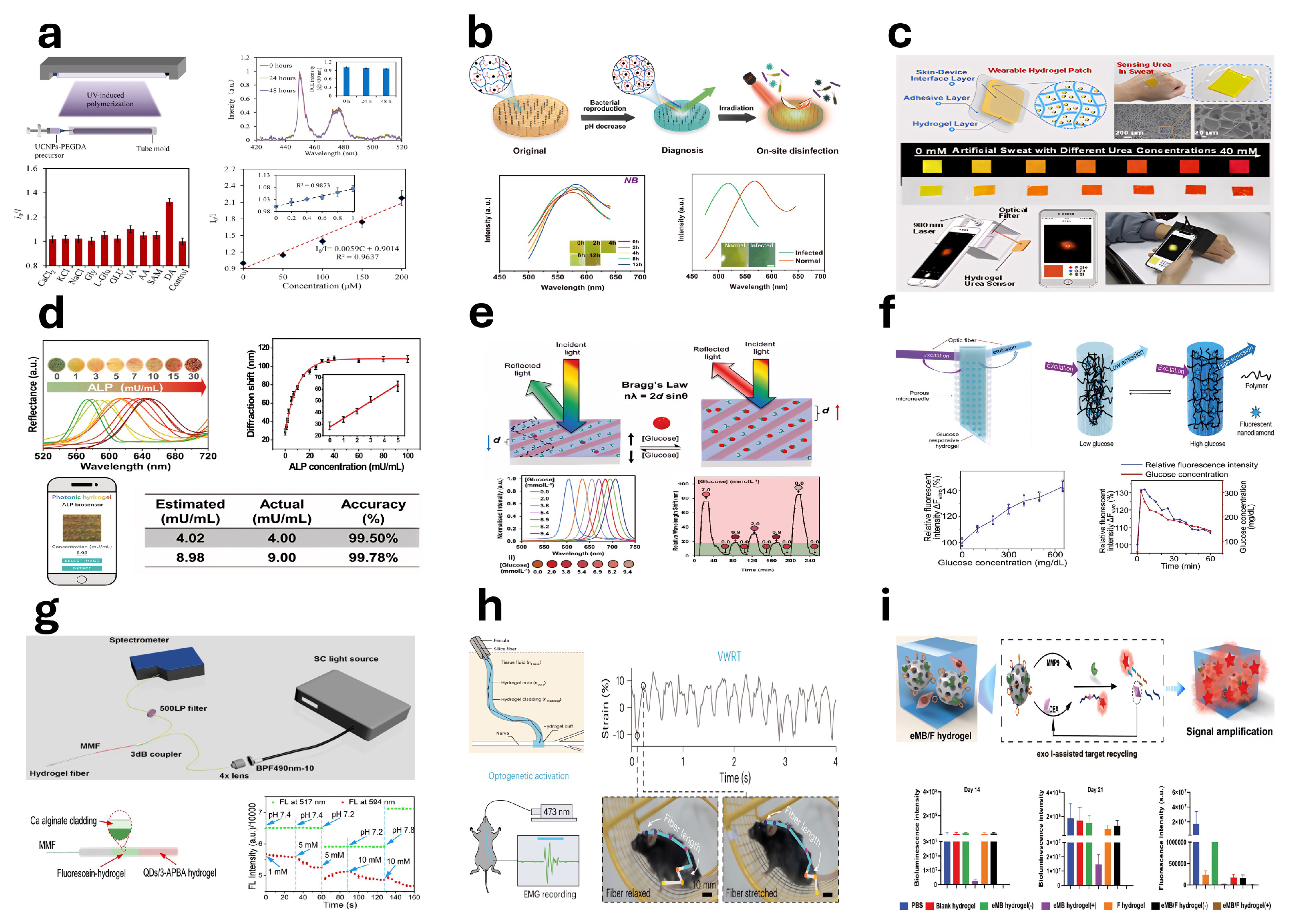
| Research Area | Research Direction | Hydrogel | Ref. |
|---|---|---|---|
| Flexible Electronics | Ionic hydrogel sensors | Collagen-PAAm | [53] |
| Enhanced mechanical properties | Metal-coordination HGs | [54] | |
| Environmental Applications | Solar-driven water purification | LSAG | [55] |
| Pollutant adsorption mechanisms | Double-network HGs | [57] | |
| Soft Robotics | Extreme-environment robotics | Multifunctional ionic HGs | [56] |
| Gradient-responsive robotics | Gradient-structured HGs | [58] | |
| Medical simulation robotics | Carbon-nanomaterial HGs | [59] | |
| Fire-resistant robotic skins | MMT-biocompatible HGs | [60] | |
| Microfabrication and Security | Micro/nano photonics fabrication | Photonic HGs | [51] |
| 3D encryption/decryption | pH-responsive degradable HGs | [61,62] |
| Research Methods | Indications | Hydrogel | Ref. |
|---|---|---|---|
| Vivo | Infected wound | PN-AP | [63] |
| Acutely injured retina | Cur@PDA@GelCA | [68] | |
| Wounds in infants and diabetics | PGA-GelMA | [76] | |
| Gastrointestinal Treatment | e-GLUE | [77] | |
| Fostering bone regeneration | O2-PSSG | [69] | |
| Contact lenses | PVA | [73] | |
| Tumor treatment | CR–PLD–Fe3O4–Ag | [78] | |
| Oral insulin delivery | A-C-DNA | [79] | |
| Oral cavity wound | PAM-G-CS | [80] | |
| Hemostasis and Wound Sealing | RAAS | [81] | |
| Magnetic controlled drug release | SA-NHS@CS | [71] | |
| Active target delivery | NEs@EM@nanogels | [72] | |
| Vitro | Sensor/Intelligent manipulator | Nacl@PAAm | [82] |
| Sensor/Continuum robots | Na-Alg@PDMS | [83] |
| Field | Application | Hydrogel | Ref. |
|---|---|---|---|
| Agriculture | Fertilizer and water management | Superabsorbent hydrogel | [95] |
| Pesticide detection | Wearable hydrogel sensor | [96] | |
| Urban agriculture | Hydrogel system | [97] | |
| Atmospheric water harvesting | Biopolymer hydrogel | [98] | |
| Drought resistance | Functional hydrogel | [99] | |
| Fungicide delivery | Polysaccharide supramolecular hydrogel | [100] | |
| Soil moisture retention | Lignin-based hydrogel | [101] | |
| Nitrate storage | PNIPAM hydrogel | [102] | |
| Food Science | Food processing | Pectin hydrogel | [115] |
| Bioactive delivery | Chitosan hydrogel | [103] | |
| Bioactive release | Starch hydrogel | [104] | |
| Structural diversity | Hydrogel crosslinking (Review) | [116] | |
| Food application | Peptide hydrogels (Review) | [117] | |
| Food safety | Functional hydrogels (Review) | [105,106] | |
| Food packaging enhancement | Edible polymer hydrogel | [107] | |
| Sustainable packaging | Gelatin-based hydrogel | [108] | |
| Food preservation | Carboxymethyl hydrogel film | [109] | |
| pH-responsive packaging | Polysaccharide hydrogel | [110] | |
| Cosmetics | Formulation development | Chitosan, hyaluronic acid, alginate | [118] |
| Dermatology | Multifunctional hydrogel | [111] | |
| Moisturizer | Hyaluronic–silicon hydrogel | [112] | |
| Anti-aging | Fullerene-polysaccharide hydrogel | [113] | |
| Safety testing | Hyaluronic acid hydrogel film | [119] | |
| Exfoliating product | Enzyme-based hydrogel | [120] | |
| Moisturizing additives | Green tea nanoparticle hydrogel | [121] | |
| Sunscreen | Yeast–gelatin hydrogel | [114] |
| Crosslinking Type | Crosslinking Mechanism | Reaction Conditions | Ref. |
|---|---|---|---|
| Chemical | Radical Polymerization | Photoinitiator | [129] |
| Dynamic covalent (1,2-dithiolane) | Photoinduced | [130] | |
| Enzyme(TGase) | Temperature/pH | [131] | |
| UV | 2,2-Dimethoxy-2-phenylacetophenone | [132] | |
| Physical | Ionic | CaCO3 | [133] |
| Hydrogen Bonds | HCl | [134] | |
| Hydrogen Bonds | Dimethyl Sulfoxid/Hydrogen-bond Acceptor | [135] | |
| Ionic/Hydrogen Bonds | 2-Amino-4-hydroxy-6-methylpyrimidine/Dimethyl Sulfoxid | [136] | |
| Ionic/Hydrogen Bonds | H2SO4 | [137] | |
| Collaborative Hydrogen Bonds | 2-methyl-2-benzyloxy carbonyl propylene carbonate | [138] | |
| Both | Free Radical/Hydrogen Bond | sGOa/mGOa | [139] |
| Enzymatically Catalyzed Crosslinking | Horseradish Peroxidase/H2O2 | [140] | |
| Ionic/UV | KCl/I2959 | [141] | |
| Freezing/UV | Irgacure 2959 | [142] | |
| Ionic/Enzyme(TG) | TG/Gacl | [143] | |
| Hydrogen Bonds/UV | HCL/I2959 | [144] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Du, J.; Mao, Y. Hydrogel-Based Continuum Soft Robots. Gels 2025, 11, 254. https://doi.org/10.3390/gels11040254
Wang H, Du J, Mao Y. Hydrogel-Based Continuum Soft Robots. Gels. 2025; 11(4):254. https://doi.org/10.3390/gels11040254
Chicago/Turabian StyleWang, Honghong, Jingli Du, and Yi Mao. 2025. "Hydrogel-Based Continuum Soft Robots" Gels 11, no. 4: 254. https://doi.org/10.3390/gels11040254
APA StyleWang, H., Du, J., & Mao, Y. (2025). Hydrogel-Based Continuum Soft Robots. Gels, 11(4), 254. https://doi.org/10.3390/gels11040254









