Unveiling the Compression Mechanical Properties of AMPS–APTAC–DMAAm Terpolymeric Hydrogels
Abstract
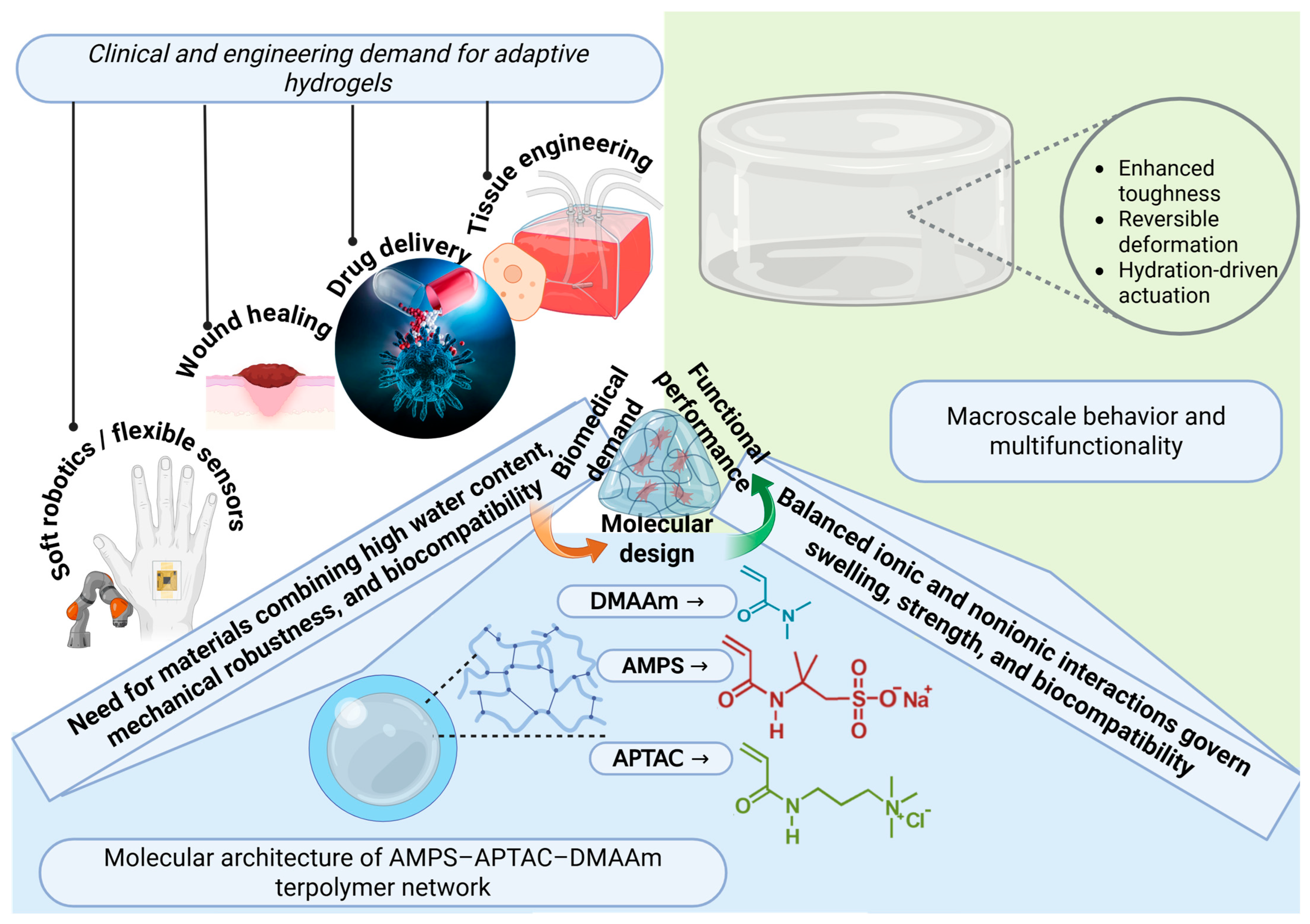
1. Introduction
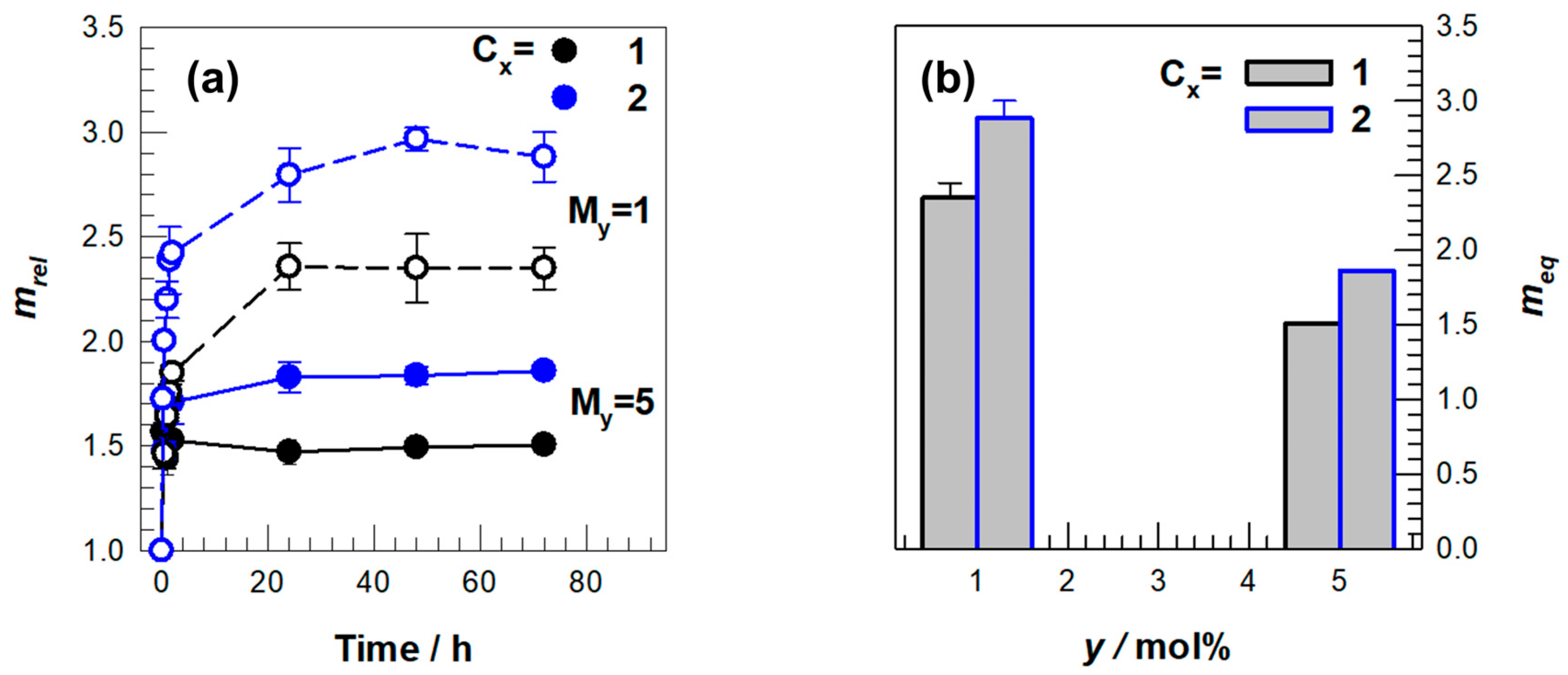
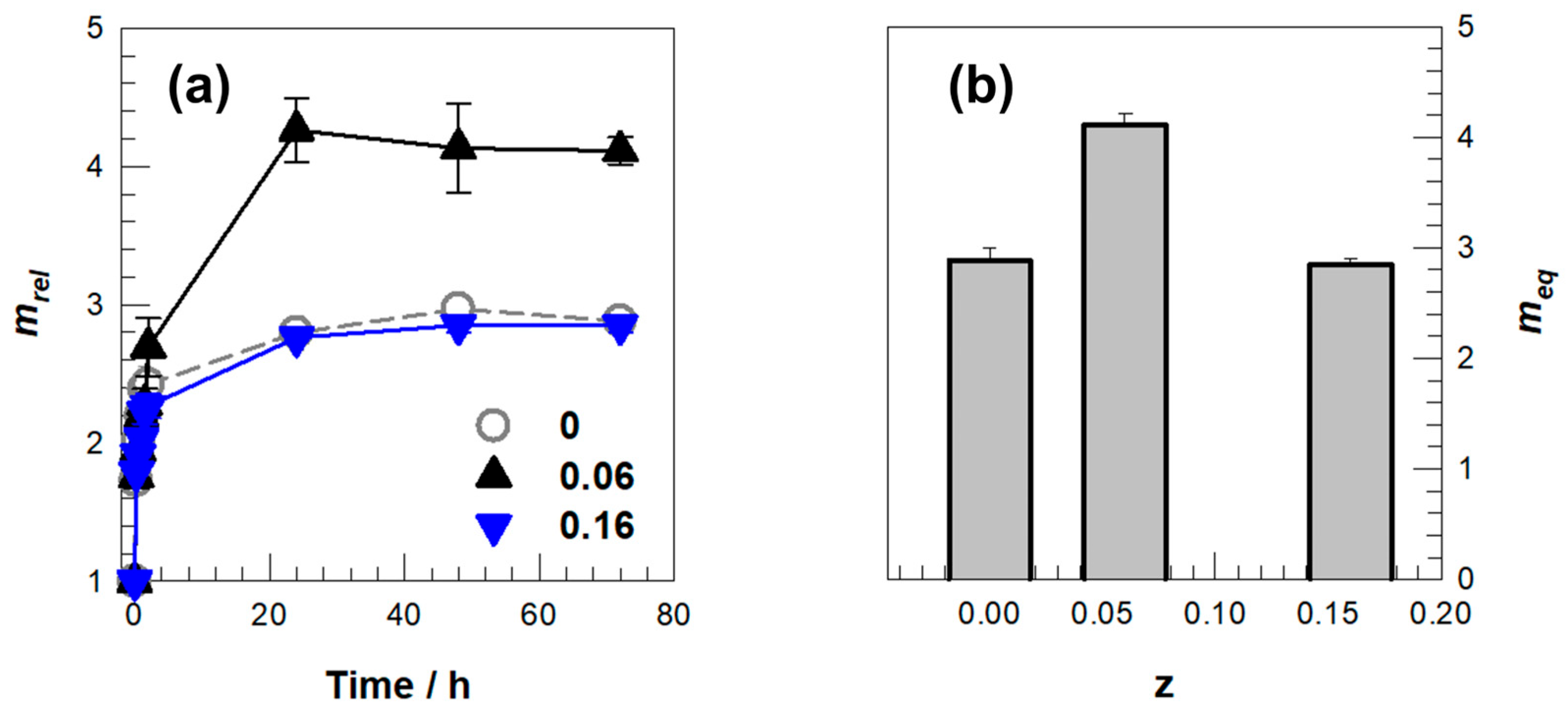
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of AMPS–APTAC–DMAAm Hydrogels
4.3. Swelling Tests
4.4. Mechanical Tests
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gundogan, N.; Okay, O.; Oppermann, W. Swelling, Elasticity and Spatial Inhomogeneity of Poly(N,N-dimethylacrylamide) Hydrogels Formed at Various Polymer Concentrations. Macromol. Chem. Phys. 2004, 205, 814–823. [Google Scholar] [CrossRef]
- Papadakis, C.M.; Tsitsilianis, C. Responsive Hydrogels from Associative Block Copolymers: Physical Gelling through Polyion Complexation. Gels 2017, 3, 2–18. [Google Scholar] [CrossRef]
- Qiao, C.D.; Cao, X.L.; Wang, F. Swelling Behavior Study of Physically Cross-Linked Gelatin Hydrogels. Polym. Polym. Compos. 2012, 20, 53–57. [Google Scholar] [CrossRef]
- Altuncu, S.; Duman, F.D.; Gulyuz, U.; Acar, H.Y.; Okay, O.; Avci, D. Structure–Property Relationships of Novel Phosphonate-Functionalized Networks and Gels of Poly(β-Amino Esters). Eur. Polym. J. 2019, 113, 155–164. [Google Scholar] [CrossRef]
- Asayama, S.; Seno, K.; Kawakami, H. Synthesis of Carboxymethyl Poly(1-vinylimidazole) as a Polyampholyte for Biocompatibility. Chem. Lett. 2013, 42, 358–360. [Google Scholar] [CrossRef]
- Haag, S.L.; Bernards, M.T. Enhanced Biocompatibility of Polyampholyte Hydrogels. Langmuir 2020, 36, 3292–3299. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.P. Why Are Double Network Hydrogels So Tough? Soft Matter 2010, 6, 2583–2590. [Google Scholar] [CrossRef]
- King, D.R.; Sun, T.L.; Huang, Y.W.; Kurokawa, T.; Nonoyama, T.; Crosby, A.J.; Gong, J.P. Extremely Tough Composites from Fabric Reinforced Polyampholyte Hydrogels. Mater. Horiz. 2015, 2, 584–591. [Google Scholar] [CrossRef]
- Toleutay, G.; Su, E.; Kudaibergenov, S.; Okay, O. Highly Stretchable and Thermally Healable Polyampholyte Hydrogels via Hydrophobic Modification. Colloid Polym. Sci. 2020, 298, 273–284. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, H.L.; Liu, Y.; Tan, H.M. Preparation and Study on the Volume Phase Transition Properties of Novel Carboxymethyl Chitosan Grafted Polyampholyte Superabsorbent Polymers. J. Taiwan Inst. Chem. Eng. 2016, 59, 569–577. [Google Scholar] [CrossRef]
- Tian, D.T.; Li, S.R.; Liu, X.P.; Wang, J.S.; Liu, C.M. Synthesis and Properties of Konjac Glucomannan-Graft-Poly(Acrylic Acid-Co-Trimethylallyl Ammonium Chloride) as a Novel Polyampholytic Superabsorbent. Adv. Polym. Technol. 2013, 32, E131–E140. [Google Scholar] [CrossRef]
- Barcellona, M.N.; Johnson, N.; Bernards, M.T. Characterizing Drug Release from Nonfouling Polyampholyte Hydrogels. Langmuir 2015, 31, 13402–13409. [Google Scholar] [CrossRef]
- Bingol, B.; Altuncu, S.; Duman, F.D.; Ak, A.; Gulyuz, U.; Acar, H.Y.; Avci, D. One-Step Injectable and Bioreducible Poly(β-Amino Ester) Hydrogels as Controlled Drug Delivery Platforms. ACS Appl. Polym. Mater. 2019, 1, 1724–1734. [Google Scholar] [CrossRef]
- Chen, Y.M.; Sun, P.J. pH-Sensitive Polyampholyte Microgels of Poly(Acrylic Acid-Co-Vinylamine) as Injectable Hydrogel for Controlled Drug Release. Polymers 2019, 11, 285. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; La Flamme, K.; Peppas, N.A. Dynamic Swelling Behavior of pH-Sensitive Anionic Hydrogels Used for Protein Delivery. J. Appl. Polym. Sci. 2003, 89, 1606–1613. [Google Scholar] [CrossRef]
- Batchelor, M.; Paci, E. Helical Polyampholyte Sequences Have Unique Thermodynamic Properties. J. Phys. Chem. B 2018, 122, 11784–11791. [Google Scholar] [CrossRef] [PubMed]
- Blackman, L.D.; Gunatillake, P.A.; Cass, P.; Locock, K.S. An Introduction to Zwitterionic Polymer Behavior and Applications in Solution and at Surfaces. Chem. Soc. Rev. 2019, 48, 757–770. [Google Scholar] [CrossRef]
- Haag, S.L.; Bernards, M.T. Polyampholyte Hydrogels in Biomedical Applications. Gels 2017, 3, 41. [Google Scholar] [CrossRef]
- Qu, Z.Y.; Xu, H.; Gu, H.C. Synthesis and Biomedical Applications of Poly((Meth)Acrylic Acid) Brushes. ACS Appl. Mater. Interfaces 2015, 7, 14537–14551. [Google Scholar] [CrossRef]
- Schroeder, M.E.; Zurick, K.M.; McGrath, D.E.; Bernards, M.T. Multifunctional Polyampholyte Hydrogels with Fouling Resistance and Protein Conjugation Capacity. Biomacromolecules 2013, 14, 3112–3122. [Google Scholar] [CrossRef]
- Bin Ihsan, A.; Sun, T.L.; Kurokawa, T.; Karobi, S.N.; Nakajima, T.; Nonoyama, T.; Gong, J.P. Self-Healing Behaviors of Tough Polyampholyte Hydrogels. Macromolecules 2016, 49, 4245–4252. [Google Scholar] [CrossRef]
- Cui, K.P.; Sun, T.L.; Kurokawa, T.; Nakajima, T.; Nonoyama, T.; Chen, L.; Gong, J.P. Stretching-Induced Ion Complexation in Physical Polyampholyte Hydrogels. Soft Matter 2016, 12, 8833–8840. [Google Scholar] [CrossRef] [PubMed]
- Toleutay, G.; Dauletbekova, M.; Shakhvorostov, A.; Kudaibergenov, S. Quenched Polyampholyte Hydrogels Based on (3-Acrylamidopropyl)Trimethyl Ammonium Chloride and Sodium Salt of 2-Acrylamido-2-Methyl-1-Propanesulfonic Acid. Macromol. Symp. 2018, 385, 1800160. [Google Scholar] [CrossRef]
- Toleutay, G.; Su, E.; Kudaibergenov, S.E. Swelling and Mechanical Properties of Quenched Polyampholyte Hydrogels Based on 2-Acrylamido-2-Methyl-1-Propanesulfonic Acid Sodium Salt (AMPS) and (3-Acrylamidopropyl)Trimethylammonium Chloride (APTAC). Bull. Univ. Karaganda Chem. 2019, 96, 35–43. [Google Scholar] [CrossRef]
- Toleutay, G.; Shakhvorostov, A.; Kabdrakhmanova, S.K.; Kudaibergenov, S.E. Solution Behavior of Quenched or Strongly Charged Polyampholytes in Aqueous-Salt Solutions. Bull. Univ. Karaganda Chem. 2019, 94, 35–44. [Google Scholar] [CrossRef]
- Toleutay, G.; Su, E.; Yelemessova, G. Equimolar Polyampholyte Hydrogel Synthesis Strategies with Adaptable Properties. Polymers 2023, 15, 3131–3146. [Google Scholar] [CrossRef]
- Sekizkardes, B.; Su, E.; Okay, O. Mechanically Strong Superabsorbent Terpolymer Hydrogels Based on AMPS via Hydrogen-Bonding Interactions. ACS Appl. Polym. Mater. 2023, 5, 2043–2050. [Google Scholar] [CrossRef]
- Antipova, C.G.; Krupnin, A.E.; Zakirov, A.R.; Pobezhimov, V.V.; Romanenko, D.A.; Stolyarova, D.Y.; Chvalun, S.N.; Grigoriev, T.E. A Comprehensive Mechanical Testing of Polyacrylamide Hydrogels: The Impact of Crosslink Density. Polymers 2025, 17, 737. [Google Scholar] [CrossRef] [PubMed]
- Su, E.; Yurtsever, M.; Okay, O. A Self-Healing and Highly Stretchable Polyelectrolyte Hydrogel via Cooperative Hydrogen Bonding as a Superabsorbent Polymer. Macromolecules 2019, 52, 3257–3267. [Google Scholar] [CrossRef]
- Dingus, O.F.; Wong, J.Y.; Bryant, S.J. Architecting a Partial-Thickness Cartilage Substitute with Tissue-Like Mechanics by Monomer Selection and Crosslinking in Tough Hydrogel Networks. J. Mater. Chem. B 2025, 13, 5613–5623. [Google Scholar] [CrossRef]
- De Piano, R.; Caccavo, D.; Barba, A.A.; Lamberti, G. Swelling Behavior of Anionic Hydrogels: Experiments and Modeling. Gels 2024, 10, 813. [Google Scholar] [CrossRef]
- Çeper, T.; Mohotti, S.W.; Lange, L.X.; Schacher, F.H. Polyampholyte Hydrogels with pH-Dependent Swelling for Controlled Catch–Release of Model Dyes. Org. Mater. 2024, 6, 1–11. [Google Scholar] [CrossRef]
- Simões, B.; Rebelo, R.C.; Ledesma, S.; Pereira, P.; Moreira, R.; Ferreira, B.C.; Coelho, J.F.J.; Serra, A.C. Development of Polyampholyte Cellulose-Based Hydrogels for Diapers with Improved Biocompatibility. Gels 2025, 11, 282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.; Wei, Q.; Wang, Y.; Lei, M.; Li, M.; Li, D.; Zhang, L.; Wu, Y. Self-Healing Mechanism and Conductivity of the Hydrogel Flexible Sensors: A Review. Gels 2021, 7, 216. [Google Scholar] [CrossRef]
- Roca-Arroyo, A.F.; Gutierrez-Rivera, J.A.; Morton, L.D.; Castilla-Casadiego, D.A. Hydrogel Network Architecture Design Space: Impact on Mechanical and Viscoelastic Properties. Gels 2025, 11, 588. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Parashar, A. Effect of the Degree of Polymerization and Water Content on the Thermal Transport Phenomena in PEGDA Hydrogel: A Molecular-Dynamics-Based Study. Phys. Chem. Chem. Phys. 2023, 25, 18960–18972. [Google Scholar] [CrossRef]
- Yang, P.; Boer, G.; Snow, F.; Williamson, A.; Cheeseman, S.; Samarasinghe, R.M.; Rifai, A.; Priyam, A.; Elnathan, R.; Guijt, R.; et al. Test and Tune: Evaluating, Adjusting and Optimising the Stiffness of Hydrogels to Influence Cell Fate. Chem. Eng. J. 2025, 505. [Google Scholar] [CrossRef]
- Leng, Y.; Britten, C.N.; Tarannum, F.; Foley, K.; Billings, C.; Liu, Y.; Walters, K.B. Stimuli-Responsive Phosphate Hydrogel: A Study on Swelling Behavior, Mechanical Properties, and Application in Expansion Microscopy. ACS Omega 2024, 9, 37687–37701. [Google Scholar] [CrossRef]
- Yelemessova, G.; Gussenov, I.; Ayazbayeva, A.; Shakhvorostov, A.; Orazzhanova, L.; Klivenko, A.; Kudaibergenov, S. Preparation and Characterization of Preformed Polyelectrolyte and Polyampholyte Gel Particles for Plugging of High-Permeability Porous Media. Gels 2024, 10, 562. [Google Scholar] [CrossRef]
- Choi, S.; Pyo, M.; Lee, S.; Jeong, Y.; Nam, Y.; Park, S.; Jang, Y.-A.; Kim, K.; Park, C.H. Engineering Thermo-Responsive Hydrogels with Tailored Mechanics for Biomedical Integration. Polymers 2025, 17, 2424. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Meng, X.; Liu, S.; Poisson, J.; Vana, P.; Zhang, K. Dehydration Regulates Structural Reorganization of Dynamic Hydrogels. Nat. Commun. 2024, 15, 6886. [Google Scholar] [CrossRef] [PubMed]
- Karageorgos, F.F.; Alexiou, M.; Tsoulfas, G.; Alexopoulos, A.H. Hydrogel-Based Vascularized Organ Tissue Engineering: A Systematized Review on Abdominal Organs. Gels 2024, 10, 653. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yu, X.; Cao, Z.; Liu, Y.; Jiang, X.; Gu, X. Synergic Enhancement of Multifunctional Performance in Tough Multi-Network Hydrogels with Multi-Level Dynamic Hydrogen Bonds. Chem. Eng. J. 2024, 489, 151249. [Google Scholar] [CrossRef]
- Petelinšek, N.; Mommer, S. Tough Hydrogels for Load-Bearing Applications. Adv. Sci. 2024, 11, 2307404. [Google Scholar] [CrossRef]
- Ma, C.; Du, C.; Tong, Q.B.; Zhang, X.N.; Du, M.; Zheng, Q.; Wu, Z.L. Tough Supramolecular Hydrogels of Poly(N,N-Dimethylacrylamide)-Grafted Poly(Methacrylic Acid) with Cooperative Hydrogen Bonds as Physical Crosslinks. Soft Matter 2024, 20, 7448–7456. [Google Scholar] [CrossRef]
- Sha, Z.; Chen, X.; Song, H.; Zheng, Y.; Cui, W.; Ran, R. Toughening Hydrogels with Small Molecules: Tiny Matter, Big Impact. Mater. Horiz. 2025, 12, 7244–7276. [Google Scholar] [CrossRef]
- Xi, S.; Zhang, H.J.; Liu, X.; Zhu, X.; Guo, H.; Chang, B.P.; You, X. Effect of Hydrogen Bonding Strength on the Structure and Properties of Hydrogels. Polymer 2025, 287, 126553. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Guo, Y.; Qian, H.; Chen, Y.; Chen, Y.; Wang, J.; Wang, Y.; Martins, M.C.L.; Hu, X.; et al. Non-Swelling Polyelectrolyte Complex Hydrogels with Tissue-Matchable Mechanical Properties for Versatile Wet Wound Closure. Compos. Part B Eng. 2024, 279, 111456. [Google Scholar] [CrossRef]
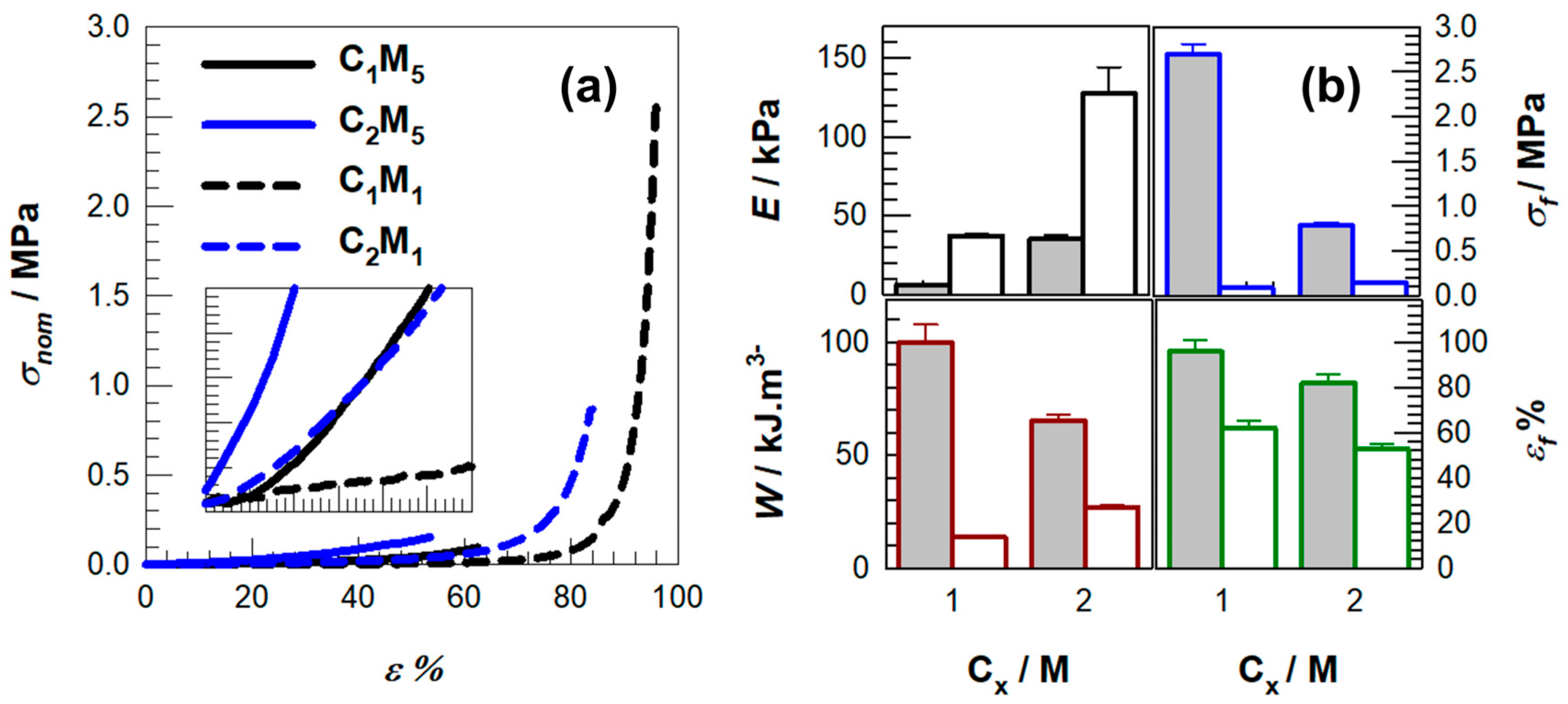
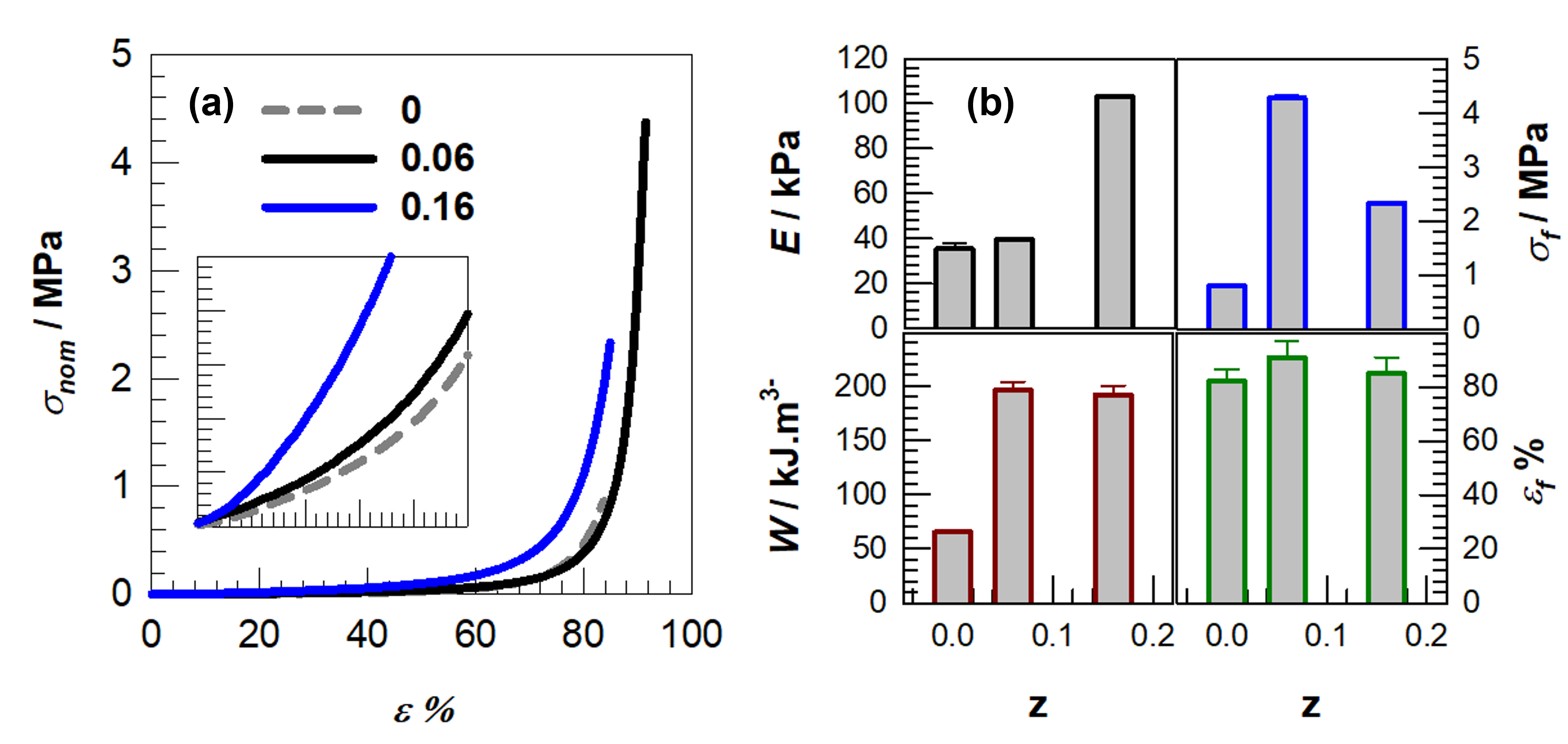
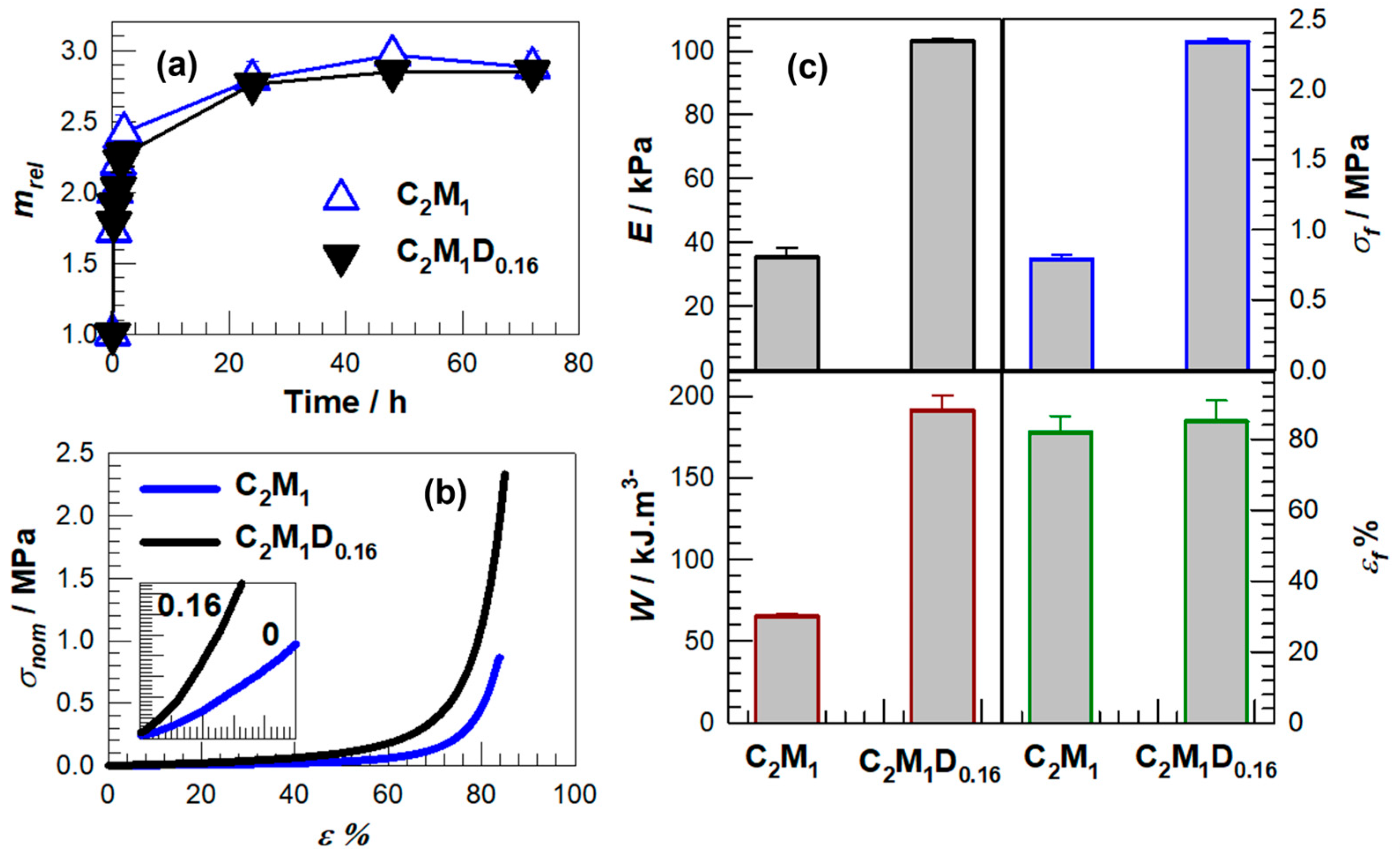
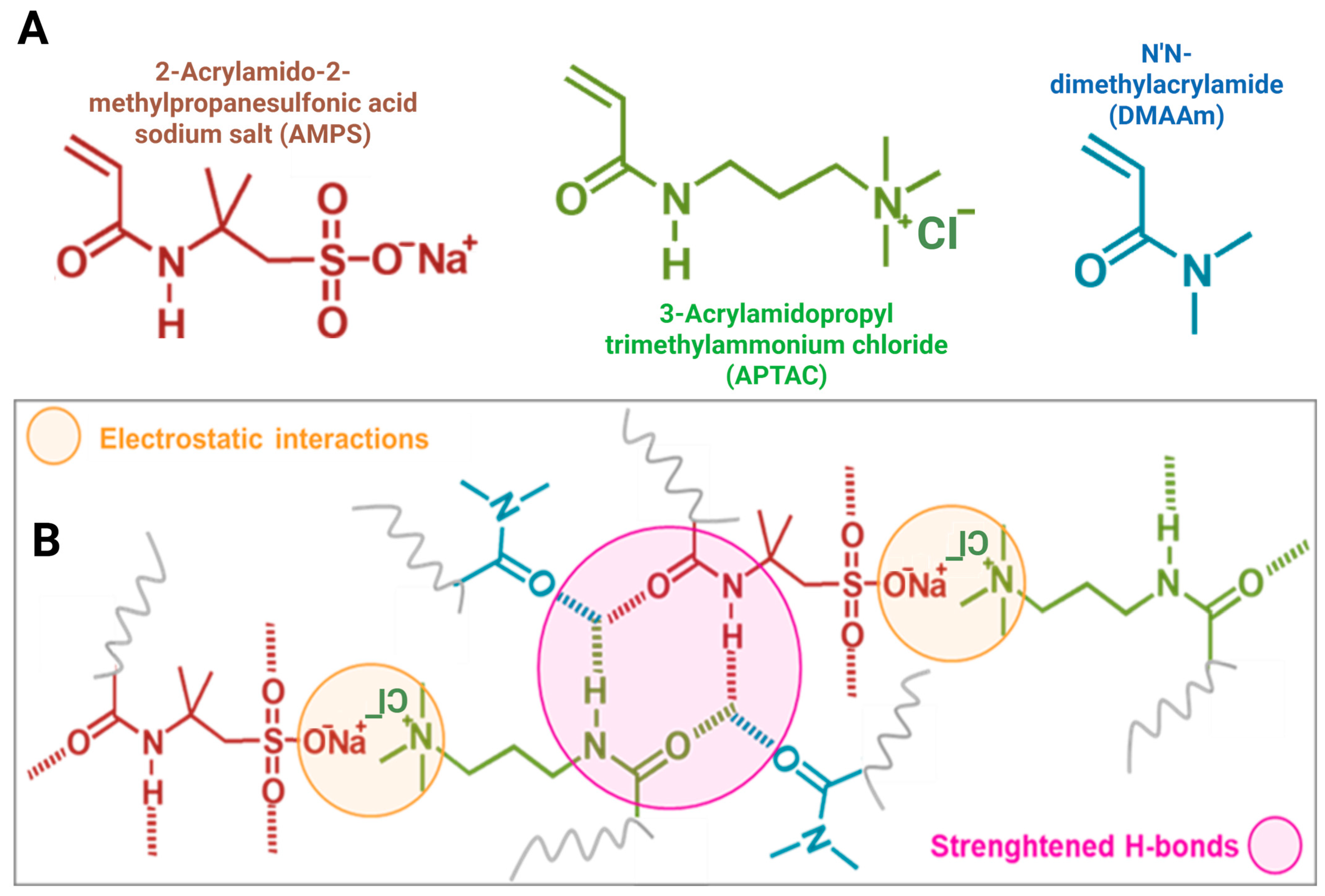
| Samples | E/kPa | σf/kPa | εf% | W/kJ.m−3 |
|---|---|---|---|---|
| C1M5 | 37.3 ± 1.5 | 90.0 ± 9.5 | 62.0 | 13.6 ± 0.6 |
| C1M1 | 6.6 ± 0.4 | 2700.0 ± 110.0 | 96.0 | 100.2 ± 7.7 |
| C2M5 | 127.3 ± 16.7 | 150.0 ± 10.0 | 53.0 | 27.1 ± 0.7 |
| C2M1 | 35.4 ± 2.8 | 790.0 ± 30.0 | 82.0 | 65.6 ± 2.5 |
| C2M1D0.06 | 39.5 ± 1.0 | 4280.0 ± 50.0 | 91.0 | 196.0 ± 8.0 |
| C2M1D0.16 | 103.0 ± 0.8 | 2340.0 ± 20.0 | 85.0 | 191.6 ± 9.0 |
| Effect | Code | Total Ionic Monomers (mol) | DMAAm (mol) | MBAAm (mol) | Cx (M) | Mγ (mol%) |
|---|---|---|---|---|---|---|
| Concentration | C1M5 | 0.0918 | — | 0.0046 | 1.15 | 5 |
| C1M1 | 0.0918 | — | 0.0092 | 1.15 | 1 | |
| C2M5 | 0.1376 | — | 0.0069 | 1.72 | 5 | |
| C2M1 | 0.1376 | — | 0.0014 | 1.72 | 1 | |
| DMAAm | C2M1D0.06 | 0.1376 | 0.0083 | 0.0015 | 1.72 | 1 |
| C2M1D0.16 | 0.1376 | 0.0258 | 0.0016 | 1.72 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mussalimova, M.; Gizatullina, N.; Yelemessova, G.; Taubatyrova, A.; Aitkaliyeva, G.; Shynykul, Z.; Su, E.; Toleutay, G. Unveiling the Compression Mechanical Properties of AMPS–APTAC–DMAAm Terpolymeric Hydrogels. Gels 2025, 11, 941. https://doi.org/10.3390/gels11120941
Mussalimova M, Gizatullina N, Yelemessova G, Taubatyrova A, Aitkaliyeva G, Shynykul Z, Su E, Toleutay G. Unveiling the Compression Mechanical Properties of AMPS–APTAC–DMAAm Terpolymeric Hydrogels. Gels. 2025; 11(12):941. https://doi.org/10.3390/gels11120941
Chicago/Turabian StyleMussalimova, Madina, Nargiz Gizatullina, Gaukhargul Yelemessova, Anel Taubatyrova, Gulzat Aitkaliyeva, Zhanserik Shynykul, Esra Su, and Gaukhar Toleutay. 2025. "Unveiling the Compression Mechanical Properties of AMPS–APTAC–DMAAm Terpolymeric Hydrogels" Gels 11, no. 12: 941. https://doi.org/10.3390/gels11120941
APA StyleMussalimova, M., Gizatullina, N., Yelemessova, G., Taubatyrova, A., Aitkaliyeva, G., Shynykul, Z., Su, E., & Toleutay, G. (2025). Unveiling the Compression Mechanical Properties of AMPS–APTAC–DMAAm Terpolymeric Hydrogels. Gels, 11(12), 941. https://doi.org/10.3390/gels11120941






