Preparation and Characterization of an Acid-Responsive ZIF-8 Hydrogel Dressing with Sustained-Release Function for Targeted Therapy of Periodontitis
Abstract
1. Introduction
2. Results and Discussion
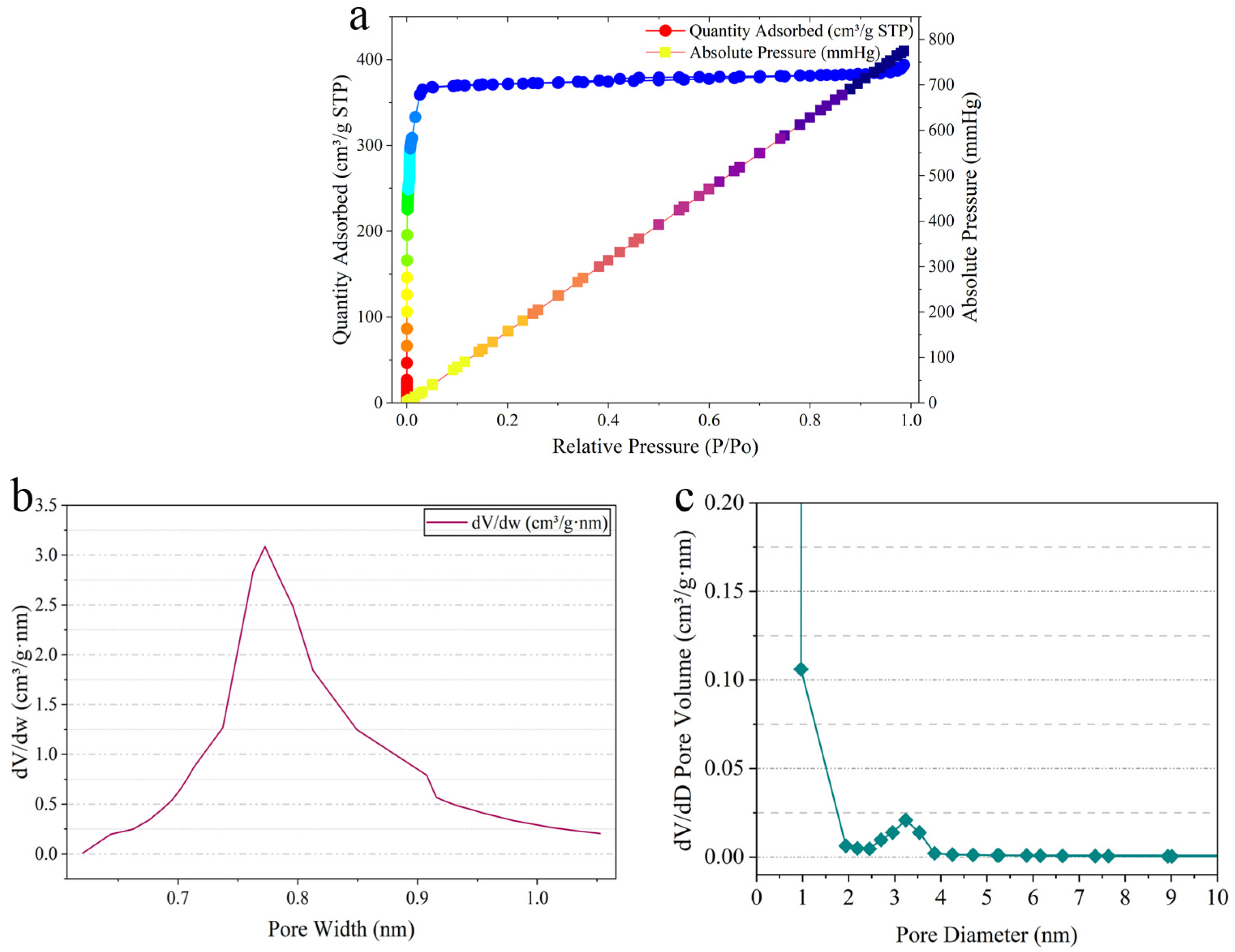
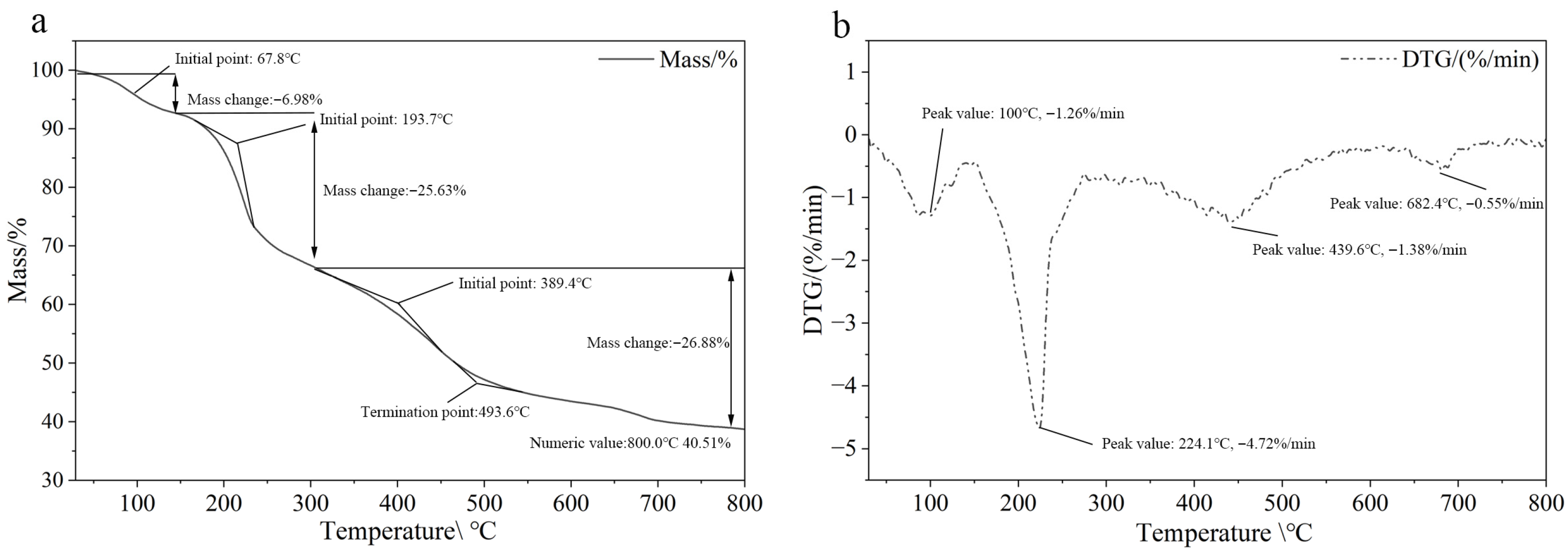
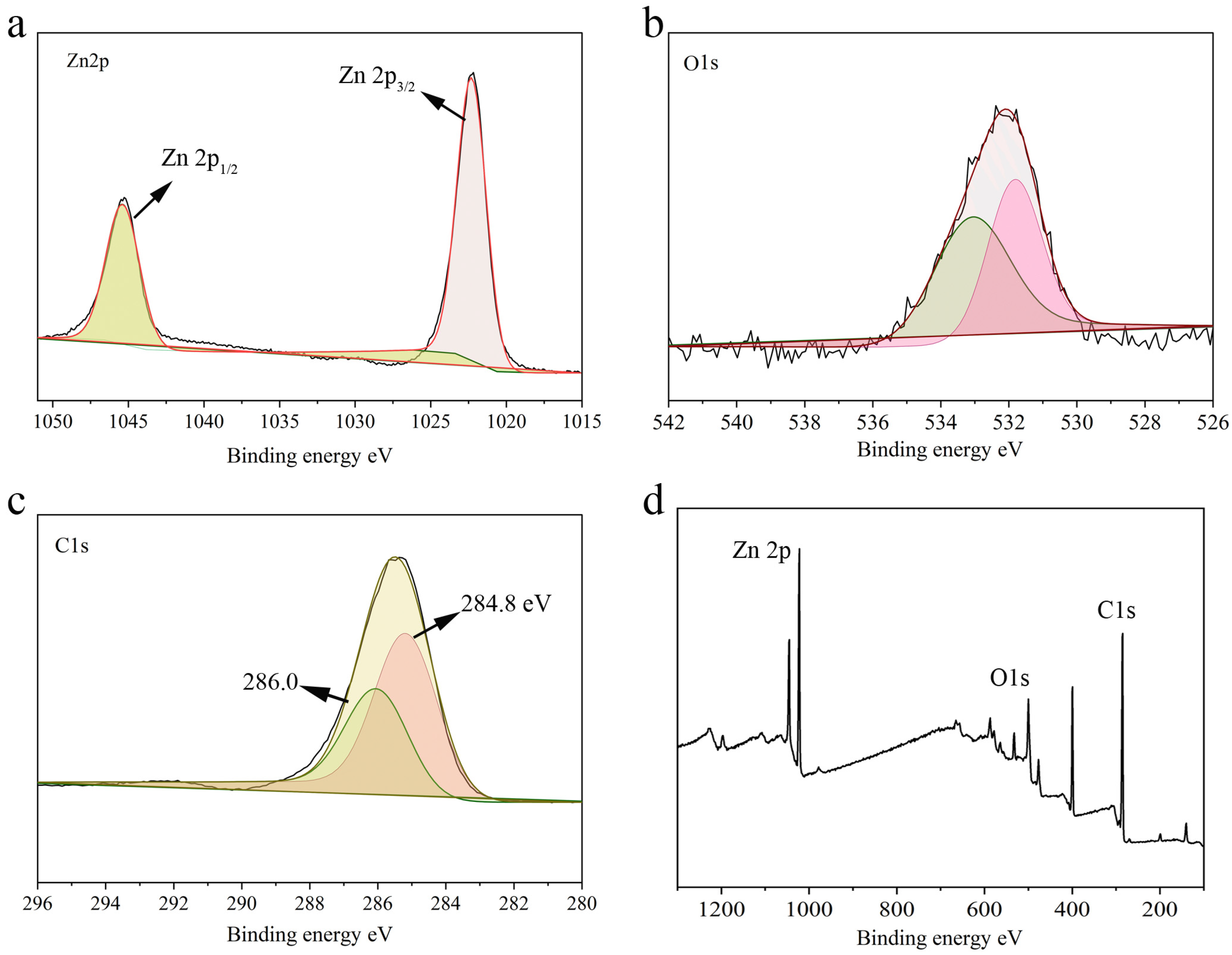
2.1. Characterization of Hydrogel@ZIF-8@MNZ
2.2. Adsorption Performance
2.2.1. Adsorption Isotherm Fitting and Mechanism Analysis
2.2.2. Adsorption Kinetic Model Analysis
2.2.3. Thermodynamic Analysis
2.3. Antibacterial Effect
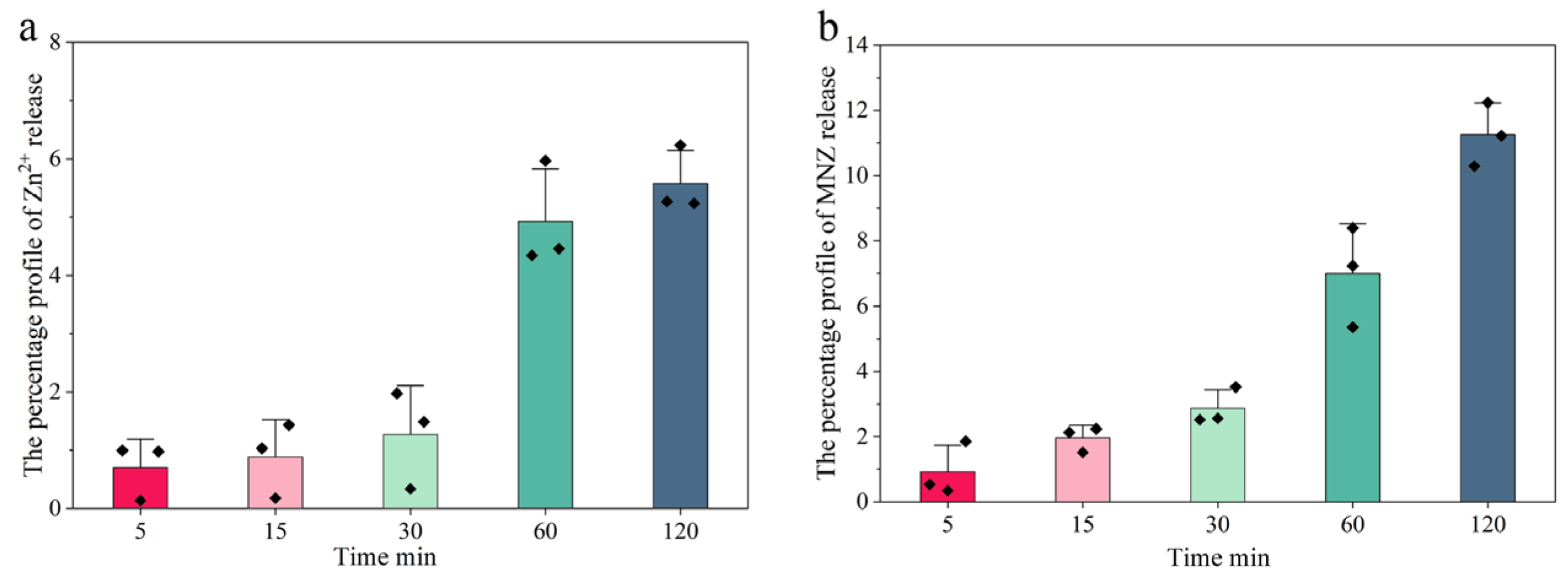
2.3.1. Sustained Release Rate
2.3.2. Mortality (Minimum Inhibitory Concentration)
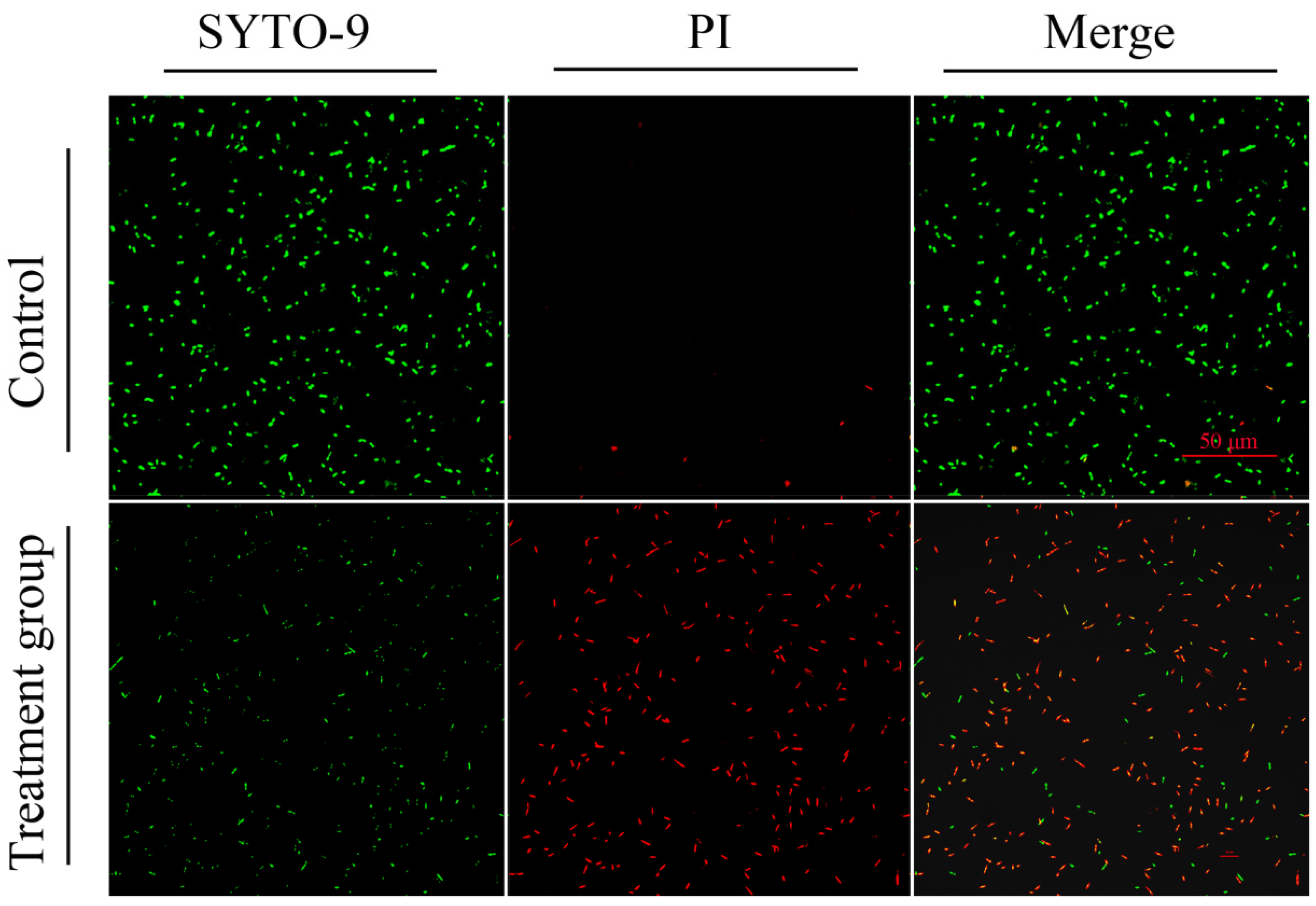
2.3.3. Live/Dead Fluorescence Staining to Assess Extracellular Antibacterial Activity
2.4. Antibacterial Effect
2.5. Biological Safety of ZIF-8@MNZ
2.6. Stability of Hydrogel@ZIF-8@MNZ Under Simulated Oral Conditions
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of ZIF-8 Nanoparticles
4.3. Loading of Metronidazole into ZIF-8
4.4. Fabrication of Hydrogel@ZIF-8@MNZ
4.5. Adsorption Studies
Integrated Form (Assuming ΔH Is Constant over T)
4.6. Biological Evaluation Experiments
4.6.1. Swelling Test
4.6.2. Rheological Behavior
4.6.3. Live/Dead Fluorescence Staining to Assess Extracellular Antibacterial Activity
4.6.4. Cytotoxicity Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Fang, Y.; Liang, W.; Wong, C.C.; Qin, H.; Gao, Y.; Liang, M.; Song, L.; Zhang, Y.; Fan, M.; et al. Fusobacterium nucleatum facilitates anti-PD-1 therapy in microsatellite stable colorectal cancer. Cancer Cell 2024, 42, 1729–1746.e1728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, D.; Liu, C.; Tang, B.; Cui, Y.; Guo, D.; Duan, M.; Tu, Y.; Zheng, H.; Ning, X.; et al. Outer Membrane Vesicles Derived From Fusobacterium nucleatum Trigger Periodontitis Through Host Overimmunity. Adv. Sci. 2024, 11, 2400882. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, T.; Li, X.; Jin, L. Metronidazole-Treated Porphyromonas gingivalis Persisters Invade Human Gingival Epithelial Cells and Perturb Innate Responses. Antimicrob. Agents Chemother. 2020, 64, e02529-19. [Google Scholar] [CrossRef]
- Arjunan, P. Eye on the Enigmatic Link: Dysbiotic Oral Pathogens in Ocular Diseases; The Flip Side. Int. Rev. Immunol. 2021, 40, 409–432. [Google Scholar] [CrossRef]
- Haar, E.L.V.; So, J.; Gyamfi-Bannerman, C.; Han, Y.W. Fusobacterium nucleatum and adverse pregnancy outcomes: Epidemiological and mechanistic evidence. Anaerobe 2018, 50, 55–59. [Google Scholar] [CrossRef]
- Catalan, E.A.; Seguel-Fuentes, E.; Fuentes, B.; Aranguiz-Varela, F.; Castillo-Godoy, D.P.; Rivera-Asin, E.; Bocaz, E.; Fuentes, J.A.; Bravo, D.; Schinnerling, K.; et al. Oral Pathobiont-Derived Outer Membrane Vesicles in the Oral-Gut Axis. Int. J. Mol. Sci. 2024, 25, 11141. [Google Scholar] [CrossRef]
- Chaudhary, P.P.; Kaur, M.; Myles, I.A. Does “all disease begin in the gut”? The gut-organ cross talk in the microbiome. Appl. Microbiol. Biotechnol. 2024, 108, 339. [Google Scholar] [CrossRef]
- Vivekanandan, K.E.; Kumar, P.V.; Jaysree, R.C.; Rajeshwari, T. Exploring molecular mechanisms of drug resistance in bacteria and progressions in CRISPR/Cas9-based genome expurgation solutions. Glob. Med. Genet. 2025, 12, 100042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Y.; Yan, Z.-B.; Meng, Y.-M.; Hong, X.-Y.; Shao, G.; Ma, J.-J.; Cheng, X.-R.; Liu, J.; Kang, J.; Fu, C.-Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Hurtado-Celotti, D.; Martínez-Rodríguez, N.; Ruiz-Sáenz, P.L.; Barona-Dorado, C.; Santos-Marino, J.; Martínez-González, J.M. Piperacillin-Tazobactam as an Adjuvant in the Mechanical Treatment of Patients with Periodontitis: A Randomized Clinical Study. Antibiotics 2022, 11, 1689. [Google Scholar] [CrossRef] [PubMed]
- Ramón, G.-S.J.; Antonio, R.-C.J.; Ofelia, H.-G.S.; Claudia, E.-B.M.; Rocío, M.-S.; Vianeth, M.-R.; Celia, M.-C.K.; Roldán, M.-S.A. Efficacy of clindamycin compared with amoxicillin-metronidazole after a 7-day regimen in the treatment of periodontitis in patients with diabetes: A randomized clinical trial. BMJ Open Diabetes Res. Care 2020, 8, 665. [Google Scholar] [CrossRef] [PubMed]
- Cusumano, G.; Flores, G.A.; Venanzoni, R.; Angelini, P. The Impact of Antibiotic Therapy on Intestinal Microbiota: Dysbiosis. Antibiotic Resistance, and Restoration Strategies. Antibiotics 2025, 14, 371. [Google Scholar] [CrossRef]
- Laforgia, A.; Inchingolo, A.D.; Piras, F.; Colonna, V.; Giorgio, R.V.; Carone, C.; Rapone, B.; Malcangi, G.; Inchingolo, A.M.; Inchingolo, F.; et al. Therapeutic Strategies and Genetic Implications for Periodontal Disease Management: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 7217. [Google Scholar] [CrossRef] [PubMed]
- Deria, P.; Chung, Y.G.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. Water stabilization of Zr6-based metal-organic frameworks via solvent-assisted ligand incorporation. Chem. Sci. 2015, 6, 5172–5176. [Google Scholar] [CrossRef]
- Wu, M.-X.; Yang, Y.-W. Metal-Organic Framework (MOF)-Based Drug/Cargo Delivery and Cancer Therapy. Adv. Mater. 2017, 29, 1606134. [Google Scholar] [CrossRef]
- Reshmi, R.; Jiju, K.R.; Suma, S.; Anoop, S.N. Folic acid grafted aminated zeolitic imidazolate framework (ZIF-8) as pH responsive drug carrier for targeted delivery of curcumin. J. Drug Deliv. Sci. Technol. 2023, 79, 104098. [Google Scholar] [CrossRef]
- Zhong, F.; He, Y.; Wang, P.; Chen, C.; Wu, Y. Novel pH-responsive self-healing anti-corrosion coating with high barrier and corrosion inhibitor loading based on reduced graphene oxide loaded zeolite imidazole framework. Colloids Surf. Physicochem. Eng. Asp. 2022, 642, 128641. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Zhang, X.; Lu, Y.; Azeez, N.A.; Zhang, L.; Krishnakumar, G.S.; Wang, M.-H. Exploring the synthesis; properties, and potential of chitosan-functionalized metal-organic frameworks in emerging applications. Prog. Mater. Sci. 2025, 148, 101387. [Google Scholar] [CrossRef]
- Salazar, J.; Hidalgo-Rosa, Y.; Burboa, P.C.; Wu, Y.-N.; Escalona, N.; Leiva, A.; Zarate, X.; Schott, E. UiO-66(Zr) as drug delivery system for non-steroidal anti-inflammatory drugs. J. Control. Release 2024, 370, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Cai, K.; Zhao, J.; Liu, C.; Li, H.; Tan, P.; Li, Y.; Li, D.; Ma, X. Mannosylated MOF Encapsulated in Lactobacillus Biofilm for Dual-Targeting Intervention Against Mammalian Escherichia coli Infections. Adv. Mater. 2025, 37, 2503056. [Google Scholar] [CrossRef]
- Zhou, Z.; Ke, Q.; Wu, M.; Zhang, L.; Jiang, K. Pore Space Partition Approach of ZIF-8 for pH Responsive Codelivery of Ursolic Acid and 5-Fluorouracil. ACS Mater. Lett. 2023, 5, 466–472. [Google Scholar] [CrossRef]
- Sha, Z.; Wu, Y.; Zheng, Y.; Yang, K.; Gong, X.; Xuan, L.; Li, X.; Chen, X. Advances in pH-responsive drug delivery systems for periodontitis treatment. Drug Deliv. 2025, 32, 2522109. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Liu, Y.; Qi, J.; Yu, M.; Wang, Y.; Qiu, Y.; Ma, Z.; Liu, S. Dual photothermal-photocatalytic Au@ZIF-8/Ti3C2Tx nanocomposite for enhanced solar steam sterilization and biofouling-resistant hydrogel membrane. Mater. Today Chem. 2025, 43, 102467. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Tang, M.; Peng, C.; Wang, G.; Wang, J.; Wang, X.; Chang, X.; Guo, J.; Gui, S. Smart stimuli-responsive hydrogels for drug delivery in periodontitis treatment. Biomed. Pharmacother. 2023, 162, 114688. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the potential of hydrogels for advanced therapeutic applications: Current achievements and future directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef]
- Kaur, H.; Gogoi, B.; Sharma, I.; Das, D.K.; Azad, M.A.; Pramanik, D.D.; Pramanik, A. Hydrogels as a Potential Biomaterial for Multimodal Therapeutic Applications. Mol. Pharm. 2024, 21, 4827–4848. [Google Scholar] [CrossRef]
- Hu, M.; Ge, X.; Chen, X.; Mao, W.; Qian, X.; Yuan, W.-E. Micro/Nanorobot: A Promising Targeted Drug Delivery System. Pharmaceutics 2020, 12, 665. [Google Scholar] [CrossRef]
- di Nicola, N.; Di Pelino, M.; Foschi, M.; Passalacqua, R.; Lazzarini, A.; Ruggieri, F. ZIF-8 as Potential Pesticide Adsorbent Medium for Wastewater Treatment: The Case Study of Model Linuron Extraction Conditions Optimization via Design of Experiment. Molecules 2025, 30, 2480. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Lu, M.; Yang, M.; Li, J.; Wang, Z.; Li, G.; Wang, H. Application of a novel adsorbent UiO-66 modified by Ce to tetracycline removal in water bodies. J. Environ. Chem. Eng. 2023, 11, 110478. [Google Scholar] [CrossRef]
- Uddin, M.S.; Khand, S.; Dong, C. Effect of Crosslinking Agents on Chitosan Hydrogel Carriers for Drug Loading and Release for Targeted Drug Delivery. Gels 2024, 10, 421. [Google Scholar] [CrossRef]
- Li, H.; Cheng, Y.; Li, J.; Li, T.; Zhu, J.; Deng, W.; Zhu, J.; He, D. Preparation and Adsorption Performance Study of Graphene Quantum Dots@ZIF-8 Composites for Highly Efficient Removal of Volatile Organic Compounds. Nanomaterials 2022, 12, 4008. [Google Scholar] [CrossRef]
- Kaur, H.; Mohanta, G.C.; Gupta, V.; Kukkar, D.; Tyagi, S. Synthesis and characterization of ZIF-8 nanoparticles for controlled release of 6-mercaptopurine drug. J. Drug Deliv. Sci. Technol. 2017, 41, 106–112. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I.; Panayotov, D.A.; Mihaylov, M.Y.; Ivanova, E.Z.; Chakarova, K.K.; Andonova, S.M.; Drenchev, N.L. Power of Infrared and Raman Spectroscopies to Characterize Metal-Organic Frameworks and Investigate Their Interaction with Guest Molecules. Chem. Rev. 2021, 121, 1286–1424. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Su, J.; Jia, M.; Li, Z.; Liu, G.; Li, W. Vapor-phase linker exchange of metal-organic frameworks. Sci. Adv. 2020, 6, eaax7270. [Google Scholar] [CrossRef]
- Dai, J.-P.; Li, D.; He, Y.-L.; Du, S.; Li, J.-N. Pore-scale investigation on the multi-component gas adsorption and diffusion in carbon xerogel microporous structure using molecular simulation methods. Microporous Mesoporous Mater. 2022, 337, 111890. [Google Scholar] [CrossRef]
- Chmelik, C.; Bux, H.; Caro, J.; Heinke, L.; Hibbe, F.; Titze, T.; Kärger, J. Mass Transfer in a Nanoscale Material Enhanced by an Opposing Flux. Phys. Rev. Lett. 2010, 104, 085902. [Google Scholar] [CrossRef]
- Schejn, A.; Balan, L.; Falk, V.; Aranda, L.; Medjahdi, G.; Schneider, R. Controlling ZIF-8 nano- and microcrystal formation and reactivity through zinc salt variations. CrystEngComm 2014, 16, 4493–4500. [Google Scholar] [CrossRef]
- Gan, S.; Qiu, M.; Li, L.; Li, Y.; Su, D.; Jia, Z.; Xia, Y.; Xu, W.; Yang, D.; Lei, J.; et al. Fabrication of novel three-dimensional carbon aerogel of Ag@ZIF/CCA for highly enhanced visible-light photocatalytic performance. Ind. Crops Prod. 2024, 208, 117803. [Google Scholar] [CrossRef]
- Hossain, M.S.; Hossain, M.M.; Khatun, M.K.; Hossain, K.R. Hydrogel-based superadsorbents for efficient removal of heavy metals in industrial wastewater treatment and environmental conservation. Environ. Funct. Mater. 2023, 2, 142–158. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Y.; Huang, M.; Zhang, H.; Bai, F. Observation of enhanced dynamic ΔG effect near ferromagnetic resonance frequency. Appl. Phys. Lett. 2023, 123, 012406. [Google Scholar] [CrossRef]
- Modi, A.; Jiang, Z.; Kasher, R. Hydrostable ZIF-8 layer on polyacrylonitrile membrane for efficient treatment of oilfield produced water. Chem. Eng. J. 2021, 434, 133513. [Google Scholar] [CrossRef]
- Wu, D.; Lin, H.; Ren, X.; Qian, J.; Ma, N.; Dai, W. ZIF-8 derived spherical porous carbon as an efficient sustained-release carrier for nitroimidazole drugs. Mater. Today Chem. 2024, 38, 102057. [Google Scholar] [CrossRef]
- Alamgir; Talha, K.; Wang, B.; Liu, J.-H.; Ullah, R.; Feng, F.; Yu, J.; Chen, S.; Li, J.-R. Effective adsorption of metronidazole antibiotic from water with a stable Zr(IV)-MOFs: Insights from DFT. kinetics and thermodynamics studies. J. Environ. Chem. Eng. 2020, 8, 103642. [Google Scholar] [CrossRef]
- Mazloomi, S.; Amarloei, A.; Gholami, F.; Haghighat, G.A.; Gholikandi, G.B.; Nourmoradi, H.; Mohammadi, A.A.; Fattahi, M.; Le, B.N. Parametric study and process modeling for metronidazole removal by rhombic dodecahedron ZIF-67 crystals. Sci. Rep. 2023, 13, 14654. [Google Scholar] [CrossRef]
- Li, J.; Chang, H.; Li, Y.; Li, Q.; Shen, K.; Yi, H.; Zhang, J. Synthesis and adsorption performance of La@ZIF-8 composite metal-organic frameworks. RSC Adv. 2020, 10, 3380–3390. [Google Scholar] [CrossRef]
- Anbari, A.P.; Delcheh, S.R.; Kashif, M.; Ranjbari, A.; Akbari, M.K.; Zhuiykov, S.; Heynderickx, P.M.; Verpoort, F. Engineering Fe-Modified Zeolitic Imidazolate Frameworks (Fe-ZIF-8 and Fe-ZIF-67) via In Situ Thermal Synthesis for Enhanced Adsorption of Malachite Green from Aqueous Solutions: A Comprehensive Study of Isotherms, Kinetics, and Thermodynamics. Nanomaterials 2025, 15, 1097. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, J.; Zhang, W.; Yang, C.; Zhang, L.; Zhu, W.; Sun, J.; Li, G.; Li, T.; Wang, J. Amorphous Fe/Mn bimetal-organic frameworks: Outer and inner structural designs for efficient arsenic(iii) removal. J. Mater. Chem. A 2019, 7, 2845–2854. [Google Scholar] [CrossRef]
- Puccia, V.; Avena, M. On the use of the Dubinin-Radushkevich equation to distinguish between physical and chemical adsorption at the solid-water interface. Colloid. Interface Sci. Commun. 2021, 41, 100376. [Google Scholar] [CrossRef]
- Gao, J.; Chu, W.; Ding, X.; Ding, L.; Guo, Q.; Fu, Y. Degradation Kinetic Studies of BSA@ZIF-8 Nanoparticles with Various Zinc Precursors, Metal-to-Ligand Ratios, and pH Conditions. ACS Omega 2023, 8, 44601–44610. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Singh, A.; Garg, N.; Randhawa, J.K. Curcumin encapsulated zeolitic imidazolate frameworks as stimuli responsive drug delivery system and their interaction with biomimetic environment. Sci. Rep. 2017, 7, 12598. [Google Scholar] [CrossRef] [PubMed]
- Sharafinia, S.; Rashidi, A.; Tabarkhoon, F.; Dehghan, F.; Tabarkhoon, F.; Bazmi, M. Effective adsorption of amoxicillin by using UIO-66@ Cr-MIL-101 nanohybrid: Isotherm. kinetic, thermodynamic, and optimization by central composite design. Sci. Rep. 2023, 13, 22689. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Simchi, A.; Far, H.S. Nanoporous composites of activated carbon-metal organic frameworks for organic dye adsorption: Synthesis. adsorption mechanism and kinetics studies. J. Ind. Eng. Chem. 2020, 81, 405–414. [Google Scholar] [CrossRef]
- Chu, K.H.; Hashim, M.A.; Zawawi, M.H.; Bollinger, J.-C. The Weber-Morris model in water contaminant adsorption: Shattering long-standing misconceptions. J. Environ. Chem. Eng. 2025, 13, 117266. [Google Scholar] [CrossRef]
- Mehrizad, A. Prompt loading and prolonged release of metronidazole by calcium ferrite-carbon nanotubes carrier: Optimization and modeling of the process by RSM and ANN. Diam. Relat. Mater. 2023, 135, 109899. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Kim, Y.; Lee, J.; Choe, W.; Kim, J. Understanding the Structural Collapse during Activation of Metal-Organic Frameworks with Copper Paddlewheels. Inorg. Chem. 2022, 61, 9702–9709. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, M.; Lin, Y.S. Stability of ZIF-8 in water under ambient conditions. Microporous Mesoporous Mater. 2019, 279, 201–210. [Google Scholar] [CrossRef]
- Xia, Z.; Zhao, B.; Xiang, J.; Xu, K.; Luo, K.; Yu, L.-X.; Yang, K.-K.; Wang, Y.-Z. Injectable pH-Responsive Hydrogel Adapted to Gingival Crevicular Fluid Microenvironment for Periodontitis Therapy. ACS Appl. Mater. Interfaces 2025, 17, 31357–31367. [Google Scholar] [CrossRef]
- Zhu, R.; Du, W.; Mi, X. Injectable, pH-responsive hybrid hydrogels for the treatment of periodontitis: The role of hybrid hydrogels on periodontitis. Cell. Mol. Biol. 2023, 69, 210–216. [Google Scholar] [CrossRef]
- Tran, V.A.; Lee, S.-W. pH-triggered degradation and release of doxorubicin from zeolitic imidazolate framework-8 (ZIF8) decorated with polyacrylic acid. RSC Adv. 2021, 11, 9222–9234. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Chang, S.; Kim, N.-E.; Choi, S.-O.; Song, Y.-J.; Yuan, Y.; Kim, J. Curcumin/Zeolitic Imidazolate Framework-8 Nanoparticle-Integrated Microneedles for pH-Responsive Treatment of Skin Disorders. ACS Appl. Nano Mater. 2022, 5, 13671–13679. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, K.; Liu, J.; Tian, Y.; Li, H.; Wang, H.; Lin, Z.; Qiu, M.; Tang, B. A ZIF-8-based multifunctional intelligent drug release system for chronic osteomyelitis. Colloids Surf. B Biointerfaces 2022, 212, 112354. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, R.; Kovács, A.; Berkó, S.; Budai-Szűcs, M. Smart biomaterial gels for periodontal therapy: A novel approach. Biomed. Pharmacother. 2025, 183, 117836. [Google Scholar] [CrossRef] [PubMed]
- Onopiuk, B.; Onopiuk, P.; Dąbrowska, Z.; Dąbrowska, E.; Pietruska, M.; Car, H. Effect of Metronidazole on the Oxidoreductive Processes in the Submandibular and Parotid Glands in Experimental Research. Oxid. Med. Cell. Longev. 2018, 2018, 7083486. [Google Scholar] [CrossRef]
- Dingsdag, S.A.; Hunter, N. Metronidazole: An update on metabolism, structure-cytotoxicity and resistance mechanisms. J. Antimicrob. Chemother. 2017, 73, 265–279. [Google Scholar] [CrossRef]
- Afshan, N.; Tariq, R.; Riaz, I.; Manan, A.; Iqbal, A.; Ejaz, M.; Sohail, A.; Bari, A.; Mahmood, S.; Iqbal, S.; et al. Antimicrobial framework nucleic acid-based DNAzyme cluster with high catalytic efficiency. J. Chem. Technol. Biotechnol. 2024, 99, 2027–2034. [Google Scholar] [CrossRef]
- Chrystal, E.J.; Koch, R.L.; McLafferty, M.A.; Goldman, P. Relationship between metronidazole metabolism and bactericidal activity. Antimicrob. Agents Chemother. 1980, 18, 566–573. [Google Scholar] [CrossRef]
- Ihalin, R.; Nuutila, J.; Loimaranta, V.; Lenander, M.; Tenovuo, J.; Lilius, E.-M. Susceptibility of Fusobacterium nucleatum to killing by peroxidase-iodide-hydrogen peroxide combination in buffer solution and in human whole saliva. Anaerobe 2003, 9, 23–30. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, T.; Li, Y.; Huang, L.; Yin, D. Fusobacterium nucleatum: The Opportunistic Pathogen of Periodontal and Peri-Implant Diseases. Front. Microbiol. 2022, 13, 860149. [Google Scholar] [CrossRef]
- Galbadage, T.; Liu, D.; Alemany, L.B.; Pal, R.; Tour, J.M.; Gunasekera, R.S.; Cirillo, J.D. Molecular Nanomachines Disrupt Bacterial Cell Wall, Increasing Sensitivity of Extensively Drug-Resistant Klebsiella pneumoniae to Meropenem. ACS Nano 2019, 13, 14377–14387. [Google Scholar] [CrossRef]
- Chen, E.H.L.; Wang, C.-H.; Liao, Y.-T.; Chan, F.-Y.; Kanaoka, Y.; Uchihashi, T.; Kato, K.; Lai, L.; Chang, Y.-W.; Ho, M.-C.; et al. Visualizing the membrane disruption action of antimicrobial peptides by cryo-electron tomography. Nat. Commun. 2023, 14, 5464. [Google Scholar] [CrossRef] [PubMed]
- Löfmark, S.; Edlund, C.; Nord, C.E. Metronidazole Is Still the Drug of Choice for Treatment of Anaerobic Infections. Clin. Infect. Dis. 2010, 50, S16–S23. [Google Scholar] [CrossRef]
- Van Zuylen, E.M.; Ferguson, S.A.; Hughes, A.; Rennison, D.; Brimble, M.A.; Cook, G.M. Disruption of Metallostasis in the Anaerobic Human Pathogen Fusobacterium nucleatum by the Zinc Ionophore PBT2. ACS Infect. Dis. 2021, 7, 2285–2298. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Z.; Tang, Z.; Huang, Y.; Huang, M.; Liu, H.; Ziebolz, D.; Schmalz, G.; Jia, B.; Zhao, J. More Than Just a Periodontal Pathogen -the Research Progress on Fusobacterium nucleatum. Front. Cell Infect. Microbiol. 2022, 12, 815318. [Google Scholar] [CrossRef]
- Yang, L.; Wen, J.; Wang, Q.; Cui, H. Effect of solution chemistry on the stability and transport of ZIF-8 in saturated porous media. J. Environ. Chem. Eng. 2022, 10, 107562. [Google Scholar] [CrossRef]
- Van Cleuvenbergen, S.; Smith, Z.J.; Deschaume, O.; Bartic, C.; Wachsmann-Hogiu, S.; Verbiest, T.; van der Veen, M.A. Morphology and structure of ZIF-8 during crystallisation measured by dynamic angle-resolved second harmonic scattering. Nat. Commun. 2018, 9, 3418. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, X.; Cai, Y.; Zhao, S.; Wang, S. Effects of metronidazole on mesophilic and thermophilic fermentation: Biodegradation mechanisms, microbial communities, and reversibility. Bioresour. Technol. 2022, 362, 127795. [Google Scholar] [CrossRef]
- Sun, J.; Chu, R.; Khan, Z.U.H. A Theoretical Study on the Degradation Mechanism, Kinetics, and Ecotoxicity of Metronidazole (MNZ) in •OH- and SO(4)(•-)-Assisted Advanced Oxidation Processes. Toxics 2023, 11, 796. [Google Scholar] [CrossRef] [PubMed]
- Steckiewicz, K.P.; Dmochowska, M.; Megiel, E.; Barcińska, E.; Inkielewicz-Stępniak, I. Functionalization-Dependent Cytotoxicity of Silver Nanoparticles: A Comparative Study of Chlorhexidine and Metronidazole Conjugates. Biomolecules 2025, 15, 850. [Google Scholar] [CrossRef] [PubMed]
- Carnamucio, F.; Foti, C.; Micale, N.; Van Pelt, N.; Matheeussen, A.; Caljon, G.; Giuffrè, O. Metronidazole Interaction with Cu2+ and Zn2+: Speciation Study in Aqueous Solution and Biological Activity Evaluation. ACS Omega 2024, 9, 29000–29008. [Google Scholar] [CrossRef]
- Buschini, A.; Ferrarini, L.; Franzoni, S.; Galati, S.; Lazzaretti, M.; Mussi, F.; Northfleet de Albuquerque, C.; Maria Araújo Domingues Zucchi, T.; Poli, P. Genotoxicity Revaluation of Three Commercial Nitroheterocyclic Drugs: Nifurtimox, Benznidazole, and Metronidazole. J. Parasitol. Res. 2009, 2009, 463575. [Google Scholar] [CrossRef]
- Cui, H.; Tang, S.; Huang, S.; Lei, L.; Jiang, Z.; Li, L.; Wei, S. Simultaneous mitigation of arsenic and cadmium accumulation in rice grains by foliar inhibitor with ZIF-8@Ge-132. Sci. Total Environ. 2023, 860, 160307. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Wang, X.; Chen, Z.; Liu, Y.; Yang, W. An S2- responsive nanocontainer for inhibiting microbial corrosion caused by sulfate-reducing bacteria. Colloids Surf. Physicochem. Eng. Asp. 2023, 663, 131110. [Google Scholar] [CrossRef]
- Cheng, H.; Newton, M.A.A.; Rajib, M.; Zhang, Q.; Gao, W.; Lu, Z.; Zheng, Y.; Dai, Z.; Zhu, J. A ZIF-8-encapsulated interpenetrated hydrogel/nanofiber composite patch for chronic wound treatment. J. Mater. Chem. B 2024, 12, 2042–2053. [Google Scholar] [CrossRef]
- Tomić, S.L.; Radić, M.M.B.; Vuković, J.S.; Filipović, V.V.; Nikodinovic-Runic, J.; Vukomanović, M. Alginate-Based Hydrogels and Scaffolds for Biomedical Applications. Mar. Drugs 2023, 21, 177. [Google Scholar] [CrossRef] [PubMed]
- Fakhree, M.A.; Nordin, N.A.; Nazmi, N.; Mazlan, S.A.; Aziz, S.A.; Ubaidillah, U.; Ahmad, F.; Choi, S.-B. Field-Dependent Rheological Properties of Magnetorheological Elastomer with Fountain-Like Particle Chain Alignment. Micromachines 2022, 13, 492. [Google Scholar] [CrossRef] [PubMed]


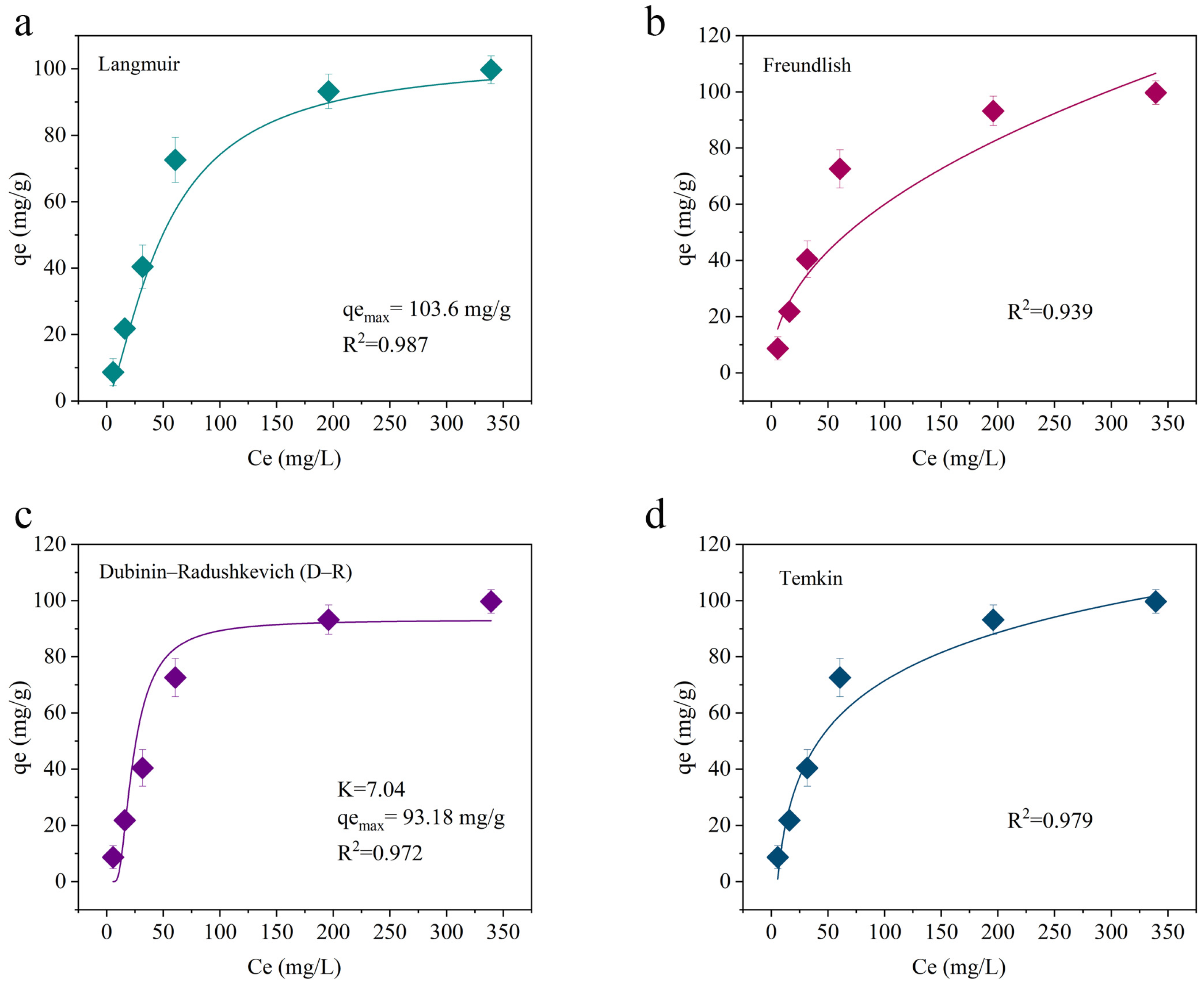
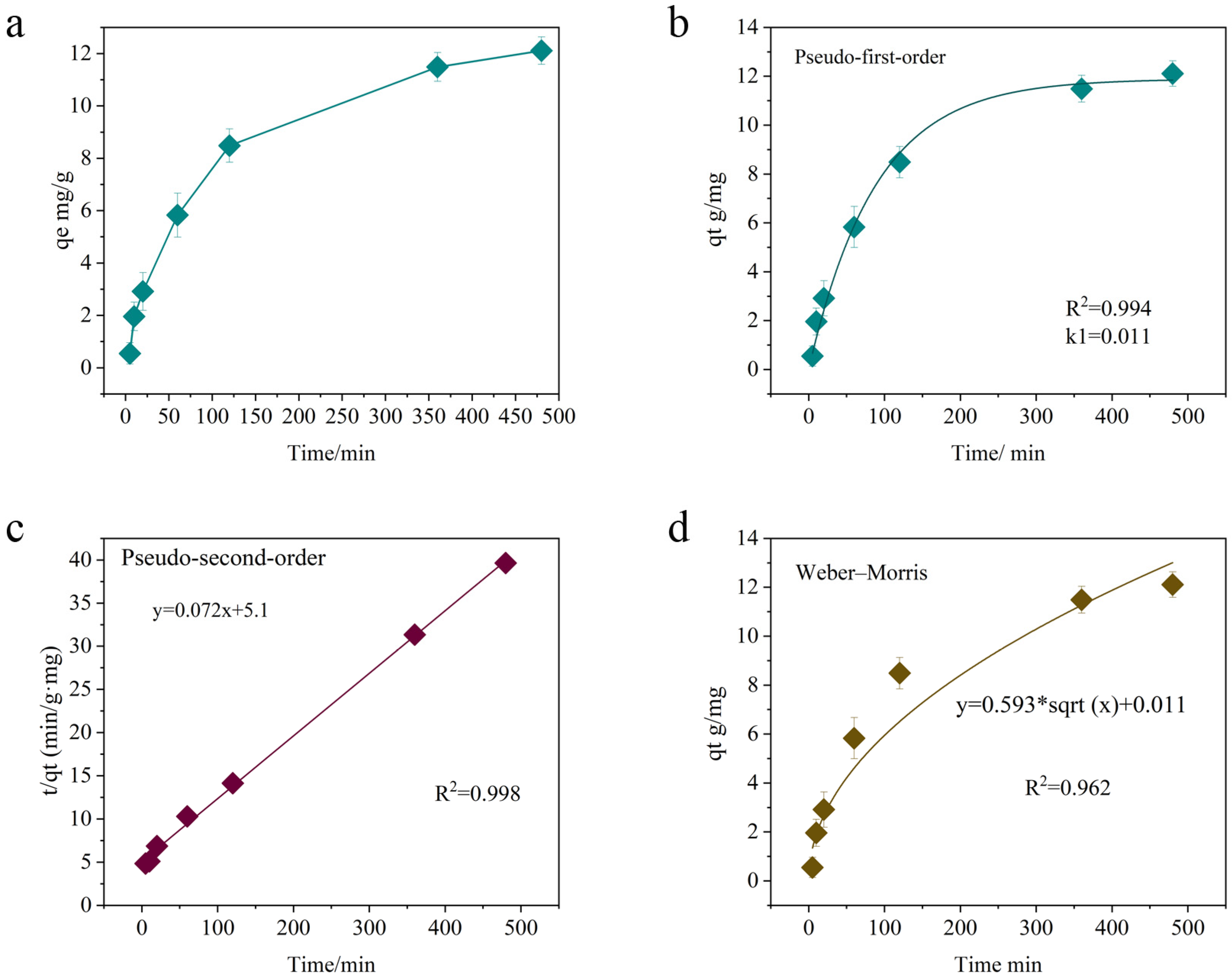
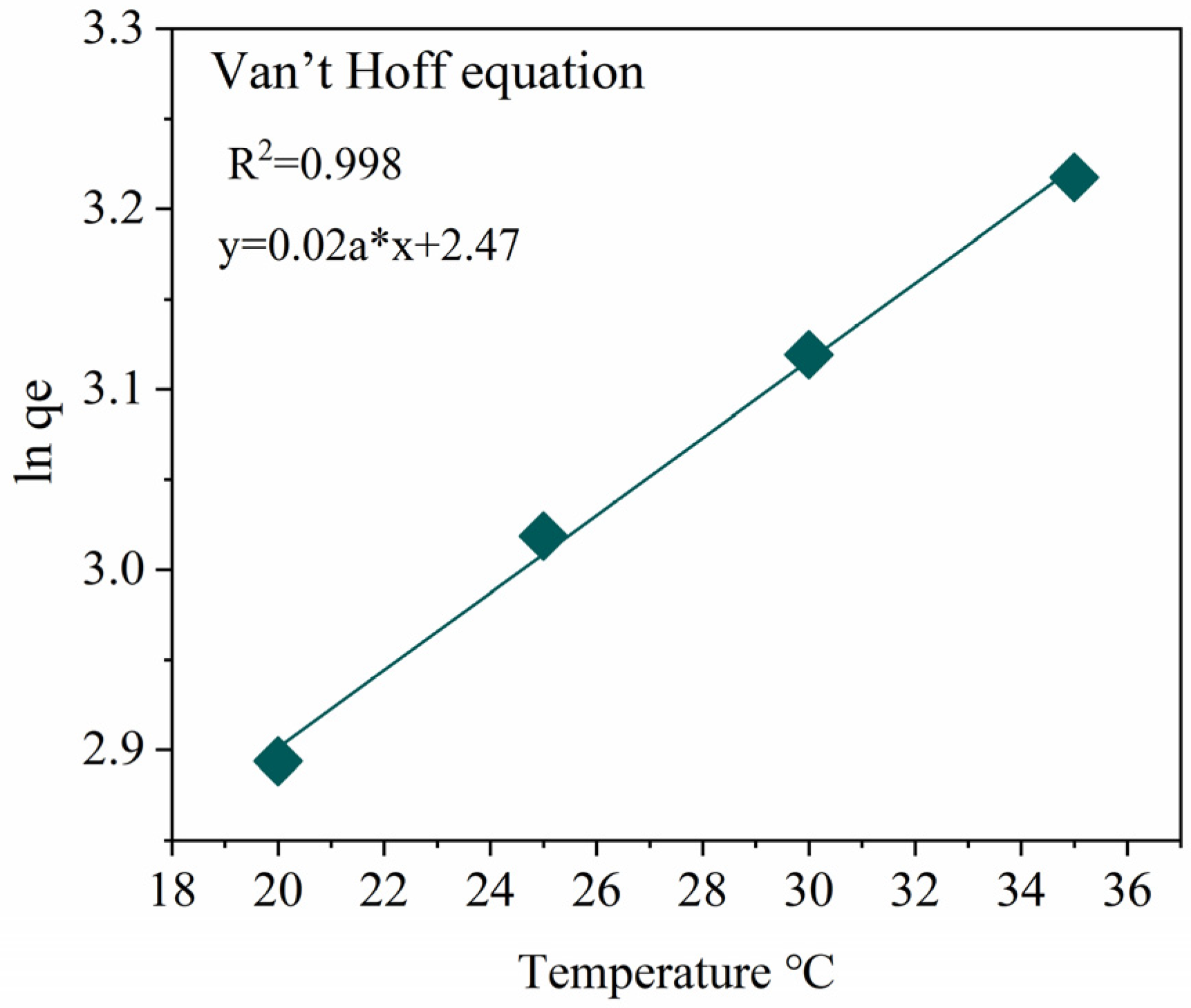
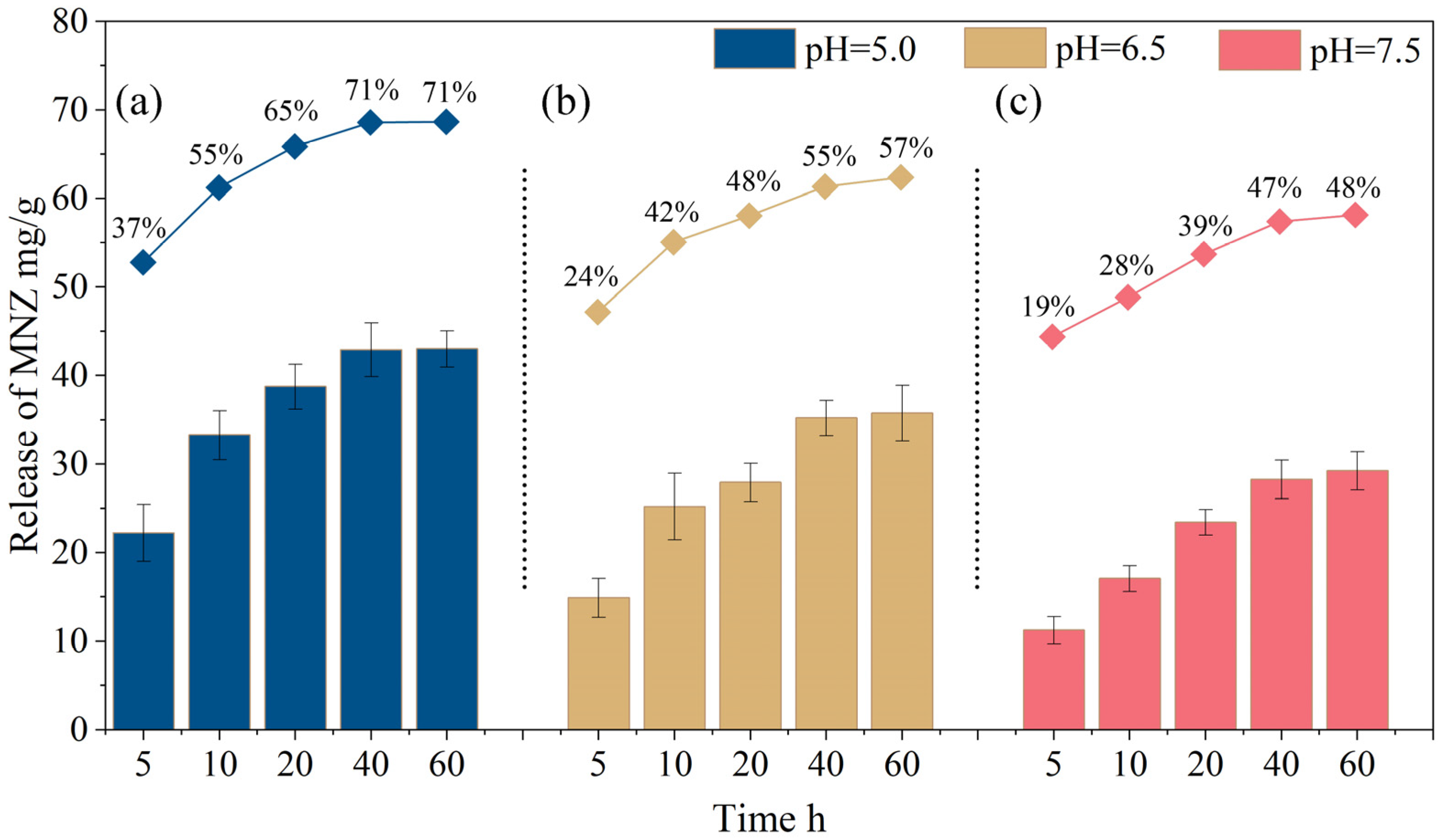
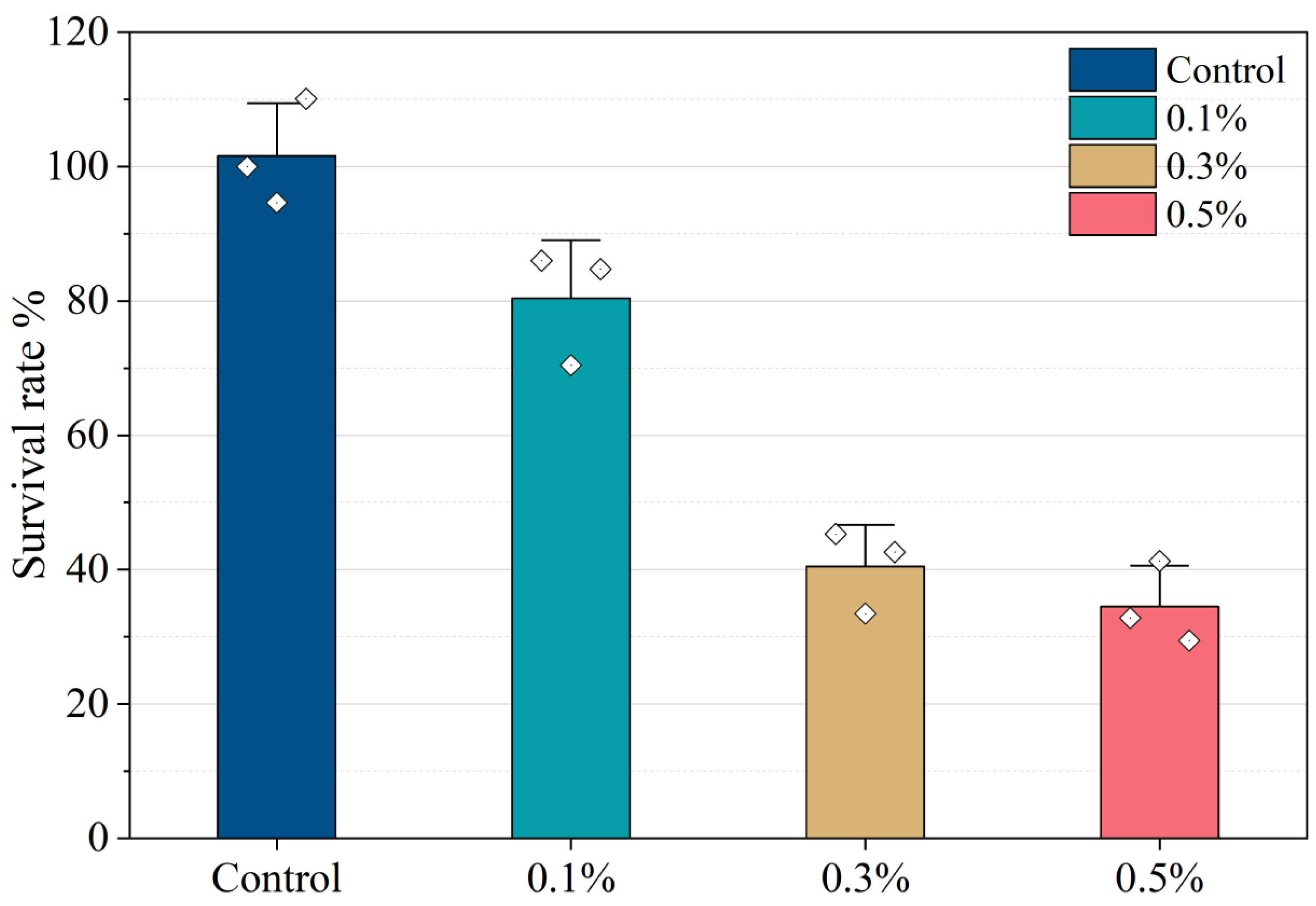

| Model | Index | Value | R2 | Explanation |
|---|---|---|---|---|
| Langmuir | qmax (mg/g) | 103.6 | 0.987 | Saturation adsorption capacity; model fit |
| KL (L/mg) | 0.04 | Represents adsorption affinity | ||
| Freundlich | KF | 3.02 | 0.939 | Multilayer adsorption model; slightly less applicable than the Langmuir model |
| n | 2.3 | 1 < n < 10 indicates favorable adsorption | ||
| D-R | qmax (mg/g) | 93.18 | 0.972 | Slightly lower adsorption capacity |
| E (kJ/mol) | 6.21 | E < 8 kJ/mol indicates a physical adsorption process | ||
| Temkin | A (L/g) | 1.54 | 0.979 | Adsorption heat varies with surface coverage |
| B (J/mol) | 214.36 | Larger B values indicate higher adsorption heat |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; Hao, M.; Cui, H.; Zeng, R.; Ma, H.; Long, A.; Li, X. Preparation and Characterization of an Acid-Responsive ZIF-8 Hydrogel Dressing with Sustained-Release Function for Targeted Therapy of Periodontitis. Gels 2025, 11, 813. https://doi.org/10.3390/gels11100813
Chen B, Hao M, Cui H, Zeng R, Ma H, Long A, Li X. Preparation and Characterization of an Acid-Responsive ZIF-8 Hydrogel Dressing with Sustained-Release Function for Targeted Therapy of Periodontitis. Gels. 2025; 11(10):813. https://doi.org/10.3390/gels11100813
Chicago/Turabian StyleChen, Bingbing, Mengqi Hao, Hao Cui, Rui Zeng, Hang Ma, Anying Long, and Xuegang Li. 2025. "Preparation and Characterization of an Acid-Responsive ZIF-8 Hydrogel Dressing with Sustained-Release Function for Targeted Therapy of Periodontitis" Gels 11, no. 10: 813. https://doi.org/10.3390/gels11100813
APA StyleChen, B., Hao, M., Cui, H., Zeng, R., Ma, H., Long, A., & Li, X. (2025). Preparation and Characterization of an Acid-Responsive ZIF-8 Hydrogel Dressing with Sustained-Release Function for Targeted Therapy of Periodontitis. Gels, 11(10), 813. https://doi.org/10.3390/gels11100813







