Influence of Calcium Crosslinker Form on Alginate Hydrogel Properties
Abstract
1. Introduction
2. Results and Discussion
3. Conclusions
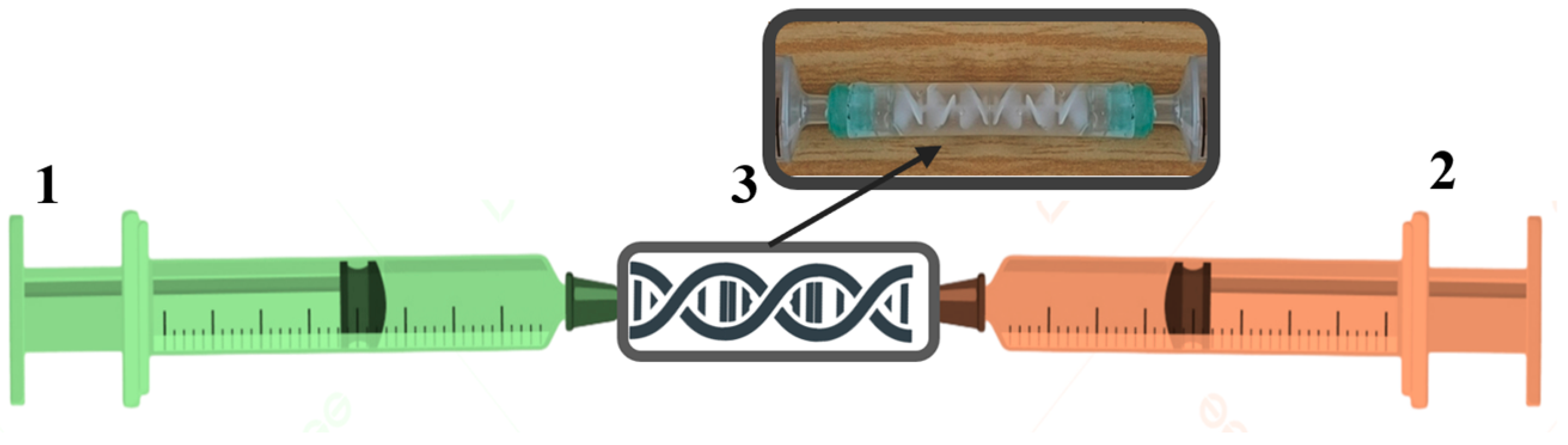
4. Materials and Methods
4.1. Materials
4.2. Crosslinker Synthesis
4.3. Hydrogel Synthesis
4.4. Equilibrium Swelling in Water
4.5. Calcium Salt Density
4.6. Mesh Size Evaluation
4.7. Gel Fraction
4.8. Dynamic Mechanical Test
4.9. Scanning Electron Microscopy
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GF | Gel fraction |
| SA | Sodium alginate |
| PAA | Poly(acrylic acid) |
References
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the Potential of Hydrogels for Advanced Therapeutic Applications: Current Achievements and Future Directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef]
- Zhu, T.; Ni, Y.; Biesold, G.M.; Cheng, Y.; Ge, M.; Li, H.; Huang, J.; Lin, Z.; Lai, Y. Recent Advances in Conductive Hydrogels: Classifications, Properties, and Applications. Chem. Soc. Rev. 2023, 52, 473–509. [Google Scholar] [CrossRef]
- Liu, B.; Chen, K. Advances in Hydrogel-Based Drug Delivery Systems. Gels 2024, 10, 262. [Google Scholar] [CrossRef]
- Petrina, R.O.; Kurka, M.S.; Holubovska, Y.I.; Suberlyak, S.A.; Fedorova, O.V.; Hrytsenko, O.M. Research of Complex of Calendula Officinalis Extract–Hydrogel for Application in Cosmeceuticals. Vopr. Khim. Khim. Tekhnol. 2022, 3, 53–59. [Google Scholar] [CrossRef]
- Grytsenko, O.; Pukach, P.; Suberlyak, O.; Shakhovska, N.; Karovič, V., Jr. Usage of Mathematical Modeling and Optimization in Development of Hydrogel Medical Dressings Production. Electronics 2021, 10, 620. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, Y.; Zhang, J.; Liang, H.; Chen, X.; Tan, H. Natural Polymer-Based Hydrogels: From Polymer to Biomedical Applications. Pharmaceutics 2023, 15, 2514. [Google Scholar] [CrossRef] [PubMed]
- Tomić, S.L.; Babić Radić, M.M.; Vuković, J.S.; Filipović, V.V.; Nikodinovic-Runic, J.; Vukomanović, M. Alginate-Based Hydrogels and Scaffolds for Biomedical Applications. Mar. Drugs 2023, 21, 177. [Google Scholar] [CrossRef]
- Berradi, A.; Aziz, F.; Achaby, M.E.; Ouazzani, N.; Mandi, L. A Comprehensive Review of Polysaccharide-Based Hydrogels as Promising Biomaterials. Polymers 2023, 15, 2908. [Google Scholar] [CrossRef]
- Abka-khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef]
- Chen, X.; Wu, T.; Bu, Y.; Yan, H.; Lin, Q. Fabrication and Biomedical Application of Alginate Composite Hydrogels in Bone Tissue Engineering: A Review. Int. J. Mol. Sci. 2024, 25, 7810. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, Z.; Wang, H.; Song, Z.; Yu, D.; Li, G.; Liu, X.; Liu, W. Progress in Research on Metal Ion Crosslinking Alginate-Based Gels. Gels 2024, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-Box Model-Based Gelation of Alginate and Pectin: A Review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef]
- Hu, C.; Lu, W.; Sun, C.; Zhao, Y.; Zhang, Y.; Fang, Y. Gelation Behavior and Mechanism of Alginate with Calcium: Dependence on Monovalent Counterions. Carbohydr. Polym. 2022, 294, 119788. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; He, J.; Sun, C.; Lu, W.; Zhang, Y.; Fang, Y. Doubling Growth of Egg-Box Structure during Calcium-Mediated Molecular Assembly of Alginate. J. Colloid Interface Sci. 2023, 634, 747–756. [Google Scholar] [CrossRef]
- Donati, I.; Christensen, B.E. Alginate-Metal Cation Interactions: Macromolecular Approach. Carbohydr. Polym. 2023, 321, 121280. [Google Scholar] [CrossRef]
- Joshi, A.; Choudhury, S.; Majhi, A.; Parasuram, S.; Baghel, V.S.; Chauhan, S.; Khanra, S.; Lahiri, D.; Chatterjee, K. 4D-Printed Multifunctional Hydrogels as Flexible Strain Sensors and Nerve Conduits. Biomater. Sci. 2025, 13, 4706–4716. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Du, J.; Mao, Y. Hydrogel-Based Continuum Soft Robots. Gels 2025, 11, 254. [Google Scholar] [CrossRef]
- Gounden, V.; Singh, M. Hydrogels and Wound Healing: Current and Future Prospects. Gels 2024, 10, 43. [Google Scholar] [CrossRef]
- Charbonier, F.; Indana, D.; Chaudhuri, O. Tuning Viscoelasticity in Alginate Hydrogels for 3D Cell Culture Studies. Current Protoc. 2021, 1, e124. [Google Scholar] [CrossRef]
- Ji, D.; Park, J.M.; Oh, M.S.; Nguyen, T.L.; Shin, H.; Kim, J.S.; Kim, D.; Park, H.S.; Kim, J. Superstrong, Superstiff, and Conductive Alginate Hydrogels. Nat. Commun. 2022, 13, 3019. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Rowley, J.A.; Eiselt, P.; Moy, E.M.; Bouhadir, K.H.; Mooney, D.J. Controlling Mechanical and Swelling Properties of Alginate Hydrogels Independently by Cross-Linker Type and Cross-Linking Density. Macromolecules 2000, 33, 4291–4294. [Google Scholar] [CrossRef]
- Malektaj, H.; Drozdov, A.D.; deClaville Christiansen, J. Mechanical Properties of Alginate Hydrogels Cross-Linked with Multivalent Cations. Polymers 2023, 15, 3012. [Google Scholar] [CrossRef]
- Besco, S.; Brisotto, M.; Gianoncelli, A.; Depero, L.E.; Bontempi, E.; Lorenzetti, A.; Modesti, M. Processing and Properties of Polypropylene-based Composites Containing Inertized Fly Ash from Municipal Solid Waste Incineration. J. Appl. Polym. Sci. 2013, 130, 4157–4164. [Google Scholar] [CrossRef]
- Nahorniak, M.; Fihurka, N.; Nosova, N.; Varvarenko, S.; Bendoraitiene, J.; Peciulyte, L.; Rutkaite, R.; Samaryk, V. Synthesis and Study on the Properties of Polysaccharides Modified via the Steglich Reaction. Appl. Nanosci. 2023, 13, 7413–7423. [Google Scholar] [CrossRef]
- Shen, K.-H.; Yeh, Y.-Y.; Chiu, T.-H.; Wang, R.; Yeh, Y.-C. Dual Dynamic Covalently Crosslinked Alginate Hydrogels with Tunable Properties and Multiple Stimuli-Responsiveness. ACS Biomater. Sci. Eng. 2022, 8, 4249–4261. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Wang, Q.; Han, Y.; Chen, H.; Tan, Y. Doubly Dynamic Hydrogel Formed by Combining Boronate Ester and Acylhydrazone Bonds. Polymers 2020, 12, 487. [Google Scholar] [CrossRef]
- Wijayapala, R.; Hashemnejad, S.M.; Kundu, S. Carbon Nanodots Crosslinked Photoluminescent Alginate Hydrogels. RSC Adv. 2017, 7, 50389–50395. [Google Scholar] [CrossRef]
- Vlizlo, V.; Ostapiv, D.; Kuzmina, N.; Mysak, A.; Nosova, N.; Varvarenko, S.; Samaryk, V.; Stybel, V.; Ostapiv, R. Sorptive Properties of Hydrogel Dressings and Their Antimicrobial Action after Saturation with Tetracyclines. Regul. Mech. Biosyst. 2025, 16, e25088. [Google Scholar] [CrossRef]
- Hashemnejad, S.M.; Kundu, S. Rheological Properties and Failure of Alginate Hydrogels with Ionic and Covalent Crosslinks. Soft Matter 2019, 15, 7852–7862. [Google Scholar] [CrossRef]
- Rees, D.A.; Welsh, E.J. Secondary and Tertiary Structure of Polysaccharides in Solutions and Gels. Angew. Chem. Int. Ed. Engl. 1977, 16, 214–224. [Google Scholar] [CrossRef]
- Xu, P.; Song, J.; Dai, Z.; Xu, Y.; Li, D.; Wu, C. Effect of Ca2+ Cross-Linking on the Properties and Structure of Lutein-Loaded Sodium Alginate Hydrogels. Int. J. Biol. Macromol. 2021, 193, 53–63. [Google Scholar] [CrossRef]
- Savić Gajić, I.M.; Savić, I.M.; Svirčev, Z. Preparation and Characterization of Alginate Hydrogels with High Water-Retaining Capacity. Polymers 2023, 15, 2592. [Google Scholar] [CrossRef]
- Girón-Hernández, J.; Gentile, P.; Benlloch-Tinoco, M. Impact of Heterogeneously Crosslinked Calcium Alginate Networks on the Encapsulation of β-Carotene-Loaded Beads. Carbohydr. Polym. 2021, 271, 118429. [Google Scholar] [CrossRef] [PubMed]
- Growney Kalaf, E.A.; Flores, R.; Bledsoe, J.G.; Sell, S.A. Characterization of Slow-Gelling Alginate Hydrogels for Intervertebral Disc Tissue-Engineering Applications. Mater. Sci. Eng. C 2016, 63, 198–210. [Google Scholar] [CrossRef]
- Hidayat, E.; Sarbani, N.M.M.; Lahiri, S.K.; Samitsu, S.; Yonemura, S.; Mitoma, Y.; Harada, H. Effects of Sodium Alginate-Poly(Acrylic Acid) Cross-Linked Hydrogel Beads on Soil Conditioner in the Absence and Presence of Phosphate and Carbonate Ions. Case Stud. Chem. Environ. Eng. 2024, 9, 100642. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Kirar, N. Swelling and Drug Release Behavior of Calcium Alginate/Poly (Sodium Acrylate) Hydrogel Beads. Des. Monomers Polym. 2016, 19, 89–98. [Google Scholar] [CrossRef]
- Bukartyk, M.M.; Nosova, N.H.; Maikovych, O.V.; Bukartyk, N.M.; Stasiuk, A.V.; Dron, I.A.; Fihurka, N.V.; Khomiak, S.V.; Ostapiv, D.D.; Vlislo, V.V.; et al. Preparation and research of properties of combined alginate/gelatin hydrogels. J. Chem. Tech. 2022, 30, 11–20. [Google Scholar] [CrossRef]
- Maikovych, O.; Nosova, N.; Bukartyk, N.; Fihurka, N.; Ostapiv, D.; Samaryk, V.; Pasetto, P.; Varvarenko, S. Gelatin-Based Hydrogel with Antiseptic Properties: Synthesis and Properties. Appl. Nanosci. 2023, 13, 7611–7623. [Google Scholar] [CrossRef]
- Giz, A.S.; Berberoglu, M.; Bener, S.; Aydelik-Ayazoglu, S.; Bayraktar, H.; Alaca, B.E.; Catalgil-Giz, H. A Detailed Investigation of the Effect of Calcium Crosslinking and Glycerol Plasticizing on the Physical Properties of Alginate Films. Int. J. Biol. Macromol. 2020, 148, 49–55. [Google Scholar] [CrossRef]
- Požar, M.; Lovrinčević, B. Structure and Dynamics in Aqueous Mixtures of Glycerol: Insights from Molecular Dynamics Simulations. Soft Matter 2024, 20, 8061–8067. [Google Scholar] [CrossRef]
- Eslami, Z.; Elkoun, S.; Robert, M.; Adjallé, K. A Review of the Effect of Plasticizers on the Physical and Mechanical Properties of Alginate-Based Films. Molecules 2023, 28, 6637. [Google Scholar] [CrossRef]
- Mei, B.; Lin, T.-W.; Sheridan, G.S.; Evans, C.M.; Sing, C.E.; Schweizer, K.S. How Segmental Dynamics and Mesh Confinement Determine the Selective Diffusivity of Molecules in Crosslinked Dense Polymer Networks. ACS Cent. Sci. 2023, 9, 508–518. [Google Scholar] [CrossRef]
- Lee, B.-H.; Li, B.; Guelcher, S.A. Gel Microstructure Regulates Proliferation and Differentiation of MC3T3-E1 Cells Encapsulated in Alginate Beads. Acta Biomater. 2012, 8, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Neves, B.S.; Gonçalves, R.C.; Mano, J.F.; Oliveira, M.B. Controlling the Diffusion of Small Molecules from Matrices Processed by All-Aqueous Methodologies: Towards the Development of Green Pharmaceutical Products. Green. Chem. 2024, 26, 4417–4431. [Google Scholar] [CrossRef]
- Cuccia, N.L.; Pothineni, S.; Wu, B.; Méndez Harper, J.; Burton, J.C. Pore-Size Dependence and Slow Relaxation of Hydrogel Friction on Smooth Surfaces. Proc. Natl. Acad. Sci. USA 2020, 117, 11247–11256. [Google Scholar] [CrossRef] [PubMed]
- Richbourg, N.R.; Peppas, N.A. The Swollen Polymer Network Hypothesis: Quantitative Models of Hydrogel Swelling, Stiffness, and Solute Transport. Prog. Polym. Sci. 2020, 105, 101243. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical Mechanics of Cross-Linked Polymer Networks II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Amsden, B.G. Hydrogel Mesh Size and Its Impact on Predictions of Mathematical Models of the Solute Diffusion Coefficient. Macromolecules 2022, 55, 8399–8408. [Google Scholar] [CrossRef]
- Borges, F.T.P.; Papavasiliou, G.; Teymour, F. Characterizing the Molecular Architecture of Hydrogels and Crosslinked Polymer Networks beyond Flory–Rehner—I. Theory. Biomacromolecules 2020, 21, 5104–5118. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.W.; Neufeld, R.J. Modeling the Controllable pH-Responsive Swelling and Pore Size of Networked Alginate Based Biomaterials. Biomaterials 2009, 30, 6119–6129. [Google Scholar] [CrossRef]
- Rehmann, M.S.; Skeens, K.M.; Kharkar, P.M.; Ford, E.M.; Maverakis, E.; Lee, K.H.; Kloxin, A.M. Tuning and Predicting Mesh Size and Protein Release from Step Growth Hydrogels. Biomacromolecules 2017, 18, 3131–3142. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-Responsive Hydrogels: Theory, Modern Advances, and Applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Bordenyuk, O.Y.; Kapatsila, S.M.; Tsykunkov, S.S.; Nadashkevych, Z.Y.; Fihurka, N.V.; Samaryk, V. Synthesis and Study of the Structure of Copolymers of Rarely Crosslinked Polyacrylic Acid. CTAS 2024, 7, 196–201. [Google Scholar] [CrossRef]
- Richbourg, N.R.; Wancura, M.; Gilchrist, A.E.; Toubbeh, S.; Harley, B.A.C.; Cosgriff-Hernandez, E.; Peppas, N.A. Precise Control of Synthetic Hydrogel Network Structure via Linear, Independent Synthesis-Swelling Relationships. Sci. Adv. 2021, 7, eabe3245. [Google Scholar] [CrossRef] [PubMed]
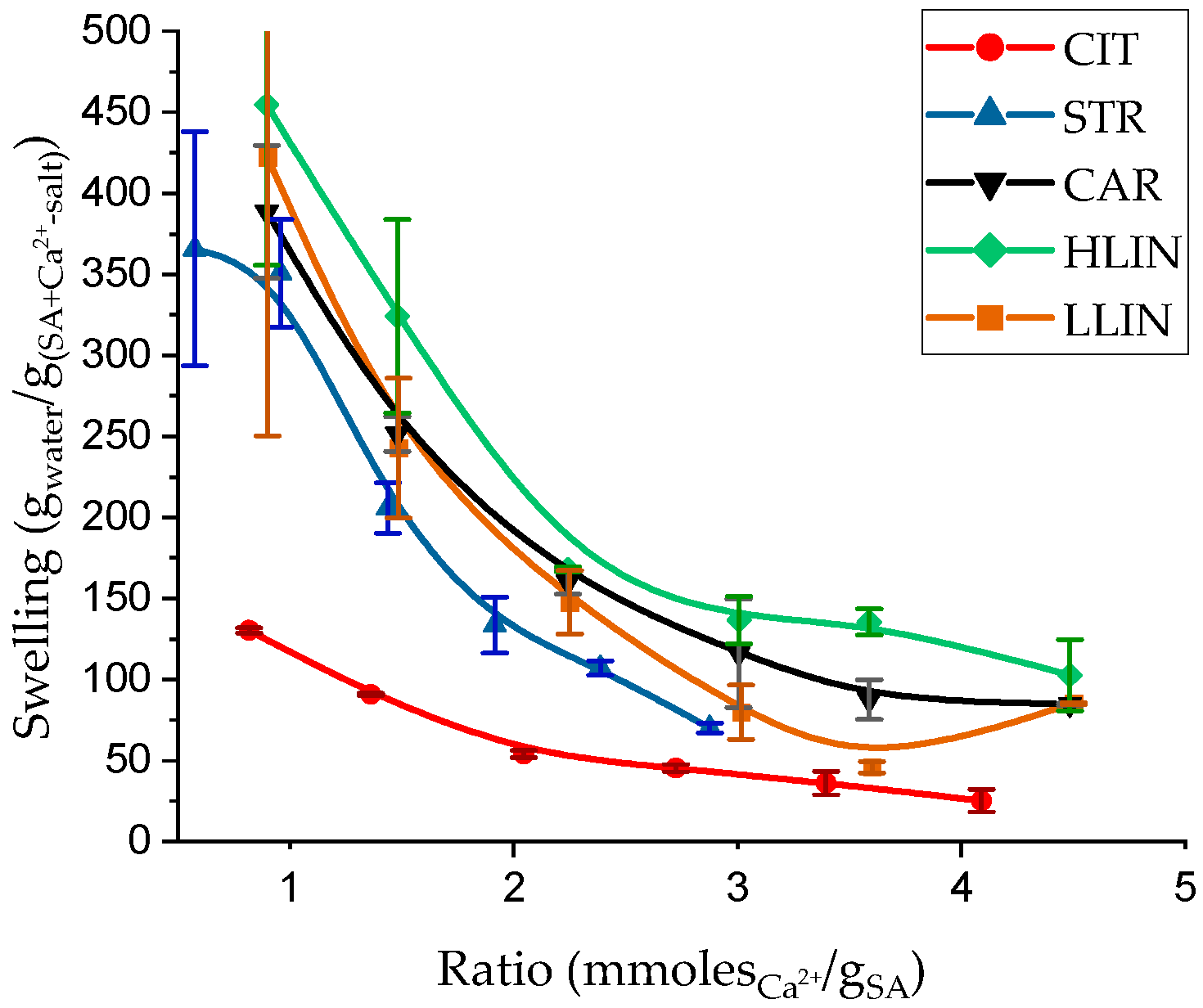

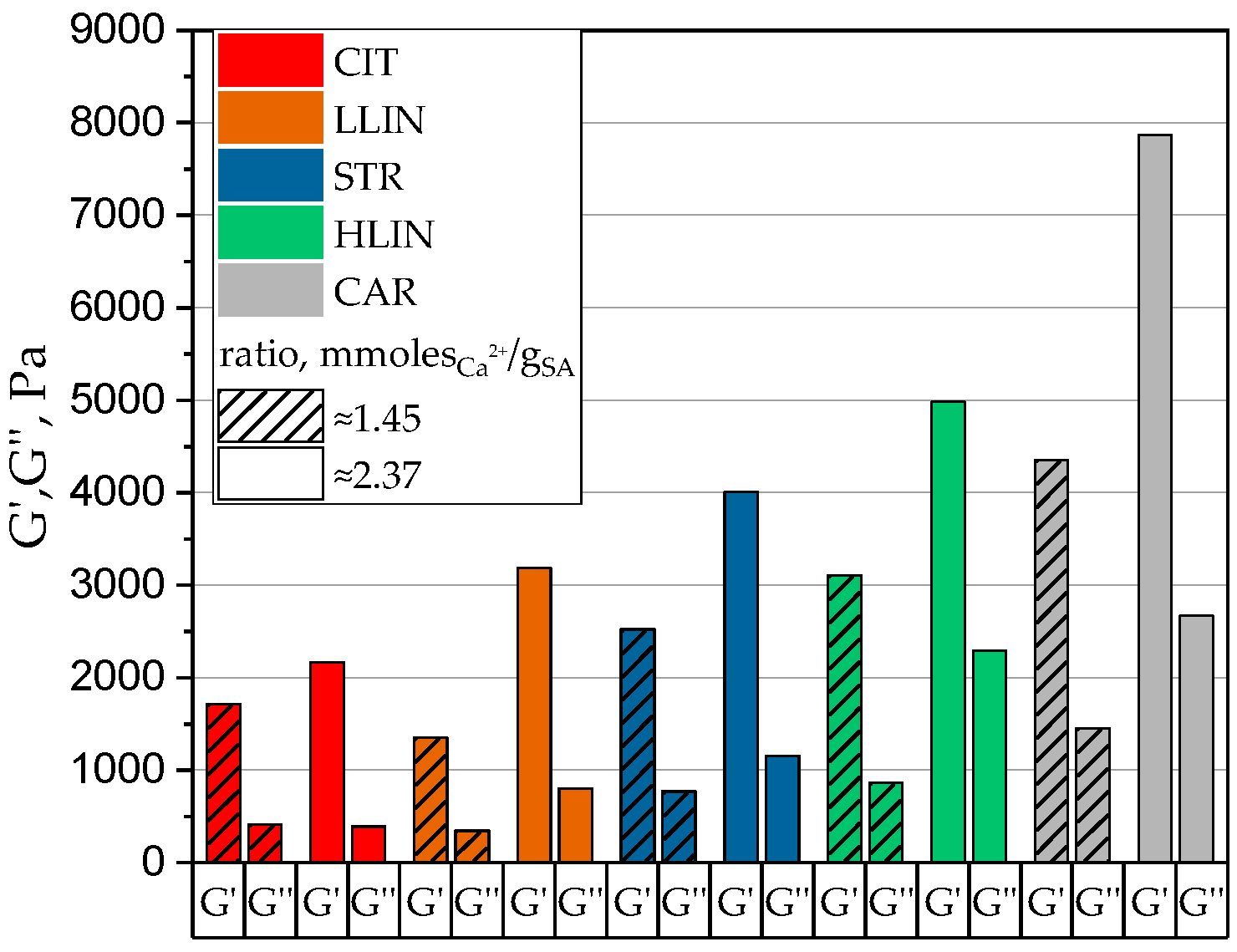
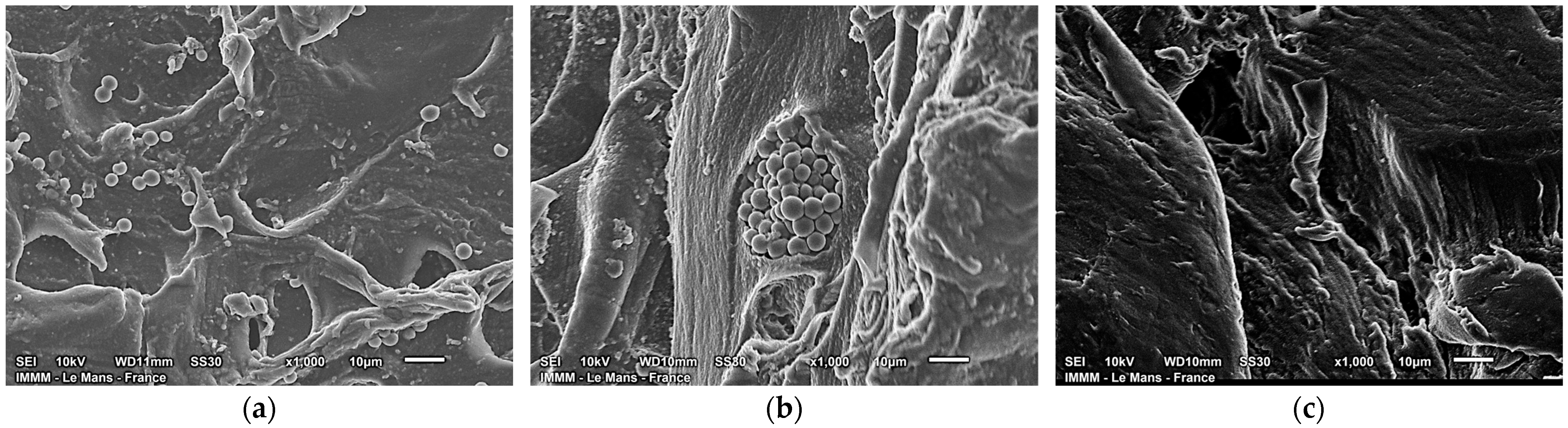
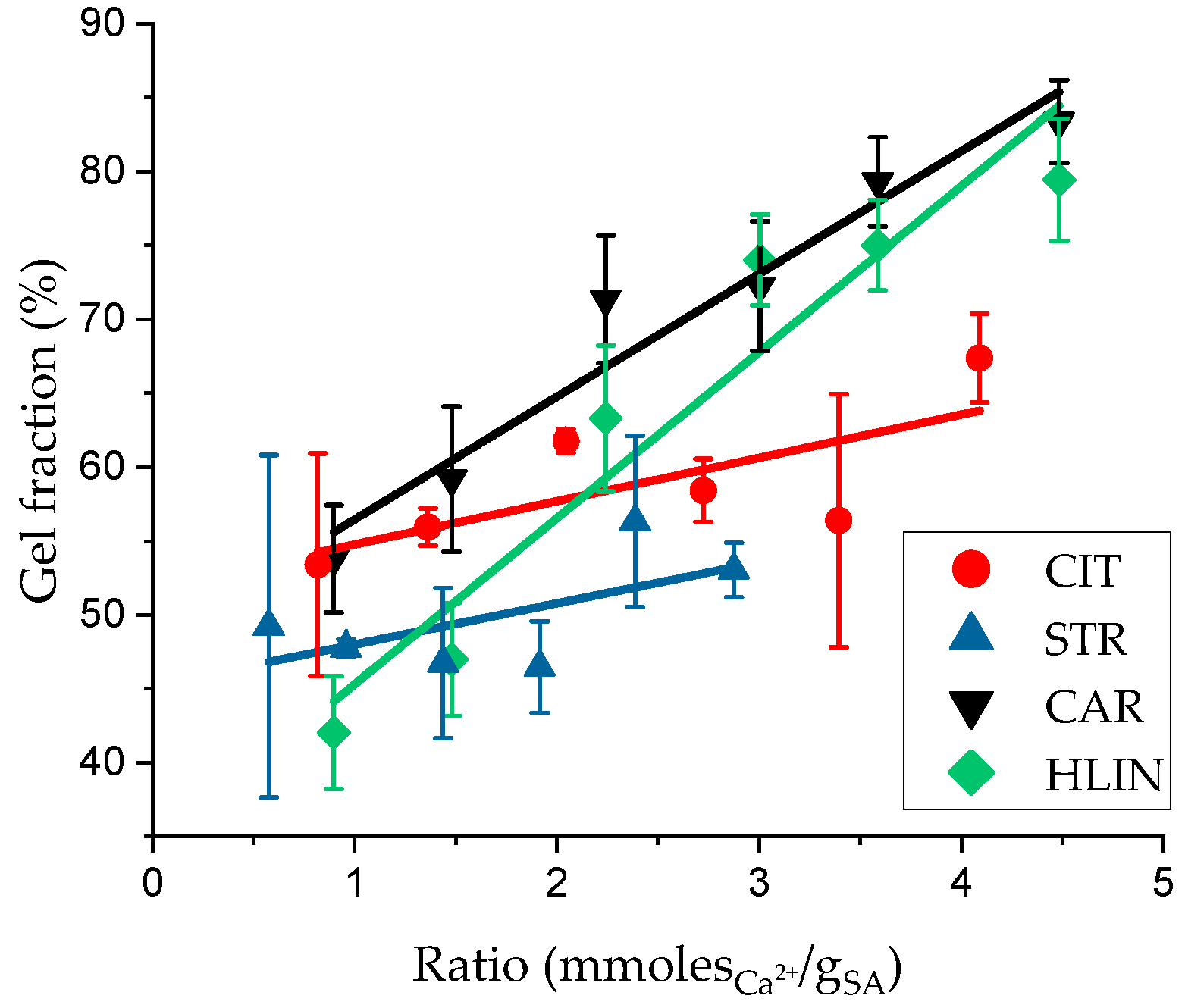

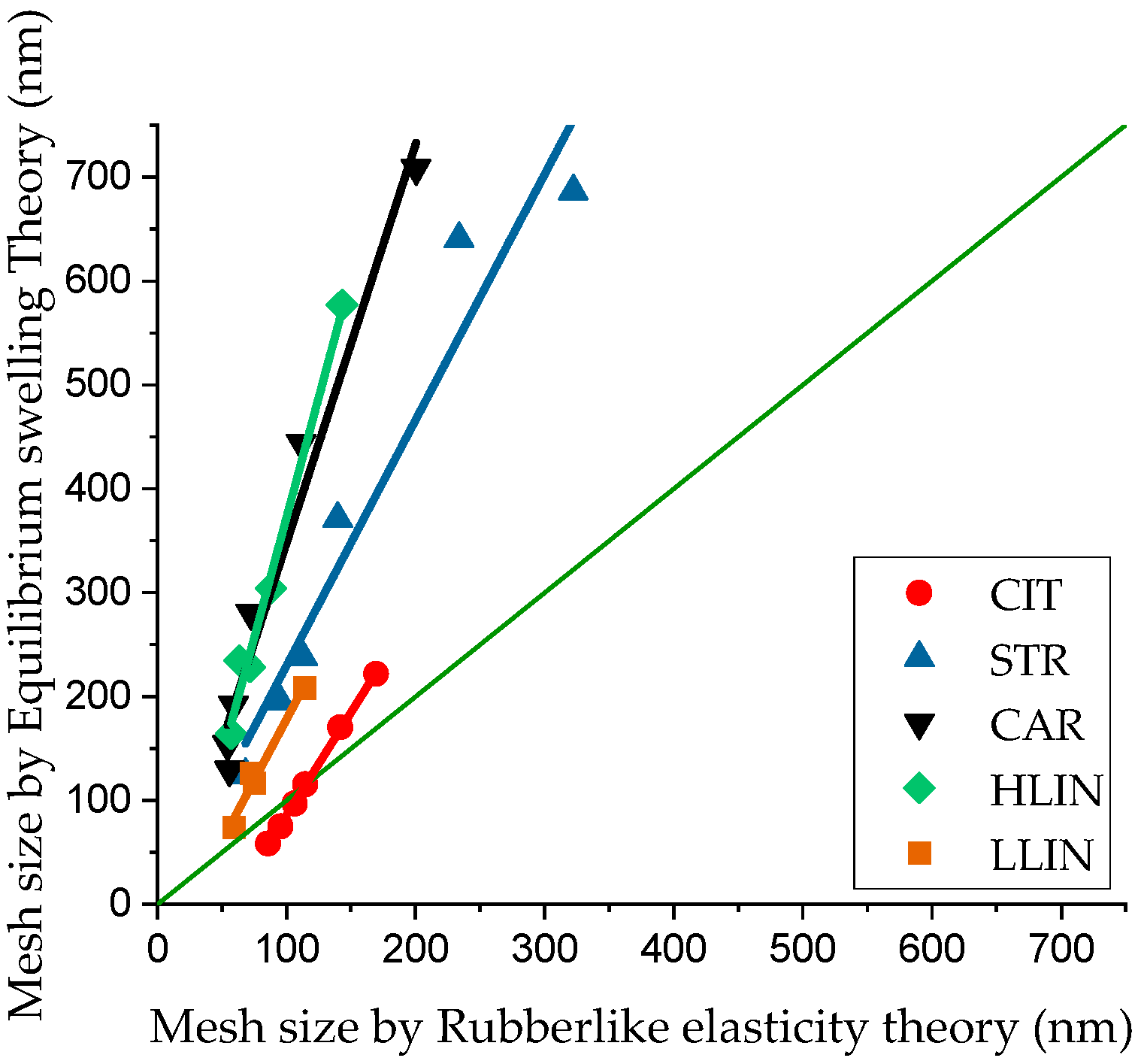
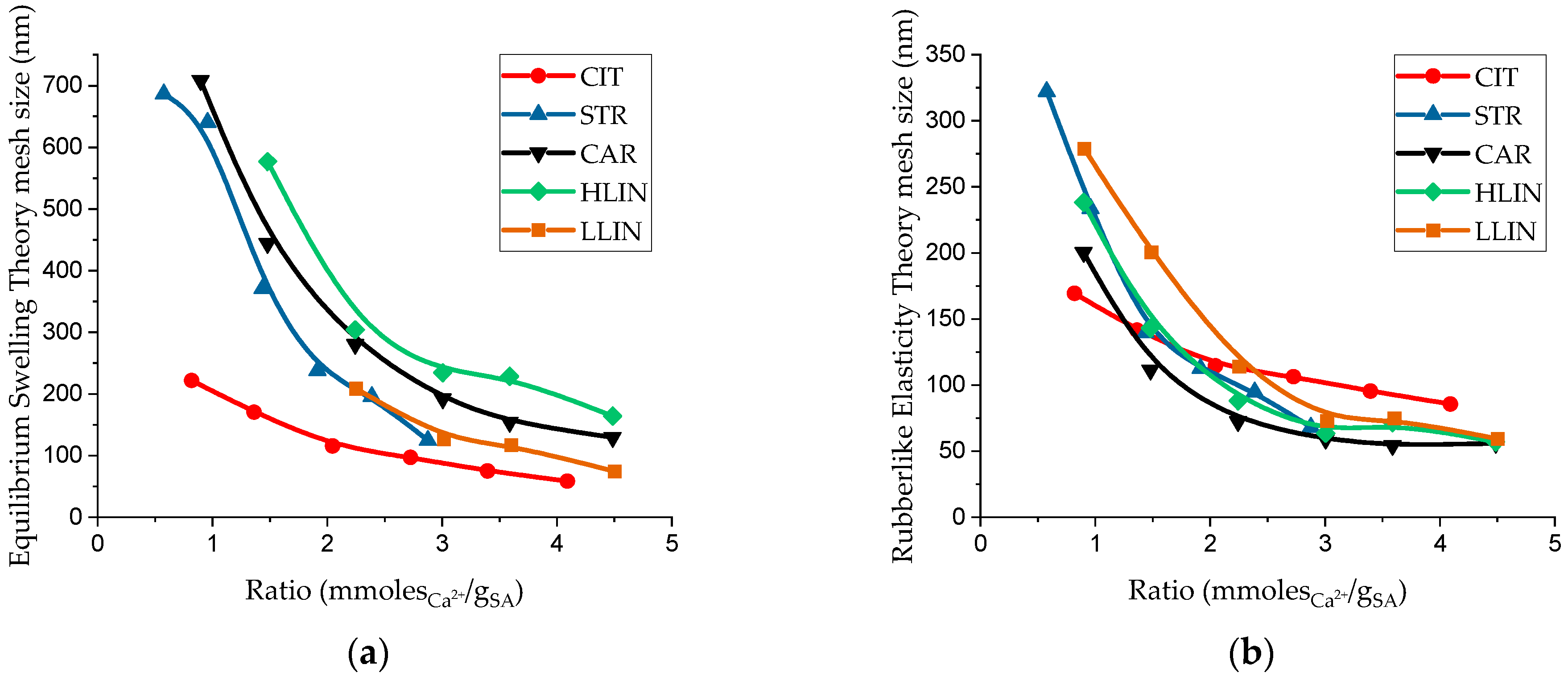
| Sample Name | ρp, g⋅cm−3 | Equilibrium Swelling Theory | Rubber-like Elasticity Theory | |||||
|---|---|---|---|---|---|---|---|---|
| υ2, % | ns 105, mol cm−3 | , kDa | ζ, nm | G, kPa | , kDa | ζ, nm | ||
| CIT1 | 1.564 | 2.91 | 16.3 | 9.6 | 59 | 2.61 | 20.54 | 86 |
| CIT2 | 1.516 | 2.33 | 11.1 | 13.6 | 75 | 2.44 | 22.01 | 95 |
| CIT3 | 1.702 | 1.97 | 8.37 | 20.3 | 97 | 2.20 | 24.40 | 106 |
| CIT4 | 1.721 | 1.71 | 6.56 | 26.3 | 116 | 2.08 | 25.75 | 115 |
| CIT5 | 1.521 | 1.17 | 3.44 | 44.2 | 170 | 1.76 | 30.51 | 142 |
| CIT6 | 1.294 | 0.87 | 2.10 | 61.6 | 222 | 1.49 | 35.96 | 169 |
| STR1 | 1.507 | 1.51 | 5.34 | 28.2 | 125 | 6.38 | 8.41 | 68 |
| STR2 | 1.674 | 1.08 | 3.02 | 55.4 | 196 | 4.12 | 13.01 | 95 |
| STR3 | 1.578 | 0.89 | 2.19 | 72.2 | 238 | 3.32 | 16.15 | 113 |
| STR4 | 1.597 | 0.61 | 1.17 | 136.8 | 371 | 2.77 | 19.38 | 140 |
| STR5 | 1.539 | 0.38 | 0.52 | 295.6 | 641 | 1.36 | 39.38 | 234 |
| STR6 | 1.584 | 0.36 | 0.48 | 329.1 | 687 | 0.74 | 72.48 | 322 |
| CAR1 | 1.382 | 1.41 | 4.76 | 29.0 | 129 | 9.90 | 5.42 | 56 |
| CAR2 | 1.730 | 1.34 | 4.36 | 39.6 | 154 | 10.87 | 4.93 | 54 |
| CAR3 | 1.511 | 1.05 | 2.89 | 52.3 | 192 | 10.83 | 4.95 | 59 |
| CAR4 | 1.628 | 0.79 | 1.77 | 91.9 | 280 | 8.77 | 6.11 | 72 |
| CAR5 | 1.559 | 0.52 | 0.89 | 175.5 | 444 | 4.85 | 11.06 | 111 |
| CAR6 | 1.531 | 0.35 | 0.45 | 340.8 | 708 | 1.97 | 27.27 | 200 |
| HLIN1 | 1.444 | 1.18 | 3.50 | 41.3 | 164 | 10.74 | 4.99 | 57 |
| HLIN2 | 1.562 | 0.92 | 2.30 | 67.9 | 229 | 8.07 | 6.64 | 72 |
| HLIN3 | 1.564 | 0.90 | 2.22 | 70.4 | 234 | 10.36 | 5.17 | 64 |
| HLIN4 | 1.677 | 0.74 | 1.61 | 104.2 | 304 | 6.12 | 8.76 | 88 |
| HLIN5 | 1.509 | 0.41 | 0.60 | 253.1 | 577 | 3.44 | 15.59 | 143 |
| HLIN6 | * | 0.30 | 0.35 | * | * | 1.54 | 34.89 | 238 |
| LLIN1 | 1.333 | 2.22 | 10.3 | 13.0 | 74 | 6.53 | 8.21 | 59 |
| LLIN2 | 1.197 | 1.45 | 4.96 | 24.1 | 117 | 5.46 | 9.83 | 75 |
| LLIN3 | 1.174 | 1.35 | 4.38 | 26.8 | 126 | 6.05 | 8.86 | 73 |
| LLIN4 | 1.074 | 0.85 | 2.01 | 53.5 | 209 | 3.35 | 16.02 | 114 |
| LLIN5 | * | 0.54 | 0.94 | * | * | 1.46 | 36.68 | 201 |
| LLIN6 | * | 0.32 | 0.40 | * | * | 1.06 | 50.46 | 279 |
| Code | CIT1 | CIT2 | CIT3 | CIT4 | CIT5 | CIT6 |
| Glycerin, % | 12.79 | 13.04 | 13.29 | 13.54 | 13.79 | 13.99 |
| Calcium citrate salt, % | 1.50 | 1.25 | 1.00 | 0.75 | 0.50 | 0.30 |
| Code | STR1 | STR2 | STR3 | STR4 | STR5 | STR6 |
| Glycerin, % | 12.79 | 13.04 | 13.29 | 13.54 | 13.79 | 13.99 |
| Rarely structured PAA calcium salt, % | 1.50 | 1.25 | 1.00 | 0.75 | 0.50 | 0.30 |
| Code | CAR1 | CAR2 | CAR3 | CAR4 | CAR5 | CAR6 |
| Glycerin, % | 11.7 | 12.22 | 12.55 | 12.99 | 13.43 | 13.77 |
| Carbomer-940 calcium salt, % | 2.59 | 2.07 | 1.73 | 1.29 | 0.85 | 0.52 |
| Code | HLIN1 | HLIN2 | HLIN3 | HLIN4 | HLIN5 | HLIN6 |
| Glycerin, % | 12.13 | 12.56 | 12.84 | 13.21 | 13.57 | 13.85 |
| High-molecular-weight linear PAA calcium salt, % | 2.16 | 1.73 | 1.45 | 1.08 | 0.71 | 0.43 |
| Code | LLIN1 | LLIN2 | LLIN3 | LLIN4 | LLIN5 | LLIN6 |
| Glycerin, % | 12.00 | 12.46 | 12.75 | 13.14 | 13.53 | 13.83 |
| Low-molecular-weight linear PAA calcium salt, % | 2.29 | 1.83 | 1.53 | 1.14 | 0.75 | 0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapatsila, S.; Taras, R.; Varchuk, D.; Nosova, N.; Varvarenko, S.; Samaryk, V. Influence of Calcium Crosslinker Form on Alginate Hydrogel Properties. Gels 2025, 11, 885. https://doi.org/10.3390/gels11110885
Kapatsila S, Taras R, Varchuk D, Nosova N, Varvarenko S, Samaryk V. Influence of Calcium Crosslinker Form on Alginate Hydrogel Properties. Gels. 2025; 11(11):885. https://doi.org/10.3390/gels11110885
Chicago/Turabian StyleKapatsila, Solomiia, Roman Taras, Diana Varchuk, Nataliia Nosova, Serhii Varvarenko, and Volodymyr Samaryk. 2025. "Influence of Calcium Crosslinker Form on Alginate Hydrogel Properties" Gels 11, no. 11: 885. https://doi.org/10.3390/gels11110885
APA StyleKapatsila, S., Taras, R., Varchuk, D., Nosova, N., Varvarenko, S., & Samaryk, V. (2025). Influence of Calcium Crosslinker Form on Alginate Hydrogel Properties. Gels, 11(11), 885. https://doi.org/10.3390/gels11110885








