Ultrasound-Enhanced Gelation of Stimuli-Responsive and Biocompatible Phenylalanine-Derived Hydrogels
Abstract
1. Introduction
2. Results and Discussion
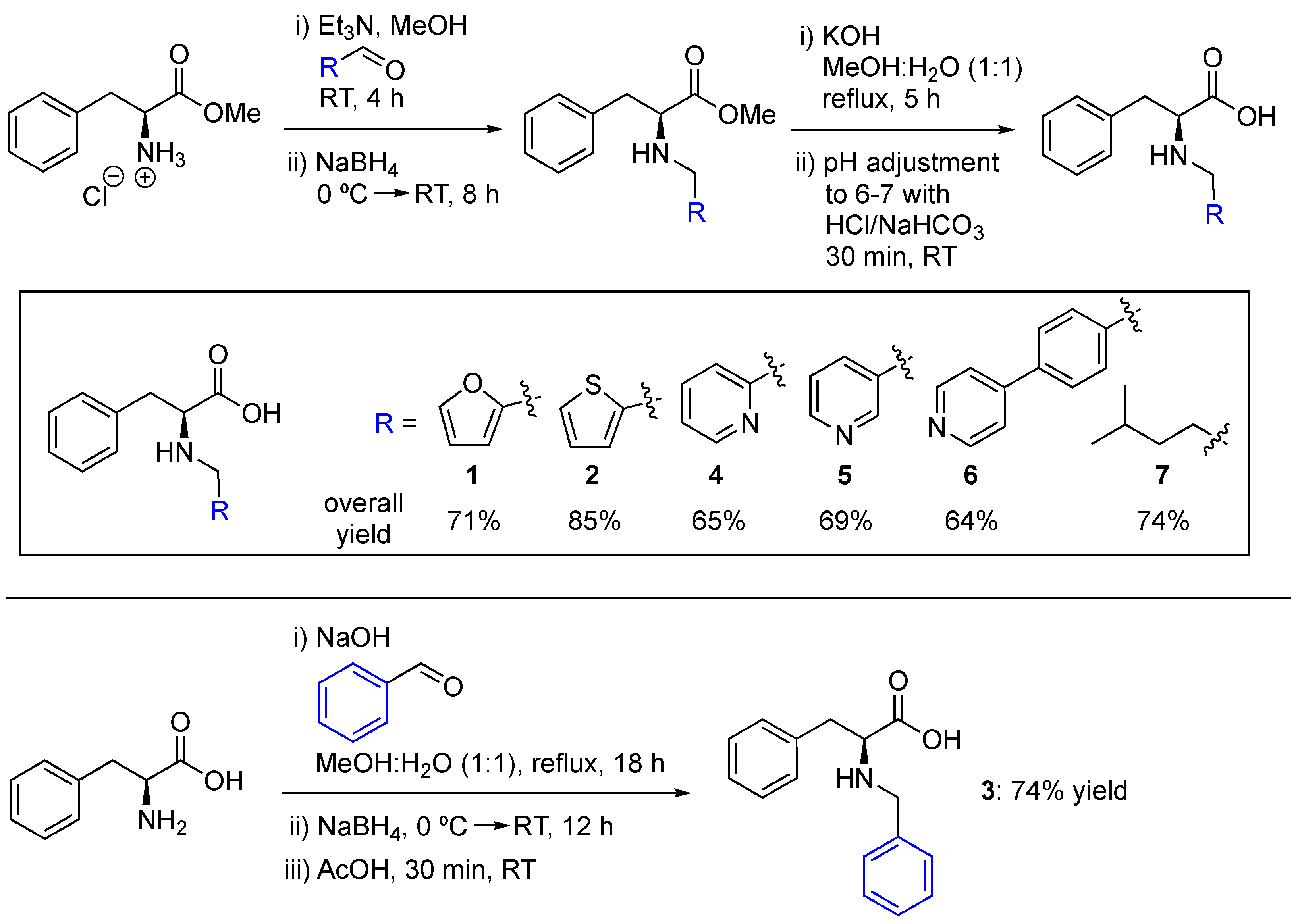
2.1. Synthesis of N-Substituted Phe Derivatives
2.2. Solubility and Gelation Properties
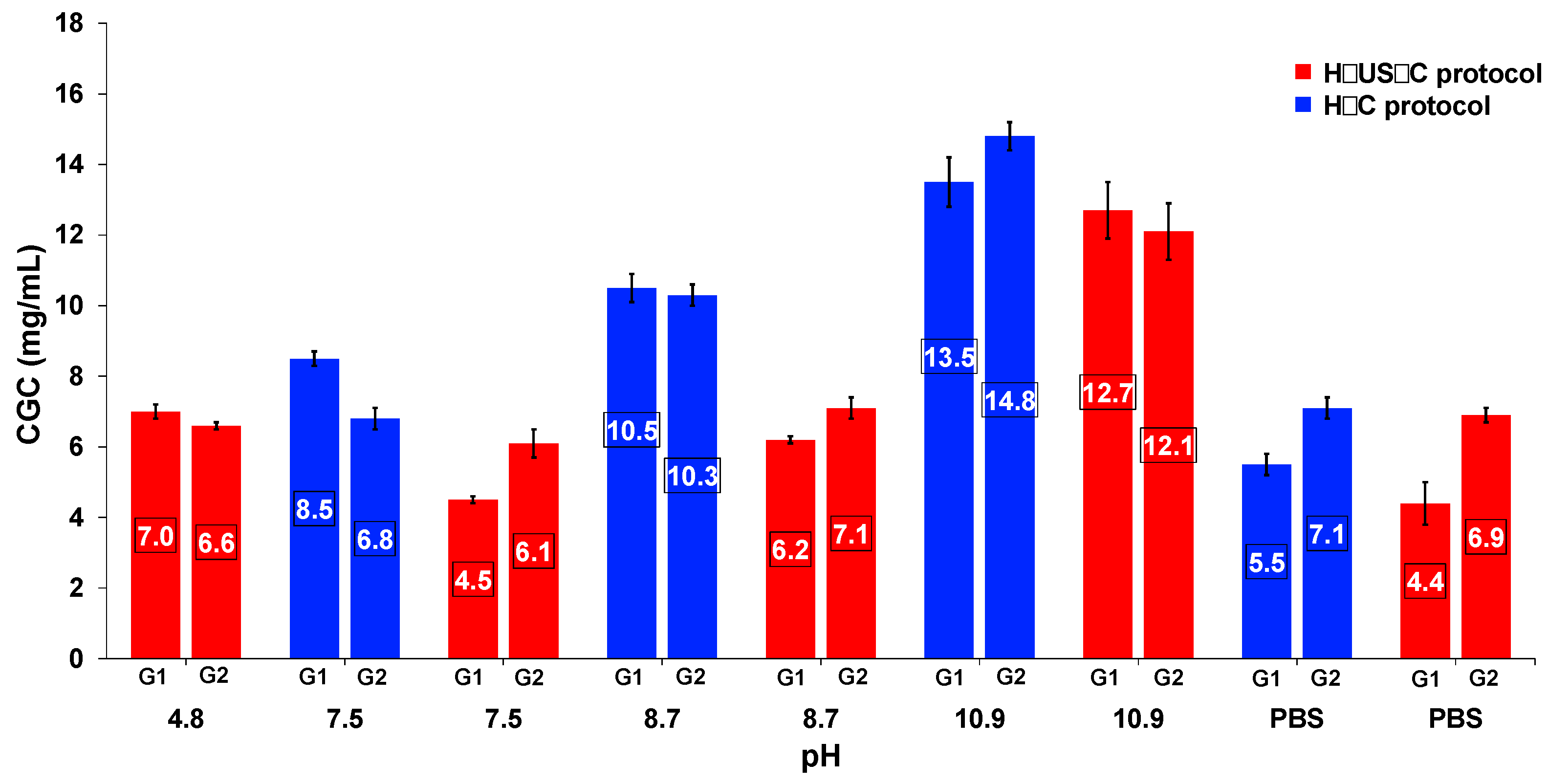
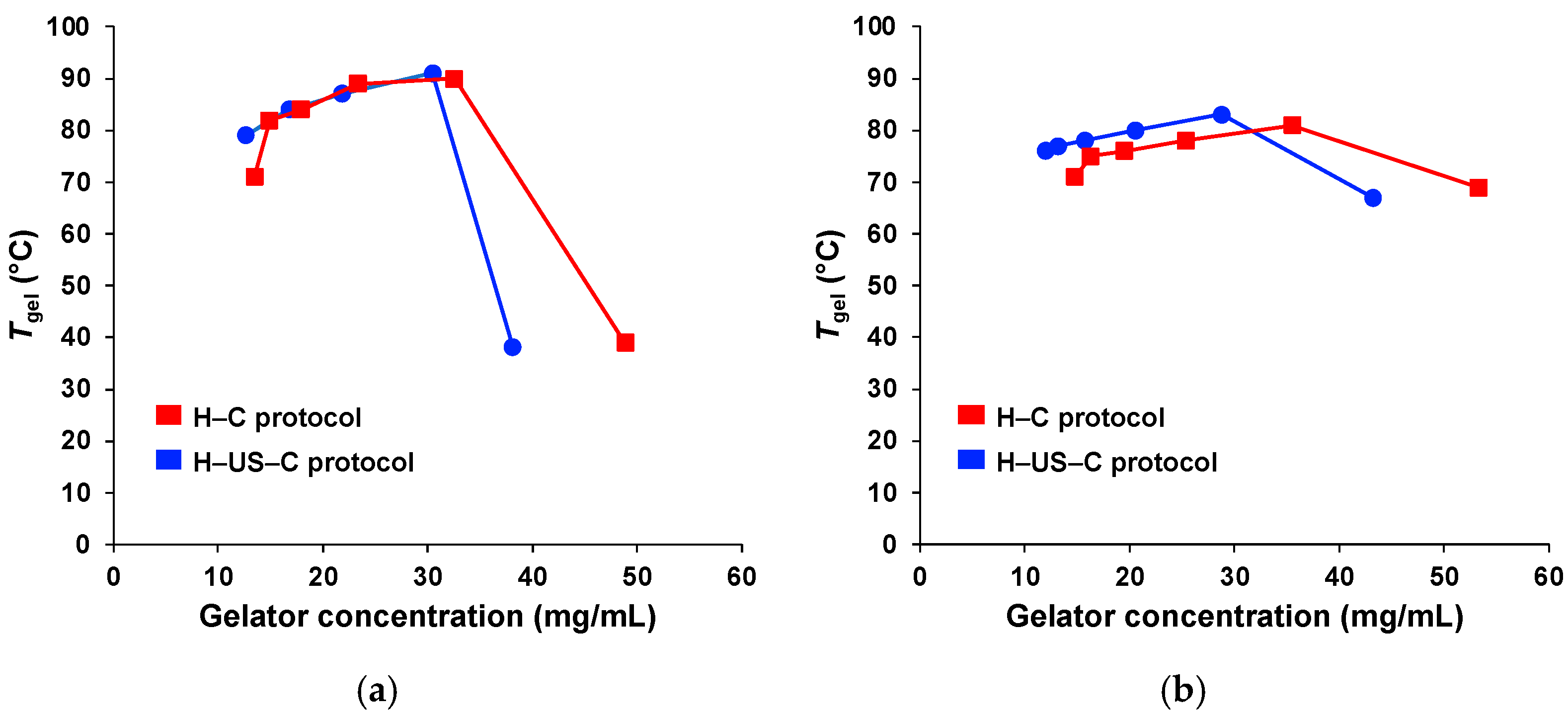
2.3. Effect of Ultrasound on Gelation Ability and Critical Gelation Concentration
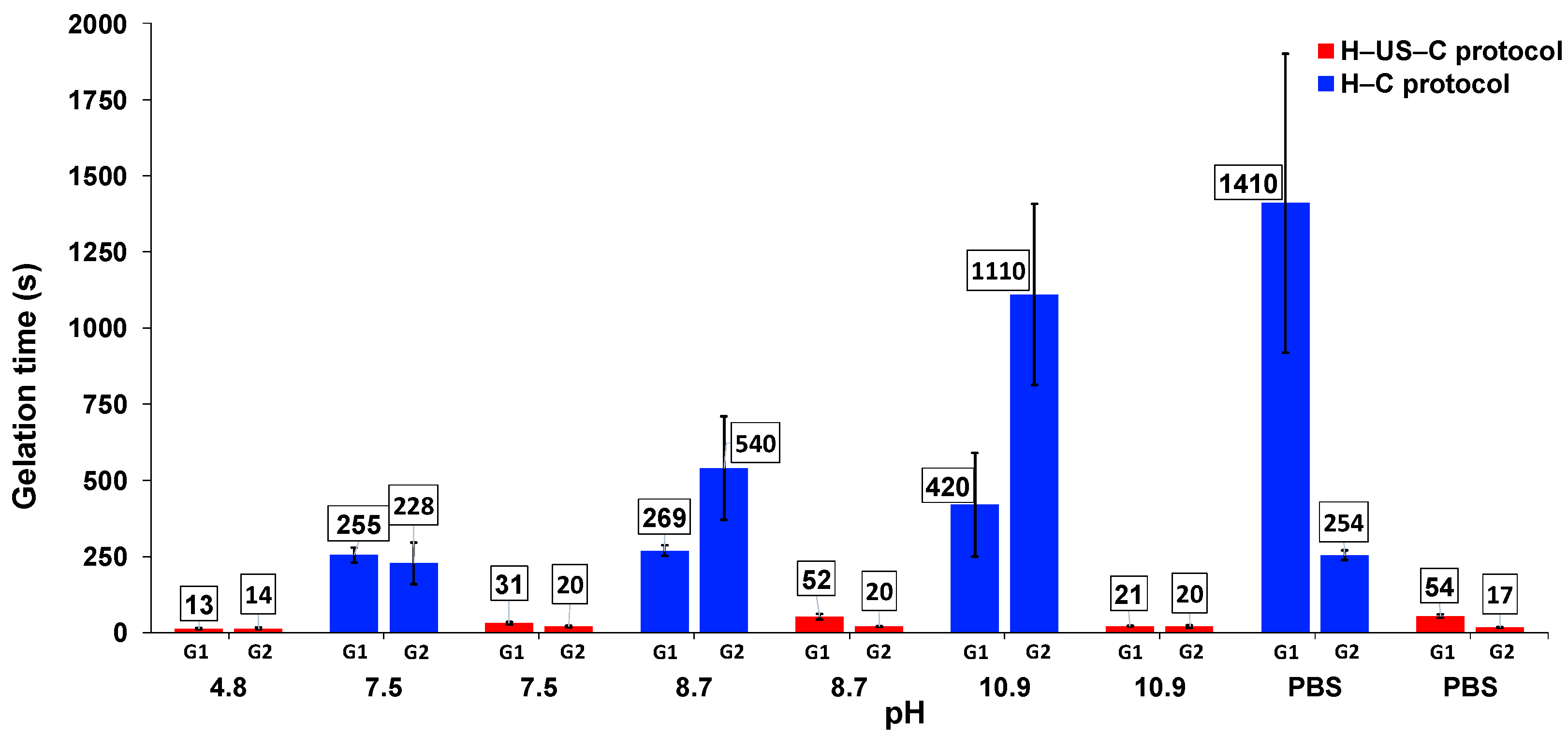
2.4. Effect of Ultrasound on Gelation Kinetics
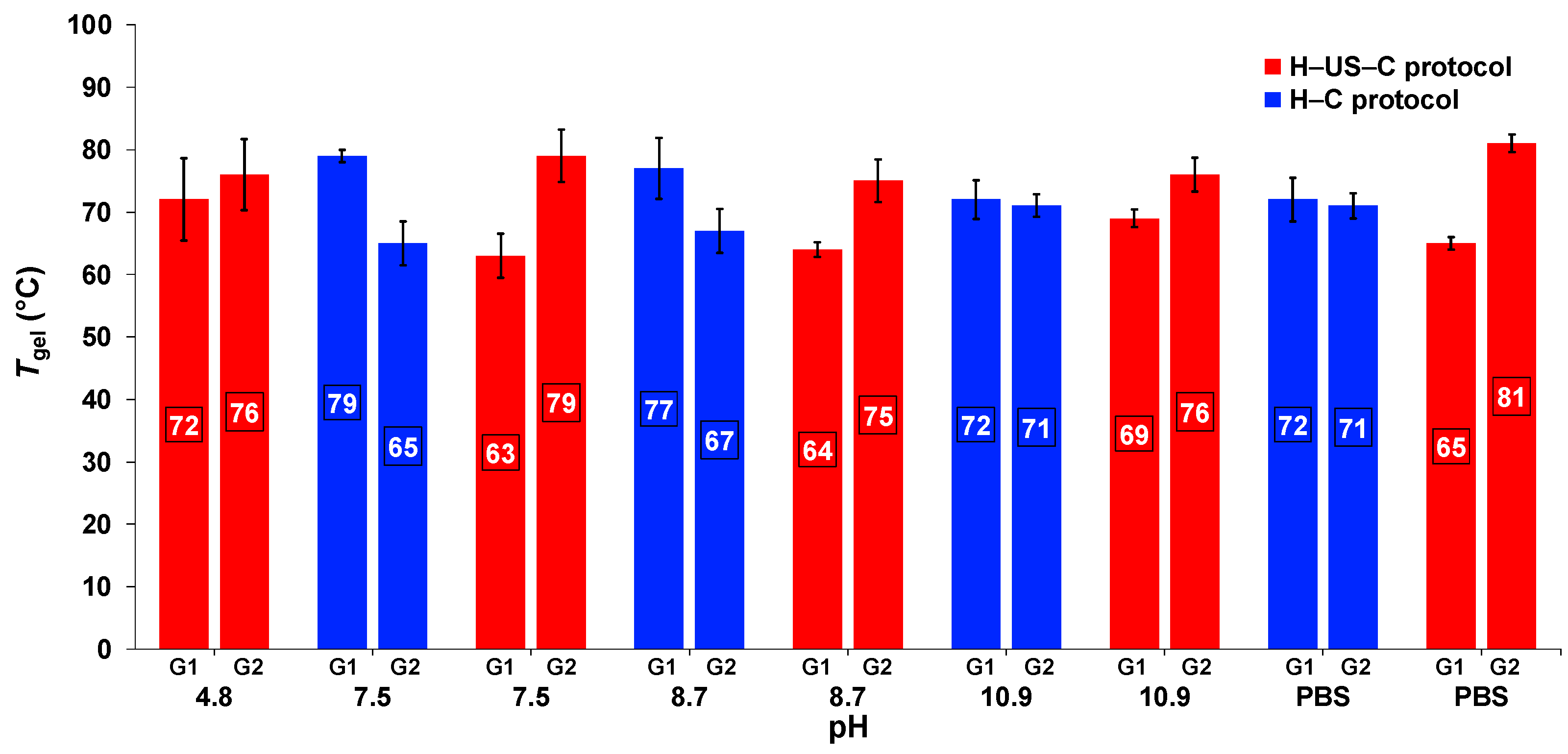
2.5. Effect of Ultrasound on the Thermal Stability
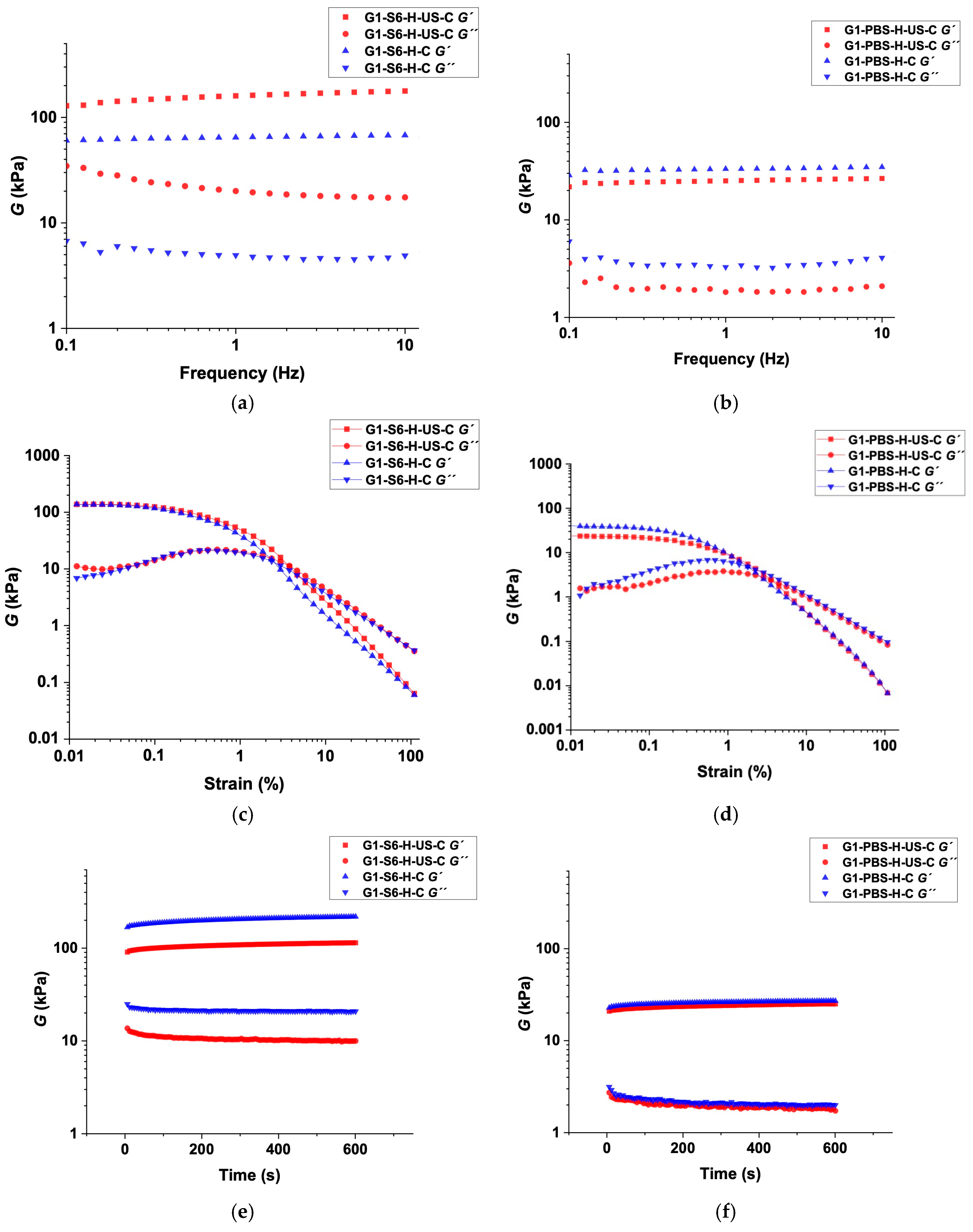
2.6. Rheological Behavior
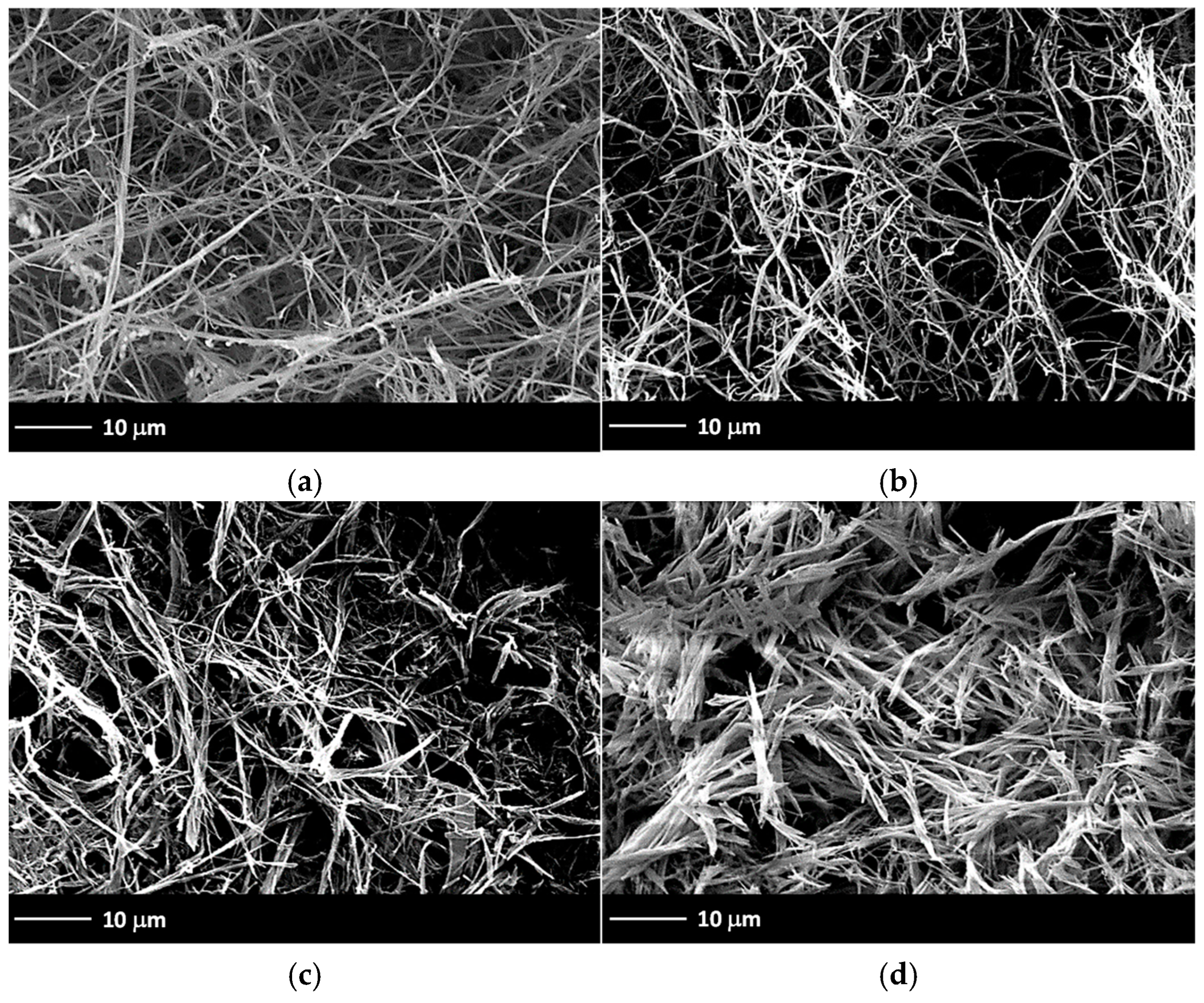
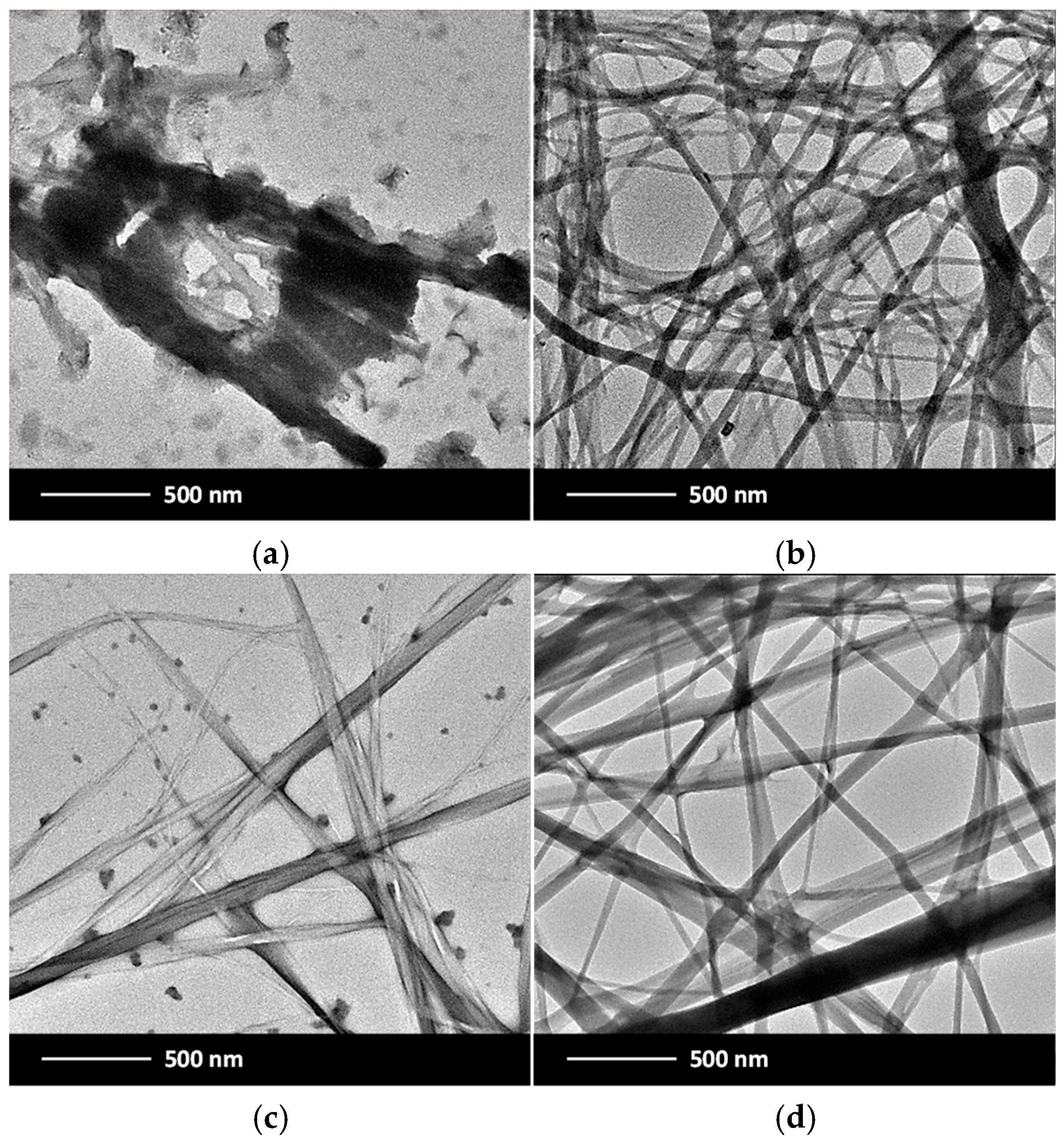
2.7. Morphological Characterization
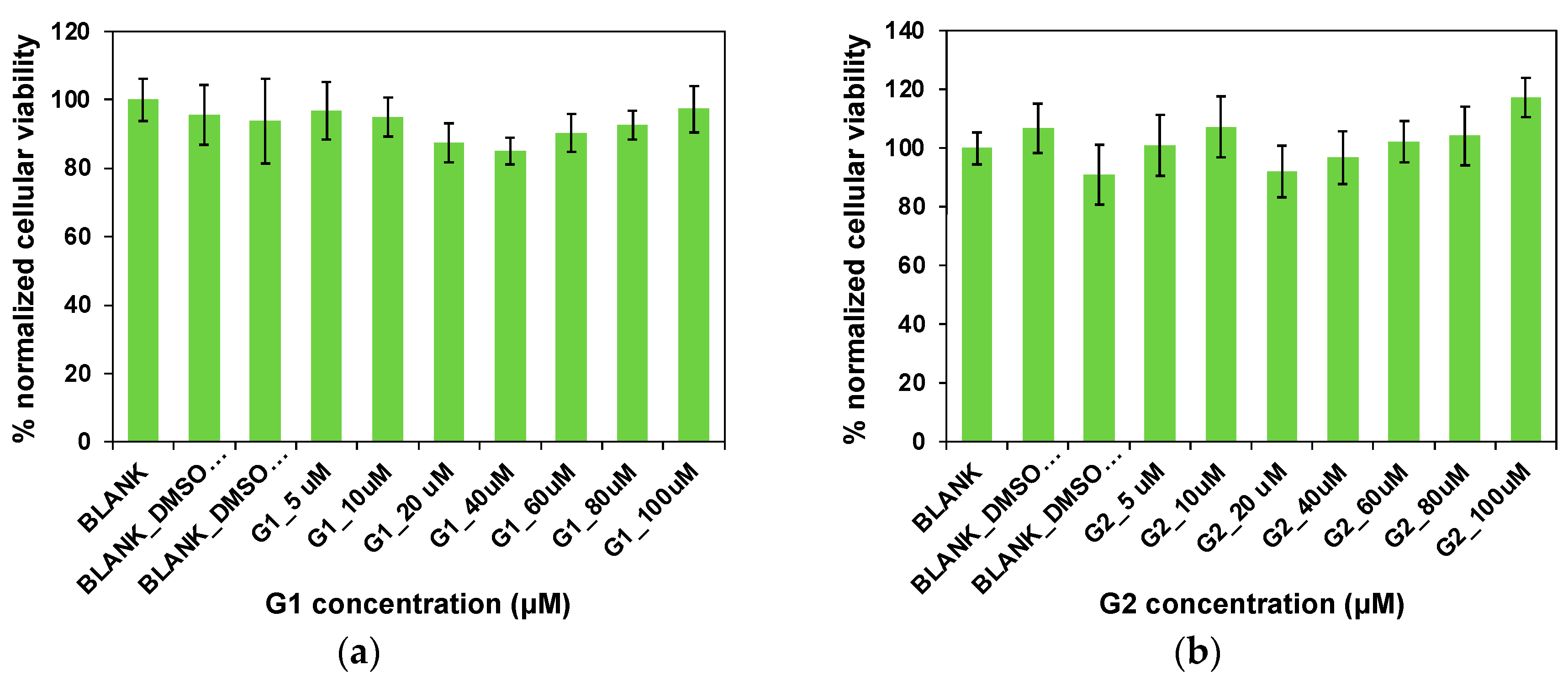
2.8. Cytotoxicity
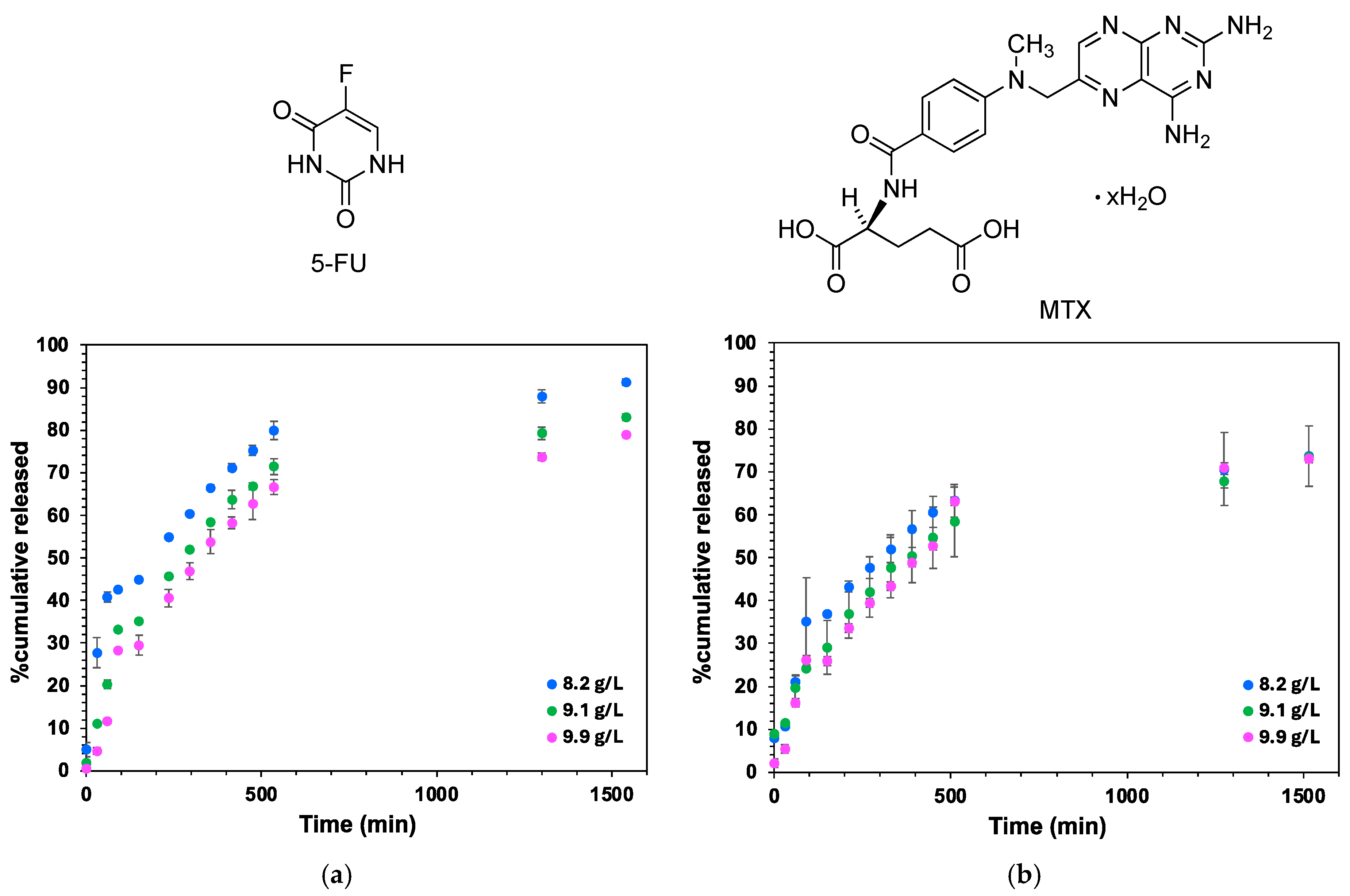
2.9. In Vitro Drug Release
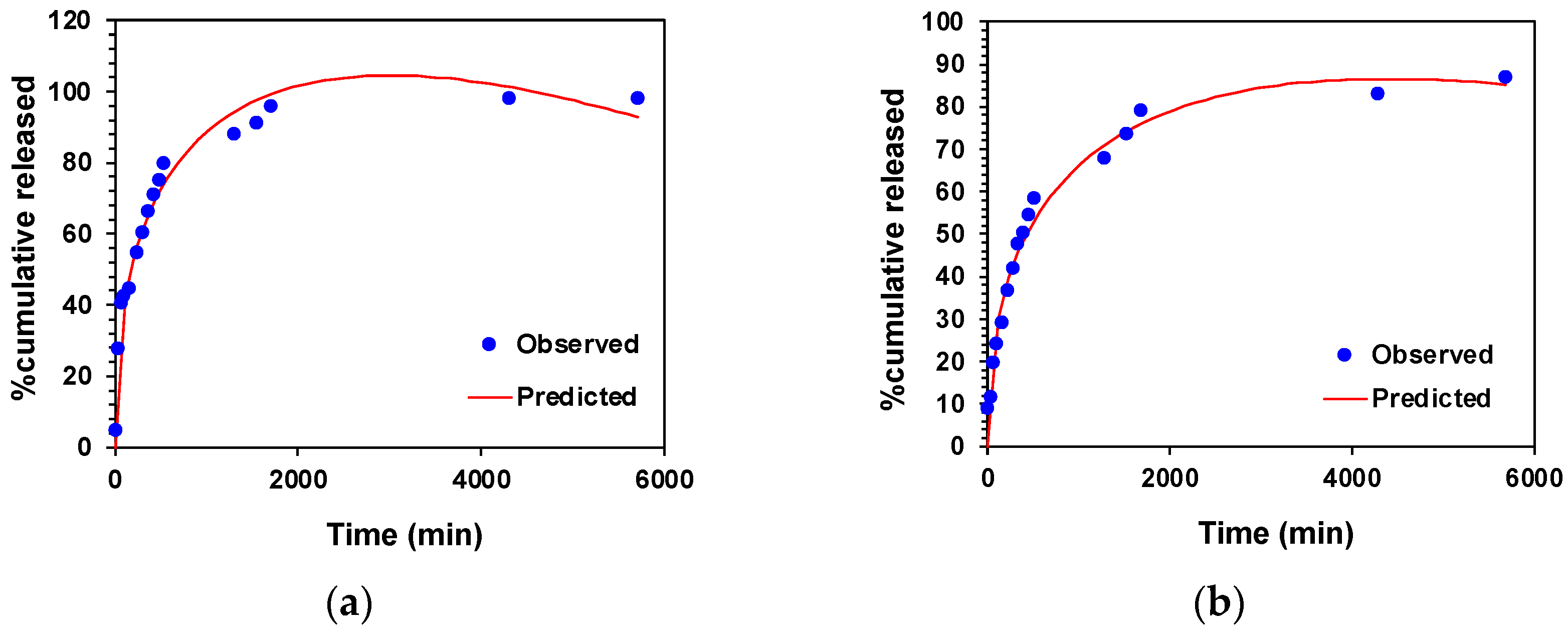
2.10. Drug Release Kinetics
3. Conclusions and Outlook
4. Materials and Methods
4.1. Synthesis and Characterization of Compounds
4.1.1. Materials
4.1.2. Characterization Methods
4.1.3. Synthetic Procedures
- General procedure for the synthesis of compounds 1, 2, 4, 5, 6, and 7
- General procedure for the synthesis of compound 3
4.2. Preparation and Characterization of Hydrogels
4.2.1. Preparation of Hydrogels
4.2.2. Physicochemical Characterization of Hydrogels
4.3. Cytotoxicity Assays
4.3.1. Materials
4.3.2. Experimental Protocol
4.4. In Vitro Drug Release
4.4.1. Experimental Procedure
4.4.2. Statistical Analysis and Mathematical Modeling
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIC | Akaike’s information criterion |
| ANOVA | Analysis of variance |
| CGC | Critical gelation concentration |
| DCM | Dichloromethane |
| DFS | Dynamic frequency sweep |
| DMEM | Dubelcco’s modified eagle medium |
| DMSO | Dimethyl sulfoxide |
| DSC | Differential scanning calorimetry |
| DSS | Dynamic strain sweep |
| DTS | Dynamic time sweep |
| EE | Encapsulation efficiency |
| FBS | Fetal bovine serum |
| FTIR-ATR | Fourier transform infrared spectroscopy-attenuated total reflectance |
| H–C | Heating–cooling |
| H–US–C | Heating–ultrasound–cooling |
| 5-FU | 5-Fluorouracil |
| MSC | Model selection criterion |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NMR | Nuclear magnetic resonance |
| PBS | Phosphate-buffered saline |
| Phe | Phenylalanine |
| RT | Room temperature |
| SEM | Scanning electron microscopy |
| TEA | Triethylamine |
| TEM | Transmission electron microscopy |
| Tgel | Gel-to-sol transition temperature |
| UV | Ultraviolet |
References
- Yan, D.; Wang, Z.; Zhang, Z. Stimuli-responsive crystalline smart materials: From rational design and fabrication to applications. Acc. Chem. Res. 2022, 55, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Bril, M.; Fredrich, S.; Kurniawan, N.A. Stimuli-responsive materials: A smart way to study dynamic cell responses. Smart Mater. Med. 2022, 3, 257–273. [Google Scholar] [CrossRef]
- Trehan, K.; Saini, M.; Thakur, S. Stimuli-responsive material in controlled release of drug. In Engineered biomaterials: Synthesis and applications; Malviya, R., Sundram, S., Eds.; Springer: Singapore, 2023; pp. 535–561. [Google Scholar]
- Municoy, S.; Álvarez Echazú, M.I.; Antezana, P.E.; Galdopórpora, J.M.; Olivetti, C.; Mebert, A.M.; Foglia, M.L.; Tuttolomondo, M.V.; Alvarez, G.S.; Hardy, J.G.; et al. Stimuli-responsive materials for tissue engineering and drug delivery. Int. J. Mol. Sci. 2020, 21, 4724. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.D.; Steed, J.W. Gels with sense: Supramolecular materials that respond to heat, light and sound. Chem. Soc. Rev. 2016, 45, 6546–6596. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.K. Applications of supramolecular gels. In Molecular Gels, 2nd ed.; Weiss, R.G., Ed.; Royal Society of Chemistry: Cambridge, UK, 2018; pp. 300–371. [Google Scholar]
- Chelu, M.; Musuc, A.M. Polymer gels: Classification and recent developments in biomedical applications. Gels 2023, 9, 161. [Google Scholar] [CrossRef]
- Smith, D.K. Supramolecular gels—A panorama of low-molecular-weight gelators from ancient origins to next-generation technologies. Soft Matter 2024, 20, 10–70. [Google Scholar] [CrossRef] [PubMed]
- Chivers, P.R.A.; Smith, D.K. Shaping and structuring supramolecular gels. Nat. Rev. Mater. 2019, 4, 463–478. [Google Scholar] [CrossRef]
- Zöller, K.; To, D.; Bernkop-Schnürch, A. Biomedical applications of functional hydrogels: Innovative developments, relevant clinical trials and advanced products. Biomaterials 2025, 312, 122718. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Song, W.J.; Sun, J.-Y. Hydrogel soft robotics. Mater. Today Phys. 2020, 15, 100258. [Google Scholar] [CrossRef]
- Ritik; Sarkar, S.; Pragti; Varshney, N.; Kumar, A.; Kuznetsov, M.L.; Jha, H.C.; Mukhopadhyay, S. Nano-structured gel materials for environmental remediation and biomedical applications. ACS Appl. Nano Mater. 2024, 7, 22292–22303. [Google Scholar] [CrossRef]
- Bachl, J.; Hohenleutner, A.; Dhar, B.B.; Cativiela, C.; Maitra, U.; König, B.; Díaz, D.D. Organophotocatalysis in nanostructured soft gel materials as tunable reaction vessels: Comparison with homogeneous and micellar solutions. J. Mater. Chem. A 2013, 1, 4577–4588. [Google Scholar] [CrossRef]
- Maiti, B.; Abramov, A.; Pérez-Ruiz, R.; Díaz, D.D. The prospect of photochemical reactions in confined gel media. Acc. Chem. Res. 2019, 52, 1865–1876. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.-Q.; Feng, C.-L. Amino acids and peptide-based supramolecular hydrogels for three-dimensional cell culture. Adv. Mater. 2017, 29, 1604062. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Häring, M.; Haldar, D.; Díaz, D.D. Phenylalanine and derivatives as versatile low-molecular weight gelators: Design, structure and tailored function. Biomater. Sci. 2017, 6, 38–59. [Google Scholar] [CrossRef] [PubMed]
- Ginesi, R.E.; Draper, E.R. Methods of changing low molecular weight gel properties through gelation kinetics. Soft Matter 2024, 20, 3887–3896. [Google Scholar] [CrossRef]
- Gibaud, T.; Dagès, N.; Lidon, P.; Jung, G.; Ahouré, L.C.; Sztucki, M.; Poulesquen, A.; Hengl, N.; Pignon, F.; Mannevielle, S. Rheoacoustic gels: Tuning mechanical and flow properties of colloidal gels with ultrasonic vibrations. Phys. Rev. X 2020, 10, 011028. [Google Scholar] [CrossRef]
- Bardelang, D. Ultrasound induced gelation: A paradigm shift. Soft Matter 2009, 5, 1969–1971. [Google Scholar] [CrossRef]
- Bachl, J.; Sampedro, D.; Mayr, J.; Díaz, D.D. Ultrasonication-enhanced gelation properties of a versatile amphiphilic formamidine-based gelator exhibiting both organogelation and hydrogelation abilities. Phys. Chem. Chem. Phys. 2017, 19, 22981–22994. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.; Alegre-Requena, J.V.; Marqués-López, E.; Herrera, R.P.; Casanovas, J.; Alemán, C.; Díaz, D.D. Self-assembled fibrillar networks of a multifaceted chiral squaramide: Supramolecular multistimuli-responsive alcogels. Soft Matter 2016, 12, 4361–4374. [Google Scholar] [CrossRef] [PubMed]
- Cravotto, G.; Cintas, P. Molecular self-assembly and patterning induced by sound waves. The case of gelation. Chem. Soc. Rev. 2009, 38, 2684–2697. [Google Scholar] [CrossRef]
- Thomas, R.G.; Surendran, S.P.; Jeong, Y.Y. Tumor microenvironment-stimuli responsive nanoparticles for anticancer therapy. Front. Mol. Biosci. 2020, 7, 610533. [Google Scholar]
- Liu, Y.; Ran, Y.; Ge, Y.; Raza, F.; Li, S.; Zafar, H.; Wu, Y.; Paiva-Santos, A.C.; Yu, C.; Sun, M.; et al. pH-sensitive peptide hydrogels as a combination drug delivery system for cancer treatment. Pharmaceutics 2022, 14, 652. [Google Scholar] [CrossRef] [PubMed]
- Lao, L.L.; Peppas, N.A.; Boey, F.Y.C.; Venkatraman, S.S. Modeling of drug release from bulk-degrading polymers. Int. J. Pharm. 2011, 418, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Pinho, E.; Machado, S.; Soares, G. Smart hydrogel for the pH-selective drug delivery of antimicrobial compounds. Macromol. Symp. 2019, 385, 1800182. [Google Scholar] [CrossRef]
- Papadopoulou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the use of the Weibull function for the discernment of drug release mechanisms. Int. J. Pharm. 2006, 309, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Gao, J.; Qiu, Y.; Wu, L.; Guo, F.; Lo, K.K.-W.; Li, D. Design, synthesis, and characterization of piperazinedione-based dual protein inhibitors for both farnesyltransferase and geranylgeranyltransferase-I. Eur. J. Med. Chem. 2011, 46, 2264–2273. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Khullar, S.; Mandal, S.K. Controlling the self-assembly of homochiral coordination architectures of CuII by substitution in amino acid based ligands: Synthesis, crystal structures and physicochemical properties. Dalton Trans. 2015, 44, 5672–5687. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Weiss, R.G. Chemically reversible organogels via “latent” gelators. Aliphatic amines with carbon dioxide and their ammonium carbamates. Langmuir 2002, 18, 7124–7135. [Google Scholar] [CrossRef]
- Gong, C.Y.; Shi, S.; Dong, P.W.; Kan, B.; Gou, M.L.; Wang, X.H.; Li, X.Y.; Luo, F.; Zhao, X.; Wei, Y.Q.; et al. Synthesis and characterization of PEG-PCL-PEG thermosensitive hydrogel. Int. J. Pharm. 2009, 365, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef]
- Bermejo-López, A.; Raeder, M.; Martínez-Castro, E.; Martín-Matute, B. Selective and quantitative functionalization of unprotected a-amino acids using a recyclable homogeneous catalyst. Chem 2022, 8, 3302–3323. [Google Scholar] [CrossRef]
- Wei, C.-W.; Wang, X.-J.; Gao, S.-Q.; Wen, G.-B.; Lin, Y.-W. A Phenylalanine derivative containing a 4-pyridine group can construct both single crystals and a selective Cu-Ag bimetallohydrogel. Eur. J. Inorg. Chem. 2019, 1349–1353. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buxaderas, E.; Moglie, Y.; Figueroa, A.B.; Alegre-Requena, J.V.; Grijalvo, S.; Saldías, C.; Herrera, R.P.; Marqués-López, E.; Díaz Díaz, D. Ultrasound-Enhanced Gelation of Stimuli-Responsive and Biocompatible Phenylalanine-Derived Hydrogels. Gels 2025, 11, 160. https://doi.org/10.3390/gels11030160
Buxaderas E, Moglie Y, Figueroa AB, Alegre-Requena JV, Grijalvo S, Saldías C, Herrera RP, Marqués-López E, Díaz Díaz D. Ultrasound-Enhanced Gelation of Stimuli-Responsive and Biocompatible Phenylalanine-Derived Hydrogels. Gels. 2025; 11(3):160. https://doi.org/10.3390/gels11030160
Chicago/Turabian StyleBuxaderas, Eduardo, Yanina Moglie, Aarón Baz Figueroa, Juan V. Alegre-Requena, Santiago Grijalvo, César Saldías, Raquel P. Herrera, Eugenia Marqués-López, and David Díaz Díaz. 2025. "Ultrasound-Enhanced Gelation of Stimuli-Responsive and Biocompatible Phenylalanine-Derived Hydrogels" Gels 11, no. 3: 160. https://doi.org/10.3390/gels11030160
APA StyleBuxaderas, E., Moglie, Y., Figueroa, A. B., Alegre-Requena, J. V., Grijalvo, S., Saldías, C., Herrera, R. P., Marqués-López, E., & Díaz Díaz, D. (2025). Ultrasound-Enhanced Gelation of Stimuli-Responsive and Biocompatible Phenylalanine-Derived Hydrogels. Gels, 11(3), 160. https://doi.org/10.3390/gels11030160









