Simultaneous Removal of Heavy Metals and Dyes on Sodium Alginate/Polyvinyl Alcohol/κ-Carrageenan Aerogel Beads
Abstract
1. Introduction
2. Results and Discussion
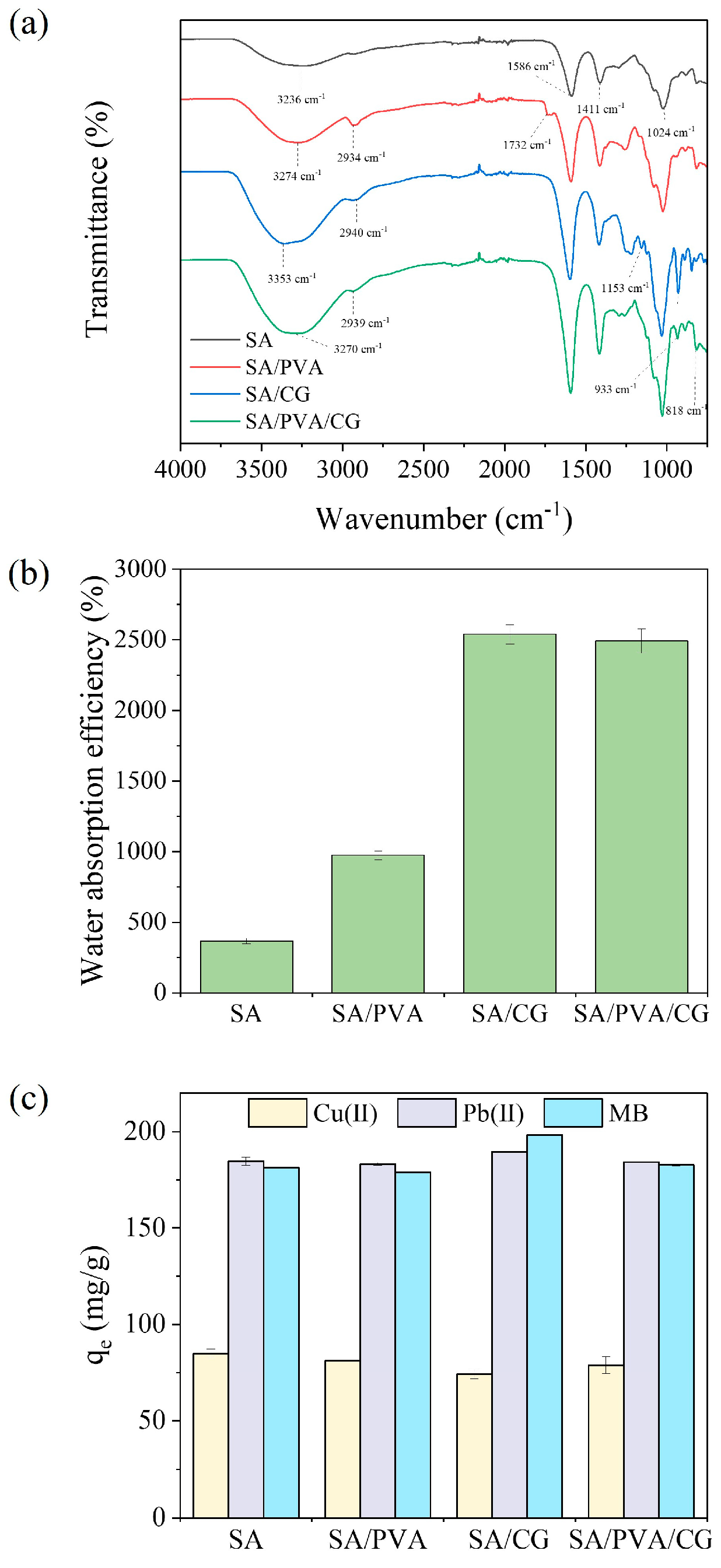
2.1. Comparison of the Characteristics and Adsorption Capacity of the SA-Based Aerogel Beads
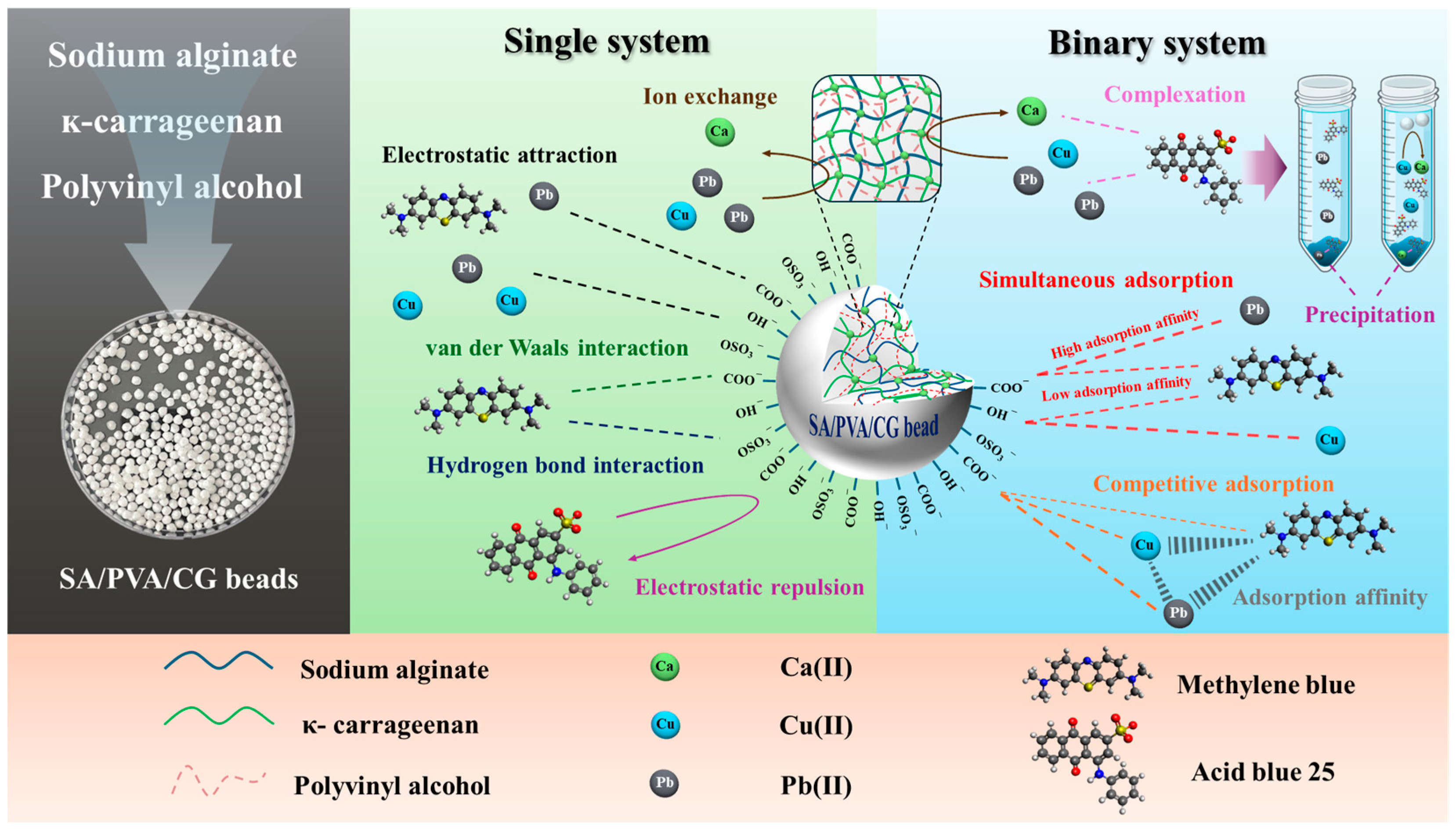
2.2. Adsorption Mechanisms for the SA/PVA/CG Aerogel Beads in Single-Pollutant Removal
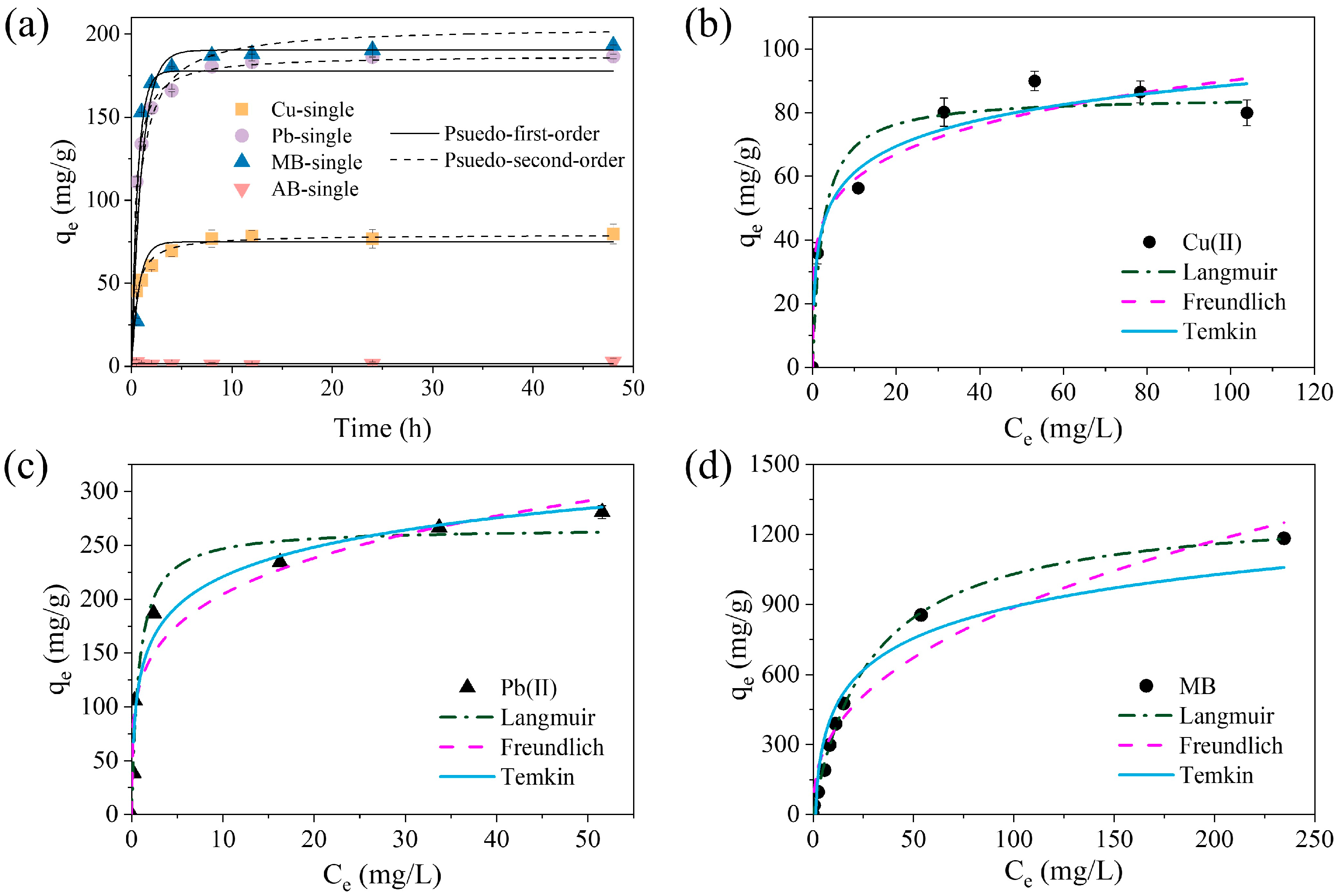
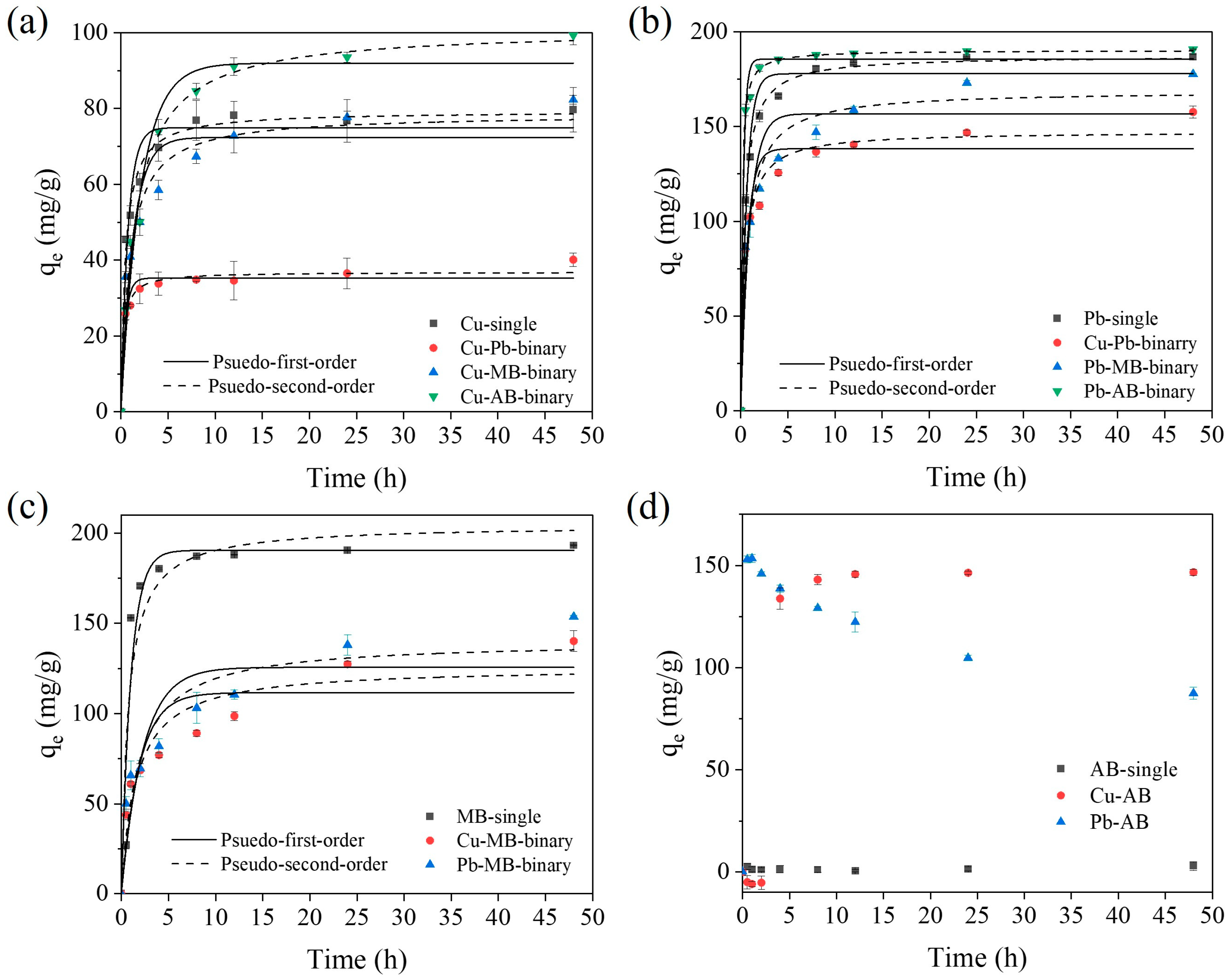
2.2.1. Adsorption Kinetics
2.2.2. Adsorption Isotherms
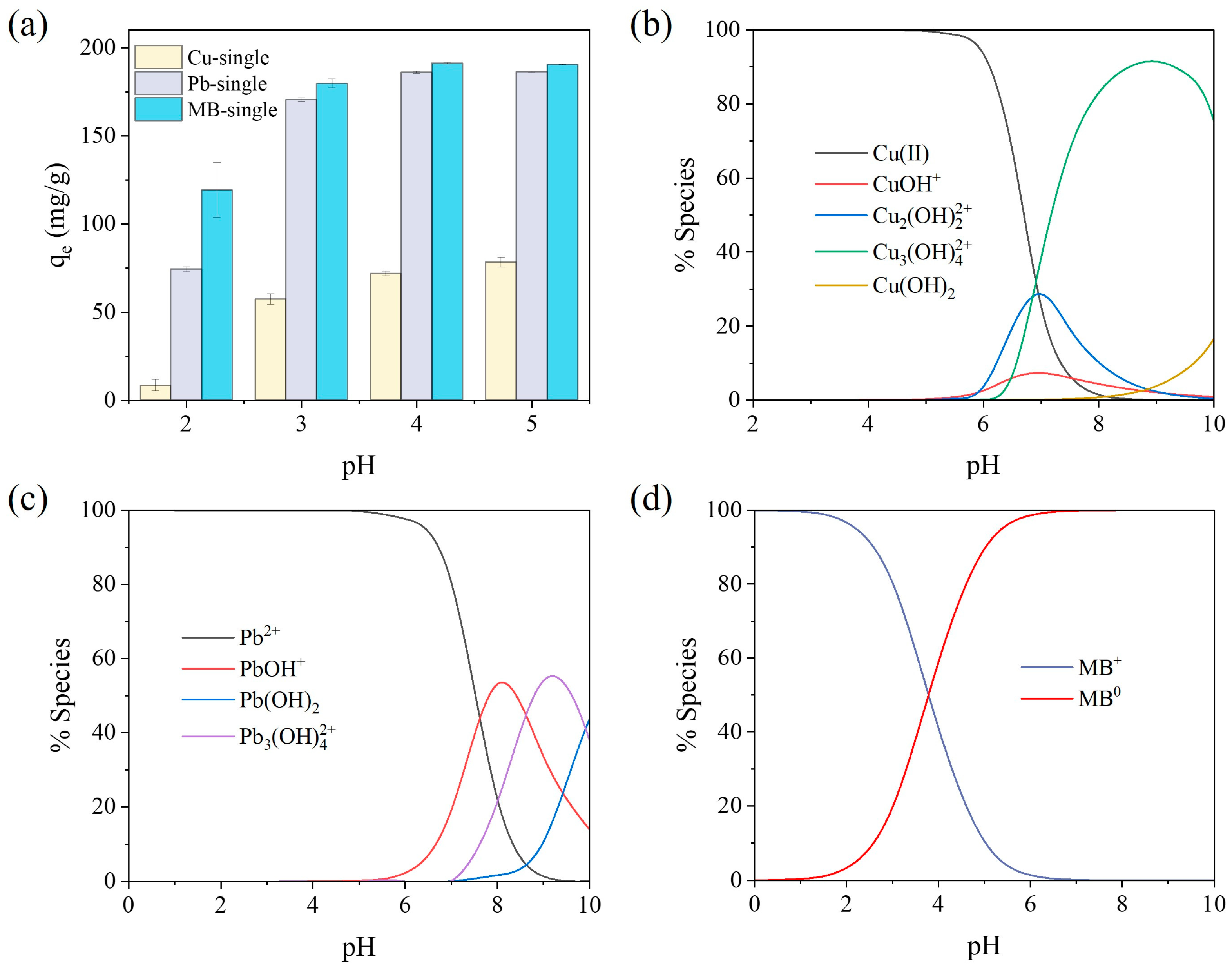
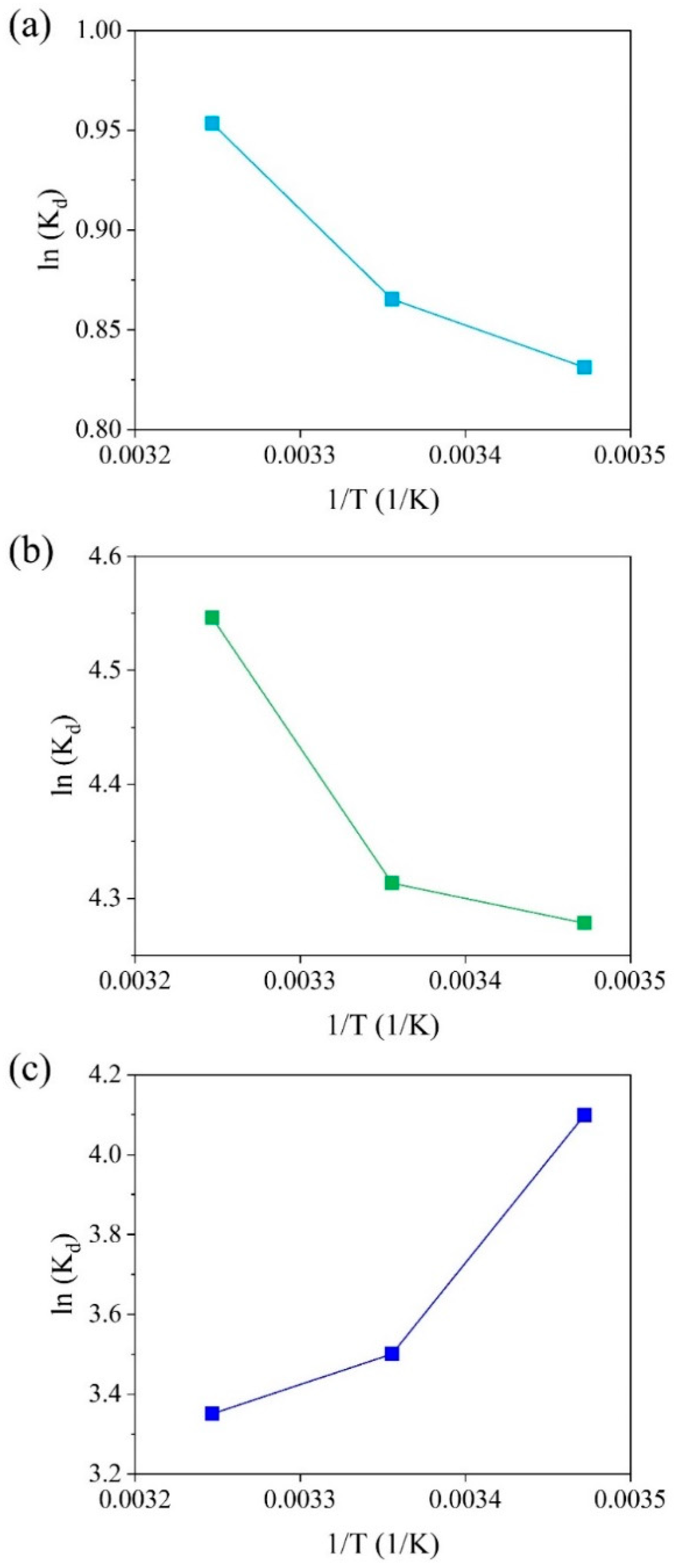
2.2.3. Effects of pH and Temperature
2.3. Adsorption Mechanisms for the SA/PVA/CG Aerogel Beads in Binary-Pollutant Removal
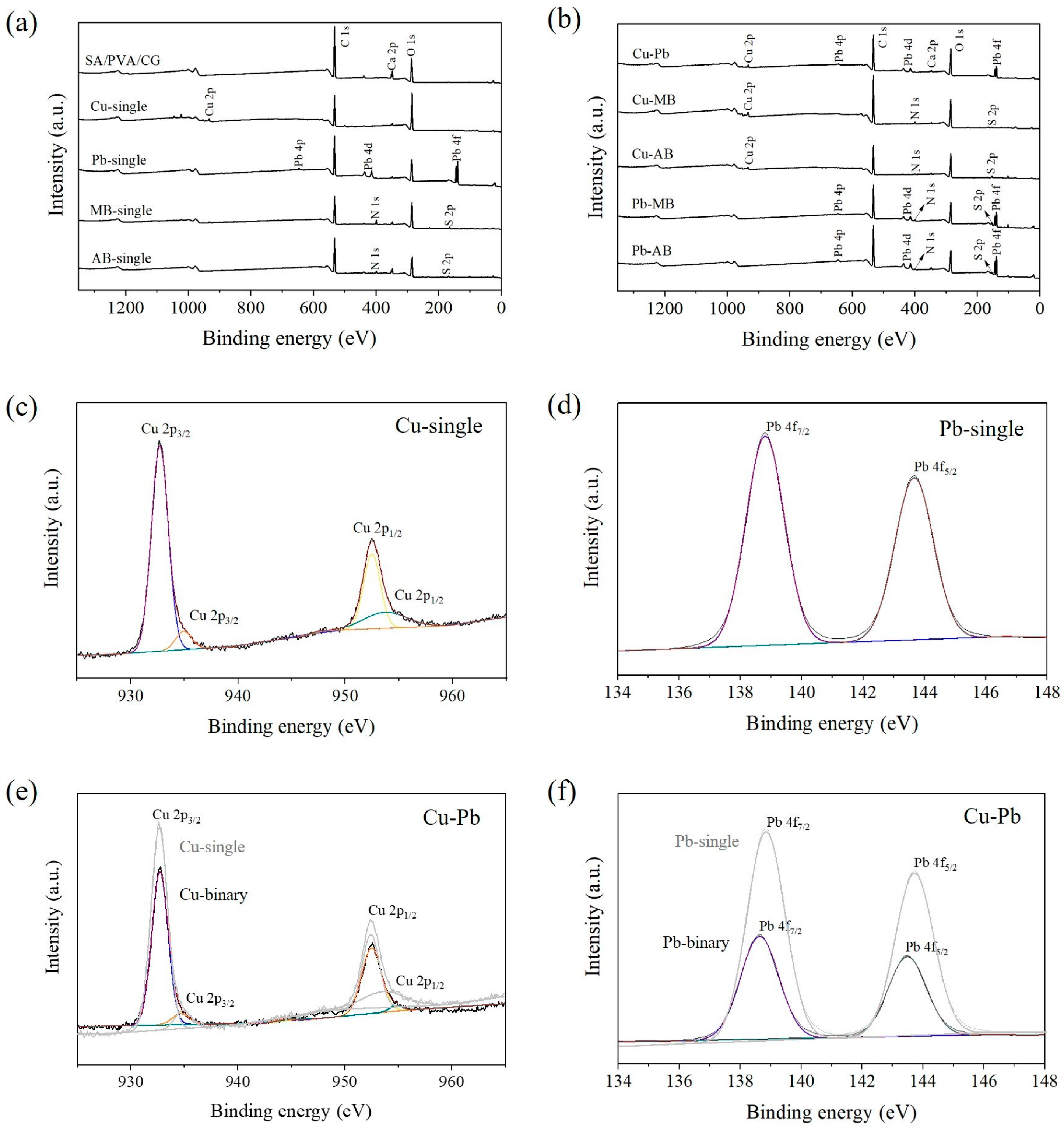
2.4. Verification of the Adsorption Mechanisms Using XPS Analysis
2.5. Acute Toxicity and Biodegradability Assessment of the SA/PVA/CG Aerogel Beads
3. Conclusions
4. Materials and Methods
4.1. Materials
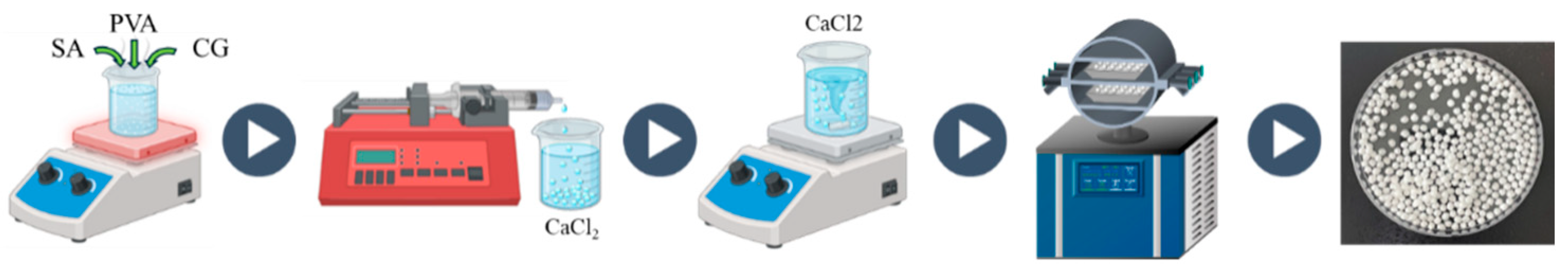
4.2. Synthesis of SA-Based Aerogel Beads
4.3. Comparison of the Characteristics and Adsorption Capacity of the SA-Based Beads
4.4. Adsorption Mechanisms for the SA/PVA/CG Beads in Single-Pollutant Systems
4.5. Adsorption Performance of the SA/PVA/CG Beads Under Binary-Pollutant Conditions
4.6. Acute Toxicity and Biodegradation of the SA/PVA/CG Beads
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adel, M.; Ahmed, M.A.; Elabiad, M.A.; Mohamed, A.A. Removal of heavy metals and dyes from wastewater using graphene oxide-based nanomaterials: A critical review. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100719. [Google Scholar] [CrossRef]
- Sinha, A.; Osborne, W.J. Biodegradation of reactive green dye (RGD) by indigenous fungal strain VITAF-1. Int. Biodeterior. Biodegrad. 2016, 114, 176–183. [Google Scholar] [CrossRef]
- Islam, M.A.; Ali, I.; Karim, S.M.A.; Hossain Firoz, M.S.; Chowdhury, A.-N.; Morton, D.W.; Angove, M.J. Removal of dye from polluted water using novel nano manganese oxide-based materials. J. Water Process Eng. 2019, 32, 100911. [Google Scholar] [CrossRef]
- Hammood, Z.A.; Chyad, T.F.; Al-Saedi, R. Adsorption Performance of Dyes Over Zeolite for Textile Wastewater Treatment. Ecol. Chem. Eng. S 2021, 28, 329–337. [Google Scholar] [CrossRef]
- Velusamy, S.; Roy, A.; Sundaram, S.; Kumar Mallick, T. A Review on Heavy Metal Ions and Containing Dyes Removal Through Graphene Oxide-Based Adsorption Strategies for Textile Wastewater Treatment. Chem. Rec. 2021, 21, 1570–1610. [Google Scholar] [CrossRef]
- Chen, X.; Hossain, M.F.; Duan, C.; Lu, J.; Tsang, Y.F.; Islam, M.S.; Zhou, Y. Isotherm models for adsorption of heavy metals from water—A review. Chemosphere 2022, 307, 135545. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, T.I.; Radwan, E.K.; El-Wakeel, S.T. Unary and binary adsorption of anionic dye and toxic metal from wastewater using 3-aminopropyltriethoxysilane functionalized porous cellulose acetate microspheres. Microporous Mesoporous Mater. 2022, 338, 111996. [Google Scholar] [CrossRef]
- Aslam, A.A.; Hassan, S.U.; Saeed, M.H.; Kokab, O.; Ali, Z.; Nazir, M.S.; Siddiqi, W.; Aslam, A.A. Cellulose-based adsorbent materials for water remediation: Harnessing their potential in heavy metals and dyes removal. J. Clean. Prod. 2023, 421, 138555. [Google Scholar] [CrossRef]
- Hernandez-Montoya, V.; Perez-Cruz, M.A.; Mendoza-Castillo, D.I.; Moreno-Virgen, M.R.; Bonilla-Petriciolet, A. Competitive adsorption of dyes and heavy metals on zeolitic structures. J. Environ. Manag. 2013, 116, 213–221. [Google Scholar] [CrossRef]
- Zhao, F.; Repo, E.; Yin, D.; Meng, Y.; Jafari, S.; Sillanpaa, M. EDTA-Cross-Linked beta-Cyclodextrin: An Environmentally Friendly Bifunctional Adsorbent for Simultaneous Adsorption of Metals and Cationic Dyes. Environ. Sci. Technol. 2015, 49, 10570–10580. [Google Scholar] [CrossRef]
- Liu, Y.; Ke, Y.; Shang, Q.; Yang, X.; Wang, D.; Liao, G. Fabrication of multifunctional biomass-based aerogel with 3D hierarchical porous structure from waste reed for the synergetic adsorption of dyes and heavy metal ions. Chem. Eng. J. 2023, 451, 138934. [Google Scholar] [CrossRef]
- Zeng, X.; Zhu, J.; Zhang, G.; Wu, Z.; Lu, J.; Ji, H. Molecular-level understanding on complexation-adsorption-degradation during the simultaneous removal of aqueous binary pollutants by magnetic composite aerogels. Chem. Eng. J. 2023, 468, 143536. [Google Scholar] [CrossRef]
- Tovar-Gomez, R.; Rivera-Ramirez, D.A.; Hernandez-Montoya, V.; Bonilla-Petriciolet, A.; Duran-Valle, C.J.; Montes-Moran, M.A. Synergic adsorption in the simultaneous removal of acid blue 25 and heavy metals from water using a Ca(PO3)2-modified carbon. J. Hazard. Mater. 2012, 199–200, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Yue, W.; Zhao, H.; Long, F.; Cao, Y.; Pan, X. Simultaneous capture of methyl orange and chromium(vi) from complex wastewater using polyethylenimine cation decorated magnetic carbon nanotubes as a recyclable adsorbent. RSC Adv. 2019, 9, 4722–4734. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, G.; Wen, J.; Li, X.; Zhu, J.; Wu, Z. Simultaneous removal of aqueous same ionic type heavy metals and dyes by a magnetic chitosan/polyethyleneimine embedded hydrophobic sodium alginate composite: Performance, interaction and mechanism. Chemosphere 2023, 318, 137869. [Google Scholar] [CrossRef] [PubMed]
- Soltani, R.; Marjani, A.; Shirazian, S. A hierarchical LDH/MOF nanocomposite: Single, simultaneous and consecutive adsorption of a reactive dye and Cr(vi). Dalton Trans. 2020, 49, 5323–5335. [Google Scholar] [CrossRef]
- Saravanan, A.; Karishma, S.; Kumar, P.S.; Varjani, S.; Yaashikaa, P.R.; Jeevanantham, S.; Ramamurthy, R.; Reshma, B. Simultaneous removal of Cu(II) and reactive green 6 dye from wastewater using immobilized mixed fungal biomass and its recovery. Chemosphere 2021, 271, 129519. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Hu, S.; Sun, J.; Du, Q.; Yang, X.; Ji, Q.; Wang, Z.; Wang, D.; Xia, Y. Removal of methylene blue from water by cellulose/graphene oxide fibres. J. Exp. Nanosci. 2016, 11, 1156–1170. [Google Scholar] [CrossRef]
- Li, L.; Zhao, J.; Sun, Y.; Yu, F.; Ma, J. Ionically cross-linked sodium alginate/ĸ-carrageenan double-network gel beads with low-swelling, enhanced mechanical properties, and excellent adsorption performance. Chem. Eng. J. 2019, 372, 1091–1103. [Google Scholar] [CrossRef]
- Yi, X.; Sun, F.; Han, Z.; Han, F.; He, J.; Ou, M.; Gu, J.; Xu, X. Graphene oxide encapsulated polyvinyl alcohol/sodium alginate hydrogel microspheres for Cu (II) and U (VI) removal. Ecotoxicol. Environ. Saf. 2018, 158, 309–318. [Google Scholar] [CrossRef]
- Voron’ko, N.G.; Derkach, S.R.; Vovk, M.A.; Tolstoy, P.M. Complexation of kappa-carrageenan with gelatin in the aqueous phase analysed by (1)H NMR kinetics and relaxation. Carbohydr. Polym. 2017, 169, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Cui, T.; Yang, C.; Dai, X.; Ma, J. kappa-Carrageenan/Sodium alginate double-network hydrogel with enhanced mechanical properties, anti-swelling, and adsorption capacity. Chemosphere 2019, 237, 124417. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Guo, C.; Hao, J.; Zhao, Z.; Long, H.; Li, M. Adsorption of heavy metal ions by sodium alginate based adsorbent-a review and new perspectives. Int. J. Biol. Macromol. 2020, 164, 4423–4434. [Google Scholar] [CrossRef]
- Tonnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef]
- Mohd Zain, N.A.; Mohd Suardi, S.; Idris, A. Hydrolysis of liquid pineapple waste by invertase immobilized in PVA–alginate matrix. Biochem. Eng. J. 2010, 50, 83–89. [Google Scholar] [CrossRef]
- Mahdavinia, G.R.; Massoudi, A.; Baghban, A.; Shokri, E. Study of adsorption of cationic dye on magnetic kappa-carrageenan/PVA nanocomposite hydrogels. J. Environ. Chem. Eng. 2014, 2, 1578–1587. [Google Scholar] [CrossRef]
- Croitoru, C.; Pop, M.A.; Bedo, T.; Cosnita, M.; Roata, I.C.; Hulka, I. Physically Crosslinked Poly (Vinyl Alcohol)/Kappa-Carrageenan Hydrogels: Structure and Applications. Polymers 2020, 12, 560. [Google Scholar] [CrossRef]
- Radoor, S.; Kandel, D.R.; Park, K.; Jayakumar, A.; Karayil, J.; Lee, J. Low-cost and eco-friendly PVA/carrageenan membrane to efficiently remove cationic dyes from water: Isotherms, kinetics, thermodynamics, and regeneration study. Chemosphere 2024, 350, 140990. [Google Scholar] [CrossRef]
- Farooq, U.; Teuwen, J.; Dransfeld, C. Toughening of Epoxy Systems with Interpenetrating Polymer Network (IPN): A Review. Polymers 2020, 12, 1908. [Google Scholar] [CrossRef]
- Liu, X.J.; Ren, X.Y.; Guan, S.; Li, H.Q.; Song, Z.K.; Gao, G.H. Highly stretchable and tough double network hydrogels via molecular stent. Eur. Polym. J. 2015, 73, 149–161. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Wang, X.; Zong, L.; Kang, Y.; Wang, A. Fast and Highly Efficient Adsorption Removal of Toxic Pb(II) by a Reusable Porous Semi-IPN Hydrogel Based on Alginate and Poly(Vinyl Alcohol). Front. Chem. 2021, 9, 662482. [Google Scholar] [CrossRef]
- Kim, Y.J.; Min, J. Property modulation of the alginate-based hydrogel via semi-interpenetrating polymer network (semi-IPN) with poly(vinyl alcohol). Int. J. Biol. Macromol. 2021, 193, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Ayouch, I.; Barrak, I.; Kassab, Z.; El Achaby, M.; Barhoun, A.; Draoui, K. Impact of the drying process on the efficiency of alginate beads for cadmium removal from water: Kinetic, isotherm and thermodynamic study. Environ. Technol. Innov. 2020, 20. [Google Scholar] [CrossRef]
- Lim, H.-P.; Ooi, C.-W.; Tey, B.-T.; Chan, E.-S. Controlled delivery of oral insulin aspart using pH-responsive alginate/κ-carrageenan composite hydrogel beads. React. Funct. Polym. 2017, 120, 20–29. [Google Scholar] [CrossRef]
- Rather, R.A.; Bhat, M.A.; Shalla, A.H. Multicomponent interpenetrating metal based Alginate-Carrageenan biopolymer hydrogel beads substantiated by graphene oxide for efficient removal of methylene blue from waste water. Chem. Eng. Res. Des. 2022, 182, 604–615. [Google Scholar] [CrossRef]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Panão, C.O.; Campos, E.L.S.; Lima, H.H.C.; Rinaldi, A.W.; Lima-Tenório, M.K.; Tenório-Neto, E.T.; Guilherme, M.R.; Asefa, T.; Rubira, A.F. Ultra-absorbent hybrid hydrogel based on alginate and SiO2 microspheres: A high-water-content system for removal of methylene blue. J. Mol. Liq. 2019, 276, 204–213. [Google Scholar] [CrossRef]
- Chen, S.C.; Wu, Y.C.; Mi, F.L.; Lin, Y.H.; Yu, L.C.; Sung, H.W. A novel pH-sensitive hydrogel composed of N,O-carboxymethyl chitosan and alginate cross-linked by genipin for protein drug delivery. J. Control. Release 2004, 96, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Shilpa, A.; Agrawal, S.S.; Ray, A.R. Controlled Delivery of Drugs from Alginate Matrix. J. Macromol. Sci. Part C Polym. Rev. 2003, 43, 187–221. [Google Scholar] [CrossRef]
- Cai, W.; Tan, L.; Yu, J.; Jaroniec, M.; Liu, X.; Cheng, B.; Verpoort, F. Synthesis of amino-functionalized mesoporous alumina with enhanced affinity towards Cr(VI) and CO2. Chem. Eng. J. 2014, 239, 207–215. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Yue, R.; Gao, F.; Ren, R.; Wei, J.; Wang, X.; Kong, Z. Functional group-rich hyperbranched magnetic material for simultaneous efficient removal of heavy metal ions from aqueous solution. J. Hazard. Mater. 2020, 384, 121288. [Google Scholar] [CrossRef] [PubMed]
- Kothavale, V.P.; Sharma, A.; Dhavale, R.P.; Chavan, V.D.; Shingte, S.R.; Selyshchev, O.; Dongale, T.D.; Park, H.H.; Zahn, D.R.T.; Salvan, G.; et al. Carboxyl and thiol-functionalized magnetic nanoadsorbents for efficient and simultaneous removal of Pb(II), Cd(II), and Ni(II) heavy metal ions from aqueous solutions: Studies of adsorption, kinetics, and isotherms. J. Phys. Chem. Solids 2023, 172, 111089. [Google Scholar] [CrossRef]
- Granados-Correa, F.; Corral-Capulin, N.G.; Olguín, M.T.; Acosta-León, C.E. Comparison of the Cd(II) adsorption processes between boehmite (γ-AlOOH) and goethite (α-FeOOH). Chem. Eng. J. 2011, 171, 1027–1034. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, C.; Choi, I.; Rengaraj, S.; Yi, J. Arsenic removal using mesoporous alumina prepared via a templating method. Environ. Sci. Technol. 2004, 38, 924–931. [Google Scholar] [CrossRef]
- Zhou, N.; Chen, H.; Xi, J.; Yao, D.; Zhou, Z.; Tian, Y.; Lu, X. Biochars with excellent Pb(II) adsorption property produced from fresh and dehydrated banana peels via hydrothermal carbonization. Bioresour. Technol. 2017, 232, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.S.; Gusain, R.; Kumar, N. Adsorption equilibrium isotherms, kinetics and thermodynamics. In Carbon Nanomaterial-Based Adsorbents for Water Purification, 1st ed.; Kumar, N., Gusain, R., Sinha, R.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 101–118. [Google Scholar]
- Oladoja, N.; Ololade, I.; Idiaghe, J.; Egbon, E. Equilibrium isotherm analysis of the sorption of congo red by palm kernel coat. Open Chem. 2009, 7, 760–768. [Google Scholar] [CrossRef]
- Nharingo, T.; Shoniwa, V.; Hunga, O.; Shumba, M. Exploring the biosorption of methylene blue dye onto acid treated sugarcane bagasse. Int. J. Curr. Res. 2013, 5, e2175. [Google Scholar]
- Deng, L.; Zeng, H.; Shi, Z.; Zhang, W.; Luo, J. Sodium dodecyl sulfate intercalated and acrylamide anchored layered double hydroxides: A multifunctional adsorbent for highly efficient removal of Congo red. J. Colloid Interface Sci. 2018, 521, 172–182. [Google Scholar] [CrossRef]
- Jiao, C.; Xiong, J.; Tao, J.; Xu, S.; Zhang, D.; Lin, H.; Chen, Y. Sodium alginate/graphene oxide aerogel with enhanced strength-toughness and its heavy metal adsorption study. Int. J. Biol. Macromol. 2016, 83, 133–141. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, Y.; Lin, Z.; Zhao, B.; Wang, J.; Xie, J.; Zhang, A. Preparation of a novel bio-adsorbent of sodium alginate grafted polyacrylamide/graphene oxide hydrogel for the adsorption of heavy metal ion. Sci. Total Environ. 2020, 744, 140653. [Google Scholar] [CrossRef]
- Rahmani, Z.; Ghaemy, M.; Olad, A. Removal of heavy metals from polluted water using magnetic adsorbent based on κ-carrageenan and N-doped carbon dots. Hydrometallurgy 2022, 213, 105915. [Google Scholar] [CrossRef]
- Zhang, W.; Deng, Q.; He, Q.; Song, J.; Zhang, S.; Wang, H.; Zhou, J.; Zhang, H. A facile synthesis of core-shell/bead-like poly (vinyl alcohol)/alginate@PAM with good adsorption capacity, high adaptability and stability towards Cu(II) removal. Chem. Eng. J. 2018, 351, 462–472. [Google Scholar] [CrossRef]
- Bai, C.; Wang, L.; Zhu, Z. Adsorption of Cr(III) and Pb(II) by graphene oxide/alginate hydrogel membrane: Characterization, adsorption kinetics, isotherm and thermodynamics studies. Int. J. Biol. Macromol. 2020, 147, 898–910. [Google Scholar] [CrossRef]
- Godiya, C.B.; Xiao, Y.; Lu, X. Amine functionalized sodium alginate hydrogel for efficient and rapid removal of methyl blue in water. Int. J. Biol. Macromol. 2020, 144, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, X.; Qi, Y.; Sun, W.; Men, Y. Preparation of kappa-carrageenan/graphene oxide gel beads and their efficient adsorption for methylene blue. J. Colloid Interface Sci. 2017, 506, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qin, Y. Equilibrium sorption isotherms for of Cu2+ on rice bran. Process Biochem. 2005, 40, 677–680. [Google Scholar] [CrossRef]
- Senobari, S.; Nezamzadeh-Ejhieh, A. A comprehensive study on the photocatalytic activity of coupled copper oxide-cadmium sulfide nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 196, 334–343. [Google Scholar] [CrossRef]
- Mirsalari, S.A.; Nezamzadeh-Ejhieh, A. Focus on the photocatalytic pathway of the CdS-AgBr nano-catalyst by using the scavenging agents. Sep. Purif. Technol. 2020, 250, 117235. [Google Scholar] [CrossRef]
- El Jery, A.; Aldrdery, M.; Shirode, U.R.; Gavilán, J.C.O.; Elkhaleefa, A.; Sillanpää, M.; Sammen, S.S.; Tizkam, H.H. An Efficient Investigation and Machine Learning-Based Prediction of Decolorization of Wastewater by Using Zeolite Catalyst in Electro-Fenton Reaction. Catalysts 2023, 13, 1085. [Google Scholar] [CrossRef]
- Isawi, H. Using Zeolite/Polyvinyl alcohol/sodium alginate nanocomposite beads for removal of some heavy metals from wastewater. Arab. J. Chem. 2020, 13, 5691–5716. [Google Scholar] [CrossRef]
- Mohammed, N.; Grishkewich, N.; Berry, R.M.; Tam, K.C. Cellulose nanocrystal–alginate hydrogel beads as novel adsorbents for organic dyes in aqueous solutions. Cellulose 2015, 22, 3725–3738. [Google Scholar] [CrossRef]
- Almuslem, A.S.; Alnaim, N.; Ibrahim, S.S.; Ibrahim, M.A. Green Synthesis and Characteristics of Cellulose Nanocrystal/Poly Acrylic Acid Nanocomposite Thin Film for Organic Dye Adsorption during Water Treatment. Polymers 2023, 15, 2154. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Chatterjee, S.; Chatterjee, B.P.; Guha, A.K. Adsorptive removal of congo red, a carcinogenic textile dye by chitosan hydrobeads: Binding mechanism, equilibrium and kinetics. Colloids Surfaces A Physicochem. Eng. Asp. 2007, 299, 146–152. [Google Scholar] [CrossRef]
- Ram, R.; Morrisroe, L.; Etschmann, B.; Vaughan, J.; Brugger, J. Lead (Pb) sorption and co-precipitation on natural sulfide, sulfate and oxide minerals under environmental conditions. Miner. Eng. 2021, 163, 106801. [Google Scholar] [CrossRef]
- Cano, E.; Torres, C.L.; Bastidas, J.M. An XPS study of copper corrosion originated by formic acid vapour at 40% and 80% relative humidity. Mater. Corros. 2001, 52, 667–676. [Google Scholar] [CrossRef]
- d’Halluin, M.; Rull-Barrull, J.; Bretel, G.; Labrugère, C.; Le Grognec, E.; Felpin, F.-X. Chemically Modified Cellulose Filter Paper for Heavy Metal Remediation in Water. ACS Sustain. Chem. Eng. 2017, 5, 1965–1973. [Google Scholar] [CrossRef]
- Jiang, X.; An, Q.-D.; Xiao, Z.-Y.; Zhai, S.-R.; Shi, Z. Versatile core/shell-like alginate@polyethylenimine composites for efficient removal of multiple heavy metal ions (Pb2+, Cu2+, CrO42−): Batch and fixed-bed studies. Mater. Res. Bull. 2019, 118. [Google Scholar] [CrossRef]
- Sheng, P.X.; Ting, Y.P.; Chen, J.P.; Hong, L. Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: Characterization of biosorptive capacity and investigation of mechanisms. J. Colloid Interface Sci. 2004, 275, 131–141. [Google Scholar] [CrossRef]
- Chen, P.; Cao, Z.-f.; Wen, X.; Wang, J.; Yang, F.; Qiu, P.; Yue, Y.-j.; Liu, G.-y.; Wang, S.; Zhong, H. A novel mesoporous silicate material (MS) preparation from dolomite and enhancing methylene blue removal by electronic induction. J. Taiwan Inst. Chem. Eng. 2017, 80, 128–136. [Google Scholar] [CrossRef]
- Lima, P.H.; Pereira, S.V.; Rabello, R.B.; Rodriguez-Castellon, E.; Beppu, M.M.; Chevallier, P.; Mantovani, D.; Vieira, R.S. Blood protein adsorption on sulfonated chitosan and kappa-carrageenan films. Colloids Surf. B Biointerfaces 2013, 111, 719–725. [Google Scholar] [CrossRef]
- Berton, S.B.R.; de Jesus, G.A.M.; Sabino, R.M.; Monteiro, J.P.; Venter, S.A.S.; Bruschi, M.L.; Popat, K.C.; Matsushita, M.; Martins, A.F.; Bonafe, E.G. Properties of a commercial kappa-carrageenan food ingredient and its durable superabsorbent hydrogels. Carbohydr. Res. 2020, 487, 107883. [Google Scholar] [CrossRef] [PubMed]
- Fantauzzi, M.; Elsener, B.; Atzei, D.; Rigoldi, A.; Rossi, A. Exploiting XPS for the identification of sulfides and polysulfides. RSC Adv. 2015, 5, 75953–75963. [Google Scholar] [CrossRef]
- Kumar, A.; Sood, A.; Han, S.S. Poly (vinyl alcohol)-alginate as potential matrix for various applications: A focused review. Carbohydr. Polym. 2022, 277, 118881. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Z.; Zheng, H.; Chen, L.; Li, F. Biodegradable and re-usable sponge materials made from chitin for efficient removal of microplastics. J. Hazard. Mater. 2021, 420, 126599. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test. In OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2004. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, T.; Yoon, S.; Choi, J.-H.; Kim, N.; Park, J.-A. Simultaneous Removal of Heavy Metals and Dyes on Sodium Alginate/Polyvinyl Alcohol/κ-Carrageenan Aerogel Beads. Gels 2025, 11, 211. https://doi.org/10.3390/gels11030211
Jang T, Yoon S, Choi J-H, Kim N, Park J-A. Simultaneous Removal of Heavy Metals and Dyes on Sodium Alginate/Polyvinyl Alcohol/κ-Carrageenan Aerogel Beads. Gels. 2025; 11(3):211. https://doi.org/10.3390/gels11030211
Chicago/Turabian StyleJang, Taesoon, Soyeong Yoon, Jin-Hyuk Choi, Narae Kim, and Jeong-Ann Park. 2025. "Simultaneous Removal of Heavy Metals and Dyes on Sodium Alginate/Polyvinyl Alcohol/κ-Carrageenan Aerogel Beads" Gels 11, no. 3: 211. https://doi.org/10.3390/gels11030211
APA StyleJang, T., Yoon, S., Choi, J.-H., Kim, N., & Park, J.-A. (2025). Simultaneous Removal of Heavy Metals and Dyes on Sodium Alginate/Polyvinyl Alcohol/κ-Carrageenan Aerogel Beads. Gels, 11(3), 211. https://doi.org/10.3390/gels11030211





