Advancements in Tissue-Equivalent Gel Dosimeters
Abstract
1. Introduction
2. Gel Dosimeters
2.1. Fricke Gel Dosimeters and Hydrogels
2.2. Polymer Dosimeters
2.3. Solid Plastic Dosimeters
2.4. Radiofluoregenic or Radiophotoluminescence Dosimeters
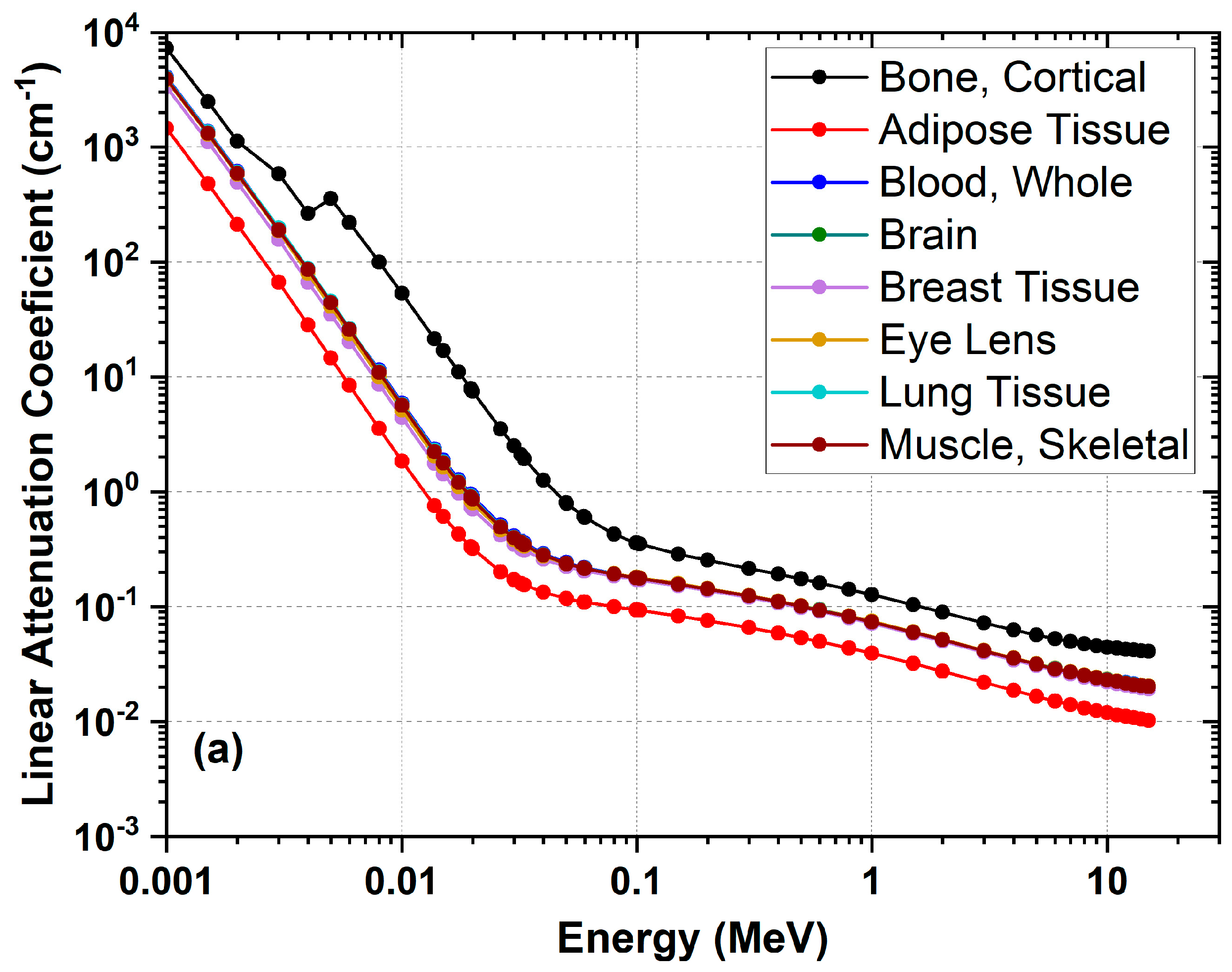
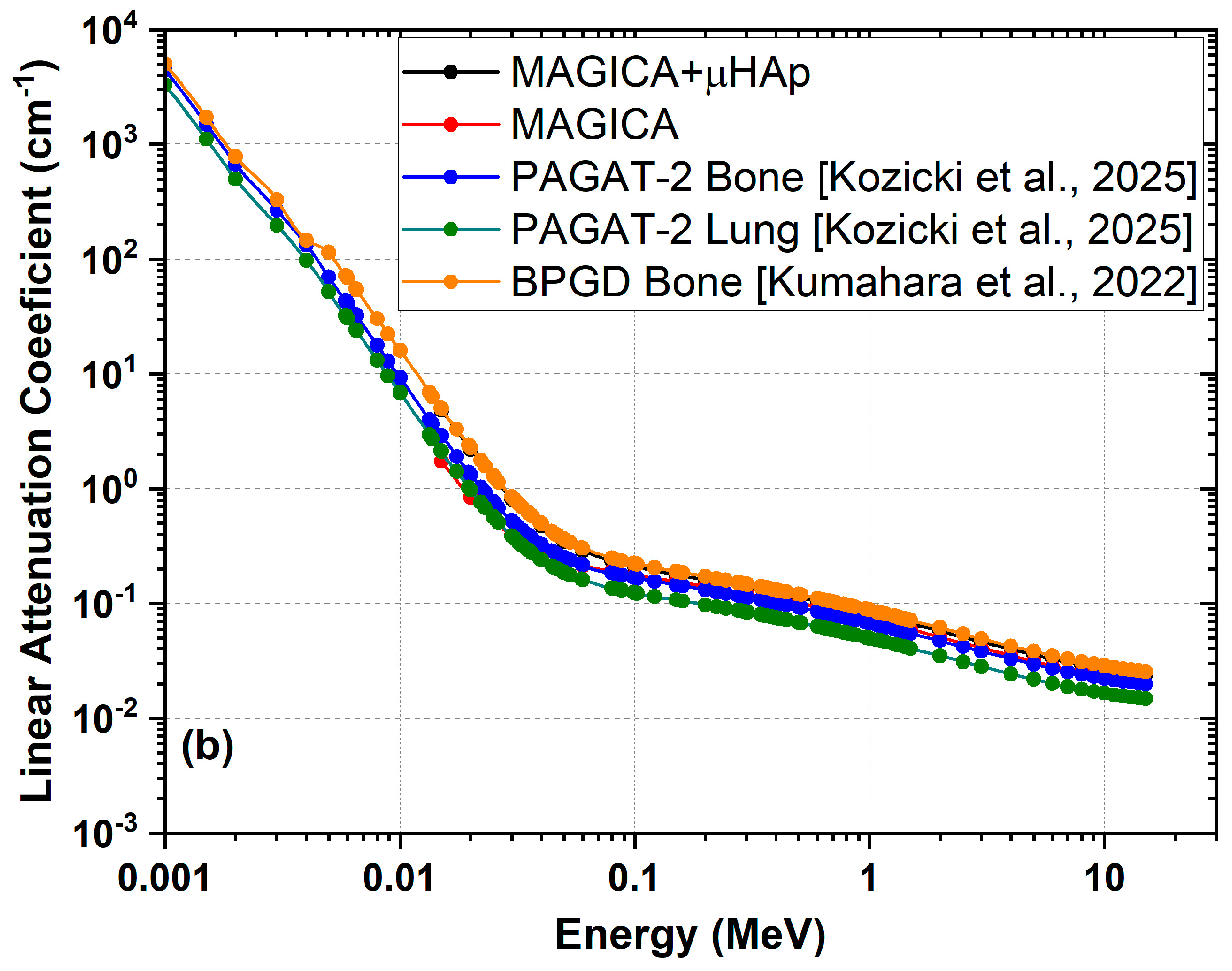
3. Tissue Equivalence
| Dosimeter Type | Samples | Approximate Chemical Formula * | ρ [g/cm3] | <Z/A> | Zeff |
|---|---|---|---|---|---|
| Polymer Gel Dosimeters | PAGAT, MAGAT, NIPAM | C15H16O116N3PS1Cl1K1 MgCl doped PAGAT2 [44] | 1.12 | 0.542 | 10.71 |
| Radiochromic Polymer Gels | PRESAGE | C29H57O14N9S [47] | 1.05 | 0.540 | 7.3 |
| Solid Plastic Dosimeters | PMMA | C5H8O2 | 1.190 | 0.539 | 6.47 |
| Radiophotoluminescence | RPLDs | C596H741038O370212N156Na3P1 [48] | 2.20 | 0.500 | 12.00 |
| Solid Phase Organic Dosimeters | TLDs | LiF | 2.635 | 0.463 | 3.92 |
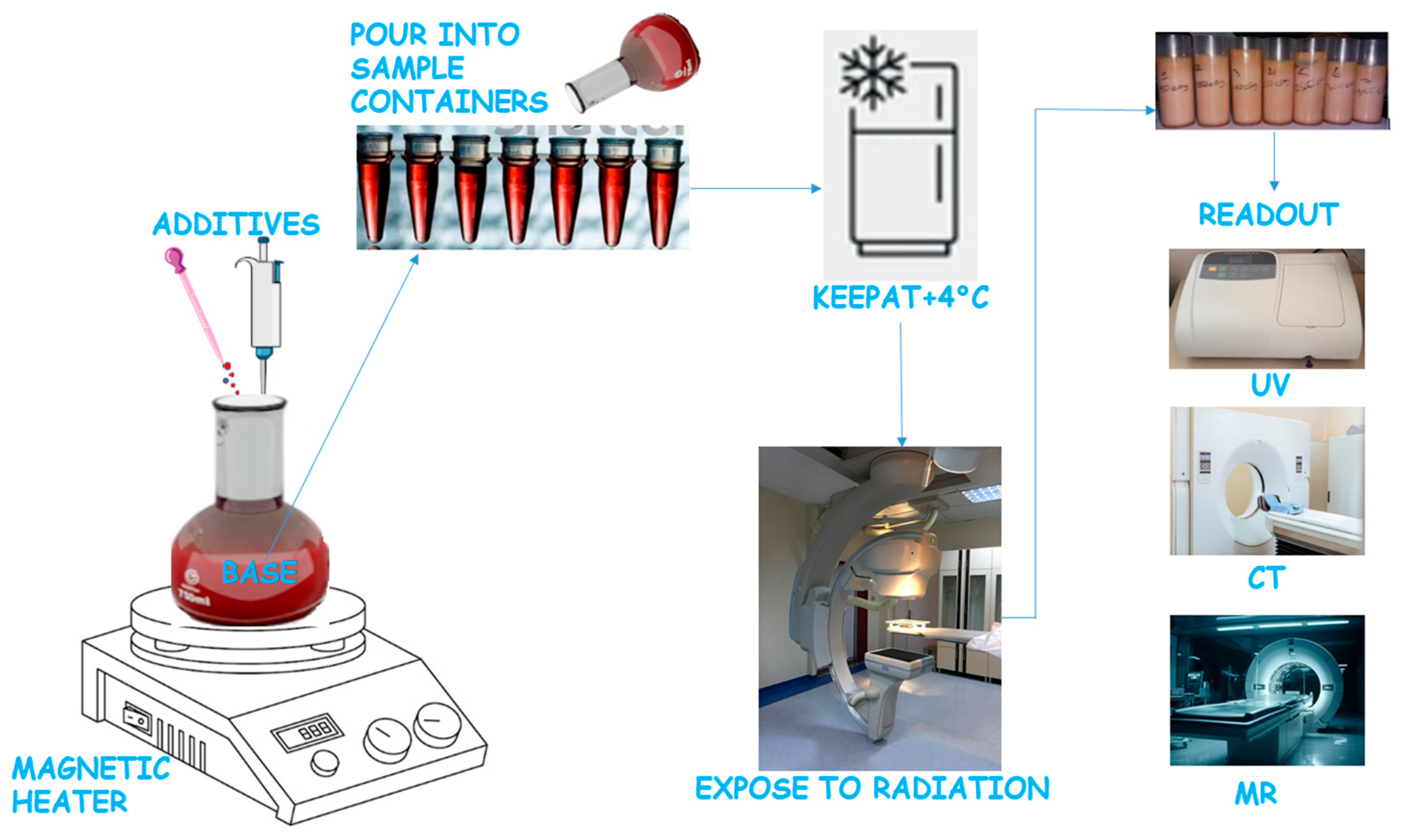
4. Measurement Procedures
4.1. NMR and MRI Scanning Methods
4.2. X-Ray CT Scanning
4.3. Optical Scanning
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Podgoršak, E.B. Radiation Physics for Medical Physicists, 3rd ed.; Springer Nature: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-319-25380-0. [Google Scholar]
- Yadav, N. Tissue-Equivalent Materials Used to Develop Phantoms in Radiation Dosimetry: A Review. Mater. Today Proc. 2021, 47, 7170–7173. [Google Scholar] [CrossRef]
- Marques, T.; Schwarcke, M.; Garrido, C.; Zucolot, V.; Baffa, O.; Nicolucci, P. Gel Dosimetry Analysis of Gold Nanoparticle Application in Kilovoltage Radiation Therapy. J. Phys. Conf. Ser. 2010, 250, 012084. [Google Scholar] [CrossRef]
- Powers, M.; Baines, J.; Crane, R.; Fisher, C.; Gibson, S.; Marsh, L.; Oar, B.; Shoobridge, A.; Simpson-Page, E.; Van der Walt, M.; et al. Commissioning measurements on an Elekta Unity MR-Linac. Phys. Eng. Sci. Med. 2022, 45, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Magugliani, G.; Marranconi, M.; Liosi, G.; Locatelli, F.; Gambirasio, A.; Trombetta, L.; Hertsyk, V.; Torri, V.; Galluccio, F.; Macerata, E.; et al. Pilot Scale Validation Campaign of Gel Dosimetry for Pre-Treatment Quality Assurance in Stereotactic Radiotherapy. Phys. Med. 2023, 114, 103158. [Google Scholar] [CrossRef]
- Kudrevicius, L.; Jaselske, E.; Adliene, D.; Rudzianskas, V.; Radziunas, A.; Tamasauskas, A. Application of 3D Gel Dosimetry as a Quality Assurance Tool in Functional Leksell Gamma Knife Radiosurgery. Gels 2022, 8, 69. [Google Scholar] [CrossRef]
- Guadarrama-Huerta, P.J.; Arzaga-Barajas, E.; Rodríguez-Laguna, A.; Jiménez-Acosta, J.A.; Poitevin-Chacón, M.A.; Massillon-Jl, G. Patient-Specific Quality Assurance in SBRT Treatments Using 3D Polymer Gel Dosimetry. Radiat. Meas. 2024, 175, 107166. [Google Scholar] [CrossRef]
- Ermeneux, L.; Petitfils, A.; Marage, L.; Gschwind, R.; Huet, C. Dosimetry with the TruView Gel on a 0.35 T MR-Linac: A Feasibility Study. Radiat. Meas. 2024, 175, 107170. [Google Scholar] [CrossRef]
- Nierer, L.; Kamp, F.; Reiner, M.; Corradini, S.; Rabe, M.; Dietrich, O.; Parodi, K.; Belka, C.; Kurz, C.; Landry, G. Evaluation of an Anthropomorphic Ion Chamber and 3D Gel Dosimetry Head Phantom at a 0.35 T MR-Linac Using Separate 1.5 T MR-Scanners for Gel Readout. Z. Med. Phys. 2022, 32, 312–325. [Google Scholar] [CrossRef]
- Pappas, E.; Kalaitzakis, G.; Boursianis, T.; Zoros, E.; Zourari, K.; Pappas, E.P.; Makris, D.; Seimenis, I.; Efstathopoulos , E.; Maris, T.G.; et al. Dosimetric performance of the Elekta Unity MR-linac system: 2D and 3D dosimetry in anthropomorphic inhomogeneous geometry. Phys. Med. Biol. 2019, 64, 225009. [Google Scholar]
- Du, D.; Kim, J.; Glide-Hurst, C.; Doemer, A.; Wen, N.; Movsas, B.; Dragovic, J.; Chetty, I. Commissioning and Validation of Patient-Specific Quality Assurance on an MR-Linac. Med. Phys. 2018, 45, e143. [Google Scholar]
- Maras, P.; Kozicki, M. Fast Isocenter Determination Using 3D Polymer Gel Dosimetry with Kilovoltage Cone-Beam CT Reading and the PolyGeVero-CT Software Package for Linac Quality Assurance in Radiotherapy. Materials 2022, 15, 6807. [Google Scholar] [CrossRef] [PubMed]
- Sunbul, N.B.; Oraiqat, I.; Rosen, B.; Miller, C.; Meert, C.; Matuszak, M.M.; Clarke, S.; Pozzi, S.; Moran, J.M.; El Naqa, I. Application of Radiochromic Gel Dosimetry to Commissioning of a Megavoltage Research Linear Accelerator for Small-Field Animal Irradiation Studies. Med. Phys. 2021, 48, 1404–1416. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.L.; Ruda, H.E. Impact of Scattering Foil Composition on Electron Energy Distribution in a Clinical Linear Accelerator Modified for FLASH Radiotherapy: A Monte Carlo Study. Materials 2024, 17, 3355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Almajidi, Y.Q.; Awad, S.A.; Alhachami, F.R.; Gatea, M.A.; Kadhum, W.R. Dosimetric Properties of PASSAG Polymer Gel Dosimeter in Electron Beam Radiotherapy Using Magnetic Resonance Imaging. J. X-Ray Sci. Technol. 2023, 31, 825–836. [Google Scholar] [CrossRef]
- De Deene, Y. Radiation Dosimetry by Use of Radiosensitive Hydrogels and Polymers: Mechanisms, State-of-the-Art and Perspective from 3D to 4D. Gels 2022, 8, 599. [Google Scholar] [CrossRef]
- Nezhad, Z.A.; Geraily, G. A Review Study on Application of Gel Dosimeters in Low Energy Radiation Dosimetry. Appl. Radiat. Isot. 2022, 179, 110015. [Google Scholar] [CrossRef]
- Romeo, M.; Cottone, G.; D’Oca, M.C.; Bartolotta, A.; Gallo, S.; Miraglia, R.; Gerasia, R.; Milluzzo, G.; Romano, F.; Gagliardo, C.; et al. Diffusion Correction in Fricke Hydrogel Dosimeters: A Deep Learning Approach with 2D and 3D Physics-Informed Neural Network Models. Gels 2024, 10, 565. [Google Scholar] [CrossRef]
- Penev, K.I.; Mequanint, K. New directions for tetrazolium—Gellan gum gel dosimeters. J. Phys. Conf. Ser. 2022, 2167, 012031. [Google Scholar] [CrossRef]
- Korkut, Ö.; Aktaş, S.; Sağsöz, M. Dosimetric Fricke Gel Systems Improved with CaCl2 and Gluconic Acid. Int. Adv. Res. Eng. J. 2018, 2, 143–146. [Google Scholar]
- Aktaş, S.; Korkut, Ö.; Sağsöz, M.E. Dose Response of Gluconic Acid-Doped Fricke Gels Irradiated with X-Rays. Int. Adv. Res. Eng. J. 2021, 5, 47–52. [Google Scholar] [CrossRef]
- Sağsöz, M.E.; Korkut, Ö.; Alemdar, N.; Aktaş, S.; Çalı, E.B.; Kantarcı, M. Comparison of Dosimetry Gels Prepared by Agar and Bovine Gelatine. J. Phys. Conf. Ser. 2016, 707, 012037. [Google Scholar] [CrossRef]
- Krauleidis, A.; Adliene, D.; Rutkuniene, Z. The Impact of Temporal Changes in Irradiated nMAG Polymer Gels on Their Applicability in Small Field Dosimetry in Radiotherapy. Gels 2022, 8, 629. [Google Scholar] [CrossRef] [PubMed]
- Cinq-Mars, M.; Jutras, J.-D.; Beaulieu, L. Evaluation of Novel Gel Dosimeters in Radiotherapy. J. Phys. Conf. Ser. 2023, 2630, 012032. [Google Scholar] [CrossRef]
- Farahani, S.; Mosleh-Shirazi, M.A.; Riyahi Alam, N.; Mahdavi, S.R.; Raeisi, F. Global and Spatial Dosimetric Characteristics of N-Vinylpyrrolidone-Based Polymer Gel Dosimeters as a Function of Medium-Term Post-Preparation and Post-Irradiation Time. Radiat. Phys. Chem. 2022, 198, 110280. [Google Scholar] [CrossRef]
- Farhood, B.; Abtahi, S.M.M.; Geraily, G.; Ghorbani, M.; Mahdavi, S.R.; Zahmatkesh, M.H. Dosimetric characteristics of PASSAG as a new polymer gel dosimeter with negligible toxicity. Radiat. Phys. Chem. 2018, 147, 91–100. [Google Scholar] [CrossRef]
- Penev, K.I.; Mulligan, M.; Mequanint, K. Optimization of the Dose Rate Effect in Tetrazolium Gellan Gel Dosimeters. Gels 2023, 9, 334. [Google Scholar] [CrossRef]
- Jaszczak-Kuligowska, M.; Maras, P.; Kozicki, M. Preliminary study on a bifunctional, elastic NBT–PVA radiochromic gel acting as a bolus and dosimeter in radiotherapy. J. Phys. Conf. Ser. 2024, 2799, 012007. [Google Scholar] [CrossRef]
- Mohyedin, M.Z.; Zin, H.M.; Adenan, M.Z.; Abdul Rahman, A.T. A review of PRESAGE radiochromic polymer and the compositions for application in radiotherapy dosimetry. Polymers 2022, 14, 2887. [Google Scholar] [CrossRef]
- Mohyedin, M.Z.; Zin, H.M.; Abubakar, A.; Rahman, A.T.A. Study of PRESAGE® dosimeter for end-to-end 3D radiotherapy verification using an anthropomorphic phantom with bespoke dosimeter insert. Phys. Eng. Sci. Med. 2024, 47, 955–966. [Google Scholar] [CrossRef]
- Du, Y.; Wang, R.; Yue, H.; Zhang, Y.; Wu, H.; Wang, W. Dose response and stability of silicone-based deformable radiochromic dosimeters (FlexyDos3D) using spectrophotometer and flatbed scanner. Radiat. Phys. Chem. 2020, 168, 108574. [Google Scholar] [CrossRef]
- Wheatley, M.J.; De Deene, Y. Loss and reintroduction of the radical initiator into the FlexyDos3D silicone dosimeter for 3D printing. J. Phys. Conf. Ser. 2023, 2630, 012027. [Google Scholar] [CrossRef]
- Koshimizu, M. Tissue-Equivalent Radiophotoluminescence Dosimetry Materials Based on Production of Luminescent Molecules via Radiation Chemical Reactions. Radiat. Meas. 2024, 176, 107222. [Google Scholar] [CrossRef]
- Skowyra, M.M.; Ankjærgaard, C.; Yu, L.; Lindvold, L.R.; Skov, A.L.; Miller, A. Characterization of a Radiofluorogenic Polymer for Low-Energy Electron Beam Penetration Depth Visualization. Polymers 2022, 14, 1015. [Google Scholar] [CrossRef]
- Eyadeh, M.M.; Rabaeh, K.A.; Issa, A.S.B.; Diamond, K.R. Evaluation of a Novel N-(Hydroxymethyl)Acrylamide Polymer Gel Dosimeter Formulation with Organic Glucose Additive for Radiotherapy. Radiat. Meas. 2023, 166, 106983. [Google Scholar] [CrossRef]
- Kozicki, M.; Maras, P. An Optical Reusable 2D Radiochromic Gel-Based System for Ionising Radiation Measurements in Radiotherapy. Molecules 2024, 29, 2558. [Google Scholar] [CrossRef]
- Rafiei, M.M.; Tavakoli-Anbaran, H.; Kurudirek, M. A detailed investigation of gamma-ray energy absorption and dose buildup factor for soft tissue and tissue equivalents using Monte Carlo simulation. Radiat. Phys. Chem. 2020, 177, 109118. [Google Scholar] [CrossRef]
- Kumahara, N.; Takemura, A.; Ishihara, S.; Noto, K.; Kojima, H.; Isomura, N.; Yokoyama, H.; Goto, I. Sensitivity of a Bone-Equivalent Polymer Gel Dosimeter for Measuring the Dose to Bone During Radiation Therapy. Radiol. Phys. Technol. 2023, 16, 227–234. [Google Scholar] [CrossRef]
- Hubbell, J.H.; Seltzer, S.M. Tables of X-Ray Mass Attenuation Coefficients and Mass Energy-Absorption Coefficients; Version 1.03; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2004. Available online: http://physics.nist.gov/xaamdi (accessed on 21 December 2024).
- White, D.R.; Booz, J.; Griffith, R.V.; Spokas, J.J.; Wilson, I.J. Tissue Substitutes in Radiation Dosimetry and Measurement. J. Radiol. Prot. 1989, 23, 1. [Google Scholar]
- Sakar, E.; Ozpolat, O.F.; Alım, B.; Sayyed, M.I.; Kurudirek, M. Phy-X/PSD: Development of a User-Friendly Online Software for Calculation of Parameters Relevant to Radiation Shielding and Dosimetry. Radiat. Phys. Chem. 2020, 166, 108496. [Google Scholar] [CrossRef]
- Çalı, E.B.; Korkut, O.; Gundes, A.; Gallo, S.; Sagsoz, M.E. Dosimetric and Physical Characteristics of a Bone-Equivalent Normoxic Polymer Gel. unpublished.
- Macchione, M.A.; Lechón Páez, S.; Strumia, M.C.; Valente, M.; Mattea, F. Chemical Overview of Gel Dosimetry Systems: A Comprehensive Review. Gels 2022, 8, 663. [Google Scholar] [CrossRef]
- Kunkyab, T.; Hilts, M.; Jirasek, A.; Hyde, D. Spatial and Dosimetric Accuracy of 3D Polymer Gel with CBCT Readout—Varian HyperArc® SRS Implementation. J. Phys. Conf. Ser. 2023, 2630, 012015. [Google Scholar] [CrossRef]
- Kozicki, M.; Jaszczak-Kuligowska, M.; Maras, P. Measurement of Ionising Radiation Dose Absorbed by Bones Using a Bone-Imitating Polymer Gel Dosimeter. Measurement 2025, 240, 115633. [Google Scholar] [CrossRef]
- Jaszczak-Kuligowska, M.; Kozicki, M.; Maras, P. Towards Optimization of the Chemical Composition of a Bone-Imitating Dosimeter as a Potential Component of Multiphase Dosimeters. J. Phys. Conf. Ser. 2024, 2799, 012006. [Google Scholar] [CrossRef]
- Farajzadeh, E.; Sina, S. Developing a radiochromic dosimeter for dosimetry in blood irradiation chambers. Radiat. Phys. Chem. 2021, 188, 109637. [Google Scholar] [CrossRef]
- Sandwall, P.A.; Bastow, B.P.; Spitz, H.B.; Elson, H.R.; Lamba, M.; Connick, W.B.; Fenichel, H. Radio-Fluorogenic Gel Dosimetry with Coumarin. Bioengineering 2018, 5, 53. [Google Scholar] [CrossRef]
- Zhang, P.; Jiang, L.; Chen, H.; Hu, L. Recent Advances in Hydrogel-Based Sensors Responding to Ionizing Radiation. Gels 2022, 8, 238. [Google Scholar] [CrossRef]
- Campbell, W.G.; Rudko, D.A.; Braam, N.A.; Wells, D.M.; Jirasek, A. Validation of Dosimetry Using Hydrogel Systems. Med. Phys. 2013, 40, 061712. [Google Scholar] [CrossRef]
- Doran, S.J. 3D Dosimetry Readout Techniques. J. Phys. Conf. Ser. 2019, 1305, 012029. [Google Scholar] [CrossRef]
- De Deene, Y.; Mason, D. Optimization of MRI Pulse Sequences and Gadobutrol-Doped Polymer Gel for Real-Time 4D Radiation Dosimetry on the MRI-Linac. J. Phys. Conf. Ser. 2023, 2630, 012014. [Google Scholar] [CrossRef]
- Keshtkar, M.; ATakavar Zahmatkesh, M.H.; Montazerabadi, A.R. Uncertainty Analysis in MRI-based Polymer Gel Dosimetry. J. Biomed. Phys. Eng. 2017, 7, 299–304. [Google Scholar]
- Khan, M.; Heilemann, G.; Lechner, W.; Georg, D.; Berg, A.G. Basic Properties of a New Polymer Gel for 3D-Dosimetry at High Dose-Rates Typical for FFF Irradiation Based on Dithiothreitol and Methacrylic Acid (MAGADIT): Sensitivity, Range, Reproducibility, Accuracy, Dose Rate Effect and Impact of Oxygen Scavenger. Polymers 2019, 11, 1717. [Google Scholar] [CrossRef] [PubMed]
- Fitilis, I.; Grigoriadis, A.; Tazes, I.; Petrakis, S.; Andrianaki, G.; Dimitriou, V.; Bakarezos, E.; Benis, E.P.; Tsiapa, I.; Boursianis, T.; et al. Polymer-Gel Radiation Dosimetry of Laser-Based Relativistic Electron Sources for Biomedical Applications: First Qualitative Results and Experimental Challenges. Front. Phys. 2022, 10, 727511. [Google Scholar] [CrossRef]
- Baldock, C.; De Deene, Y.; Doran, S.; Ibbott, G.; Jirasek, A.; Lepage, M.; McAuley, K.B.; Oldham, M.; Schreiner, L.J. Polymer gel dosimetry. Phys. Med. Biol. 2010, 55, R1–R63. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Warmington, L.; Gopishankar, N. Three-dimensional radiation dosimetry using polymer gel and solid radiochromic polymer: From basics to clinical applications. World J. Radiol. 2017, 9, 112–125. [Google Scholar] [CrossRef]
- Goosheha, A.; Abtahi, S.M.; Akhond, A.; Mahdavi, S.R. A review of clinical imaging techniques in polymer gel dosimeters. Radiat. Phys. Eng. 2024, 5, 15–23. [Google Scholar]
- Rabaeh, K.A.; Basfar, A.A.; Almousa, A.A.; Devic, S.; Moftah, B. New normoxic N-(Hydroxymethyl)acrylamide based polymer gel for 3D dosimetry in radiation therapy. Phys. Medica PM Int. J. Devoted Appl. Phys. Med. Biol. Off. J. Ital. Assoc. Biomed. Phys. (AIFB) 2017, 33, 121–126. [Google Scholar] [CrossRef]
- Moftah, B.; Basfar, A.A.; Almousa, A.A.; Al Kafi, A.M.; Rabaeh, K.A. Novel 3D polymer gel dosimeters based on N-(3-Methoxypropyl)acrylamide (NMPAGAT) for quality assurance in radiation oncology. Radiat. Meas. 2020, 135, 106372. [Google Scholar] [CrossRef]
- Sagsoz, M.E.; Pirimoglu, R.B. Radiation Dose to Breasts from a Cardiac Computed Tomography Angiography Scanogram Can Be Reduced by Switching Tube Position. Turk. J. Med. Sci. 2016, 46, 5. [Google Scholar] [CrossRef]
- Sagsoz, M.E.; Bayraktutan, U.; Ogul, H.; Kantarci, M. Chest Circumference as a Predictive Parameter of Computed Tomography Coronary Angiography Radiation Doses from Dual-Source Computed Tomography. Eurasian J. Med. 2013, 45, 43–46. [Google Scholar] [CrossRef]
- Javaheri, N.; Yarahmadi, M.; Refaei, A.; Aghamohammadi, A. Investigating the Sensitivity of New Formulation MAGAT and NIPAM Polymer Gels in the Radiation Therapy Dosimetry. J. Biomed. Phys. Eng. 2022, 12, 489–496. [Google Scholar] [CrossRef]
- Özbay, T.; Yurt, A.; Özsoykal, İ. Simulation of Water Equivalency of Polymer Gel Dosimeters with GAMOS. J. Basic Clin. Health Sci. 2020, 1, 51–58. [Google Scholar] [CrossRef]
- Jirasek, A. Considerations for x-ray CT polymer gel dosimetry. J. Phys. Conf. Ser. 2013, 444, 012005. [Google Scholar] [CrossRef]
- Ceberg, S.; Olding, T.; Baldock, C. Gel dosimetry has a viable future for dosimetry in the radiation oncology clinic. Phys. Eng. Sci. Med. 2024, 47, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, H.; Oshika, R.; Tachibana, R.; Seki, K. Toward “on-line” X-ray computed tomography-based dosimetry using a new polymer gel with rapid response. Radiat. Phys. Chem. 2024, 218, 111570. [Google Scholar] [CrossRef]
- Rousseau, A.; Stien, C.; Gouriou, J.; Bordy, J.M.; Boissonnat, G.; Blideanu, V. End-to-End Quality Assurance for Stereotactic Radiotherapy with Fricke-Xylenol Orange-Gelatin Gel Dosimeter and Dual-Wavelength Cone-Beam Optical CT Readout. Phys. Med. Eur. J. Med. Phys. 2023, 113, 102656. [Google Scholar] [CrossRef]
- Abtahi, S.M.M.; Habibi, F. Investigation of the Beam Quality and Dose Rate Dependence of PAKAG Polymer Gel Dosimeter in Optical Readout Technique. J. Phys. Conf. Ser. 2023, 2630, 012032. [Google Scholar] [CrossRef]
- de Lera-Garrido, F.J.; Vázquez-Villar, V.; Fernández-Liencres, M.P.; Sánchez-Ruiz, A.; Navarro, A.; Tolosa, J.; García-Martínez, J.C. Design of Large Stokes Shift Fluorescent Ortho-Bis-Styrylbenzenes: Optical Characterization and Fluoride Sensing in Logical Gates. Dye. Pigment. 2024, 225, 112035. [Google Scholar] [CrossRef]
- Moluchi, O.; Mulligan, M.; Jordan, K. Bare Spherical Gel Dosimeter with Optical Computed Tomography Scanning. J. Phys. Conf. Ser. 2023, 2630, 012024. [Google Scholar] [CrossRef]
- Silveira, M.A.; Pavoni, J.F.; Bruno, A.C.; Arruda, G.V.; Baffa, O. Three-Dimensional Dosimetry by Optical-CT and Radiochromic Gel Dosimeter of a Multiple Isocenter Craniospinal Radiation Therapy Procedure. Gels 2022, 8, 582. [Google Scholar] [CrossRef]
- Chacón, D.; Vedelago, J.; Strumia, M.C.; Valente, M.; Mattea, F. Raman spectroscopy as a tool to evaluate oxygen effects on the response of polymer gel dosimetry. Appl. Radiat. Isot. 2019, 150, 43–52. [Google Scholar] [CrossRef]
- Kozicki, M.; Maras, P.; Jaszczak-Kuligowska, M. 3D Polymer Gel Dosimeters with iCBCT 3D Reading and polyGeVero-CT Software Package for Quality Assurance in Radiotherapy. Materials 2024, 17, 1283. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Vrielinck, H.; Jacobsohn, L.G.; Smet, P.F.; Poelman, D. Passive Dosimeters for Radiation Dosimetry: Materials, Mechanisms, and Applications. Adv. Funct. Mater. 2024, 34, 2406186. [Google Scholar] [CrossRef]
- Colnot, J.; Chiavassa, S.; Delpon, G.; Huet, C. Study of the use of gel dosimetry in combination with 3D printing phantom for personalized pretreatment QA in radiotherapy. J. Phys. Conf. Ser. 2021, 2167, 012017. [Google Scholar] [CrossRef]
- Locarno, S.; Gallo, S.; Arosio, P.; Biordi, C.; Dallasega, D.; Gargano, M.; Ludwig, N.; Orsini, F.; Pignoli, E.; Veronese, I.; et al. Dosimetric double network hydrogel based on Poly (vinyl-alcohol)/Phenylalanine-derivatives with enhanced mechanical properties. ACS Appl. Polym. Mater. 2023, 5, 1902–1914. [Google Scholar] [CrossRef]
- de Almeida, W.D.S.; Alves, A.V.S.; Oliveira, W.F.; da Silveira, M.A.L.; de Souza, S.O.; d’Errico, F.; Sussuchi, E.M. Radiochromic Fricke gels with eriochrome cyanine R for radiotherapy dosimetry. Radiat. Phys. Chem. 2022, 191, 109830. [Google Scholar] [CrossRef]
- Gallo, S.; Locarno, S.; Brambilla, E.; Lenardi, C.; Pignoli, E.; Veronese, I. Dosimetric characterization of double network Fricke hydrogel based on PVA-GTA and phenylalanine peptide derivative. J. Phys. D Appl. Phys. 2023, 57, 075303. [Google Scholar] [CrossRef]
- Alves, A.V.S.; de Almeida, W.S.; Sussuchi, E.M.; Lazzeri, L.; d’Errico, F.; de Souza, S.O. Investigation of chelating agents/ligands for Fricke gel dosimeters. Radiat. Phys. Chem. 2018, 150, 151–156. [Google Scholar] [CrossRef]
- Aboelezz, E.; Pogue, B.W. Review of nanomaterial advances for ionizing radiation dosimetry. Appl. Phys. Rev. 2023, 10, 021312. [Google Scholar] [CrossRef]
- Merkis, M.; Griskonis, E.; Laurikaitiene, J.; Puiso, J.; Pikas, I.; Palvanov, S.; Adliene, D. Investigation of dose sensitivity and dose enhancement effect in silver nanoparticle enriched dose gels. Radiat. Phys. Chem. 2023, 213, 111213. [Google Scholar] [CrossRef]
- Locarno, S.; Arosio, P.; Curtoni, F.; Piazzoni, M.; Pignoli, E.; Gallo, S. Microscopic and macroscopic characterization of hydrogels based on poly (vinyl-alcohol)–glutaraldehyde mixtures for Fricke gel dosimetry. Gels 2024, 10, 172. [Google Scholar] [CrossRef]
- De Deene, Y.; Jirasek, A. Gel dosimetry: An overview of dosimetry systems and read out methods. Radiat. Meas. 2024, 179, 107321. [Google Scholar] [CrossRef]
| Material | Approximate Chemical Formula | ρ [g/cm3] | <Z/A> | Zeff |
|---|---|---|---|---|
| Adipose Tissue | C1143H2594N11O401Na1S1Cl1 | 0.555 | 0.554 | 3.27 |
| Blood, Whole | C212H2322N54O1071Na1S1Cl1Fe1K1 | 1.060 | 0.550 | 3.66 |
| Bone, Cortical | C297H959N169O624Na1Mg2S74Ca129 | 1.920 | 0.515 | 5.08 |
| Brain | C195H1724N25O718Na1P2S1Cl1K1 | 1.040 | 0.552 | 3.62 |
| Breast Tissue | C1111H4249N86O1322Na2Mg2S3Ca1 | 1.020 | 0.552 | 3.48 |
| Eye Lens | C576H3375 N144O1433Na2P1S3Cl1 | 1.070 | 0.547 | 3.58 |
| Ferrous Sulfate Standard Fricke | FeSO4·7H2O | 1.024 | 0.553 | 5.61 |
| Gadolinium Oxysulfide | Gd2O2S10 | 7.440 | 0.423 | 11.92 |
| Gafchromic Sensor | C7HNO2 | 1.300 | 0.544 | 3.47 |
| Lithium Tetraborate | Li2B4O7 | 2.440 | 0.485 | 3.64 |
| Lung Tissue | C171H1996N43O914Na2P1S2Cl2K1 | 1.050 | 0.550 | 3.66 |
| Muscle, Skeletal | C425H3615N87O1585Na2P2S3Cl1K4 | 1.050 | 0.550 | 3.64 |
| Ovary | C152H2043 N34O941Na2P1S1Cl1K1 | 1.050 | 0.551 | 3.65 |
| Polystyrene | C8H8 | 1.060 | 0.538 | 5.7 |
| Polytetrafluoroethylene (Teflon) | C2F4 | 2.250 | 0.480 | 8.43 |
| Polyvinyl Chloride | C2H3Cl | 1.406 | 0.512 | 13.86 |
| Radiochromic Dye Film | C9H16N1O2 | 1.080 | 0.550 | 6.2 |
| Testis | C15H16O116N3P1S1Cl1K1 | 1.040 | 0.552 | 9.02 |
| Tissue, Soft | C23H34O119N5P1S1Cl1K1 | 1.060 | 0.550 | 8.85 |
| Tissue, Four-Component | C6H13NO7 | 1.000 | 0.550 | 7.02 |
| Water, Liquid | H2O | 1.000 | 0.555 | 7.42 |
| Fricke Gel Dosimeters | Polymer Dosimeters | Radiochromic Polymer Dosimeters | Solid Plastic Dosimeters | RPL Dosimeters | |
|---|---|---|---|---|---|
| Bases | Water, Gelatine, Fe2+ | Water, Gelatine, Monomer | Water, Gelatine, Surfactant/PVA | Plastic/Elastomer | Solutions ff Coumarin/Aqueous Benzoic Acid/Terephthalic Acid/Trimesic Acid |
| Additives | Xo, Mtb, Pva, Nano Gels | Crosslinker Antioxidant | Hydrophobic Dye, Organic Halogen, Tetrazolium Salts/Iodine | Dye, Halogen | Gold Nanoparticles, Mpy, Tbua, Nanoclay, Rd 123, Dhr 123, Halogen, Fe3+ Pyridine, Nanoclay, Gelatine, Agarose |
| Readout | OCT, MRI, UV-Vis | OCT, MRI, Raman | OCT, XCT, Raman | OCT, Raman | Optical |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagsoz, M.E.; Korkut, O.; Gallo, S. Advancements in Tissue-Equivalent Gel Dosimeters. Gels 2025, 11, 81. https://doi.org/10.3390/gels11020081
Sagsoz ME, Korkut O, Gallo S. Advancements in Tissue-Equivalent Gel Dosimeters. Gels. 2025; 11(2):81. https://doi.org/10.3390/gels11020081
Chicago/Turabian StyleSagsoz, Mustafa Erdem, Ozlem Korkut, and Salvatore Gallo. 2025. "Advancements in Tissue-Equivalent Gel Dosimeters" Gels 11, no. 2: 81. https://doi.org/10.3390/gels11020081
APA StyleSagsoz, M. E., Korkut, O., & Gallo, S. (2025). Advancements in Tissue-Equivalent Gel Dosimeters. Gels, 11(2), 81. https://doi.org/10.3390/gels11020081








