A Polyvinyl Alcohol Hydrogel Based on a Polypyrrole/Biomass Carbon Nanosphere Synergistic Network for Flexible Pressure Sensors
Abstract
1. Introduction
2. Results and Discussion
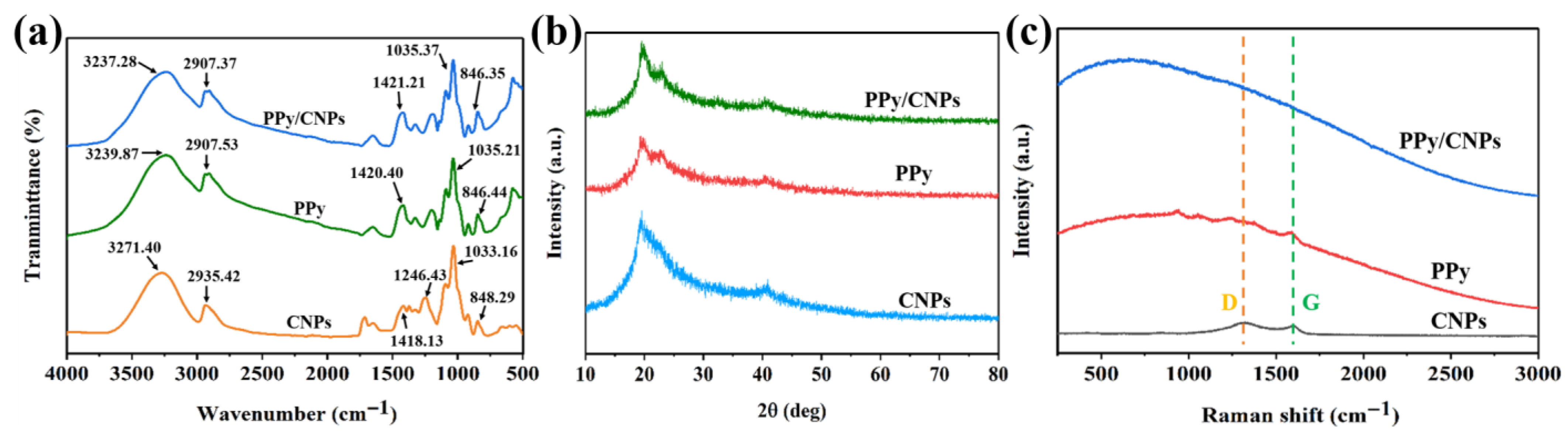
2.1. Material Characterization
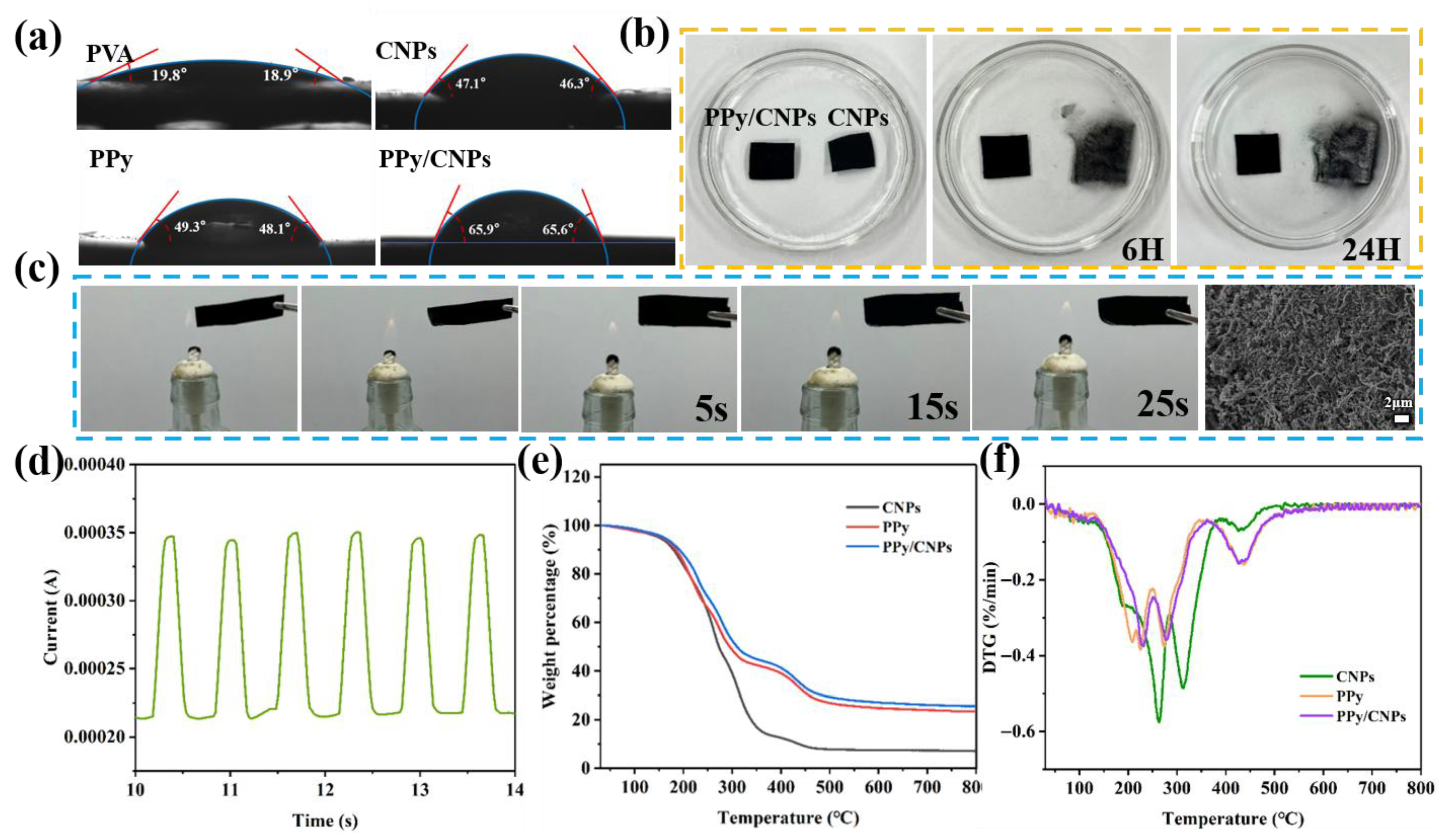
2.2. Properties of the Hydrogel Film
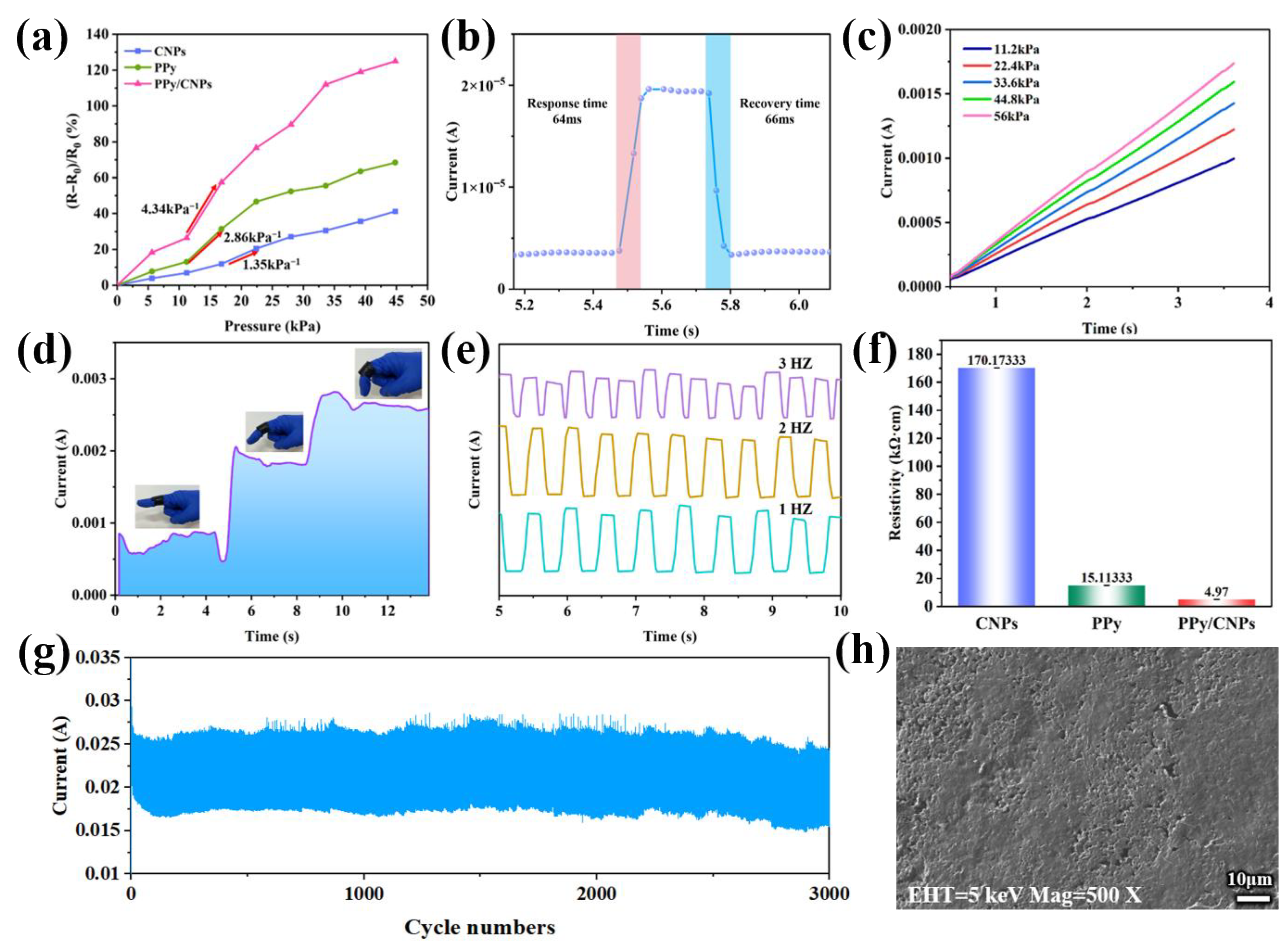
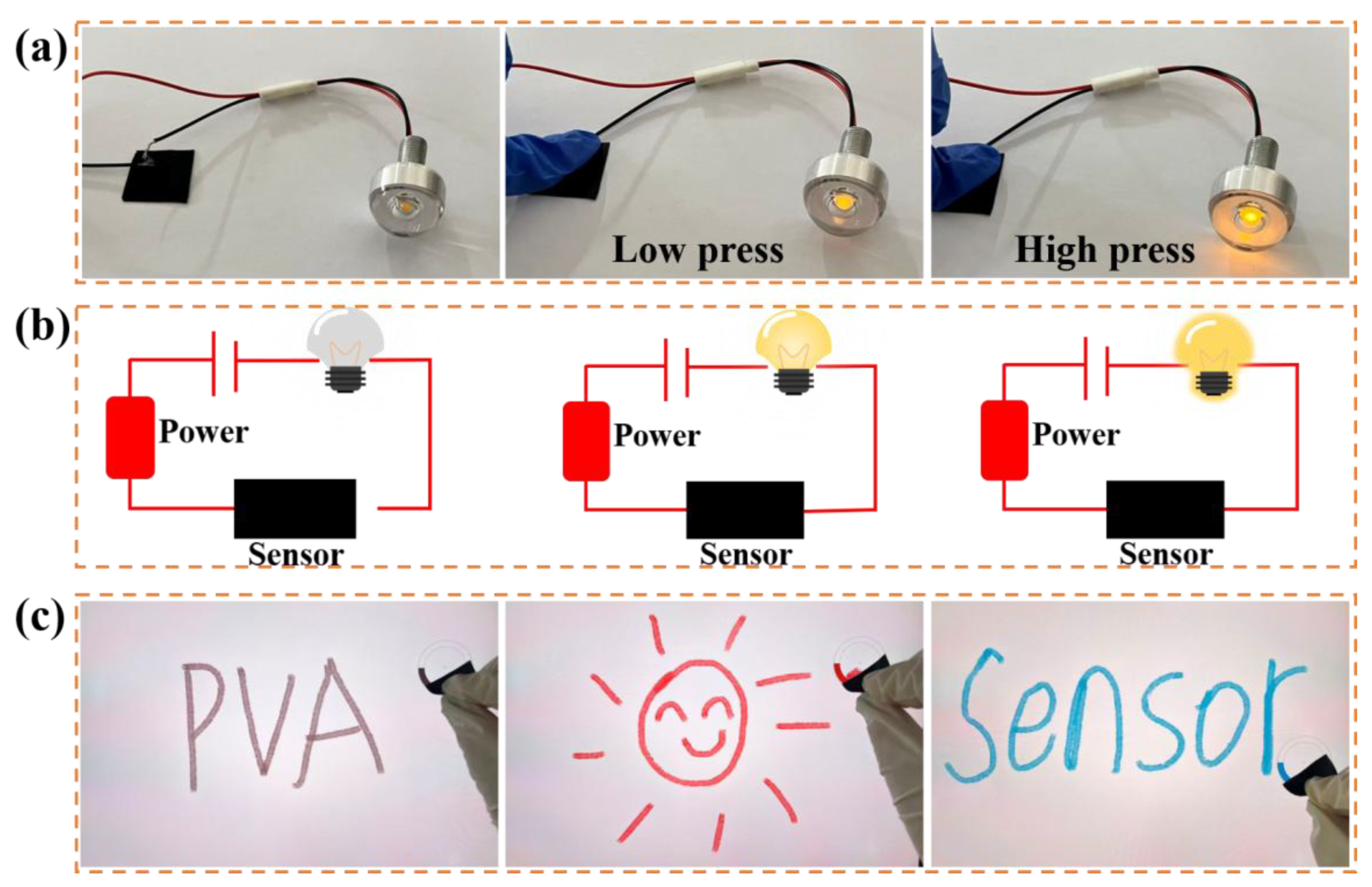
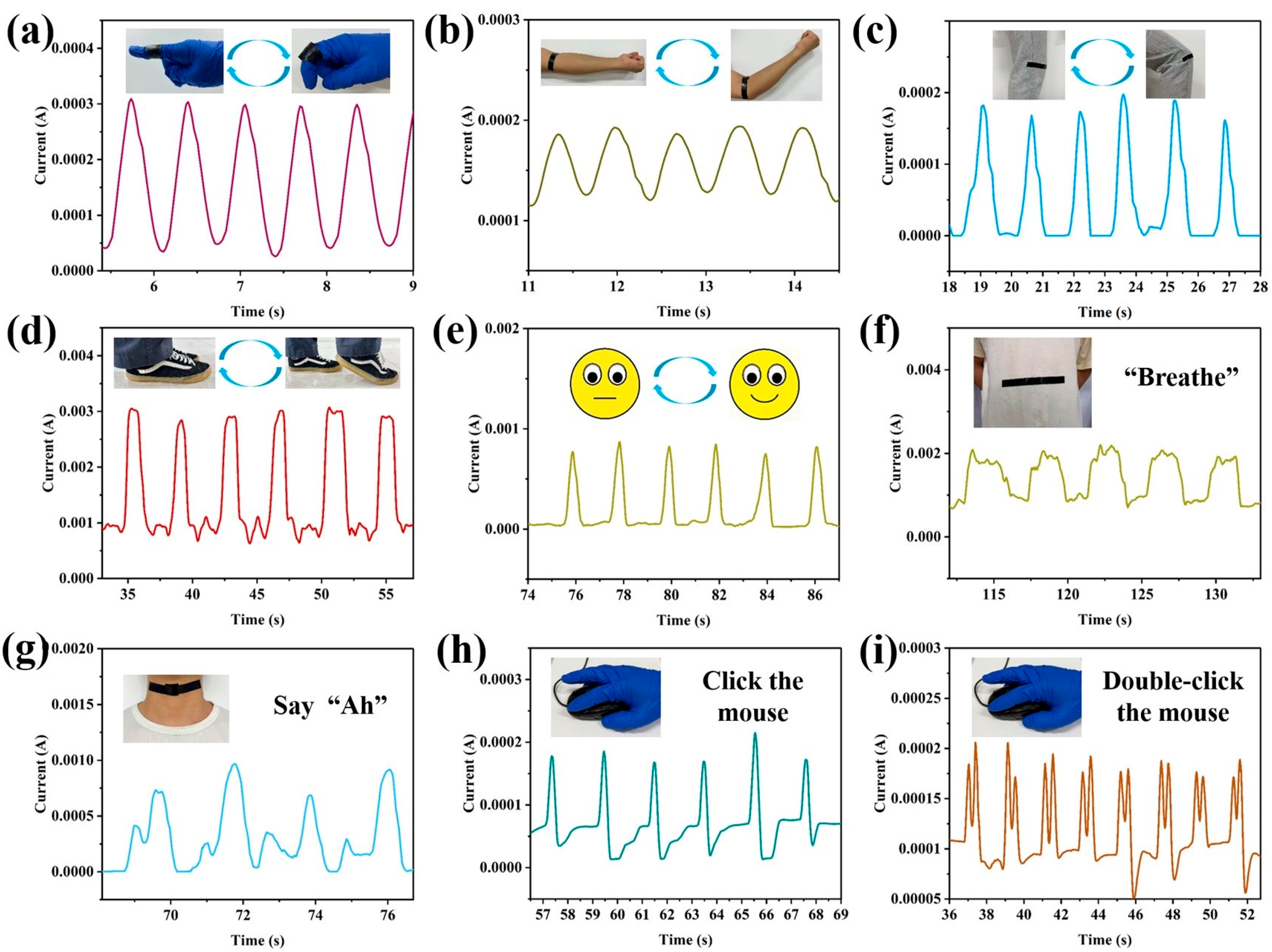
2.3. Applications of Hydrogel Film Pressure Sensors
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of CNPs
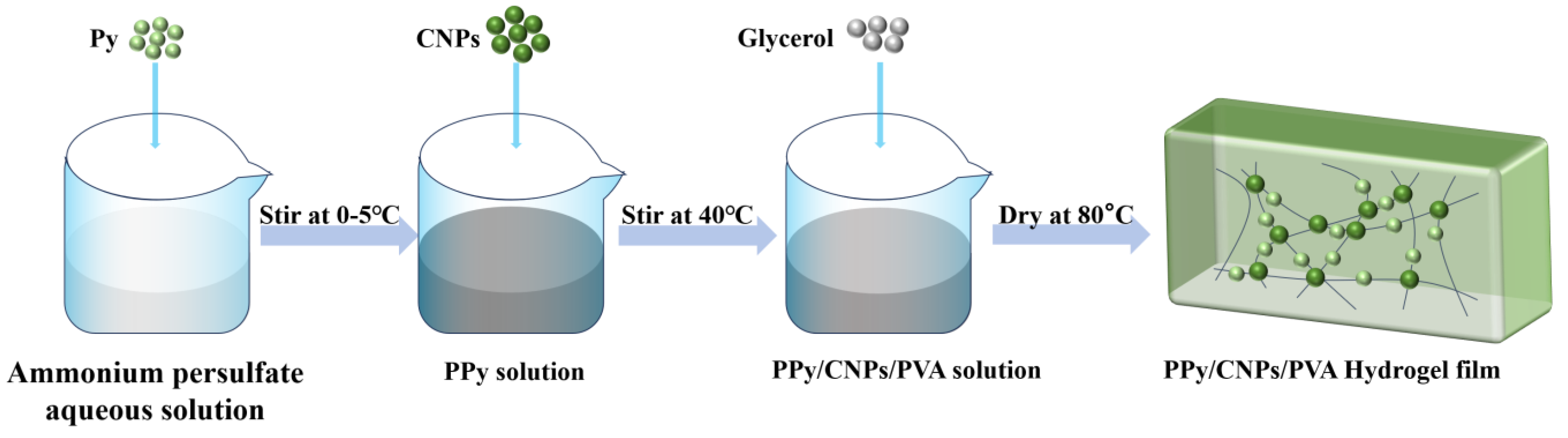
4.3. Preparation of PPy/CNP/PVA Composites
4.4. Characterization
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PVA | Polyvinyl alcohol |
| PPy | Polypyrrole |
| CNPs | Carbon nanospheres |
| Py | Pyrrole monomer |
| APS | Ammonium persulfate |
References
- Ibrahim, Z.; Asif, A.; Hassan, G.; Shuja, A. Fabrication of UV-curable and self-adhesive strain sensor based on 2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone and PVA composite for biomedical applications. Sens. Actuators A Phys. 2025, 392, 116754. [Google Scholar] [CrossRef]
- Seleka, W.M.; Phasha, M.M.; Kganyakgo, L.K.; Makwakwa, D.; Makhado, E. Preparation of a conductive hydrogel based on carrageenan, polyvinyl alcohol, and polypyrrole as a potential room temperature electrochemical hydrogen gas sensor. Microchem. J. 2025, 214, 114091. [Google Scholar] [CrossRef]
- Fang, J.; Meng, C.; Yuan, F.; Zhang, G.; Xu, Z.; Ni, Q. Sandpaper-assisted preparation of microstructured multifunctional PANI/PVA composite hydrogel film for high-sensitivity pressure sensors with accurate pressure identification and motion monitoring. Mater. Today Commun. 2024, 38, 107783. [Google Scholar] [CrossRef]
- Firouz, M.M.; Salehi, M.B.; Baniasadi, M.; Mokhtarani, B. Artemisia ciniformis-enhanced multifunctional hydrogel with ionic conductivity and anti-freezing properties for wearable strain sensors. Int. J. Biol. Macromol. 2025, 330, 147892. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Xu, S.; Yu, S.; Li, P.; Zhan, J.; Yi, L.; Liu, J.; Liu, F. Laser confined printing of microstructured MXene-graphene film for highly sensitive flexible pressure sensor. Sens. Actuators A Phys. 2025, 394, 116951. [Google Scholar] [CrossRef]
- Chenani, H.; Razaghi, Z.; Saeidi, M.; Aghaii, A.H.; Rastkhiz, M.A.; Orouji, M.; Ekrami, A.; Simchi, A. A stretchable, adhesive, and wearable hydrogel-based patches based on a bilayer PVA composite for online monitoring of sweat by artificial intelligence-assisted smartphones. Talanta 2025, 287, 127640. [Google Scholar] [CrossRef]
- Viswanathan, P.; Chandrasekhar, A. Stretch-Responsive Triboelectric Nanogenerator Based on Conductive PVA/AC Hydrogel for Human Activity Monitoring. Results Eng. 2025, 28, 107734. [Google Scholar] [CrossRef]
- Lu, K.; Post, C.; Hu, J.; Maniar, D.; Folkersma, R.; Voet, V.S.; Loos, K. Structure and properties of biodegradable self-healing starch/PVA/chitosan hydrogels. Polymer 2025, 336, 128864. [Google Scholar] [CrossRef]
- Entifar, N.A.E.; Azizi, M.J.; Kim, G.W.; Entifar, S.A.N.; Slamet, M.N.; Kim, J.H.; Kim, Y.H. High-performance PVA/xanthan gum hydrogel via dual cross-linking with ionic treatment for wearable sensing and hydrovoltaic energy generation. Chem. Eng. J. 2025, 520, 165637. [Google Scholar] [CrossRef]
- Tangoh, A.F.; Park, J.; Same, N.N.; Yakub, A.O.; Chaulagain, D.; Roh, J.W.; Suh, D.; Lim, J.O.; Huh, J.S. ZnO nanorods/Graphene/CNT nanocomposite gas sensors for enhanced VOC gas response and selectivity: Selective analysis of formaldehyde and ethanol. Mater. Sci. Eng. B 2025, 323, 118783. [Google Scholar] [CrossRef]
- Chaudhuri, B.; Bhadra, D. Novel electrospun fibrous films of PVA–PVP–AgNP, PVP-PVA-GO and PVA–PVP–AgNP–GO nanocomposites for biomedical and technological applications. Mater. Lett. 2025, 405, 139771. [Google Scholar] [CrossRef]
- Thiruchelvam, P.; Dasmahapatra, A.K. Supramolecular Self-Assembled, Conductive, Mechanically Flexible MXene Cross-Linked Polypyrrole Hydrogel for Wearable Energy Storage Applications. Small 2025, 21, e2502286. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, Z.; Wang, J.; Wei, W.; Quan, J.; Huang, Y. Natural bamboo fiber composite sensor with low pressure, high sensitivity, and surface interlocking structure. Sens. Actuators A Phys. 2025, 384, 116219. [Google Scholar] [CrossRef]
- Sharma, S.; Bhende, M.; Verma, H.R.; Kumar, S. Physically cross-linked PVA/f-MWCNTs nanocomposite hydrogel with enhanced thermal, mechanical, and dielectric properties. Mater. Today Commun. 2024, 40, 109400. [Google Scholar] [CrossRef]
- Dong, X.; Song, T.; Ren, D.; Dai, K.; Leng, Y.; Wang, T.; Wang, S.; Sun, M.; Liu, D.; Zhang, Q.; et al. Preparation of wearable strain sensor based on PVA/MWCNTs hydrogel composite. Mater. Today Commun. 2022, 31, 103278. [Google Scholar] [CrossRef]
- Ai, J.; Li, K.; Li, J.; Yu, F.; Ma, J. Super flexible, fatigue resistant, self-healing PVA/xylan/borax hydrogel with dual-crosslinked network. Int. J. Biol. Macromol. 2021, 172, 66–73. [Google Scholar] [CrossRef]
- Chen, S.; Dong, T.; Lan, Y. A Highly Sensitive and Stretchable Flexible Strain Sensor Based on a PVC/PVA Dual-Substrate Composite for Wearable Electronics. Polymer 2025, 337, 128964. [Google Scholar] [CrossRef]
- Ao, J.; Li, D.; Ding, L.; Xu, J.; Chen, Y.; Liu, X.; Wu, L.; Xu, J.; Jiang, S.; Zhang, S. Plasmonic silver nanorod-reinforced flexible polyvinyl alcohol hydrogel SERS sensors for ultrasensitive detection of food colorants in fruit juice. Food Chem. 2025, 495, 146405. [Google Scholar] [CrossRef]
- Xu, Q.; Wu, Z.; Zhao, W.; He, M.; Guo, N.; Weng, L.; Lin, Z.; Taleb, M.F.A.; Ibrahim, M.M.; Singh, M.V.; et al. Strategies in the preparation of conductive polyvinyl alcohol hydrogels for applications in flexible strain sensors, flexible supercapacitors, and triboelectric nanogenerator sensors: An overview. Adv. Compos. Hybrid Mater. 2023, 6, 203. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, L.; Ling, Q.; Liu, J.; Gu, H. Mussel-induced nano-silver antibacterial, self-healing, self-adhesive, anti-freezing, and moisturizing dual-network organohydrogel based on SA-PBA/PVA/CNTs as flexible wearable strain sensors. Polymer 2022, 256, 125270. [Google Scholar] [CrossRef]
- Ding, Y.; Hou, Y.; Liu, W.; Liu, Y.; Liu, Q.; Yao, Y.; Chen, D. Wearable Strain Sensor Based on Double Network PVA/SA/CNT Hydrogel for Reliable Electrocardiography and Motion Monitoring. Sens. Actuators A Phys. 2025, 396, 117196. [Google Scholar] [CrossRef]
- Liu, X.; Ma, J.; Li, B.; Du, L.; Wang, C.; Ji, Y.; Zheng, W.; Dong, Q.; Ma, Y.; Li, T.; et al. Highly sensitive, anti-freeze, repairable, and conductive double-network organohydrogel for flexible pressure sensors. Polymer 2024, 298, 126892. [Google Scholar] [CrossRef]
- Zhao, Y.; Mu, S.; Hu, B.; Hao, J.; Xia, Y.; Zhongling, Y.; Han, Z.; Kong, X.; Zhu, X.; Xu, Z.; et al. Polyvinyl alcohol/silk fibroin/graphene hydrogel-based flexible sensor with self-healing, ultra-stretchable, and eco-friendly properties for advanced wearable electronics and human-machine interaction applications. Sens. Actuators B Chem. 2025, 445, 138566. [Google Scholar] [CrossRef]
- Wu, F.; Lin, X.; Xu, Y.; Chen, Y.; He, Y.; Wang, J.; Liu, M. Bilayer PVA composite film with structural color for high-performance and multifunctional sensing. Compos. Sci. Technol. 2023, 241, 110106. [Google Scholar] [CrossRef]
- Hasan, M.Z.; Xu, C.; Motaleb, K.A.; Ahmed, M.F.; Zhuang, J.; Tan, S.; Janutėnienė, J.; Bashar, M.M.; Tu, H.; Luo, L.; et al. Extraction and incorporation of cellulose microfibers from textile wastes into MXene-enhanced PVA-borax hydrogel for multifunctional wearable sensors. Int. J. Biol. Macromol. 2025, 295, 139640. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, Y.; Gao, S.; Jiang, B.; Liu, M.; Huang, J.; Fan, J.; Wu, Z.; Zhang, X. Organic solvent-free and high-productive fabrication of lignin nanospheres via soft templating and their mechanical enhancement on PVA hydrogels. Ind. Crop. Prod. 2024, 210, 118211. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, Z.; Fei, G.; Lavorgna, M.; Xia, H. Polyimide derived carbon/graphene hybrid aerogel microspheres for strong and wide bandwidth microwave absorption. Adv. Funct. Mater. 2025, 35, 2419252. [Google Scholar] [CrossRef]
- Tao, S.; Yang, Q.; Zhou, W.; Zhu, J.; Pan, H.; Xu, L.; Wang, J.; Zhao, H.; Zhou, T.; Wang, J. Incorporation of polyvinyl alcohol in bacterial cellulose/polypyrrole flexible conductive films to enhance the mechanical and conductive performance. Int. J. Biol. Macromol. 2024, 282, 137571. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, B.; Yu, J.; Yan, Z.; Liu, R.; Yu, C.; Zhao, Z.; Zhang, H.; Yao, F.; Li, J. polypyrrole-dopamine/poly (vinyl alcohol) anisotropic hydrogel for strain sensor and bioelectrodes. Chem. Eng. J. 2024, 486, 150182. [Google Scholar] [CrossRef]
- Bai, Z.; Zeng, W.; Deng, J.; Zhou, S.; Yang, C.; Jin, T.; Wei, H. Piezoresistive sensors based on bacterial cellulose/ZnO/PPy composite materials for human signal monitoring and sound detection. Sens. Actuators A Phys. 2024, 377, 115714. [Google Scholar] [CrossRef]
- Qin, Z.; Zhao, G.; Zhang, Y.; Gu, Z.; Tang, Y.; Aladejana, J.T.; Ren, J.; Jiang, Y.; Guo, Z.; Peng, X.; et al. A simple and effective physical ball-milling strategy to prepare super-tough and stretchable PVA@ MXene@ PPy hydrogel for flexible capacitive electronics. Small 2023, 19, 2303038. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shi, H.; Song, F.; Yang, W.; Yang, B.; Ding, D.; Liu, Z.; Hui, L.; Zhang, F. A highly sensitive and anti-freezing conductive strain sensor based on polypyrrole/cellulose nanofiber crosslinked polyvinyl alcohol hydrogel for human motion detection. Int. J. Biol. Macromol. 2024, 257, 128800. [Google Scholar] [CrossRef]
- Zainul, R.; Hsu, C.Y.; Basem, A.; Jasim, D.J.; Conde, Á.A.S.; Ajaj, Y.; Muzammil, K.; Islam, S.; Elawady, A. In-situ growth of MCo2O4 nanospheres (M: Mn, Ni) over g-C3N4@ PPy as high-performance and novel composites for sustainable supercapacitors. J. Energy Storage 2024, 90, 111727. [Google Scholar] [CrossRef]
- Tan, L.; Yang, Y.D.; Li, N.; Chen, S.; Liu, Z.Q. Enhanced activity and stability of Co 3 O 4-decorated nitrogen-doped carbon hollow sphere catalysts for microbial fuel cells. Catal. Sci. Technol. 2017, 7, 1315–1323. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Q.; Wei, T.; Jiang, L.; Zhang, M.; Jing, X.; Fan, Z. Template-assisted low temperature synthesis of functionalized graphene for ultrahigh volumetric performance supercapacitors. ACS Nano 2014, 8, 4720–4729. [Google Scholar] [CrossRef]
- El Nokab, M.E.H.; Sayed, J.E.; De Witte, F.; Dewettinck, K.; Elshewy, A.; Zhang, Z.; Van Steenberge, P.H.; Wang, T.; Sebakhy, K.O. A comparative analytical study for the different water pools present in alginate hydrogels: Qualitative vs. quantitative approaches. Food Hydrocoll. 2024, 154, 110159. [Google Scholar] [CrossRef]
- Ma, W.; Jiang, Z.; Lu, T.; Xiong, R.; Huang, C. Lightweight, elastic and superhydrophobic multifunctional nanofibrous aerogel for self-cleaning, oil/water separation and pressure sensing. Chem. Eng. J. 2022, 430, 132989. [Google Scholar] [CrossRef]
- Yu, W.; Chen, J.; Gao, Q.; Guo, Y.; Zhang, S.; Pan, Y.; Nie, B.; Zhang, X.; Jiang, L.; Qiu, J.; et al. Multifunctional PVA/SA-based hydrogels integrating high stretchability, conductivity, and antibacterial activity for human-machine interactive flexible sensors. Chem. Eng. J. 2025, 519, 164695. [Google Scholar] [CrossRef]
- Guo, D.; Li, Y.; Zhou, Q.; Yu, Z.; Liu, X.; Dong, S.; Zhang, S.; Sung, H.-K.; Yao, Z.; Li, Y.; et al. Degradable, biocompatible, and flexible capacitive pressure sensor for intelligent gait recognition and rehabilitation training. Nano Energy 2024, 127, 109750. [Google Scholar] [CrossRef]
- He, S.; Cui, Z.; Fang, H.; Wei, X.; Shao, W. Incorporation of multi-dynamic interactions into carboxyl graphene oxide-enhanced PVA/PAA/agarose hydrogels for multifunctional wearable sensors. Int. J. Biol. Macromol. 2025, 314, 144336. [Google Scholar] [CrossRef]
- Eslami, R.; Azizi, N.; Santhirakumaran, P.; Mehrvar, M.; Zarrin, H. 3D dual network effect of alkalinized MXene and hBN in PVA for wearable strain/pressure sensor applications. Chem. Eng. J. 2024, 480, 148063. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, Z.; Yi, C.; Yin, C.; Zhang, X.; Peng, C. A Polyvinyl Alcohol Hydrogel Based on a Polypyrrole/Biomass Carbon Nanosphere Synergistic Network for Flexible Pressure Sensors. Gels 2025, 11, 956. https://doi.org/10.3390/gels11120956
Shu Z, Yi C, Yin C, Zhang X, Peng C. A Polyvinyl Alcohol Hydrogel Based on a Polypyrrole/Biomass Carbon Nanosphere Synergistic Network for Flexible Pressure Sensors. Gels. 2025; 11(12):956. https://doi.org/10.3390/gels11120956
Chicago/Turabian StyleShu, Ziyan, Chunqiang Yi, Cailiu Yin, Xinjiang Zhang, and Chengcheng Peng. 2025. "A Polyvinyl Alcohol Hydrogel Based on a Polypyrrole/Biomass Carbon Nanosphere Synergistic Network for Flexible Pressure Sensors" Gels 11, no. 12: 956. https://doi.org/10.3390/gels11120956
APA StyleShu, Z., Yi, C., Yin, C., Zhang, X., & Peng, C. (2025). A Polyvinyl Alcohol Hydrogel Based on a Polypyrrole/Biomass Carbon Nanosphere Synergistic Network for Flexible Pressure Sensors. Gels, 11(12), 956. https://doi.org/10.3390/gels11120956






