Effect of Vanillin and Chitin Particles on the Chitosan-Based Oleogels Produced by the Emulsion-Templated Method
Abstract
1. Introduction
2. Results and Discussion
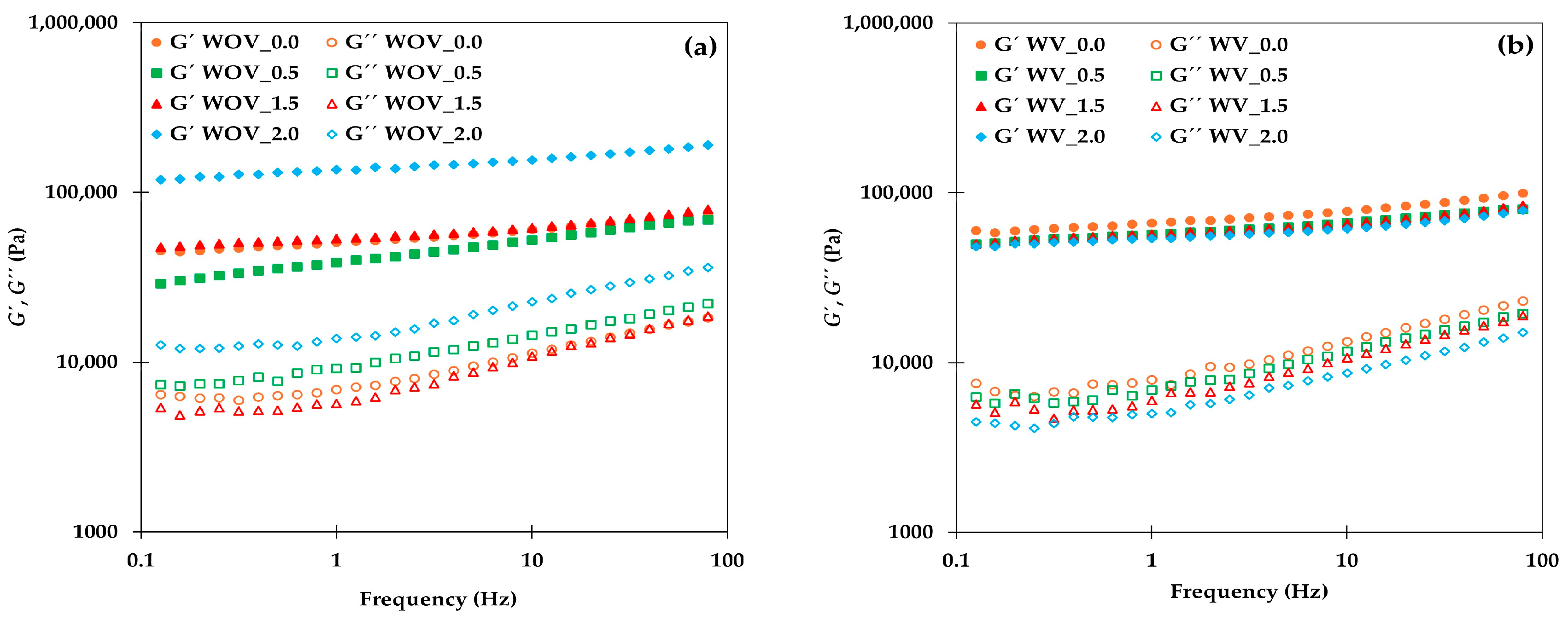
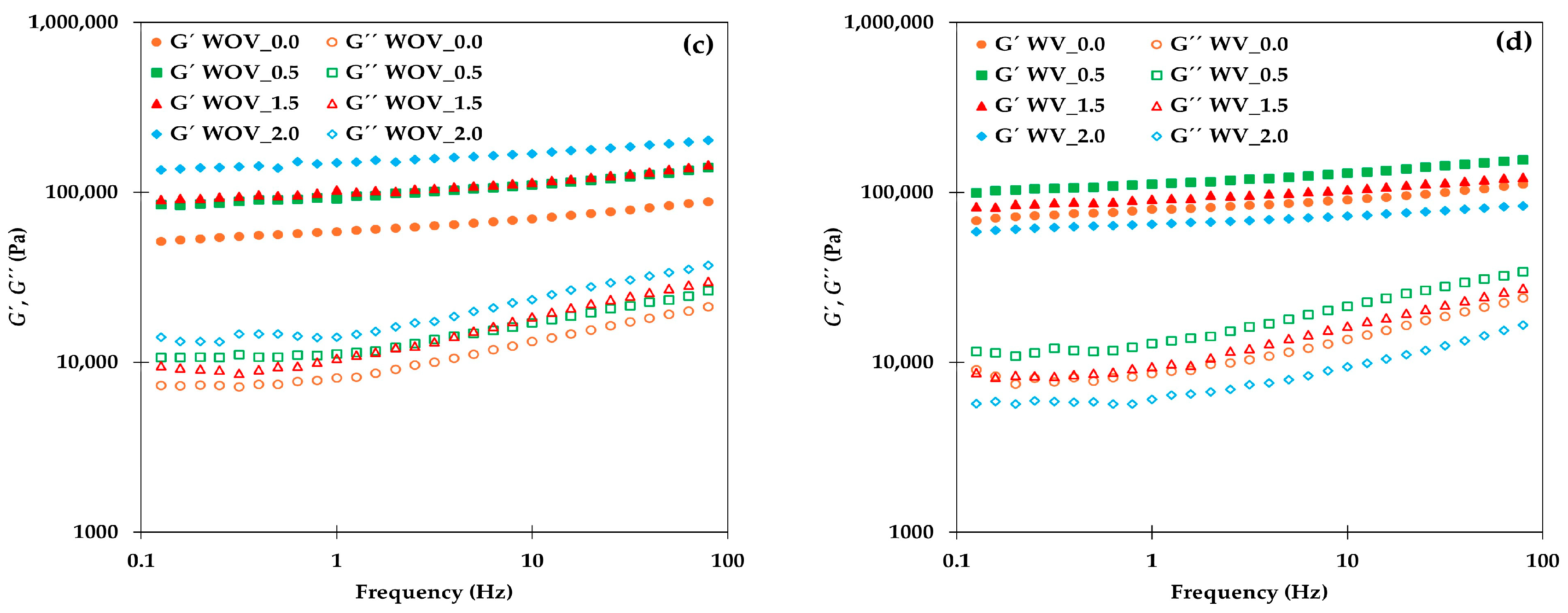
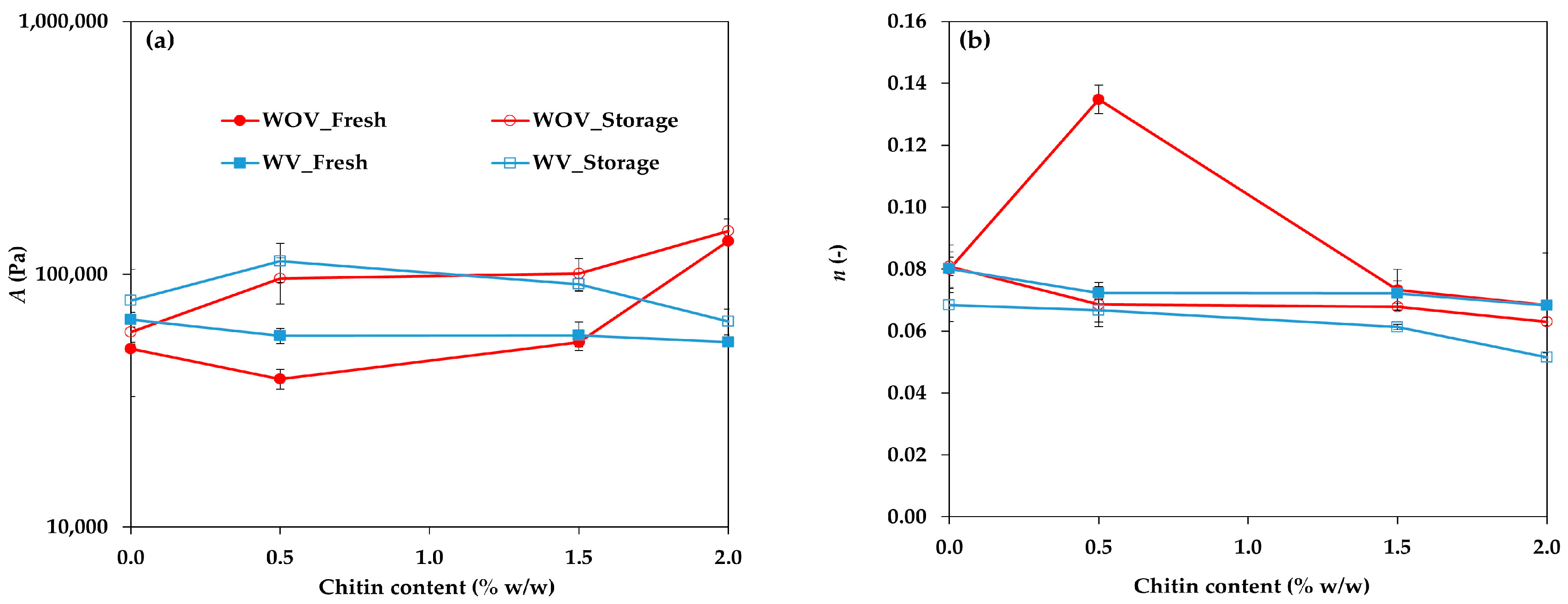
2.1. Rheological Characterization
2.2. Oil Binding Capacity
2.3. Textural Properties
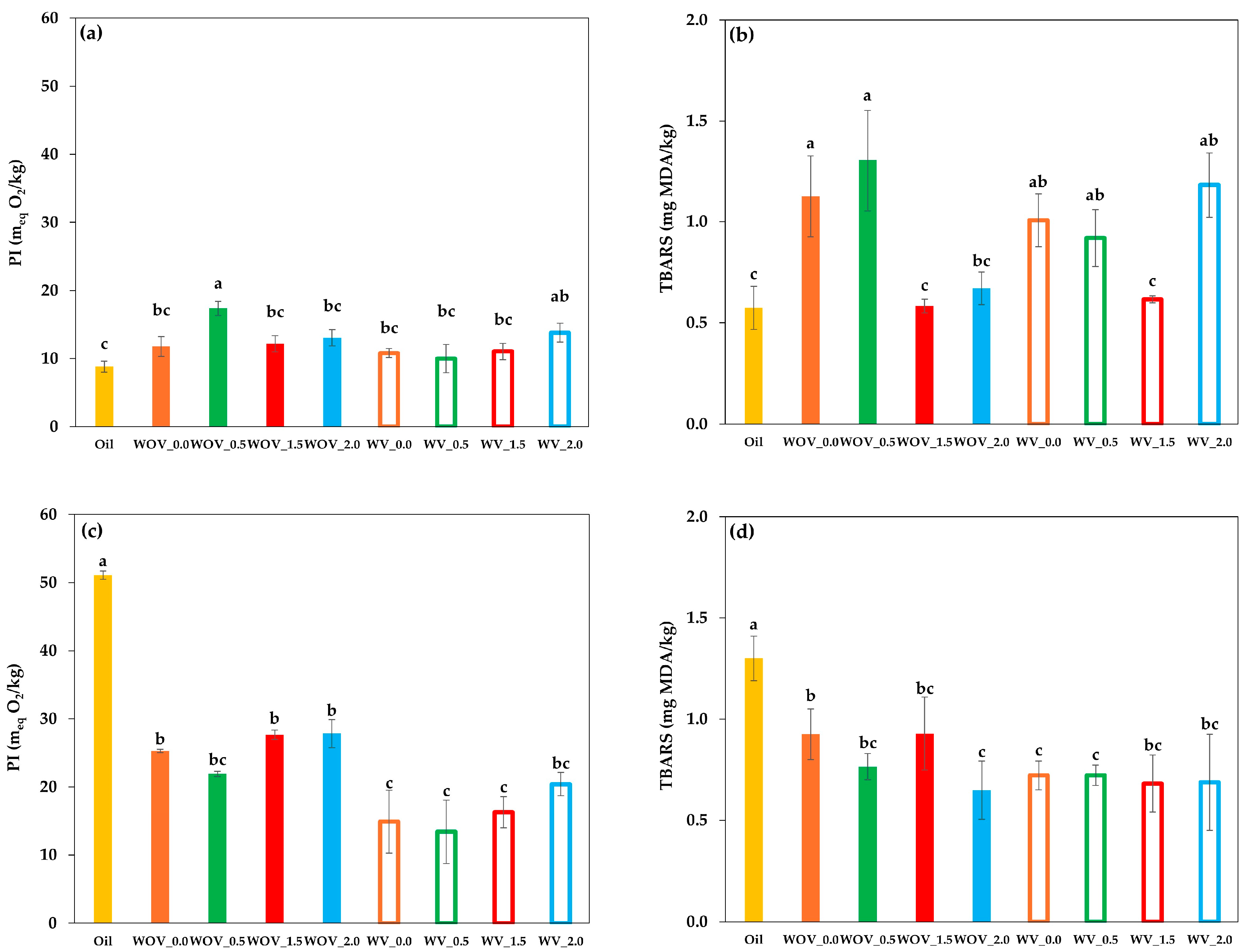
2.4. Oxidative Stability
2.5. Relationships Among Rheological, Textural, Oil Binding Capacity, and Oxidation Parameters
3. Conclusions
4. Materials and Methods
4.1. Materials and Reagents
4.2. Emulsion and Oleogel Preparation
4.3. Oleogel Characterization
4.3.1. Rheological Characterization
4.3.2. Oil Binding Capacity
4.3.3. Textural Properties
4.3.4. Oxidative Stability
4.4. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reynolds, A.N.; Hodson, L.; De Souza, R.; Diep, H.Y.; Vlietstra, L.; Mann, J. Saturated Fat and Trans-Fat Intakes and Their Replacement with Other Macronutrients: A Systematic Review and Meta-Analysis of Prospective Observational Studies; World Health Organization: Geneva, Switzerland, 2022; pp. 1–164. ISBN 978-92-4-006166-8. Available online: https://iris.who.int/bitstream/handle/10665/366301/9789240061668-eng.pdf (accessed on 3 June 2025).
- Yilmaz, E.; Demirci, Ş. Preparation and Evaluation of Virgin Olive Oil Oleogels Including Thyme and Cumin Spices with Sunflower Wax. Gels 2021, 7, 95. [Google Scholar] [CrossRef]
- Marangoni, F.; Galli, C.; Ghiselli, A.; Lercker, G.; La Vecchia, C.; Maffeis, C.; Poli, A. Palm oil and human health. Meeting report of Nutrition Foundation of Italy symposium. Int. J. Food Sci. Nutr. 2017, 68, 643–655. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Chen, K.; Deng, D. Improvement strategies for fats and oils used in future food processing based on health orientation and sustainability: Research progress, challenges and solutions. Crit. Rev. Food Sci. Nutr. 2023, 65, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Gengatharan, A.; Mohamad, N.V.; Che Zahari, C.N.M.; Vijayakumar, R. Oleogels: Innovative formulations as fat substitutes and bioactive delivery systems in food and beyond. Food Struct. 2023, 38, 100356. [Google Scholar] [CrossRef]
- Silva, P.M.; Cerqueira, M.A.; Martins, A.J.; Fasolin, L.H.; Cunha, R.L.; Vicente, A.A. Oleogels and bigels as alternatives to saturated fats: A review on their application by the food industry. J. Am. Oil Chem. Soc. 2022, 99, 1025–1046. [Google Scholar] [CrossRef]
- Shahidi, F.; Varatharajan, V.; Peng, H.; Senadheera, R. Utilization of marine by-products for the recovery of value-added products. J. Food Bioact. 2019, 6, 10–61. [Google Scholar] [CrossRef]
- Farooq, S.; Ahmad, I.A.; Zhang, Y.; Chen, M.; Zhang, H. Preparation, characterization and digestive mechanism of plant-derived oil bodies-based oleogels structured by chitosan and vanillin. Food Hydrocoll. 2023, 136, 108247. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Hsu, S.H. Hydrogels based on Schiff base linkages for biomedical applications. Molecules 2019, 24, 3005. [Google Scholar] [CrossRef]
- Walton, N.J.; Mayer, M.J.; Narbad, A. Vanillin. Phytochemistry 2003, 63, 505–515. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, T.; Chen, X.; Tian, D.; Wang, L.; Gao, R. Preparation and characterization of vanillin-conjugated chitosan-stabilized emulsions via a Schiff-base reaction. Food Sci. Biotechnol. 2023, 32, 1489–1499. [Google Scholar] [CrossRef]
- Brito, G.B.; Di Sarli Peixoto, V.O.; Martins, M.T.; Rosário, D.K.A.; Ract, J.N.; Conte-Júnior, C.A.; Torres, A.G.; Castelo-Branco, V.N. Development of chitosan-based oleogels via crosslinking with vanillin using an emulsion templated approach: Structural characterization and their application as fat-replacer. Food Struct. 2022, 32, 100264. [Google Scholar] [CrossRef]
- Xu, C.; Zhan, W.; Tang, X.; Mo, F.; Fu, L.; Lin, B. Self-Healing Chitosan/Vanillin Hydrogels Based on Schiff-Base Bond/Hydrogen Bond Hybrid Linkages. Polym. Test. 2018, 66, 155–163. [Google Scholar] [CrossRef]
- Sacco, P.; Furlani, F.; de Marzo, G.; Marsich, E.; Paoletti, S.; Donati, I. Concepts for developing physical gels of chitosan and of chitosan derivatives. Gels 2018, 4, 67. [Google Scholar] [CrossRef]
- Bai, L.; Huan, S.; Xiang, W.; Liu, L.; Yang, Y.; Nugroho, R.W.N.; Fan, Y.; Rojas, O.J. Self-assembled networks of short and long chitin nanoparticles for oil/water interfacial superstabilization. ACS Sustain. Chem. Eng. 2019, 7, 6497–6511. [Google Scholar] [CrossRef]
- de Alcântara, N.E.; Siqueira, L.G.; Brito, G.B.; Guimarães, J.T.; da Cruz, A.G.; Perrone, D.; Castelo-Branco, V.N. Effect of different incorporation processes of chitosan-based oleogel as a fat substitute on the structural characteristics of vanilla ice cream. Int. J. Dairy Technol. 2025, 78, e70020. [Google Scholar] [CrossRef]
- Pinho-Jr, J.S.; Moreira, B.S.; Brito, G.B.; Torres, L.G.A.; Eiriz, D.N.; Torres, A.G.; Teodoro, A.J.; Perrone, D.; Castelo-Branco, V.N. Impact of chitosan-based oleogels crosslinked with vanillin containing different oil phases on lipid digestion and bioaccessibility of carotenoids and tocols. Food Chem. 2025, 487, 144740. [Google Scholar] [CrossRef]
- Lama, M.; Montes, L.; Franco, D.; Franco-Uría, A.; Moreira, R. Chitosan-based oleogels: Emulsion drying kinetics and physical, rheological, and textural characteristics of olive oil oleogels. Mar. Drugs 2024, 22, 318. [Google Scholar] [CrossRef]
- Chevalier, Y.; Bolzinger, M.A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Gallego, R.; González, M.; Arteaga, J.F.; Valencia, C.; Franco, J.M. Influence of functionalization degree on the rheological properties of isocyanate-functionalized chitin- and chitosan-based chemical oleogels for lubricant applications. Polymers 2014, 6, 1929–1947. [Google Scholar] [CrossRef]
- Silva, T.J.; Barrera-Arellano, D.; Ribeiro, A.P.B. Oleogel-based emulsions: Concepts, structuring agents, and applications in food. J. Food Sci. 2021, 86, 2901–2916. [Google Scholar] [CrossRef]
- Tavernier, I.; Doan, C.D.; Van de Walle, D.; Danthine, S.; Rimaux, T.; Dewettinck, K. Sequential crystallization of high and low melting waxes to improve oil structuring in wax-based oleogels. RSC Adv. 2017, 7, 12113–12125. [Google Scholar] [CrossRef]
- Wang, Z.; Chandrapala, J.; Truong, T.; Farahnaky, A. Multicomponent oleogels prepared with high- and low-molecular-weight oleogelators: Ethylcellulose and waxes. Foods 2023, 12, 3093. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, N.; Sharma, H.K. Development and characterization of groundnut oil-based oleogels for replacing saturated fats in cookies formulations. Discov. Food 2025, 5, 30. [Google Scholar] [CrossRef]
- Su, C.; Li, Y.; Zhu, J.; Gao, Y.; Li, Q.; Du, S.; Yu, X. Effect of flaxseed gum on the brittleness of oleogels based on candelilla wax. RSC Adv. 2022, 12, 30734–30741. [Google Scholar] [CrossRef]
- Su, S.; Qin, S.; Xia, H.; Li, P.; Li, H.; Li, C.; Guo, S.; Zeng, C. The impact of oil type on the performance of β-amyrin-based oleogels: Formation, physicochemical properties, and potential correlation analysis. Foods 2024, 13, 876. [Google Scholar] [CrossRef] [PubMed]
- Dimakopoulou-Papazoglou, D.; Zampouni, K.; Prodromidis, P.; Moschakis, T.; Katsanidis, E. Microstructure, physical properties, and oxidative stability of olive oil oleogels composed of sunflower wax and monoglycerides. Gels 2024, 10, 195. [Google Scholar] [CrossRef]
- Ursachi, C.S.; Perța-Crisan, S.; Tolan, I.; Chambre, D.R.; Chereji, B.D.; Condrat, D.; Munteanu, F.D. Development and characterization of ethylcellulose oleogels based on pumpkin seed oil and rapeseed oil. Gels 2024, 10, 384. [Google Scholar] [CrossRef]
- Lim, J.; Hwang, H.S.; Lee, S. Oil-structuring characterization of natural waxes in canola oil oleogels: Rheological, thermal, and oxidative properties. Appl. Biol. Chem. 2017, 60, 17–22. [Google Scholar] [CrossRef]
- Park, C.; Maleky, F. A critical review of the last 10 years of oleogels in food. Front. Sustain. Food Syst. 2020, 4, 139. [Google Scholar] [CrossRef]
- Nikiforidis, C.V.; Scholten, E. Polymer organogelation with chitin and chitin nanocrystals. RSC Adv. 2015, 5, 37789–37798. [Google Scholar] [CrossRef]
- Morales, E.; Iturra, N.; Contardo, I.; Quilaqueo, M.; Franco, D.; Rubilar, M. Fat replacers based on oleogelation of beeswax/shellac wax and healthy vegetable oils. LWT 2023, 185, 115144. [Google Scholar] [CrossRef]
- AOCS, American Oil Chemists’ Society. Anisidine Value, Official Method Cd 8b-90; AOCS Official Method; AOCS: Urbana, IL, USA, 2017. [Google Scholar]
- Zhao, Q.; Selomulya, C.; Wang, S.; Xiong, H.; Chen, X.D.; Li, W.; Peng, H.; Xie, J.; Sun, W.; Zhou, Q. Enhancing the oxidative stability of food emulsions with rice dreg protein hydrolysate. Food Res. Int. 2012, 48, 876–884. [Google Scholar] [CrossRef]

| Oleogel | Storage (Days) | Hardness (kPa) | Adhesiveness (N s) | Cohesiveness (-) | Elasticity (%) |
|---|---|---|---|---|---|
| WOV_0.0 | 0 | 5.6 ± 0.8 bc | −0.41 ± 0.11 a | 0.34 ± 0.03 abc | 50.1 ± 11.1 ab |
| WOV_0.5 | 4.7 ± 1.2 c | −0.35 ± 0.09 a | 0.36 ± 0.04 ab | 50.0 ± 8.6 ab | |
| WOV_1.5 | 5.7 ± 0.6 bc | −0.65 ± 0.09 ab | 0.37 ± 0.02 a | 58.0 ± 6.3 a | |
| WOV_2.0 | 8.0 ± 1.1 a | −0.61 ± 0.17 ab | 0.29 ± 0.02 c | 38.3 ± 3.1 b | |
| WV_0.0 | 7.5 ± 1.3 ab | −0.59 ± 0.18 ab | 0.32 ± 0.02 bc | 47.8 ± 13.1 ab | |
| WV_0.5 | 7.4 ± 1.2 ab | −0.54 ± 0.28 ab | 0.32 ± 0.04 abc | 49.4 ± 10.8 ab | |
| WV_1.5 | 7.1 ± 0.5 ab | −0.98 ± 0.16 c | 0.37 ± 0.02 a | 57.8 ± 9.8 a | |
| WV_2.0 | 6.4 ± 1.3 abc | −0.80 ± 0.12 bc | 0.33 ± 0.03 abc | 45.3 ± 6.1 ab | |
| WOV_0.0 | 15 | 6.7 ± 0.8 C | −0.34 ± 0.12 A | 0.32 ± 0.04 AB | 46.9 ± 9.4 A |
| WOV_0.5 | 7.2 ± 1.5 C | −0.45 ± 0.21 AB | 0.31 ± 0.04 AB | 60.9 ± 12.8 A | |
| WOV_1.5 | 7.9 ± 1.4 BC | −0.63 ± 0.05 ABC | 0.38 ± 0.08 A | 56.4 ± 15.7 A | |
| WOV_2.0 | 10.4 ± 0.3 A | −0.71 ± 0.09 BCD | 0.27 ± 0.01 B | 42.8 ± 4.7 A | |
| WV_0.0 | 9.5 ± 1.5 AB | −0.49 ± 0.17 AB | 0.30 ± 0.03 AB | 44.7 ± 7.3 A | |
| WV_0.5 | 9.5 ± 1.5 AB | −0.52 ± 0.25 AB | 0.30 ± 0.05 AB | 45.5 ± 8.3 A | |
| WV_1.5 | 7.8 ± 0.9 BC | −0.99 ± 0.19 D | 0.38 ± 0.03 A | 57.3 ± 9.5 A | |
| WV_2.0 | 8.6 ± 0.3 ABC | −0.91 ± 0.12 ACD | 0.32 ± 0.02 AB | 46.9 ± 5.0 A |
| Hardness | Adhesiveness | Cohesiveness | Elasticity | PI | TBARs | OBC | tan δ | A | n | |
|---|---|---|---|---|---|---|---|---|---|---|
| Hardness (kPa) | - | 0.034 | −0.032 | 0.223 | −0.337 | −0.223 | 0.675 ** | −0.400 | 0.634 ** | −0.539 ** |
| Adhesiveness (N s) | 0.151 | - | 0.196 | 0.275 | 0.390 | 0.510 * | −0.280 | 0.640 ** | 0.013 | 0.507 * |
| Cohesiveness | 0.070 | 0.035 | - | 0.909 ** | 0.259 | 0.068 | −0.258 | 0.333 | −0.408 * | 0.231 |
| Elasticity (%) | 0.122 | 0.286 | 0.831 ** | - | 0.043 | −0.021 | 0.016 | 0.201 | −0.223 | 0.054 |
| PI (meq O2 kg−1) | −0.059 | 0.175 | 0.076 | 0.143 | - | 0.576 ** | −0.702 ** | 0.666 ** | −0.072 | 0.687 ** |
| TBARs (mg MDA/kg) | −0.199 | 0.451 * | 0.396 | 0.352 | 0.454 * | - | −0.266 | 0.577 ** | −0.368 | 0.516 ** |
| OBC (%) | 0.412 * | 0.193 | −0.159 | −0.175 | −0.657 ** | −0.106 | - | −0.725 ** | 0.303 | −0.776 ** |
| tan δ (-) | −0.172 | 0.796 ** | 0.152 | 0.400 | 0.000 | 0.395 | 0.142 | - | −0.343 | 0.924 ** |
| A (Pa) | 0.745 ** | 0.246 | 0.220 | 0.378 | 0.251 | −0.048 | −0.022 | 0.027 | - | −0.388 |
| n (-) | −0.316 | 0.660 ** | −0.104 | −0.045 | 0.213 | 0.349 | −0.054 | 0.628 ** | −0.146 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montes, L.; Viciana, S.; Franco, D.; Sineiro, J.; Moreira, R. Effect of Vanillin and Chitin Particles on the Chitosan-Based Oleogels Produced by the Emulsion-Templated Method. Gels 2025, 11, 799. https://doi.org/10.3390/gels11100799
Montes L, Viciana S, Franco D, Sineiro J, Moreira R. Effect of Vanillin and Chitin Particles on the Chitosan-Based Oleogels Produced by the Emulsion-Templated Method. Gels. 2025; 11(10):799. https://doi.org/10.3390/gels11100799
Chicago/Turabian StyleMontes, Leticia, Sofía Viciana, Daniel Franco, Jorge Sineiro, and Ramón Moreira. 2025. "Effect of Vanillin and Chitin Particles on the Chitosan-Based Oleogels Produced by the Emulsion-Templated Method" Gels 11, no. 10: 799. https://doi.org/10.3390/gels11100799
APA StyleMontes, L., Viciana, S., Franco, D., Sineiro, J., & Moreira, R. (2025). Effect of Vanillin and Chitin Particles on the Chitosan-Based Oleogels Produced by the Emulsion-Templated Method. Gels, 11(10), 799. https://doi.org/10.3390/gels11100799








