Multifunctional Nanomaterial-Integrated Hydrogels for Sustained Drug Delivery: From Synthesis and Characterization to Biomedical Application
Abstract
1. Introduction
1.1. Importance of Controlled/Sustained Drug Delivery
1.2. Hydrogels as Key Platforms for Biomedical Applications
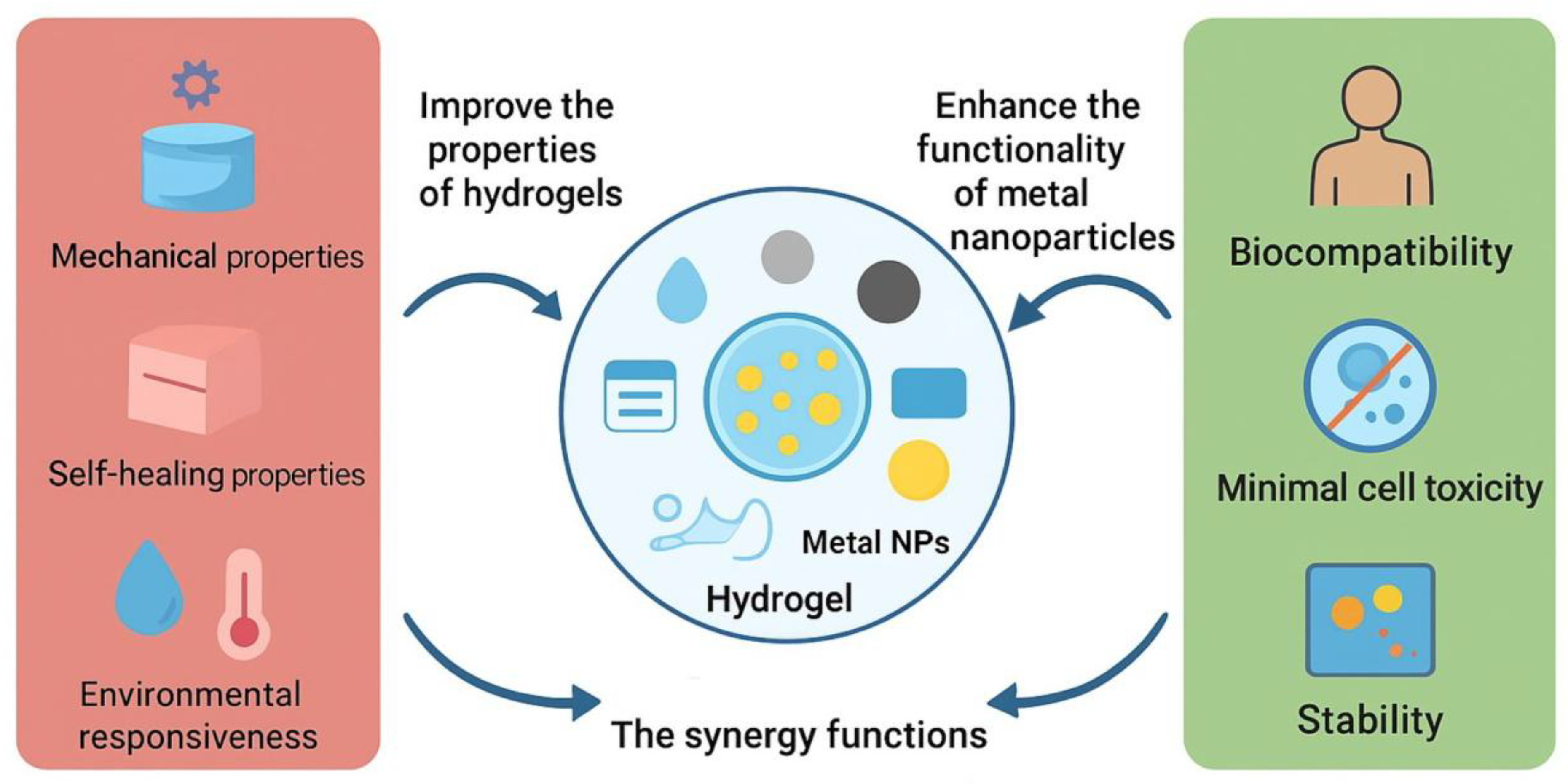
1.3. Nanomaterial–Hydrogel Composites: Overcoming Limitations
1.4. Biomedical Relevance, Research Landscape, and Methodology of this Review
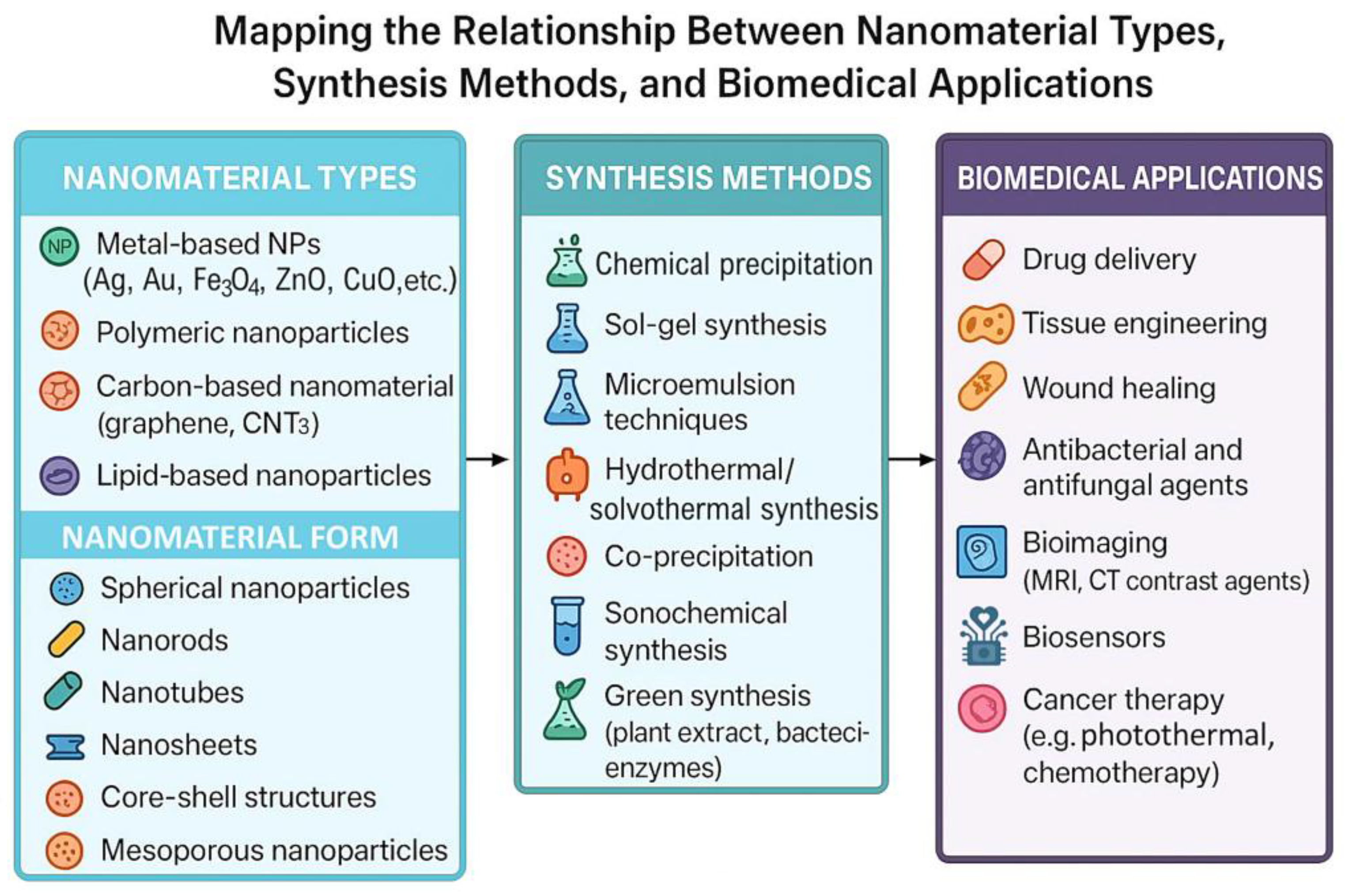
2. Synthesis of Nanomaterials for Hydrogel Integration
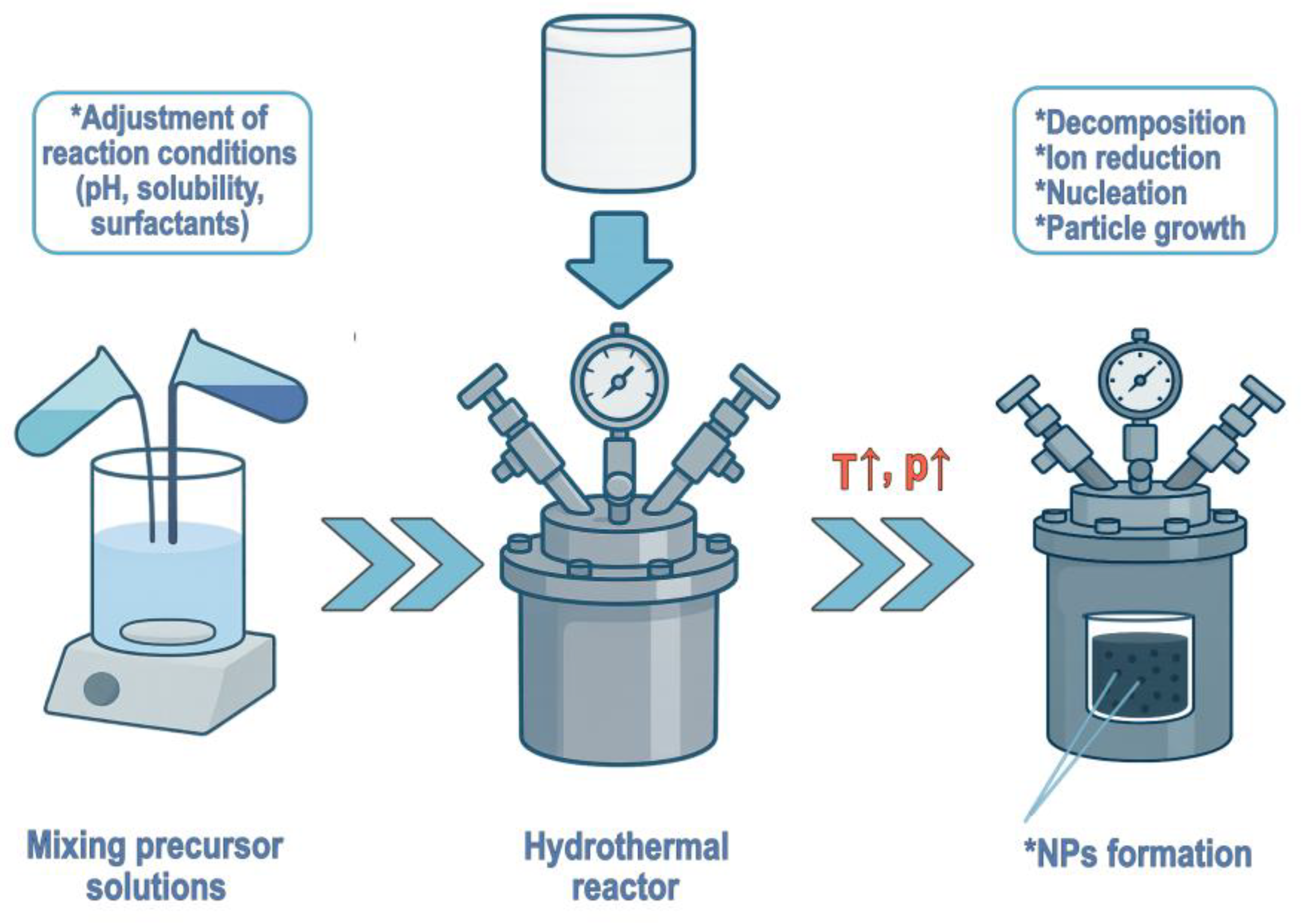
2.1. Nanoparticle Fabrication Techniques
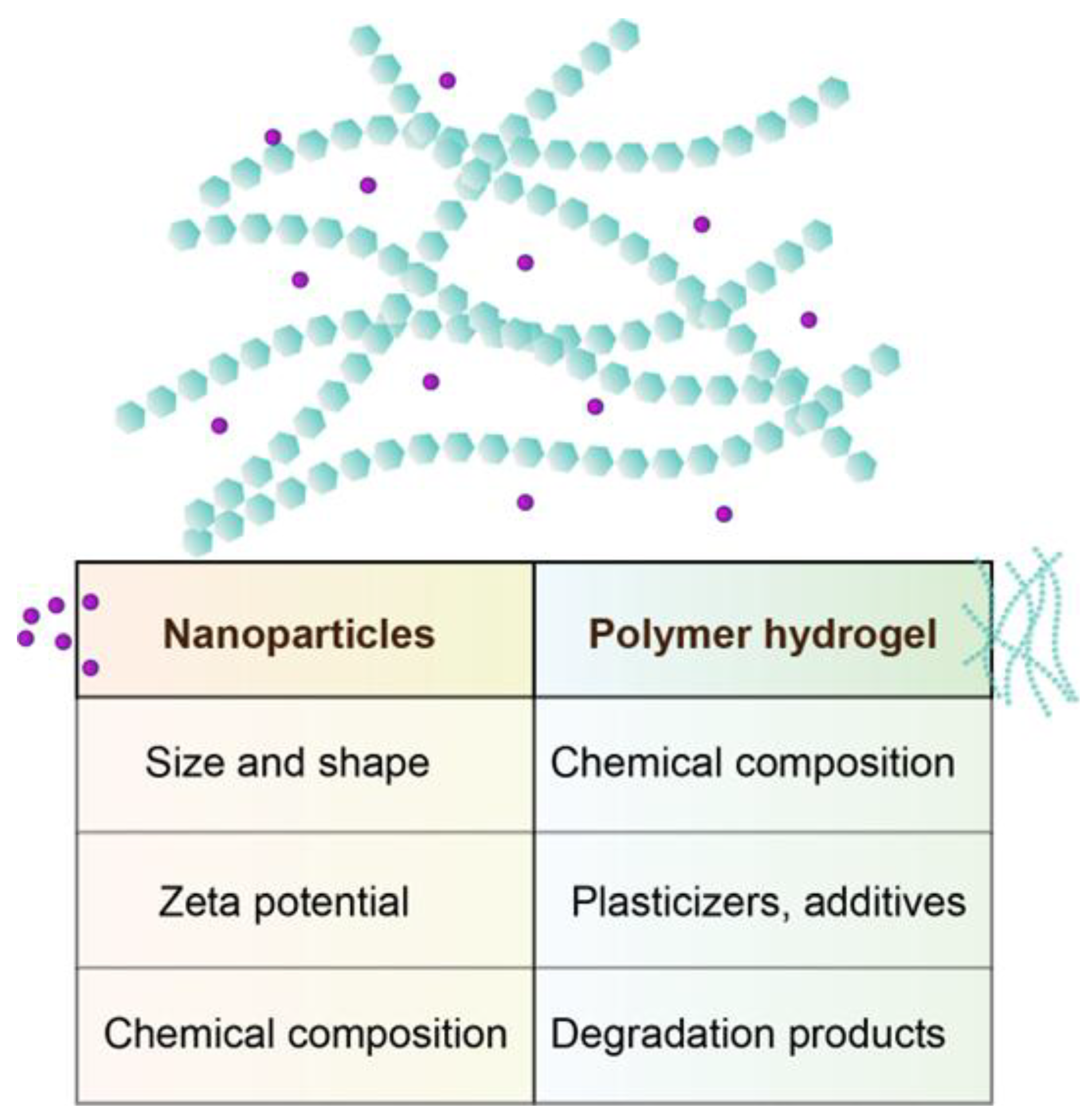
2.2. Functionalization Strategies for Compatibility with Hydrogel Systems
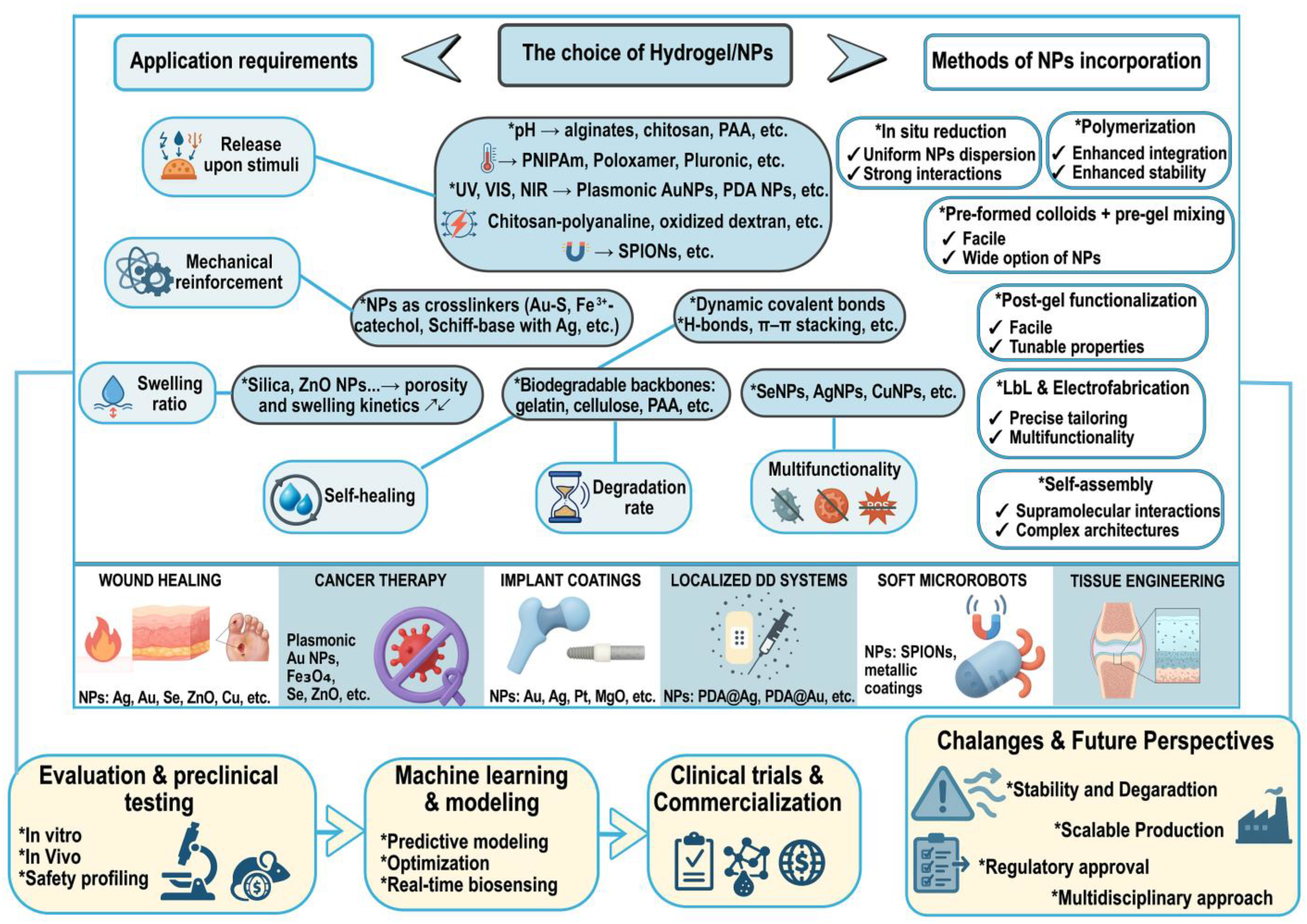
3. Design and Fabrication of Nanocomposite Hydrogels
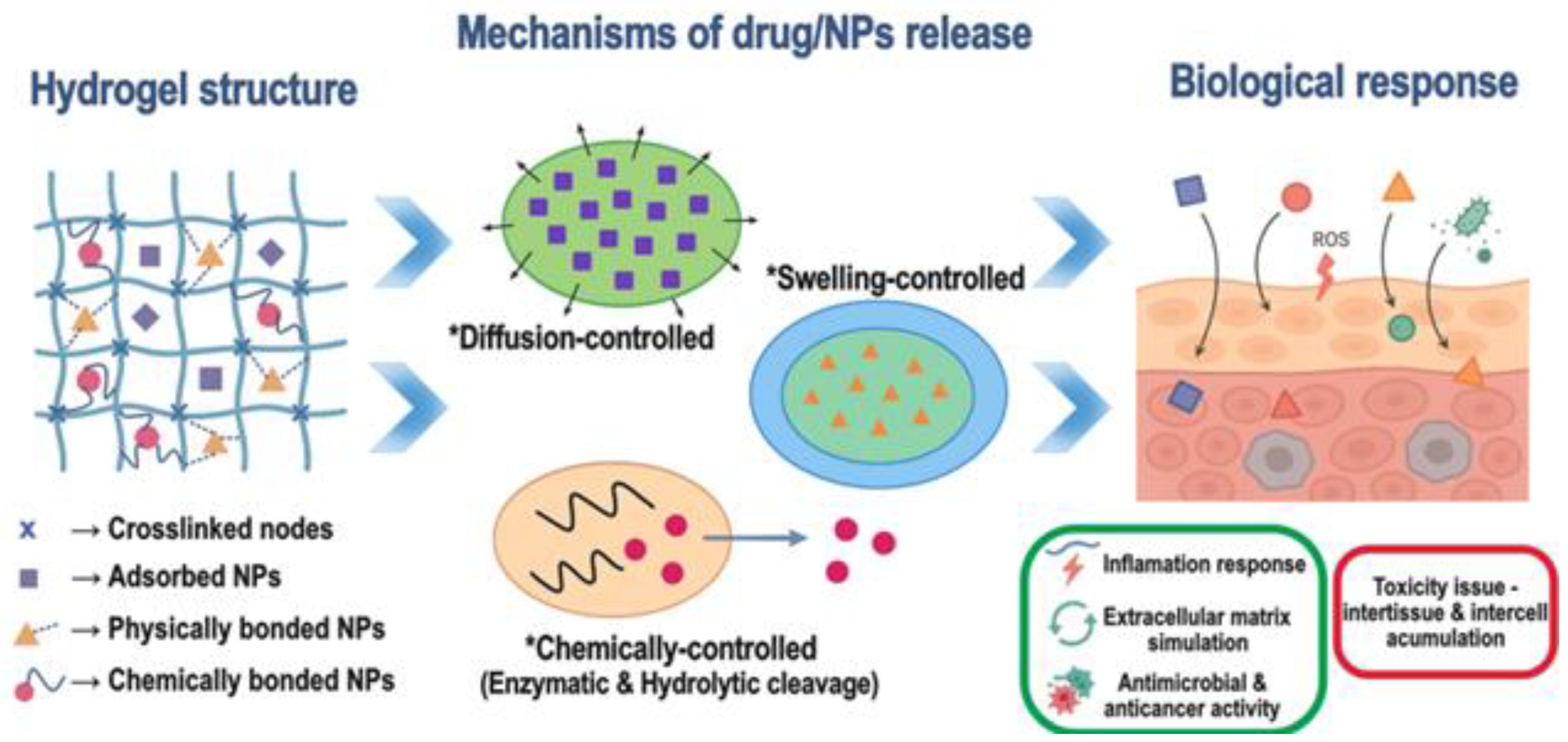
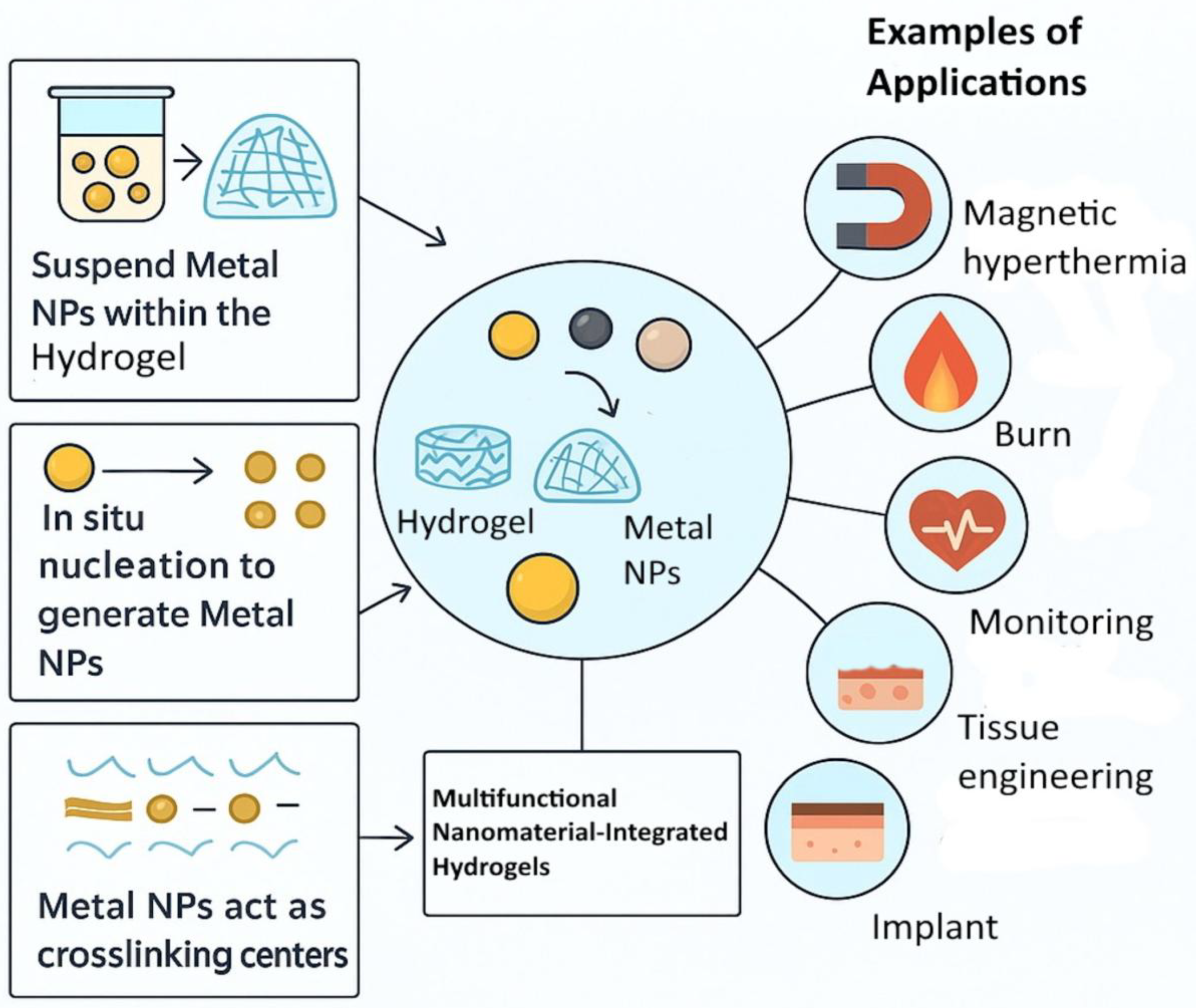
3.1. Incorporation of Metalloid, Metal, or Metalloid/Metal Oxide Nanoparticles
3.2. Tunability of Mechanical, Swelling, and Release Properties
4. Physicochemical Characterization Techniques
4.1. Microscopy Techniques
4.2. Spectroscopy Techniques
4.3. Thermal Methods
4.4. X-Ray-Based Techniques
4.5. Electrochemical Techniques
4.6. Rheology
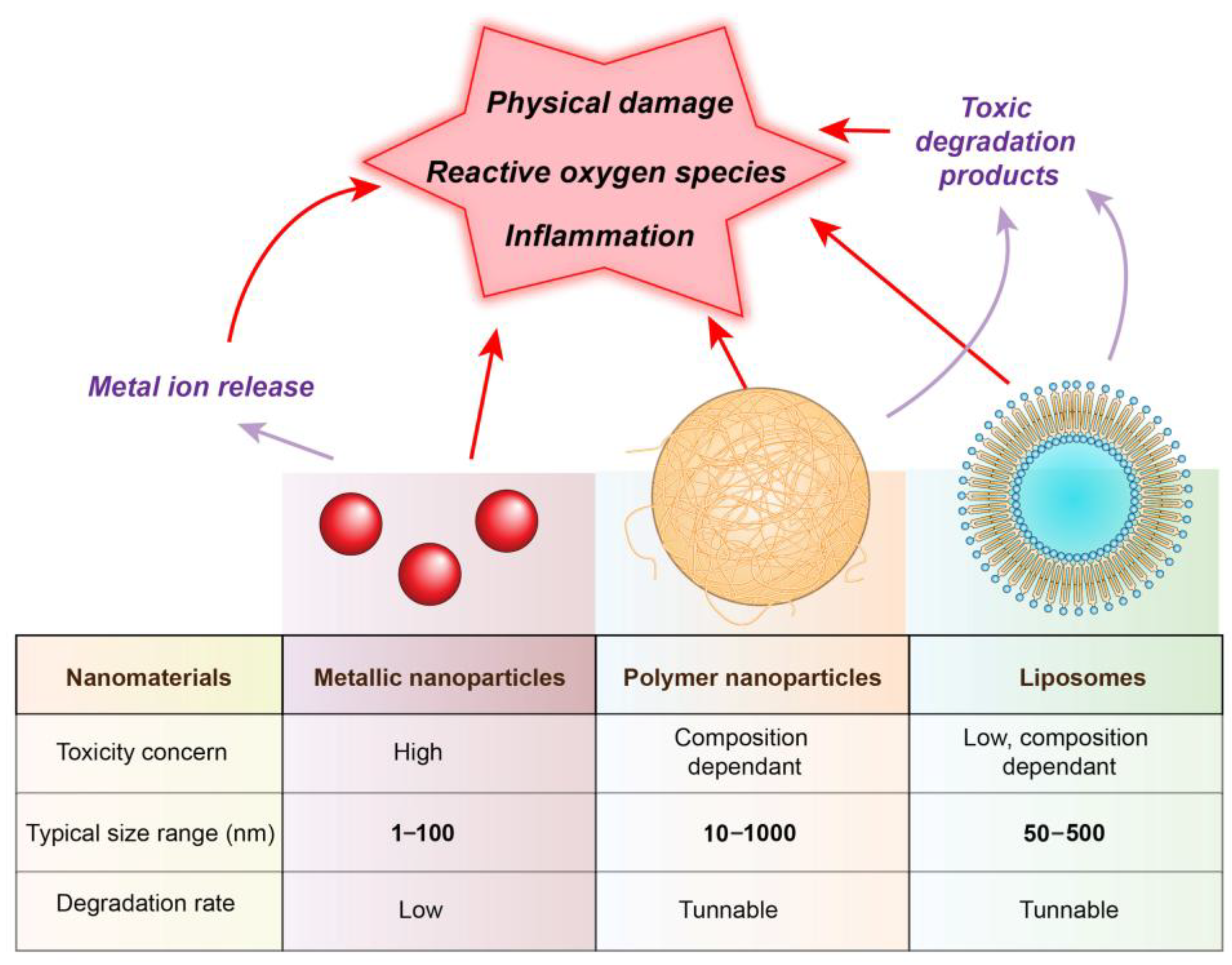
5. Biological Interactions and Safety Assessment
5.1. Cytotoxicity and Genotoxicity Assays
5.2. Inflammation Response
5.3. Antioxidative and Anti-Inflammatory Capacity
5.4. Antibacterial Activity
6. Smart and Stimuli-Responsive Hydrogels
6.1. Hydrogels with Nanomaterials for Electrophysiology and Electrical Stimulation (EP/ES)
6.1.1. Material Platforms
Conducting-Polymer Hydrogels
MXene–Polymer Networks
Graphene/CNT Organohydrogels
Ionic & Adhesive Gels
4D-Printed Hydrogels
6.1.2. Recording Performance & Integration
6.2. Electrical Stimulation with Hydrogels
6.3. Failure Modes and Mitigation Strategies for Nanomaterial-Doped Hydrogel Electrodes in Electrophysiology and Stimulation
6.4. Translational Roadmap for Hydrogel EP/ES
7. Data-Driven Design and Optimisation
7.1. The Role of Data Engineering
7.2. Machine Learning (ML) in Hydrogel Modelling
7.3. Role of Artificial Intelligence (AI) in Personalised Medicine and Hydrogel Design
8. Challenges and Future Perspectives
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sahu, B.; Maity, S.; Jain, A.; Banerjee, S. Next-Generation Stimuli-Responsive Polymers for a Sustainable Tomorrow. Chem. Commun. 2025, 61, 12265–12282. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Stevanović, M.; Filipović, N. A Review of Recent Developments in Biopolymer Nano-Based Drug Delivery Systems with Antioxidative Properties: Insights into the Last Five Years. Pharmaceutics 2024, 16, 670. [Google Scholar] [CrossRef]
- Stevanović, M. Polymeric Micro-and Nanoparticles for Controlled and Targeted Drug Delivery. In Nanostructures for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2017; pp. 355–378. [Google Scholar]
- Panigrahi, S.K.; Das, S.; Majumdar, S. Unveiling the Potentials of Hydrophilic and Hydrophobic Polymers in Microparticle Systems: Opportunities and Challenges in Processing Techniques. Adv. Colloid Interface Sci. 2024, 326, 103121. [Google Scholar] [CrossRef] [PubMed]
- Filipović, N.; Veselinović, L.; Ražić, S.; Jeremić, S.; Filipič, M.; Žegura, B.; Tomić, S.; Čolić, M.; Stevanović, M. Poly (ε-Caprolactone) Microspheres for Prolonged Release of Selenium Nanoparticles. Mater. Sci. Eng. C 2019, 96, 776–789. [Google Scholar] [CrossRef] [PubMed]
- Păduraru, L.; Panainte, A.-D.; Peptu, C.-A.; Apostu, M.; Vieriu, M.; Bibire, T.; Sava, A.; Bibire, N. Smart Drug Delivery Systems Based on Cyclodextrins and Chitosan for Cancer Therapy. Pharmaceuticals 2025, 18, 564. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.; Bao, Q.; Burgess, D. Long-Acting PLGA Microspheres: Advances in Excipient and Product Analysis toward Improved Product Understanding. Adv. Drug Deliv. Rev. 2023, 198, 114857. [Google Scholar] [CrossRef]
- Li, W.; Tang, J.; Lee, D.; Tice, T.R.; Schwendeman, S.P.; Prausnitz, M.R. Clinical Translation of Long-Acting Drug Delivery Formulations. Nat. Rev. Mater. 2022, 7, 406–420. [Google Scholar] [CrossRef]
- Khattak, S.; Ullah, I.; Yousaf, M.T.; Ullah, S.; Yousaf, H.; Li, Y.; Jin, H.; Shen, J.; Xu, H.-T. Advancements in Hydrogels: A Comprehensive Review of Natural, Synthetic, and Hybrid Innovations for Wound Healing. Int. J. Biol. Macromol. 2025, 327, 147270. [Google Scholar] [CrossRef]
- Revete, A.; Aparicio, A.; Cisterna, B.A.; Revete, J.; Luis, L.; Ibarra, E.; Segura González, E.A.; Molino, J.; Reginensi, D. Advancements in the Use of Hydrogels for Regenerative Medicine: Properties and Biomedical Applications. Int. J. Biomater. 2022, 2022, 3606765. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, X.; Wu, Y.; Gao, J. Locally Injectable Hydrogels for Tumor Immunotherapy. Gels 2021, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Alberts, A.; Moldoveanu, E.-T.; Niculescu, A.-G.; Grumezescu, A.M. Hydrogels for Wound Dressings: Applications in Burn Treatment and Chronic Wound Care. J. Compos. Sci. 2025, 9, 133. [Google Scholar] [CrossRef]
- Farasati Far, B.; Safaei, M.; Nahavandi, R.; Gholami, A.; Naimi-Jamal, M.R.; Tamang, S.; Ahn, J.E.; Ramezani Farani, M.; Huh, Y.S. Hydrogel Encapsulation Techniques and Its Clinical Applications in Drug Delivery and Regenerative Medicine: A Systematic Review. ACS Omega 2024, 9, 29139–29158. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, A.; Haag, R.; Schedler, U. Hydrogels and Their Role in Biosensing Applications. Adv. Healthc. Mater. 2021, 10, 2100062. [Google Scholar] [CrossRef]
- Aghajani, M.; Garshasbi, H.R.; Naghib, S.M.; Mozafari, M.R. 3D Printing of Hydrogel Polysaccharides for Biomedical Applications: A Review. Biomedicines 2025, 13, 731. [Google Scholar] [CrossRef]
- Liu, B.; Chen, K. Advances in Hydrogel-Based Drug Delivery Systems. Gels 2024, 10, 262. [Google Scholar] [CrossRef]
- Mostafavi, A.; Quint, J.; Russell, C.; Tamayol, A. Nanocomposite Hydrogels for Tissue Engineering Applications. In Biomaterials for Organ and Tissue Regeneration; Elsevier: Amsterdam, The Netherlands, 2020; pp. 499–528. [Google Scholar]
- Stevanović, M.; Lukić, M.J.; Stanković, A.; Filipović, N.; Kuzmanović, M.; Janićijević, Ž. Biomedical Inorganic Nanoparticles: Preparation, Properties, and Perspectives. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–46. [Google Scholar]
- Ewii, U.E.; Attama, A.A.; Olorunsola, E.O.; Onugwu, A.L.; Nwakpa, F.U.; Anyiam, C.; Chijioke, C.; Ogbulie, T. Nanoparticles for Drug Delivery: Insight into in Vitro and in Vivo Drug Release from Nanomedicines. Nano TransMed 2025, 4, 100083. [Google Scholar] [CrossRef]
- Jahanbekam, S.; Asare-Addo, K.; Alipour, S.; Nokhodchi, A. Smart Hydrogels and the Promise of Multi-Responsive in-Situ Systems. J. Drug Deliv. Sci. Technol. 2025, 107, 106758. [Google Scholar] [CrossRef]
- Vannaladsaysy, V.; Choudhury, S.; Datta, S.; Chatterjee, K. NIR-Responsive Shape Memory Composite Nanofibers as Deployable Matrices for Biomedical Applications. Smart Mater. Struct. 2025, 34, 055004. [Google Scholar] [CrossRef]
- Pei, Y.; Wang, Y.; Chen, J.; Zhou, J.; Han, Y.; Liu, X.; Chen, S.; Chen, S.; He, D.; Wu, Y.; et al. Bionic Nanostructures Create Mechanical Signals to Mediate the Composite Structural Bone Regeneration Through Multi--System Regulation. Adv. Sci. 2025, 12, e02299. [Google Scholar] [CrossRef]
- Kurul, F.; Turkmen, H.; Cetin, A.E.; Topkaya, S.N. Nanomedicine: How Nanomaterials Are Transforming Drug Delivery, Bio-Imaging, and Diagnosis. Next Nanotechnol. 2025, 7, 100129. [Google Scholar] [CrossRef]
- Delgado-Pujol, E.J.; Martínez, G.; Casado-Jurado, D.; Vázquez, J.; León-Barberena, J.; Rodríguez-Lucena, D.; Torres, Y.; Alcudia, A.; Begines, B. Hydrogels and Nanogels: Pioneering the Future of Advanced Drug Delivery Systems. Pharmaceutics 2025, 17, 215. [Google Scholar] [CrossRef] [PubMed]
- Minh Hoang, C.N.; Nguyen, S.H.; Tran, M.T. Nanoparticles in Cancer Therapy: Strategies to Penetrate and Modulate the Tumor Microenvironment–A Review. Smart Mater. Med. 2025, 6, 270–284. [Google Scholar] [CrossRef]
- Pangli, H.; Vatanpour, S.; Hortamani, S.; Jalili, R.; Ghahary, A. Incorporation of Silver Nanoparticles in Hydrogel Matrices for Controlling Wound Infection. J. Burn Care Res. 2021, 42, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Z.; Zhao, C.; Xu, C.; Shin, A.; Wu, J.; Li, D.; Lin, K.; Liu, J. A Sustained-Release PDGF-BB Nanocomposite Hydrogel for DM-Associated Bone Regeneration. J. Mater. Chem. B 2023, 11, 974–984. [Google Scholar] [CrossRef]
- Shi, Z.; Zhong, Q.; Chen, Y.; Gao, J.; Pan, X.; Lian, Q.; Chen, R.; Wang, P.; Wang, J.; Shi, Z.; et al. Nanohydroxyapatite, Nanosilicate-Reinforced Injectable, and Biomimetic Gelatin-Methacryloyl Hydrogel for Bone Tissue Engineering. Int. J. Nanomed. 2021, 16, 5603–5619. [Google Scholar] [CrossRef]
- Sheng, R.; Chen, J.; Wang, H.; Luo, Y.; Liu, J.; Chen, Z.; Mo, Q.; Chi, J.; Ling, C.; Tan, X.; et al. Nanosilicate--Reinforced Silk Fibroin Hydrogel for Endogenous Regeneration of Both Cartilage and Subchondral Bone. Adv. Healthc. Mater. 2022, 11, 2200602. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Xu, J.; Tai, C.-H.; Wen, T.-K.; Hsu, S. Biodegradable, Electroconductive Self-Healing Hydrogel Based on Polydopamine-Coated Polyurethane Nano-Crosslinker for Parkinson’s Disease Therapy. Biomaterials 2025, 320, 123268. [Google Scholar] [CrossRef]
- Sheikh-Oleslami, S.; Tao, B.; D’Souza, J.; Butt, F.; Suntharalingam, H.; Rempel, L.; Amiri, N. A Review of Metal Nanoparticles Embedded in Hydrogel Scaffolds for Wound Healing In Vivo. Gels 2023, 9, 591. [Google Scholar] [CrossRef]
- Stevanović, M.M.; Filipović, N.; Kuzmanović, M.; Tomić, N.; Ušjak, D.; Milenković, M.; Zheng, K.; Stampfl, J.; Boccaccini, A.R. Synthesis and Characterization of a Collagen-Based Composite Material Containing Selenium Nanoparticles. J. Biomater. Appl. 2022, 36, 1800–1811. [Google Scholar] [CrossRef]
- Li, Y.; Pan, Y.; Kong, L.; Long, H.; Teng, J.; Zhen, H.; Ding, Q.; Pan, R.; Tian, X. Black Phosphorus Nanosheet Hydrogels Elicit a Thermogenic Effect and Enhance Diabetic Wound Healing through Controlled Drug Release. Mater. Des. 2025, 254, 113967. [Google Scholar] [CrossRef]
- Kuan, C.-H.; Chang, L.; Ho, C.-Y.; Tsai, C.-H.; Liu, Y.-C.; Huang, W.-Y.; Wang, Y.-N.; Wang, W.-H.; Wang, T.-W. Immunomodulatory Hydrogel Orchestrates Pro-Regenerative Response of Macrophages and Angiogenesis for Chronic Wound Healing. Biomaterials 2025, 314, 122848. [Google Scholar] [CrossRef]
- Stevanović, M. Assembly of Polymers/Metal Nanoparticles and Their Applications as Medical Devices. In Advanced Biomaterials and Biodevices; Tiwari, A., Nordin, A.N., Eds.; Wiley: Hoboken, NJ, USA, 2014; pp. 343–366. ISBN 9781118773635. [Google Scholar]
- Stevanović, M. Biomedical Applications of Nanostructured Polymeric Materials. In Nanostructured Polymer Composites for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–19. [Google Scholar]
- Yukawa, H.; Sato, K.; Baba, Y. Theranostics Applications of Quantum Dots in Regenerative Medicine, Cancer Medicine, and Infectious Diseases. Adv. Drug Deliv. Rev. 2023, 200, 114863. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Newton, M.A.A.; Cheng, H.; Zhang, Q.; Gao, W.; Zheng, Y.; Lu, Z.; Dai, Z.; Zhu, J. Progress of Hydrogel Dressings with Wound Monitoring and Treatment Functions. Gels 2023, 9, 694. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Tang, Q.; Li, M.; Yang, Q.; Zhang, Y.; Lei, L.; Li, S. Manganese-Derived Biomaterials for Tumor Diagnosis and Therapy. J. Nanobiotechnol. 2024, 22, 335. [Google Scholar] [CrossRef] [PubMed]
- Loukelis, K.; Helal, Z.A.; Mikos, A.G.; Chatzinikolaidou, M. Nanocomposite Bioprinting for Tissue Engineering Applications. Gels 2023, 9, 103. [Google Scholar] [CrossRef]
- Yoon, M.S.; Lee, J.M.; Jo, M.J.; Kang, S.J.; Yoo, M.K.; Park, S.Y.; Bong, S.; Park, C.-S.; Park, C.-W.; Kim, J.-S.; et al. Dual-Drug Delivery Systems Using Hydrogel–Nanoparticle Composites: Recent Advances and Key Applications. Gels 2025, 11, 520. [Google Scholar] [CrossRef]
- Duman, H.; Eker, F.; Akdaşçi, E.; Witkowska, A.M.; Bechelany, M.; Karav, S. Silver Nanoparticles: A Comprehensive Review of Synthesis Methods and Chemical and Physical Properties. Nanomaterials 2024, 14, 1527. [Google Scholar] [CrossRef]
- Stevanović, M.; Bračko, I.; Milenković, M.; Filipović, N.; Nunić, J.; Filipič, M.; Uskoković, D.P. Multifunctional PLGA Particles Containing Poly(l-Glutamic Acid)-Capped Silver Nanoparticles and Ascorbic Acid with Simultaneous Antioxidative and Prolonged Antimicrobial Activity. Acta Biomater. 2014, 10, 151–162. [Google Scholar] [CrossRef]
- Stevanović, M.; Uskoković, V.; Filipović, M.; Škapin, S.D.; Uskoković, D. Composite PLGA/AgNpPGA/AscH Nanospheres with Combined Osteoinductive, Antioxidative, and Antimicrobial Activities. ACS Appl. Mater. Interfaces 2013, 5, 9034–9042. [Google Scholar] [CrossRef]
- Gul, M.; Kashif, M.; Muhammad, S.; Azizi, S.; Sun, H. Various Methods of Synthesis and Applications of Gold-Based Nanomaterials: A Detailed Review. Cryst. Growth Des. 2025, 25, 2227–2266. [Google Scholar] [CrossRef]
- Pal, P.; Pareek, A. Zinc Oxide Nanoparticles: A Comprehensive Review on Its Synthesis, Characterization, and Role in Biomedical Applications as Well as Health Risks. Inorg. Chem. Commun. 2025, 181, 115314. [Google Scholar] [CrossRef]
- Janićijević, Ž.; Stanković, A.; Žegura, B.; Veljović, Đ.; Djekić, L.; Krajišnik, D.; Filipič, M.; Stevanović, M.M. Safe-by-Design Gelatin-Modified Zinc Oxide Nanoparticles. J. Nanoparticle Res. 2021, 23, 203. [Google Scholar] [CrossRef]
- Ghotekar, S.; Pansambal, S.; Bilal, M.; Pingale, S.S.; Oza, R. Environmentally Friendly Synthesis of Cr2O3 Nanoparticles: Characterization, Applications and Future Perspective—A Review. Case Stud. Chem. Environ. Eng. 2021, 3, 100089. [Google Scholar] [CrossRef]
- Oliveira, R.L.M.S.; Barbosa, L.; Hurtado, C.R.; Ramos, L.d.P.; Montanheiro, T.L.A.; Oliveira, L.D.; Tada, D.B.; Trichês, E.d.S. Bioglass--based Scaffolds Coated with Silver Nanoparticles: Synthesis, Processing and Antimicrobial Activity. J. Biomed. Mater. Res. Part A 2020, 108, 2447–2459. [Google Scholar] [CrossRef]
- Filipović, N.; Ušjak, D.; Milenković, M.T.; Zheng, K.; Liverani, L.; Boccaccini, A.R.; Stevanović, M.M. Comparative Study of the Antimicrobial Activity of Selenium Nanoparticles With Different Surface Chemistry and Structure. Front. Bioeng. Biotechnol. 2021, 8, 624621. [Google Scholar] [CrossRef]
- Tomić, N.; Stevanović, M.M.; Filipović, N.; Ganić, T.; Nikolić, B.; Gajić, I.; Ćulafić, D.M. Resveratrol/Selenium Nanocomposite with Antioxidative and Antibacterial Properties. Nanomaterials 2024, 14, 368. [Google Scholar] [CrossRef]
- Meng, Y.Q.; Shi, Y.N.; Zhu, Y.P.; Liu, Y.Q.; Gu, L.W.; Liu, D.D.; Ma, A.; Xia, F.; Guo, Q.Y.; Xu, C.C.; et al. Recent Trends in Preparation and Biomedical Applications of Iron Oxide Nanoparticles. J. Nanobiotechnol. 2024, 22, 24. [Google Scholar] [CrossRef]
- Stojanović, Z.; Otoničar, M.; Lee, J.; Stevanović, M.M.; Hwang, M.P.; Lee, K.H.; Choi, J.; Uskoković, D. The Solvothermal Synthesis of Magnetic Iron Oxide Nanocrystals and the Preparation of Hybrid Poly(l-Lactide)–Polyethyleneimine Magnetic Particles. Colloids Surf. B Biointerfaces 2013, 109, 236–243. [Google Scholar] [CrossRef]
- Filipović, N.; Tomić, N.; Kuzmanović, M.; Stevanović, M.M. Nanoparticles. Potential for Use to Prevent Infections. In Urinary Stents; Springer International Publishing: Cham, Germany, 2022; pp. 325–339. [Google Scholar]
- Ijaz, I.; Gilani, E.; Nazir, A.; Bukhari, A. Detail Review on Chemical, Physical and Green Synthesis, Classification, Characterizations and Applications of Nanoparticles. Green Chem. Lett. Rev. 2020, 13, 223–245. [Google Scholar] [CrossRef]
- Vinukonda, A.; Bolledla, N.; Jadi, R.K.; Chinthala, R.; Devadasu, V.R. Synthesis of Nanoparticles Using Advanced Techniques. Next Nanotechnol. 2025, 8, 100169. [Google Scholar] [CrossRef]
- Salehirozveh, M.; Dehghani, P.; Mijakovic, I. Synthesis, Functionalization, and Biomedical Applications of Iron Oxide Nanoparticles (IONPs). J. Funct. Biomater. 2024, 15, 340. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.A.; Elshahawy, M.F.; Raafat, A.I.; Abdou, F.Y.; Tahar, H.A. Rat Model Evaluation for Healing-Promoting Effectiveness and Antimicrobial Activity of Electron Beam Synthesized (Polyvinyl Alcohol-Pectin)-Silver Doped Zinc Oxide Hydrogel Dressings Enriched with Lavender Oil. Int. J. Biol. Macromol. 2025, 288, 138618. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, M.; Das, D.B.; Salem, Z.A.; Beherei, H.H. Nanomaterials for Biomedical Applications: Production, Characterisations, Recent Trends and Difficulties. Molecules 2021, 26, 1077. [Google Scholar] [CrossRef] [PubMed]
- John, J. Cutting-Edge Nanoparticle Innovations in Biomedical Science: Synthesis, Applications, Challenges, and Future Prospects. J. Nanotechnol. Nanomater. 2025, 6, 43–66. [Google Scholar] [CrossRef]
- Dey, S.; Mohanty, D.L.; Divya, N.; Bakshi, V.; Mohanty, A.; Rath, D.; Das, S.; Mondal, A.; Roy, S.; Sabui, R. A Critical Review on Zinc Oxide Nanoparticles: Synthesis, Properties and Biomedical Applications. Intell. Pharm. 2025, 3, 53–70. [Google Scholar] [CrossRef]
- Ielo, I.; Rando, G.; Giacobello, F.; Sfameni, S.; Castellano, A.; Galletta, M.; Drommi, D.; Rosace, G.; Plutino, M.R. Synthesis, Chemical–Physical Characterization, and Biomedical Applications of Functional Gold Nanoparticles: A Review. Molecules 2021, 26, 5823. [Google Scholar] [CrossRef]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; de Lima, T.M.T.; Delbem, A.C.B.; Monteiro, D.R. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef]
- Qin, X.; Wang, Z.; Lai, J.; Liang, Y.; Qian, K. The Synthesis of Selenium Nanoparticles and Their Applications in Enhancing Plant Stress Resistance: A Review. Nanomaterials 2025, 15, 301. [Google Scholar] [CrossRef]
- Wei, G.; Qu, J.; Yu, Z.; Li, Y.; Guo, Q.; Qi, T. Mineralizer Effects on the Synthesis of Amorphous Chromium Hydroxide and Chromium Oxide Green Pigment Using Hydrothermal Reduction Method. Dye. Pigment. 2015, 113, 487–495. [Google Scholar] [CrossRef]
- Ruiz-Jorge, F.; Portela, J.R.; Sánchez-Oneto, J.; Martínez de la Ossa, E.J. Synthesis of Micro-and Nanoparticles in Sub- and Supercritical Water: From the Laboratory to Larger Scales. Appl. Sci. 2020, 10, 5508. [Google Scholar] [CrossRef]
- Bîrcă, A.C.; Minculescu, M.A.; Niculescu, A.-G.; Hudiță, A.; Holban, A.M.; Alberts, A.; Grumezescu, A.M. Nanoparticle-Enhanced Collagen Hydrogels for Chronic Wound Management. J. Funct. Biomater. 2025, 16, 91. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Krishnan, N.; Heo, J.; Fang, R.H.; Zhang, L. Nanoparticle–Hydrogel Superstructures for Biomedical Applications. J. Control. Release 2020, 324, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Kohane, D.S. Hybrid Nanoparticle–Hydrogel Systems for Drug Delivery Depots and Other Biomedical Applications. ACS Nano 2024, 18, 22780–22792. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhong, H.-J.; Ding, H.; Yu, B.; Ma, X.; Liu, X.; Chong, C.-M.; He, J. Polyvinyl Alcohol (PVA)-Based Hydrogels: Recent Progress in Fabrication, Properties, and Multifunctional Applications. Polymers 2024, 16, 2755. [Google Scholar] [CrossRef]
- Wahid, F.; Zhao, X.-J.; Jia, S.-R.; Bai, H.; Zhong, C. Nanocomposite Hydrogels as Multifunctional Systems for Biomedical Applications: Current State and Perspectives. Compos. Part B Eng. 2020, 200, 108208. [Google Scholar] [CrossRef]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef]
- Xing, W.; Tang, Y. On Mechanical Properties of Nanocomposite Hydrogels: Searching for Superior Properties. Nano Mater. Sci. 2022, 4, 83–96. [Google Scholar] [CrossRef]
- Rana, M.M.; De la Hoz Siegler, H. Evolution of Hybrid Hydrogels: Next-Generation Biomaterials for Drug Delivery and Tissue Engineering. Gels 2024, 10, 216. [Google Scholar] [CrossRef]
- Hickey, J.W.; Santos, J.L.; Williford, J.-M.; Mao, H.-Q. Control of Polymeric Nanoparticle Size to Improve Therapeutic Delivery. J. Control. Release 2015, 219, 536–547. [Google Scholar] [CrossRef]
- Stornes, M.; Blanco, P.M.; Dias, R.S. Polyelectrolyte-Nanoparticle Mutual Charge Regulation and Its Influence on Their Complexation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127258. [Google Scholar] [CrossRef]
- Ribeiro, M.M.; Simões, M.; Vitorino, C.; Mascarenhas-Melo, F. Physical Crosslinking of Hydrogels: The Potential of Dynamic and Reversible Bonds in Burn Care. Coord. Chem. Rev. 2025, 542, 216868. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Ma, Y.; Wang, M.; Pan, G. Nano-Crosslinked Dynamic Hydrogels for Biomedical Applications. Mater. Today Bio. 2023, 20, 100640. [Google Scholar] [CrossRef]
- Rumon, M.M.H.; Rahman, M.S.; Akib, A.A.; Sohag, M.S.; Rakib, M.R.A.; Khan, M.A.R.; Yesmin, F.; Shakil, M.S.; Rahman Khan, M.M. Progress in Hydrogel Toughening: Addressing Structural and Crosslinking Challenges for Biomedical Applications. Discov. Mater. 2025, 5, 5. [Google Scholar] [CrossRef]
- Yammine, P.; El Safadi, A.; Kassab, R.; El-Nakat, H.; Obeid, P.J.; Nasr, Z.; Tannous, T.; Sari-Chmayssem, N.; Mansour, A.; Chmayssem, A. Types of Crosslinkers and Their Applications in Biomaterials and Biomembranes. Chemistry 2025, 7, 61. [Google Scholar] [CrossRef]
- Kamel, S.; El-Sayed, N.S. Cross-Linking Strategies for the Design of Smart Injectable Hydrogels. In Injectable Smart Hydrogels for Biomedical Applications; Royal Society of Chemistry: London, UK, 2024; pp. 128–149. [Google Scholar]
- Xue, L.; An, R.; Zhao, J.; Qiu, M.; Wang, Z.; Ren, H.; Yu, D.; Zhu, X. Self--Healing Hydrogels: Mechanisms and Biomedical Applications. MedComm 2025, 6, e70181. [Google Scholar] [CrossRef] [PubMed]
- Nam, M.; Lee, J.W.; Cha, G.D. Biomedical Application of Enzymatically Crosslinked Injectable Hydrogels. Gels 2024, 10, 640. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.S.; Premanand, R.; Ragupathi, I.; Bhaviripudi, V.R.; Aepuru, R.; Kannan, K.; Shanmugaraj, K. Comprehensive Review of Hydrogel Synthesis, Characterization, and Emerging Applications. J. Compos. Sci. 2024, 8, 457. [Google Scholar] [CrossRef]
- Guo, Z.; Lu, J.; Gao, J.; Zhang, Y.; Xing, F.; Li, Y.; Chen, S.; Xie, W.; Sun, S.; Qi, G.; et al. Metal Ion-Driven Assembly for Constructing a Metal–Phenolic Network Nanoparticle-Loaded Hydrogel as a Tumor Photothermal-Immunotherapy Agent. J. Mater. Chem. B 2025, 13, 9865–9875. [Google Scholar] [CrossRef]
- Vinnacombe-Willson, G.A.; Núñez-Martínez, M.; Herrero-Ruiz, A.; Bevilacqua, F.; Pazos, R.; Troncoso-Afonso, L.; Gallego-González, M.; Scarabelli, L.; Liz-Marzán, L.M. Plasmonic-Hydrogel Hybrid Biomaterials Via In Situ Seeded Growth. Angew. Chem. Int. Ed. 2025, 64, e202501854. [Google Scholar] [CrossRef]
- Gholamali, I.; Hosseini, S.N.; Alipour, E. Doxorubicin-Loaded Oxidized Starch/Poly (Vinyl Alcohol)/CuO Bio-Nanocomposite Hydrogels as an Anticancer Drug Carrier Agent. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 967–980. [Google Scholar] [CrossRef]
- Nagaraja, K.; Rao, K.M.; Hemalatha, D.; Zo, S.; Han, S.S.; Rao, K.S.V.K. Strychnos potatorum L. Seed Polysaccharide-Based Stimuli-Responsive Hydrogels and Their Silver Nanocomposites for the Controlled Release of Chemotherapeutics and Antimicrobial Applications. ACS Omega 2022, 7, 12856–12869. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Srivastava, S.K.; Shrivastava, S.L.; Mandal, A.K. Hierarchical Assembly of Nanodimensional Silver–Silver Oxide Physical Gels Controlling Nosocomial Infections. ACS Omega 2020, 5, 32617–32631. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, Y.F.; Guo, X.; Chen, Y.; Li, J.; Xu, X.; Li, Y.; Cun, D.; Bera, H.; Yang, M. High Molecular Weight Laminarin/AgNPs-Impregnated PVA Based in Situ Hydrogels Accelerated Diabetic Wound Healing. Carbohydr. Polym. 2025, 367, 123991. [Google Scholar] [CrossRef]
- Xue, Y.; Yang, F.; He, Y.; Wang, F.; Xia, D.; Liu, Y. Multifunctional Hydrogel with Photothermal ROS Scavenging and Antibacterial Activity Accelerates Diabetic Wound Healing. Adv. Healthc. Mater. 2025, 14, e2402236. [Google Scholar] [CrossRef]
- Veeramani, S.; Thulasimuthu, E.; Mayakrishnan, V.; Ilangovan, R. Development and Characterization of ZnO-, CuO-, and CuO–ZnO-Incorporated Porous Sodium Alginate–Polyvinyl Alcohol–Gelatin Hybrid Bio-Nanocomposite Scaffolds for Wound Healing Applications. Polym. Bull. 2025, 82, 8301–8323. [Google Scholar] [CrossRef]
- Cabral, F.V.; Santana, B.d.M.; Lange, C.N.; Batista, B.L.; Seabra, A.B.; Ribeiro, M.S. Pluronic F-127 Hydrogels Containing Copper Oxide Nanoparticles and a Nitric Oxide Donor to Treat Skin Cancer. Pharmaceutics 2023, 15, 1971. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiu, Z.; Jiang, X.; Zhang, H.; Li, X.; Feng, Y.; Li, B.; Cai, R.; Li, C.; Tao, G. Injectable Hydrogels with ROS-Triggered Drug Release Enable the Co-Delivery of Antibacterial Agent and Anti-Inflammatory Nanoparticle for Periodontitis Treatment. J. Nanobiotechnol. 2025, 23, 205. [Google Scholar] [CrossRef]
- Dong, Q.; Zu, D.; Kong, L.; Chen, S.; Yao, J.; Lin, J.; Lu, L.; Wu, B.; Fang, B. Construction of Antibacterial Nano-Silver Embedded Bioactive Hydrogel to Repair Infectious Skin Defects. Biomater. Res. 2022, 26, 36. [Google Scholar] [CrossRef]
- Raveendran, R.L.; Lekshmi, G.S.; Anirudhan, T.S. Self-Assembled Sustainable Bionanocomposite Hydrogels from Chitosan for the Combination Chemotherapy of Hydrophobic and Hydrophilic Drugs. Int. J. Biol. Macromol. 2024, 283, 137881. [Google Scholar] [CrossRef]
- Gounden, V.; Singh, M. Gold Nanoparticle-Based Hydrogel: Application in Anticancer Drug Delivery and Wound Healing In Vitro. Pharmaceutics 2025, 17, 633. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chu, R.; Li, H.; Hua, T.; Chen, H.; Li, R.; Zhou, D.; Cao, S.; Ye, S.; Li, H. A Novel Wound Dressing Based on a Gold Nanoparticle Self-Assembled Hydrogel to Promote Wound Healing. Mater. Adv. 2023, 4, 2918–2925. [Google Scholar] [CrossRef]
- Cao, Y.; Li, W.; Quan, F.; Xia, Y.; Xiong, Z. Green–Light–Driven Poly(N-Isopropylacrylamide-Acrylamide)/Fe3O4 Nanocomposite Hydrogel Actuators. Front. Mater. 2022, 9, 827608. [Google Scholar] [CrossRef]
- Rahmani, P.; Shojaei, A.; Sakorikar, T.; Wang, M.; Mendoza-Apodaca, Y.; Dickey, M.D. Liquid Metal Nanoparticles Physically Hybridized with Cellulose Nanocrystals Initiate and Toughen Hydrogels with Piezoionic Properties. ACS Nano 2024, 18, 8038–8050. [Google Scholar] [CrossRef]
- Aguilar, N.M.; Sanchez-Gaytan, B.L.; Soriano-Moro, G. Polyacrylamide-Based Nanocomposite Hydrogel Prepared by Synergistic Solar Light-Induced Polymerization. Macromol. Res. 2025, 33, 399–405. [Google Scholar] [CrossRef]
- Kılıç, H.; Ceylan, D. Multi-Responsive Shape Memory and Self-Healing Hydrogels with Gold and Silver Nanoparticles. J. Mater. Chem. B 2025, 13, 336–353. [Google Scholar] [CrossRef]
- Yan, K.; Xu, F.; Wei, W.; Yang, C.; Wang, D.; Shi, X. Electrochemical Synthesis of Chitosan/Silver Nanoparticles Multilayer Hydrogel Coating with PH-Dependent Controlled Release Capability and Antibacterial Property. Colloids Surf. B Biointerfaces 2021, 202, 111711. [Google Scholar] [CrossRef]
- Singh, B.; Chejara, M.R.; Park, M.-H. Light-Responsive Layer-By-Layer Film Containing Gold Nanorods for Sequential Drug Release. ACS Omega 2023, 8, 48405–48412. [Google Scholar] [CrossRef]
- Miao, Y.; Xu, M.; Zhang, L. Electrochemistry-Induced Improvements of Mechanical Strength, Self-Healing, and Interfacial Adhesion of Hydrogels. Adv. Mater. 2021, 33, 2102308. [Google Scholar] [CrossRef]
- Kim, S.; Regitsky, A.U.; Song, J.; Ilavsky, J.; McKinley, G.H.; Holten-Andersen, N. In Situ Mechanical Reinforcement of Polymer Hydrogels via Metal-Coordinated Crosslink Mineralization. Nat. Commun. 2021, 12, 667. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Yan, Z.; Ji, S.; Xiao, S.; Gao, J. Metal Nanoparticle Hybrid Hydrogels: The State-of-the-Art of Combining Hard and Soft Materials to Promote Wound Healing. Theranostics 2024, 14, 1534–1560. [Google Scholar] [CrossRef]
- Shahid, N.; Erum, A.; Hanif, S.; Malik, N.S.; Tulain, U.R.; Syed, M.A. Nanocomposite Hydrogels-A Promising Approach towards Enhanced Bioavailability and Controlled Drug Delivery. Curr. Pharm. Des. 2024, 30, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, Z.; Kazeminava, F.; Afrouz, M.; Abbaszadeh, M.; Mehr, N.T.; Shiran, J.A.; Gouda, C.; Adeli, M.; Kafil, H.S. A Review on the Impacts of Metal/Metal Nanoparticles on Characteristics of Hydrogels: Special Focus on Carbohydrate Polymers. Int. J. Biol. Macromol. 2023, 253, 126535. [Google Scholar] [CrossRef] [PubMed]
- Khodami, S.; Gharakhloo, M.; Dagdelen, S.; Fita, P.; Romanski, J.; Karbarz, M.; Stojek, Z.; Mackiewicz, M. Rapid Photoinduced Self-Healing, Controllable Drug Release, Skin Adhesion Ability, and Mechanical Stability of Hydrogels Incorporating Linker-Modified Gold Nanoparticles and Nanogels. ACS Appl. Mater. Interfaces 2024, 16, 57659–57671. [Google Scholar] [CrossRef] [PubMed]
- Charlet, A.; Lutz-Bueno, V.; Mezzenga, R.; Amstad, E. Shape Retaining Self-Healing Metal-Coordinated Hydrogels. Nanoscale 2021, 13, 4073–4084. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, Y.; Xi, M.; Li, G.; Jiang, Y. One-Step Preparation of Adhesive Composite Hydrogels through Fast and Simultaneous In Situ Formation of Silver Nanoparticles and Crosslinking. Gels 2022, 8, 256. [Google Scholar] [CrossRef]
- Awasthi, S. Unveiling the Development Principles and Mechanistic Understanding of Controlled Drug Delivery Strategies for Chronic Bone Defects and Diabetic Wound Management. Mater. Adv. 2025, 6, 5831–5856. [Google Scholar] [CrossRef]
- Awasthi, S.; Gaur, J.K.; Pandey, S.K.; Bobji, M.S.; Srivastava, C. High-Strength, Strongly Bonded Nanocomposite Hydrogels for Cartilage Repair. ACS Appl. Mater. Interfaces 2021, 13, 24505–24523. [Google Scholar] [CrossRef]
- Nezami, S.; Sadeghi, M.; Mohajerani, H. A Novel PH-Sensitive and Magnetic Starch-Based Nanocomposite Hydrogel as a Controlled Drug Delivery System for Wound Healing. Polym. Degrad. Stab. 2020, 179, 109255. [Google Scholar] [CrossRef]
- Rezanejade Bardajee, G.; Mahmoodian, H.; Shafiei, N.; Amiri, B. Development of a Multi-Stimuli-Responsive Magnetic Nanogel–Hydrogel Nanocomposite for Prolonged and Controlled Doxorubicin Release. Bioconjug. Chem. 2025, 36, 1604–1627. [Google Scholar] [CrossRef]
- Karimi-Rastehkenari, A.; Youseftabar-Miri, L.; Askarizadeh, E.; Divsar, F. PH-Sensitive Magnetic Nanocomposite Hydrogels Chitosan/Hyaluronic Acid/Fe3O4: Ciprofloxacin Release, Kinetics and Antibacterial Activity. Int. J. Biol. Macromol. 2025, 319, 145211. [Google Scholar] [CrossRef]
- Awasthi, S. Ferrogels towards Nanotheranostics. Mater. Today Chem. 2024, 35, 101877. [Google Scholar] [CrossRef]
- Awasthi, S. A Review on Hydrogels and Ferrogels for Biomedical Applications. JOM 2021, 73, 2440–2451. [Google Scholar] [CrossRef]
- Zhang, F.; Feng, Q.; Zhan, J.; Chen, S.; Yang, G.; Li, T.; Zhou, X.; He, C. NIR-Activatable Antibacterial 3D-Printed Hydrogel Scaffold with Controllable Drug Release for Enhanced Vascularized Bone Regeneration. ACS Appl. Mater. Interfaces 2025, 17, 40035–40051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, X.; Wang, J.; Zhang, Y.; Dong, M.; Bu, T.; Li, L.; Liu, Y.; Wang, L. Multifunctional Injectable Hydrogel Dressings for Effectively Accelerating Wound Healing: Enhancing Biomineralization Strategy. Adv. Funct. Mater. 2021, 31, 2100093. [Google Scholar] [CrossRef]
- Ren, H.; Guo, Q.; Lin, B.; Liu, Y.; Wang, X.; Huang, K.; Wu, K. Biological Applications on a Novel Composite Hydrogel Containing Mesoporous Silica Nanoparticles Loaded with Insulin-like Growth Factor-1 and Vancomycin. J. Mater. Sci. Mater. Med. 2025, 36, 35. [Google Scholar] [CrossRef]
- Pablos, J.L.; Lozano, D.; Manzano, M.; Vallet-Regí, M. Regenerative Medicine: Hydrogels and Mesoporous Silica Nanoparticles. Mater. Today Bio 2024, 29, 101342. [Google Scholar] [CrossRef]
- Bernasconi, R.; Pizzetti, F.; Rossetti, A.; Butler, B.; Levi, M.; Pané, S.; Rossi, F.; Magagnin, L. Layer-by-Layer Fabrication of Hydrogel Microsystems for Controlled Drug Delivery From Untethered Microrobots. Front. Bioeng. Biotechnol. 2021, 9, 692648. [Google Scholar] [CrossRef]
- Atli, I.; Ilgin, P.; Karabayir, E.S.; Ozay, H.; Ozay, O. Enhanced Antimicrobial and Anticancer Activities of Zein Protein-Agarose@Au Composite Hydrogel for Controlled Release of Silibinin in Colon Cancer Therapy. Int. J. Biol. Macromol. 2025, 321, 146286. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, B.; Yu, D.; Ji, T.; Nie, K.; Tian, J.; Shen, X.; Zhang, K.; Ou, J.; Yang, X.; et al. Electrochemical--Genetic Programming of Protein--Based Magnetic Soft Robots for Active Drug Delivery. Adv. Sci. 2025, 12, e2503404. [Google Scholar] [CrossRef]
- Ju, X.; Kong, J.; Qi, G.; Duan, X.; Peng, C.; Wang, E.; Dong, S.; Li, J.; Jin, Y. Dual-Function Polyionic Liquid Piezoelectric Hydrogel: Combining Antibacterial Activity and Electrical Stimulation for Infected Wound Treatment. J. Colloid. Interface Sci. 2025, 700, 138317. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Ma, R.; Liu, G.; Li, X.; Xu, K.; Liu, P.; Cai, K. Fabrication of a New Hyaluronic Acid/Gelatin Nanocomposite Hydrogel Coating on Titanium-Based Implants for Treating Biofilm Infection and Excessive Inflammatory Response. ACS Appl. Mater. Interfaces 2023, 15, 13783–13801. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mu, W.; Wang, Y.; Liu, T.; Peng, Z.; Chen, X. Nanocomposite Hydrogel with Multiple Metal Ions Coupling for Effective Treatment of Bacteria Infected Wound via Cascade Catalysis and Tissue Regeneration. Chem. Eng. J. 2025, 511, 161783. [Google Scholar] [CrossRef]
- Wang, X.; Hu, J.; Chen, C.; Lu, J.; Liu, C.; Ning, Y.; Lu, F. Berberine@AgNPs@Carboxylated Chitosan Hydrogel Dressing with Immunomodulatory and Anti-Biofilm Properties Promotes Wound Repair in Drug-Resistant Bacterial Infections. Int. J. Biol. Macromol. 2025, 315, 144496. [Google Scholar] [CrossRef]
- Ziaei, A.A.; Erfan-Niya, H.; Fathi, M.; Amiryaghoubi, N. In Situ Fast Gelling Modified Polyvinyl Alcohol/Polyethylene Glycol Hybrid Hydrogels Incorporating Doxorubicin-Loaded Chitosan/AuNPs Nanogels for Localized Treatment of Breast Cancer. Int. J. Biol. Macromol. 2025, 315, 144381. [Google Scholar] [CrossRef]
- Ghosh, D.; Deka, D.; Das, G. Leveraging Metal Oxide-Fenugreek Hydrogel Nanocomposites for Enhanced Structural and Biological Properties. Soft Matter 2025, 21, 4069–4077. [Google Scholar] [CrossRef]
- Khodami, S.; Gharakhloo, M.; Dagdelen, S.; Fita, P.; Karbarz, M.; Stojek, Z. Poly(N-Isopropylacrylamide) Hydrogel Crosslinked with Laponite and Loaded with Au Nanoparticles for Sensitive Motion Sensor with Improved Mechanical Stability, Conductivity, and Controllable Releasing Ability. Sens. Actuators A Phys. 2025, 394, 116909. [Google Scholar] [CrossRef]
- Augustine, R.; Zahid, A.A.; Hasan, A.; Dalvi, Y.B.; Jacob, J. Cerium Oxide Nanoparticle-Loaded Gelatin Methacryloyl Hydrogel Wound-Healing Patch with Free Radical Scavenging Activity. ACS Biomater. Sci. Eng. 2021, 7, 279–290. [Google Scholar] [CrossRef]
- Zhang, C.; Fei, Y.; Li, M.; Li, J.; Tang, M.; Wang, G.; Li, J.; Wang, Y.; Ding, Y.; Peng, C.; et al. Chitosan-P407-PNIPAM Hydrogel Loaded with AgNPs/Lipid Complex for Antibacterial, Inflammation Regulation and Alveolar Bone Regeneration in Periodontitis Treatment. Int. J. Biol. Macromol. 2025, 307, 142080. [Google Scholar] [CrossRef]
- Razack, S.A.; Kim, Y.E.; Kang, H.W. κ-Carrageenan–Gelatin Hydrogel Embedding Carvacrol Loaded Gold Nanobipyramids for Treating Prostate Cancer via Fractionated Photothermal-Chemotherapy. Int. J. Biol. Macromol. 2025, 291, 138974. [Google Scholar] [CrossRef]
- Liu, H.; Ai, R.; Liu, B.; He, L. Tea Polyphenol Nano-Crosslinked Dynamical Hyaluronic Acid-Based Hydrogel for Diabetic Wound Healing. Int. J. Biol. Macromol. 2024, 282, 136856. [Google Scholar] [CrossRef]
- Luanda, A.; Mahadev, M.; Charyulu, R.N.; Badalamoole, V. Locust Bean Gum-Based Silver Nanocomposite Hydrogel as a Drug Delivery System and an Antibacterial Agent. Int. J. Biol. Macromol. 2024, 282, 137097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Jian, M.; Pei, Y.; Liu, J.; Zheng, X.; Tang, K. Sustained-Release, Antibacterial, Adhesive Gelatin Composite Hydrogel with AgNPs Double-Capped with Curdlan Derivatives. Int. J. Biol. Macromol. 2024, 277, 134222. [Google Scholar] [CrossRef] [PubMed]
- Heidari, F.; Raoufi, Z.; Abdollahi, S.; Asl, H.Z. Antibiotic Delivery in the Presence of Green AgNPs Using Multifunctional Bilayer Carrageenan Nanofiber/Sodium Alginate Nanohydrogel for Rapid Control of Wound Infections. Int. J. Biol. Macromol. 2024, 277, 134109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gu, G.; Xu, Y.; Luan, X.; Liu, J.; He, P.; Wei, G. Injectable Self--Healing Antibacterial Hydrogels with Tailored Functions by Loading Peptide Nanofiber--Biomimetic Silver Nanoparticles. Macromol. Rapid Commun. 2024, 45, e2400173. [Google Scholar] [CrossRef]
- Sajid, A.; Amjad, M.; Manzoor, Q.; Wazir, S.; Sajid, A.; Alwadai, N.; Iqbal, M.; Tamam, N. Synthesis of Bimetallic Oxides (SrO-CoO) Nanoparticles Decorated Polyacrylamide Hydrogels for Controlled Drug Release and Wound Healing Applications. Int. J. Biol. Macromol. 2024, 274, 133194. [Google Scholar] [CrossRef]
- Ferrag, C.; Li, S.; Jeon, K.; Andoy, N.M.; Sullan, R.M.A.; Mikhaylichenko, S.; Kerman, K. Polyacrylamide Hydrogels Doped with Different Shapes of Silver Nanoparticles: Antibacterial and Mechanical Properties. Colloids Surf. B Biointerfaces 2021, 197, 111397. [Google Scholar] [CrossRef]
- Li, W.; Shi, Z.; Jing, H.; Dou, Y.; Liu, X.; Zhang, M.; Qiu, Z.; Heger, Z.; Li, N. Streamlined Metal-Based Hydrogel Facilitates Stem Cell Differentiation, Extracellular Matrix Homeostasis and Cartilage Repair in Male Rats. Nat. Commun. 2025, 16, 4344. [Google Scholar] [CrossRef]
- Rani, I.; Warkar, S.G.; Kumar, A. Nano ZnO Embedded Poly (Ethylene Glycol) Diacrylate Cross-Linked Carboxymethyl Tamarind Kernel Gum (CMTKG)/Poly (Sodium Acrylate) Composite Hydrogels for Oral Delivery of Ciprofloxacin Drug and Their Antibacterial Properties. Mater. Today Commun. 2023, 35, 105635. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, Y.; Chen, Y.; Fan, H.; Zhang, J.; Li, J.; Lin, W.; Yi, G.; Feng, X. Adhesive Polydopamine-Based Photothermal Hybrid Hydrogel for on-Demand Lidocaine Delivery, Effective Anti-Bacteria, and Prolonged Local Long-Lasting Analgesia. Int. J. Biol. Macromol. 2024, 259, 129266. [Google Scholar] [CrossRef]
- Mao, L.; Wang, L.; Zhang, M.; Ullah, M.W.; Liu, L.; Zhao, W.; Li, Y.; Ahmed, A.A.Q.; Cheng, H.; Shi, Z.; et al. In Situ Synthesized Selenium Nanoparticles--Decorated Bacterial Cellulose/Gelatin Hydrogel with Enhanced Antibacterial, Antioxidant, and Anti--Inflammatory Capabilities for Facilitating Skin Wound Healing. Adv. Healthc. Mater. 2021, 10, 2100402. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, F.D.; Fischer, T.; Hayn, A.; Mierke, C.T.; Burgess, J.K.; Harmsen, M.C. A Beginner’s Guide to the Characterization of Hydrogel Microarchitecture for Cellular Applications. Gels 2022, 8, 535. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Włodarczyk-Biegun, M.K. Faithful Scanning Electron Microscopic (SEM) Visualization of 3D Printed Alginate-Based Scaffolds. Bioprinting 2020, 20, e00098. [Google Scholar] [CrossRef]
- Denzer, B.R.; Kulchar, R.J.; Huang, R.B.; Patterson, J. Advanced Methods for the Characterization of Supramolecular Hydrogels. Gels 2021, 7, 158. [Google Scholar] [CrossRef]
- Aigoin, J.; Payré, B.; Minvielle Moncla, J.; Escudero, M.; Goudouneche, D.; Ferri-Angulo, D.; Calmon, P.-F.; Vaysse, L.; Kemoun, P.; Malaquin, L.; et al. Comparative Analysis of Electron Microscopy Techniques for Hydrogel Microarchitecture Characterization: SEM, Cryo-SEM, ESEM, and TEM. ACS Omega 2025, 10, 14687–14698. [Google Scholar] [CrossRef]
- Ito, E.; Takase, H.; Yamamoto, K. TEM Observation of Inorganic Substances Distributed in Gel Materials for Medical Devices Using Ultra-Thin Cryosectioning. Microscopy 2020, 69, 408–410. [Google Scholar] [CrossRef]
- Kiyama, R.; Yoshida, M.; Nonoyama, T.; Sedlačík, T.; Jinnai, H.; Kurokawa, T.; Nakajima, T.; Gong, J.P. Nanoscale TEM Imaging of Hydrogel Network Architecture. Adv. Mater. 2023, 35, 2208902. [Google Scholar] [CrossRef]
- Nikolić, L.; Stojanović, T.; Nikolić, V.; Urošević, M.; Ilić-Stojanović, S.; Tačić, A.; Gajić, I.; Savić, V.; Zdravković, A. Synthesis and Characterisation of Hydrogels Based on Starch and Citric Acid. Adv. Technol. 2020, 9, 50–57. [Google Scholar] [CrossRef]
- Ashames, A.; Ullah, K.; Al-Tabakha, M.; Khan, S.A.; Hassan, N.; Mannan, A.; Ikram, M.; Buabeid, M.; Murtaza, G. Development, Characterization and In-Vitro Evaluation of Guar Gum Based New Polymeric Matrices for Controlled Delivery Using Metformin HCl as Model Drug. PLoS ONE 2022, 17, e0271623. [Google Scholar] [CrossRef]
- Krakovský, I.; Hanyková, L.; Štastná, J. Phase Transition in Polymer Hydrogels Investigated by Swelling, DSC, FTIR and NMR. J. Therm. Anal. Calorim. 2025, 150, 1245–1262. [Google Scholar] [CrossRef]
- Garnica-Palafox, I.M.; Velázquez-Benítez, A.M.; Sánchez-Arévalo, F.M.; Qureshi, N. Optical Characterization of Chitosan/Poly(Vinyl Alcohol) Hybrid Hydrogels and the Effect of Genipin Crosslinking and Multiwalled Carbon Nanotube Fillers. Opt. Mater. 2025, 160, 116686. [Google Scholar] [CrossRef]
- Martin-Saldaña, S.; Al Waeel, M.; Alsharabasy, A.M.; Daly, A.; Pandit, A. An Interdisciplinary Framework for the Characterization of Extracellular Matrix-Hydrogels for Biomedical Applications. Matter 2022, 5, 3659–3705. [Google Scholar] [CrossRef]
- Sakhaii, P.; Bohorc, B.; Olpp, T.; Mohnicke, M.; Rieke-Zapp, J.; Dhal, P.K. Radio Frequency Gradient Enhanced Diffusion-Edited Semi-Solid State NMR Spectroscopy for Detailed Structural Characterization of Chemically Modified Hyaluronic Acid Hydrogels. Sci. Rep. 2024, 14, 28612. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, S.; Biermann, M.; Kara, S.; Jopp, S.; Meyer, J. A Novel Characterization Technique for Hydrogels–in Situ Rheology-Raman Spectroscopy for Gelation and Polymerization Tracking. Mater. Adv. 2024, 5, 6957–6966. [Google Scholar] [CrossRef]
- Affatato, S.; Trucco, D.; Taddei, P.; Vannozzi, L.; Ricotti, L.; Nessim, G.; Lisignoli, G. Wear Behavior Characterization of Hydrogels Constructs for Cartilage Tissue Replacement. Materials 2021, 14, 428. [Google Scholar] [CrossRef]
- Fernández-Galiana, Á.; Bibikova, O.; Vilms Pedersen, S.; Stevens, M.M. Fundamentals and Applications of Raman-Based Techniques for the Design and Development of Active Biomedical Materials. Adv. Mater. 2024, 36, e2210807. [Google Scholar] [CrossRef]
- Malkovskiy, A.V.; Tom, A.; Joubert, L.-M.; Bao, Z. Visualization of the Distribution of Covalently Cross-Linked Hydrogels in CLARITY Brain-Polymer Hybrids for Different Monomer Concentrations. Sci. Rep. 2022, 12, 13549. [Google Scholar] [CrossRef]
- Azkune, M.; Ayesta, I.; Ruiz-Rubio, L.; Arrospide, E.; Vilas-Vilela, J.L.; Zubia, J. Hydrogel-Core Microstructured Polymer Optical Fibers for Selective Fiber Enhanced Raman Spectroscopy. Sensors 2021, 21, 1845. [Google Scholar] [CrossRef]
- Straksys, A.; Abouhagger, A.; Kirsnytė-Šniokė, M.; Kavleiskaja, T.; Stirke, A.; Melo, W.C.M.A. Development and Characterization of a Gelatin-Based Photoactive Hydrogel for Biomedical Application. J. Funct. Biomater. 2025, 16, 43. [Google Scholar] [CrossRef]
- Cona, C.; Bailey, K.; Barker, E. Characterization Methods to Determine Interpenetrating Polymer Network (IPN) in Hydrogels. Polymers 2024, 16, 2050. [Google Scholar] [CrossRef]
- Zheng, Y.; Ma, Y.; Ukwatta, R.H.; Xue, F.; Li, C. Development of Novel Cornstarch Hydrogel-Based Food Coolant and Its Characterization. Polymers 2024, 16, 569. [Google Scholar] [CrossRef]
- Olate--Moya, F.; Palza, H. Effect of Graphene Oxide on the <scp>pH--responsive</Scp> Drug Release from Supramolecular Hydrogels. J. Appl. Polym. Sci. 2022, 139, 51420. [Google Scholar] [CrossRef]
- Rajawasam, C.W.H.; Dodo, O.J.; Weerasinghe, M.A.S.N.; Raji, I.O.; Wanasinghe, S.V.; Konkolewicz, D.; De Alwis Watuthanthrige, N. Educational Series: Characterizing Crosslinked Polymer Networks. Polym. Chem. 2024, 15, 219–247. [Google Scholar] [CrossRef]
- Kedir, C.N.; Salinas-Torres, D.; Quintero-Jaime, A.F.; Benyoucef, A.; Morallon, E. Hydrogels Obtained from Aniline and Piperazine: Synthesis, Characterization and Their Application in Hybrid Supercapacitors. J. Mol. Struct. 2022, 1248, 131445. [Google Scholar] [CrossRef]
- Han, J.; Jang, S.; Kim, B.-K.; Park, K. Electrochemical Study of Agarose Hydrogels for Natural Convection on Macroelectrodes and Ultramicroelectrodes. J. Anal. Sci. Technol. 2023, 14, 10. [Google Scholar] [CrossRef]
- Melnik, E.; Kurzhals, S.; Mutinati, G.C.; Beni, V.; Hainberger, R. Electrochemical Diffusion Study in Poly(Ethylene Glycol) Dimethacrylate-Based Hydrogels. Sensors 2024, 24, 3678. [Google Scholar] [CrossRef] [PubMed]
- Alam, K.; Iqbal, M.; Hasan, A.; Al-Maskari, N. Rheological Characterization of Biological Hydrogels in Aqueous State. J. Appl. Biotechnol. Rep. 2020, 7, 171–175. [Google Scholar] [CrossRef]
- Karvinen, J.; Kellomäki, M. Characterization of Self-Healing Hydrogels for Biomedical Applications. Eur. Polym. J. 2022, 181, 111641. [Google Scholar] [CrossRef]
- Ahmad, N.; Bukhari, S.N.A.; Hussain, M.A.; Ejaz, H.; Munir, M.U.; Amjad, M.W. Nanoparticles Incorporated Hydrogels for Delivery of Antimicrobial Agents: Developments and Trends. RSC Adv. 2024, 14, 13535–13564. [Google Scholar] [CrossRef]
- Egorikhina, M.N.; Timofeeva, L.B.; Linkova, D.D.; Rubtsova, Y.P.; Bugrova, M.L.; Charykova, I.N.; Ryabkov, M.G.; Kobyakova, I.I.; Farafontova, E.A.; Aleynik, D.Y. Biocompatibility Study of Hydrogel Biopolymer Scaffold with Encapsulated Mesenchymal Stem Cells. Polymers 2023, 15, 1337. [Google Scholar] [CrossRef]
- Patel, G.; Dalwadi, C. Recent Patents on Stimuli Responsive Hydrogel Drug Delivery System. Recent Pat. Drug Deliv. Formul. 2013, 7, 206–215. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current Hydrogel Advances in Physicochemical and Biological Response-Driven Biomedical Application Diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef] [PubMed]
- Segneanu, A.-E.; Bejenaru, L.E.; Bejenaru, C.; Blendea, A.; Mogoşanu, G.D.; Biţă, A.; Boia, E.R. Advancements in Hydrogels: A Comprehensive Review of Natural and Synthetic Innovations for Biomedical Applications. Polymers 2025, 17, 2026. [Google Scholar] [CrossRef] [PubMed]
- Stojkovska, J.; Zvicer, J.; Obradovic, B. Preclinical Functional Characterization Methods of Nanocomposite Hydrogels Containing Silver Nanoparticles for Biomedical Applications. Appl. Microbiol. Biotechnol. 2020, 104, 4643–4658. [Google Scholar] [CrossRef] [PubMed]
- Karami, P.; Stampoultzis, T.; Guo, Y.; Pioletti, D.P. A Guide to Preclinical Evaluation of Hydrogel-Based Devices for Treatment of Cartilage Lesions. Acta Biomater. 2023, 158, 12–31. [Google Scholar] [CrossRef]
- Pimton, P.; Ratphibun, P.; Tassaneesuwan, N.; Chiangnoon, R.; Uttayarat, P. Cytotoxicity Evaluation of Hydrogel Sheet Dressings Fabricated by Gamma Irradiation: Extract and Semi-Direct Contact Tests. Trends Sci. 2022, 19, 4583. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, S.; Mi, F.; Yang, Y.; Song, Q.; Gao, Y.; Wu, C.; Wen, W. Nanoparticle-Reinforced Hydrogel with a Well-Defined Pore Structure for Sustainable Drug Release and Effective Wound Healing. ACS Appl. Bio Mater. 2025, 8, 1406–1417. [Google Scholar] [CrossRef]
- Lohrasbi, S.; Mirzaei, E.; Karimizade, A.; Takallu, S.; Rezaei, A. Collagen/Cellulose Nanofiber Hydrogel Scaffold: Physical, Mechanical and Cell Biocompatibility Properties. Cellulose 2020, 27, 927–940. [Google Scholar] [CrossRef]
- Margot, A.M.; Engels, A.; Sittinger, M.; Dehne, T.; Hemmati-Sadeghi, S. Quantitatively Measuring the Cytotoxicity of Viscous Hydrogels with Direct Cell Sampling in a Micro Scale Format “MicroDrop” and Its Comparison to CCK8. J. Mater. Sci. Mater. Med. 2024, 35, 34. [Google Scholar] [CrossRef]
- Sukumaran, A.; Sweety, V.K.; Vikas, B.; Joseph, B. Cytotoxicity and Cell Viability Assessment of Biomaterials. In Cytotoxicity-Understanding Cellular Damage and Response; IntechOpen: London, UK, 2023. [Google Scholar]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Markossian, S., Grossman, A., Baskir, H., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2013. [Google Scholar]
- ISO 10993-5.2009; Biological Evaluation of Medical Devices, Part 5: Tests for in Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral Red Uptake Assay for the Estimation of Cell Viability/Cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Sæbø, I.; Bjørås, M.; Franzyk, H.; Helgesen, E.; Booth, J. Optimization of the Hemolysis Assay for the Assessment of Cytotoxicity. Int. J. Mol. Sci. 2023, 24, 2914. [Google Scholar] [CrossRef]
- Lee, K.; Ra, H.; Grogan, S.; D’Lima, D. Genotoxicity Study of Carboxymethyl Chitosan-Based Hydrogel for Clinical Use. Med. Res. Arch. 2022, 10. [Google Scholar] [CrossRef]
- Šramková, L.B.A.P.A.M.M. Genotoxicity of Selected Hydrogels Loaded with Iron Oxide Nanoparticles for Potential Application in Regenerative Medicine. Span. J. Environ. Mutagen. Genom. 2023, 27, 107. [Google Scholar]
- Mosesso, P.; Cinelli, S.; Natarajan, A.T.; Palitti, F. In Vitro Cytogenetic Assays: Chromosomal Aberrations and Micronucleus Tests. Methods Mol. Biol. 2013, 1044, 123–146. [Google Scholar] [PubMed]
- Takić Miladinov, D.; Tomić, S.; Stojanović, S.; Najdanović, J.; Filipović, J.; Trajanović, M.; Najman, S. Synthesis, Swelling Properties and Evaluation of Genotoxicity of Hydrogels Based on (Meth)Acrylates and Itaconic Acid. Mater. Res. 2016, 19, 1070–1079. [Google Scholar] [CrossRef][Green Version]
- Abdel-Wahhab, M.A.; Salman, A.S.; Ibrahim, M.I.M.; El-Kady, A.A.; Abdel-Aziem, S.H.; Hassan, N.S.; Waly, A.I. Curcumin Nanoparticles Loaded Hydrogels Protects against Aflatoxin B1-Induced Genotoxicity in Rat Liver. Food Chem. Toxicol. 2016, 94, 159–171. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Y.; Deng, L.; Shen, Z.; Huang, Q.; Shah, N.G.; Chen, W.; Zhang, Y.; Wang, X.; Yu, L.; et al. Antibacterial and Antioxidative Hydrogel Dressings Based on Tannic Acid-Gelatin/Oxidized Sodium Alginate Loaded with Zinc Oxide Nanoparticles for Promoting Wound Healing. Int. J. Biol. Macromol. 2024, 279, 135177. [Google Scholar] [CrossRef]
- Kapusta, O.; Jarosz, A.; Stadnik, K.; Giannakoudakis, D.A.; Barczyński, B.; Barczak, M. Antimicrobial Natural Hydrogels in Biomedicine: Properties, Applications, and Challenges—A Concise Review. Int. J. Mol. Sci. 2023, 24, 2191. [Google Scholar] [CrossRef]
- Chen, Q.; He, Y.; Li, Q.; Yang, K.; Sun, L.; Xu, H.; Wang, R. Intelligent Design and Medical Applications of Antimicrobial Hydrogels. Colloid Interface Sci. Commun. 2023, 53, 100696. [Google Scholar] [CrossRef]
- Stevanović, M.; Kovačević, B.; Petković, J.; Filipič, M.; Uskoković, D. Effect of Poly-α, γ, L-Glutamic Acid as a Capping Agent on Morphology and Oxidative Stress-Dependent Toxicity of Silver Nanoparticles. Int. J. Nanomed. 2011, 6, 2837–2847. [Google Scholar] [CrossRef][Green Version]
- Liu, J.; Jiang, W.; Xu, Q.; Zheng, Y. Progress in Antibacterial Hydrogel Dressing. Gels 2022, 8, 503. [Google Scholar] [CrossRef]
- Hu, L.; Chee, P.L.; Sugiarto, S.; Yu, Y.; Shi, C.; Yan, R.; Yao, Z.; Shi, X.; Zhi, J.; Kai, D.; et al. Hydrogel--Based Flexible Electronics. Adv. Mater. 2023, 35, e2205326. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Shen, Z.; Sun, X.; Yu, D.; Liu, K.; Mugo, S.M.; Chen, W.; Wang, D.; Zhang, Q. Electron Conductive and Transparent Hydrogels for Recording Brain Neural Signals and Neuromodulation. Adv. Mater. 2023, 35, e2211159. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Zhang, C.; Guo, T.; Tian, Y.; Song, W.; Lei, J.; Li, Q.; Wang, A.; Zhang, M.; Bai, S.; et al. Hydrogel Nanoarchitectonics of a Flexible and Self--Adhesive Electrode for Long--Term Wireless Electroencephalogram Recording and High--Accuracy Sustained Attention Evaluation. Adv. Mater. 2023, 35, e2209606. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; Xue, J.; Huang, G.; Zheng, S.; Zhao, K.; Huang, J.; Wang, Y.; Zhang, Y.; Yin, T.; et al. Body Temperature Enhanced Adhesive, Antibacterial, and Recyclable Ionic Hydrogel for Epidermal Electrophysiological Monitoring. Adv. Healthc. Mater. 2022, 11, e2200653. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Ren, J.; Pan, Y.; Cheng, L.; Xu, X.; Tan, C.L.; Sun, H.; Shi, Y.; Yan, S. Scaled Elastic Hydrogel Interfaces for Brain Electrophysiology. Adv. Funct. Mater. 2024, 34, 2407926. [Google Scholar] [CrossRef]
- Yu, J.; Wan, R.; Tian, F.; Cao, J.; Wang, W.; Liu, Q.; Yang, H.; Liu, J.; Liu, X.; Lin, T.; et al. 3D Printing of Robust High--Performance Conducting Polymer Hydrogel--Based Electrical Bioadhesive Interface for Soft Bioelectronics. Small 2024, 20, e2308778. [Google Scholar] [CrossRef]
- Li, N.; Wang, X.; Liu, Y.; Li, Y.; Li, J.; Qin, Z.; Jiao, T. Ultrastretchable, Self-Adhesive and Conductive MXene Nanocomposite Hydrogel for Body-Surface Temperature Distinguishing and Electrophysiological Signal Monitoring. Chem. Eng. J. 2024, 483, 149303. [Google Scholar] [CrossRef]
- Su, H.; Mao, L.; Chen, X.; Liu, P.; Pu, J.; Mao, Z.; Fujiwara, T.; Ma, Y.; Mao, X.; Li, T. A Complementary Dual-Mode Ion-Electron Conductive Hydrogel Enables Sustained Conductivity for Prolonged Electroencephalogram Recording. Adv. Sci. 2024, 11, e2405273. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Xue, Y.; Lei, I.M.; Chen, X.; Zhang, P.; Cai, C.; Liang, X.; Lu, Y.; Liu, J. Engineering Electrodes with Robust Conducting Hydrogel Coating for Neural Recording and Modulation. Adv. Mater. 2023, 35, e2209324. [Google Scholar] [CrossRef]
- Li, X.; He, L.; Li, Y.; Chao, M.; Li, M.; Wan, P.; Zhang, L. Healable, Degradable, and Conductive MXene Nanocomposite Hydrogel for Multifunctional Epidermal Sensors. ACS Nano 2021, 15, 7765–7773. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Wang, B.; Ren, Q.; Nie, J.; Guo, B.; Lu, Y.; Lu, X.; Zhang, Y.; Ji, D.; Lv, Y.; et al. Fully Implantable Wireless Cardiac Pacing and Sensing System Integrated with Hydrogel Electrodes. Adv. Sci. 2024, 11, e2401982. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, Y.; Shi, S.; Zheng, Y.; Ye, Z.; Liao, J.; Sun, Q.; Dang, B.; Shen, X. Myelin Sheath-Inspired Hydrogel Electrode for Artificial Skin and Physiological Monitoring. ACS Nano 2024, 18, 27420–27432. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xu, H.; Liu, L.; Zheng, Y.; Han, W.; Wang, L. MXene--Induced Flexible, Water-Retention, Semi-Interpenetrating Network Hydrogel for Ultra-Stable Strain Sensors with Real-Time Gesture Recognition. Adv. Sci. 2023, 10, e2303922. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, Z.; Zhang, N.; Li, J.; Zhou, P.; Hu, F.; Rong, Y.; Lu, B.; Gu, G. High--Stretchability, Ultralow--Hysteresis ConductingPolymer Hydrogel Strain Sensors for Soft Machines. Adv. Mater. 2022, 34, e2203650. [Google Scholar] [CrossRef]
- Miao, J.; Tian, M.; Qu, L.; Zhang, X. Flexible, Transparent and Conductive Wearable Electronic Skin Based on 2D Titanium Carbide (MXene) Ink. Carbon 2024, 222, 118950. [Google Scholar] [CrossRef]
- You, L.; Shi, X.; Cheng, J.; Yang, J.; Xiong, C.; Ding, Z.; Zheng, Z.; Wang, S.; Wang, J. Flexible Porous Gelatin/Polypyrrole/Reduction Graphene Oxide Organohydrogel for Wearable Electronics. J. Colloid Interface Sci. 2022, 625, 197–209. [Google Scholar] [CrossRef]
- Lin, Z.; Kireev, D.; Liu, N.; Gupta, S.; LaPiano, J.; Obaid, S.N.; Chen, Z.; Akinwande, D.; Efimov, I.R. Graphene Biointerface for Cardiac Arrhythmia Diagnosis and Treatment. Adv. Mater. 2023, 35, e2212190. [Google Scholar] [CrossRef]
- Yan, C.; Hu, S.; Fei, Q.; Zhang, B.; Wu, W. Advancing Drug Delivery: Design and Applications of MOF-Polyurethane Composites for Controlled Release Systems. ACS Omega 2025, 10, 43363–43378. [Google Scholar] [CrossRef]
- Kush, P.; Singh, R.; Kumar, P. Recent Advances in Metal–Organic Framework-Based Anticancer Hydrogels. Gels 2025, 11, 76. [Google Scholar] [CrossRef]
- Joshi, A.; Choudhury, S.; Baghel, V.S.; Ghosh, S.; Gupta, S.; Lahiri, D.; Ananthasuresh, G.K.; Chatterjee, K. 4D Printed Programmable Shape--Morphing Hydrogels as Intraoperative Self--Folding Nerve Conduits for Sutureless Neurorrhaphy. Adv. Healthc. Mater. 2023, 12, e2300701. [Google Scholar] [CrossRef]
- Joshi, A.; Choudhury, S.; Majhi, A.; Parasuram, S.; Baghel, V.S.; Chauhan, S.; Khanra, S.; Lahiri, D.; Chatterjee, K. 4D-Printed Multifunctional Hydrogels as Flexible Strain Sensors and Nerve Conduits. Biomater. Sci. 2025, 13, 4706–4716. [Google Scholar] [CrossRef]
- Distler, T.; Boccaccini, A.R. 3D Printing of Electrically Conductive Hydrogels for Tissue Engineering and Biosensors–A Review. Acta Biomater. 2020, 101, 1–13. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, L.; Zheng, Y.; Zhao, S.; Wei, W.; Zhang, D.; Fu, X.; Jiang, K.; Shen, G.; Han, W. Highly-Stable Polymer-Crosslinked 2D MXene-Based Flexible Biocompatible Electronic Skins for in Vivo Biomonitoring. Nano Energy 2021, 84, 105921. [Google Scholar] [CrossRef]
- Sun, Z.; Song, C.; Zhou, J.; Hao, C.; Liu, W.; Liu, H.; Wang, J.; Huang, M.; He, S.; Yang, M. Rapid Photothermal Responsive Conductive MXene Nanocomposite Hydrogels for Soft Manipulators and Sensitive Strain Sensors. Macromol. Rapid Commun. 2021, 42, 2100499. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Gong, S.; Xia, Y.; Xu, N.; Yu, J.; Wang, C.; Wang, J.; Yong, Q.; Chu, F. Muscle-like Self-Strengthening Poly(Vinyl Alcohol)/Cellulose/MXene Composite Hydrogel by Mechanical Training for Wearable Electronics. Int. J. Biol. Macromol. 2025, 322, 146706. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, X.; Rong, C.; Ma, X.; Zhang, B.; Xuan, F. Layered Structured MXene/PVA Conductive Hydrogels with Excellent Mechanical Properties for Flexible Strain and Temperature Sensing. Small 2025, 21, e06824. [Google Scholar] [CrossRef]
- Kaur, H.; Gogoi, B.; Sharma, I.; Das, D.K.; Azad, M.A.; Pramanik, D.D.; Pramanik, A. Hydrogels as a Potential Biomaterial for Multimodal Therapeutic Applications. Mol. Pharm. 2024, 21, 4827–4848. [Google Scholar] [CrossRef]
- Gong, S. The Significance of Materials Informatics on Material Science. Appl. Comput. Eng. 2024, 58, 208–214. [Google Scholar] [CrossRef]
- Zivic, F.; Malisic, A.K.; Grujovic, N.; Stojanovic, B.; Ivanovic, M. Materials Informatics: A Review of AI and Machine Learning Tools, Platforms, Data Repositories, and Applications to Architectured Porous Materials. Mater. Today Commun. 2025, 48, 113525. [Google Scholar] [CrossRef]
- Rangineni, S.; Bhanushali, A.; Marupaka, D.; Venkata, S.; Suryadevara, M. Analysis of Data Engineering Techniques With Data Quality in Multilingual Information Recovery. Int. J. Comput. Sci. Eng. 2023, 11, 29–36. [Google Scholar] [CrossRef]
- Negut, I.; Bita, B. Exploring the Potential of Artificial Intelligence for Hydrogel Development—A Short Review. Gels 2023, 9, 845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Chandra Sekhar, P.D.; Singh, M.; Tong, Y.; Kucukdeger, E.; Yoon, H.Y.; Haring, A.P.; Roman, M.; Kong, Z.J.; et al. Rapid, Autonomous High-Throughput Characterization of Hydrogel Rheological Properties via Automated Sensing and Physics-Guided Machine Learning. Appl. Mater. Today 2023, 30, 101720. [Google Scholar] [CrossRef]
- Seifermann, M.; Reiser, P.; Friederich, P.; Levkin, P.A. High--Throughput Synthesis and Machine Learning Assisted Design of Photodegradable Hydrogels. Small Methods 2023, 7, e2300553. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yue, X.; Zhang, J.; Zhai, Z.; Moammeri, A.; Edgar, K.J.; Berahas, A.S.; Al Kontar, R.; Johnson, B.N. Scalable Accelerated Materials Discovery of Sustainable Polysaccharide-Based Hydrogels by Autonomous Experimentation and Collaborative Learning. ACS Appl. Mater. Interfaces 2024, 16, 70310–70321. [Google Scholar] [CrossRef]
- Moosavi, S.M.; Jablonka, K.M.; Smit, B. The Role of Machine Learning in the Understanding and Design of Materials. J. Am. Chem. Soc. 2020, 142, 20273–20287. [Google Scholar] [CrossRef]
- Khosravi, B.; Weston, A.D.; Nugen, F.; Mickley, J.P.; Maradit Kremers, H.; Wyles, C.C.; Carter, R.E.; Taunton, M.J. Demystifying Statistics and Machine Learning in Analysis of Structured Tabular Data. J. Arthroplast. 2023, 38, 1943–1947. [Google Scholar] [CrossRef]
- Li, Z.; Song, P.; Li, G.; Han, Y.; Ren, X.; Bai, L.; Su, J. AI Energized Hydrogel Design, Optimization and Application in Biomedicine. Mater. Today Bio 2024, 25, 101014. [Google Scholar] [CrossRef]
- Bai, L.; Wu, Y.; Li, G.; Zhang, W.; Zhang, H.; Su, J. AI-Enabled Organoids: Construction, Analysis, and Application. Bioact. Mater. 2024, 31, 525–548. [Google Scholar] [CrossRef]
- Fareed, M.M.; Shityakov, S. Next-Generation Hydrogel Design: Computational Advances in Synthesis, Characterization, and Biomedical Applications. Polymers 2025, 17, 1373. [Google Scholar] [CrossRef]
- Xu, L.; Liu, S.; Lin, A.; Su, Z.; Liang, D. Interpretable Prediction and Analysis of PVA Hydrogel Mechanical Behavior Using Machine Learning. Gels 2025, 11, 550. [Google Scholar] [CrossRef]
- Protopapa, C.; Siamidi, A.; Eneli, A.A.; Elbadawi, M.; Vlachou, M. Machine Learning Predicts Drug Release Profiles and Kinetic Parameters Based on Tablets’ Formulations. AAPS J. 2025, 27, 124. [Google Scholar] [CrossRef]
- AL-Rajabi, M.M.; Alzyod, S.; Patel, A.; Teow, Y.H. A Hybrid Machine Learning Framework for Predicting Drug-Release Profiles, Kinetics, and Mechanisms of Temperature-Responsive Hydrogels. Polym. Bull. 2025, 82, 2911–2932. [Google Scholar] [CrossRef]
- Liao, H.; Hu, S.; Yang, H.; Wang, L.; Tanaka, S.; Takigawa, I.; Li, W.; Fan, H.; Gong, J.P. Data-Driven de Novo Design of Super-Adhesive Hydrogels. Nature 2025, 644, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Prado, V.C.; de Carvalho, B.R.F.; Moenke, K.; Zamberlan, A.M.; Atuati, S.F.; Assis, A.C.P.; Brum, E.d.S.; Lazo, R.E.L.; Adams, A.I.H.; Ferreira, L.M.; et al. Repeated Administration of Guar Gum Hydrogel Containing Sesamol-Loaded Nanocapsules Reduced Skin Inflammation in Mice in an Irritant Contact Dermatitis Model. Pharmaceutics 2025, 17, 1029. [Google Scholar] [CrossRef] [PubMed]
- Woodring, R.N.; Gurysh, E.G.; Pulipaka, T.; Shilling, K.E.; Stiepel, R.T.; Pena, E.S.; Bachelder, E.M.; Ainslie, K.M. Supervised Machine Learning for Predicting Drug Release from Acetalated Dextran Nanofibers. Biomater. Sci. 2025, 13, 2806–2823. [Google Scholar] [CrossRef]
- Sourroubille, M.; Miranda-Valdez, I.Y.; Mäkinen, T.; Koivisto, J.; Alava, M.J. Thermogelation of Methylcellulose: A Rheological Approach with Gaussian Process Regression. Colloids Surfaces A Physicochem. Eng. Asp. 2025, 709, 136057. [Google Scholar] [CrossRef]
- Kuenneth, C.; Ramprasad, R. PolyBERT: A Chemical Language Model to Enable Fully Machine-Driven Ultrafast Polymer Informatics. Nat. Commun. 2023, 14, 4099. [Google Scholar] [CrossRef]
- Mohammad, S.; Akand, R.; Cook, K.M.; Nilufar, S.; Chowdhury, F. Leveraging Deep Learning and Generative AI for Predicting Rheological Properties and Material Compositions of 3D Printed Polyacrylamide Hydrogels. Gels 2024, 10, 660. [Google Scholar] [CrossRef]
- Gao, Q.; Dukker, T.; Schweidtmann, A.M.; Weber, J.M. Self-Supervised Graph Neural Networks for Polymer Property Prediction. Mol. Syst. Des. Eng. 2024, 9, 1130–1143. [Google Scholar] [CrossRef]
- Cadamuro, F.; Piazzoni, M.; Gamba, E.; Sonzogni, B.; Previdi, F.; Nicotra, F.; Ferramosca, A.; Russo, L. Artificial Intelligence Tool for Prediction of ECM Mimics Hydrogel Formulations via Click Chemistry. Biomater. Adv. 2025, 175, 214323. [Google Scholar] [CrossRef]
- Abdolghader, P.; Ridsdale, A.; Grammatikopoulos, T.; Resch, G.; Légaré, F.; Stolow, A.; Pegoraro, A.F.; Tamblyn, I. Unsupervised Hyperspectral Stimulated Raman Microscopy Image Enhancement: Denoising and Segmentation via One-Shot Deep Learning. Opt. Express 2021, 29, 34205. [Google Scholar] [CrossRef]
- Koronaki, E.D.; Kaven, L.F.; Faust, J.M.M.; Kevrekidis, I.G.; Mitsos, A. Nonlinear Manifold Learning Determines Microgel Size from Raman Spectroscopy. AIChE J. 2024, 70, e18494. [Google Scholar] [CrossRef]
- Redolfi-Bristol, D.; Yamamoto, K.; Zhu, W.; Mazda, O.; Riello, P.; Marin, E.; Pezzotti, G. Mapping Selenium Nanoparticles Distribution Inside Cells through Confocal Raman Microspectroscopy. ACS Appl. Mater. Interfaces 2025, 17, 18124–18133. [Google Scholar] [CrossRef] [PubMed]
- Piovarči, M.; Foshey, M.; Xu, J.; Erps, T.; Babaei, V.; Didyk, P.; Rusinkiewicz, S.; Matusik, W.; Bickel, B. Closed-Loop Control of Direct Ink Writing via Reinforcement Learning. ACM Trans. Graph. 2022, 41, 1–10. [Google Scholar] [CrossRef]
- Gao, Q.; Schweidtmann, A.M. Deep Reinforcement Learning for Process Design: Review and Perspective. Curr. Opin. Chem. Eng. 2024, 44, 101012. [Google Scholar] [CrossRef]
- Jiang, Z.; Feng, J.; Wang, F.; Wang, J.; Wang, N.; Zhang, M.; Hsieh, C.; Hou, T.; Cui, W.; Ma, L. AI--Guided Design of Antimicrobial Peptide Hydrogels for Precise Treatment of Drug--resistant Bacterial Infections. Adv. Mater. 2025, 37, e2500043. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Aliabouzar, M.; Fabiilli, M.L. Acoustically Responsive Scaffolds: Unraveling Release Kinetics and Mechanisms for Sustained, Steady Drug Delivery. J. Control. Release 2024, 374, 205–218. [Google Scholar] [CrossRef]
- Valentino, A.; Yazdanpanah, S.; Conte, R.; Calarco, A.; Peluso, G. Smart Nanocomposite Hydrogels as Next-Generation Therapeutic and Diagnostic Solutions. Gels 2024, 10, 689. [Google Scholar] [CrossRef]
- Hu, Y.; Pan, Z.; De Bock, M.; Tan, T.X.; Wang, Y.; Shi, Y.; Yan, N.; Yetisen, A.K. A Wearable Microneedle Patch Incorporating Reversible FRET-Based Hydrogel Sensors for Continuous Glucose Monitoring. Biosens. Bioelectron. 2024, 262, 116542. [Google Scholar] [CrossRef]
- Huang, W.; Pang, I.; Bai, J.; Cui, B.; Qi, X.; Zhang, S. Artificial Intelligence-Enhanced, Closed-Loop Wearable Systems Toward Next-Generation Diabetes Management. Adv. Intell. Syst. 2025, 7, 2400822. [Google Scholar] [CrossRef]
- Sarkar, S.K.; Takei, K. Toward Environmentally Friendly Hydrogel-Based Flexible Intelligent Sensor Systems. Adv. Intell. Discov. 2025, 202500041. [Google Scholar] [CrossRef]
- Finster, R.; Sankaran, P.; Bihar, E. Computational and AI-Driven Design of Hydrogels for Bioelectronic Applications. Adv. Electron. Mater. 2025, 11, 2400763. [Google Scholar] [CrossRef]
- Goetz, L.H.; Schork, N.J. Personalized Medicine: Motivation, Challenges, and Progress. Fertil. Steril. 2018, 109, 952–963. [Google Scholar] [CrossRef]
- Su, J.; Yang, L.; Sun, Z.; Zhan, X. Personalized Drug Therapy: Innovative Concept Guided With Proteoformics. Mol. Cell. Proteomics 2024, 23, 100737. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, M.; Xu, T.; Zhang, X. Multifunctional Hydrogel as Wound Dressing for Intelligent Wound Monitoring. Chem. Eng. J. 2022, 433, 134625. [Google Scholar] [CrossRef]
- Niu, Y.; Zhao, Z.; Yang, L.; Lv, D.; Sun, R.; Zhang, T.; Li, Y.; Bao, Q.; Zhang, M.; Wang, L.; et al. Towards Intelligent Wound Care: Hydrogel-Based Wearable Monitoring and Therapeutic Platforms. Polymers 2025, 17, 1881. [Google Scholar] [CrossRef]
- Yao, C.; Liu, Z.; Yang, C.; Wang, W.; Ju, X.; Xie, R.; Chu, L. Poly(N-isopropylacrylamide)-Clay Nanocomposite Hydrogels with Responsive Bending Property as Temperature-Controlled Manipulators. Adv. Funct. Mater. 2015, 25, 2980–2991. [Google Scholar] [CrossRef]
- Chen, M.; Liu, H.; Chen, X.; Kang, L.; Yao, X.; Tan, L.; Zhu, W.; Yu, J.; Qin, X.; Wu, D. A Novel Multifunction of Wearable Ionic Conductive Hydrogel Sensor for Promoting Infected Wound Healing. Appl. Mater. Today 2024, 39, 102298. [Google Scholar] [CrossRef]
- Wu, J.; Li, Y.; Duan, S.; Wang, Z.; Jing, X.; Lin, Y.; Zhu, D.; Lei, W.; Shi, Q.; Tao, L. Bioinspired Stretchable MXene Deformation-Insensitive Hydrogel Temperature Sensors for Plant and Skin Electronics. Research 2023, 6, 106. [Google Scholar] [CrossRef]
- Li, W.; Yu, Y.; Huang, R.; Wang, X.; Lai, P.; Chen, K.; Shang, L.; Zhao, Y. Multi--Bioinspired Functional Conductive Hydrogel Patches for Wound Healing Management. Adv. Sci. 2023, 10, e2301479. [Google Scholar] [CrossRef]
- Abri, S.; Durr, H.; Barton, H.A.; Adkins-Travis, K.; Shriver, L.P.; Pukale, D.D.; Fulton, J.A.; Leipzig, N.D. Chitosan-Based Multifunctional Oxygenating Antibiotic Hydrogel Dressings for Managing Chronic Infection in Diabetic Wounds. Biomater. Sci. 2024, 12, 3458–3470. [Google Scholar] [CrossRef] [PubMed]
- Wiraja, C.; Ning, X.; Cui, M.; Xu, C. Hydrogel-Based Technologies for the Diagnosis of Skin Pathology. Technologies 2020, 8, 47. [Google Scholar] [CrossRef]
- Xia, J.; Sonkusale, S. Flexible Thread-Based Electrochemical Sensors for Oxygen Monitoring. Analyst 2021, 146, 2983–2990. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, Z.; Wei, Y.; Ding, Q.; Zilberman, M.; Tao, K.; Xie, X.; Wu, J. Self-Healing, Self-Adhesive and Stable Organohydrogel-Based Stretchable Oxygen Sensor with High Performance at Room Temperature. Nano-Micro Lett. 2022, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Buchberger, A.; Peterka, S.; Coclite, A.M.; Bergmann, A. Fast Optical Humidity Sensor Based on Hydrogel Thin Film Expansion for Harsh Environment. Sensors 2019, 19, 999. [Google Scholar] [CrossRef]
- Wu, Z.; Ding, Q.; Li, Z.; Zhou, Z.; Luo, L.; Tao, K.; Xie, X.; Wu, J. Ultrasensitive, Stretchable, and Transparent Humidity Sensor Based on Ion-Conductive Double-Network Hydrogel Thin Films. Sci. China Mater. 2022, 65, 2540–2552. [Google Scholar] [CrossRef]
- Eskilson, O.; Zattarin, E.; Berglund, L.; Oksman, K.; Hanna, K.; Rakar, J.; Sivlér, P.; Skog, M.; Rinklake, I.; Shamasha, R.; et al. Nanocellulose Composite Wound Dressings for Real-Time PH Wound Monitoring. Mater. Today Bio 2023, 19, 100574. [Google Scholar] [CrossRef]
- Gamerith, C.; Luschnig, D.; Ortner, A.; Pietrzik, N.; Guse, J.-H.; Burnet, M.; Haalboom, M.; van der Palen, J.; Heinzle, A.; Sigl, E.; et al. PH-Responsive Materials for Optical Monitoring of Wound Status. Sens. Actuators B Chem. 2019, 301, 126966. [Google Scholar] [CrossRef]
- Rahimi, R.; Brener, U.; Chittiboyina, S.; Soleimani, T.; Detwiler, D.A.; Lelièvre, S.A.; Ziaie, B. Laser-Enabled Fabrication of Flexible and Transparent PH Sensor with near-Field Communication for in-Situ Monitoring of Wound Infection. Sens. Actuators B Chem. 2018, 267, 198–207. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Yang, X.; Shi, Z.; Li, J.; Xue, L.; Liu, S.; Lei, Y. A Responsive Hydrogel-Based Microneedle System for Minimally Invasive Glucose Monitoring. Smart Mater. Med. 2023, 4, 69–77. [Google Scholar] [CrossRef]
- Zhang, S.; Ge, G.; Qin, Y.; Li, W.; Dong, J.; Mei, J.; Ma, R.; Zhang, X.; Bai, J.; Zhu, C.; et al. Recent Advances in Responsive Hydrogels for Diabetic Wound Healing. Mater. Today Bio 2023, 18, 100508. [Google Scholar] [CrossRef]
- Giovannini, G.; Cinelli, P.; Boesel, L.F.; Rossi, R.M. Thioflavin-Modified Molecularly Imprinted Hydrogel for Fluorescent-Based Non-Enzymatic Glucose Detection in Wound Exudate. Mater. Today Bio 2022, 14, 100258. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Liu, F.; Yang, X.; Xia, Y. The Key Role of Uric Acid in Oxidative Stress, Inflammation, Fibrosis, Apoptosis, and Immunity in the Pathogenesis of Atrial Fibrillation. Front. Cardiovasc. Med. 2021, 8, 641136. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.L.; Upton, Z.; Edwards, H.; Finlayson, K.; Shooter, G.K. Elevated Uric Acid Correlates with Wound Severity. Int. Wound J. 2012, 9, 139–149. [Google Scholar] [CrossRef] [PubMed]
- RoyChoudhury, S.; Umasankar, Y.; Jaller, J.; Herskovitz, I.; Mervis, J.; Darwin, E.; Hirt, P.A.; Borda, L.J.; Lev-Tov, H.A.; Kirsner, R.; et al. Continuous Monitoring of Wound Healing Using a Wearable Enzymatic Uric Acid Biosensor. J. Electrochem. Soc. 2018, 165, B3168–B3175. [Google Scholar] [CrossRef]
- Kassal, P.; Kim, J.; Kumar, R.; de Araujo, W.R.; Steinberg, I.M.; Steinberg, M.D.; Wang, J. Smart Bandage with Wireless Connectivity for Uric Acid Biosensing as an Indicator of Wound Status. Electrochem. Commun. 2015, 56, 6–10. [Google Scholar] [CrossRef]
- Wang, Z.; Hao, Z.; Yu, S.; Huang, C.; Pan, Y.; Zhao, X. A Wearable and Deformable Graphene-Based Affinity Nanosensor for Monitoring of Cytokines in Biofluids. Nanomaterials 2020, 10, 1503. [Google Scholar] [CrossRef]
- Lee, K.K.; Go, K.; Lee, E.; Kim, H.; Kim, S.; Kim, J.-H.; Chae, M.S.; Jeong, J.-O. Multifunctional Hydrogels for Advanced Cancer Treatment: Diagnostic Imaging and Therapeutic Modalities. Gels 2025, 11, 426. [Google Scholar] [CrossRef]
- Kaladharan, K.; Ouyang, C.-H.; Yang, H.-Y.; Tseng, F.-G. Selectively Cross-Linked Hydrogel-Based Cocktail Drug Delivery Micro-Chip for Colon Cancer Combinatorial Drug Screening Using AI-CSR Platform for Precision Medicine. Lab. Chip 2024, 24, 4766–4777. [Google Scholar] [CrossRef]
- Abdullah, M.; Obayedullah, M.; Shariful Islam Shuvo, M.; Abul Khair, M.; Hossain, D.; Nahidul Islam, M. A Review on Multifunctional Applications of Nanoparticles: Analyzing Their Multi-Physical Properties. Results Surf. Interfaces 2025, 21, 100635. [Google Scholar] [CrossRef]
| Method | Principle | Advantages | Limitations |
|---|---|---|---|
| In situ synthesis ofNPs within hydrogels | Metal precursor ions diffuse into the polymer network and are reduced in situ to form NPs inside the gel. | Uniform particle distribution, strong integration with the matrix, and controlled size. | Requires careful reaction optimization; harsh conditions may damage the gel. |
| Mixing pre-synthesizedNPs before gelation | Stable colloidal dispersions are mixed with the polymer solution, followed by gelation. | Simple and fast; suitable for sensitive biomaterials. | Weaker interaction with the matrix; risk of aggregation. |
| Post-gel functionalization | The hydrogel is formed first and then decorated with NPs through covalent bonding or physical adsorption. | Flexible surface modification; tunable surface properties. | NPs remain mostly at the surface; the procedure can be complex. |
| Self-assembly/supramolecular assembly | NPs and polymers self-assemble into a network via supramolecular interactions. | Enables complex architectures, reversible interactions, and self-healing potential. | Sensitive to environmental changes; requires precise conditions. |
| Polymerization in the presence ofNPs | NPs act as nucleation or anchoring sites during monomer polymerization. | Strong integration into the network; enhanced stability. | Possible toxicity of initiators; requires careful control. |
| Layer-by-layer assembly and electrofabrication | Hydrogels are formed in multiple layers or with multiple networks incorporating NPs. | Advanced functionalities; precise tailoring; improved stability and durability; multifunctionality. | More complex and time-consuming preparation. |
| Hydrogel (Matrix) | NPs Type | Key Improvements | Application | Reference |
|---|---|---|---|---|
| Carboxylated chitosan hydrogel | Ag NPs (in situ, with berberine) | Stronger antibacterial/anti-biofilm activity; improved stability in infected wounds; mechanical integrity | Wound dressing for drug-resistant bacterial infections | [131] |
| Thiolated polyvinyl alcohol/polyethylene glycol diacrylate | Drug-loaded nanogels (CS/Au nanogels) | Rapid in situ gelation; higher mechanical strength; improved drug retention and localized delivery | Localized breast cancer therapy (injectable in situ gel) | [132] |
| Fenugreek polysaccharide hydrogel | Metal-oxide NPs (MnO2, Fe2O3, CuO formed in situ) | Enhanced thermal stability and mechanical robustness; long-lasting antibacterial and antioxidant activity | Wound healing, antimicrobial coatings, drug delivery | [133] |
| pNIPAm/nanogel composite hydrogel | Linker-modified Au NPs | Higher tensile strength; rapid NIR-triggered self-healing; thermo-responsive swelling; strong skin adhesion | Wearable wound dressings, on-demand therapeutics | [134] |
| Gelatin methacryloyl | CeO2 NPs | Improved mechanical stability; antioxidant activity; sustained therapeutic effect; enhanced wound closure | Diabetic wound-healing patch | [135] |
| Chitosan-P407-PNIPAm composite hydrogel | Ag NPs (lipid-complexed) | Enhanced injectability; higher mechanical strength; antibacterial and anti-inflammatory effects; bone regeneration | Periodontitis/alveolar bone regeneration | [136] |
| Carrageenan gelatin hydrogel | Au nanobipyramids (carvacrol loaded) | Photothermal enhancement of chemotherapy; improved local heating and drug release | Cancer therapy (photothermal chemotherapy) | [137] |
| Hyaluronic acid-phenylboronic acid hydrogel | Tea polyphenol-stabilized Ag NPs | Dynamic nano-crosslinking; self-healing; antioxidant and antibacterial activity; controlled release | Diabetic wound healing dressings | [138] |
| Locust bean gum/PVA hydrogel | Ag NPs (green synthesis) | Increased porosity; pH-dependent swelling; improved antibacterial performance; better release control | Antibacterial drug delivery/wound care | [139] |
| Laminarin/PVA hydrogel | Ag NPs (laminarin-reduced) | Improved mechanical stability; accelerated diabetic wound healing; controlled antibacterial release | Diabetic wound healing | [91] |
| Gelatin/chitosan hydrogel | Ag NPs (double-capped with curdlan derivatives) | Enhanced antibacterial efficacy; stronger adhesion; sustained release; improved mechanical robustness | Antibacterial wound dressings | [140] |
| Bilayer carrageenan/alginate hydrogel | Ag NPs (green synthesis) | Effective antibacterial activity; rapid drug delivery; multilayer-controlled release | Rapid wound infection control | [141] |
| Hyaluronic acid and quaternized chitosan | Biomimetic Ag NPs | Injectable and self-healing; antibacterial activity; tailored mechanics | Injectable wound therapy | [142] |
| Polyacrylamide hydrogel | SrO-CoO bimetallic oxide NPs | Improved controlled drug release; stronger mechanics; reduced leaching | Wound healing and controlled release scaffolds | [143] |
| Polyacrylamide (PAA) hydrogel crosslinked with MBA (PAA-MBA) | AgNPs of different shapes: spherical, triangular, rod | Significant increase in storage modulus & Young’s modulus versus NP-free hydrogel; enhanced mechanical strength | Wound dressing, antimicrobial hydrogel materials | [144] |
| Methacrylated gelatin hydrogel | Streamlined-ZnO (non-spherical high-curvature ZnO NPs), PEI/miR-17 complexes | Improved mechanical/rheological properties, MMP-responsive and sustained release of miR-17, enhanced stability, better injectability | Injectable hydrogel for cartilage repair, delivery of miR-17 for osteoarthritis treatment | [145] |
| Carboxymethyl tamarind kernel gum (CMTKG)/Poly(sodium acrylate), crosslinked with PEGDA | ZnO NPs | Improved swelling capacity and thermal stability; increased porosity and gel content; enhanced antibacterial activity; controlled release of ciprofloxacin following Korsmeyer–Peppas (Fickian diffusion) model | Antibacterial hydrogel for controlled drug delivery (ciprofloxacin) | [146] |
| PAA@Gelatin | PDA@Ag (Ag NPs grown on PDA nanospheres) | Enhanced mechanical strength, self-healing, adhesion, reversibility; uniform NP dispersion; higher photothermal conversion efficiency; synergistic antibacterial and analgesic effects | Sustained delivery of lidocaine hydrochloride (LiH); localized long-acting analgesia with NIR-triggered on-demand photothermal pain relief | [147] |
| Bacterial cellulose + Gelatin | Selenium NPs | Enhanced mechanical strength, swelling, flexibility, and biodegradability; slow and sustained release of SeNPs; strong antioxidant, anti-inflammatory, and antibacterial activity (including against multidrug-resistant strains) | Wound dressing for infection prevention and accelerated skin regeneration | [148] |
| Type of NPs | Mechanical Properties | Release Profile | Biocompatibility | Application | Ref. |
|---|---|---|---|---|---|
| Ag NPs | ↑ Stiffness & toughness; enhanced wet adhesion | Sustained release; prolonged antibacterial effect | No cytotoxicity up to 50 µg/mL; >90% cell viability | Wound dressing; antibacterial coatings | [113,140] |
| Au NPs | Tensile strength ↑ 2×; ~85% self-healing in 2 min under NIR | Precise thermo-responsive release; NIR-triggered delivery | Viability > 85% up to 100 µg/mL; good cytocompatibility | Wound healing; wearable therapeutics; bioinks | [92,111] |
| Fe3O4 NPs | ↑ Elastic modulus; puncture resistance; swelling ↑; strong magnetic responsiveness | Tunable release with external magnetic field (54 → 90%); sustained antibiotic release | Cytocompatible at <200 µg/mL; cell proliferation supported | Ferrogels; cartilage repair; guided drug delivery | [117,118] |
| TiO2, ZrO2 (TMOs) | ↑ Compressive strength, elastic modulus; self-healing | Enhanced release under enzymatic or pH stimuli | Good cytocompatibility; non-toxic at tested levels | Diabetic wound healing; bone/cartilage regeneration | [114,115] |
| Se NPs | Improved flexibility, biodegradability, and toughness | Slow, sustained Se release; antioxidant effect | No significant cytotoxicity; promotes angiogenesis | Wound healing; antimicrobial and anti-inflammatory dressings | [148] |
| ZnO NPs | ↑ Porosity and swelling capacity, thermal stability | Ciprofloxacin release fitted Korsmeyer–Peppas model followed by Fickian diffusion | Safe in low–moderate loading; antibacterial action enhanced | Controlled antibiotic release; infection prevention | [146] |
| MSNs (SiO2) | ↑ Network density; structural stability | Reduced burst release; prolonged IGF-1 release > 144 h | High biocompatibility; no acute toxicity reported | Sustained multidrug delivery; stability of labile drugs | [123] |
| Method | Type | Short Description of the Method |
|---|---|---|
| MTT | Tetrazolium dye indicator 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide | Tetrazolium dyes react with the live cell enzymes, which convert them to intensely colored formazan crystals. Crystals are then dissolved, and the absorbance is measured [187,188,189]. |
| MTS | Tetrazolium dye indicator 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium | |
| XTT | Tetrazolium dye indicator 2,3-Bis-(2-Methoxy-4-Nitro-5-Sulfophenyl)-2H-Tetrazolium-5-Carboxanilide | |
| Cell counting kit-8 | Tetrazolium dye indicator WST-8 (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt) | |
| Neutral red uptake | Cationic dye indicator (Dimethyldiaminotoluphenazine) | Viable cells absorb the dye, after which it is located in the lysosomes. After washing out the unabsorbed color, the dye is solubilized, and absorbance is measured [187,189,190]. |
| Resazurin | Phenoxazine dye, blue | Redox indicator, cell-permeable. In viable cells is reduced to a fluorescent, pink product, resorufin. Quantification of signal is done by fluorescent spectrophotometry [187,188]. |
| LDH assays | Lactate dehydrogenase (LDH) based assays. | LDH is released from dead or damaged cells; therefore, its amount is inversely proportional to the number of healthy cells. Detection can be fluorometric, colorimetric, or bioluminescent [187,189]. |
| Hemolysis assay | Measuring the release of hemoglobin | Released hemoglobin is an indicator of the lysis of erythrocytes. It is detected using spectrophotometry [187,191]. |
| Live/Dead staining techniques | A combination of fluorescent dyes | The principle of the fluorescent live/dead staining is based on a combination of two different fluorescent dyes, one of which stains live cells, while the other stains dead cells. The detection and quantification are then performed by fluorescent microscopy or cytofluorimetry [184]. |
| Test Name | Purpose/Description |
|---|---|
| Bacterial reverse mutation test | Detects the ability of a test substance to induce reverse mutations in specialized bacterial strains that possess easily detectable mutations for certain traits [192]. |
| Chromosome aberration test | Testing of the potential of induction of structural damage in chromosomes in metaphase in mammalian cells (clastogenesis). Uses specialized stains and is based on microscopy. Can be performed on the in vitro or in vivo samples [194]. |
| Micronucleus assay | Testing of damage to chromosomes, structural as well as numerical (aneuploidy). Uses specialized stains and is based on microscopic detection of micronuclei (interphase chromosomes, or chromosome fragments). Can be performed on the in vitro or in vivo samples [194]. |
| Comet assay | Detection of induction of DNA breaks in vitro or in vivo. Based on the electrophoresis of DNA fragments and microscopic evaluation [195]. |
| Parameter | Typical Target/Range (Application-Dependent) | Why it Matters (EP/ES) | Design Levers (Nanomaterials & Chemistry) | Verification/Notes | Recent Examples (Last ≤5 yrs) |
|---|---|---|---|---|---|
| Water content | 50–90% (w/w) | High water lowers skin–electrode impedance, improves comfort & ion transport | Organohydrogels (water–glycerol), zwitterionic/ionic gels; humectants | Gravimetry; mass loss vs. time under use-like RH/temp | [205,206] |
| Elastic (Young’s) modulus | 1–100 kPa (skin-like) | Conformality → stable contact, less motion artifact & hot spots | Soft ionogels; catechol/boronate networks; nanofillers to tune | Uniaxial compression/tension; DMA | [202,207] |
| Conductivity (ionic +/− electronic) | 10−4–10−2 S·cm−1 (ionic gels) to ≥100 S·cm−1 (mixed electronic/ionic) | Low interface Z, stable bi-phasic stimulation, low noise | PEDOT:PSS, MXene, graphene/CNT networks; salt/ILs for ionic pathways | 4-point probe/EIS in wet state; temp & frequency sweeps | [203,207,208] |
| Skin–electrode impedance (10–100 Hz) | ECG/EMG: ≤1 kΩ; EEG (hair): typically few–tens kΩ with good SNR | Sets noise floor & stimulation efficiency | Larger area; conductive fillers; chloride buffering; soft fit | EIS on body; report area, pressure, hair/skin prep | [205,209] |
| Interfacial impedance @1 kHz | ≲102–103 Ω·cm2 (recording); lower preferred for ES | Minimizes polarization, improves charge transfer | Mixed conduction (PEDOT:PSS/MXene), Ag/AgCl buffering | EIS with physiological electrolyte | [207,210] |
| Peel adhesion (180°) | ~0.3–1.5 N·cm−1 | Secure but gentle removal; stable long-wear contact | Catechol/zwitterion/boronate motifs; microtexture | ASTM/ISO peel at controlled rate; cyclic reuse | [205] |
| “Reversible” adhesion | Multi-use on/off with minimal residue & stable Z | Re-positioning; repeated sessions without irritation | Thermo-/moisture-switchable bonds; ionic/zwitterionic gels | Track Z & peel over cycles (10–100×) | [205] |
| Dehydration stability | Minimal drift over 4–72 h | Stable impedance & SNR during wear | Organohydrogels (glycerol/IL), barrier films with tuned WVTR | Mass/impedance drift under wear-like conditions | [203] |
| Charge injection capacity & polarization (ES) | Stable under biphasic pulses; low DC offset | Avoids tissue damage & drift | PEDOT:PSS/MXene networks; chloride reservoirs; smooth edges | Voltage transient analysis; CIC vs. pulse width & density | [207,210] |
| Current density uniformity (ES) | No edge hot-spots (validated maps) | Prevents burns & pain; uniform fields | Filleted geometries; current-spreading meshes/inks | FEA + thermal mapping under load | [210] |
| Biocompatibility | Meets ISO 10993-5/-10/-23 | Safety for long-term skin/implants | High-purity monomers; thorough wash; antibacterial without polarization | Cytotox/irritation/sensitization panels | [205,211] |
| Application scope | EP (EEG/ECG/EMG), ES (TENS/EMS), neuromodulation | Versatility across recording + stimulation | Tailor filler type/loading to band & duty | Report per-modality benchmarks | [206,212] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stevanović, M.; Jović, M.; Filipović, N.; Lukač, S.; Tomić, N.; Popović Maneski, L.; Stojanović, Z. Multifunctional Nanomaterial-Integrated Hydrogels for Sustained Drug Delivery: From Synthesis and Characterization to Biomedical Application. Gels 2025, 11, 892. https://doi.org/10.3390/gels11110892
Stevanović M, Jović M, Filipović N, Lukač S, Tomić N, Popović Maneski L, Stojanović Z. Multifunctional Nanomaterial-Integrated Hydrogels for Sustained Drug Delivery: From Synthesis and Characterization to Biomedical Application. Gels. 2025; 11(11):892. https://doi.org/10.3390/gels11110892
Chicago/Turabian StyleStevanović, Magdalena, Maja Jović, Nenad Filipović, Sara Lukač, Nina Tomić, Lana Popović Maneski, and Zoran Stojanović. 2025. "Multifunctional Nanomaterial-Integrated Hydrogels for Sustained Drug Delivery: From Synthesis and Characterization to Biomedical Application" Gels 11, no. 11: 892. https://doi.org/10.3390/gels11110892
APA StyleStevanović, M., Jović, M., Filipović, N., Lukač, S., Tomić, N., Popović Maneski, L., & Stojanović, Z. (2025). Multifunctional Nanomaterial-Integrated Hydrogels for Sustained Drug Delivery: From Synthesis and Characterization to Biomedical Application. Gels, 11(11), 892. https://doi.org/10.3390/gels11110892










