Hydrogels for Peripheral Nerve Repair: Emerging Materials and Therapeutic Applications
Abstract
1. Background
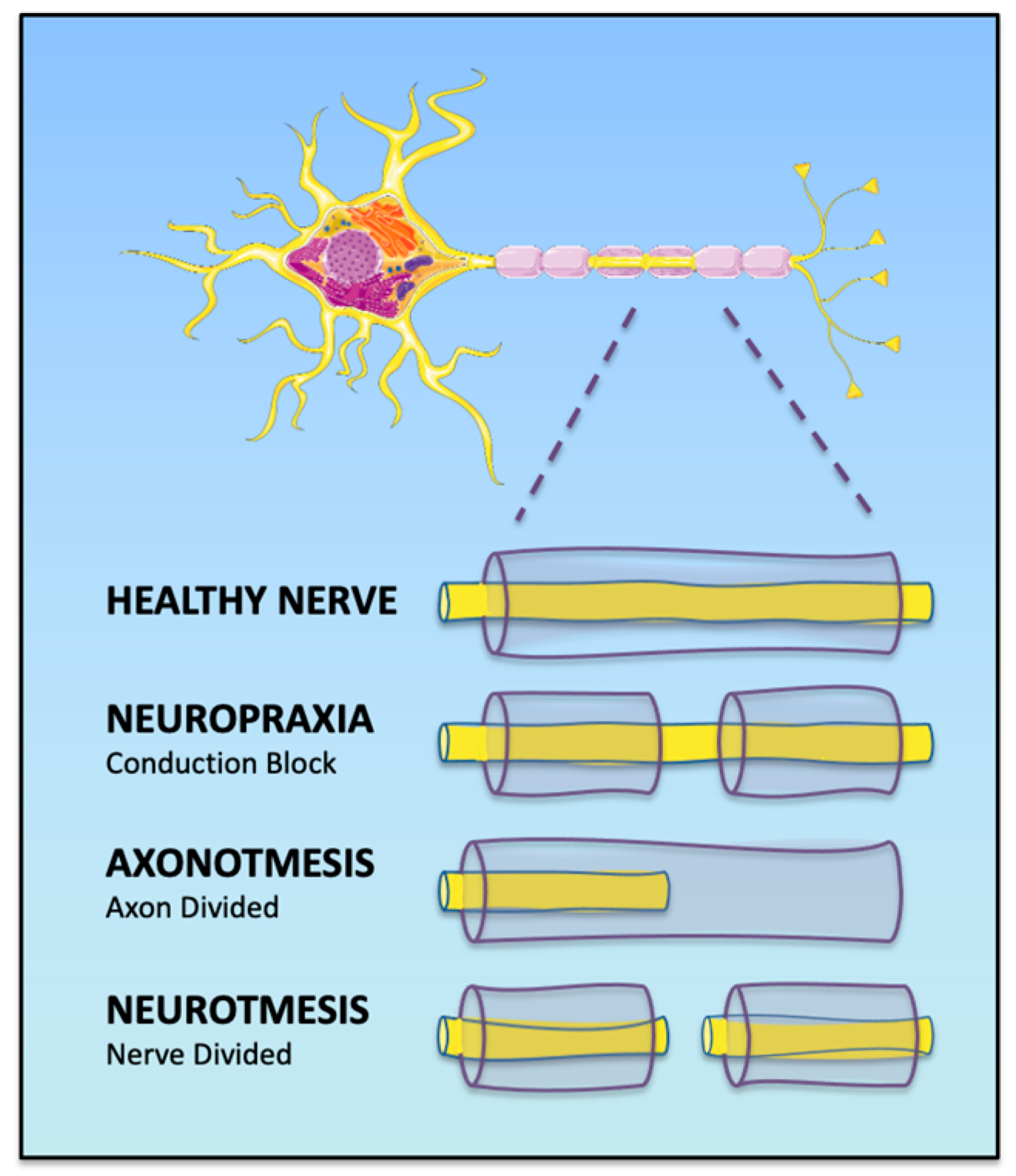
1.1. Classification of Peripheric Nerve Injury
1.2. Common Pathologies and Occupational Implications
1.3. Therapeutic Strategies
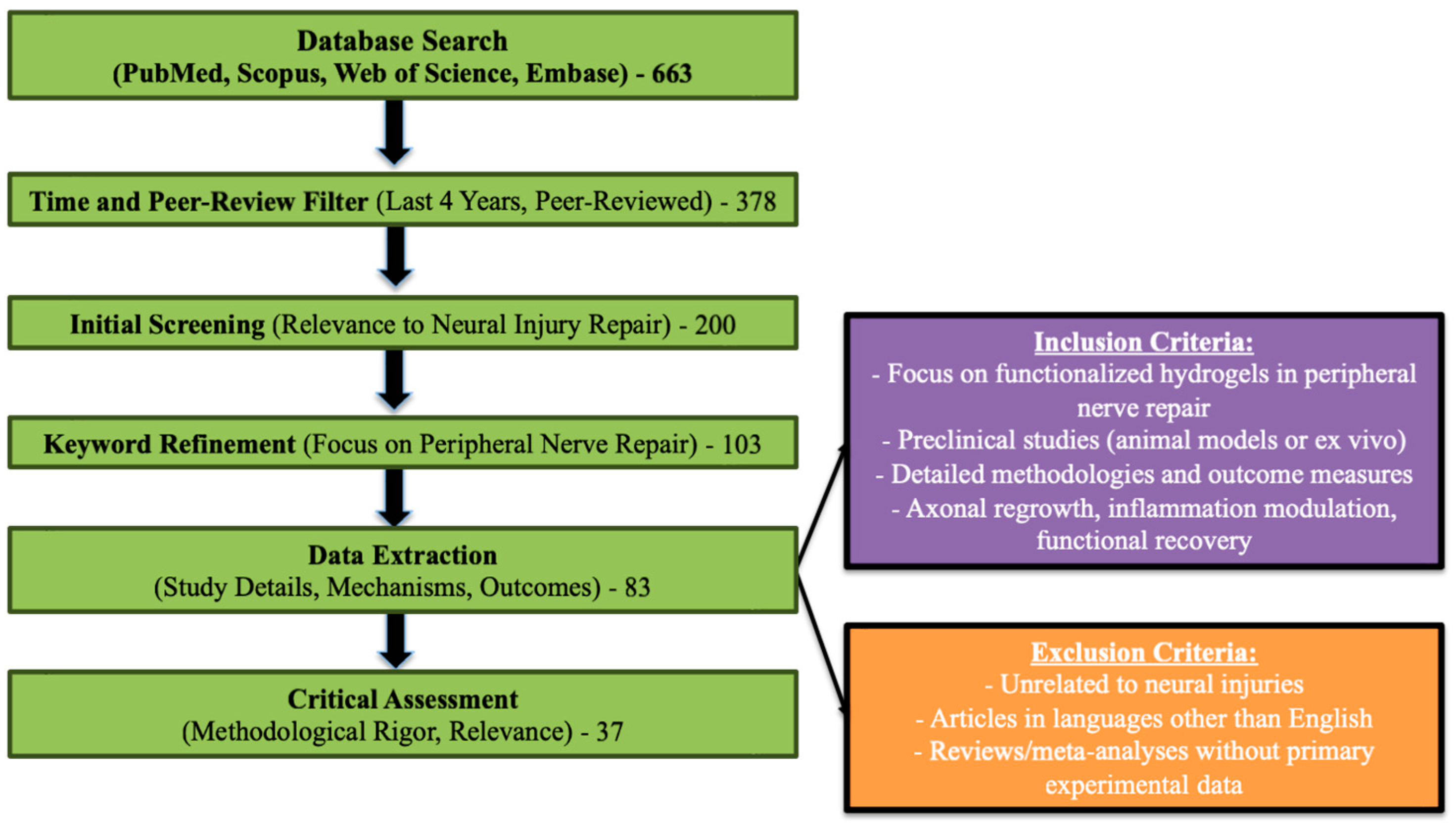
2. Material and Methods
3. Mechanisms of Action in Peripheral Nerve Regeneration
3.1. Hydrogel-Based Conduits for Peripheral Nerve Repair: Advantages, Emerging Materials, Mechanical Properties, and Fabrication Techniques
3.1.1. Advantages of Hydrogel-Based Conduits in Peripheral Nerve Repair
3.1.2. Emerging Materials and Mechanical Properties
3.1.3. Fabrication Techniques, Compatibility, and Evaluation
4. Applications of Hydrogels in Peripheral Nerve Repair
4.1. Guiding Axonal Growth: Scaffold Hydrogels
4.2. Reducing Scar Tissue: Barrier Hydrogels
4.3. Enhancing Electrical Conductivity: Conductive Hydrogels
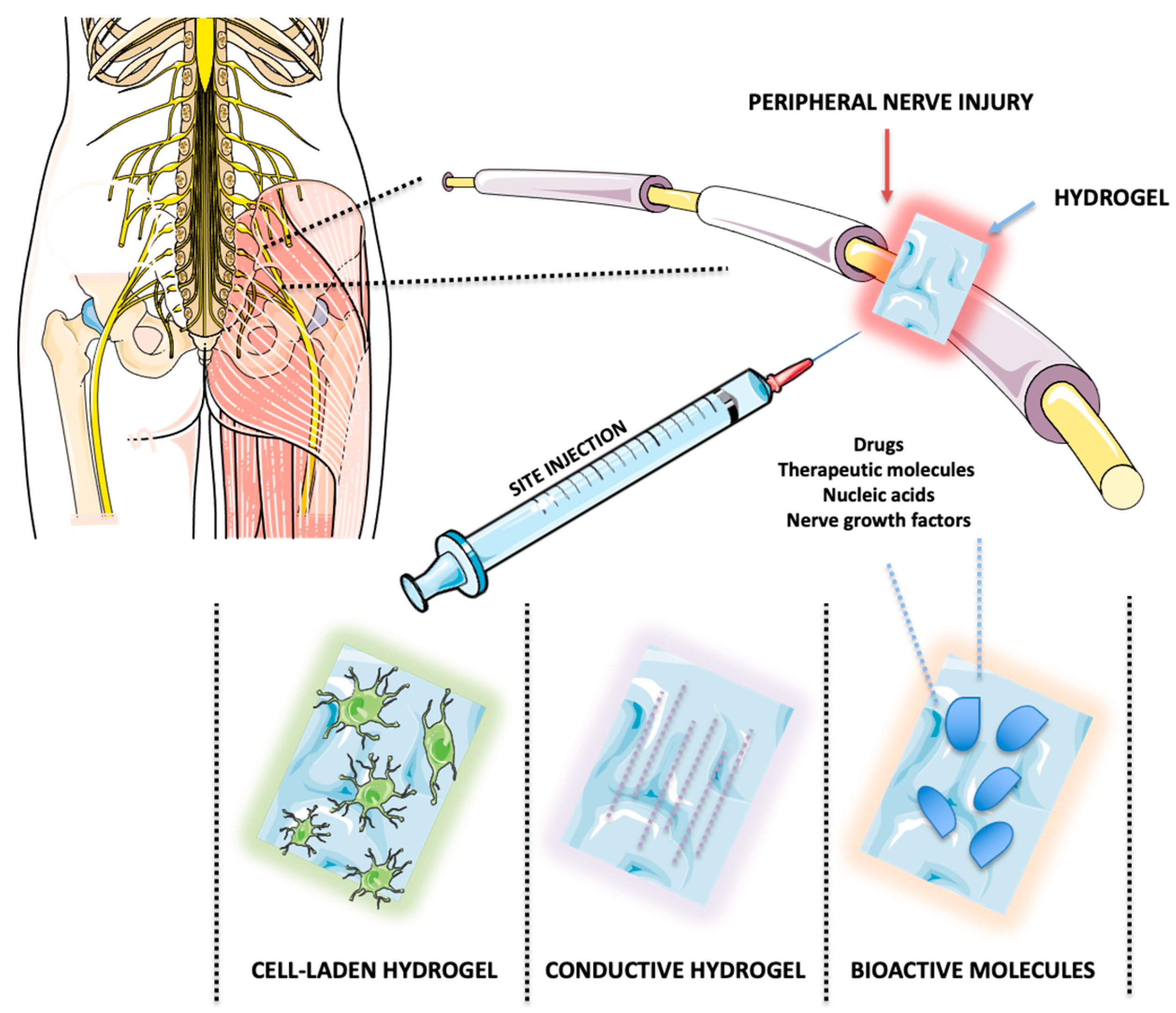
4.4. Promoting Cellular Healing: Drug Delivery and Cell-Encapsulating Hydrogels
| Active Compounds/Features | Hydrogel Materials | Loaded Drugs/Bioactives | Study Model | Effects on Recovery | Ref. |
|---|---|---|---|---|---|
| Laminin and NGF | Laminin-modified gellan gum | NGF | In vitro and ex vivo | Enhanced neuronal proliferation, differentiation, reduced apoptosis | [108] |
| Extracellular vesicles (EVs) | Thermosensitive hydrogel (pluronic–alginate–lysine–dextran) | EVs from adipose-derived stem cells | In vitro and in vivo (rat model) | Promoted Schwann cell migration and proliferation, improved axonal outgrowth and nerve conduction | [109] |
| bFGF and DPSCs | Gelatin methacryloyl (GelMA) | bFGF and DPSCs | In vitro and in vivo (rat sciatic nerve) | Accelerated Schwann cell migration and functional recovery similar to autografts | [106] |
| Laminin, collagen | Peripheral nerve matrix (PNM) hydrogel | None | In vitro and in vivo | Enhanced axon extension and electrophysiological recovery, reduced muscle atrophy | [104] |
| Curcumin | Keratin–chitosan hydrogels | Curcumin | In vitro and in vivo (rat sciatic nerve) | Reduced inflammation, improved axonal regeneration and functional recovery | [80] |
| Magnesium nanoparticles | Silk fibroin IPN hydrogels | Magnesium nanoparticles | In vitro (SCs and macrophages) | Enhanced myelination, reduced muscle atrophy, improved sensory and motor function | [69] |
| Decellularized ECM | Decellularized peripheral nerve hydrogel | None | In vitro | Enhanced Schwann cell viability, improved structural and functional recovery | [105] |
| Functionalized peptides | Aligned fibrin/self-assembling peptide hydrogel | None | In vitro and in vivo | Promoted Schwann cell alignment, axonal regeneration, functional recovery comparable to autografts | [68] |
| NGF-loaded microspheres | Chitosan/polycaprolactone hydrogels | Dopamine-modified NGF | In vitro | Sustained NGF release, enhanced axonal growth, Schwann cell migration | [109] |
| Magnesium and bisphosphonates | Nanocomposite hydrogels | Magnesium and bisphosphonate | In vitro and in vivo (rat sciatic nerve) | Enhanced myelination and axonal regrowth, reduced inflammation and muscle atrophy | [70] |
| Collagen and laminin | Decellularized ECM hydrogels derived from nerves | None | In vitro | Improved neuronal differentiation and axonal guidance, retained key ECM proteins for structural support | [72] |
5. Translational Barriers in Peripheral Nerve Repair
FDA-Approved Materials
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopes, B.; Sousa, P.; Alvites, R.; Branquinho, M.; Sousa, A.C.; Mendonça, C.; Atayde, L.M.; Luís, A.L.; Varejão, A.S.P.; Maurício, A.C. Peripheral Nerve Injury Treatments and Advances: One Health Perspective. Int. J. Mol. Sci. 2022, 23, 918. [Google Scholar] [CrossRef]
- Menorca, R.M.; Fussell, T.S.; Elfar, J.C. Nerve physiology: Mechanisms of injury and recovery. Hand Clin. 2013, 29, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Grinsell, D.; Keating, C.P. Peripheral nerve reconstruction after injury: A review of clinical and experimental therapies. Biomed. Res. Int. 2014, 2014, 698256. [Google Scholar] [CrossRef] [PubMed]
- Habre, S.B.; Bond, G.; Jing, X.L.; Kostopoulos, E.; Wallace, R.D.; Konofaos, P. The Surgical Management of Nerve Gaps: Present and Future. Ann. Plast. Surg. 2018, 80, 252–261. [Google Scholar] [CrossRef]
- Shi, S.; Ou, X.; Cheng, D. How Advancing is Peripheral Nerve Regeneration Using Nanofiber Scaffolds? A Comprehensive Review of the Literature. Int. J. Nanomed. 2023, 18, 6763–6779. [Google Scholar] [CrossRef] [PubMed]
- Omidian, H.; Chowdhury, S.D.; Cubeddu, L.X. Hydrogels for Neural Regeneration: Exploring New Horizons. Materials 2024, 17, 3472. [Google Scholar] [CrossRef] [PubMed]
- Revete, A.; Aparicio, A.; Cisterna, B.A.; Revete, J.; Luis, L.; Ibarra, E.; Segura González, E.A.; Molino, J.; Reginensi, D. Advancements in the Use of Hydrogels for Regenerative Medicine: Properties and Biomedical Applications. Int. J. Biomater. 2022, 2022, 3606765. [Google Scholar] [CrossRef]
- Ali, S.; Saik, J.E.; Gould, D.J.; Dickinson, M.E.; West, J.L. Immobilization of Cell-Adhesive Laminin Peptides in Degradable PEGDA Hydrogels Influences Endothelial Cell Tubulogenesis. BioRes. Open Access 2013, 2, 241–249. [Google Scholar] [CrossRef]
- El Ouaamari, Y.; Van den Bos, J.; Willekens, B.; Cools, N.; Wens, I. Neurotrophic Factors as Regenerative Therapy for Neurodegenerative Diseases: Current Status, Challenges and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 3866. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, M.; Liu, N. Interactions between Schwann cell and extracellular matrix in peripheral nerve regeneration. Front. Neurol. 2024, 15, 1372168. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, E.; Seifalian, A.; Mellati, A.; Saremi, J.; Asadpour, S.; Enderami, S.E.; Nekounam, H.; Mahmoodi, N. Injectable hydrogels in central nervous system: Unique and novel platforms for promoting extracellular matrix remodeling and tissue engineering. Mater. Today Bio 2023, 20, 100614. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Xiao, C.; Liu, B. Engineered hydrogels for peripheral nerve repair. Mater. Today Bio 2023, 20, 100668. [Google Scholar] [CrossRef]
- Stocco, E.; Barbon, S.; Emmi, A.; Tiengo, C.; Macchi, V.; De Caro, R.; Porzionato, A. Bridging Gaps in Peripheral Nerves: From Current Strategies to Future Perspectives in Conduit Design. Int. J. Mol. Sci. 2023, 24, 9170. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://smart.servier.com/smart_image/neuron-ov/ (accessed on 15 January 2025).
- Bergmeister, K.D.; Große-Hartlage, L.; Daeschler, S.C.; Rhodius, P.; Böcker, A.; Beyersdorff, M.; Kern, A.O.; Kneser, U.; Harhaus, L. Acute and long-term costs of 268 peripheral nerve injuries in the upper extremity. PLoS ONE 2020, 15, e0229530. [Google Scholar] [CrossRef] [PubMed]
- Kouyoumdjian, J.A.; Graça, C.R.; Ferreira, V.F.M. Peripheral nerve injuries: A retrospective survey of 1124 cases. Neurol. India 2017, 65, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.A.; Olsson, D.; Burdorf, A.; Punnett, L.; Järvholm, B.; Wahlström, J. Occupational biomechanical risk factors for radial nerve entrapment in a 13-year prospective study among male construction workers. Occup. Environ. Med. 2019, 76, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Andrasfay, T.; Raymo, N.; Goldman, N.; Pebley, A.R. Physical work conditions and disparities in later life functioning: Potential pathways. SSM-Popul. Health 2021, 16, 100990. [Google Scholar] [CrossRef] [PubMed]
- Rotaru-Zavaleanu, A.D.; Lungulescu, C.V.; Bunescu, M.G.; Vasile, R.C.; Gheorman, V.; Gresita, A.; Dinescu, V.C. Occupational Carpal Tunnel Syndrome: A scoping review of causes, mechanisms, diagnosis, and intervention strategies. Front. Public Health 2024, 12, 1407302. [Google Scholar] [CrossRef]
- Miettinen, L.; Ryhänen, J.; Shiri, R.; Karppinen, J.; Miettunen, J.; Auvinen, J.; Hulkkonen, S. Work-related risk factors for ulnar nerve entrapment in the Northern Finland Birth Cohort of 1966. Sci. Rep. 2021, 11, 10010. [Google Scholar] [CrossRef]
- Raza, C.; Riaz, H.A.; Anjum, R.; Shakeel, N.U.A. Repair strategies for injured peripheral nerve: Review. Life Sci. 2020, 243, 117308. [Google Scholar] [CrossRef] [PubMed]
- Supra, R.; Agrawal, D.K. Peripheral Nerve Regeneration: Opportunities and Challenges. J. Spine Res. Surg. 2023, 5, 10–18. [Google Scholar] [CrossRef]
- Rasappan, K.; Rajaratnam, V.; Wong, Y.R. Conduit-based Nerve Repairs Provide Greater Resistance to Tension Compared with Primary Repairs: A Biomechanical Analysis on Large Animal Samples. Plast. Reconstr. Surg.-Glob. Open 2018, 6, e1981. [Google Scholar] [CrossRef] [PubMed]
- Tada, K.; Nakada, M.; Matsuta, M.; Yamauchi, D.; Ikeda, K.; Tsuchiya, H. Long-Term Outcomes of Donor Site Morbidity After Sural Nerve Graft Harvesting. J. Hand Surg. Glob. Online 2020, 2, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.M.; MacEwan, M.; Santosa, K.B.; Chenard, K.E.; Ray, W.Z.; Hunter, D.A.; Mackinnon, S.E.; Johnson, P.J. Acellular nerve allografts in peripheral nerve regeneration: A comparative study. Muscle Nerve 2011, 44, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Fornasari, B.E.; Carta, G.; Gambarotta, G.; Raimondo, S. Natural-Based Biomaterials for Peripheral Nerve Injury Repair. Front. Bioeng. Biotechnol. 2020, 8, 554257. [Google Scholar] [CrossRef]
- Zhao, W.; Tu, H.; Chen, J.; Wang, J.; Liu, H.; Zhang, F.; Li, J. Functionalized hydrogels in neural injury repairing. Front. Neurosci. 2023, 17, 1199299. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, T.L.; Zhang, H.J.; Gao, J.; Yang, P.F. A Promising Application of Injectable Hydrogels in Nerve Repair and Regeneration for Ischemic Stroke. Int. J. Nanomed. 2024, 19, 327–345. [Google Scholar] [CrossRef]
- Madhusudanan, P.; Raju, G.; Shankarappa, S. Hydrogel systems and their role in neural tissue engineering. J. R. Soc. Interface 2020, 17, 20190505. [Google Scholar] [CrossRef] [PubMed]
- Nectow, A.R.; Marra, K.G.; Kaplan, D.L. Biomaterials for the development of peripheral nerve guidance conduits. Tissue Eng. Part B Rev. 2012, 18, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, R.D.; Martínez, J.J.; Burdick, J.A.; Winkelstein, B.A. Controlled release of GDNF reduces nerve root-mediated behavioral hypersensitivity. J. Orthop. Res. 2009, 27, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Enriquez-Ochoa, D.; Robles-Ovalle, P.; Mayolo-Deloisa, K.; Brunck, M.E.G. Immobilization of Growth Factors for Cell Therapy Manufacturing. Front. Bioeng. Biotechnol. 2020, 8, 620. [Google Scholar] [CrossRef]
- Nilasaroya, A.; Kop, A.M.; Morrison, D.A. Heparin-functionalized hydrogels as growth factor-signaling substrates. J. Biomed. Mater. Res. Part A 2021, 109, 374–384. [Google Scholar] [CrossRef]
- Carballo-Molina, O.A.; Velasco, I. Hydrogels as scaffolds and delivery systems to enhance axonal regeneration after injuries. Front. Cell. Neurosci. 2015, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Morwood, A.J.; El-Karim, I.A.; Clarke, S.A.; Lundy, F.T. The Role of Extracellular Matrix (ECM) Adhesion Motifs in Functionalised Hydrogels. Molecules 2023, 28, 4616. [Google Scholar] [CrossRef]
- Sun, Z.; Guo, S.S.; Fässler, R. Integrin-mediated mechanotransduction. J. Cell Biol. 2016, 215, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Miguel, F.; Barbosa, F.; Ferreira, F.C.; Silva, J.C. Electrically Conductive Hydrogels for Articular Cartilage Tissue Engineering. Gels 2022, 8, 710. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Liu, J.; Ye, Z.; Huang, J.; He, F.; Xiao, W.; Hu, X.; Luo, Z. CaMKII-Mediated CREB Phosphorylation Is Involved in Ca2+-Induced BDNF mRNA Transcription and Neurite Outgrowth Promoted by Electrical Stimulation. PLoS ONE 2016, 11, e0162784. [Google Scholar] [CrossRef]
- Bu, W.; Wu, Y.; Ghaemmaghami, A.M.; Sun, H.; Mata, A. Rational design of hydrogels for immunomodulation. Regen. Biomater. 2022, 9, rbac009. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive oxygen species (ROS) scavenging biomaterials for anti-inflammatory diseases: From mechanism to therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.L.; Valdez, H.; Conde, M.; Viaña-Mendieta, P.; Boccaccini, A.R. Polymers and Bioactive Compounds with a Macrophage Modulation Effect for the Rational Design of Hydrogels for Skin Regeneration. Pharmaceutics 2023, 15, 1655. [Google Scholar] [CrossRef]
- Maeso, L.; Antezana, P.E.; Hvozda Arana, A.G.; Evelson, P.A.; Orive, G.; Desimone, M.F. Progress in the Use of Hydrogels for Antioxidant Delivery in Skin Wounds. Pharmaceutics 2024, 16, 524. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Iranpanah, A.; Kooshki, L.; Moradi, S.Z.; Saso, L.; Fakhri, S.; Khan, H. The Exosome-Mediated PI3K/Akt/mTOR Signaling Pathway in Neurological Diseases. Pharmaceutics 2023, 15, 1006. [Google Scholar] [CrossRef] [PubMed]
- Kuan, C.H.; Chang, L.; Ho, C.Y.; Tsai, C.H.; Liu, Y.C.; Huang, W.Y.; Wang, Y.N.; Wang, W.H.; Wang, T.W. Immunomodulatory hydrogel orchestrates pro-regenerative response of macrophages and angiogenesis for chronic wound healing. Biomaterials 2025, 314, 122848. [Google Scholar] [CrossRef]
- Wang, Y.; Kankala, R.K.; Ou, C.; Chen, A.; Yang, Z. Advances in hydrogel-based vascularized tissues for tissue repair and drug screening. Bioact. Mater. 2022, 9, 198–220. [Google Scholar] [CrossRef]
- Rosales, A.M.; Anseth, K.S. The design of reversible hydrogels to capture extracellular matrix dynamics. Nat. Rev. Mater. 2016, 1, 15012. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Qi, Z.; Pan, S.; Xia, P.; Kong, W.; Sun, B.; Du, H.; Zhang, R.; Zhu, L.; Zhou, D.; et al. Advances in Conductive Hydrogel for Spinal Cord Injury Repair and Regeneration. Int. J. Nanomed. 2023, 18, 7305–7333. [Google Scholar] [CrossRef] [PubMed]
- Zöller, K.; To, D.; Bernkop-Schnürch, A. Biomedical applications of functional hydrogels: Innovative developments, relevant clinical trials and advanced products. Biomaterials 2025, 312, 122718. [Google Scholar] [CrossRef] [PubMed]
- Tsou, Y.H.; Khoneisser, J.; Huang, P.C.; Xu, X. Hydrogel as a bioactive material to regulate stem cell fate. Bioact. Mater. 2016, 1, 39–55. [Google Scholar] [CrossRef]
- Walker, B.W.; Lara, R.P.; Mogadam, E.; Yu, C.H.; Kimball, W.; Annabi, N. Rational Design of Microfabricated Electroconductive Hydrogels for Biomedical Applications. Prog. Polym. Sci. 2019, 92, 135–157. [Google Scholar] [CrossRef] [PubMed]
- Gresita, A.; Raja, I.; Petcu, E.; Hadjiargyrou, M. Collagen-Coated Hyperelastic Bone Promotes Osteoblast Adhesion and Proliferation. Materials 2023, 16, 6996. [Google Scholar] [CrossRef]
- Krishani, M.; Shin, W.Y.; Suhaimi, H.; Sambudi, N.S. Development of Scaffolds from Bio-Based Natural Materials for Tissue Regeneration Applications: A Review. Gels 2023, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Kumar, V.; Joo, S.W.; Mandal, T.K. Cutting-Edge Hydrogel Technologies in Tissue Engineering and Biosensing: An Updated Review. Materials 2024, 17, 4792. [Google Scholar] [CrossRef] [PubMed]
- Younes, H.M.; Kadavil, H.; Ismail, H.M.; Adib, S.A.; Zamani, S.; Alany, R.G.; Al-Kinani, A.A. Overview of Tissue Engineering and Drug Delivery Applications of Reactive Electrospinning and Crosslinking Techniques of Polymeric Nanofibers with Highlights on Their Biocompatibility Testing and Regulatory Aspects. Pharmaceutics 2023, 16, 32. [Google Scholar] [CrossRef]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Aurand, E.R.; Lampe, K.J.; Bjugstad, K.B. Defining and designing polymers and hydrogels for neural tissue engineering. Neurosci. Res. 2012, 72, 199–213. [Google Scholar] [CrossRef]
- Barcena, A.J.R.; Dhal, K.; Patel, P.; Ravi, P.; Kundu, S.; Tappa, K. Current Biomedical Applications of 3D-Printed Hydrogels. Gels 2023, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Hong, H.; Hu, R.; Liu, J.; Liu, C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact. Mater. 2022, 10, 15–31. [Google Scholar] [CrossRef]
- Mankavi, F.; Ibrahim, R.; Wang, H. Advances in Biomimetic Nerve Guidance Conduits for Peripheral Nerve Regeneration. Nanomaterials 2023, 13, 2528. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Chen, X.F.; Zhou, L.P.; Rao, F.; Zhang, D.Y.; Wang, Y.H. A nerve conduit filled with Wnt5a-loaded fibrin hydrogels promotes peripheral nerve regeneration. CNS Neurosci. Ther. 2022, 28, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ma, B.; Li, Q.; Zhang, M.; Kou, Y. Chitin Conduits with Different Inner Diameters at Both Ends Combined with Dual Growth Factor Hydrogels Promote Nerve Transposition Repair in Rats. J. Funct. Biomater. 2023, 14, 442. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Qu, F.; Pei, Y.; Lei, S.; Xia, S.; Liang, J.; Li, S.; Sun, X.; Liu, L. Repairing sciatic nerve injury with self-assembling peptide nanofiber scaffold-containing chitosan conduit. Front. Neurol. 2022, 13, 867711. [Google Scholar] [CrossRef]
- Meder, T.; Prest, T.; Skillen, C.; Marchal, L.; Yupanqui, V.T.; Soletti, L.; Gardner, P.; Cheetham, J.; Brown, B.N. Nerve-specific extracellular matrix hydrogel promotes functional regeneration following nerve gap injury. NPJ Regen. Med. 2021, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, C.; Fattahpour, H.; Fong, D.; Hadjiargyrou, M.; Sanaei, P. Effects of Elasticity on Cell Proliferation in a Tissue-Engineering Scaffold Pore. Bull. Math. Biol. 2023, 85, 25. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Silva, T.L.; Cristobal, C.D.; Lai, C.S.E.; Leyva-Aranda, V.; Lee, H.K.; Hartgerink, J.D. Self-assembling multidomain peptide hydrogels accelerate peripheral nerve regeneration after crush injury. Biomaterials 2021, 265, 120401. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, C.; Zhu, J.; Lu, C.; Li, H.; Chen, F.; Lu, J.; Zhang, Z.; Yan, X.; Zhao, H.; et al. Self-assembling peptide hydrogels functionalized with LN- and BDNF- mimicking epitopes synergistically enhance peripheral nerve regeneration. Theranostics 2020, 10, 8227–8249. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y.; Zhang, J.; Zhang, M.; Dai, C.; Zhang, Y.; Zhang, L.; Bian, L.; Yang, Y.; Zhang, K.; et al. Advancing neural regeneration via adaptable hydrogels: Enriched with Mg2+ and silk fibroin to facilitate endogenous cell infiltration and macrophage polarization. Bioact. Mater. 2024, 33, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Yuan, W.; Xu, J.; Tong, W.; Mi, J.; Ho, P.C.; Chow, D.H.K.; Li, Y.; Yao, H.; Li, X.; et al. Magnesium-Encapsulated Injectable Hydrogel and 3D-Engineered Polycaprolactone Conduit Facilitate Peripheral Nerve Regeneration. Adv. Sci. 2022, 9, e2202102. [Google Scholar] [CrossRef] [PubMed]
- Xuan, H.; Wu, S.; Jin, Y.; Wei, S.; Xiong, F.; Xue, Y.; Li, B.; Yang, Y.; Yuan, H. A Bioinspired Self-Healing Conductive Hydrogel Promoting Peripheral Nerve Regeneration. Adv. Sci. 2023, 10, e2302519. [Google Scholar] [CrossRef]
- Gregory, E.; Baek, I.H.; Ala-Kokko, N.; Dugan, R.; Pinzon-Herrera, L.; Almodóvar, J.; Song, Y.H. Peripheral Nerve Decellularization for In Vitro Extracellular Matrix Hydrogel Use: A Comparative Study. ACS Biomater. Sci. Eng. 2022, 8, 2574–2588. [Google Scholar] [CrossRef] [PubMed]
- Kellaway, S.C.; Roberton, V.; Jones, J.N.; Loczenski, R.; Phillips, J.B.; White, L.J. Engineered neural tissue made using hydrogels derived from decellularised tissues for the regeneration of peripheral nerves. Acta Biomater. 2023, 157, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Yang, Z.; Chen, S.; Zhang, F.; Rao, Z.; Zhao, C.; Quan, D.; Bai, Y.; Shen, J. Nanofibrous nerve guidance conduits decorated with decellularized matrix hydrogel facilitate peripheral nerve injury repair. Theranostics 2021, 11, 2917–2931. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, T.; Li, Y. 3D Printing and Bioprinting Nerve Conduits for Neural Tissue Engineering. Polymers 2020, 12, 1637. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Jeon, E.Y.; Nam, J.J.; Park, J.H.; Choi, I.C.; Kim, S.H.; Chung, J.J.; Lee, K.; Park, J.W.; Jung, Y. Development of a regenerative porous PLCL nerve guidance conduit with swellable hydrogel-based microgrooved surface pattern via 3D printing. Acta Biomater. 2022, 141, 219–232. [Google Scholar] [CrossRef]
- Liu, C.; Fan, L.; Tian, Z.; Wen, H.; Zhou, L.; Guan, P.; Luo, Y.; Chan, C.; Tan, G.; Ning, C.; et al. Self-curling electroconductive nerve dressing for enhancing peripheral nerve regeneration in diabetic rats. Bioact. Mater. 2021, 6, 3892–3903. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, W.; Lin, Z.; Lu, Y.; Chen, H.; Li, B.; Li, Z.; Xia, H.; Li, L.; Zhang, T. Conducting molybdenum sulfide/graphene oxide/polyvinyl alcohol nanocomposite hydrogel for repairing spinal cord injury. J. Nanobiotechnol. 2022, 20, 210. [Google Scholar] [CrossRef] [PubMed]
- Elkhenany, H.; Bonilla, P.; Giraldo, E.; Alastrue Agudo, A.; Edel, M.J.; Vicent, M.J.; Roca, F.G.; Ramos, C.M.; Doblado, L.R.; Pradas, M.M.; et al. A Hyaluronic Acid Demilune Scaffold and Polypyrrole-Coated Fibers Carrying Embedded Human Neural Precursor Cells and Curcumin for Surface Capping of Spinal Cord Injuries. Biomedicines 2021, 9, 1928. [Google Scholar] [CrossRef]
- Sun, X.; Huang, X.; Liang, Q.; Wang, N.; Zheng, X.; Zhang, Q.; Yu, D. Curcumin-loaded keratin-chitosan hydrogels for enhanced peripheral nerve regeneration. Int. J. Biol. Macromol. 2024, 272, 132448. [Google Scholar] [CrossRef]
- Kim, W.R.; Kang, M.; Park, H.; Ham, H.J.; Lee, H.; Geum, D. Functional Test Scales for Evaluating Cell-Based Therapies in Animal Models of Spinal Cord Injury. Stem Cells Int. 2017, 2017, 5160261. [Google Scholar] [CrossRef] [PubMed]
- Boboc, I.K.S.; Rotaru-Zavaleanu, A.D.; Calina, D.; Albu, C.V.; Catalin, B.; Turcu-Stiolica, A. A Preclinical Systematic Review and Meta-Analysis of Behavior Testing in Mice Models of Ischemic Stroke. Life 2023, 13, 567. [Google Scholar] [CrossRef]
- Deuis, J.R.; Dvorakova, L.S.; Vetter, I. Methods Used to Evaluate Pain Behaviors in Rodents. Front. Mol. Neurosci. 2017, 10, 284. [Google Scholar] [CrossRef] [PubMed]
- García-García, Ó.D.; Carriel, V.; Chato-Astrain, J. Myelin histology: A key tool in nervous system research. Neural Regen. Res. 2024, 19, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the potential of hydrogels for advanced therapeutic applications: Current achievements and future directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef]
- Xie, C.; Chen, Y.; Wang, L.; Liao, K.; Xue, B.; Han, Y.; Li, L.; Jiang, Q. Recent research of peptide-based hydrogel in nervous regeneration. Bioact. Mater. 2024, 40, 503–523. [Google Scholar] [CrossRef]
- Nawrotek, K.; Mąkiewicz, M.; Zawadzki, D. Fabrication and Characterization of Polycaprolactone/Chitosan—Hydroxyapatite Hybrid Implants for Peripheral Nerve Regeneration. Polymers 2021, 13, 775. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, J.; Lu, C.; Chai, Y.; Cao, Z.; Lu, J.; Zhang, Z.; Zhao, H.; Huang, Y.Y.; Yao, S.; et al. Aligned fibrin/functionalized self-assembling peptide interpenetrating nanofiber hydrogel presenting multi-cues promotes peripheral nerve functional recovery. Bioact. Mater. 2022, 8, 529–544. [Google Scholar] [CrossRef]
- Amagat, J.; Su, Y.; Svejsø, F.H.; Le Friec, A.; Sønderskov, S.M.; Dong, M.; Fang, Y.; Chen, M. Self-snapping hydrogel-based electroactive microchannels as nerve guidance conduits. Mater. Today Bio 2022, 16, 100437. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Dong, Y.; Liu, H.; Jiang, X.; Yang, L.; Luo, J.; Hu, Y.; Gou, M. 3D printed elastic hydrogel conduits with 7,8-dihydroxyflavone release for peripheral nerve repair. Mater. Today Bio 2023, 20, 100652. [Google Scholar] [CrossRef] [PubMed]
- Takeya, H.; Itai, S.; Kimura, H.; Kurashina, Y.; Amemiya, T.; Nagoshi, N.; Iwamoto, T.; Sato, K.; Shibata, S.; Matsumoto, M.; et al. Schwann cell-encapsulated chitosan-collagen hydrogel nerve conduit promotes peripheral nerve regeneration in rodent sciatic nerve defect models. Sci. Rep. 2023, 13, 11932. [Google Scholar] [CrossRef]
- Kuna, V.K.; Lundgren, A.; Anerillas, L.O.; Kelk, P.; Brohlin, M.; Wiberg, M.; Kingham, P.J.; Novikova, L.N.; Andersson, G.; Novikov, L.N. Efficacy of Nerve-Derived Hydrogels to Promote Axon Regeneration Is Influenced by the Method of Tissue Decellularization. Int. J. Mol. Sci. 2022, 23, 8746. [Google Scholar] [CrossRef]
- Yang, P.; Peng, Y.; Dai, X.; Jie, J.; Kong, D.; Gu, X.; Yang, Y. Bionic peptide scaffold in situ polarization and recruitment of M2 macrophages to promote peripheral nerve regeneration. Bioact. Mater. 2023, 30, 85–97. [Google Scholar] [CrossRef]
- Xue, W.; Shi, W.; Kuss, M.; Kong, Y.; Alimi, O.A.; Wang, H.; DiMaio, D.J.; Yu, C.; Duan, B. A Dual-network Nerve Adhesive with Enhanced Adhesion Strength Promotes Transected Peripheral Nerve Repair. Adv. Funct. Mater. 2023, 33, 2209971. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, H.; Duan, P.; Liu, K.; Yu, Y.; Wei, W.; Wang, W.; Liu, Y.; Cheng, Q.; Liang, X.; et al. An injectable and adaptable hydrogen sulfide delivery system for modulating neuroregenerative microenvironment. Sci. Adv. 2023, 9, eadi1078. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Zhou, Z.; Chen, M.; Gong, X. Photothermal Treatment of Polydopamine Nanoparticles@Hyaluronic Acid Methacryloyl Hydrogel Against Peripheral Nerve Adhesion in a Rat Model of Sciatic Nerve. Int. J. Nanomed. 2023, 18, 2777–2793. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.; Kobayashi, N.R.; Higgins, M.J.; Quigley, A.F.; Jamali, S.; Moulton, S.E.; Kapsa, R.M.; Wallace, G.G.; Crook, J.M. Electrical stimulation using conductive polymer polypyrrole promotes differentiation of human neural stem cells: A biocompatible platform for translational neural tissue engineering. Tissue Eng. Part C Methods 2015, 21, 385–393. [Google Scholar] [CrossRef]
- Liang, F.; Yang, Y.; Chen, Y.; Xie, J.; Liu, S.; Tan, Z.; Tian, L.; Yu, Z.; Shi, Z.; Xie, P.; et al. Ropivacaine microsphere-loaded electroconductive nerve dressings for long-acting analgesia and functional recovery following diabetic peripheral nerve injury. Mater. Today Bio 2023, 21, 100712. [Google Scholar] [CrossRef]
- Thakur, R.; Aplin, F.P.; Fridman, G.Y. A Hydrogel-Based Microfluidic Nerve Cuff for Neuromodulation of Peripheral Nerves. Micromachines 2021, 12, 1522. [Google Scholar] [CrossRef]
- Srinivasan, S.S.; Gfrerer, L.; Karandikar, P.; Som, A.; Alshareef, A.; Liu, S.; Higginbotham, H.; Ishida, K.; Hayward, A.; Kalva, S.P.; et al. Adaptive conductive electrotherapeutic scaffolds for enhanced peripheral nerve regeneration and stimulation. Med 2023, 4, 541–553.e545. [Google Scholar] [CrossRef] [PubMed]
- Olguín, Y.; Selva, M.; Benavente, D.; Orellana, N.; Montenegro, I.; Madrid, A.; Jaramillo-Pinto, D.; Otero, M.C.; Corrales, T.P.; Acevedo, C.A. Effect of Electrical Stimulation on PC12 Cells Cultured in Different Hydrogels: Basis for the Development of Biomaterials in Peripheral Nerve Tissue Engineering. Pharmaceutics 2023, 15, 2760. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Rao, S.; Chen, W.; Felix, K.; Ni, J.; Sahasrabudhe, A.; Lin, S.; Wang, Q.; Liu, Y.; He, Z.; et al. Fatigue-resistant hydrogel optical fibers enable peripheral nerve optogenetics during locomotion. Nat. Methods 2023, 20, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Choi, W.S.; Jeong, J.O. A Review of Advanced Hydrogel Applications for Tissue Engineering and Drug Delivery Systems as Biomaterials. Gels 2024, 10, 693. [Google Scholar] [CrossRef]
- Bernard, M.; McOnie, R.; Tomlinson, J.E.; Blum, E.; Prest, T.A.; Sledziona, M.; Willand, M.; Gordon, T.; Borschel, G.H.; Soletti, L.; et al. Peripheral Nerve Matrix Hydrogel Promotes Recovery after Nerve Transection and Repair. Plast. Reconstr. Surg. 2023, 152, 458e–467e. [Google Scholar] [CrossRef] [PubMed]
- Bousalis, D.; McCrary, M.W.; Vaughn, N.; Hlavac, N.; Evering, A.; Kolli, S.; Song, Y.H.; Morley, C.; Angelini, T.E.; Schmidt, C.E. Decellularized peripheral nerve as an injectable delivery vehicle for neural applications. J. Biomed. Mater. Res. Part A 2022, 110, 595–611. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; He, Y.; Jin, L.; Zhang, Y.; Guastaldi, F.P.; Albashari, A.A.; Hu, F.; Wang, X.; Wang, L.; Xiao, J.; et al. Corrigendum to Application of bioactive hydrogels combined with dental pulp stem cells for the repair of large gap peripheral nerve injuries’ [Bioactive Mater. 6 (3) (2021) 638–654]. Bioact. Mater. 2022, 7, 2. [Google Scholar] [CrossRef]
- Chen, S.H.; Kao, H.K.; Wun, J.R.; Chou, P.Y.; Chen, Z.Y.; Hsieh, S.T.; Fang, H.W.; Lin, F.H. Thermosensitive hydrogel carrying extracellular vesicles from adipose-derived stem cells promotes peripheral nerve regeneration after microsurgical repair. APL Bioeng. 2022, 6, 046103. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, A.; Zhong, Y.; Huang, L.; Yang, J.; Zhou, C.; Zhou, L.; Zhang, Y.; Fu, G. Laminin-modified gellan gum hydrogels loaded with the nerve growth factor to enhance the proliferation and differentiation of neuronal stem cells. RSC Adv. 2020, 10, 17114–17122. [Google Scholar] [CrossRef]
- Nawrotek, K.; Kubicka, M.; Gatkowska, J.; Wieczorek, M.; Michlewska, S.; Bekier, A.; Wach, R.; Rudnicka, K. Controlling the Spatiotemporal Release of Nerve Growth Factor by Chitosan/Polycaprolactone Conduits for Use in Peripheral Nerve Regeneration. Int. J. Mol. Sci. 2022, 23, 2852. [Google Scholar] [CrossRef]
- McBenedict, B.; Hauwanga, W.N.; Escudeiro, G.; Petrus, D.; Onabanjo, B.B.; Johnny, C.; Omer, M.; Amaravadhi, A.R.; Felix, A.; Dang, N.B.; et al. A Review and Bibliometric Analysis of Studies on Advances in Peripheral Nerve Regeneration. Cureus 2024, 16, e69515. [Google Scholar] [CrossRef] [PubMed]
- Rahimi Darehbagh, R.; Seyedoshohadaei, S.A.; Ramezani, R.; Rezaei, N. Stem cell therapies for neurological disorders: Current progress, challenges, and future perspectives. Eur. J. Med. Res. 2024, 29, 386. [Google Scholar] [CrossRef]
- Nuelle, J.A.V.; Bozynski, C.; Stoker, A. Innovations in Peripheral Nerve Injury: Current Concepts and Emerging Techniques to Improve Recovery. Mo. Med. 2022, 119, 129–135. [Google Scholar] [PubMed]
- Yang, Q.; Su, S.; Liu, S.; Yang, S.; Xu, J.; Zhong, Y.; Yang, Y.; Tian, L.; Tan, Z.; Wang, J.; et al. Exosomes-loaded electroconductive nerve dressing for nerve regeneration and pain relief against diabetic peripheral nerve injury. Bioact. Mater. 2023, 26, 194–215. [Google Scholar] [CrossRef] [PubMed]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.R.; Walker, M.J.; Gilbert, F. Ethical and regulatory issues of stem cell-derived 3-dimensional organoid and tissue therapy for personalised regenerative medicine. BMC Med. 2022, 20, 499. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, J.R.; Mulenga, C.M.; Tran, K.; Feinberg, K.; Santerre, J.P.; Borschel, G.H. Biohacking Nerve Repair: Novel Biomaterials, Local Drug Delivery, Electrical Stimulation, and Allografts to Aid Surgical Repair. Bioengineering 2024, 11, 776. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhang, G.; Yang, Y.; Huang, H.; Li, Z.; Chen, X.; Zheng, D.; Lu, Y.G.; Niu, G. Advanced techniques and innovations in peripheral nerve repair: A comprehensive review for clinical and experimental reference. Rev. Neurosci. 2024. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wei, H.; Cheng, H.; Qi, Y.; Gu, Y.; Ma, X.; Sun, J.; Ye, F.; Guo, F.; Cheng, C. Advances in nerve guidance conduits for peripheral nerve repair and regeneration. Am. J. Stem Cells 2023, 12, 112–123. [Google Scholar]
- Li, Y.; Ma, Z.; Ren, Y.; Lu, D.; Li, T.; Li, W.; Wang, J.; Ma, H.; Zhao, J. Tissue Engineering Strategies for Peripheral Nerve Regeneration. Front. Neurol. 2021, 12, 768267. [Google Scholar] [CrossRef]
- Phutane, P.; Telange, D.; Agrawal, S.; Gunde, M.; Kotkar, K.; Pethe, A. Biofunctionalization and Applications of Polymeric Nanofibers in Tissue Engineering and Regenerative Medicine. Polymers 2023, 15, 1202. [Google Scholar] [CrossRef] [PubMed]
- Rotaru-Zăvăleanu, A.D.; Dinescu, V.C.; Aldea, M.; Gresita, A. Hydrogel-Based Therapies for Ischemic and Hemorrhagic Stroke: A Comprehensive Review. Gels 2024, 10, 476. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; Goronzy, J.J. Aging of the Immune System. Mechanisms and Therapeutic Targets. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. S5), S422–S428. [Google Scholar] [CrossRef] [PubMed]
| Active Components/Features | Hydrogel Materials | Loaded Drugs/Active Components | Study Models | Effects on Recovery | Ref. |
|---|---|---|---|---|---|
| Polycaprolactone, chitosan, hydroxyapatite | Polycaprolactone/chitosan–hydroxyapatite | None | Preclinical, in vitro studies | Enhanced nerve guidance, axonal regrowth, improved implant stability and mechanical properties | [87] |
| RAD peptides (IKV, RGI, IKV/RGI) | RAD/IKV, RAD/RGI, RAD/IKV/RGI | None | Animal study (rat model) | Enhanced Schwann cell adhesion, myelination, neurotrophin secretion, axonal regeneration, functional recovery | [88] |
| Peptide nanofiber scaffold with chitosan conduits | Self-assembling peptide nanofiber scaffold (RAD/KLT, RAD/IKVAV, RAD/KLT/IKVAV), chitosan conduits | None | In vitro (Schwann cell assays), in vivo (rat model) | Improved nerve healing, remyelination, axonal regeneration, better muscle innervation and weight. | [64] |
| Decellularized ECM derived from bone, liver, and small-intestinal submucosa | Decellularized extracellular matrix (dECM) hydrogels | None | In vitro (DRG neurite extension), in vivo (rat sciatic nerve injury model) | Improved structural integrity, enhanced Schwann cell alignment, promoted vascularization | [73] |
| Visible light-crosslinked gelatin | PLCL with visible light-crosslinked gelatin hydrogel | None | In vivo (rat sciatic nerve defect model) | Demonstrated axonal regeneration, remyelination, functional recovery | [76] |
| Decellularized porcine nerve-derived ECM | Decellularized porcine nerve-derived ECM hydrogels | None | Preclinical (rat model, 24-week study) | Improved axon count, electrophysiological recovery, functional gait recovery comparable to autografts. | [65] |
| VEGF, NGF | Gelatin methacrylate (GM) hydrogels combined with chitin conduits | VEGF and NGF | In vitro (cell proliferation, migration, apoptosis), in vivo (rat model) | Enhanced nerve regeneration, improved nerve conduction velocity, reduced muscle atrophy, functional recovery | [63] |
| Wnt5a | Wnt5a-loaded fibrin hydrogels | Wnt5a | In vitro (Schwann cell assays), in vivo (rat model) | Improved axonal growth, myelination, Schwann cell proliferation, VEGF and NGF secretion | [62] |
| Multidomain peptides (K2, K2-IIKDI, K2-IKVAV) | Multidomain peptide (MDP) hydrogels | None | In vitro (neurite outgrowth), in vivo (rat crush injury model) | Accelerated functional recovery, enhanced macrophage recruitment, faster axonal regeneration | [67] |
| Graphitic carbon nitride (g-C3N4), reduced graphene oxide (rGO) | Graphitic carbon nitride (g-C3N4) and reduced graphene oxide (rGO)-based hydrogels | None | In vitro (PC12 cell studies), in vivo (nerve guidance conduit models) | Enhanced neurite extension, anisotropic guidance, optimal mechanical properties, biocompatibility | [89] |
| Gelatin methacryloyl (GelMA), silk fibroin methacrylate (SF-MA) | Gelatin methacryloyl (GelMA) and silk fibroin methacrylate (SF-MA) hydrogels | None | In vitro (Schwann cell assays), in vivo (rat sciatic nerve defect model) | Promoted axonal elongation, enhanced Schwann cell adhesion, proliferation, migration, functional recovery | [90] |
| Schwann cells encapsulated in chitosan–collagen hydrogel | Schwann cell-encapsulated chitosan–collagen hydrogel nerve conduit | None | In vitro (SC survival), in vivo (rat sciatic nerve defect model) | Promoted axonal regrowth, enhanced remyelination, better motor functional recovery | [91] |
| Decellularized porcine vagus nerve tissue | Peripheral nerve-derived hydrogels | None | In vitro (Schwann cell and sensory neuron culture), in vivo (rat sciatic nerve gap model) | Promoted axonal regeneration, supported Schwann cell viability, enhanced neurite outgrowth | [92] |
| Decellularized nerve matrix | Decellularized nerve matrix hydrogel-coated nanofibrous scaffolds | None | In vitro (Schwann cell migration, axonal outgrowth), in vivo (rat sciatic nerve defect model) | Promoted axonal growth, Schwann cell migration, enhanced myelination, functional recovery similar to autografts | [74] |
| Active Components/Features | Hydrogel Materials | Loaded Drugs/Active Components | Study Models | Effects on Recovery | Ref. |
|---|---|---|---|---|---|
| Dopamine–isothiocyanate and decellularized nerve matrix | Dual-network nerve-adhesive (DNNA) | None | In vitro (SC proliferation, axonal outgrowth), in vivo (rat sciatic nerve transection model) | Enhanced axonal outgrowth, reduced inflammation and fibrosis, improved motor and sensory recovery | [94] |
| M2-derived cytokines and extracellular vesicles (EVs) | Bionic peptide hydrogel scaffolds | M2-derived cytokines and EVs | In vitro (SC and macrophage behavior), in vivo (rat sciatic nerve gap model) | Promoted M2 macrophage polarization, enhanced SC migration, axonal regeneration, functional recovery | [93] |
| Reactive oxygen species (ROS)-triggered H2S release | Thermosensitive poly(amino acid) hydrogel (mPEG-PA-PP) | H2S | In vitro (SC, macrophage, endothelial cells), in vivo (rat sciatic nerve transection model) | Enhanced nerve regeneration, reduced oxidative stress and inflammation, promoted angiogenesis, functional recovery | [95] |
| Polydopamine nanoparticles (PDA NPs) | Polydopamine nanoparticles@hyaluronic acid methacryloyl (PDA NPs@HAMA) hydrogel | None | In vivo (rat sciatic nerve adhesion model) | Reduced nerve adhesion, improved motor nerve conduction, reduced inflammatory response, better nerve functionality | [96] |
| Active Components/Features | Hydrogel Materials | Loaded Drugs/Active Compounds | Study Models | Effects on Recovery | Ref. |
|---|---|---|---|---|---|
| Conductive polymer | Electroconductive hydrogel | None | In vivo (diabetic sciatic nerve injury model) | Promoted axonal regeneration, remyelination, improved motor function, reduced muscle atrophy | [77] |
| Polypyrrole (PPy), tannic acid (TA), ropivacaine microspheres | Electroconductive hydrogel with PPy and TA | Ropivacaine microspheres | In vitro (SC, PC12 cell assays), in vivo | Enhanced axonal regeneration, myelination, reduced muscle atrophy, long-acting analgesia, functional recovery | [98] |
| Conductive electrolytic material | Conductive electrolytic hydrogel integrated in a nerve cuff | None | In vivo (rat sciatic nerve model) | Effective neuromodulation, reversible nerve block, comparable stimulation and recording to traditional electrodes | [99] |
| Gold nanoparticles | Alginate/poly-acrylamide hydrogel | Gold nanoparticles | Rodent and porcine nerve injury models | Enhanced motor/sensory recovery, axonogenesis, muscle mass preservation, atraumatic electrode removal | [100] |
| Collagen, alginate, GelMA, PEGDA | Multi-component hydrogel | None | In vitro (PC12 cell differentiation studies) | Enhanced PC12 cell differentiation and neurite outgrowth, dependent on hydrogel type and stimulation | [101] |
| Fatigue-resistant nanocrystals | Polyvinyl alcohol (PVA) nanocrystalline hydrogel optical fibers | None | In vivo (mouse models, optogenetics) | Enabled stable optogenetic nerve modulation, reduced pain hypersensitivity, facilitated motor recovery | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taisescu, O.; Dinescu, V.C.; Rotaru-Zavaleanu, A.D.; Gresita, A.; Hadjiargyrou, M. Hydrogels for Peripheral Nerve Repair: Emerging Materials and Therapeutic Applications. Gels 2025, 11, 126. https://doi.org/10.3390/gels11020126
Taisescu O, Dinescu VC, Rotaru-Zavaleanu AD, Gresita A, Hadjiargyrou M. Hydrogels for Peripheral Nerve Repair: Emerging Materials and Therapeutic Applications. Gels. 2025; 11(2):126. https://doi.org/10.3390/gels11020126
Chicago/Turabian StyleTaisescu, Oana, Venera Cristina Dinescu, Alexandra Daniela Rotaru-Zavaleanu, Andrei Gresita, and Michael Hadjiargyrou. 2025. "Hydrogels for Peripheral Nerve Repair: Emerging Materials and Therapeutic Applications" Gels 11, no. 2: 126. https://doi.org/10.3390/gels11020126
APA StyleTaisescu, O., Dinescu, V. C., Rotaru-Zavaleanu, A. D., Gresita, A., & Hadjiargyrou, M. (2025). Hydrogels for Peripheral Nerve Repair: Emerging Materials and Therapeutic Applications. Gels, 11(2), 126. https://doi.org/10.3390/gels11020126







