Optimization of Celery Tail Waste-Based Hydrogels and Application in Soil Water Retention
Abstract
1. Introduction
2. Results and Discussion
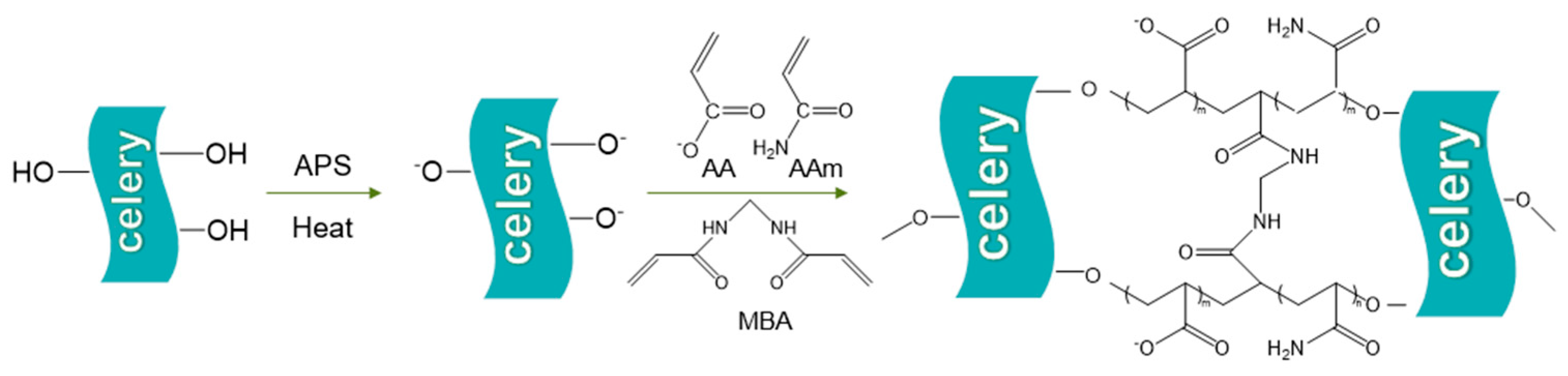
2.1. Synthesis of the Hydrogel
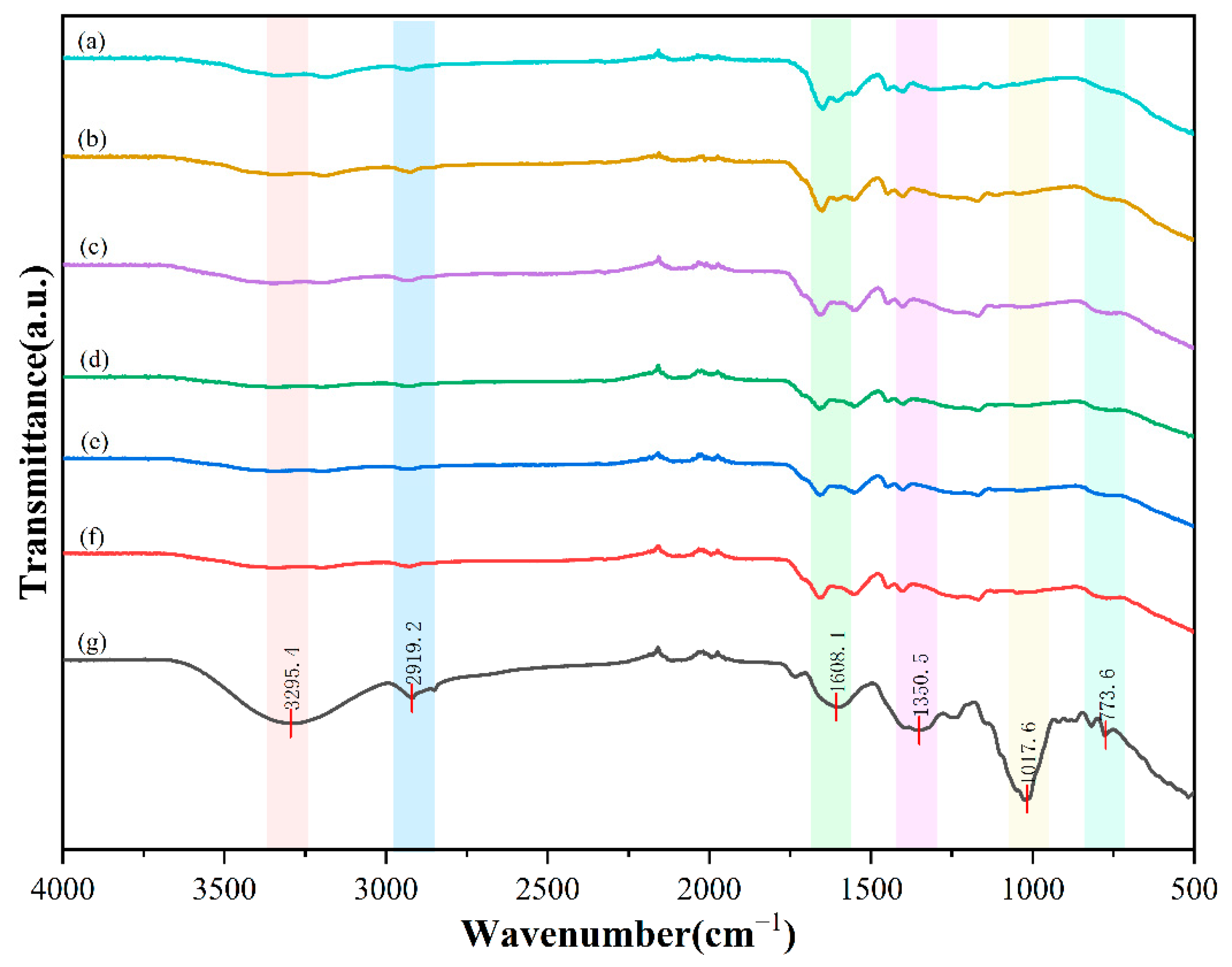
2.2. Analysis of Infrared Characteristics
2.3. Analysis of Microscopic Morphological Characteristics
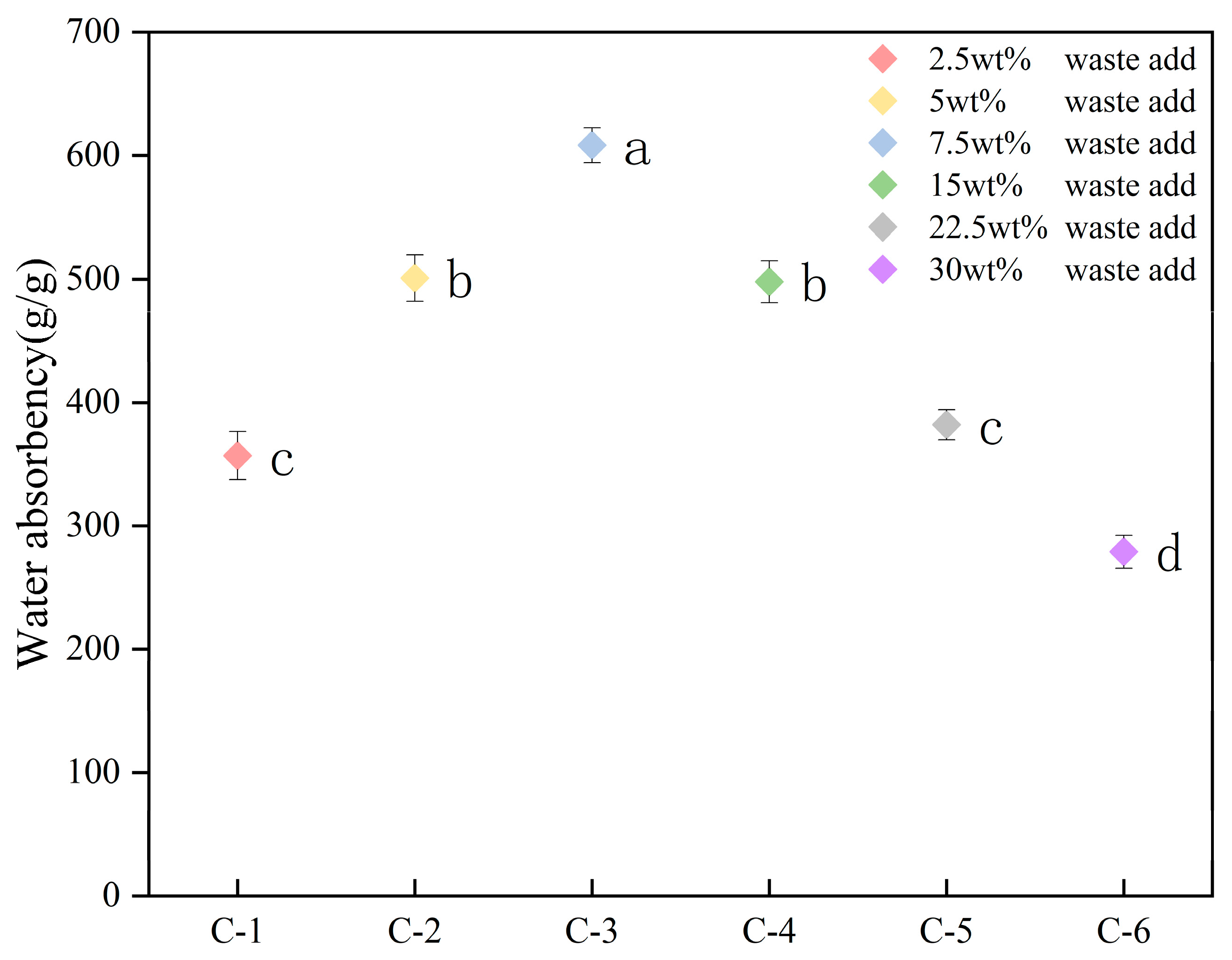
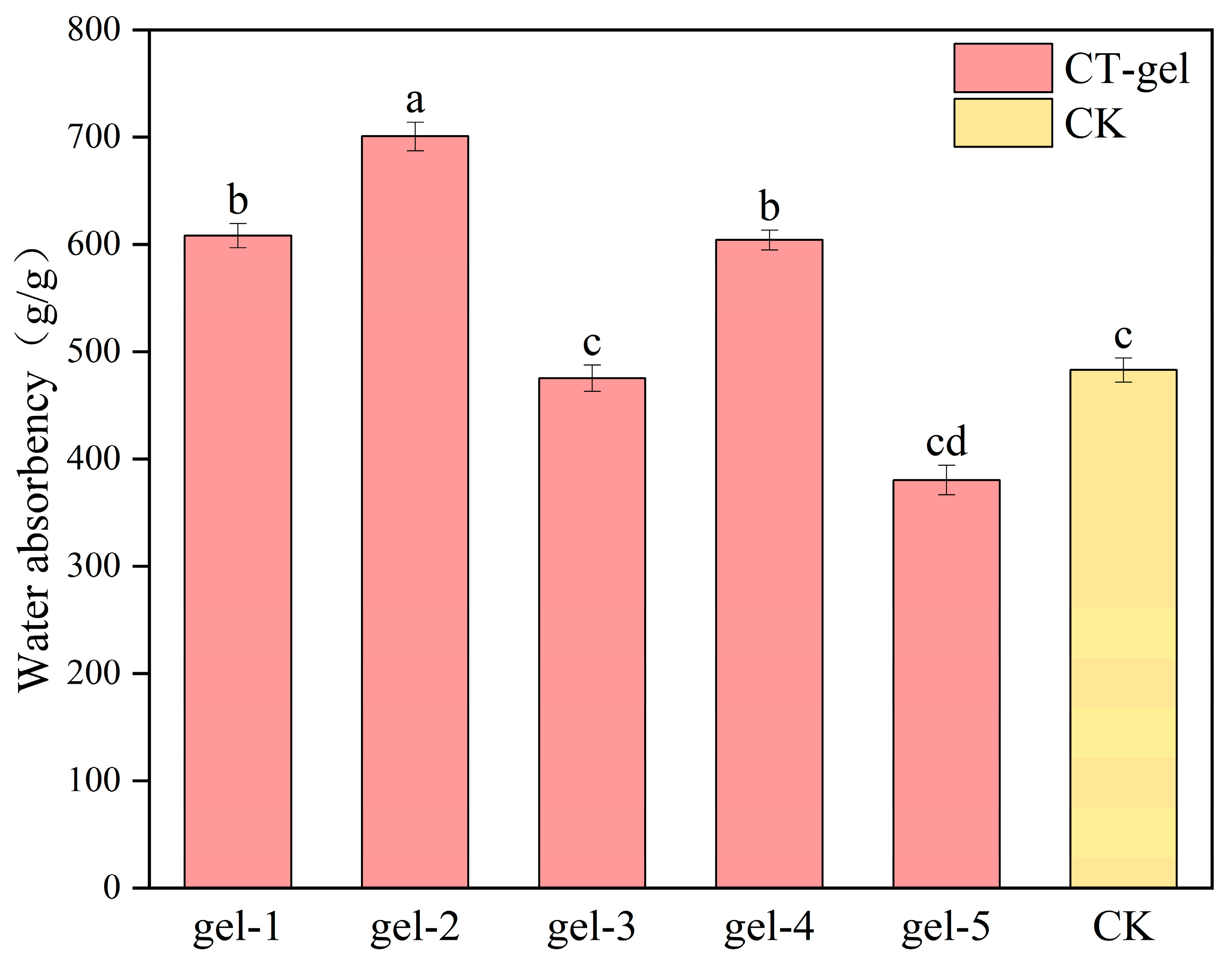
2.4. Analysis of the Water Absorption Rate of the Hydrogel
2.4.1. Hydrogels with Different Addition Amounts of Celery Tail Waste
2.4.2. Hydrogels with Different Proportions
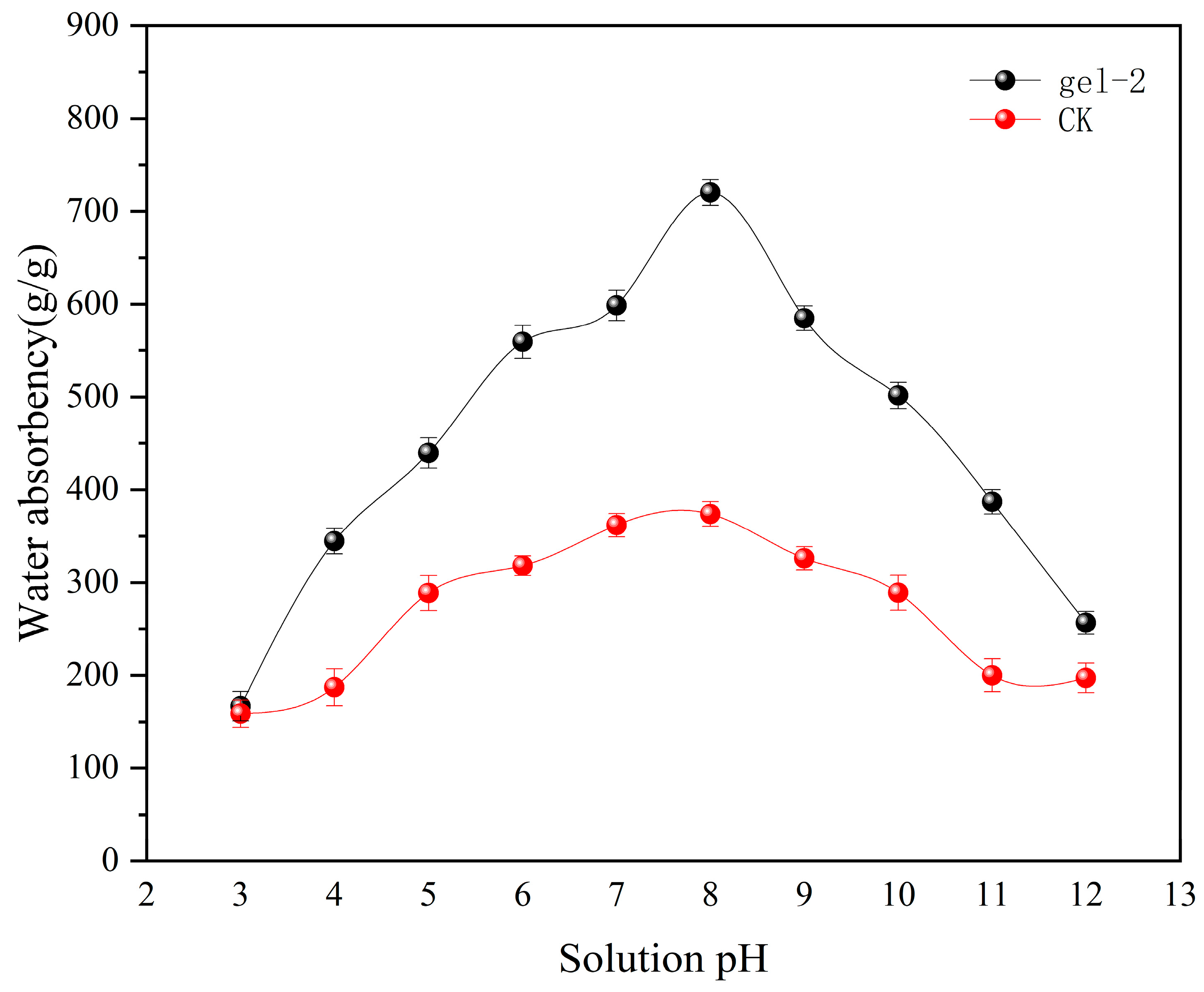
2.5. Analysis of Swelling Capacity of Hydrogels at Different pH
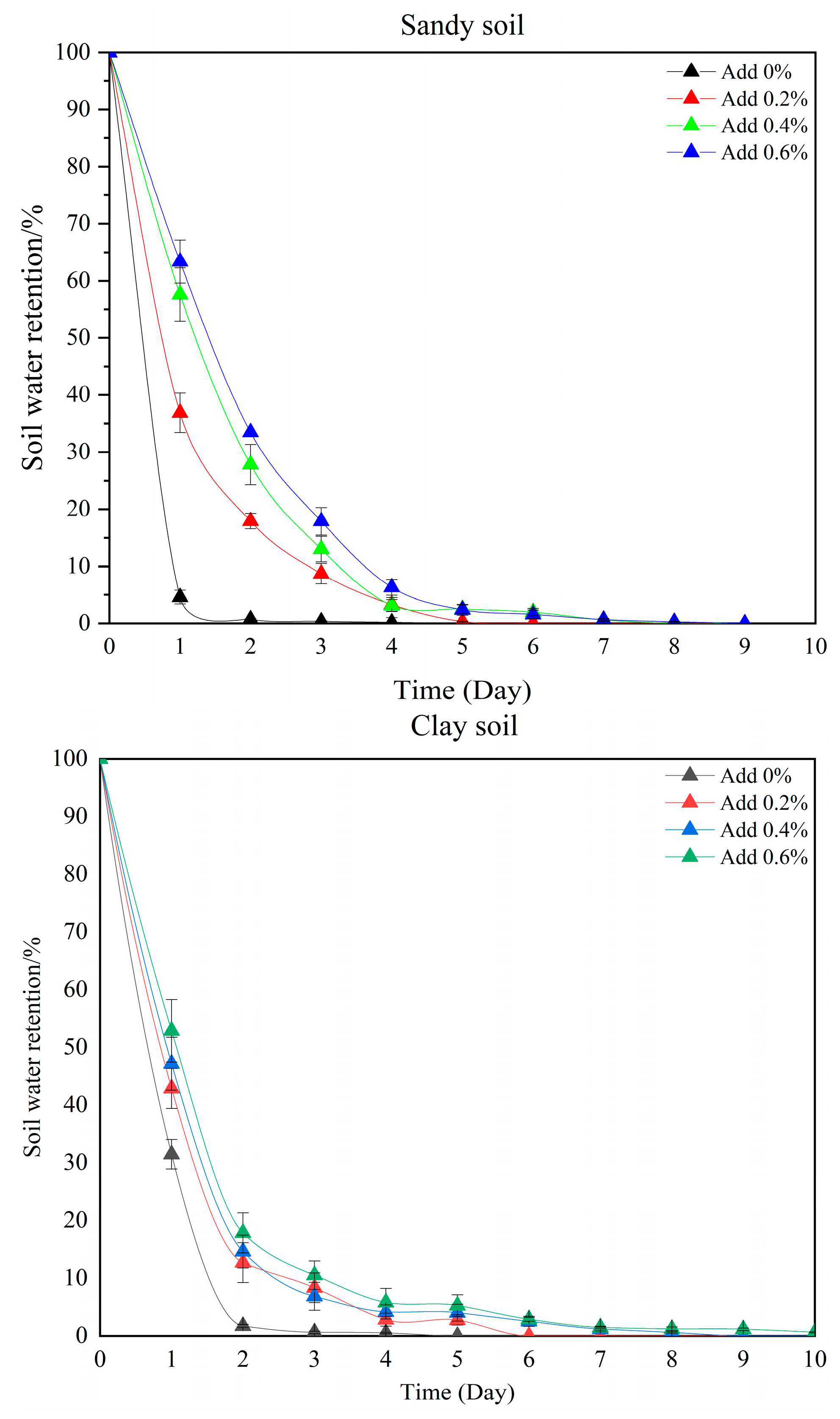
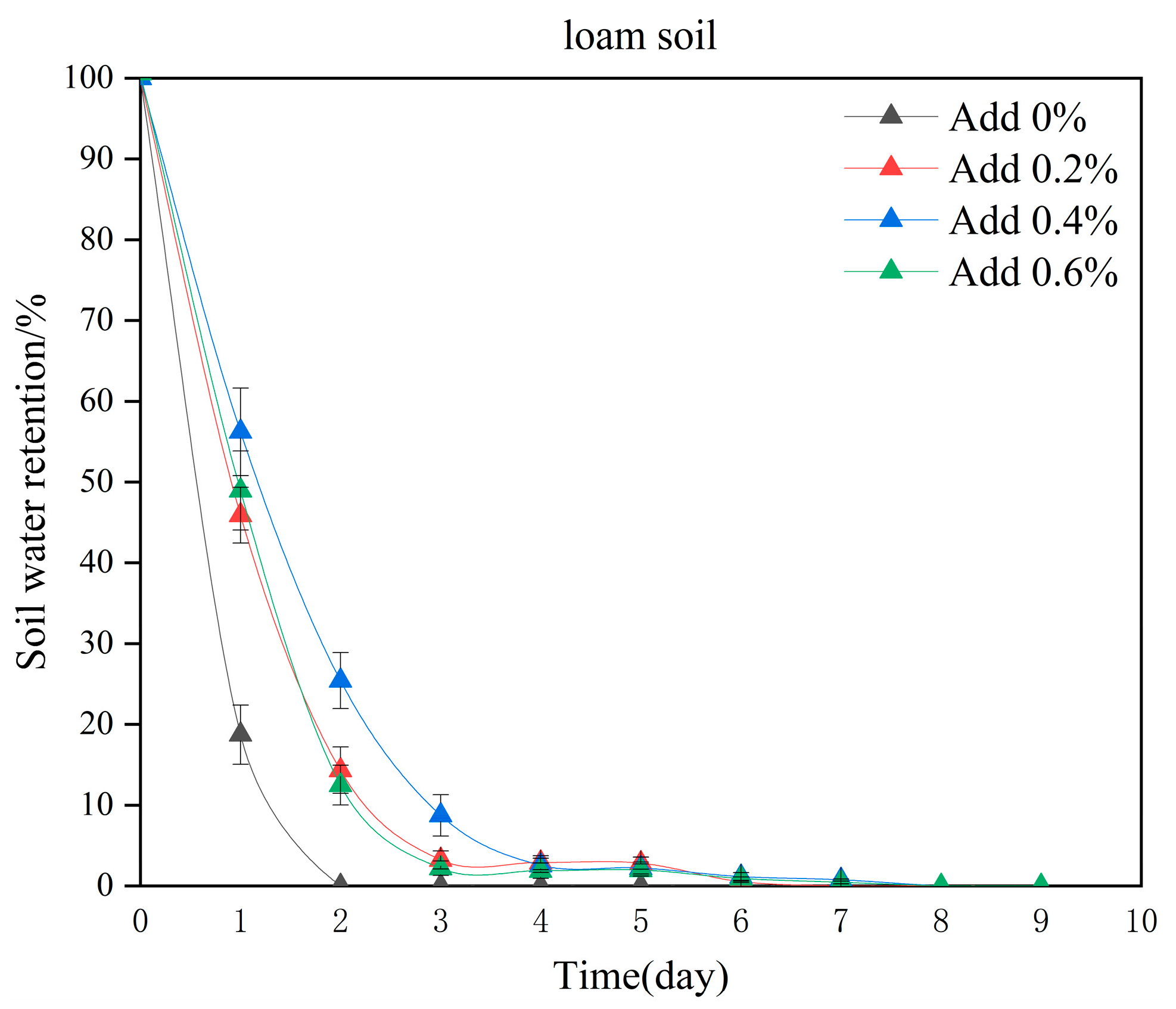
2.6. Analysis of Water Retention and Reusability Characteristics
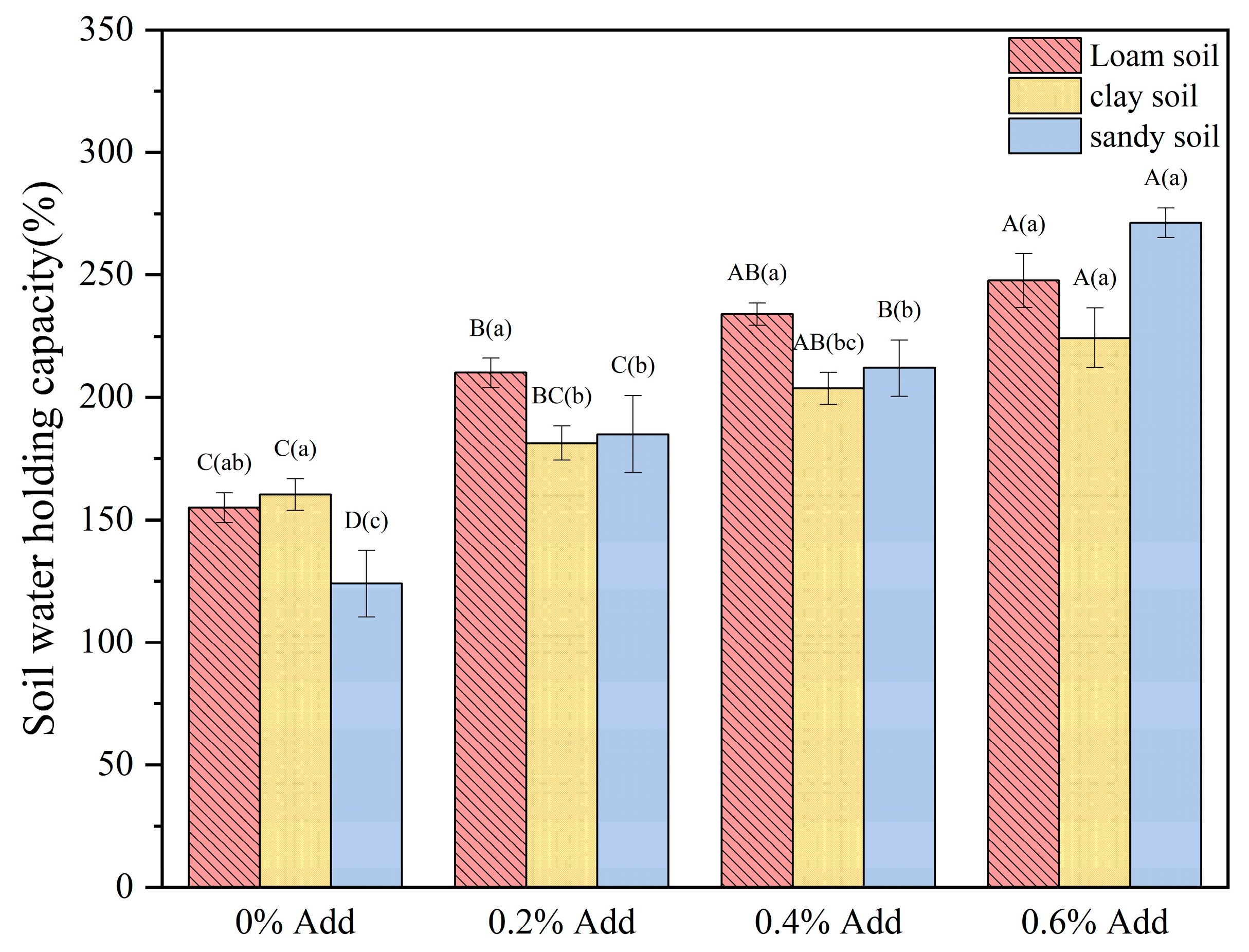
2.7. Analysis of the Water Retention and Water-Holding Capacities of Hydrogels in Different Soils
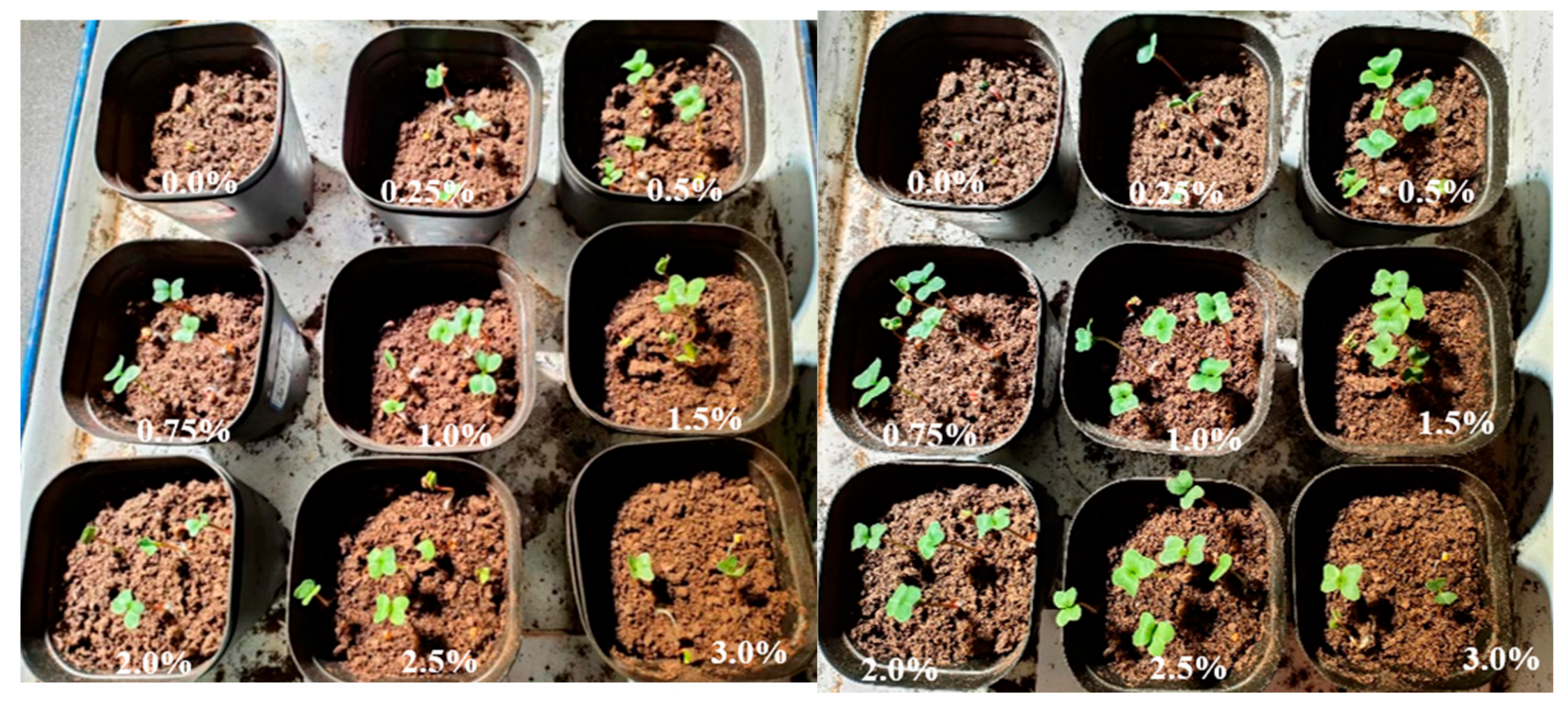
2.8. Evaluation of the Plant Growth Performance of Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Hydrogels
4.3. Determination of the Swelling Ratio of Hydrogel
4.4. Infrared Spectroscopy
4.5. SEM
4.6. Determination of the Reusability of the Hydrogel
4.7. Determination of the Water Retention and Water-Holding Capacity of the Hydrogel
4.8. Pot Experiment
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pattnaik, A.; Ghosh, P.; Poonia, A.K. An overview on advancements in hydrogels for effective wastewater treatment. J. Mol. Liq. 2025, 424, 127120. [Google Scholar] [CrossRef]
- Nguyen Van, T.; Thuy, C.N.; Thi Thu, H.N.; K Thai, P.; Dang, X.T.; Kuwahara, Y. Coconut biochar doped with graphitic carbon nanosheets and α-Fe2O3 shows high adsorption rate for multiple toxic elements in contaminated water. Clean Technol. Environ. Policy 2025, prepublish. [Google Scholar] [CrossRef]
- Huang, D.; Gao, Y.; Zhang, L.; Zhang, R.; Wu, Y.; Guan, H.; Luo, D. Enhancing waste management and nutrient recovery: Preparation of N slow-release fertilizer using sewage sludge and its release behavior, effects on ryegrass (Lolium perenne L.) growth. Biochem. Eng. J. 2025, 216, 109664. [Google Scholar] [CrossRef]
- Kalami, S.; Kalami, S.; Noorbakhsh, R.; Shirani, M.; Koohi, M.K. Development of nanoscale zero-valent iron embedded on polyaniline reinforced with sodium alginate hydrogel microbeads for effective adsorption of arsenic from apatite soil leachate water. Int. J. Biol. Macromol. 2025, 304, 140841. [Google Scholar] [CrossRef]
- Chaurasiya, A.; Pande, P.P.; Shankar, R.; Khare, P.; Kumar, P.; Yadav, N.K.; Dey, K.K. Synthesis and characterization of chemically functionalized novel smart guar gum xanthate based hydrogel: Swelling, isotherm, kinetics, thermodynamic and reusability studies. J. Phys. Chem. Solids 2025, 2001, 12584. [Google Scholar] [CrossRef]
- Teng, B.; Wu, J.; Zhong, Y.; Wang, Y.; Qiao, D.; Quan, R.; Zhou, Z.; Cai, L.; Qi, P.; Luo, Z.; et al. Innovative Utilization of Citrus Sinensis Peel Hydrogels: Enhancing Soil Water Retention and Efficient Removal of Methylene Blue from Wastewater. Polymers 2025, 17, 428. [Google Scholar] [CrossRef]
- Patel, T.; Lata, R.; Arikibe, J.E.; Rohindra, D. Towards sustainable microplastic cleanup: Al/Fe ionotropic chitosan hydrogels for efficient PET removal. Environ. Monit. Assess. 2025, 197, 228. [Google Scholar] [CrossRef]
- Verma, A.; Aljohani, K.; Aljohani, B.S.; Lal, B.; Jadeja, Y.; Ballal, S.; Chahar, M.; Suman, R. Innovations in cellulose-based hydrogels for enhanced wastewater treatment through adsorption. Int. J. Biol. Macromol. 2025, 303, 140660. [Google Scholar] [CrossRef]
- Sowbhagya, H.B. Chemistry, technology, and nutraceutical functions of celery (Apium graveolens L.): An overview. Crit. Rev. Food Sci. Nutr. 2014, 54, 389–398. [Google Scholar] [CrossRef]
- Wang, L.; Hemmatpour, H.; Rudolf, P.; Gerlach, D.; Euverink, G.J.W.; Picchioni, F. Swollen hydrogels with strong mechanical characteristics: A superior adsorbent for the sustainable removal of diclofenac sodium. J. Colloid Interface Sci. 2025, 686, 754–763. [Google Scholar] [CrossRef]
- Wei, Z.; Xi, H.; Pei, X.; Zhang, X.; Qiu, M.; Huang, T.; Wang, Z.; Jiang, J.; Du, J.; Jian, D. Methods and mechanisms for enhancing the water retention properties of Jiuzhaigou disintegrated rubble soils. J. Mt. Sci. 2025, 22, 1–18. [Google Scholar] [CrossRef]
- Mokshina, N.; Sautkina, O.; Gorshkov, O.; Mikshina, P. A Fresh Look at Celery Collenchyma and Parenchyma Cell Walls Through a Combination of Biochemical, Histochemical, and Transcriptomic Analyses. Int. J. Mol. Sci. 2025, 26, 738. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Choudhary, P.; Majeed, A.; Guleria, S.; Kumar, M.; Rana, A.K.; Rajauria, G. Cellulose based membranes, hydrogels and aerogels for water treatment application. Ind. Crops Prod. 2025, 225, 120474. [Google Scholar] [CrossRef]
- Simion, A.-I.; Grigoraș, C.-G.; Favier, L. Batch Adsorption of Orange II Dye on a New Green Hydrogel—Study on Working Parameters and Process Enhancement. Gels 2025, 11, 79. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, X.; Liu, J.; Zhang, Z.; Qin, C.; Zhao, Y.; Wu, G.; Wu, N.; Xu, W. Synergistic Remediation of Cadmium Pollution in Saline-Alkali Soil by Hydrogel and Suaeda salsa. ACS Appl. Mater. Interfaces 2025, 17, 3911–3923. [Google Scholar] [CrossRef]
- Hou, X.; Chu, R.; Zhang, N.; Yan, J. Synergistic function of abscisic acid and β-amylase contributed to relatively high biomass accumulation in wild ancestral green foxtail under water deficit. Plant Physiol. Biochem. 2025, 221, 109620. [Google Scholar] [CrossRef]
- Harighi, H.; Salehi, M.B.; Taghikhani, V.; Mirzaei, M. Optimizing Environmentally—Friendly oil Recovery: Synergies of Imidazolium—Based ionic liquids and carboxymethylcellulose hydrogels. J. Mol. Liq. 2024, 415, 126320. [Google Scholar] [CrossRef]
- Mubark, A.E.; Zaki, E.G.; Hakem, H.A.; Abdel-Rahman, A.A.; Elsaeed, S.M.; Elkelish, A. Guar Gum Hydrogel as a Biosorbent for Cd(II) and Cu(II) Ions: Kinetic, Equilibrium, and Thermodynamic Insights. ChemistrySelect 2025, 10, e202404817. [Google Scholar] [CrossRef]
- Gosukonda, S.J.; Degala, N.V.; Singh, P.H. Assessment of energy cane bagasse—Derived cellulosic microfiber hydrogels on the growth of potted chili peppers. Heliyon 2024, 10, e28972. [Google Scholar] [CrossRef]
- He, Y.; Lin, D.; Jiang, G.; Jiang, L.; Xiao, J.; Li, H.; Zhou, L.; Gou, S. Carboxyl-functionalized montmorillonite composite hydrogel containing alginate as a highly efficient adsorbent for simultaneous removal of cationic dyes and heavy metal ions. J. Water Process Eng. 2025, 69, 106875. [Google Scholar] [CrossRef]
- Yang, Y.; Li, G.; Yue, X.; Zhang, K.; Zhang, Z.; Zheng, H.; Zhao, X.; Li, H.; Zhou, P.; Wu, F.; et al. Advances in biochar composites for environmental sustainability. Adv. Compos. Hybrid Mater. 2024, 8, 74. [Google Scholar] [CrossRef]
- Hryb, W.; Alshawaf, M.; Krysztofiński, P. A Preliminary Assessment of Utilizing Solid Waste Fractions as an Agricultural Growth Medium in Arid Soils. Int. J. Environ. Sci. Dev. 2023, 14, 195–201. [Google Scholar] [CrossRef]
- Ghasemzadeh, H.; Shidrang, S.; Keshtkar Vanashi, A. Nanocomposite magnetic hydrogel based on κ-carrageenan and acrylic acid for the removal of Cd(II), Co(II), Cu(II), and Ni(II); Efficient adsorption enhanced by activated carbon and magnetic nanoparticles. Int. J. Biol. Macromol. 2025, 292, 139164. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Bai, X.; Zhang, P.; Jia, X.; Yao, Y. Soil water retention and release in a small catchment affected by erosion and deposition on China’s Loess Plateau. Catena 2025, 249, 108683. [Google Scholar] [CrossRef]
- Liu, J.; Leung, A.K.; Dong, H.; Jiang, Z. Pore-scale investigation of the hysteretic water retention behaviour of unsaturated soils by tracking preferential water transport. Comput. Geotech. 2025, 179, 107049. [Google Scholar] [CrossRef]
- Armelin, E.; Lanzalaco, S. Insights on the use of biobased hydrogels in electrochemical water treatment. Curr. Opin. Electrochem. 2025, 50, 101635. [Google Scholar] [CrossRef]
- Liu, X.L.; Sun, Y.L.; Hu, Z.Z.; Zhu, C.F. Acryloyl starch/carboxymethyl cellulose grafting copolymerization composite hydrogel for efficient adsorption of methylene blue. J. Polym. Eng. 2025, 45, 73–81. [Google Scholar] [CrossRef]
- Diksha, K.; Bhavanam, A.; Giribabu, D. Lignin and black liquor based composite hydrogels for the enhanced adsorption of malachite green dye from aqueous solutions: Kinetics, rheology and isotherm studies. Int. J. Biol. Macromol. 2025, 296, 139613. [Google Scholar] [CrossRef]
- He, C.; Huang, Y.; Shao, Q.; Kong, F.; Zheng, D.; Qiu, X. Lignin-based ternary composite hydrogel for slow-release of fertilizer and soil water retention. Int. J. Biol. Macromol. 2025, 296, 139679. [Google Scholar] [CrossRef]
- Chen, Z.; Intraravimonmata, C.; Kamchoom, V.; Chen, R.; Sinsamutpadung, N. Biochar Amendment as a Mitigation Against Freezing–Thawing Effects on Soil Hydraulic Properties. Agronomy 2025, 15, 137. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Qiao, S.; Wang, B.; Zeng, X.; Ren, B.; Yang, X. Modified corn stalk carboxymethyl cellulose, acrylic acid and SBA-15 based composite hydrogel used for agricultural water retention. J. Appl. Polym. Sci. 2023, 140, e54613. [Google Scholar] [CrossRef]
- Zhang, P.; Chang, F.; Huo, L.; Yao, Z.; Luo, J. Impacts of Biochar Pyrolysis Temperature, Particle Size, and Application Rate on Water Retention of Loess in the Semiarid Region. Water 2025, 17, 69. [Google Scholar] [CrossRef]
- Nazzari, E.C.; Wernke, G.; Magalhães Ghiotto, G.A.V.; Bergamasco, R.; Gomes, R.G. Hydrogel Biocomposite of Alginate and Mucilage of Opuntia ficus-indica Cactus in the Adsorption of Methylene Blue in Aqueous Solution. ACS Omega 2025, 10, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Xia, J.; Xu, W.; Hashan, D.; Zhen, Q.; She, D. Optimizing soil remediation with multi-functional L-PH hydrogel: Enhancing water retention and heavy metal stabilization in farmland soil. Sci. Total Environ. 2024, 959, 178154. [Google Scholar] [CrossRef]
- Zhou, J.; Cao, B.; Esakkimuthu, S.; Mu, M.; Wang, S. Preparation and Characterization of Hydrogel Materials Based on the Hydrothermal Liquefaction of Enteromorpha prolifera. J. Renew. Mater. 2024, 12, 2069–2078. [Google Scholar] [CrossRef]
- Cao, S.; Zhang, X.; Xie, S.; Wang, C.; Bai, J.; Li, X.; Zhang, R.; Xiao, X.; Hu, J.; Jiang, X. Mechanistic insights into efficient phosphorus adsorption and recovery from water using functional ZnO/ZnFe-LDHs alginate hydrogels. J. Environ. Chem. Eng. 2025, 13, 115091. [Google Scholar] [CrossRef]
- He, C.; Wang, X.; Mou, H.; Hou, W.; He, Q.; Kang, Y.; Kong, H.; Li, R.; Chen, W.; Ao, T.; et al. Sustainable soil rehabilitation with multifunctional mandarin orange peel/tobermorite composite hydrogels: Water retention, immobilization of heavy metals, fertilizer release and bacterial community composition. Chem. Eng. J. 2024, 502, 158030. [Google Scholar] [CrossRef]
- Keshawy, M.; Kamal, R.S.; Abdelhamid, A.E.; Labena, A.; Amin, A.; Hasan, A.M.; Abdel-Raouf, M.E. Novel green sustainable hydrogel composites based on guar gum and algal species for wastewater remediation. Int. J. Environ. Sci. Technol. 2024, prepublish. [Google Scholar] [CrossRef]
- Saje, M.B.; Uthman, T.O.; Surgun, S. Preparation and application of sodium alginate-chitosan hydrogel polyelectrolyte complex adsorbent for treatment of oil spillage. Chem. Data Collect. 2024, 54, 101174. [Google Scholar] [CrossRef]
- Muñoz Martinez, M.X.; Macías Quiroga, I.F.; Sanabria-González, N.R. Adsorption of Cr(VI) Using Organoclay/Alginate Hydrogel Beads and Their Application to Tannery Effluent. Gels 2024, 10, 779. [Google Scholar] [CrossRef]
- Siryk, O.; Tomczyk, A.; Nosalewicz, A.; Szewczuk-Karpisz, K. Novel biochar-filled hydrogel composites: Assessment of multifunctionality and potential in environmental applications. J. Environ. Manag. 2024, 371, 123345. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Xu, Z.; Wu, M.; Cheng, Y.; Zhao, L.; Chen, Z. Insights into the occurrence, distribution and dissipation of widespread agrochemicals in celery agrosystems for joint risk assessment. Environ. Res. 2024, 263, 120036. [Google Scholar] [CrossRef]
- Huang, W.; Jia, Y.; Niu, C.; Zhang, H.; Wang, Y.; Feng, C. Effects of Carbon-Based Modified Materials on Soil Water and Fertilizer Retention and Pollution Control in Rice Root Zone. Sustainability 2024, 16, 6750. [Google Scholar] [CrossRef]
- Lu, W.; Tang, H.; He, T.; Rao, W.; Wang, J. Enhanced UIO-66@PDA/SA hydrogel composite bead soil conditioner exhibiting good water retention, adsorption and efficient recyclability capabilities. J. Mol. Struct. 2024, 1316, 138979. [Google Scholar] [CrossRef]
- Liu, Y.; Xin, Z.; Tian, L.; Villa-Gomez, D.; Wang, W.; Cao, Y. Fabrication of peptide-encapsulated sodium alginate hydrogel for selective gallium adsorption. Int. J. Biol. Macromol. 2024, 263, 130436. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, W.; Pan, G.; Chen, N.; Xie, Q. Sodium alginate/diatom-based blotting hydrogel material for Pb(II) removal: Effect of crosslinker optimization on its composition, selectivity, and desorption rate. Colloids Surf. A Physicochem. Eng. Asp. 2024, 688, 133593. [Google Scholar] [CrossRef]
- Gaur, K.; Siddique, Y.H. The Role of Apigenin in Alleviating the Impact of Environmental Pollutants. Curr. Bioact. Compd. 2024, 21, 70–80. [Google Scholar] [CrossRef]
- Guha, R.D.; Rahmani, F.; Berkowitz, K.; Pasquinelli, M.; Grace, L.R. Temporal evolution of the behavior of absorbed moisture in a damaged polymer-quartz composite: A molecular dynamics study. Comput. Mater. Sci. 2022, 214, 111690. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, W.; Xu, D.; Guan, X.; Wang, S. Occurrence, uptake, and health risk assessment of nonylphenol in soil-celery system simulating long-term reclaimed water irrigation. J. Hazard. Mater. 2021, 406, 124773. [Google Scholar] [CrossRef]




| Number | AA (wt %) | AAm (wt %) | MBA (wt %) | APS (wt %) | Water (g) |
|---|---|---|---|---|---|
| CK | 7 | 3 | 0.025 | 0.2 | 124.144 |
| Gel-1 | 7 | 3 | 0.05 | 0.2 | 124.000 |
| Gel-2 | 7 | 3 | 0.025 | 0.2 | 124.144 |
| Gel-3 | 7 | 3 | 0.1 | 0.2 | 123.712 |
| Gel-4 | 5 | 5 | 0.05 | 0.2 | 126.560 |
| Gel-5 | 3 | 7 | 0.05 | 0.2 | 129.120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhong, Y.; Wu, J.; Xie, Y.; Fang, S.; Cai, L. Optimization of Celery Tail Waste-Based Hydrogels and Application in Soil Water Retention. Gels 2025, 11, 248. https://doi.org/10.3390/gels11040248
Wang Y, Zhong Y, Wu J, Xie Y, Fang S, Cai L. Optimization of Celery Tail Waste-Based Hydrogels and Application in Soil Water Retention. Gels. 2025; 11(4):248. https://doi.org/10.3390/gels11040248
Chicago/Turabian StyleWang, Yuqin, Yuan Zhong, Jun Wu, Yufan Xie, Shiwei Fang, and Liqun Cai. 2025. "Optimization of Celery Tail Waste-Based Hydrogels and Application in Soil Water Retention" Gels 11, no. 4: 248. https://doi.org/10.3390/gels11040248
APA StyleWang, Y., Zhong, Y., Wu, J., Xie, Y., Fang, S., & Cai, L. (2025). Optimization of Celery Tail Waste-Based Hydrogels and Application in Soil Water Retention. Gels, 11(4), 248. https://doi.org/10.3390/gels11040248







