Platelet-Rich Plasma in Veterinary Orthopedic Surgery: A Systematic Review and Quality Evaluation on Liquid- and Gel-Based Therapies in Dogs
Abstract
1. Introduction
2. Results and Discussion
2.1. Intervention Protocol and Outcome Assessment
2.2. Complications
2.3. Type of PRP and Associated Materials
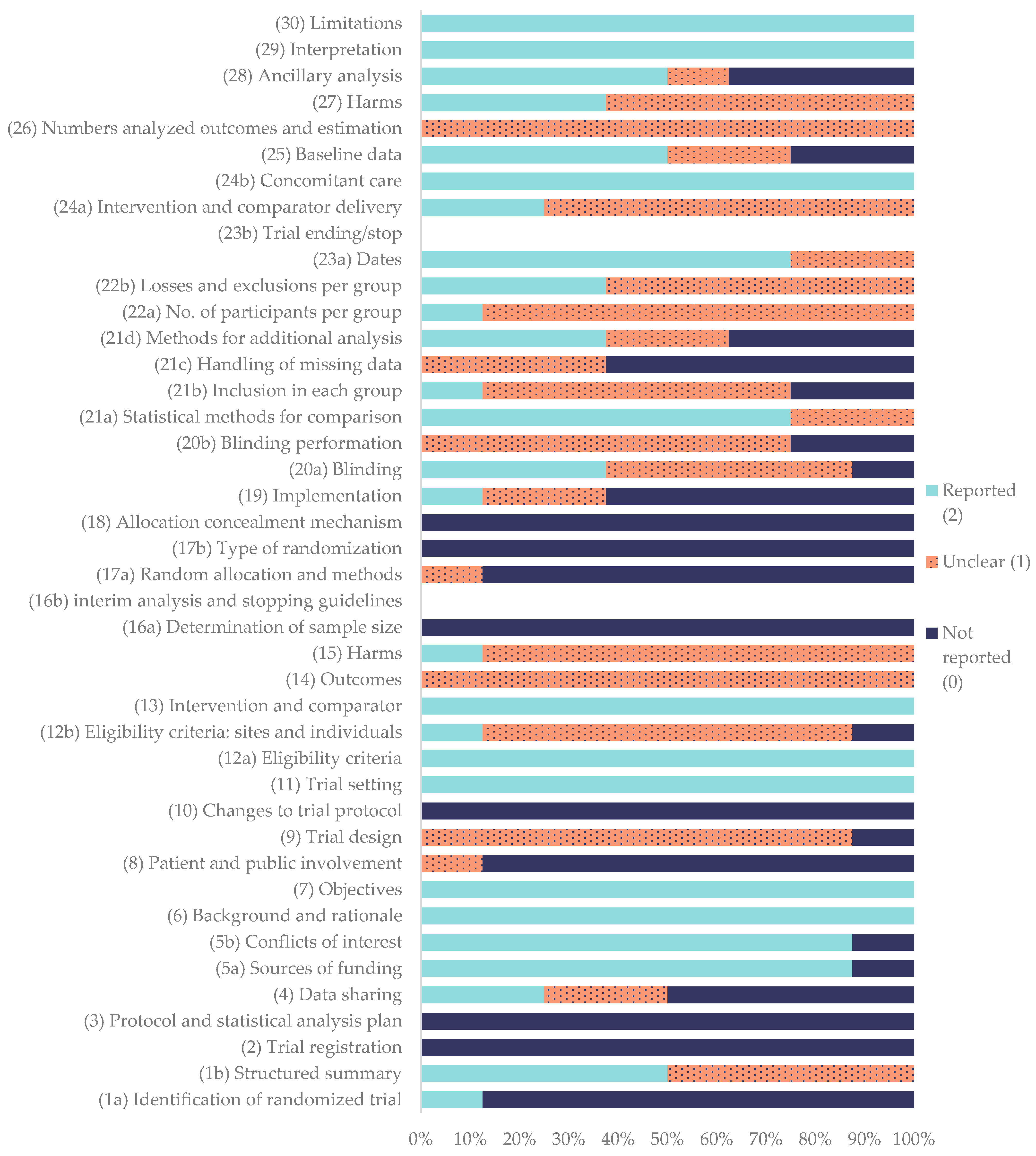
2.4. Quality Assesment in In Vivo Studies
2.5. Quality Assesment in Clinical Studies
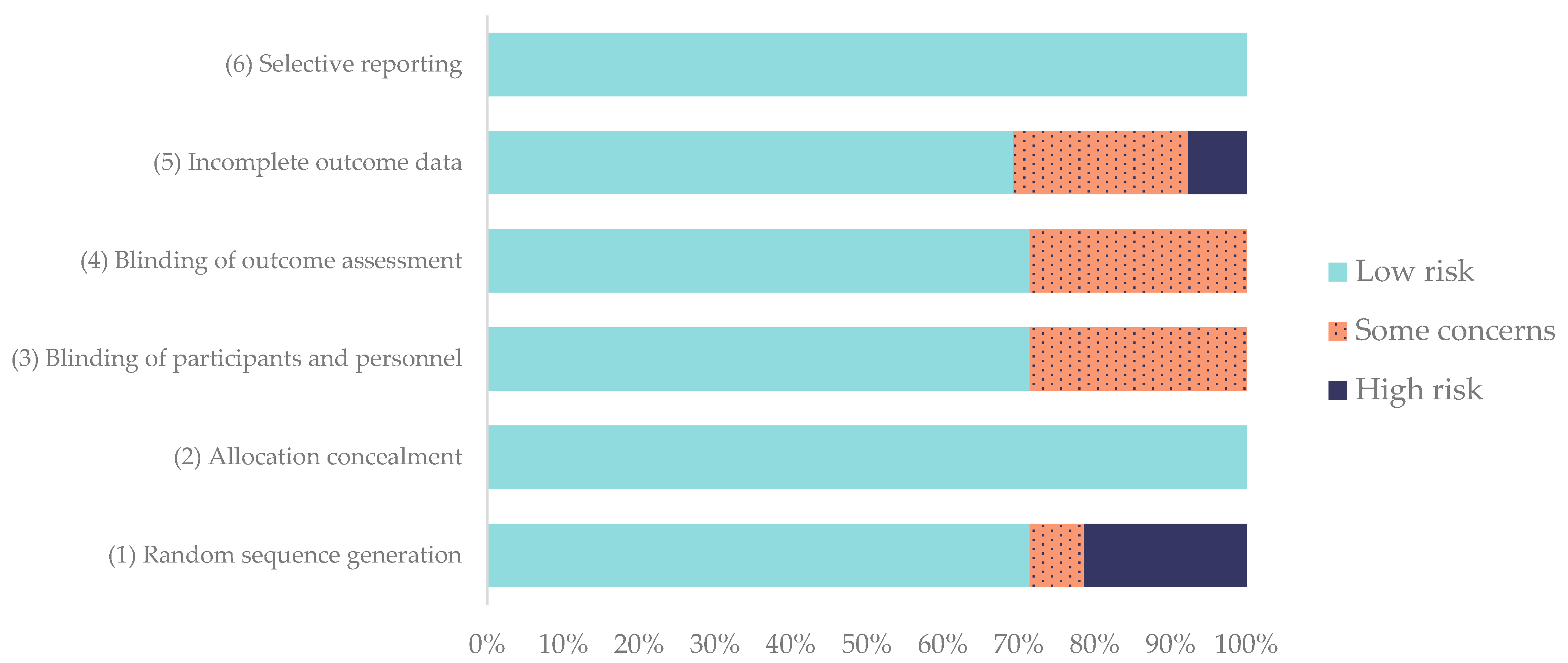
2.6. Risk of Bias
2.7. Discussion
2.7.1. Effects of PRP on the Bone
2.7.2. Effects of PRP on Ligament/Tendon Tissue
2.7.3. Methodological Quality
2.7.4. Risk of Bias
2.7.5. Clinical Research Implications and Limitations
3. Conclusions
4. Materials and Methods
4.1. Search Strategy
4.2. PICO Methology
4.3. Inclusion and Exclusion Criteria
4.4. Analysis and Extraction of Parameters of Interest
4.5. Quality Assesment of In Vivo Studies
4.6. Quality Assesment of Clinical Studies
4.7. Risk-of-Bias Assesment
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARRIVE | Animal Research: Reporting of In Vivo Experiments |
| BMC | Bone Marrow Concentrate |
| CCLR | Cranial Cruciate Ligament Rupture |
| CONSORT | Consolidated Standards of Reporting Trials |
| GFs | Growth Factors |
| IGF | Insulin Growth Factor |
| JCR | Journal Citation Reports |
| lPRP | Leukoreduced PRP |
| MIPO | Minimally Invasive Plate Osteosynthesis |
| MMT | Modified Maquet Technique |
| OA | Osteoarthritis |
| PDGF | Platelet-Derived Growth Factor |
| PLA | Polylactic Acid |
| PRGF | Plasma Rich in Growth Factors |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PRP | Platelet-Rich Plasma |
| RoB 2.0 | Risk of Bias |
| SYRCLE | Systematic Review Centre for Laboratory Animal Experimentation |
| TGF- b1 | Transforming Growth Factor |
| TPLO | Tibial Plateau Leveling Osteotomy |
| VEGF | Vascular Endothelial Growth Factor |
| WOS | World of Science |
References
- Stief, M.; Gottschalk, J.; Ionita, J.-C.; Einspanier, A.; Oechtering, G.; Böttcher, P. Concentration of Platelets and Growth Factors in Canine Autologous Conditioned Plasma. Vet. Comp. Orthop. Traumatol. 2011, 24, 122–125. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Bonacucina, G.; Pucciarelli, S.; Cespi, M.; Serri, E.; Polzonetti, V.; Tambella, A.M.; Vincenzetti, S. Rheological Properties and Growth Factors Content of Platelet-Rich Plasma: Relevance in Veterinary Biomedical Treatments. Biomedicines 2020, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Intini, G. The Use of Platelet-Rich Plasma in Bone Reconstruction Therapy. Biomaterials 2009, 30, 4956–4966. [Google Scholar] [CrossRef] [PubMed]
- Yung, Y.-L.; Fu, S.-C.; Cheuk, Y.-C.; Qin, L.; Ong, M.T.-Y.; Chan, K.-M.; Yung, P.S.-H. Optimisation of Platelet Concentrates Therapy: Composition, Localisation, and Duration of Action. Asia-Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2017, 7, 27–36. [Google Scholar] [CrossRef]
- Marck, R.E.; Gardien, K.L.M.; Vlig, M.; Breederveld, R.S.; Middelkoop, E. Growth Factor Quantification of Platelet-Rich Plasma in Burn Patients Compared to Matched Healthy Volunteers. Int. J. Mol. Sci. 2019, 20, 288. [Google Scholar] [CrossRef] [PubMed]
- Shen, E.; Chou, T.; Gau, C.; Tu, H.; Chen, Y.; Fu, E. Releasing Growth Factors from Activated Human Platelets after Chitosan Stimulation: A Possible Bio-material for Platelet-rich Plasma Preparation. Clin. Oral. Implants Res. 2006, 17, 572–578. [Google Scholar] [CrossRef]
- Frelinger, A.L., III; Torres, A.S.; Caiafa, A.; Morton, C.A.; Berny-Lang, M.A.; Gerrits, A.J.; Carmichael, S.L.; Neculaes, V.B.; Michelson, A.D. Platelet-Rich Plasma Stimulated by Pulse Electric Fields: Platelet Activation, Procoagulant Markers, Growth Factor Release and Cell Proliferation. Platelets 2015, 27, 128–135. [Google Scholar] [CrossRef]
- Semple, E.; Speck, E.R.; Aslam, R.; Kim, M.; Kumar, V.; Semple, J.W. Evaluation of Platelet Gel Characteristics Using Thrombin Produced by the Thrombin Processing Device: A Comparative Study. J. Oral. Maxillofac. Surg. 2008, 66, 632–638. [Google Scholar] [CrossRef]
- Anitua, E.; Alkhraisat, M.H.; Orive, G. Perspectives and Challenges in Regenerative Medicine Using Plasma Rich in Growth Factors. J. Control. Release 2012, 157, 29–38. [Google Scholar] [CrossRef]
- Marques, L.F.; Stessuk, T.; Camargo, I.C.C.; Sabeh Junior, N.; Santos, L.D.; Ribeiro-Paes, J.T. Platelet-Rich Plasma (PRP): Methodological Aspects and Clinical Applications. Platelets 2015, 26, 101–113. [Google Scholar] [CrossRef]
- Sánchez, M.; Beitia, M.; Pompei, O.; Jorquera, C.; Sánchez, P.; Knörr, J.; Soldado, F.; López, L.; Oraa, J.; Miren Bilbao, A.; et al. Isolation, Activation, and Mechanism of Action of Platelet-Rich Plasma and Its Applications for Joint Repair. In Regenerative Medicine; S Choudhery, M., Ed.; IntechOpen: London, UK, 2020; ISBN 978-1-83881-927-9. [Google Scholar]
- Jia, K.; You, J.; Zhu, Y.; Li, M.; Chen, S.; Ren, S.; Chen, S.; Zhang, J.; Wang, H.; Zhou, Y. Platelet-Rich Fibrin as an Autologous Biomaterial for Bone Regeneration: Mechanisms, Applications, Optimization. Front. Bioeng. Biotechnol. 2024, 12, 1286035. [Google Scholar] [CrossRef]
- Reis, I.L.; Lopes, B.; Sousa, P.; Sousa, A.C.; Caseiro, A.R.; Mendonça, C.M.; Santos, J.M.; Atayde, L.M.; Alvites, R.D.; Maurício, A.C. Equine Musculoskeletal Pathologies: Clinical Approaches and Therapeutical Perspectives—A Review. Vet. Sci. 2024, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.H.; Jeong, H.-S.; Kim, J.P.; Koh, E.-H.; Lee, S.U.; Jin, S.M.; Kim, D.H.; Sohn, J.H.; Lee, S.H. Favorable Vocal Fold Wound Healing Induced by Platelet-Rich Plasma Injection. Clin. Exp. Otorhinolaryngol. 2014, 7, 47. [Google Scholar] [CrossRef]
- Anitua, E.; Nurden, P.; Prado, R.; Nurden, A.T.; Padilla, S. Autologous Fibrin Scaffolds: When Platelet- and Plasma-Derived Biomolecules Meet Fibrin. Biomaterials 2019, 192, 440–460. [Google Scholar] [CrossRef] [PubMed]
- López, S.; Vilar, J.M.; Sopena, J.J.; Damià, E.; Chicharro, D.; Carrillo, J.M.; Cuervo, B.; Rubio, M. Assessment of the Efficacy of Platelet-Rich Plasma in the Treatment of Traumatic Canine Fractures. Int. J. Mol. Sci. 2019, 20, 1075. [Google Scholar] [CrossRef]
- Zhu, L.; Li, P.; Qin, Y.; Xiao, B.; Li, J.; Xu, W.; Yu, B. Platelet-Rich Plasma in Orthopedics: Bridging Innovation and Clinical Applications for Bone Repair. J. Orthop. Surg. 2024, 32, 10225536231224952. [Google Scholar] [CrossRef]
- Souza, T.F.B.; Andrade, A.L.; Ferrreira, G.T.N.M.; Sakamoto, S.S.; Albuquerque, V.B.; Bonfim, S.R.M.; Luvizotto, M.C.R.; Louzada, M.J.Q. Healing and Expression of Growth Factors (TGF-β and PDGF) in Canine Radial Ostectomy Gap Containing Platelet-Rich Plasma. Vet. Comp. Orthop. Traumatol. 2012, 25, 445–452. [Google Scholar] [CrossRef]
- Panda, S.; Doraiswamy, J.; Malaiappan, S.; Varghese, S.S.; Del Fabbro, M. Additive Effect of Autologous Platelet Concentrates in Treatment of Intrabony Defects: A Systematic Review and Meta-analysis. J. Investig. Clin. Dent. 2016, 7, 13–26. [Google Scholar] [CrossRef]
- Marcazzan, S.; Weinstein, R.L.; Del Fabbro, M. Efficacy of Platelets in Bone Healing: A Systematic Review on Animal Studies. Platelets 2018, 29, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Griffin, X.L.; Smith, C.M.; Costa, M.L. The Clinical Use of Platelet-Rich Plasma in the Promotion of Bone Healing: A Systematic Review. Injury 2009, 40, 158–162. [Google Scholar] [CrossRef]
- Guerra-Gomes, M.; Ferreira-Baptista, C.; Barros, J.; Alves-Pimenta, S.; Gomes, P.; Colaço, B. Exploring the Potential of Non-Cellular Orthobiologic Products in Regenerative Therapies for Stifle Joint Diseases in Companion Animals. Animals 2025, 15, 589. [Google Scholar] [CrossRef] [PubMed]
- McDougall, R.A.; Canapp, S.O.; Canapp, D.A. Ultrasonographic Findings in 41 Dogs Treated with Bone Marrow Aspirate Concentrate and Platelet-Rich Plasma for a Supraspinatus Tendinopathy: A Retrospective Study. Front. Vet. Sci. 2018, 5, 98. [Google Scholar] [CrossRef]
- Canapp, S.O.; Leasure, C.S.; Cox, C.; Ibrahim, V.; Carr, B.J. Partial Cranial Cruciate Ligament Tears Treated with Stem Cell and Platelet-Rich Plasma Combination Therapy in 36 Dogs: A Retrospective Study. Front. Vet. Sci. 2016, 3, 112. [Google Scholar] [CrossRef]
- Boharski, R.A.; Wheeler, J.L.; Cross, A.R.; Jackson, J.; Peterson, S. Hybrid, Transarticular External Fixation with Platelet-rich Plasma Injection as a Treatment for Partial Calcaneal Tendon Disruption in Dogs without Primary Tenorrhaphy. Vet. Surg. 2024, 53, 1390–1398. [Google Scholar] [CrossRef]
- Alves, J.C.; Santos, A.; Lafuente, P. Evaluation of Different Substance Combinations in a Multiple-Session Mesotherapy Protocol for the Management of Osteoarthritis in Dogs: A Retrospective Study. J. Am. Vet. Med. Assoc. 2024, 262, 1–7. [Google Scholar] [CrossRef]
- Alves, J.C.; Santos, A.; Carreira, L.M. A Preliminary Report on the Combined Effect of Intra-Articular Platelet-Rich Plasma Injections and Photobiomodulation in Canine Osteoarthritis. Animals 2023, 13, 3247. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.C.; Santos, A.; Jorge, P. Platelet-Rich Plasma Therapy in Dogs with Bilateral Hip Osteoarthritis. BMC Vet. Res. 2021, 17, 207. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, B.; Rubio, M.; Chicharro, D.; Damiá, E.; Santana, A.; Carrillo, J.M.; Romero, A.D.; Vilar, J.M.; Cerón, J.J.; Sopena, J.J. Objective Comparison between Platelet Rich Plasma Alone and in Combination with Physical Therapy in Dogs with Osteoarthritis Caused by Hip Dysplasia. Animals 2020, 10, 175. [Google Scholar] [CrossRef]
- Parlak, K.; Arican, M. Effect of Intra-Articular Administration of Autologous PRP and Activated PRP on Inflammatory Mediators in Dogs with Osteoarthritis. Vet. Med. 2020, 65, 62–70. [Google Scholar] [CrossRef]
- Venator, K.; Frye, C.W.; Gamble, L.-J.; Wakshlag, J.J. Assessment of a Single Intra-Articular Stifle Injection of Pure Platelet Rich Plasma on Symmetry Indices in Dogs with Unilateral or Bilateral Stifle Osteoarthritis from Long-Term Medically Managed Cranial Cruciate Ligament Disease. Vet. Med. 2020, 11, 31–38. [Google Scholar] [CrossRef]
- Upchurch, D.A.; Renberg, W.C.; Roush, J.K.; Milliken, G.A.; Weiss, M.L. Effects of Administration of Adipose-Derived Stromal Vascular Fraction and Platelet-Rich Plasma to Dogs with Osteoarthritis of the Hip Joints. Am. J. Vet. Res. 2016, 77, 940–951. [Google Scholar] [CrossRef]
- Brinker, W.O.; Piermattei, D.L.; Flo, G.L. Handbook of Small Animal Orthopedics and Fracture Repair, 5th ed.; Elsevier: St. Louis, MO, USA, 2016; ISBN 978-1-4377-2364-9. [Google Scholar]
- Moore, E.V.; Weeren, R.; Paek, M. Extended Long-term Radiographic and Functional Comparison of Tibial Plateau Leveling Osteotomy vs. Tibial Tuberosity Advancement for Cranial Cruciate Ligament Rupture in the Dog. Vet. Surg. 2020, 49, 146–154. [Google Scholar] [CrossRef]
- Spinella, G.; Arcamone, G.; Valentini, S. Cranial Cruciate Ligament Rupture in Dogs: Review on Biomechanics, Etiopathogenetic Factors and Rehabilitation. Vet. Sci. 2021, 8, 186. [Google Scholar] [CrossRef]
- Wemmers, A.C.; Charalambous, M.; Harms, O.; Volk, H.A. Surgical Treatment of Cranial Cruciate Ligament Disease in Dogs Using Tibial Plateau Leveling Osteotomy or Tibial Tuberosity Advancement–A Systematic Review with a Meta-Analytic Approach. Front. Vet. Sci. 2022, 9, 1004637. [Google Scholar] [CrossRef]
- Duerr, F.M.; Martin, K.W.; Rishniw, M.; Palmer, R.H.; Selmic, L.E. Treatment of Canine Cranial Cruciate Ligament Disease: A Survey of ACVS Diplomates and Primary Care Veterinarians. Vet. Comp. Orthop. Traumatol. 2014, 27, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Slocum, B.; Slocum, T.D. Tibial Plateau Leveling Osteotomy for Repair of Cranial Cruciate Ligament Rupture in the Canine. Vet. Clin. N. Am. Small Anim. Pract. 1993, 23, 777–795. [Google Scholar] [CrossRef] [PubMed]
- Ness, M.G. The Modified Maquet Procedure (MMP) in Dogs: Technical Development and Initial Clinical Experience. J. Am. Anim. Hosp. Assoc. 2016, 52, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, L.X.; Repac, J.A.; Kirkby Shaw, K.; Compton, N. Systematic Review of Postoperative Rehabilitation Interventions after Cranial Cruciate Ligament Surgery in Dogs. Vet. Surg. 2022, 51, 233–243. [Google Scholar] [CrossRef]
- Rupreht, M.; Jevtič, V.; Serša, I.; Vogrin, M.; Jevšek, M. Evaluation of the Tibial Tunnel after Intraoperatively Administered Platelet-rich Plasma Gel during Anterior Cruciate Ligament Reconstruction Using Diffusion Weighted and Dynamic Contrast-enhanced MRI. Magn. Reson. Imaging 2013, 37, 928–935. [Google Scholar] [CrossRef]
- Seijas, R.; Ares, O.; Catala, J.; Alvarez-Diaz, P.; Cusco, X.; Cugat, R. Magnetic Resonance Imaging Evaluation of Patellar Tendon Graft Remodelling after Anterior Cruciate Ligament Reconstruction with or without Platelet-Rich Plasma. J. Orthop. Surg. 2013, 21, 10–14. [Google Scholar] [CrossRef]
- Morizaki, Y.; Zhao, C.; An, K.-N.; Amadio, P.C. The Effects of Platelet-Rich Plasma on Bone Marrow Stromal Cell Transplants for Tendon Healing In Vitro. J. Hand Surg. 2010, 35, 1833–1841. [Google Scholar] [CrossRef]
- Silva, R.F.; Carmona, J.U.; Rezende, C.M.F. Use of Intra-Articular Autologous Platelet Concentrates as Coadjutants in the Surgical Arthroscopy Treatment of Elbow Dysplasia in a Bitch. Arch. Med. Vet. 2013, 45, 213–217. [Google Scholar] [CrossRef][Green Version]
- Raulinaite, K.; Zelvyte, R.; Skemiene, K.; Monkeviciene, I. Treatment Tactic of Canine Cranial Cruciate Ligament Rupture Management: A 28-Day Comparative Analysis of ACP and NSAID Induced Effects on the Serum MMP-3 Levels and Clinical Outcomes. Vet. Med. 2025, 70, 124–133. [Google Scholar] [CrossRef]
- Barbaro, K.; Marconi, G.; Innocenzi, E.; Altigeri, A.; Zepparoni, A.; Monteleone, V.; Alimonti, C.; Marcoccia, D.; Ghisellini, P.; Rando, C.; et al. Regenerative Treatment of Canine Osteogenic Lesions with Platelet-Rich Plasma and Hydroxyapatite: A Case Report. Front. Vet. Sci. 2024, 11, 1459714. [Google Scholar] [CrossRef]
- Franini, A.; Entani, M.G.; Colosio, E.; Melotti, L.; Patruno, M. Case Report: Flexor Carpi Ulnaris Tendinopathy in a Lure-Coursing Dog Treated with Three Platelet-Rich Plasma and Platelet Lysate Injections. Front. Vet. Sci. 2023, 10, 1003993. [Google Scholar] [CrossRef]
- Volz, F.; Eberle, D.; Kornmayer, M.; Zablotski, Y.; Meyer-Lindenberg, A. Effect of Intra-articular Platelet-rich Plasma or Hyaluronic Acid on Limb Function Recovery in Dogs with TPLO for Cranial Cruciate Ligament Rupture: A Randomised Controlled Trial. J. Small Anim. Pract. 2024, 65, 223–233. [Google Scholar] [CrossRef]
- Gines, J.A. Effect of Leukoreduced Platelet Rich Plasma on Intra-Articular Pro-Inflammatory Cytokines in a Canine Pilot Study. Animals 2022, 12, 2163. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Kim, J.M.; Kim, M.H.; Kang, S.S.; Kim, G.; Choi, S.H. Scintigraphic Evaluation of Osseointegrative Response around Calcium Phosphate-Coated Titanium Implants in Tibia Bone: Effect of Platelet-Rich Plasma on Bone Healing in Dogs. Eur. Surg. Res. 2013, 51, 138–145. [Google Scholar] [CrossRef] [PubMed]
- King, W.; Cawood, K.; Bookmiller, M. The Use of Autologous Protein Solution (Pro-Stride®) and Leukocyte-Rich Platelet-Rich Plasma (Restigen®) in Canine Medicine. Vet. Med. 2021, 12, 53–65. [Google Scholar] [CrossRef]
- Szponder, T.; Wessely-Szponder, J.; Sobczyńska-Rak, A.; Żylińska, B.; Radzki, R.P.; Polkowska, I. Application of Platelet-Rich Plasma and Tricalcium Phosphate in the Treatment of Comminuted Fractures in Animals. In Vivo 2018, 32, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Filgueira, F.G.F.; Minto, B.W.; Chung, D.G.; Prada, T.C.; Rosa-Ballaben, N.M.; Campos, M.G.N. Platelet-Rich Plasma, Bone Marrow and Chitosan in Minimally Invasive Plate Osteosynthesis of Canine Tibia Fractures—A Randomized Study. Vet. Med. 2019, 64, 309–316. [Google Scholar] [CrossRef]
- Franklin, S.P.; Burke, E.E.; Holmes, S.P. The Effect of Platelet-Rich Plasma on Osseous Healing in Dogs Undergoing High Tibial Osteotomy. PLoS ONE 2017, 12, e0177597. [Google Scholar] [CrossRef]
- Raulinaitė, K.; Želvytė, R.; Škėmienė, K.; Burbaitė, E.; Karvelienė, B.; Monkevičienė, I. The Single Intra-Articular Injection of Platelet-Rich Plasma vs. Non-Steroidal Anti-Inflammatory Drugs as Treatment Options for Canine Cruciate Ligament Rupture and Patellar Luxation. Vet. Sci. 2023, 10, 555. [Google Scholar] [CrossRef]
- Sample, S.J.; Racette, M.A.; Hans, E.C.; Volstad, N.J.; Schaefer, S.L.; Bleedorn, J.A.; Little, J.P.; Waller, K.R.; Hao, Z.; Block, W.F.; et al. Use of a Platelet-Rich Plasma-Collagen Scaffold as a Bioenhanced Repair Treatment for Management of Partial Cruciate Ligament Rupture in Dogs. PLoS ONE 2018, 13, e0197204. [Google Scholar] [CrossRef]
- Valiño-Cultelli, V.; Varela-López, Ó.; González-Cantalapiedra, A. Does PRGF Work? A Prospective Clinical Study in Dogs with A Novel Polylactic Acid Scaffold Injected with PRGF Using the Modified Maquet Technique. Animals 2021, 11, 2404. [Google Scholar] [CrossRef]
- Valiño-Cultelli, V.; Varela-López, Ó.; González-Cantalapiedra, A. Incidence of Patellar Desmopathy in the Modified Maquet Technique with and without PRGF. Vet. Sci. 2022, 9, 180. [Google Scholar] [CrossRef] [PubMed]
- Aryazand, Y.; Buote, N.J.; Hsieh, Y.; Hayashi, K.; Rosselli, D. Multifactorial Assessment of Leukocyte Reduced Platelet Rich Plasma Injection in Dogs Undergoing Tibial Plateau Leveling Osteotomy: A Retrospective Study. PLoS ONE 2023, 18, e0287922. [Google Scholar] [CrossRef] [PubMed]
- Bozynski, C.; Stannard, J.; Smith, P.; Hanypsiak, B.; Kuroki, K.; Stoker, A.; Cook, C.; Cook, J. Acute Management of Anterior Cruciate Ligament Injuries Using Novel Canine Models. J. Knee. Surg. 2015, 29, 594–603. [Google Scholar] [CrossRef]
- Cook, J.L.; Smith, P.A.; Bozynski, C.C.; Kuroki, K.; Cook, C.R.; Stoker, A.M.; Pfeiffer, F.M. Multiple Injections of Leukoreduced Platelet Rich Plasma Reduce Pain and Functional Impairment in a Canine Model of ACL and Meniscal Deficiency. J. Orthop. Res. 2016, 34, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Daradka, M.H.; Malkawi, M.A.; Ismail, Z.B.; Hammouri, H.M.; Abu-Abeeleh, M.A.; Rihani, S. A Novel Surgical Technique for Cranial Cruciate Ligament Repair in Dogs Using Autologous Lateral Digital Extensor Muscle Tendon Graft Combined with Platelet-Rich Plasma: A Preliminary Experimental Study. Vet. World 2025, 18, 210–219. [Google Scholar] [CrossRef]
- Xie, X.; Wu, H.; Zhao, S.; Xie, G.; Huangfu, X.; Zhao, J. The Effect of Platelet-Rich Plasma on Patterns of Gene Expression in a Dog Model of Anterior Cruciate Ligament Reconstruction. J. Surg. Res. 2013, 180, 80–88. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, S.; Wu, H.; Xie, G.; Huangfu, X.; He, Y.; Zhao, J. Platelet-Rich Plasma Enhances Autograft Revascularization and Reinnervation in a Dog Model of Anterior Cruciate Ligament Reconstruction. J. Surg. Res. 2013, 183, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.T.; Slater, M.R.; Taylor, L.; Scott, H.M.; Kerwin, S.C. Assessing Repeatability and Validity of a Visual Analogue Scale Questionnaire for Use in Assessing Pain and Lameness in Dogs. Am. J. Vet. Res. 2004, 65, 1634–1643. [Google Scholar] [CrossRef]
- Goh, C. The Efficient Orthopedic Exam. In Proceedings of the World Small Animal Veterinary Association Congress Proceedings, Toronto, ON, Canada, 16–19 July 2019. [Google Scholar]
- Scott, H.; Witte, P. Investigation of Lameness in Dogs: 1. Forelimb. Practice 2011, 33, 20–27. [Google Scholar] [CrossRef]
- Etchepareborde, S.; Brunel, L.; Bollen, G.; Balligand, M. Preliminary Experience of a Modified Maquet Technique for Repair of Cranial Cruciate Ligament Rupture in Dogs. Vet. Comp. Orthop. Traumatol. 2011, 24, 223–227. [Google Scholar] [CrossRef]
- Cross, J.A.; Cole, B.J.; Spatny, K.P.; Sundman, E.; Romeo, A.A.; Nicholson, G.P.; Wagner, B.; Fortier, L.A. Leukocyte-Reduced Platelet-Rich Plasma Normalizes Matrix Metabolism in Torn Human Rotator Cuff Tendons. Am. J. Sport. Med. 2015, 43, 2898–2906. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.A. Intra-Articular Autologous Conditioned Plasma Injections Provide Safe and Efficacious Treatment for Knee Osteoarthritis: An FDA-Sanctioned, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Am. J. Sport. Med. 2016, 44, 884–891. [Google Scholar] [CrossRef]
- Dhurat, R.; Sukesh, M. Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author′s Perspective. J. Cutan. Aesthet. Surg. 2014, 7, 189. [Google Scholar] [CrossRef]
- Anitua, E.; Sánchez, M.; Zalduendo, M.M.; De La Fuente, M.; Prado, R.; Orive, G.; Andía, I. Fibroblastic Response to Treatment with Different Preparations Rich in Growth Factors. Cell Prolif. 2009, 42, 162–170. [Google Scholar] [CrossRef]
- Murray, M.M.; Spindler, K.P.; Abreu, E.; Muller, J.A.; Nedder, A.; Kelly, M.; Frino, J.; Zurakowski, D.; Valenza, M.; Snyder, B.D.; et al. Collagen-platelet Rich Plasma Hydrogel Enhances Primary Repair of the Porcine Anterior Cruciate Ligament. J. Orthop. Res. 2007, 25, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Oyama, T.; Nishimoto, S.; Tsugawa, T.; Shimizu, F. Efficacy of Platelet-Rich Plasma in Alveolar Bone Grafting. J. Oral. Maxillofac. Surg. 2004, 62, 555–558. [Google Scholar] [CrossRef]
- Landesberg, R.; Roy, M.; Glickman, R.S. Quantification of Growth Factor Levels Using a Simplified Method of Platelet-Rich Plasma Gel Preparation. J. Oral Maxillofac. Surg. 2000, 58, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Hopewell, S.; Chan, A.-W.; Collins, G.S.; Hróbjartsson, A.; Moher, D.; Schulz, K.F.; Tunn, R.; Aggarwal, R.; Berkwits, M.; Berlin, J.A.; et al. CONSORT 2025 Explanation and Elaboration: Updated Guideline for Reporting Randomised Trials. BMJ 2025, 389, e081124. [Google Scholar] [CrossRef] [PubMed]
- Vogrin, M.; Rupreht, M.; Dinevski, D.; Hašpl, M.; Kuhta, M.; Jevsek, M.; Knežević, M.; Rožman, P. Effects of a Platelet Gel on Early Graft Revascularization after Anterior Cruciate Ligament Reconstruction: A Prospective, Randomized, Double-Blind, Clinical Trial. Eur. Surg. Res. 2010, 45, 77–85. [Google Scholar] [CrossRef]
- Boffa, A.; Salerno, M.; Merli, G.; De Girolamo, L.; Laver, L.; Magalon, J.; Sánchez, M.; Tischer, T.; Filardo, G. Platelet-rich Plasma Injections Induce Disease-modifying Effects in the Treatment of Osteoarthritis in Animal Models. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 4100–4121. [Google Scholar] [CrossRef]
- Percie Du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting Animal Research: Explanation and Elaboration for the ARRIVE Guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- Histing, T.; Garcia, P.; Holstein, J.H.; Klein, M.; Matthys, R.; Nuetzi, R.; Steck, R.; Laschke, M.W.; Wehner, T.; Bindl, R.; et al. Small Animal Bone Healing Models: Standards, Tips, and Pitfalls Results of a Consensus Meeting. Bone 2011, 49, 591–599. [Google Scholar] [CrossRef]
- Sargeant, J.M.; Plishka, M.; Ruple, A.; Selmic, L.E.; Totton, S.C.; Vriezen, E.R. Quality of Reporting of Clinical Trials in Dogs and Cats: An Update. Vet. Intern. Med. 2021, 35, 1957–1971. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, F.; Shang, B.; Speich, J.E.; Wan, Y.-J.Y.; Hashida, H.; Braun, T.; Sadoughi, A.; Puehler, T.; Lue, T.F.; et al. Reporting Quality of Animal Research in Journals That Published the ARRIVE 1.0 or ARRIVE 2.0 Guidelines: A Cross-Sectional Analysis of 943 Studies. Cardiovasc. Diagn. Ther. 2024, 14, 1070–1082. [Google Scholar] [CrossRef]
- Rahman, E.; Rao, P.; Abu-Farsakh, H.N.; Thonse, C.; Ali, I.; Upton, A.E.; Baratikkae, S.Y.; Carruthers, J.D.A.; Mosahebi, A.; Heidari, N.; et al. Systematic Review of Platelet-Rich Plasma in Medical and Surgical Specialties: Quality, Evaluation, Evidence, and Enforcement. J. Clin. Med. 2024, 13, 4571. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.L.; Aviña, A.E.; Liu, Y.-Y.; Kao, H.-K. Stromal Vascular Fraction in Canine Osteoarthritis: Advantages, Applications, and Insights for Veterinary Practitioners. Front. Vet. Sci. 2025, 12, 1586629. [Google Scholar] [CrossRef]
- Cao, Y.; Wan, Y. Effectiveness of Platelet-Rich Plasma in Anterior Cruciate Ligament Reconstruction: A Systematic Review of Randomized Controlled Trials. Orthop. Surg. 2022, 14, 2406–2417. [Google Scholar] [CrossRef]
- Iacono, V.; Padovani, L.; Qordja, F.; De Berardinis, L.; Screpis, D.; Gigante, A.P.; Zorzi, C. Surgical and Biological Treatment with a Platelet-Rich Fibrin Matrix for Patellar Tendinopathy: Clinical Outcomes and Return to Sport at 2-Year Follow-Up. J. Pers. Med. 2024, 14, 567. [Google Scholar] [CrossRef]
- Huss, M.K.; Felt, S.A.; Pacharinsak, C. Influence of Pain and Analgesia on Orthopedic and Wound-Healing Models in Rats and Mice. Comp. Med. 2019, 69, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Carroll, C.C. Analgesic Drugs Alter Connective Tissue Remodeling and Mechanical Properties. Exerc. Sport Sci. Rev. 2016, 44, 29–36. [Google Scholar] [CrossRef]
- Hadjicharalambous, C.; Alpantaki, K.; Chatzinikolaidou, M. Effects of NSAIDs on Pre-osteoblast Viability and Osteogenic Differentiation. Exp. Ther. Med. 2021, 22, 740. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Cook, J.L.; Evans, R.; Conzemius, M.G.; Lascelles, B.D.X.; McIlwraith, C.W.; Pozzi, A.; Clegg, P.; Innes, J.; Schulz, K.; Houlton, J.; et al. Proposed Definitions and Criteria for Reporting Time Frame, Outcome, and Complications For Clinical Orthopedic Studies in Veterinary Medicine: Proposed Definitions and Criteria for Veterinary Orthopedic Studies. Vet. Surg. 2010, 39, 905–908. [Google Scholar] [CrossRef]
- García-González, M.; Muñoz, F.; González-Cantalapiedra, A.; López-Peña, M.; Saulacic, N. Systematic Review and Quality Evaluation Using ARRIVE 2.0 Guidelines on Animal Models Used for Periosteal Distraction Osteogenesis. Animals 2021, 11, 1233. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie , J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
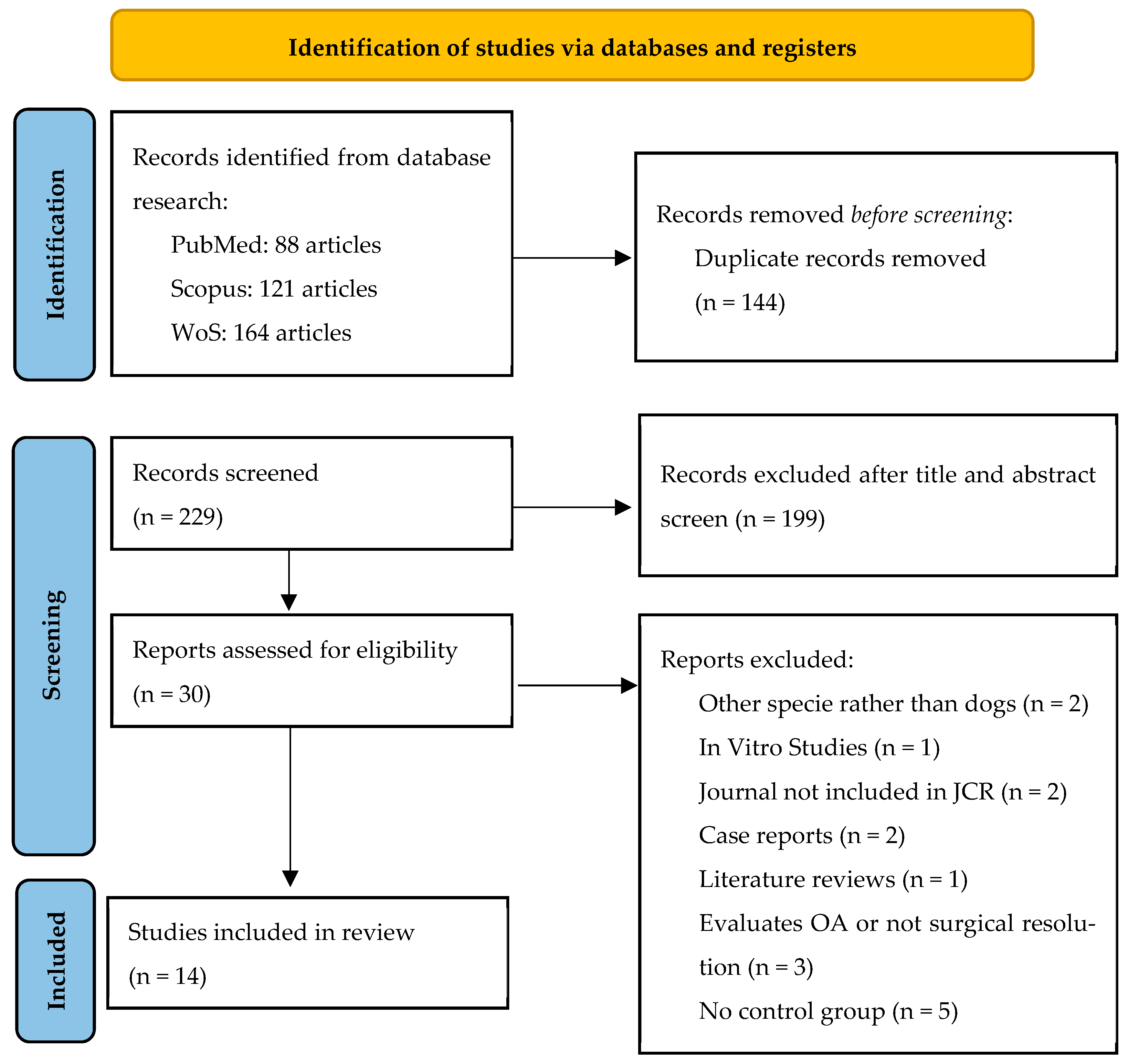

| Main Reason for Exclusion | No. | References |
|---|---|---|
| Species other than dogs | 2 | [41,42] |
| In vitro studies | 1 | [43] |
| Journal not included in JCR | 2 | [44,45] |
| Case reports | 2 | [46,47] |
| Literature reviews | 1 | [2] |
| Evaluates OA or other non-surgical applications | 3 | [48,49,50] |
| No control group | 5 | [23,24,25,51,52] |
| Author/Year | Type of Study | Study Period | Treatment Groups | No. of Patient | Sex | Age | Weight (kg) | Drop Out | |
|---|---|---|---|---|---|---|---|---|---|
| Aryazand et al., 2023 [59] | Retrospective | Jan. 2018–Dec. 2020 | PRP | 54 | 47 neutered males 2 intact males 60 neutered females 1 intact female | 6 (1–13) years | 29.7 | 0 | |
| Control | 56 | 28.3 | |||||||
| Bozynsky et al., 2015 [60] | In Vivo | NA | S.O.C. | Control | 3 | NR | Adult | 29.0 (19.7–34) | 0 |
| E. ACL | 3 | ||||||||
| Partial tear | 3 | ||||||||
| Washout | Control | 3 | |||||||
| E. ACL | 3 | ||||||||
| Partial tear | 3 | ||||||||
| PRP | Control | 3 | |||||||
| E. ACL | 3 | ||||||||
| Partial tear | 3 | ||||||||
| Cook et al., 2016 [61] | In Vivo | NA | PRP | 6 | NR | 2–5 years | 20–27 | NR | |
| Control | 6 | ||||||||
| Daradka et al., 2025 [62] | In Vivo | Jul. 2019–Jan. 2020 | PRP | 12 | Male | 11.4 years | 18 | 0 | |
| Control | 12 | ||||||||
| Filgueira et al., 2019 [53] | Prospective | 2012–2015 | PRP | 8 | 13 males 17 females | PRP: 75% > 12 months” | “47% 1–10 kg 26.5% 11–20 kg 26.5% 21–50 kg” | 2 | |
| BMC | 8 | 40% < 12 months | |||||||
| Chitosan | 8 | ||||||||
| Control | 8 | ||||||||
| Franklin et al., 2017 [54] | Prospective | 2017 | PRP | 27 | 12 neutered males 15 intact females | 4.9 ± 1.7 years | 32.2 ± 5.4 | 4 | |
| Control | 33 | 15 neutered males 17 neutered females 1 intact female | 5.7 ± 2.4 years | 31.9 ± 5.4 | |||||
| López et al., 2019 [16] | Prospective | López 2017–2018 | PRP | 20 | 11 males 9 females | 40.85 months | 16.27 | 0 | |
| Control | 23 | 10 males 13 females | 57.17 months | 13.07 | |||||
| Raulinaite et al., 2023 [55] | Prospective | 2022–2023 | PRP | 11 | 13 males 11 females | 2.7 ± 1.1 years | 18.2 ±7.5 | 0 | |
| Control | 11 | ||||||||
| Sample et al., 2018 [56] | Prospective | Apr. 2013–Jul. 2014 | PRP | 29 | 12 neutered males 3 intact males 14 neutered females | 5.5 ± 0.5 years (1.6–9.9) | 37.1 ± 1.7 (24.1–59.9) | 0 | |
| Control | 29 | ||||||||
| Souza et al., 2012 [18] | In Vivo | NA | PRP | 10 | 4 neutered males 6 neutered females | Adult | 4–6 kg | 0 | |
| Control | 11 | 4 neutered males 7 neutered females | |||||||
| Valiño-Cultelli et al., 2021 [57] | Prospective | Dec. 2017–Jul. 2020 | PRP | 29 | 16 intact males 4 neutered males 25 intact females 8 neutered females | 75.1 ± 45.63 months | 26.27 ± 7.41 | 18 | |
| Control | 24 | 70.16 ± 34.18 months | 30.13 ± 13.82 | ||||||
| Valiño-Cultelli et al., 2022 [58] | Prospective | Dec. 2017–Jul. 2020 | PRP | 29 | 16 intact males 4 neutered males 25 intact females 8 neutered | 75.1 ± 45.63 months | 26.27 ± 7.41 | 18 | |
| Control | 24 | 70.16 ± 34.18 months | 30.13 ± 13.82 | ||||||
| Xie et al., 2013a [63] | In Vivo | NA | Normal control | 18 | Male | Adult | 12.5 ± 1.48 | 0 | |
| Sham | 18 | ||||||||
| PRP control | 18 | ||||||||
| PRP | 18 | ||||||||
| Xie et al., 2013b [64] | In Vivo | NA | Normal control | 18 | Male | Adult | 12.5 ± 1.48 | 0 | |
| Sham | 18 | ||||||||
| PRP control | 18 | ||||||||
| PRP | 18 | ||||||||
| Author/Year | Injection Site/Times | V Administered (mL) | Follow-Ups | Disease | Anatomical Region |
|---|---|---|---|---|---|
| Aryazand et al., 2023 [59] | IA + osteotomy | 2 | 10–14 days + 6–10 weeks | CCLR–TPLO | Stifle |
| Bozynsky et al., 2015 [60] | IA | 2 | 24 h + 7 weeks + 7 months | CCLR | Stifle |
| Cook et al., 2016 [61] | IA/1, 2, 3, 6, and 8 weeks | 2 | 6 months | Partial CCLR + menisquectomy | Stifle |
| Daradka et al., 2025 [62] | IA + tunnels | 1 | 40 days | CCLR | Stifle |
| Filgueira et al., 2019 [53] | Fracture site | 2 | 0 + 25 + 30 + 60 + 90 + 120 days | Fracture | Tibia |
| Franklin et al., 2017 [54] | Osteotomy | 4.9 | 28 + 49 + 70 days | CCLR–TPLO | Stifle |
| López et al., 2019 [16] | Fracture site | 1.5 | 0 + 7 + 14 + 21 + 28 + 60 + 120 + 180 days | Fracture | Radius/ulna Tibia |
| Raulinaite et al., 2023 [55] | IA | 2 | 0 + 14 + 28 days | CCLR | Stifle |
| Sample et al., 2018 [56] | IA | 2 | 10 weeks + 12 months | CCLR–TPLO | Stifle |
| Souza et al., 2012 [18] | Fracture site | 1 | 14 + 21 + 28 + 35 + 45 + 60 days | Radial ostectomy model | Radius |
| Valiño-Cultelli et al., 2021 [57] | IA + osteotomy | 1.5 | 0 + 1 + 2 + 5 months | CCLR–MMT | Stifle |
| Valiño-Cultelli et al., 2022 [58] | IA + osteotomy | 1.5 | 0 + 1 + 2 + 5 months | CCLR–MMT | Stifle |
| Xie et al., 2013a [63] | Tunnels | 1 | 2 + 6 + 12 weeks | CCLR | Stifle |
| Xie et al., 2013b [64] | Tunnels | 1 | 2 + 6 + 12 weeks | CCLR | Stifle |
| Author/Year | Anesthetic Protocol | Functional Recovery Scale | Main Findings on Functional Assessment | |||
|---|---|---|---|---|---|---|
| Premedication/Sedation | Induction | Maintenance | Post-Operatory | |||
| Aryazand et al., 2023 [59] | Hydromorphone 0.1 mg/kg IV | Propofol 6 mg/kg IV | Isoflurane | AB: Cephazolin 22 mg/kg IV TID 10 days PC: NSAID 10 days Gabapentin 5–10 mg/kg 7 to 14 days | Hudson et al., 2004 [65] | Better with PRP |
| Bozynsky et al., 2015 [60] | Dexmedetomidine 5–10 mg/kg IV Morphine 0.5 mg/kg IV | Propofol 4–8 mg/kg IV | NR | AB: NR Atipamezole PC: Morphine 0.5 mg IV ×1 Tramadol 24 h | Hudson et al., 2004 [65] | Better but p > 0.05 |
| Cook et al., 2016 [61] | General anesthesia (NR) | Goniometer Hudson et al., 2004 [65] Pressure sensing walkaway | Better with PRP | |||
| Daradka et al., 2025 [62] | Xylazine 1.1 mg/kg IM Ketamine 15 mg/kg IM Meloxicam 0.2 mg/kg SC SID | AB: Amoxicillin 10 mg/kg IM BID 7 days PC: Meloxicam 0.1 mg/kg PO SID 4 days Tramadol 3 mg/kg PO BID/TID 7 days | Goh et al., 2019 [66] | Better with PRP | ||
| Filgueira et al., 2019 [53] | Chlorpromazine 0.3 mg/kg IM Morphine 0.25 mg/kg IM Meloxicam 0.2 mg/kg SC | Propofol 4 mg/kg IV | Isoflurane Epidural: Lidocaine 4 mg/kg + bupivacaine 2 mg/kg + tramadol 0.5 mg/kg | AB: Cephalexin 25 mg/kg PO BID 10 days PC: Meloxicam 0.1 mg/kg PO 5 days Dipyrone 25 mg/kg PO TID 7 days Tramadol 3 mg/kg PO TID 5 days | Scott et al., 2011 [67] | Both similar |
| Franklin et al., 2017 [54] | Dexmedetomidine 5 mg/kg IM Hydromorphone 0.1 mg/kg | Ketamine 5 mg/kg IV Diazepam 0.25 mg/kg IV Propofol 4 mg/kg in RMN | Isoflurane | AB: NR PC: Carprofen 4.4 mg/kg PO SID 7 days | NR | |
| López et al., 2019 [16] | NR | AB: NR PC: Morphine 0.2 mg IM QUID 24 h Carprofen 4 mg/kg IV SID 24 h | Own visual scale | Both similar | ||
| Raulinaite et al., 2023 [55] | Only mild sedation with dexmedetomidine 5 mg/kg IM + butorphanol 0.4 mg/kg IM | NR | Duerr et al., 2014 [37] Goniometer Own visual scale | Better with PRP | ||
| Sample et al., 2018 [56] | Dexmedetomidine 2–4 mg/kg IM Hydromorphone 0.1–0.2 mg/kg IM | Propofol 2–10 mg/kg | Isoflurane | NR | NR | |
| Souza et al. 2012 [18] | Midazolam 0.2 mg/kg IM Morphine 0.5 mg/kg IM | Propofol 4 mg/kg IV Midazolam 0.2 mg/kg | Isoflurane Brachial plex tap lidocaine + bupivacaine 7 mg/kg | AB: Cephalexin 30 mg/kg PO BID 10 days PC: Brachial plex tap repeated Morphine 0.5 mg/kg SID 12 h Tramadol 4 mg/kg PO TID 5 days Meloxicam 0.2 mg/kg PO SID 3 days | Own visual scale | Both similar |
| Valiño-Cultelli et al., 2021 [57] | Medetomidine 10 mg/kg IM Morphine 0.3 mg/kg IM Meloxicam 0.2 mg/kg IV | Propofol 2 mg/kg IV M.L.K. CRI 1 mL/kg | Sevoflurane M.L.K. CRI 1 mL/kg/h | AB: Cephazolin 22 mg/kg PO 10 days PC: Meloxicam 0.1 mg/kg PO 5 days | Etchepareborde et al., 2011 [68] | Both similar |
| Valiño-Cultelli et al., 2022 [58] | Medetomidine 10 mg/kg IM Morphine 0.3 mg/kg IM Meloxicam 0.2 mg/kg IV | Propofol 2 mg/kg IV M.L.K. CRI 1 mL/kg | Sevoflurane M.L.K. CRI 1 mL/kg/h | AB: Cephazolin 22 mg/kg PO 10 days PC: Meloxicam 0.1 mg/kg PO 5 days | Etchepareborde et al., 2011 [68] | Both similar |
| Xie et al., 2013a [63] | NR | Pentobarbital 30 mg/kg IV | NR | AB: Penicillin 3.2 million U IM SID 3 days Streptomycin 1 g IM SID 3 days PC: NR | NR | |
| Xie et al., 2013b [64] | NR | Pentobarbital 30 mg/kg IV | NR | AB: Penicillin 3.2 million U IM SID 3 days Streptomycin 1 g IM SID 3 days PC: NR | NR | |
| Author/Year | Complications | N Affected | Early/Late | Classification | Resolution |
|---|---|---|---|---|---|
| Aryazand et al., 2023 [59] | Implant infection | 16 | Late | Major | Implant removal |
| Bozynsky et al., 2015 [60] | Absence of complications | ||||
| Cook et al., 2016 [61] | Sterile acute synovitis | 1 | Early | Minor | Wash |
| Daradka et al., 2025 [62] | Mild pain and inflammation | NR | Early | Minor | Analgesic protocol |
| Filgueira et al., 2019 [53] | Mild inflammation | 1 | Early | Minor | Implant change or removal |
| Intra-articular screw placement | 1 | ||||
| Screw break | 1 | Late | Major | ||
| Franklin et al., 2017 [54] | NR | ||||
| López et al., 2019 [16] | Mild gastroenteritis | 1 | Late | Minor | Spontaneous |
| Loosening of pins | 3 | ||||
| Raulinaite et al., 2023 [55] | NR | ||||
| Sample et al., 2018 [56] | Absence of complications | ||||
| Souza et al., 2012 [18] | NR | ||||
| Valiño-Cultelli et al., 2021 [57] | Fracture of the distal cortical of tibial crest without displacement | 4 | NR | Minor | Strict rest |
| Apparition of vesicles in the incision region | 1 | AB administration | |||
| Tension band wiring rupture with or without tibial crest displacement | 4 | Major | Wire replacement | ||
| Implant rupture | 1 | Implant removal | |||
| Valiño-Cultelli et al., 2022 [58] | Fracture of the distal cortical of tibial crest without displacement | 4 | NR | Minor | Strict rest |
| Apparition of vesicles in the incision region | 1 | AB administration | |||
| Tension band wiring rupture with or without tibial crest displacement | 4 | Major | Wire replacement | ||
| Implant rupture | 1 | Implant removal | |||
| Xie et al., 2013a [63] | NR | ||||
| Xie et al., 2013b [64] | NR | ||||
| Author/Year | PRP Type | Combined | Preparation Protocol | Amount of Blood Used (mL) | Centrifugation | Platelet Count | |
|---|---|---|---|---|---|---|---|
| Speed | Time (min) | ||||||
| Aryazand et al., 2023 [59] | Liquid | No | Cross et al., 2015 [69] Smith et al., 2016 [70] Arthrex Incorporations | 10–15 | 1500 rpm | 5 | NR |
| Bozynsky et al., 2015 [60] | Liquid | No | Arthrex Incorporations | 15 | NA | P: 2.4× WB 280:1 P:L | |
| Cook et al., 2016 [61] | Liquid | No | Arthrex Incorporations | 15 | 1500 rpm | 5 | P: 2.5× WB 295:1 P:L |
| Daradka et al., 2025 [62] | Liquid | No | Dhurat et al., 2014 [71] | 20 | NR | 1 × 106 adjusted P | |
| Filgueira et al., 2019 [53] | Liquid | Activated with 10% CaCl2 | Own protocol Double centrifugation | 4.5 | 1200 rpm | 10 | NA |
| 1600 rpm | 10 | ||||||
| Franklin et al., 2017 [54] | Gel | Bovine Thrombin | Arthrex Incorporations | 120 | NR | P: 7.4× WH L: 5.45 × 103 | |
| López et al., 2019 [16] | Liquid | Activated with 10% CaCl2 | Anitua et al., 2009 [72] | 20 | 460 g | 8 | P: 2× WH L: < 0.2 × 106 L/mL |
| Raulinaite et al., 2023 [55] | Liquid | No | Arthrex Incorporations | 15 | 1500 rpm | 5 | NA |
| Sample et al., 2018 [56] | Liquid | Collagen | Murray et al., 2007 [73] SmartPReP | 32 | 100 g | 14 | P: 6.4 × WH |
| Souza et al., 2012 [18] | Gel | No | Oyama et al., 2004 [74] | 8 | 160 g | 20 | P: ≥ 338% WH |
| Valiño-Cultelli et al., 2021 [57] | Both | PLA | Anitua et al., 2009 [72] | 27 | 460 rfc | 8 | P: 1.5/2 × WH L < 0.2 × 106 |
| Valiño-Cultelli et al., 2022 [58] | Both | PLA | Anitua et al., 2009 [72] | 27 | 460 rfc | 8 | P: 1.5/2 × WH L < 0.2 × 106 |
| Xie et al., 2013a [63] | Gel | Activated with CaCl2 | Landesberg et al., 2000 [75] | 20 | 200 g | 10 | P: 5.03× WH |
| Xie et al., 2013b [64] | Gel | Activated with CaCl2 | Landesberg et al., 2000 [75] | 20 | 200 g | 10 | P: 5.03× WH |
| Author | Year | Coefficient | Quality |
|---|---|---|---|
| Bozynsky et al. [60] | 2015 | 0.83 | Excellent |
| Cook et al. [61] | 2016 | 0.81 | Excellent |
| Daradka et al. [62] | 2025 | 0.81 | Excellent |
| Souza et al. [18] | 2012 | 0.76 | Average |
| Xie et al. [63] | 2013a | 0.57 | Average |
| Xie et al. [64] | 2013b | 0.57 | Average |
| Author | Year | Coefficient | Quality |
|---|---|---|---|
| Aryazand et al. [59] | 2023 | 0.58 | Average |
| Filgueira et al. [53] | 2019 | 0.43 | Average |
| Franklin et al. [54] | 2017 | 0.56 | Average |
| Lopez et al. [16] | 2019 | 0.46 | Average |
| Raulinaite et al. [55] | 2023 | 0.60 | Good |
| Sample et al. [56] | 2018 | 0.57 | Average |
| Valiño-Cultelli et al. [57] | 2021 | 0.44 | Average |
| Valiño-Cultelli et al. [58] | 2022 | 0.44 | Average |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidal-Negreira, F.; García-González, M.; Valiño-Cultelli, V.; González-Cantalapiedra, A. Platelet-Rich Plasma in Veterinary Orthopedic Surgery: A Systematic Review and Quality Evaluation on Liquid- and Gel-Based Therapies in Dogs. Gels 2025, 11, 994. https://doi.org/10.3390/gels11120994
Vidal-Negreira F, García-González M, Valiño-Cultelli V, González-Cantalapiedra A. Platelet-Rich Plasma in Veterinary Orthopedic Surgery: A Systematic Review and Quality Evaluation on Liquid- and Gel-Based Therapies in Dogs. Gels. 2025; 11(12):994. https://doi.org/10.3390/gels11120994
Chicago/Turabian StyleVidal-Negreira, Francisco, Mario García-González, Victoria Valiño-Cultelli, and Antonio González-Cantalapiedra. 2025. "Platelet-Rich Plasma in Veterinary Orthopedic Surgery: A Systematic Review and Quality Evaluation on Liquid- and Gel-Based Therapies in Dogs" Gels 11, no. 12: 994. https://doi.org/10.3390/gels11120994
APA StyleVidal-Negreira, F., García-González, M., Valiño-Cultelli, V., & González-Cantalapiedra, A. (2025). Platelet-Rich Plasma in Veterinary Orthopedic Surgery: A Systematic Review and Quality Evaluation on Liquid- and Gel-Based Therapies in Dogs. Gels, 11(12), 994. https://doi.org/10.3390/gels11120994







