Physicochemical Characterization and Antioxidant Properties of Cellulose-Rich Extracts Obtained from Carob (Ceratonia siliqua L.) Pulp for Preparation of Cellulose-Rich Gels
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization of the Pulp from Different Carob Varieties
2.2. Characterization of the Carob Cellulose-Rich Fraction (CRF)
2.2.1. Sugar Content
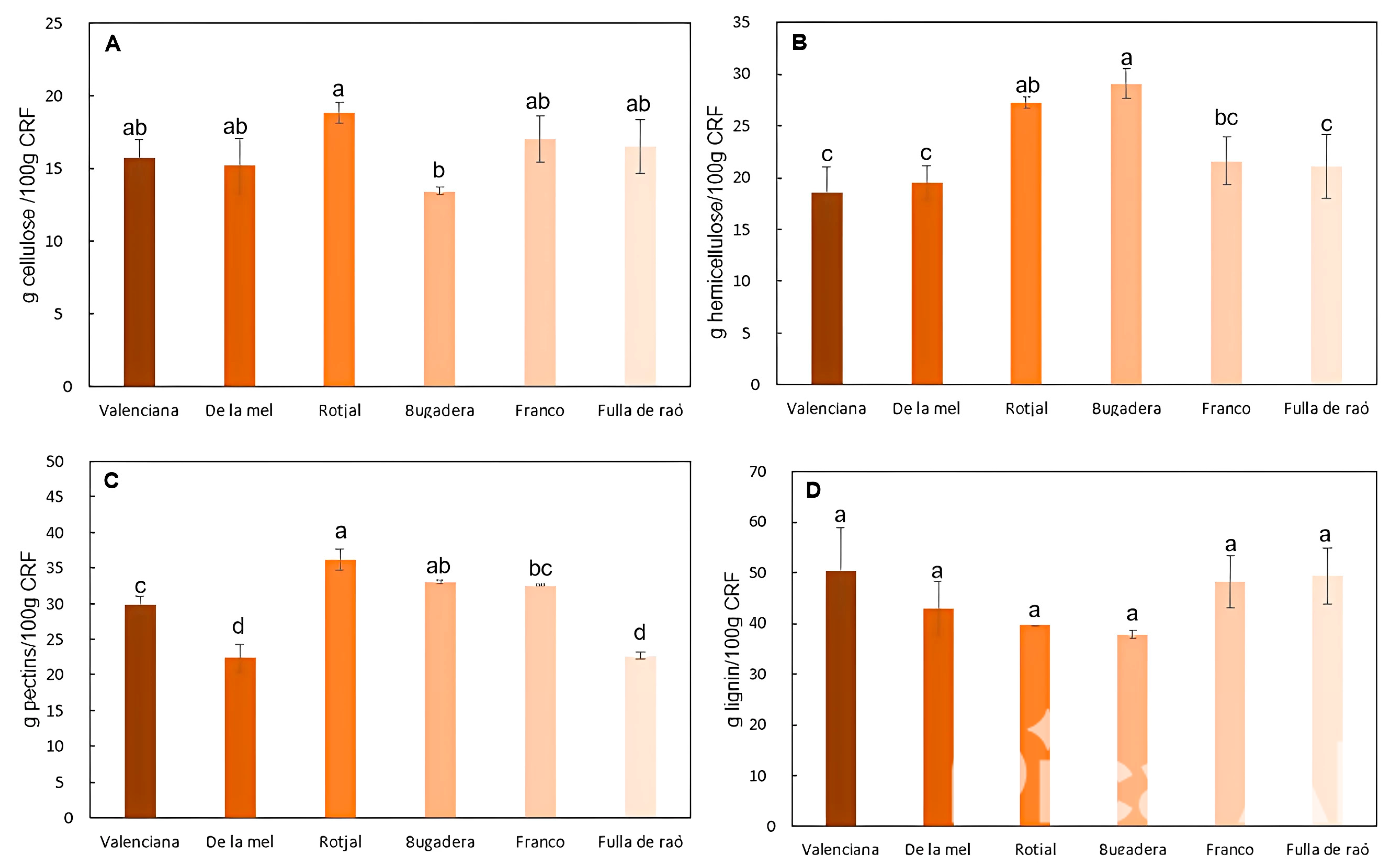
2.2.2. Polysaccharide Characterization
2.2.3. Functional Properties
2.2.4. Antioxidants Compounds
3. Conclusions
4. Materials and Methods
4.1. Physicochemical Characterisation of the Pulp from Different Carob Varieties
Moisture, pH, Acidity, Soluble Sugars and Cellulose-Rich Fraction
4.2. CRE Characterization
4.2.1. Sugar Content
4.2.2. Polysaccharides and Lignin
4.2.3. Functional Properties
4.3. Antioxidant Compounds
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brassesco, M.E.; Brandão, T.R.S.; Silva, C.L.M.; Pintado, M. Carob Bean (Ceratonia siliqua L.): A New Perspective for Functional Food. Trends Food Sci. Technol. 2021, 114, 310–322. [Google Scholar] [CrossRef]
- Correia, M.A.; Romano, P.J.; Carob, A.; Benelli, C.; Ganino, T.; Giordano, C.; Petruccelli, R.; Beghé, D.; Martins-Loução, M.A.; Correia, P.J.; et al. Carob: A Mediterranean Resource for the Future. Plants 2024, 13, 1188. [Google Scholar] [CrossRef] [PubMed]
- Yousif, A.K.; Alghzawi, H.M. Processing and Characterization of Carob Powder. Food Chem. 2000, 69, 283–287. [Google Scholar] [CrossRef]
- El Batal, H.; Hasib, A.; Ouatmane, A.; Dehbi, F.; Jaouad, A.; Boulli, A. Sugar Composition and Yield of Syrup Production from the Pulp of Moroccan Carob Pods (Ceratonia siliqua L.). Arab. J. Chem. 2016, 9, S955–S959. [Google Scholar] [CrossRef]
- Sȩczyk, Ł.; Świeca, M.; Gawlik-Dziki, U. Effect of Carob (Ceratonia siliqua L.) Flour on the Antioxidant Potential, Nutritional Quality, and Sensory Characteristics of Fortified Durum Wheat Pasta. Food Chem. 2016, 194, 637–642. [Google Scholar] [CrossRef]
- Frassoldati, A.; Ranzi, E. Modeling of Thermochemical Conversion of Biomasses. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Ebringerová, A.; Hromádková, Z.; Košťálová, Z.; Sasinková, V. Chemical Valorization of Almond Shells; BioResources: Raleigh, NC, USA, 2008; Volume 3. [Google Scholar]
- Willats, W.G.T.; Knox, J.P.; Mikkelsen, J.D. Pectin: New Insights into an Old Polymer Are Starting to Gel. Trends Food Sci. Technol. 2006, 17, 97–104. [Google Scholar] [CrossRef]
- Ström, A.; Ribelles, P.; Lundin, L.; Norton, I.; Morris, E.R.; Williams, M.A.K. Influence of Pectin Fine Structure on the Mechanical Properties of Calcium-Pectin and Acid-Pectin Gels. Biomacromolecules 2007, 8, 2668–2674. [Google Scholar] [CrossRef]
- Lopez-Sanchez, P.; Martinez-Sanz, M.; Bonilla, M.R.; Wang, D.; Gilbert, E.P.; Stokes, J.R.; Gidley, M.J. Cellulose-Pectin Composite Hydrogels: Intermolecular Interactions and Material Properties Depend on Order of Assembly. Carbohydr. Polym. 2017, 162, 71–81. [Google Scholar] [CrossRef]
- Maruyama, S.; Streletskaya, N.A.; Lim, J. Clean Label: Why This Ingredient but Not That One? Food Qual. Prefer. 2021, 87, 104062. [Google Scholar] [CrossRef]
- Ioannou, G.D.; Savva, I.K.; Christou, A.; Stavrou, I.J.; Kapnissi-Christodoulou, C.P. Phenolic Profile, Antioxidant Activity, and Chemometric Classification of Carob Pulp and Products. Molecules 2023, 28, 2269. [Google Scholar] [CrossRef]
- Correa, M.J.; Salinas, M.V.; Carbas, B.; Ferrero, C.; Brites, C.; Puppo, M.C. Technological Quality of Dough and Breads from Commercial Algarroba–Wheat Flour Blends. J. Food Sci. Technol. 2017, 54, 2104–2114. [Google Scholar] [CrossRef] [PubMed]
- Loullis, A.; Pinakoulaki, E. Carob as Cocoa Substitute: A Review on Composition, Health Benefits and Food Applications. Eur. Food Res. Technol. 2017, 244, 959–977. [Google Scholar] [CrossRef]
- Aydın, S.; Özdemir, Y. Development and Characterization of Carob Flour Based Functional Spread for Increasing Use as Nutritious Snack for Children. J. Food Qual. 2017, 2017, 5028150. [Google Scholar] [CrossRef]
- Vitali Čepo, D.; Mornar, A.; Nigović, B.; Kremer, D.; Radanović, D.; Vedrina Dragojević, I. Optimization of Roasting Conditions as an Useful Approach for Increasing Antioxidant Activity of Carob Powder. LWT-Food Sci. Technol. 2014, 58, 578–586. [Google Scholar] [CrossRef]
- Haddarah, A.; Ismail, A.; Bassal, A.; Hamieh, T.; Ioannou, I.; Ghoul, M. Morphological and chemical variability of lebanese carob varieties. Eur. Sci. J. 2013, 9, 353–369. [Google Scholar]
- Llompart Salas, B. Revalorització Dels Subproductes Derivats de La Comercialització de La Garrova (Ceratonia Síliqua L.): Fibra i Composts Antioxidants 2023; Universitat de les Illes Balears: Palma, Spain, 2023. [Google Scholar]
- Adam, I.A.A.F. Wild Fruits: Composition, Nutritional Value and Products; Springer: Cham, Switzerland, 2019; pp. 1–577. [Google Scholar] [CrossRef]
- Tounsi, L.; Mkaouar, S.; Bredai, S.; Kechaou, N. Valorization of Carob By-Product for Producing an Added Value Powder: Characterization and Incorporation into Halva Formulation. J. Food Meas. Charact. 2022, 16, 3957–3966. [Google Scholar] [CrossRef]
- Benchikh, Y.; Paris, C.; Louaileche, H.; Charbonnel, C.; Ghoul, M.; Chebil, L. Comparative Characterization of Green and Ripe Carob (Ceratonia siliqua L.): Physicochemical Attributes and Phenolic Profile. SDRP J. Food Sci. Technol. 2017, 1, 85–91. [Google Scholar] [CrossRef]
- Sciammaro, L.P. Caracterización Fisicoquímica de Vainas y Harinas de Algarrobo (Prosopis Alba y Prosopis Nigra). Ph.D. Thesis, Universidad Nacional de La Plata, La Plata, Argentina, 2015. [Google Scholar] [CrossRef]
- Khlifa, M.; Bahloul, A.; Kitane, S. Determination of Chemical Composition of Carob Pod (Ceratonia siliqua L) and Its Morphological Study. J. Mater. Environ. Sci. 2013, 4, 348–353. [Google Scholar]
- Nasar-Abbas, S.M.; E-Huma, Z.; Vu, T.H.; Khan, M.K.; Esbenshade, H.; Jayasena, V. Carob Kibble: A Bioactive-Rich Food Ingredient. Compr. Rev. Food Sci. Food Saf. 2016, 15, 63–72. [Google Scholar] [CrossRef]
- Femenia, A.; Sánchez, E.S.; Simal, S.; Rosselló, C. Compositional Features of Polysaccharides from Aloe Vera (Aloe Barbadensis Miller) Plant Tissues. Carbohydr. Polym. 1999, 39, 109–117. [Google Scholar] [CrossRef]
- Zhu, B.J.; Zayed, M.Z.; Zhu, H.X.; Zhao, J.; Li, S.P. Functional Polysaccharides of Carob Fruit: A Review. Chin. Med. 2019, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Papaefstathiou, E.; Agapiou, A.; Giannopoulos, S.; Kokkinofta, R. Nutritional Characterization of Carobs and Traditional Carob Products. Food Sci. Nutr. 2018, 6, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Franz, G.; Blaschek, W. 8—Cellulose. Methods Plant Biochem. 1990, 2, 291–322. [Google Scholar] [CrossRef]
- Kumar Gupta, P.; Sai Raghunath, S.; Venkatesh Prasanna, D.; Venkat, P.; Shree, V.; Chithananthan, C.; Choudhary, S.; Surender, K.; Geetha, K. An Update on Overview of Cellulose, Its Structure and Applications. Cellulose 2019, 4. [Google Scholar] [CrossRef]
- Szymanska-Chargot, M.; Chylinska, M.; Gdula, K.; Koziol, A.; Zdunek, A. Isolation and Characterization of Cellulose from Different Fruit and Vegetable Pomaces. Polymer 2017, 9, 495. [Google Scholar] [CrossRef]
- Umaña, M.M.; Dalmau, M.E.; Eim, V.S.; Femenia, A.; Rosselló, C. Effects of Acoustic Power and PH on Pectin-Enriched Extracts Obtained from Citrus by-Products. Modelling of the Extraction Process. J. Sci. Food Agric. 2019, 99, 6893–6902. [Google Scholar] [CrossRef]
- Gunning, A.P.; Bongaerts, R.J.M.; Morris, V.J. Recognition of Galactan Components of Pectin by Galectin-3. FASEB J. 2009, 23, 415–424. [Google Scholar] [CrossRef]
- Khamsucharit, P.; Laohaphatanalert, K.; Gavinlertvatana, P.; Sriroth, K.; Sangseethong, K. Characterization of Pectin Extracted from Banana Peels of Different Varieties. Food Sci. Biotechnol. 2017, 27, 623–629. [Google Scholar] [CrossRef]
- Prakash Maran, J.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R. Optimization of Microwave Assisted Extraction of Pectin from Orange Peel. Carbohydr. Polym. 2013, 97, 703–709. [Google Scholar] [CrossRef]
- Velásquez-Arredondo, H.I.; Ruiz-Colorado, A.A.; De Oliveira, S. Ethanol Production Process from Banana Fruit and Its Lignocellulosic Residues: Energy Analysis. Energy 2010, 35, 3081–3087. [Google Scholar] [CrossRef]
- Rodríguez-González, V.M.; Femenia, A.; González-Laredo, R.F.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Candelas-Cadillo, M.G.; Ramírez-Baca, P.; Simal, S.; Rosselló, C. Effects of Pasteurization on Bioactive Polysaccharide Acemannan and Cell Wall Polymers from Aloe Barbadensis Miller. Carbohydr. Polym. 2011, 86, 1675–1683. [Google Scholar] [CrossRef]
- Petkova, N.; Petrova, I.; Ivanov, I.; Mihov, R.; Hadjikinova, R.; Ognyanov, M.; Nikolova, V. Nutritional and Antioxidant Potential of Carob (Ceratonia siliqua) Flour and Evaluation of Functional Properties of Its Polysaccharide Fraction. J. Pharm. Sci. Res. 2017, 9, 2189–2195. [Google Scholar]
- Ganji, F.; Vasheghani Farahani, S.; Vasheghani-Farahani, E. Theoretical Description of Hydrogel Swelling: A Review. Iran. Polym. J. 2010, 19, 375–398. [Google Scholar]
- Blažic, R.; Marušić, K.; Vidović, E. Swelling and Viscoelastic Properties of Cellulose-Based Hydrogels Prepared by Free Radical Polymerization of Dimethylaminoethyl Methacrylate in Cellulose Solution. Gels 2023, 9, 94. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary Fibre and Fibre-Rich by-Products of Food Processing: Characterisation, Technological Functionality and Commercial Applications: A Review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Tofanica, B.M.; Mikhailidi, A.; Samuil, C.; Ungureanu, O.C.; Fortună, M.E.; Ungureanu, E. Advances in Cellulose-Based Hydrogels: Current Trends and Challenges. Gels 2024, 10, 842. [Google Scholar] [CrossRef]
- Ortega, N.; Macià, A.; Romero, M.-P.; Trullols, E.; Morello, J.-R.; Anglès, N.; Motilva, M.-J.; Ortega, N.; Macià, A.; Romero, M.-P.; et al. Rapid Determination of Phenolic Compounds and Alkaloids of Carob Flour by Improved Liquid Chromatography Tandem Mass Spectrometry. J. Agric. Food Chem. 2009, 57, 7239–7244. [Google Scholar] [CrossRef]
- Saci, F.; Bachir Bey, M.; Louaileche, H.; Gali, L.; Bensouici, C. Changes in Anticholinesterase, Antioxidant Activities and Related Bioactive Compounds of Carob Pulp (Ceratonia siliqua L.) during Ripening Stages. J. Food Meas. Charact. 2020, 14, 937–945. [Google Scholar] [CrossRef]
- Rtibi, K.; Selmi, S.; Grami, D.; Saidani, K.; Sebai, H.; Amri, M.; Eto, B.; Marzouki, L. Ceratonia siliqua L. (Immature Carob Bean) Inhibits Intestinal Glucose Absorption, Improves Glucose Tolerance and Protects against Alloxan-Induced Diabetes in Rat. J. Sci. Food Agric. 2017, 97, 2664–2670. [Google Scholar] [CrossRef]
- International Standard International Organization for Standardization. Meat and Meat Products-Determination of Total Fat Content. Available online: https://cdn.standards.iteh.ai/samples/6038/6b692ef064484574aec2816f3343ed68/ISO-1443-1973.pdf (accessed on 4 January 2025).
- AOAC. Método Oficial AOAC 932.14 Sólidos En Almíbar. Available online: http://files.foodmate.com/2013/files_2967.html (accessed on 4 January 2025).
- Dalmau, M.E.; Bornhorst, G.M.; Eim, V.; Rosselló, C.; Simal, S. Effects of Freezing, Freeze Drying and Convective Drying on in Vitro Gastric Digestion of Apples. Food Chem. 2017, 215, 7–16. [Google Scholar] [CrossRef]
- Coimbra, M.A.; Delgadillo, I.; Waldron, K.W.; Selvendran, R.R. Isolation and Analysis of Cell Wall Polymers from Olive Pulp. Mod. Methods Plant Anal. 1996, 17, 19–44. [Google Scholar] [CrossRef]
- González-Centeno, M.R. Caracterización de Los Subproductos de la Industria Vitivinícola Como Fuente De Fibra Dietética Y Compuestos Fenólicos. Uso de Los Ul-Trasonidos De Potencia Para La Extracción de la Fracción Fenólica; Universitat de les Illes Balears: Palma, Spain, 2013. [Google Scholar]
- Wang, W.; Ma, X.; Xu, Y.; Cao, Y.; Jiang, Z.; Ding, T.; Ye, X.; Liu, D. Ultrasound-Assisted Heating Extraction of Pectin from Grapefruit Peel: Optimization and Comparison with the Conventional Method. Food Chem. 2015, 178, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Sousa, A.G.; Crépeau, M.-J.; Sørensen, S.O.; Ralet, M.-C. Characterization of Citrus Pectin Samples Extracted under Different Conditions: Influence of Acid Type and PH of Extraction. Ann. Bot. 2014, 114, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Manrique, G.D.; Lajolo, F.M. FT-IR Spectroscopy as a Tool for Measuring Degree of Methyl Esterification in Pectins Isolated from Ripening Papaya Fruit. Postharvest Biol. Technol. 2002, 25, 99–107. [Google Scholar] [CrossRef]
- Kuniak, L.; Marchessault, R.H. Study of the Crosslinking Reaction between Epichlorohydrin and Starch. Starch 1972, 24, 110–116. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Rosselló, C.; Simal, S.; Garau, M.C.; López, F.; Femenia, A. Physico-Chemical Properties of Cell Wall Materials Obtained from Ten Grape Varieties and Their Byproducts: Grape Pomaces and Stems. LWT-Food Sci. Technol. 2010, 43, 1580–1586. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Comas-Serra, F.; Femenia, A.; Rosselló, C.; Simal, S. Effect of Power Ultrasound Application on Aqueous Extraction of Phenolic Compounds and Antioxidant Capacity from Grape Pomace (Vitis vinifera L.): Experimental Kinetics and Modeling. Ultrason. Sonochem. 2015, 22, 506–514. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Knoerzer, K.; Sabarez, H.; Simal, S.; Rosselló, C.; Femenia, A. Effect of Acoustic Frequency and Power Density on the Aqueous Ultrasonic-Assisted Extraction of Grape Pomace (Vitis vinifera L.)–A Response Surface Approach. Ultrason. Sonochem. 2014, 21, 2176–2184. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; RStudio Team: Boston, MA, USA, 2022. [Google Scholar]


| Variety | % Pulp | % Seed |
|---|---|---|
| Valenciana | 82.5 | 17.5 |
| Fulla de raó | 85.0 | 15.0 |
| Franco | 88.7 | 11.3 |
| Rotjal | 87.0 | 13.0 |
| Bugadera | 87.4 | 12.6 |
| De la mel | 84.4 | 15.6 |
| Variety | Moisture Content | pH | Acidity | Soluble Sugars | CRF |
|---|---|---|---|---|---|
| Valenciana | 14.4 ± 0.1 (a) | 5.23 ± 0.02 (a) | 3.0 ± 0.4 (cd) | 34.0 ± 1.5 (d) | 45.6 ± 2.1 (ab) |
| De la mel | 13.9 ± 0.8 (ab) | 5.23 ± 0.01 (a) | 4.4 ± 0.0 (a) | 38.2 ± 0.3 (bc) | 44.5 ± 2.0 (ab) |
| Rotjal | 12.4 ± 0.1 (ab) | 4.96 ± 0.04 (d) | 2.3 ± 0.3 (d) | 35.0 ± 1.8 (cd) | 49.1 ± 1.9 (a) |
| Bugadera | 13.2 ± 0.0 (ab) | 4.88 ± 0.01 (e) | 4.0 ± 0.2 (ab) | 41.6 ± 0.6 (ab) | 41.4 ± 2.1 (b) |
| Franco | 11.6 ± 2.3 (ab) | 5.16 ± 0.01 (b) | 3.3 ± 0.4 (bc) | 38.5 ± 0.2 (bc) | 45.7 ± 2.3 (ab) |
| Fulla de raó | 11.2 ± 0.8 (b) | 5.10 ± 0.01 (c) | 3.0 ± 0.4 (cd) | 43.4 ± 2.7 (a) | 40.9 ± 2.0 (b) |
| Variety | Rha | Fuc | Ara | Xyl | Man | Gal | Glc | UA |
|---|---|---|---|---|---|---|---|---|
| Valenciana | 12.8 ± 0.1 | 9.1 ± 0.1 | 83.1 ± 4.6 | 134.7 ± 6.2 | 24.5 ± 3.9 | 55.9 ± 4.7 | 175.1 ± 13.6 | 146.1 ± 7.7 |
| De la mel | 9.1 ± 0.1 | 6.9 ± 0.1 | 70.8 ± 4.8 | 148.4 ± 7.5 | 22.7 ± 5.7 | 48.1 ± 8.0 | 168.8 ± 13.9 | 96.1 ± 6.5 |
| Rotjal | 11.4 ± 0.1 | 9.4 ± 0.1 | 84.7 ± 2.5 | 210.4 ± 5.7 | 31.6 ± 1.34 | 46.6 ± 0.3 | 209.6 ± 8.5 | 219.2 ± 10.3 |
| Bugadera | 11.1 ± 0.1 | 6.2 ± 0.1 | 74.7 ± 1.6 | 129.6 ± 10.4 | 20.3 ± 1.5 | 45.4 ± 0.7 | 149.6 ± 3.1 | 183.3 ± 7.3 |
| Franco | 11.3 ± 0.1 | 7.2 ± 0.1 | 78.9 ± 5.2 | 165.0 ± 11.3 | 25.1 ± 0.8 | 41.8 ± 1.1 | 189.6 ± 12.6 | 193.6 ± 11.3 |
| Fulla raó | 7.8 ± 0.1 | 6.6 ± 0.1 | 77.2 ± 2.2 | 161.3 ± 70.7 | 24.5 ± 4.3 | 46.4 ± 3.2 | 183.2 ± 10.9 | 95.5 ± 6.7 |
| Variety | Linearity | Number | Length | DME (%) |
|---|---|---|---|---|
| Valenciana | 1.0 ± 0.1 (b) | 11.6 ± 2.0 (c) | 11.2 ± 3.9 (a) | 57.2 ± 1.7 (b) |
| De la mel | 0.8 ± 0.0 (b) | 10.6 ± 0.0 (c) | 13.1 ± 2.2 (a) | 56.6 ± 1.7 (b) |
| Rotjal | 1.5 ± 0.1 (a) | 19.3 ± 0.3 (ab) | 11.6 ± 1.0 (a) | 71.5 ± 2.1 (a) |
| Bugadera | 1.4 ± 0.2 (a) | 16.5 ± 2.2 (b) | 10.9 ± 0.1 (a) | 56.7 ± 1.7 (b) |
| Franco | 1.5 ± 0.2 (a) | 21.7 ± 1.3 (a) | 11.1 ± 2.4 (a) | 46.7 ± 1.4 (c) |
| Fulla de raó | 0.7 ± 0.0 (b) | 12.2 ± 0.3 (c) | 15.8 ± 1.1 (a) | 60.9 ± 1.8 (b) |
| Variety | TPC | FRAP | ABTS | CUPRAC |
|---|---|---|---|---|
| Valenciana | 38 ± 4 (b) | 25 ± 3 (b) | 610 ± 60 (b) | 300 ± 30 (c) |
| De la mel | 44 ± 4 (b) | 49 ± 4 (a) | 640 ± 80 (b) | 670 ± 60 (a) |
| Rotjal | 30 ± 3 (b) | 21 ± 2 (b) | 560 ± 60 (b) | 250 ± 20 (c) |
| Bugadera | 68 ± 6 (a) | 56 ± 6 (a) | 1070 ± 90 (a) | 700 ± 70 (a) |
| Franco | 37 ± 4 (b) | 32 ± 3 (b) | 480 ± 70 (b) | 420 ± 50 (b) |
| Fulla de raó | 38 ± 5 (b) | 29 ± 4 (b) | 590 ± 50 (b) | 370 ± 40 (bc) |
| Carob Variety | Photograph of the Fruit | Photograph of the Carob Tree | Shape | Type of Crop |
|---|---|---|---|---|
| Bugadera |  |  | Elongated semi-curved | Irrigable land |
| Fulla de raó |  |  | Elongated, thick | Dry farming |
| Valenciana |  |  | Elongated | Dry farming |
| Rotjal |  |  | Elongated, thick | Dry farming |
| Franco |  |  | Elongated curved | Dry farming |
| De la mel |  |  | Thin, small-sized | Dry farming |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llompart, B.; Dalmau, E.; Umaña, M.; Femenia, A. Physicochemical Characterization and Antioxidant Properties of Cellulose-Rich Extracts Obtained from Carob (Ceratonia siliqua L.) Pulp for Preparation of Cellulose-Rich Gels. Gels 2025, 11, 145. https://doi.org/10.3390/gels11020145
Llompart B, Dalmau E, Umaña M, Femenia A. Physicochemical Characterization and Antioxidant Properties of Cellulose-Rich Extracts Obtained from Carob (Ceratonia siliqua L.) Pulp for Preparation of Cellulose-Rich Gels. Gels. 2025; 11(2):145. https://doi.org/10.3390/gels11020145
Chicago/Turabian StyleLlompart, Bernat, Esperanza Dalmau, Mónica Umaña, and Antoni Femenia. 2025. "Physicochemical Characterization and Antioxidant Properties of Cellulose-Rich Extracts Obtained from Carob (Ceratonia siliqua L.) Pulp for Preparation of Cellulose-Rich Gels" Gels 11, no. 2: 145. https://doi.org/10.3390/gels11020145
APA StyleLlompart, B., Dalmau, E., Umaña, M., & Femenia, A. (2025). Physicochemical Characterization and Antioxidant Properties of Cellulose-Rich Extracts Obtained from Carob (Ceratonia siliqua L.) Pulp for Preparation of Cellulose-Rich Gels. Gels, 11(2), 145. https://doi.org/10.3390/gels11020145








