Antibacterial and Biocompatible Penicillin–Streptomycin Loaded Bacterial Cellulose (BC) Hydrogels for Wound Healing
Abstract
1. Introduction
2. Results
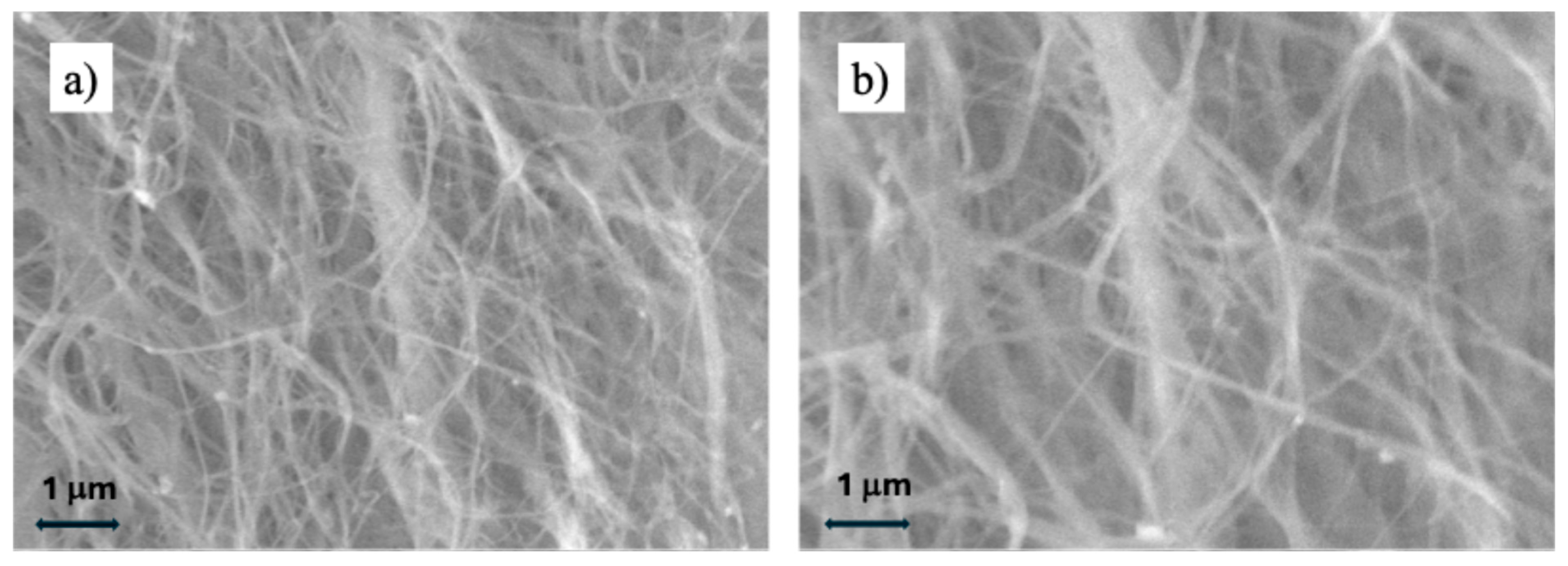
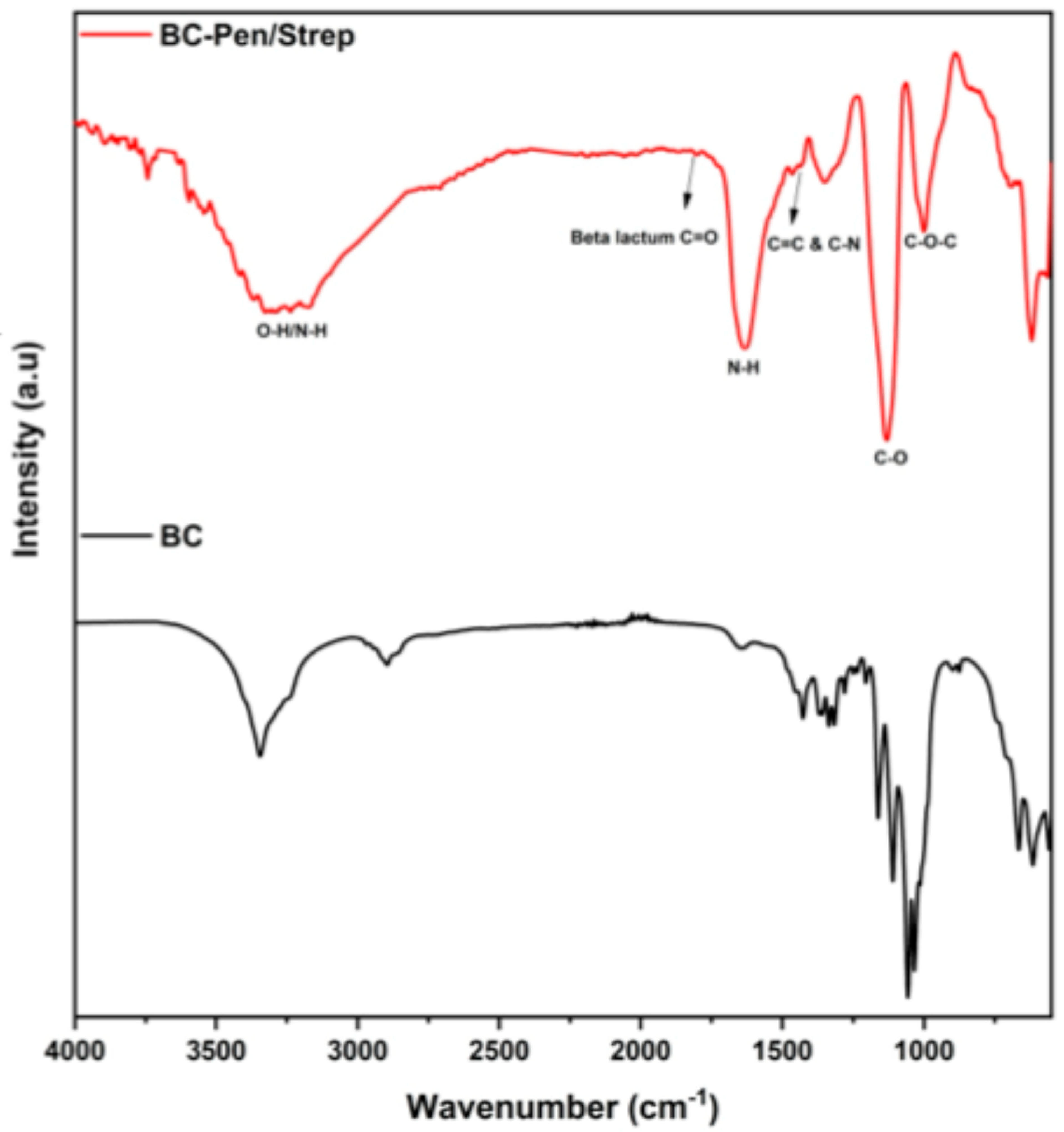
2.1. Physicochemical Analyses
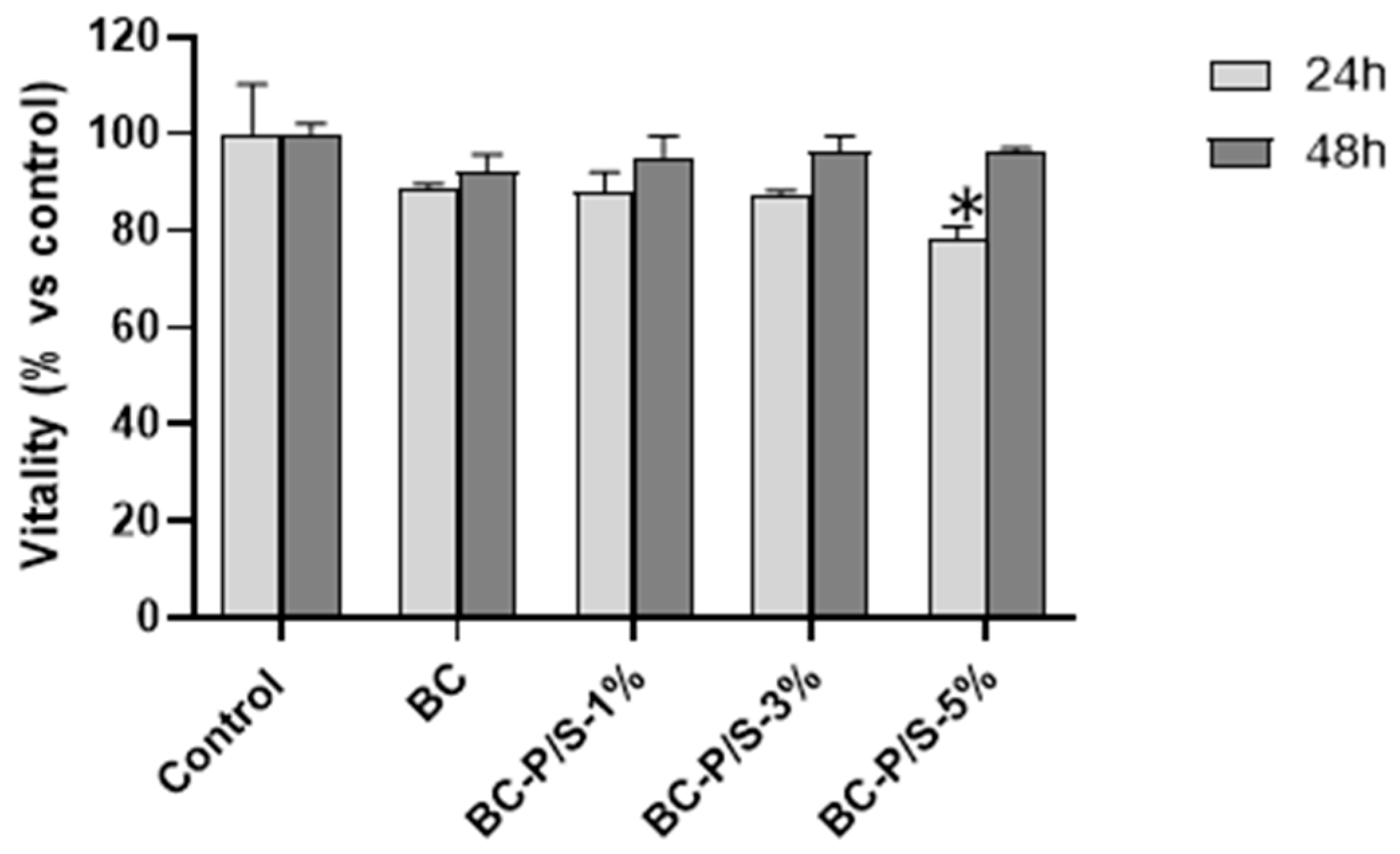
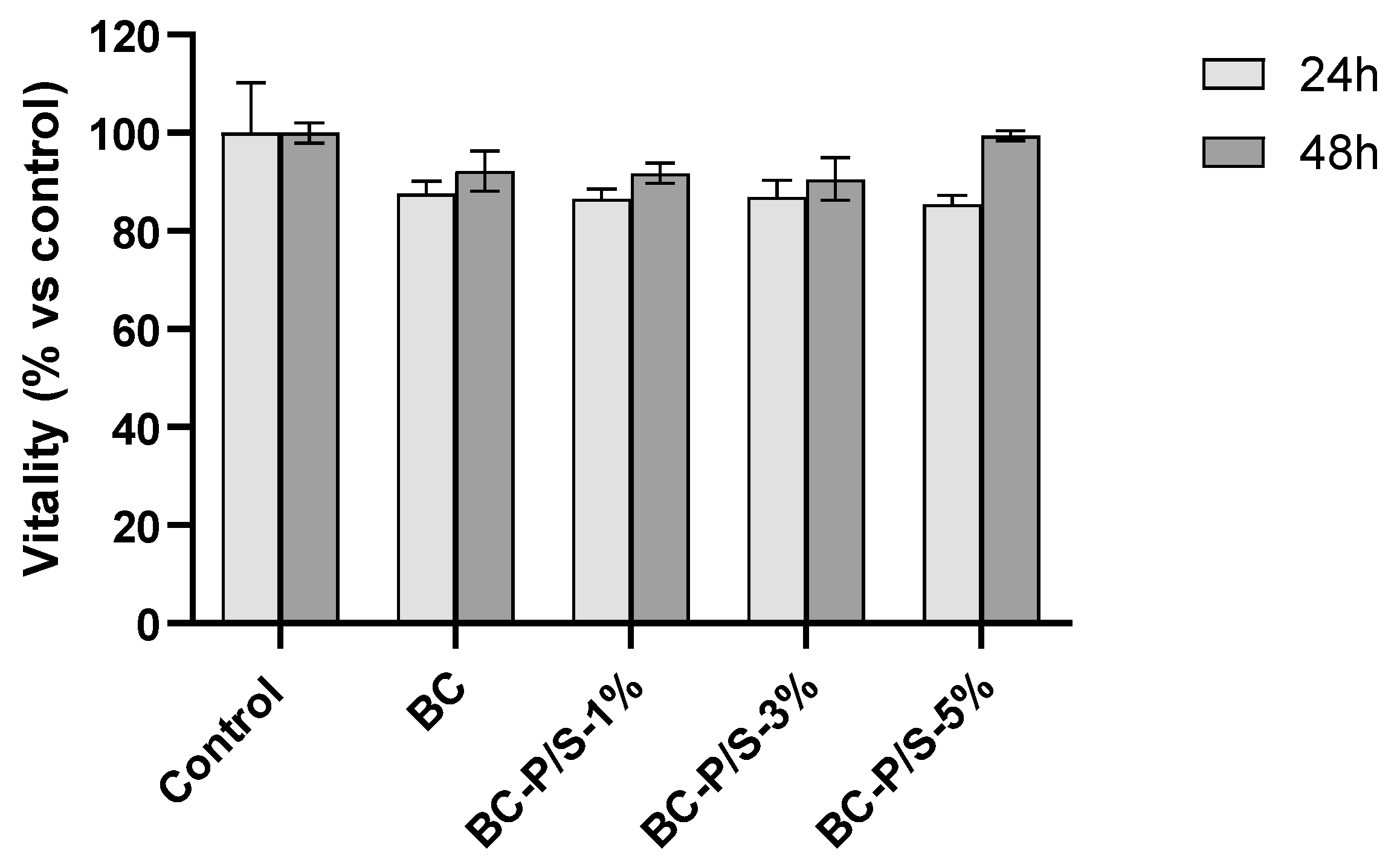
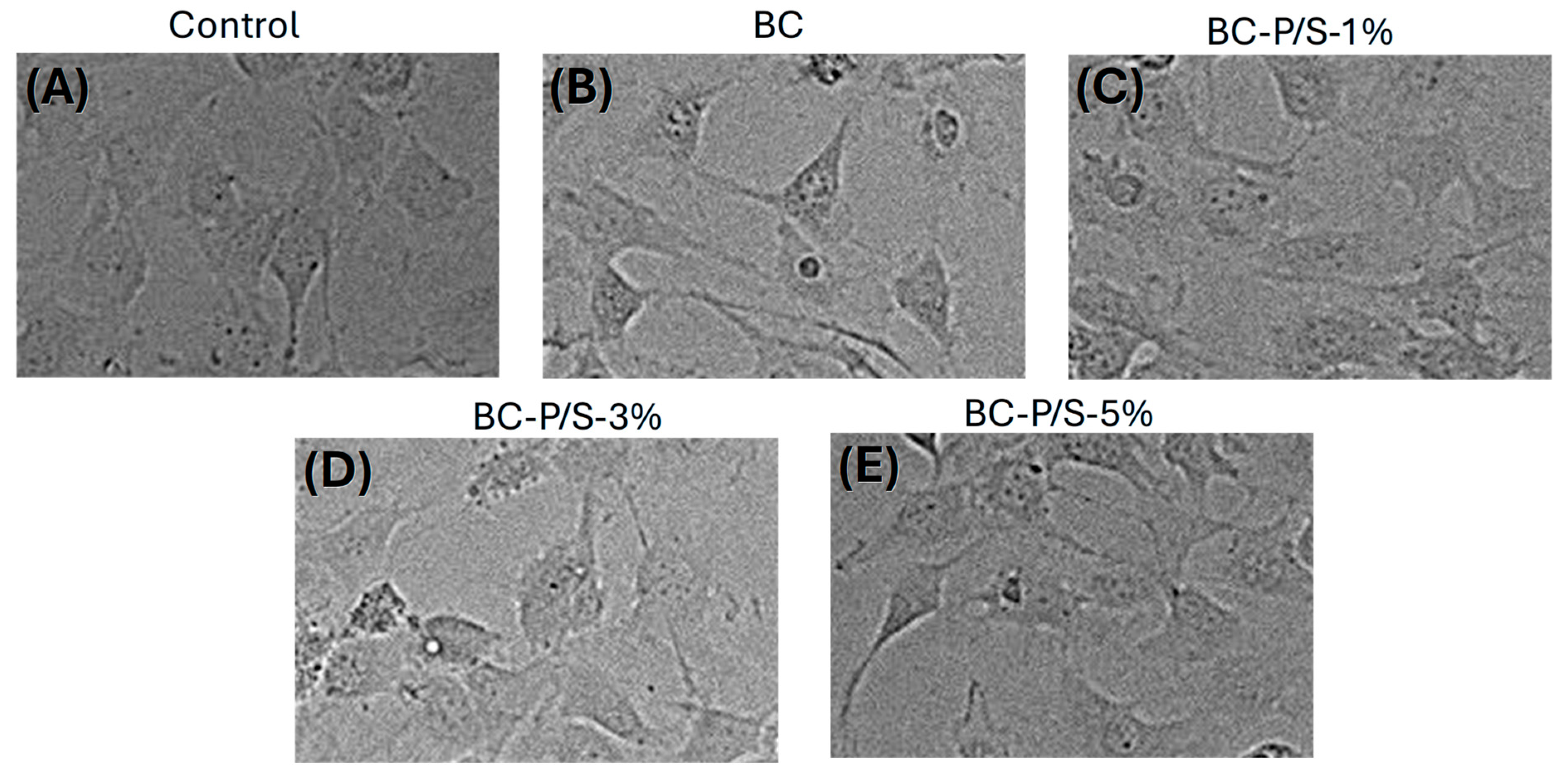
2.2. Cytotoxicity Assessment
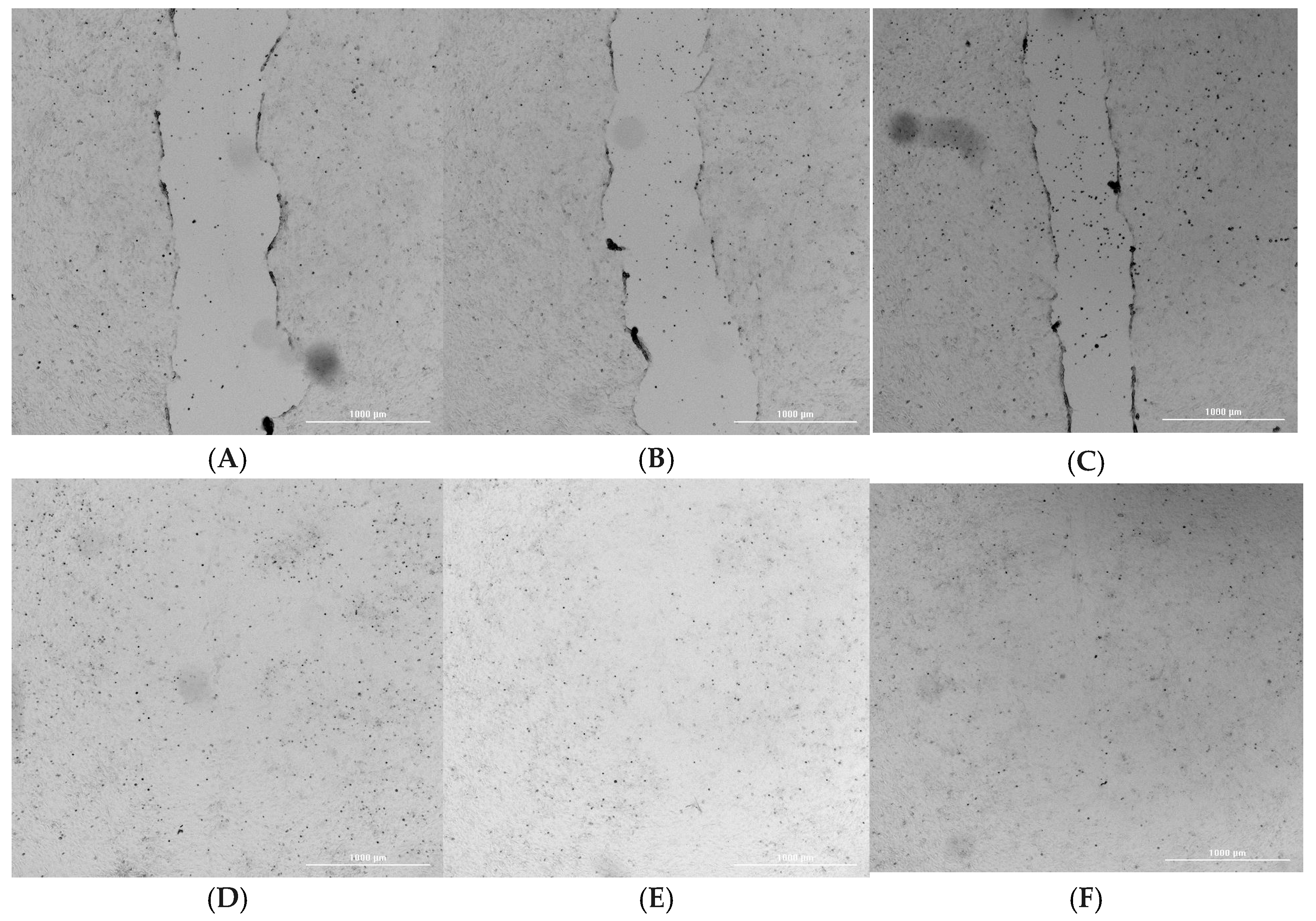
2.3. Wound Healing and Cell Migration
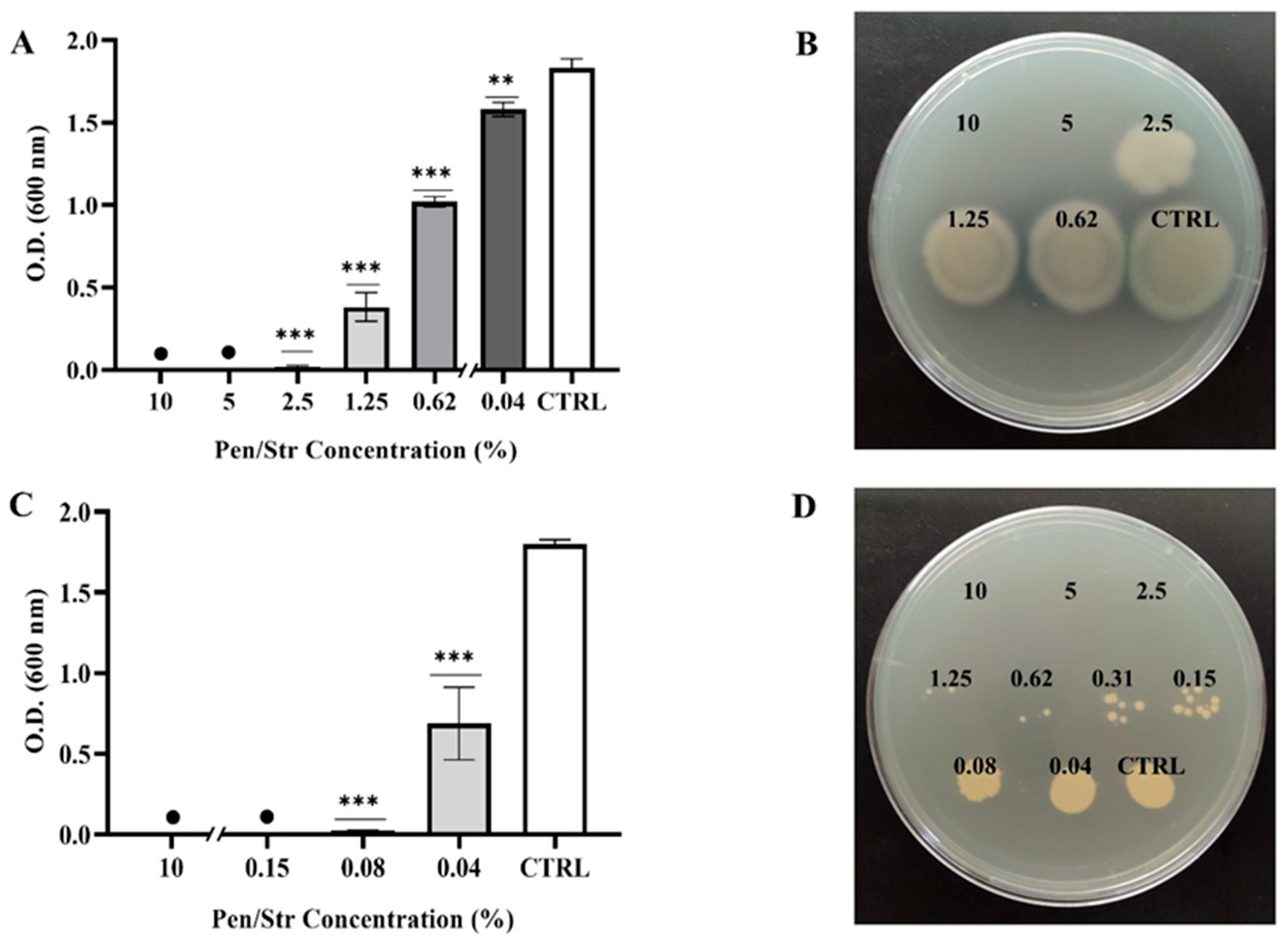
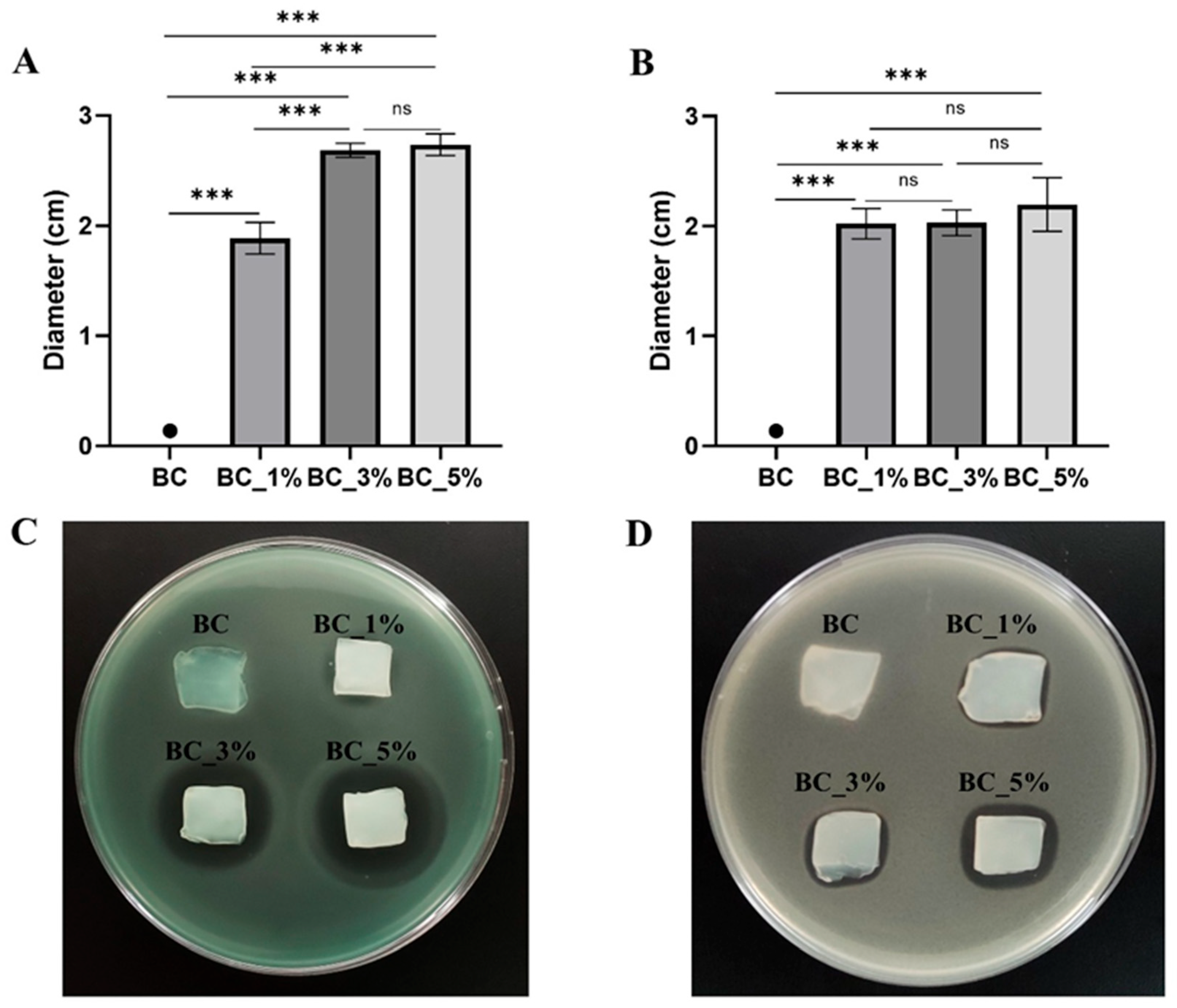
2.4. Antimicrobial Properties of Pen/Strep and Pen/Strep-Loaded BC Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Bacterial Cellulose Production
4.2.2. Pen/Strep Loaded Bacterial Cellulose Production
4.3. Characterization
4.3.1. Physicochemical Analyses
4.3.2. In Vitro Biocompatibility Assessment by MTT Assay
4.3.3. Indirect Assay
4.3.4. Direct Assay
4.3.5. Wound Healing and Cell Migration Assay
4.3.6. Study of the Antimicrobial Properties Against Pseudomonas aeruginosa and Staphylococcus aureus
4.3.7. Determination of Minimal Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC)
4.3.8. Agar Diffusion Method for Antimicrobial Activity Characterization
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baker, P.; Huang, C.; Radi, R.; Moll, S.B.; Jules, E.; Arbiser, J.L. Skin Barrier Function: The Interplay of Physical, Chemical, and Immunologic Properties. Cells 2023, 12, 2745. [Google Scholar] [CrossRef]
- Coates, M.; Blanchard, S.; MacLeod, A.S. Innate Antimicrobial Immunity in the Skin: A Protective Barrier against Bacteria, Viruses, and Fungi. PLoS Pathog. 2018, 14, e1007353. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Soulika, A.M. The Dynamics of the Skin’s Immune System. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef]
- Abdallah, F.; Mijouin, L.; Pichon, C. Skin Immune Landscape: Inside and Outside the Organism. Mediat. Inflamm. 2017, 2017, 5095293. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; De Angelis, B.; Pea, F.; Scalise, A.; Stefani, S.; Tasinato, R.; Zanetti, O.; Dalla Paola, L. Challenges in the Management of Chronic Wound Infections. J. Glob. Antimicrob. Resist. 2021, 26, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Villani, S.; Kunjalukkal Padmanabhan, S.; Stoppa, M.; Nisi, R.; Calcagnile, M.; Alifano, P.; Demitri, C.; Licciulli, A. Neem-Hypericum-Bacterial Cellulose Wound Care Paste Characterized in Vitro and in Galleria mellonella in Vivo Model. Carbohydr. Polym. Technol. Appl. 2024, 7, 100431. [Google Scholar] [CrossRef]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; de Franciscis, S. Chronic Wound Infections: The Role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert. Rev. Anti Infect. Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef]
- Mulcahy, L.R.; Isabella, V.M.; Lewis, K. Pseudomonas aeruginosa Biofilms in Disease. Microb. Ecol. 2014, 68, 1–12. [Google Scholar] [CrossRef]
- Yung, D.B.Y.; Sircombe, K.J.; Pletzer, D. Friends or Enemies? The Complicated Relationship between Pseudomonas aeruginosa and Staphylococcus aureus. Mol. Microbiol. 2021, 116, 1–15. [Google Scholar] [CrossRef]
- Kaur, H.; Gogoi, B.; Sharma, I.; Das, D.K.; Azad, M.A.; Pramanik, D.D.; Pramanik, A. Hydrogels as a Potential Biomaterial for Multimodal Therapeutic Applications. Mol. Pharm. 2024, 21, 4827–4848. [Google Scholar] [CrossRef]
- Czaja, W.; Krystynowicz, A.; Bielecki, S.; Brown, R.M. Microbial Cellulose—The Natural Power to Heal Wounds. Biomaterials 2006, 27, 145–151. [Google Scholar] [CrossRef]
- Cheng, S.; Yang, J.; Song, J.; Cao, X.; Zhou, B.; Yang, L.; Li, C.; Wang, Y. A Motion-Responsive Injectable Lubricative Hydrogel for Efficient Achilles Tendon Adhesion Prevention. Mater. Today Bio 2025, 30, 101458. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Chen, X.; Cui, Z.; Li, X.; Zhou, Y.; Wang, H.; Sun, R.; Wang, Q. Bioinspired Flexible Kevlar/Hydrogel Composites with Antipuncture and Strain-Sensing Properties for Personal Protective Equipment. ACS Appl. Mater. Interfaces 2024, 16, 45473–45486. [Google Scholar] [CrossRef]
- Wang, W.; Cai, T.; Shao, C.; Xiao, Y.; Xiang, Y.; Jiang, Y. MXene-Based Responsive Hydrogels and Applications in Wound Healing. ChemistrySelect 2024, 9, e202402073. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, B.; Li, M.; He, J.; Yin, Z.; Guo, B. Injectable Antimicrobial Conductive Hydrogels for Wound Disinfection and Infectious Wound Healing. Biomacromolecules 2020, 21, 1841–1852. [Google Scholar] [CrossRef] [PubMed]
- Bognár, B.; Spohn, R.; Lázár, V. Drug Combinations Targeting Antibiotic Resistance. npj Antimicrob. Resist. 2024, 2, 29. [Google Scholar] [CrossRef]
- Chudobova, D.; Dostalova, S.; Blazkova, I.; Michalek, P.; Ruttkay-Nedecky, B.; Sklenar, M.; Nejdl, L.; Kudr, J.; Gumulec, J.; Tmejova, K.; et al. Effect of Ampicillin, Streptomycin, Penicillin and Tetracycline on Metal Resistant and Non-Resistant Staphylococcus aureus. Int. J. Environ. Res. Public. Health 2014, 11, 3233–3255. [Google Scholar] [CrossRef] [PubMed]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef]
- Kunjalukkal Padmanabhan, S.; Protopapa, C.; Licciulli, A. Stiff and Tough Hydrophobic Cellulose-Silica Aerogels from Bacterial Cellulose and Fumed Silica. Process Biochem. 2021, 103, 31–38. [Google Scholar] [CrossRef]
- Ye, S.; He, S.; Su, C.; Jiang, L.; Wen, Y.; Zhu, Z.; Shao, W. Morphological, Release and Antibacterial Performances of Amoxicillin-Loaded Cellulose Aerogels. Molecules 2018, 23, 2082. [Google Scholar] [CrossRef]
- Padmanabhan, S.K.; Corcione, C.E.; Nisi, R.; Maffezzoli, A.; Licciulli, A. PolyDiethyleneglycol–Bisallyl Carbonate Matrix Transparent Nanocomposites Reinforced with Bacterial Cellulose Microfibrils. Eur. Polym. J. 2017, 93, 192–199. [Google Scholar] [CrossRef]
- Fuller, M.E.; Andaya, C.; McClay, K. Evaluation of ATR-FTIR for Analysis of Bacterial Cellulose Impurities. J. Microbiol. Methods 2018, 144, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Ragnoni, E.; Catalini, S.; Becucci, M.; Lapini, A.; Foggi, P. Linear and Non-Linear Middle Infrared Spectra of Penicillin G in the CO Stretching Mode Region. Symmetry 2021, 13, 106. [Google Scholar] [CrossRef]
- Kalaiarasi, S.; Jose, M. Streptomycin Loaded TiO2 Nanoparticles: Preparation, Characterization and Antibacterial Applications. J. Nanostruct Chem. 2017, 7, 47–53. [Google Scholar] [CrossRef]
- Chen, Y.M.; Xi, T.; Zheng, Y.; Guo, T.; Hou, J.; Wan, Y.; Gao, C. In Vitro Cytotoxicity of Bacterial Cellulose Scaffolds Used for Tissue-Engineered Bone. J. Bioact. Compat. Polym. 2009, 24, 137–145. [Google Scholar] [CrossRef]
- Wichai, S.; Chuysinuan, P.; Chaiarwut, S.; Ekabutr, P.; Supaphol, P. Development of Bacterial Cellulose/Alginate/Chitosan Composites Incorporating Copper (II) Sulfate as an Antibacterial Wound Dressing. J. Drug Deliv. Sci. Technol. 2019, 51, 662–671. [Google Scholar] [CrossRef]
- Salama, A.; Saleh, A.K.; Cruz-Maya, I.; Guarino, V. Bacterial Cellulose/Cellulose Imidazolium Bio-Hybrid Membranes for In Vitro and Antimicrobial Applications. J. Funct. Biomater. 2023, 14, 60. [Google Scholar] [CrossRef]
- Tunsound, V.; Krasian, T.; Daranarong, D.; Punyodom, W.; Jantanasakulwong, K.; Ross, S.; Tipduangta, P.; Rachtanapun, P.; Ross, G.; Jantrawut, P.; et al. Enhanced Mechanical Properties and Biocompatibility of Bacterial Cellulose Composite Films with Inclusion of 2D MoS2 and Helical Carbon Nanotubes for Use as Antimicrobial Drug Carriers. Int. J. Biol. Macromol. 2023, 253, 126712. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Wu, J.; Liu, H.; Ye, S.; Jiang, L.; Liu, X. Novel Bioactive Surface Functionalization of Bacterial Cellulose Membrane. Carbohydr. Polym. 2017, 178, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Bai, L.; Li, Z.; Qu, Y.; Jing, L. Review of Strategies for the Fabrication of Heterojunctional Nanocomposites as Efficient Visible-Light Catalysts by Modulating Excited Electrons with Appropriate Thermodynamic Energy. J. Mater. Chem. A 2019, 7, 10879–10897. [Google Scholar] [CrossRef]
- Knyazev, N.A.; Shmakov, S.V.; Pechkovskaya, S.A.; Filatov, A.S.; Stepakov, A.V.; Boitsov, V.M.; Filatova, N.A. Identification of Spiro-Fused [3-Azabicyclo[3.1.0]Hexane]Oxindoles as Potential Antitumor Agents: Initial In Vitro Evaluation of Anti-Proliferative Effect and Actin Cytoskeleton Transformation in 3T3 and 3T3-SV40 Fibroblast. Int. J. Mol. Sci. 2021, 22, 8264. [Google Scholar] [CrossRef] [PubMed]
- Stępnik, M.; Arkusz, J.; Smok-Pieniążek, A.; Bratek-Skicki, A.; Salvati, A.; Lynch, I.; Dawson, K.A.; Gromadzińska, J.; De Jong, W.H.; Rydzyński, K. Cytotoxic Effects in 3T3-L1 Mouse and WI-38 Human Fibroblasts Following 72 Hour and 7 Day Exposures to Commercial Silica Nanoparticles. Toxicol. Appl. Pharmacol. 2012, 263, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Plikus, M.V.; Wang, X.; Sinha, S.; Forte, E.; Thompson, S.M.; Herzog, E.L.; Driskell, R.R.; Rosenthal, N.; Biernaskie, J.; Horsley, V. Fibroblasts: Origins, Definitions, and Functions in Health and Disease. Cell 2021, 184, 3852–3872. [Google Scholar] [CrossRef]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research Techniques Made Simple: Analysis of Collective Cell Migration Using the Wound Healing Assay. J. Investig. Dermatol. 2017, 137, e11–e16. [Google Scholar] [CrossRef]
- Suarez-Arnedo, A.; Figueroa, F.T.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An Image J Plugin for the High Throughput Image Analysis of in Vitro Scratch Wound Healing Assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef]
- Giordano, M.E.; Lionetto, F.; Lionetto, M.G. Impact of Polyethylene Terephthalate Nanoplastics (PET) on Fibroblasts: A Study on NIH-3T3 Cells. Front. Physiol. 2025, 16, 1580682. [Google Scholar] [CrossRef]
- Boni, B.O.O.; Lamboni, L.; Mao, L.; Bakadia, B.M.; Shi, Z.; Yang, G. In Vivo Performance of Microstructured Bacterial Cellulose-Silk Sericin Wound Dressing: Effects on Fibrosis and Scar Formation. Eng. Sci. 2022, 19, 175–185. [Google Scholar] [CrossRef]
- Loh, E.Y.X.; Mohamad, N.; Fauzi, M.B.; Ng, M.H.; Ng, S.F.; Mohd Amin, M.C.I. Development of a Bacterial Cellulose-Based Hydrogel Cell Carrier Containing Keratinocytes and Fibroblasts for Full-Thickness Wound Healing. Sci. Rep. 2018, 8, 2875. [Google Scholar] [CrossRef]
- Zmejkoski, D.Z.; Marković, Z.M.; Mitić, D.D.; Zdravković, N.M.; Kozyrovska, N.O.; Bugárová, N.; Todorović Marković, B.M. Antibacterial Composite Hydrogels of Graphene Quantum Dots and Bacterial Cellulose Accelerate Wound Healing. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2022, 110, 1796–1805. [Google Scholar] [CrossRef]
- Pal, S.; Nisi, R.; Stoppa, M.; Licciulli, A. Silver-Functionalized Bacterial Cellulose as Antibacterial Membrane for Wound-Healing Applications. ACS Omega 2017, 2, 3632–3639. [Google Scholar] [CrossRef]
- Abdo, A.I.; Nguyen, H.T.; Kaul, L.D.; Schmitt-John, T.; Kopecki, Z.; Ogunniyi, A.D.; Richter, K. Plasma-Activated Water Accelerates Wound Healing and Reduces Staphylococcus aureus Infection in Vivo. Biofilm 2025, 10, 100308. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, Z. ROS-Scavenging Materials for Skin Wound Healing: Advancements and Applications. Front. Bioeng. Biotechnol. 2023, 11, 1304835. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Park, Y.J.; Lee, S.I.; Nam, K.C.; Lee, M.H.; Park, J.-C. Photodynamically Tunable ROS-Generating Hydrogels for Accelerated Tissue Regeneration. Bioact. Mater. 2025, 51, 977–992. [Google Scholar] [CrossRef]
- Guo, L.; Fu, Z.; Li, H.; Wei, R.; Guo, J.; Wang, H.; Qi, J. Smart Hydrogel: A New Platform for Cancer Therapy. Adv. Colloid. Interface Sci. 2025, 340, 103470. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, Y.; Ma, S.; Li, X.; Wang, W.; Chen, X.; Zheng, J.; Fan, Z.; Jiang, Y.; Liao, Y. Multifunctional DNA Hydrogels with Light-Triggered Gas-Therapy and Controlled G-Exos Release for Infected Wound Healing. Bioact. Mater. 2025, 52, 422–437. [Google Scholar] [CrossRef]
- Gao, Z.; Li, Y.; Li, X.; Chen, H.; Li, C.; Xie, X.; Zhao, Y.; Yan, H.; Yang, Z.; Hou, G. Bifunctional Modified Bacterial Cellulose-Based Hydrogel through Sequence-Dependent Crosslinking towards Enhanced Antibacterial and Cutaneous Wound Healing. Int. J. Biol. Macromol. 2025, 296, 139737. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, J.; Luo, J.; Cui, Y.; Chen, J.; Zeng, B.; Deng, Z.; Shao, L. “Double-Sided Protector” Janus Hydrogels for Skin and Mucosal Wound Repair: Applications, Mechanisms, and Prospects. J. Nanobiotechnology 2025, 23, 387. [Google Scholar] [CrossRef]
- Eskilson, O.; Zattarin, E.; Berglund, L.; Oksman, K.; Hanna, K.; Rakar, J.; Sivlér, P.; Skog, M.; Rinklake, I.; Shamasha, R.; et al. Nanocellulose Composite Wound Dressings for Real-Time pH Wound Monitoring. Mater. Today Bio 2023, 19, 100574. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Y.; Chen, P.; Zhong, S.; Yang, Y. Nucleic Acid Aptamer-Based Sensors for Bacteria Detection: A Review. BioEssays 2025, 47, e202400111. [Google Scholar] [CrossRef]
- Murtaza, G.; Rizvi, A.S.; Qiu, L.; Xue, M.; Meng, Z. Aptamer Empowered Hydrogels: Fabrication and Bio-Sensing Applications. J. Appl. Polym. Sci. 2022, 139, e52441. [Google Scholar] [CrossRef]
- Rajabimashhadi, Z.; Gallo, N.; Russo, F.; Ghiyami, S.; Mele, C.; Giordano, M.E.; Lionetto, M.G.; Salvatore, L.; Lionetto, F. Production and Physico-Chemical Characterization of Nano-Sized Collagen from Equine Tendon. Int. J. Biol. Macromol. 2024, 277, 134220. [Google Scholar] [CrossRef]
- Mohan, L.; Raja, M.D.; Uma, T.S.; Rajendran, N.; Anandan, C. In-Vitro Biocompatibility Studies of Plasma-Nitrided Titanium Alloy β-21S Using Fibroblast Cells. J. Mater. Eng. Perform. 2016, 25, 1508–1514. [Google Scholar] [CrossRef]
- Stříteský, S.; Marková, A.; Víteček, J.; Šafaříková, E.; Hrabal, M.; Kubáč, L.; Kubala, L.; Weiter, M.; Vala, M. Printing Inks of Electroactive Polymer PEDOT:PSS: The Study of Biocompatibility, Stability, and Electrical Properties. J. Biomed. Mater. Res. Part. A 2018, 106, 1121–1128. [Google Scholar] [CrossRef]
- Solìs Moré, Y.; Panella, G.; Fioravanti, G.; Perrozzi, F.; Passacantando, M.; Giansanti, F.; Ardini, M.; Ottaviano, L.; Cimini, A.; Peniche, C.; et al. Biocompatibility of Composites Based on Chitosan, Apatite, and Graphene Oxide for Tissue Applications. J. Biomed. Mater. Res. Part. A 2018, 106, 1585–1594. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices. ISO: Geneva, Switzerland, 2009.
- Villani, S.; De Matteis, V.; Calcagnile, M.; Cascione, M.; Pellegrino, P.; Vincenti, L.; Demitri, C.; Alifano, P.; Rinaldi, R. Tuning Antibacterial Efficacy against Pseudomonas aeruginosa by Using Green AgNPs in Chitosan Thin Films as a Plastic Alternative. Int. J. Biol. Macromol. 2025, 285, 138277. [Google Scholar] [CrossRef] [PubMed]
- Calcagnile, M.; Jeguirim, I.; Tredici, S.M.; Damiano, F.; Alifano, P. Spiramycin Disarms Pseudomonas aeruginosa without Inhibiting Growth. Antibiotics 2023, 12, 499. [Google Scholar] [CrossRef] [PubMed]
- Misra, R.; Sahoo, S.K. Chapter Four—Antibacterial Activity of Doxycycline-Loaded Nanoparticles. In Methods in Enzymology; Düzgüneş, N., Ed.; Nanomedicine; Academic Press: New York, NY, USA, 2012; Volume 509, pp. 61–85. [Google Scholar]
- Mohammadi, H.; Rezaeigolestani, M.; Mohsenzadeh, M. Optimization of Antimicrobial Nanocomposite Films Based on Carboxymethyl Cellulose Incorporating Chitosan Nanofibers and Guggul Gum Polysaccharide. Sci. Rep. 2024, 14, 13693. [Google Scholar] [CrossRef] [PubMed]
| Pen/Strep Concentration (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 5 | 2.5 | 1.25 | 0.62 | 0.31 | 0.25 | 0.08 | 0.04 | CTRL | |
| P. aeruginosa | − | − | − | +/− | + | + | + | + | + | + |
| S. aureus | − | − | − | − | − | − | − | − | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunjalukkal Padmanabhan, S.; Giordano, M.E.; Villani, S.; Udayan, G.; Stoppa, M.; Alifano, P.; Demitri, C.; Lionetto, M.G.; Licciulli, A. Antibacterial and Biocompatible Penicillin–Streptomycin Loaded Bacterial Cellulose (BC) Hydrogels for Wound Healing. Gels 2025, 11, 851. https://doi.org/10.3390/gels11110851
Kunjalukkal Padmanabhan S, Giordano ME, Villani S, Udayan G, Stoppa M, Alifano P, Demitri C, Lionetto MG, Licciulli A. Antibacterial and Biocompatible Penicillin–Streptomycin Loaded Bacterial Cellulose (BC) Hydrogels for Wound Healing. Gels. 2025; 11(11):851. https://doi.org/10.3390/gels11110851
Chicago/Turabian StyleKunjalukkal Padmanabhan, Sanosh, Maria Elena Giordano, Stefania Villani, Gayatri Udayan, Mariangela Stoppa, Pietro Alifano, Christian Demitri, Maria Giulia Lionetto, and Antonio Licciulli. 2025. "Antibacterial and Biocompatible Penicillin–Streptomycin Loaded Bacterial Cellulose (BC) Hydrogels for Wound Healing" Gels 11, no. 11: 851. https://doi.org/10.3390/gels11110851
APA StyleKunjalukkal Padmanabhan, S., Giordano, M. E., Villani, S., Udayan, G., Stoppa, M., Alifano, P., Demitri, C., Lionetto, M. G., & Licciulli, A. (2025). Antibacterial and Biocompatible Penicillin–Streptomycin Loaded Bacterial Cellulose (BC) Hydrogels for Wound Healing. Gels, 11(11), 851. https://doi.org/10.3390/gels11110851









