Optimized Ion-Sensitive Hydrogels Based on Gellan Gum and Arabinogalactan for the Treatment of Dry Eye Disease
Abstract
1. Introduction
2. Results and Discussion
2.1. Pre-Formulative Study
2.1.1. Optimization and Selection of the Buffer Solution
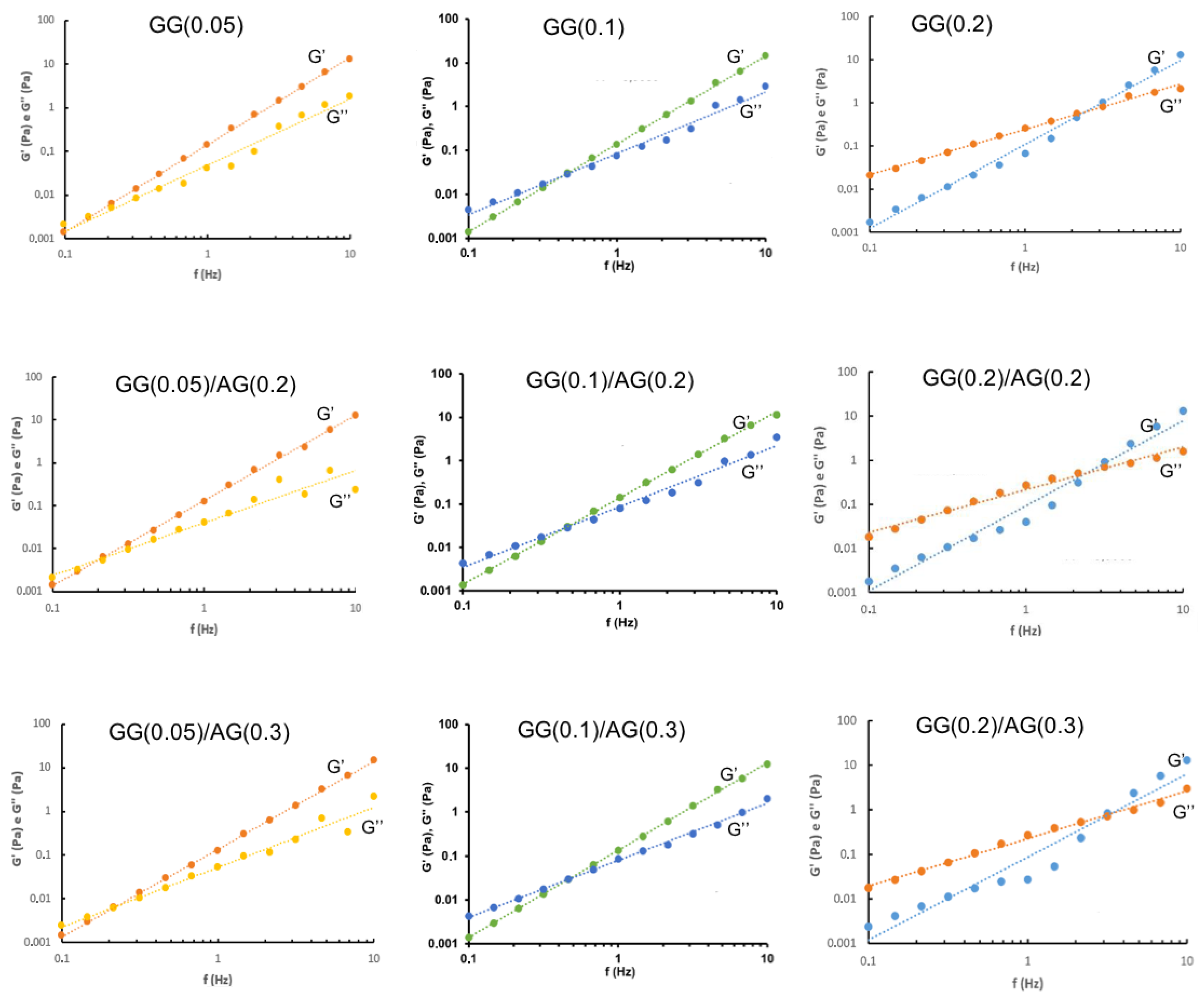
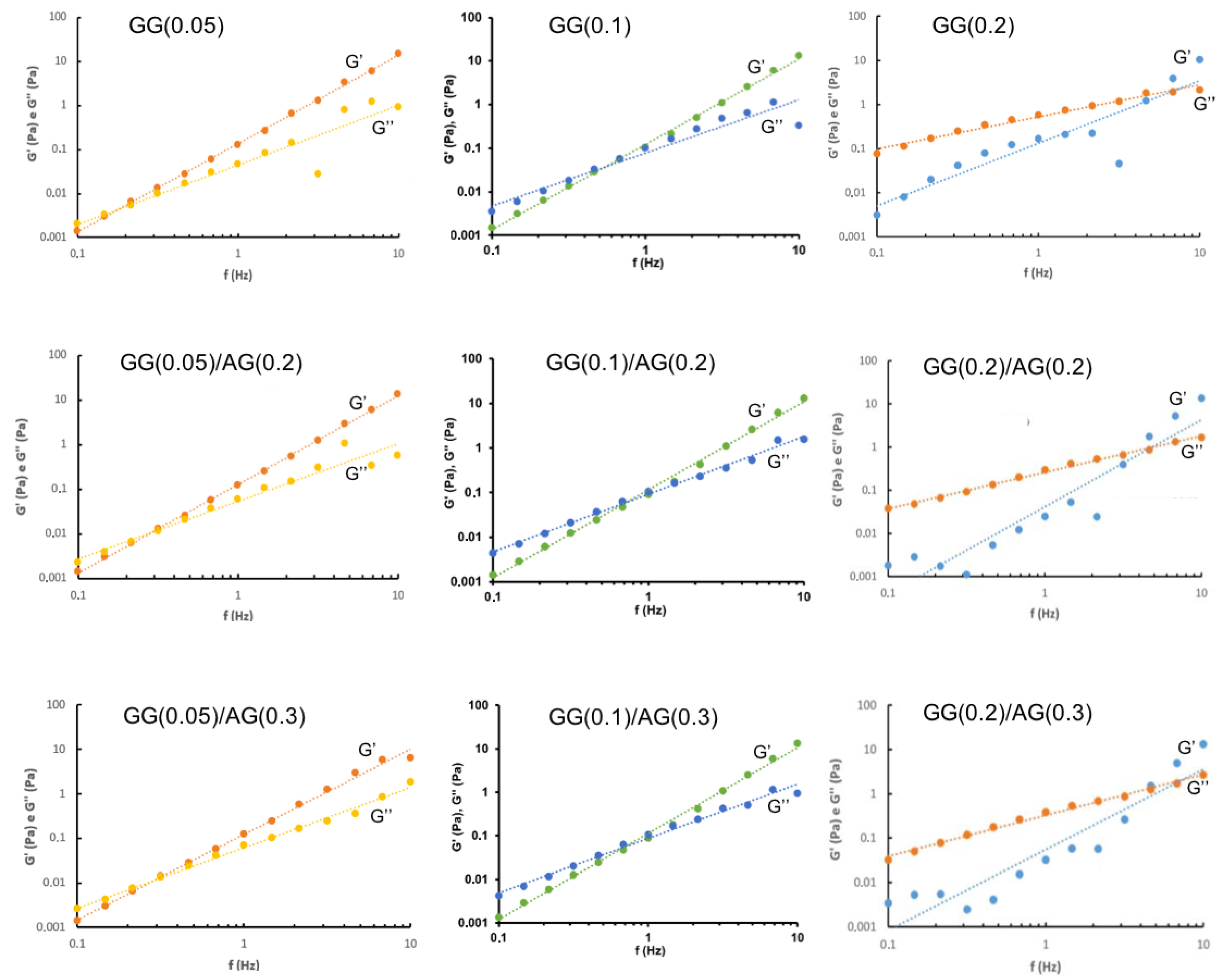
2.1.2. Characterization of GG-Based Formulations and Their Mixtures with AG
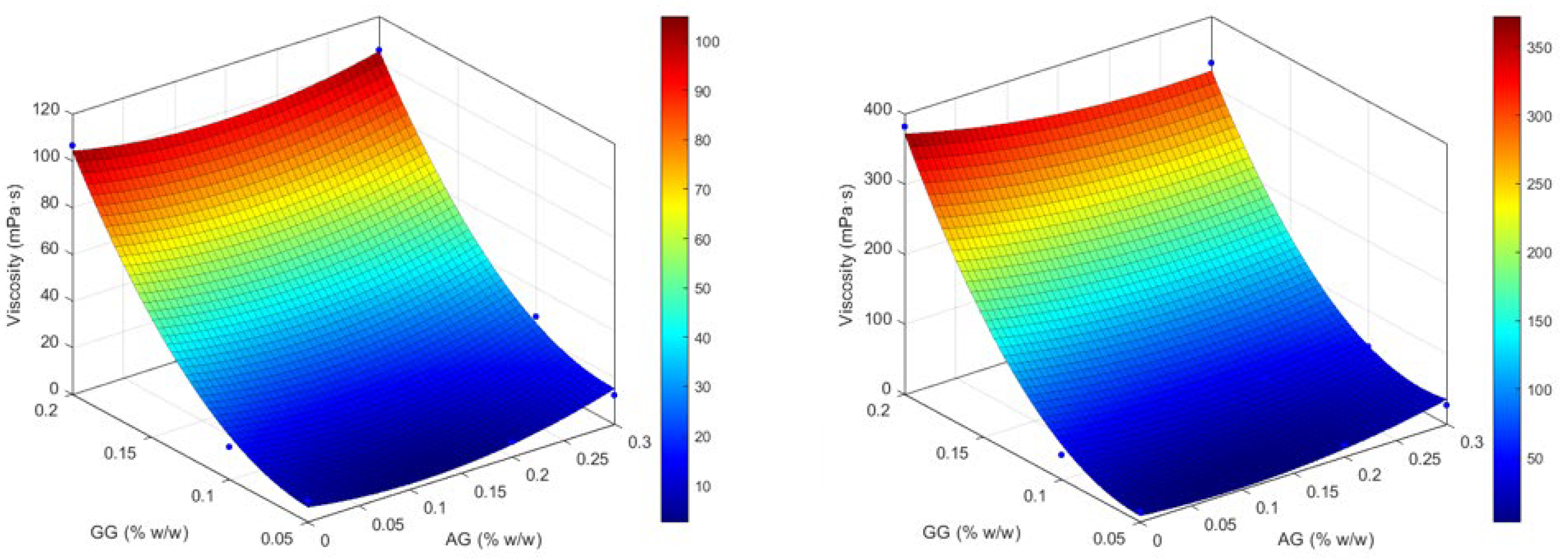
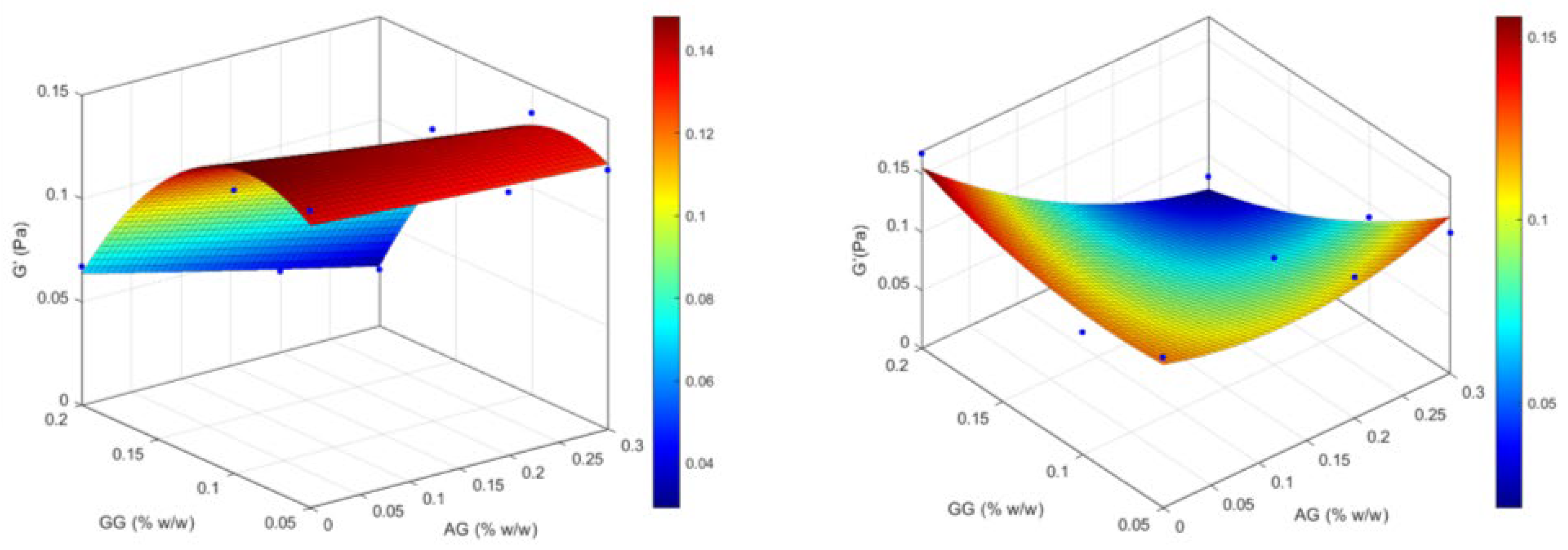
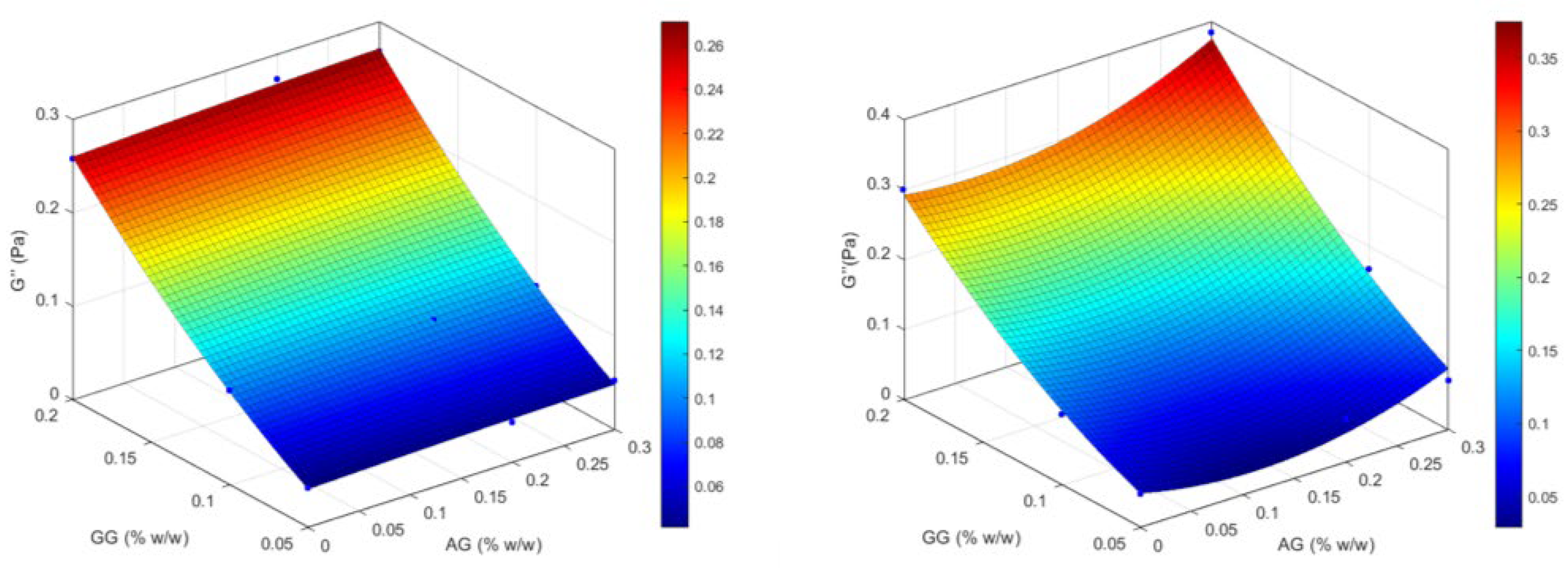
2.1.3. Design of Experiment (DoE) Optimization Study
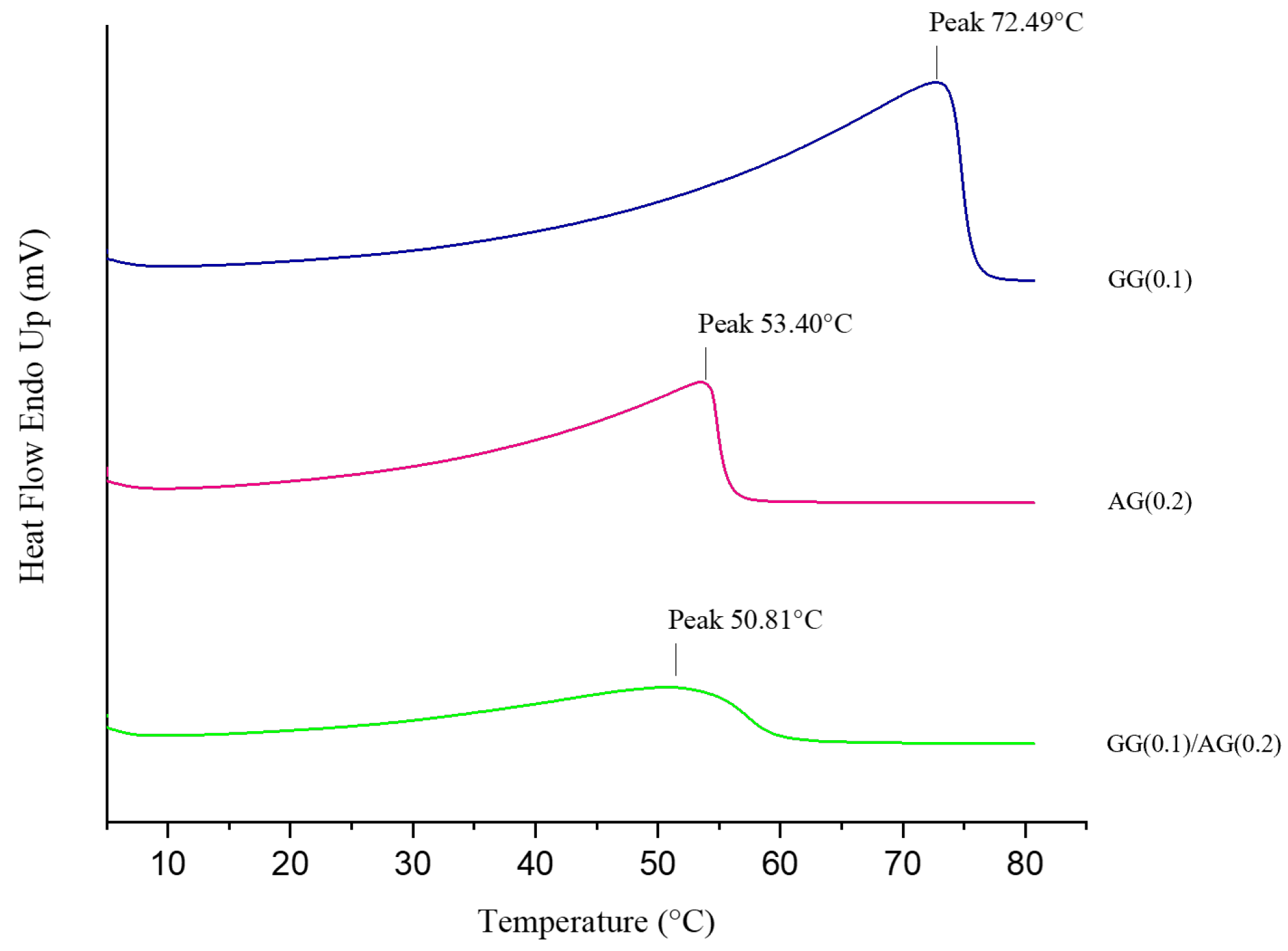
2.1.4. Interactions and Stability of the Selected Formulation
2.2. Biopharmaceutical Evaluation of the Selected Formulation
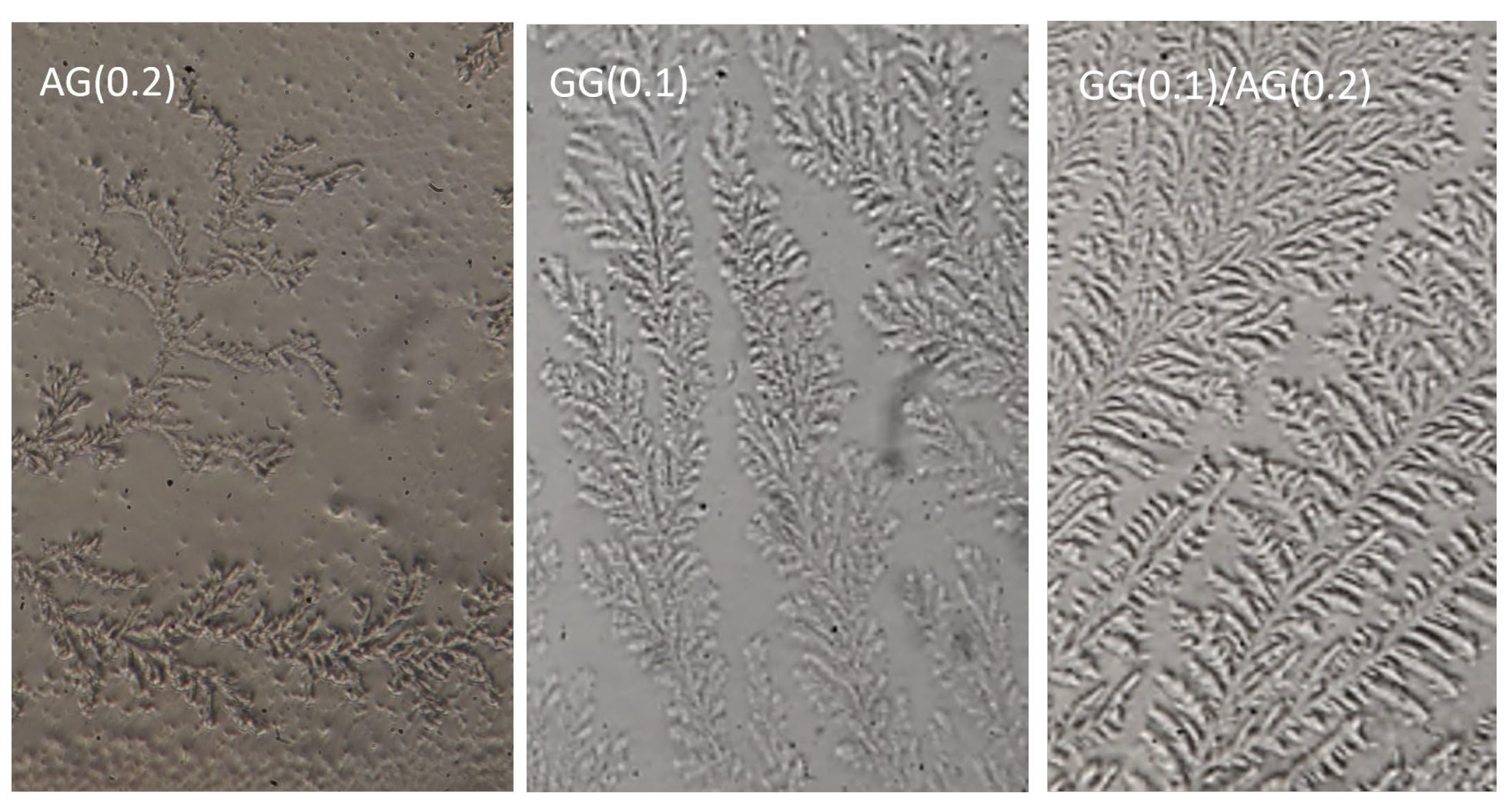
2.2.1. Ferning Test
2.2.2. Mucoadhesion
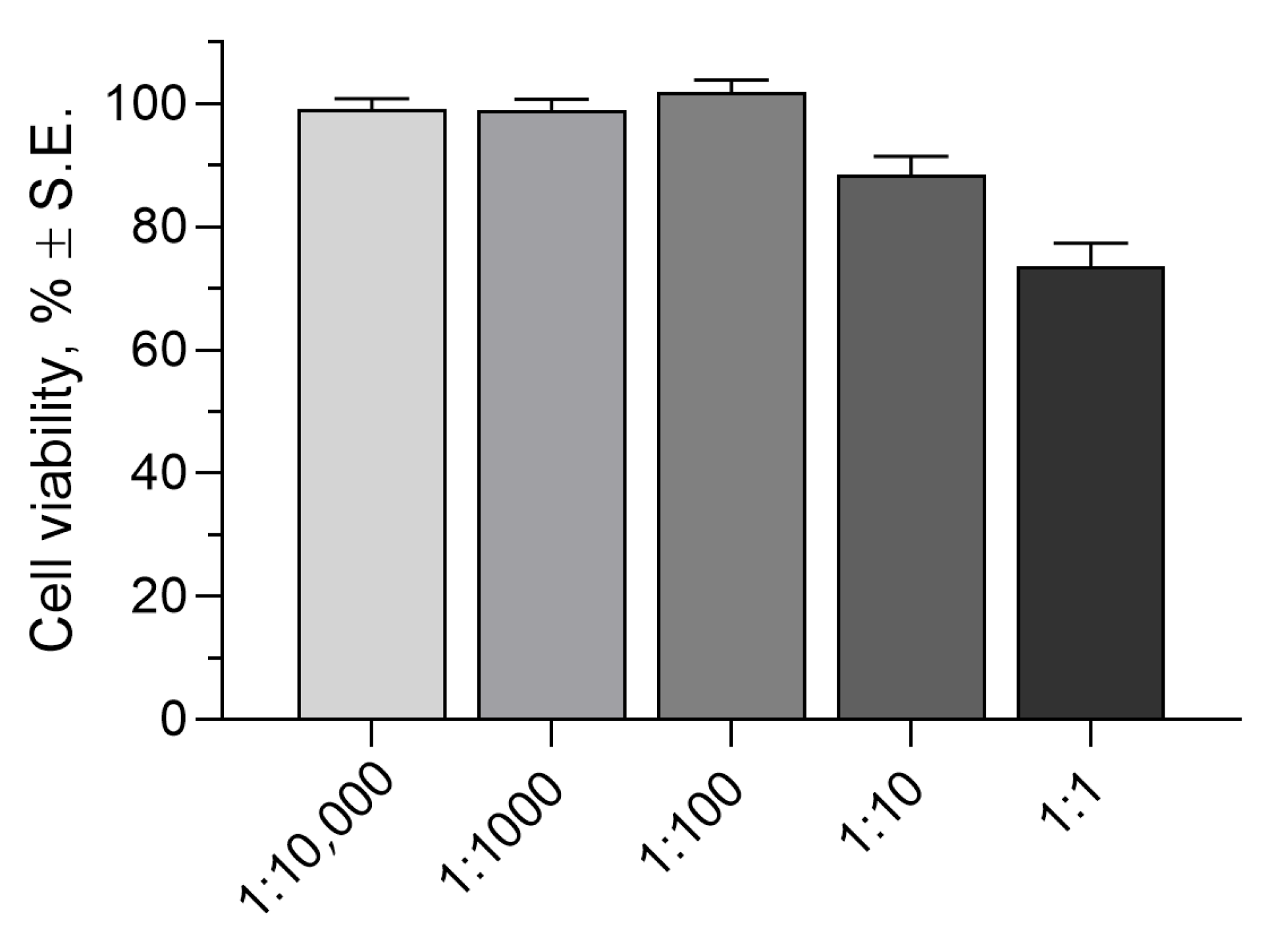
2.2.3. Cytotoxicity Assay
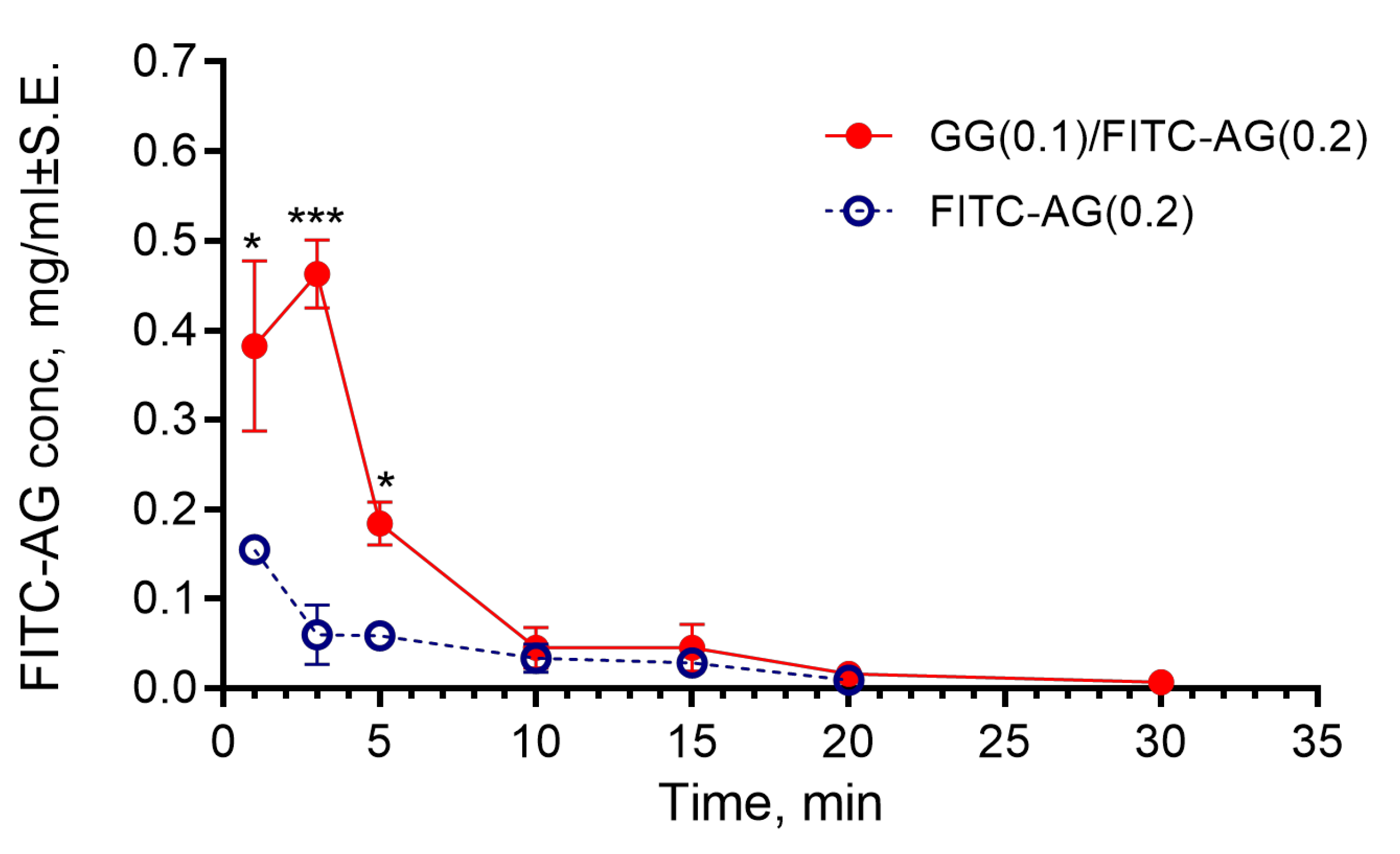
2.2.4. Evaluation of the Time of Residence of the Formulation in the Rabbit Eyes
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Osmolality and pH Measurements
4.2.2. Wettability Assessment
4.2.3. Rheological Analysis
- Stress Sweep analysis conducted at 32.0 °C ± 2.0 °C, with shear stress (τ) increasing from 0.1 to 100 Pa, while the frequency was kept at 5 Hz, to determine the linear viscoelastic region (LVR).
- Frequency Sweep analysis conducted at 32.0 ± 2.0 °C, with a frequency range of 0.1–10 Hz and a constant oscillatory stress of 1 Pa to determine the elastic modulus (G′), viscous modulus (G″), and phase angle (δ) as a function of the frequency.
4.2.4. Dynamic Light Scattering Analysis
4.2.5. Preparation of Artificial Tear Fluid (ATF)
4.2.6. Design of Experiments (DOE): Optimization Study for the Selection of the Most Performant In Situ Gelling Formulation
4.2.7. Thermal Behavior
4.2.8. Ferning Test
4.2.9. Mucoadhesion Test
4.2.10. Cytotoxicity Assay
4.2.11. Evaluation of the Time of Residence of the Formulation in the Rabbit Eyes
Fluorescein Isothiocyanate AG Synthesis
Animal Testing
4.2.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lallemand, F.; Schmitt, M.; Bourges, J.L.; Gurny, R.; Benita, S.; Garrigue, J.S. Cyclosporine A delivery to the eye: A comprehensive review of academic and industrial efforts. Eur. J. Pharm. Biopharms. 2017, 117, 14–28. [Google Scholar] [CrossRef]
- Ahmed, B.; Jaiswal, S.; Naryal, S.; Shah, R.M.; Alany, R.G.; Kaur, I.P. In situ gelling systems for ocular drug delivery. J. Control Release 2024, 371, 67–84. [Google Scholar] [CrossRef]
- Kushwaha, S.K.; Saxena, P.; Rai, A. Stimuli sensitive hydrogels for ophthalmic drug delivery: A review. Int. J. Pharm. Investig. 2012, 2, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, R.; Janaswamy, S. Morphology of Western larch arabinogalactan. Carbohydr. Res. 2002, 337, 2211–2222. [Google Scholar] [CrossRef]
- Dion, C.; Chappuis, E.; Ripoll, C. Does larch arabinogalactan enhance immune function? A review of mechanistic and clinical trials. Nutr. Metab. 2016, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Silvani, L.; Bedei, A.; De Grazia, G.; Remiddi, S. Arabinogalactan and hyaluronic acid in ophthalmic solution: Experimental effect on xanthine oxidoreductase complex as key player in ocular inflammation (in vitro study). Exp. Eye Res. 2020, 196, 108058–108066. [Google Scholar] [CrossRef]
- Di Mola, A.; Summa, F.F.; Oliva, P.; Lelj, F.; Remiddi, S.; Silvani, L.; Massa, A. Synergistic properties of Arabinogalactan (AG) and Hyaluronic Acid (HA) sodium salt mixtures. Molecules 2021, 26, 7246. [Google Scholar] [CrossRef]
- Bedei, A.; Rocha Cabrera, P.; Oliveira, L.; Castellini, L.; De Grazia, G.; Remiddi, S. Real-world treatment outcomes of an artificial tear containing arabinogalactan, hyaluronic acid and trehalose among subjects with dry eye. Clin. Ophthalmol. 2025, 19, 83–91. [Google Scholar] [CrossRef]
- Burgalassi, S.; Nicosia, N.; Monti, D.; Falcone, G.; Boldrini, E.; Chetoni, P. Larch arabinogalactan for dry eye protection and treatment of corneal lesions: Investigations in rabbits. J. Ocul. Pharmacol. Ther. 2007, 23, 541–550. [Google Scholar] [CrossRef]
- Burgalassi, S.; Nicosia, N.; Monti, D.; Falcone, G.; Boldrini, E.; Fabiani, O.; Lenzi, C.; Pirone, A.; Chetoni, P. Arabinogalactan as active compound in the management of corneal wounds: In vitro toxicity and in vivo investigations on rabbits. Curr. Eye Res. 2011, 36, 21–28. [Google Scholar] [CrossRef]
- Giavasis, I.; Harvey, L.M.; McNeil, B. Gellan gum. Crit. Rev. Biotechnol. 2000, 20, 177–211. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Zhang, H.B.; Nitta, Y.; Fang, Y.P.; Nishinari, K. Gellan. In Polysaccharides; Ramawat, K., Mérillon, J.M., Eds.; Springer: Cham, Switzerland, 2015; pp. 1–48. [Google Scholar] [CrossRef]
- Li, M.; Du, C.; Zhu, D.; Shen, M.; Cui, L.; Wang, J. Daytime variations of tear osmolarity and tear meniscus volume. Eye Contact Lens 2012, 38, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Stahl, U.; Willcox, M.; Stapleton, F. Osmolality and tear film dynamics. Clin. Exp. Optom. 2012, 95, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Aragona, P.; Di Stefano, G.; Ferreri, F.; Spinella, R.; Stilo, A. Sodium hyaluronate eye drops of different osmolarity for the treatment of dry eye in Sjögren’s syndrome patients. Br. J. Ophthalmol. 2002, 86, 879–884. [Google Scholar] [CrossRef]
- Troiano, P.; Monaco, G. Effect of hypotonic 0.4% hyaluronic acid drops in dry eye patients: A cross-over study. Cornea 2008, 27, 1126–1130. [Google Scholar] [CrossRef]
- Baeyens, V.; Bron, A.; Baudouin, C. Vismed/Hylovis Study Group. Efficacy of 0.18% hypotonic sodium hyaluronate ophthalmic solution in the treatment of signs and symptoms of dry eye disease. J. Fr. Ophtalmol. 2012, 35, 412–419. [Google Scholar] [CrossRef]
- Baudouin, C.; Aragona, P.; Messmer, E.M.; Tomlinson, A.; Calonge, M.; Boboridis, K.G.; Akova, Y.A.; Geerling, G.; Labetoulle, M.; Rolando, M. Role of Hyperosmolarity in the Pathogenesis and Management of Dry Eye Disease: Proceedings of the OCEAN Group Meeting. Ocul. Surf. 2013, 11, 246–258. [Google Scholar] [CrossRef]
- Holly, F.J.; Lamberts, D.W. Effect of nonisotonic solutions on tear film osmolality. Investig. Ophthalmol. Vis. Sci. 1981, 20, 236–245. [Google Scholar]
- Kotreka, V.L.; Davis, M.C.; Adeyeye, A. Development of topical ophthalmic in situ gel-forming estradiol delivery system intended for the prevention of age-related cataracts. PLoS ONE 2017, 12, e0170720. [Google Scholar] [CrossRef]
- García, M.C.; Trujillo, L.A.; Muñoz, J.; Alfaro, M.C. Gellan gum fluid gels: Influence of the nature and concentration of gel-promoting ions on rheological properties. Colloid Polym. Sci. 2018, 296, 1741–1748. [Google Scholar] [CrossRef]
- Pérez-Campos, S.J.; Chavarría-Hernández, N.; Tecante, A.; Ramírez-Gilly, M.; Rodríguez-Hernández, A.I. Gelation and microstructure of dilute gellan solutions with calcium ions. Food Hydrocoll. 2012, 28, 291–300. [Google Scholar] [CrossRef]
- Burgalassi, S.; Monti, D.; Tampucci, S.; Chetoni, P. In vitro evaluation of some parameters involved in mucoadhesion of aqueous polymeric dispersions. Pharm. Dev. Technol. 2015, 20, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Leong, K.C. Influences of substrate wettability and liquid viscosity on isothermal spreading of liquid droplets on solid surfaces. Exp. Fluids 2002, 33, 728–731. [Google Scholar] [CrossRef]
- Tiffany, J.M. The viscosity of human tears. Int. Ophthalmol. 1991, 15, 371–376. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Matar, O.K.; Craster, R.V. Analysis of tear film rupture: Effect of non-Newtonian rheology. J. Colloid Interface Sci. 2003, 262, 130–148. [Google Scholar] [CrossRef]
- Chrai, S.S.; Robinson, J.R. Ocular evaluation of methylcellulose vehicle in albino rabbits. J. Pharm. Sci. 1974, 63, 1218–1223. [Google Scholar] [CrossRef]
- Winfield, A.J.; Jessiman, D.; Williams, A.; Esakowitz, L. A study of the causes of non-compliance by patients prescribed eyedrops. Br. J. Ophthalmol. 1990, 74, 477–480. [Google Scholar] [CrossRef]
- Recchioni, A.; Mocciardini, E. Viscoelastic properties of the human tear film. Exp. Eye Res. 2022, 219, 109083. [Google Scholar] [CrossRef]
- Budai, L.; Budai, M.; Fülöpné Pápay, Z.E.; Vilimi, Z.; Antal, I. Rheological considerations of pharmaceutical formulations: Focus on viscoelasticity. Gels 2023, 9, 469. [Google Scholar] [CrossRef]
- Glass, D.H.; Roberts, C.J.; Litsky, A.S.; Weber, P.A. A viscoelastic biomechanical model of the cornea describing the effect of viscosity and elasticity on hysteresis. Investig. Opthalmol. Vis. Sci. 2008, 49, 3919–3926. [Google Scholar] [CrossRef]
- Vega, J.F.; Souza-Egipsy, V.; Expósito, M.T.; Ramos, J. Melting temperature depression of polymer single crystals: Application to the eco-design of tie-layers in polyolefinic-based multilayered films. Polymers 2022, 14, 1622. [Google Scholar] [CrossRef]
- Filippello, M.; Scimone, G.; Mazzone, M.G. Ferning test and artificial tears: Comparative evaluation. In Lacrimal System, Proceed Symp Lacrimal System, Toronto; Hurwitz, J., Miglior, M., Spinelli, D., Eds.; Kugler&Ghedini: New York, NY, USA, 1995; pp. 97–106. [Google Scholar]
- Masmali, A.M.; Purslow, C.; Murphy, P.J. The tear ferning test: A simple clinical technique to evaluate the ocular tear film. Clin. Exp. Optom. 2014, 97, 399–406. [Google Scholar] [CrossRef]
- Moschini, R.; Gini, F.; Cappiello, M.; Balestri, F.; Falcone, G.; Boldrini, E.; Mura, U.; Del Corso, A. Interaction of arabinogalactan with mucins. Int. J. Biol. Macromol. 2014, 67, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Tatykhanova, G.; Aseyev, V.; Kudaibergenov, S.E. Mucoadhesive properties of gellan and its modified derivatives. Rev. Adv. Chem. 2020, 10, 140–157. [Google Scholar] [CrossRef]
- Snibson, G.R.; Greaves, J.L.; Soper, N.D.; Tiffany, J.M.; Wilson, C.G.; Bron, A.J. Ocular surface residence times of artificial tear solutions. Cornea 1992, 11, 288–293. [Google Scholar] [CrossRef]
- Rupenthal, I.D.; Colin, R.; Green, C.R.; Alany, R.G. Comparison of ion-activated in situ gelling systems for ocular drug delivery. Part 1: Physicochemical characterisation and in vitro release. Int. J. Pharm. 2011, 411, 69–77. [Google Scholar] [CrossRef]
- Rupenthal, I.D.; Green, C.R.; Alany, R.G. Comparison of ion-activated in situ gelling systems for ocular drug delivery. Part 2: Precorneal retention and in vivo pharmacodynamic study. Int. J. Pharm. 2011, 411, 78–85. [Google Scholar] [CrossRef]
- Biswal, S.; Parmanik, A.; Das, D.; Sahoo, R.N.; Nayak, A.K. Gellan gum-based in-situ gel formulations for ocular drug delivery: A practical approach. Int. J. Biol. Macromol. 2025, 290, 138979. [Google Scholar] [CrossRef]
- Zaharia, A.C.; Dumitrescu, O.M.; Radu, M.; Rogoz, R.E. Adherence to Therapy in Glaucoma Treatment—A Review. J. Pers. Med. 2022, 12, 514. [Google Scholar] [CrossRef]
- Challener, C.A. Aiming for Improved Efficacy and Patient Compliance for Topical Ophthalmics. Pharm. Technol. 2023, 47, 18–19. [Google Scholar]
- Muñoz-Villegas, P.; Martínez-Bautista, H.; Olvera-Montaño, O. Determinants of adherence to treatment in patients with ophthalmic conditions. Expert Rev. Clin. Pharmacol. 2023, 16, 1249–1259. [Google Scholar] [CrossRef]
| Buffer | Na2HPO4·7H2O (mM) | Citric Acid (mM) | NaCl (mM) | CaCl2 (mM) | KCl (mM) | Mannitol (mM) | Monovalent Cations (mM) | Ca2+ (mM) | Total Cations (mM) |
|---|---|---|---|---|---|---|---|---|---|
| BS1 | 10.00 | 0.73 | 1.71 | 0.76 | 18.78 | 217.38 | 40.49 | 0.76 | 41.25 |
| BS2 | 10.00 | 0.73 | 1.71 | 2.00 | 18.78 | 217.38 | 40.49 | 2.00 | 42.49 |
| BS3 | 10.00 | 0.73 | 1.71 | - | 18.78 | 217.38 | 40.49 | - | 40.49 |
| Formulations | Cations (mM) | Mannitol (mM) | pH | Osmolality (mOsmol/kg) | Viscosity (mPa·s) | |
|---|---|---|---|---|---|---|
| Before ATF Dilution | After ATF Dilution | |||||
| GG(0.1)/BS1 | 41.25 | 217.38 | 6.45 ± 0.02 | 299 ± 0.58 | 28.62 ± 0.66 #### | 31.27 ± 0.64 * |
| GG(0.1/BS2 | 42.49 | 217.38 | 6.97 ± 0.01 | 297 ± 0.58 | 28.54 ± 2.11 ### | 26.48± 1.09 |
| GG(0.1)/BS3 | 40.49 | 217.38 | 6.73 ± 0.01 | 302 ± 1.00 | 2.18 ± 1.02 | 8.97 ± 0.99 ** |
| Formulation | GG (% w/w) | AG (% w/w) | Na2HPO4· 7H2O (mM) | Citric Acid (mM) | NaCl (mM) | CaCl2 (mM) | KCl (mM) | Mannitol (mM) | EDTA (% w/w) | BAK (% w/w) |
|---|---|---|---|---|---|---|---|---|---|---|
| GG(0.05) | 0.05 | - | 10.00 | 0.73 | 1.71 | 0.76 | 18.78 | 217.38 | 0.05 | 0.005 |
| GG(0.05)/AG(0.2) | 0.05 | 0.2 | 10.00 | 0.73 | 1.71 | 0.76 | 18.78 | 217.38 | 0.05 | 0.005 |
| GG(0.05)/AG(0.3) | 0.05 | 0.3 | 10.00 | 0.73 | 1.71 | 0.76 | 18.78 | 217.38 | 0.05 | 0.005 |
| GG(0.1) | 0.1 | - | 10.00 | 0.73 | 1.71 | 0.76 | 18.78 | 217.38 | 0.05 | 0.005 |
| GG(0.1)/AG(0.2) | 0.1 | 0.2 | 10.00 | 0.73 | 1.71 | 0.76 | 18.78 | 217.38 | 0.05 | 0.005 |
| GG(0.1)/AG(0.3) | 0.1 | 0.3 | 10.00 | 0.73 | 1.71 | 0.76 | 18.78 | 217.38 | 0.05 | 0.005 |
| GG(0.2) | 0.2 | - | 10.00 | 0.73 | 1.71 | 0.76 | 18.78 | 217.38 | 0.05 | 0.005 |
| GG(0.2)/AG(0.2) | 0.2 | 0.2 | 10.00 | 0.73 | 1.71 | 0.76 | 18.78 | 217.38 | 0.05 | 0.005 |
| GG(0.2)/AG(0.3) | 0.2 | 0.3 | 10.00 | 0.73 | 1.71 | 0.76 | 18.78 | 217.38 | 0.05 | 0.005 |
| Formulation | pH (±SE) | Osmolality (mOsmol/kg ±SE) | Wettability (θ, ° ±SE) | Viscosity (mPa. s ±SE) | Increase Factor (IF) | IF Mean | ||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | |||||
| ATF Dilution | ATF Dilution | ATF Dilution | ATF Dilution | |||||
| GG(0.05) | 6.18 ± 0.03 | 302.0 ± 1.00 | 56.10 ± 2.96 | 54.50 ± 1.01 | 9.05 ± 1.12 | 14.27 ± 0.83 | 1.58 | |
| GG(0.05)/AG(0.2) | 6.12 ± 0.02 | 297.3 ± 1.20 | 57.60 ± 1.64 | 59.10 ± 2.32 | 8.12 ± 0.86 | 16.52 ± 1.59 | 2.03 | |
| GG(0.05)/AG(0.3) | 6.28 ± 0.05 | 299.0 ± 0.58 | 54.90 ± 3.10 | 56.20 ± 3.00 | 12.04 ± 0.35 | 25.87 ± 1.31 | 2.15 | |
| 1.92 | ||||||||
| GG(0.1) | 6.42 ± 0.03 | 291.0 ± 1.00 | 49.90 ± 1.61 | 54.70 ± 1.74 | 14.07 ± 0.43 | 34.82 ± 1.02 | 2.47 | |
| GG(0.1)/AG(0.2) | 6.08 ± 0.02 | 301.3 ± 0.88 | 52.50 ± 1.12 | 51.90 ± 2.35 | 17.83 ± 1.96 | 41.66 ± 8.12 | 2.34 | |
| GG(0.1)/AG(0.3) | 5.96 ± 0.06 | 305.7 ± 0.67 | 51.30 ± 1.13 | 49.60 ± 1.38 | 19.30 ± 2.96 | 49.22 ± 2.59 | 2.55 | |
| 2.45 | ||||||||
| GG(0.2) | 6.12 ± 0.04 | 300.0 ± 0.58 | 51.70 ± 3.06 | 53.20 ± 1.46 | 106.58 ± 4.35 | 382.58 ± 6.28 | 3.59 | |
| GG(0.2)/AG(0.2) | 6.20 ± 0.01 | 298.0 ± 1.00 | 48.50 ± 1.94 | 49.40 ± 5.06 | 95.30 ± 3.86 | 302.52 ± 2.76 | 3.17 | |
| GG(0.2)/AG(0.3) | 6.32 ± 0.00 | 309.3 ± 0.67 | 51.70 ± 1.50 | 56.50 ± 1.90 | 105.41 ± 1.39 | 333.03 ± 4.21 | 3.16 | |
| 3.31 | ||||||||
| Formulation | Independent Variables Levels | Dependent Variables Values— Before ATF Dilution | Dependent Variables Values— After ATF Dilution | |||||
|---|---|---|---|---|---|---|---|---|
| X1 (AG) | X2 (GG) | Viscosity (mPa·s) | Elastic Modulus (G′, Pa) | Viscous Modulus (G″, Pa) | Viscosity (mPa·s) | Elastic Modulus (G′, Pa) | Viscous Modulus (G″, Pa) | |
| GG(0.05) | −1 | −1 | 9.05 | 0.144 | 0.042 | 14.27 | 0.129 | 0.047 |
| GG(0.05)/AG(0.2) | 0 | −1 | 8.12 | 0.127 | 0.042 | 16.52 | 0.121 | 0.061 |
| GG(0.05)/AG(0.3) | +1 | −1 | 12.04 | 0.125 | 0.052 | 25.87 | 0.121 | 0.069 |
| GG(0.1) | −1 | 0 | 14.07 | 0.137 | 0.101 | 34.82 | 0.105 | 0.101 |
| GG(0.1)/AG(0.2) | 0 | 0 | 17.83 | 0.141 | 0.106 | 41.66 | 0.092 | 0.106 |
| GG(0.1)/AG(0.3) | +1 | 0 | 19.30 | 0.136 | 0.107 | 49.22 | 0.088 | 0.168 |
| GG(0.2) | −1 | +1 | 106.58 | 0.067 | 0.258 | 382.58 | 0.167 | 0.300 |
| GG(0.2)/AG(0.2) | 0 | +1 | 95.30 | 0.039 | 0.273 | 302.52 | 0.025 | 0.298 |
| GG(0.2)/AG(0.3) | +1 | +1 | 105.41 | 0.027 | 0.268 | 333.03 | 0.032 | 0.384 |
| Formulation | Size (nm) | Polydispersity Index | Viscosity (mPa·s) |
|---|---|---|---|
| GG(0.1) | 147.3 ± 7.48 **** | 0.31 | 14.07 ± 0.43 |
| AG(0.2) | 112.1 ± 3.59 **** | 0.28 | 1.04 ± 0.054 |
| GG(0.1)/AG(0.2) | 331.9 ± 16.62 | 0.34 | 17.83 ± 1.96 |
| GG(0.1)/AG(0.2) after filtration | 295.3 ± 4.27 | 0.19 | 20.73 ± 1.71 |
| GG(0.1)/AG(0.2) after 9 months | 324.0 ± 16.30 | 0.22 | 19.57 ± 0.41 |
| Independent Variables | Levels | ||
|---|---|---|---|
| +1 | 0 | −1 | |
| X1 = AG % w/w | 0.3 | 0.2 | 0 |
| X2 = GG % w/w | 0.2 | 0.1 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paganini, V.; Tampucci, S.; Brignone, S.G.; Di Gangi, M.; Monti, D.; Burgalassi, S.; Chetoni, P. Optimized Ion-Sensitive Hydrogels Based on Gellan Gum and Arabinogalactan for the Treatment of Dry Eye Disease. Gels 2025, 11, 787. https://doi.org/10.3390/gels11100787
Paganini V, Tampucci S, Brignone SG, Di Gangi M, Monti D, Burgalassi S, Chetoni P. Optimized Ion-Sensitive Hydrogels Based on Gellan Gum and Arabinogalactan for the Treatment of Dry Eye Disease. Gels. 2025; 11(10):787. https://doi.org/10.3390/gels11100787
Chicago/Turabian StylePaganini, Valentina, Silvia Tampucci, Sofia Gisella Brignone, Mariacristina Di Gangi, Daniela Monti, Susi Burgalassi, and Patrizia Chetoni. 2025. "Optimized Ion-Sensitive Hydrogels Based on Gellan Gum and Arabinogalactan for the Treatment of Dry Eye Disease" Gels 11, no. 10: 787. https://doi.org/10.3390/gels11100787
APA StylePaganini, V., Tampucci, S., Brignone, S. G., Di Gangi, M., Monti, D., Burgalassi, S., & Chetoni, P. (2025). Optimized Ion-Sensitive Hydrogels Based on Gellan Gum and Arabinogalactan for the Treatment of Dry Eye Disease. Gels, 11(10), 787. https://doi.org/10.3390/gels11100787












