Engineering Inhalable Carboxymethyl Chitosan-Swellable Microgels for Pulmonary Delivery of Charged Hydrophilic Molecules †
Abstract
1. Introduction
2. Results and Discussion
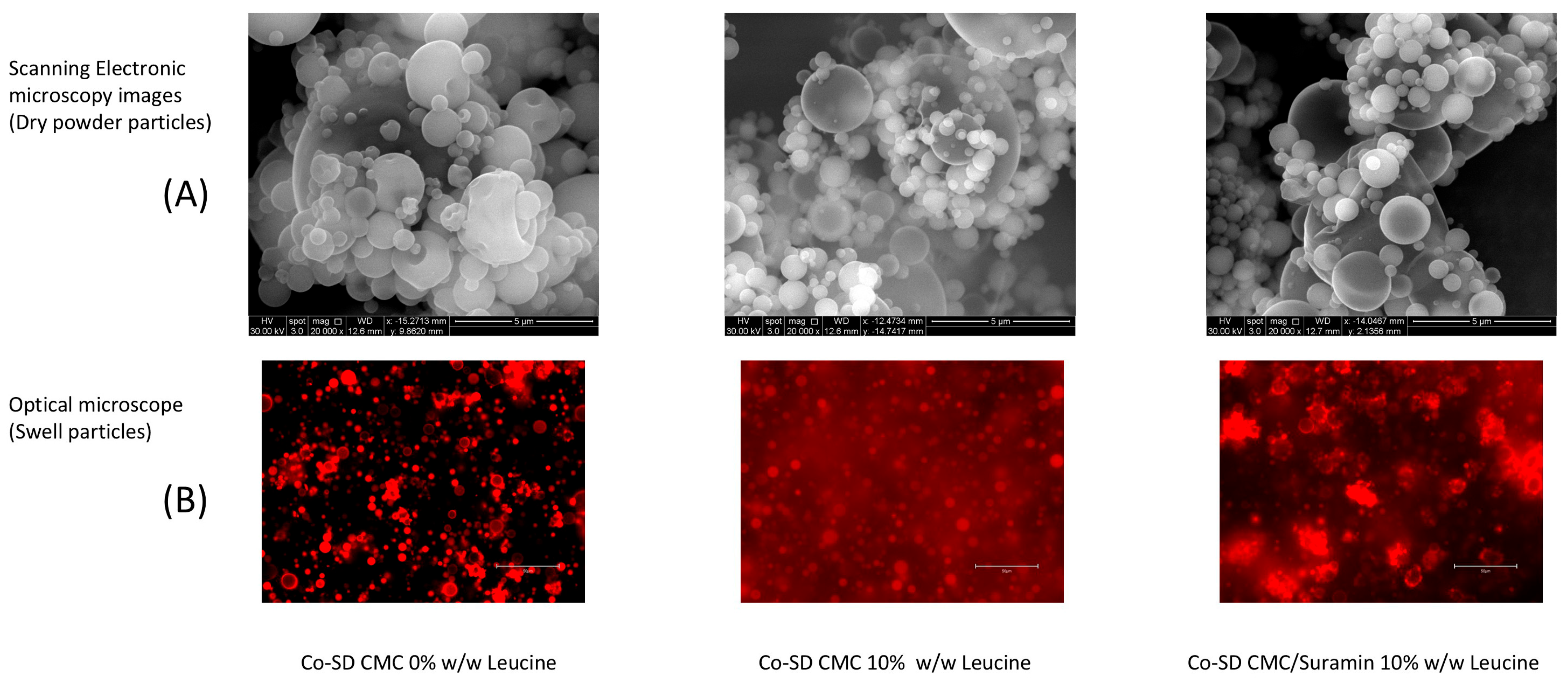
2.1. Morphology and Swelling Behavior of Spray-Dried Microparticles
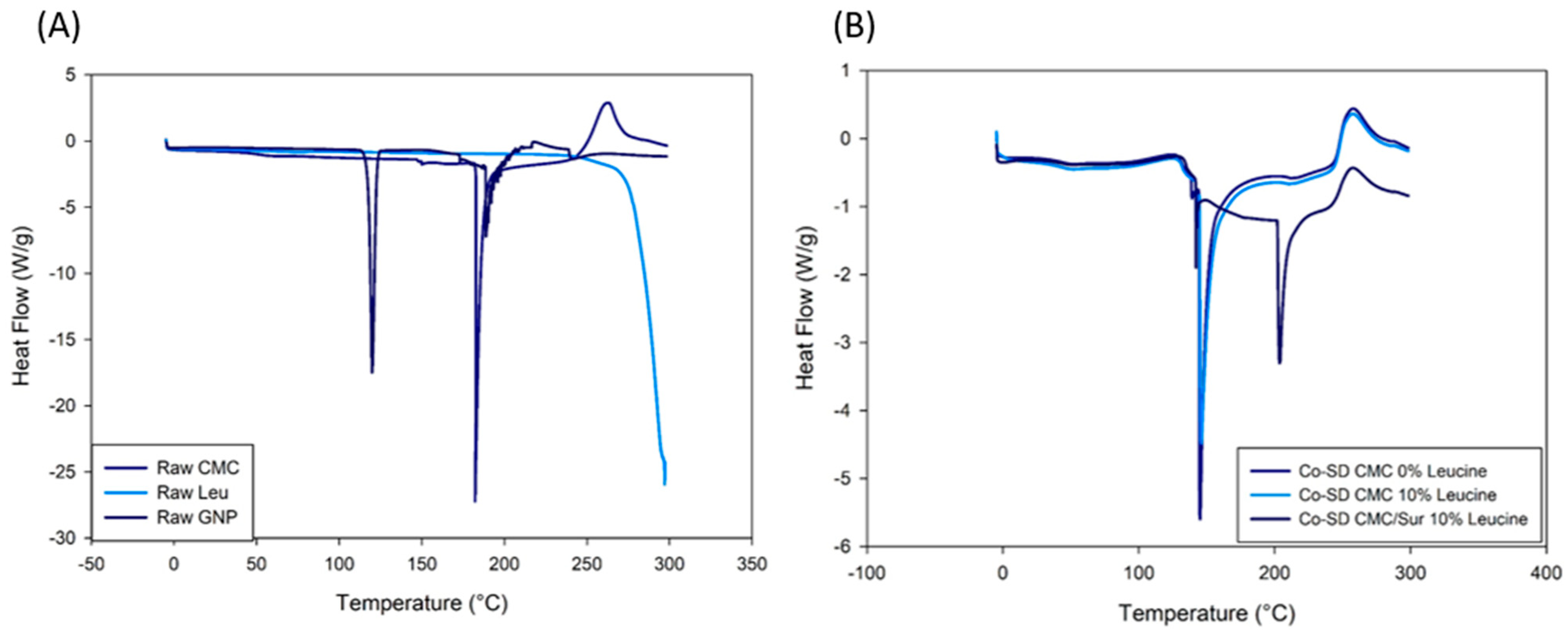
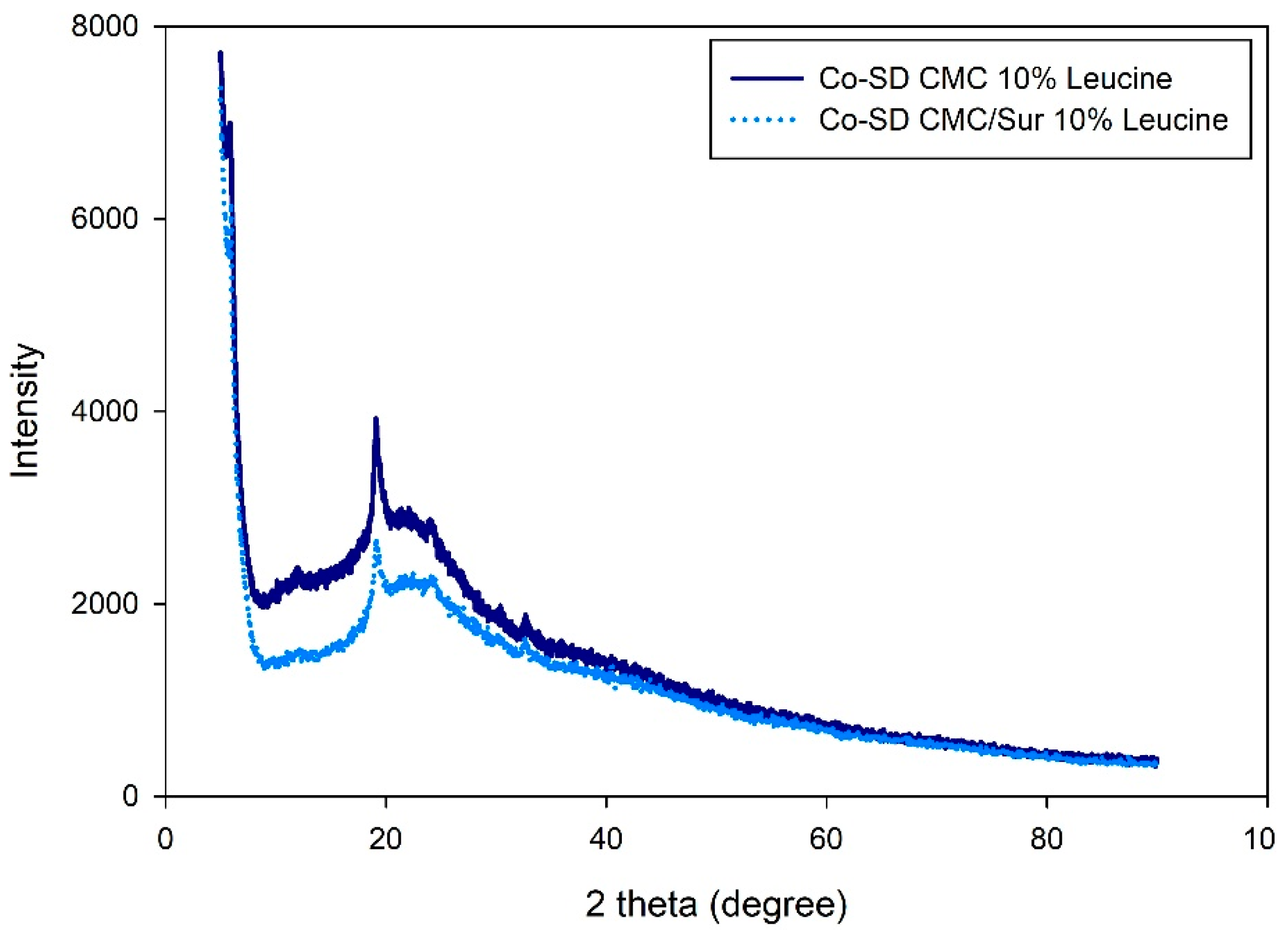
2.2. Thermal and Structural Characterization of Spray-Dried Microparticles
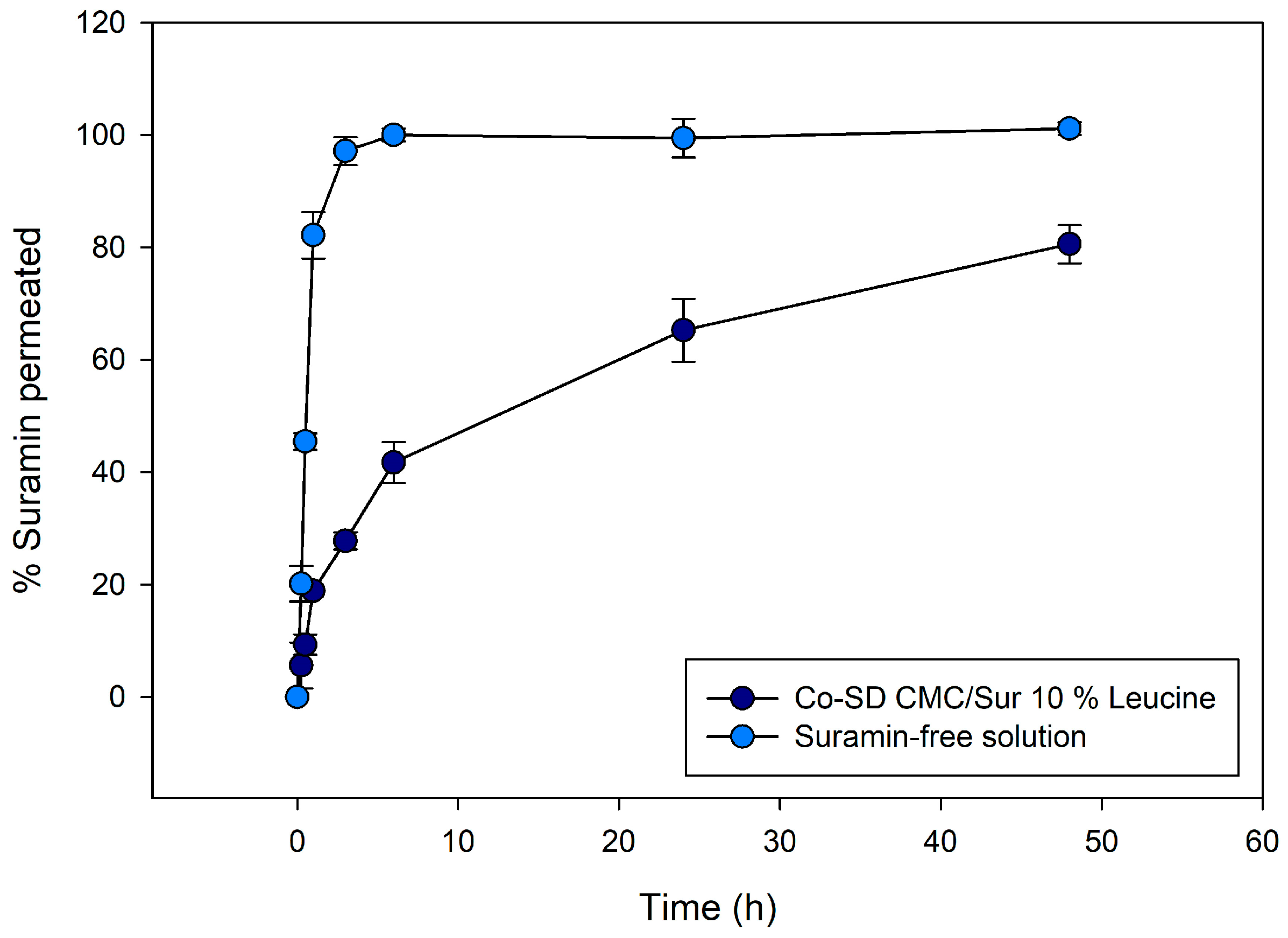
2.3. Controlled Release of Suramin from Leucine-Modified CMC Microparticles
2.4. Aerodynamic Evaluation with Next Generation Impactor (NGI)
2.5. Cytotoxicity Studies
3. Conclusions
4. Materials and Methods
4.1. Preparation of Advanced Spray Drying and Co-Spray Drying in Open Mode Using Organic Solvent
4.2. Water Content in Dry Powder
4.3. Scanning Electron Microscopy (SEM)
4.4. Laser Diffraction Particle Size and Size Distribution
4.5. Structural Characterization via X-Ray Diffraction
4.6. Differential Scanning Calorimetry (DSC)
4.7. Birefringence and Phase Transitions via Hot-Stage Microscope
4.8. Morphology Observation
4.9. In Vitro Drug Diffusion Studies Using the Franz Cell
4.10. In Vitro Aerosol Dispersion Performance
4.11. In Vitro Human Pulmonary Cell Viability
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CMC | Carboxymethyl Chitosan |
| GNP | Genipin |
| SEM | Scanning Electron Microscopy |
| DSC | Differential Scanning Calorimetry |
| XRD | X-ray Diffraction |
| HSM | Hot-Stage Microscopy |
| NGI | Next Generation Impactor |
| MMAD | Mass Median Aerodynamic Diameter |
| FPF | Fine Particle Fraction |
| RF | Respiratory Fraction |
| DPI | Dry Powder Inhaler |
| Dv10, Dv50, Dv90 | Volume-based diameter at 10%, 50%, and 90% cumulative volume |
References
- Mansour, H.M.; Muralidharan, P.; Hayes, D., Jr. Inhaled nanoparticulate systems: Composition, manufacture and aerosol delivery. J. Aerosol Med. Pulm. Drug Deliv. 2024, 37, 202–218. [Google Scholar] [CrossRef]
- Alabsi, W.; Eedara, B.B.; Encinas-Basurto, D.; Polt, R.; Mansour, H.M. Nose-to-brain delivery of therapeutic peptides as nasal aerosols. Pharmaceutics 2022, 14, 1870. [Google Scholar] [CrossRef]
- Chadha, P.; Katare, O.P.; Chhibber, S. Liposome loaded phage cocktail: Enhanced therapeutic potential in resolving Klebsiella pneumoniae mediated burn wound infections. Burns 2017, 43, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Encinas-Basurto, D.; Eedara, B.B.; Mansour, H.M. Biocompatible biodegradable polymeric nanocarriers in dry powder inhalers (DPIs) for pulmonary inhalation delivery. J. Pharm. Investig. 2024, 54, 145–160. [Google Scholar] [CrossRef]
- Hickey, A.J.; Mansour, H.M. Delivery of drugs by the pulmonary route. In Modern Pharmaceutics, Volume 2; CRC Press: Boca Raton, FL, USA, 2016; pp. 209–238. [Google Scholar]
- Lee, W.T.; Lee, H.; Kim, J.; Jung, Y.; Choi, E.; Jeong, J.H.; Jeong, J.-H.; Lee, J.H.; Youn, Y.S. Alveolar macrophage phagocytosis-evading inhaled microgels incorporating nintedanib-PLGA nanoparticles and pirfenidone-liposomes for improved treatment of pulmonary fibrosis. Bioact. Mater. 2024, 33, 262–278. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; El-Sherbiny, I.M.; Smyth, H.D. Swellable ciprofloxacin-loaded nano-in-micro hydrogel particles for local lung drug delivery. AAPS PharmSciTech 2014, 15, 1535–1544. [Google Scholar] [CrossRef]
- Baranov, M.V.; Kumar, M.; Sacanna, S.; Thutupalli, S.; Van den Bogaart, G. Modulation of immune responses by particle size and shape. Front. Immunol. 2021, 11, 607945. [Google Scholar] [CrossRef]
- Ni, R.; Zhao, J.; Liu, Q.; Liang, Z.; Muenster, U.; Mao, S. Nanocrystals embedded in chitosan-based respirable swellable microparticles as dry powder for sustained pulmonary drug delivery. Eur. J. Pharm. Sci. 2017, 99, 137–146. [Google Scholar] [CrossRef]
- Yin, Y.; Li, J. Formation of chitosan-based hydrogels network. In Chitosan-Based Hydrogels. Functions and Applications; Taylor & Francis Group: London, UK, 2012; pp. 183–197. [Google Scholar]
- Mastrangelo, E.; Mazzitelli, S.; Fabbri, J.; Rohayem, J.; Ruokolainen, J.; Nykänen, A.; Milani, M.; Pezzullo, M.; Nastruzzi, C.; Bolognesi, M. Delivery of suramin as an antiviral agent through liposomal systems. ChemMedChem 2014, 9, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Ha, E.-S.; Kim, M.-S. Surface modification strategies for high-dose dry powder inhalers. J. Pharm. Investig. 2021, 51, 635–668. [Google Scholar] [CrossRef]
- Encinas-Basurto, D.; McCombs, K.; Vallorz, E.; Mansour, H. Targeting Deep Lungs with Swellable Nano/Microgels for the Delivery of Suramin. In Proceedings of the Drug Delivery to the Lungs, Edinburgh, Scotland, UK, 8–10 December 2021; pp. 1–4. [Google Scholar]
- Ordoubadi, M.; Gregson, F.K.; Wang, H.; Nicholas, M.; Gracin, S.; Lechuga-Ballesteros, D.; Reid, J.P.; Finlay, W.H.; Vehring, R. On the particle formation of leucine in spray drying of inhalable microparticles. Int. J. Pharm. 2021, 592, 120102. [Google Scholar] [CrossRef]
- Wang, X.; Wan, W.; Lu, J.; Quan, G.; Pan, X.; Liu, P. Effects of L-leucine on the properties of spray-dried swellable microparticles with wrinkled surfaces for inhalation therapy of pulmonary fibrosis. Int. J. Pharm. 2021, 610, 121223. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Patil, C.D.; Arte, K.S.; Zhou, Q.; Qu, L. Particle surface coating for dry powder inhaler formulations. Expert Opin. Drug Deliv. 2025, 22, 711–727. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-H.; Zhang, X.; Tan, Y.-M.; Xie, R.; Wang, W.; Liu, Z.; Pan, D.-W.; Ju, X.-J.; Chu, L.-Y. Controllable Preparation and Performances of Monodisperse Genipin-Cross-Linked Gelatin–Chitosan Composite Embolic Microspheres. Langmuir 2025, 41, 4498–4511. [Google Scholar] [CrossRef]
- Uddin, M.S.; Khand, S.; Dong, C. Effect of crosslinking agents on chitosan hydrogel carriers for drug loading and release for targeted drug delivery. Gels 2024, 10, 421. [Google Scholar] [CrossRef]
- Encinas-Basurto, D.; Ruiz, V.H.; Schnellmann, R.G.; Mansour, H.M. Evaluation of Carboxymethyl Chitosan–Genipin Hydrogels as Reservoir Systems for Suramin Delivery in Epithelial Tissues. Gels 2025, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Okuda, T.; Lu, X.-Y.; Chan, H.-K. Amorphous powders for inhalation drug delivery. Adv. Drug Deliv. Rev. 2016, 100, 102–115. [Google Scholar] [CrossRef]
- Wang, Q.; Shen, Y.; Mi, G.; He, D.; Zhang, Y.; Xiong, Y.; Webster, T.J.; Tu, J.J.B. Fumaryl diketopiperazine based effervescent microparticles to escape macrophage phagocytosis for enhanced treatment of pneumonia via pulmonary delivery. Biomaterials 2020, 228, 119575. [Google Scholar] [CrossRef]
- Geiser, M. Update on macrophage clearance of inhaled micro-and nanoparticles. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 207–217. [Google Scholar] [CrossRef]
- Alhajj, N.; O’Reilly, N.J.; Cathcart, H. Leucine as an excipient in spray dried powder for inhalation. Drug Discov. Today 2021, 26, 2384–2396. [Google Scholar] [CrossRef]
- Xu, Y.; Harinck, L.; Lokras, A.G.; Gerde, P.; Selg, E.; Sjöberg, C.-O.; Franzyk, H.; Thakur, A.; Foged, C. Leucine improves the aerosol performance of dry powder inhaler formulations of siRNA-loaded nanoparticles. Int. J. Pharm. 2022, 621, 121758. [Google Scholar] [CrossRef]
- Partheniadis, I.; Nikolakakis, I. Development and characterization of co-amorphous griseofulvin/L-leucin by modified solvent processing hot-melt extrusion. Int. J. Pharm. 2024, 652, 123824. [Google Scholar] [CrossRef]
- Magramane, S.; Vlahović, K.; Gordon, P.; Kállai-Szabó, N.; Zelkó, R.; Antal, I.; Farkas, D. Inhalation Dosage Forms: A Focus on Dry Powder Inhalers and Their Advancements. Pharmaceuticals 2023, 16, 1658. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Shuai, X.; Unger, F.; Simon, M.; Bi, D.; Kissel, T. The depolymerization of chitosan: Effects on physicochemical and biological properties. Int. J. Pharm. 2004, 281, 45–54. [Google Scholar] [CrossRef]
- Mangal, S.; Nie, H.; Xu, R.; Guo, R.; Cavallaro, A.; Zemlyanov, D.; Zhou, Q.J. Physico-chemical properties, aerosolization and dissolution of co-spray dried azithromycin particles with l-leucine for inhalation. Pharm. Res. 2018, 35, 28. [Google Scholar] [CrossRef]
- Krishna Kumar, N.; Suryanarayanan, R. Crystallization propensity of amorphous pharmaceuticals: Kinetics and thermodynamics. Mol. Pharm. 2022, 19, 472–483. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, G.G.; Law, D.; Grant, D.J.; Schmitt, E.A. Physical stability of amorphous pharmaceuticals: Importance of configurational thermodynamic quantities and molecular mobility. J. Pharm. Sci. 2002, 91, 1863–1872. [Google Scholar] [CrossRef]
- Murdande, S.B.; Pikal, M.J.; Shanker, R.M.; Bogner, R.H. Solubility advantage of amorphous pharmaceuticals: I. A thermodynamic analysis. J. Pharm. Sci. 2010, 99, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Eedara, B.B.; Alabsi, W.; Encinas-Basurto, D.; Polt, R.; Hayes, D., Jr.; Black, S.M.; Mansour, H.M. Chapter: Pulmonary Drug Delivery. In Organelle and Molecular Targeting, 1st ed.; Scheherazade, L., Amiji, M.M., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: London, UK, 2022. [Google Scholar]
- Paudel, A.; Worku, Z.A.; Meeus, J.; Guns, S.; Van den Mooter, G. Manufacturing of solid dispersions of poorly water soluble drugs by spray drying: Formulation and process considerations. Int. J. Pharm. 2013, 453, 253–284. [Google Scholar] [CrossRef] [PubMed]
- Motzwickler-Németh, A.; Körmendi, E.; Farkas, Á.; Csóka, I.; Ambrus, R. Nano-Spray-Drying of Cyclodextrin/Ibuprofen Complexes with Aerosolization-Enhancing Additives for Pulmonary Drug Delivery. Int. J. Mol. Sci. 2025, 26, 4320. [Google Scholar] [CrossRef]
- Ordoubadi, M.; Shepard, K.B.; Wang, H.; Wang, Z.; Pluntze, A.M.; Churchman, J.P.; Vehring, R. On the physical stability of leucine-containing spray-dried powders for respiratory drug delivery. Pharmaceutics 2023, 15, 435. [Google Scholar] [CrossRef]
- Wilson, V.; Lou, X.; Osterling, D.J.; Stolarik, D.F.; Jenkins, G.; Gao, W.; Zhang, G.G.; Taylor, L.S. Relationship between amorphous solid dispersion in vivo absorption and in vitro dissolution: Phase behavior during dissolution, speciation, and membrane mass transport. J. Control. Release 2018, 292, 172–182. [Google Scholar] [CrossRef]
- Sun, D.D.; Lee, P.I. Probing the mechanisms of drug release from amorphous solid dispersions in medium-soluble and medium-insoluble carriers. J. Control. Release 2015, 211, 85–93. [Google Scholar] [CrossRef]
- Liu, Q.; Guan, J.; Qin, L.; Zhang, X.; Mao, S. Physicochemical properties affecting the fate of nanoparticles in pulmonary drug delivery. Drug Discov. Today 2020, 25, 150–159. [Google Scholar] [CrossRef]
- Sou, T.; Kaminskas, L.M.; Nguyen, T.-H.; Carlberg, R.; McIntosh, M.P.; Morton, D.A. The effect of amino acid excipients on morphology and solid-state properties of multi-component spray-dried formulations for pulmonary delivery of biomacromolecules. Eur. J. Pharm. Biopharm. 2013, 83, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Quemé-Peña, M.; Ricci, M.; Juhász, T.; Horváti, K.; Bosze, S.; Biri-Kovács, B.t.; Szeder, B.l.; Zsila, F.; Beke-Somfai, T.s. Old polyanionic drug suramin suppresses detrimental cytotoxicity of the host defense peptide LL-37. ACS Pharmacol. Transl. Sci. 2020, 4, 155–167. [Google Scholar] [CrossRef]
- Abd Elgadir, M.; Uddin, M.S.; Ferdosh, S.; Adam, A.; Chowdhury, A.J.K.; Sarker, M.Z.I. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: A review. J. Food Drug Anal. 2015, 23, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Mikušová, V.; Mikuš, P. Advances in chitosan-based nanoparticles for drug delivery. Int. J. Mol. Sci. 2021, 22, 9652. [Google Scholar] [CrossRef]
- Parmar, P.K.; Rao, S.G.; Bansal, A.K. Co-processing of small molecule excipients with polymers to improve functionality. Expert Opin. Drug Deliv. 2021, 18, 907–928. [Google Scholar] [CrossRef]
- Whitehead, F.A.; Kasapis, S. Modelling the mechanism and kinetics of ascorbic acid diffusion in genipin-crosslinked gelatin and chitosan networks at distinct pH. Food Biosci. 2022, 46, 101579. [Google Scholar] [CrossRef]
- Muhsin, M.D.; George, G.; Beagley, K.; Ferro, V.; Wang, H.; Islam, N. Effects of chemical conjugation of L-leucine to chitosan on dispersibility and controlled release of drug from a nanoparticulate dry powder inhaler formulation. Mol. Pharm. 2016, 13, 1455–1466. [Google Scholar] [CrossRef]
- Santos, C.; Marco, G.; Nagao, L.M.; Castro, E.; Chesworth, T. European regulatory developments for orally inhaled and nasal drug products. AAPS PharmSciTech 2018, 19, 3134–3140. [Google Scholar] [CrossRef]
- Yan, Y.; Wu, Q.; Miao, S.; Ren, P.; Wu, Y.; Shen, Y. A hydrogel microparticle with sustained release properties for pulmonary drug delivery. React. Funct. Polym. 2023, 183, 105489. [Google Scholar] [CrossRef]
- Tamboli, A.R.; Yadav, V.D.; Nadaf, S.J.; Nivekar, V.V.; Salve, K.S.; Tamboli, E.T.; Nalawade, A.M. Exploring the Frontier of Inhalation Therapy: A Review of Dry Powder Inhalers for Precision Management of Inflammatory Lung Diseases. BIO Integr. 2024, 5, 962. [Google Scholar] [CrossRef]
- Mehta, P. Imagine the superiority of dry powder inhalers from carrier engineering. J. Drug Deliv. 2018, 2018, 5635010. [Google Scholar] [CrossRef] [PubMed]
- Encinas-Basurto, D.; Acosta, M.F.; Eedara, B.B.; Fineman, J.R.; Black, S.M.; Mansour, H.M. Design and comprehensive characterization of dry powder inhalation aerosols of simvastatin DPPC/DPPG lung surfactant-mimic nanoparticles/microparticles for pulmonary nanomedicine. RSC Adv. 2024, 14, 29413–29427. [Google Scholar] [CrossRef] [PubMed]
- Acosta, M.F.; Abrahamson, M.D.; Encinas-Basurto, D.; Fineman, J.R.; Black, S.M.; Mansour, H.M. Inhalable nanoparticles/microparticles of an AMPK and Nrf2 activator for targeted pulmonary drug delivery as dry powder inhalers. AAPS J. 2021, 23, 2. [Google Scholar] [CrossRef] [PubMed]


| System Composition (% Leu w/w) | FPF < 5 µm (%) | RF (%) | MMAD (µm) | GSD |
|---|---|---|---|---|
| 0 | 13.5 ± 1.8 a | 37.7 ± 4.9 a | 11.3 ± 1.8 d | 3.0 ± 0.1 |
| 2.5 | 20.4 ± 2.3 b | 55.8 ± 0.9 b | 5.9 ± 0.4 c | 3.1 ± 0.1 |
| 5 | 29.4 ± 0.9 c | 75.2 ± 2.0 c | 4.1 ± 1.1 bc | 3.1 ± 0.7 |
| 10 | 35.2 ± 1.0 d | 85.3 ± 1.3 d | 1.0 ± 0.4 a | 3.4 ± 0.3 |
| 10 with Sur | 34.4 ± 2.79 d | 81.9 ± 0.63 d | 2.07 ± 0.3 ab | 2.85 ± 0.3 |
| Powder Composition | Feed Concentration (% w/v) | Pump Rate (%) | Inlet T (°C) | Outlet T (°C) | Karl Fisher (%) |
|---|---|---|---|---|---|
| Spray Dry CMC w/v 0% Leu | 0.2 | 25 | 125 | 49 | 16.75 ± 0.50 |
| Spray Dry CMC w/v 2.5% Leu | 0.2 | 25 | 125 | 49 | 18.73 ± 0.65 |
| Spray Dry CMC w/v 5% Leu | 0.2 | 25 | 125 | 49 | 19.76 ± 0.96 |
| Spray Dry CMC w/v 10% Leu | 0.2 | 25 | 125 | 49 | 23.63 ± 1.37 |
| Spray Dry CMC/Suramin w/v 10% Leu | 0.2 | 25 | 125 | 49 | 17.77 ± 0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Encinas-Basurto, D.; McCombs, K.; Vallorz, E.L.; Acosta, M.F.; Schnellmann, R.G.; Mansour, H.M. Engineering Inhalable Carboxymethyl Chitosan-Swellable Microgels for Pulmonary Delivery of Charged Hydrophilic Molecules. Gels 2025, 11, 1015. https://doi.org/10.3390/gels11121015
Encinas-Basurto D, McCombs K, Vallorz EL, Acosta MF, Schnellmann RG, Mansour HM. Engineering Inhalable Carboxymethyl Chitosan-Swellable Microgels for Pulmonary Delivery of Charged Hydrophilic Molecules. Gels. 2025; 11(12):1015. https://doi.org/10.3390/gels11121015
Chicago/Turabian StyleEncinas-Basurto, David, Kiley McCombs, Ernest L. Vallorz, Maria F. Acosta, Rick G. Schnellmann, and Heidi M. Mansour. 2025. "Engineering Inhalable Carboxymethyl Chitosan-Swellable Microgels for Pulmonary Delivery of Charged Hydrophilic Molecules" Gels 11, no. 12: 1015. https://doi.org/10.3390/gels11121015
APA StyleEncinas-Basurto, D., McCombs, K., Vallorz, E. L., Acosta, M. F., Schnellmann, R. G., & Mansour, H. M. (2025). Engineering Inhalable Carboxymethyl Chitosan-Swellable Microgels for Pulmonary Delivery of Charged Hydrophilic Molecules. Gels, 11(12), 1015. https://doi.org/10.3390/gels11121015








