Biofunctional Polyvinyl Alcohol/Xanthan Gum/Gelatin Hydrogel Dressings Loaded with Curcumin: Antibacterial Properties and Cell Viability
Abstract
1. Introduction
2. Results and Discussion
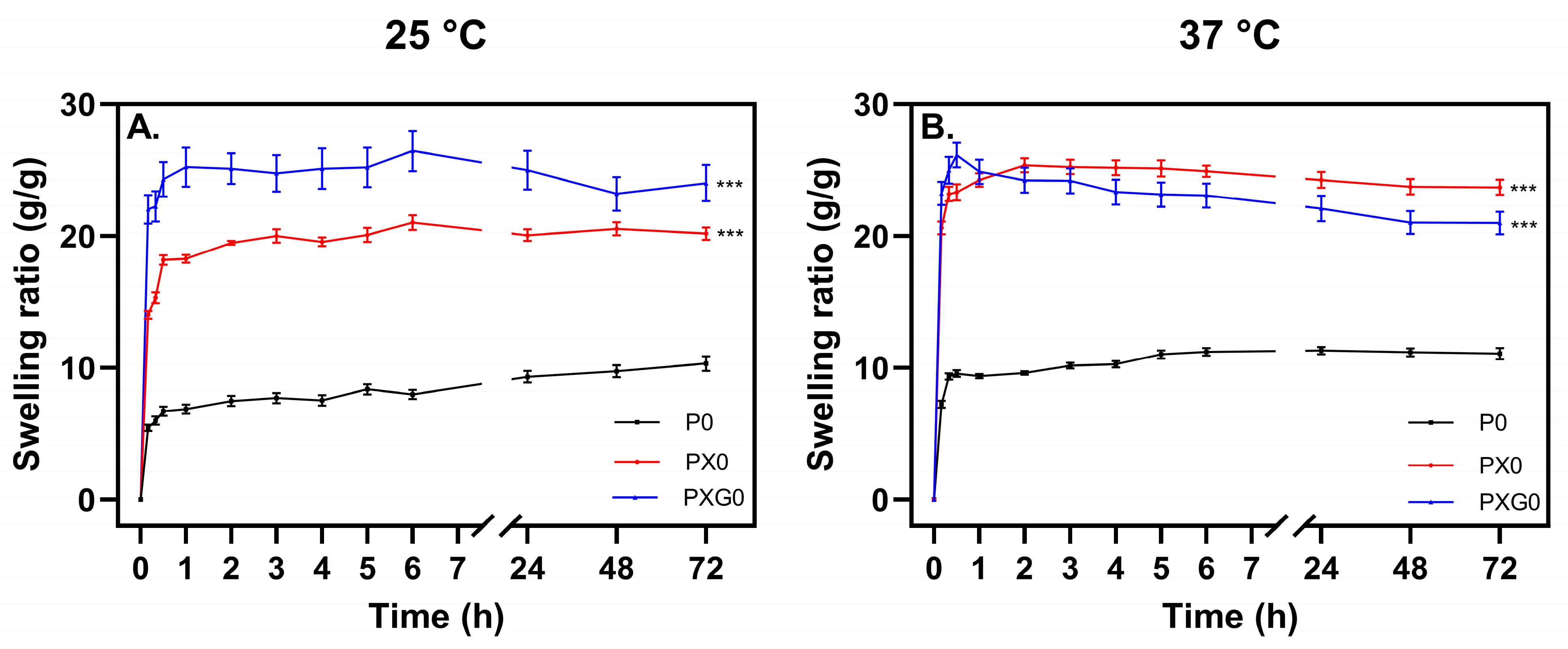
2.1. Development and Swelling Assessment of Hydrogel Dressings
2.2. Characterization of Hydrogel Dressings
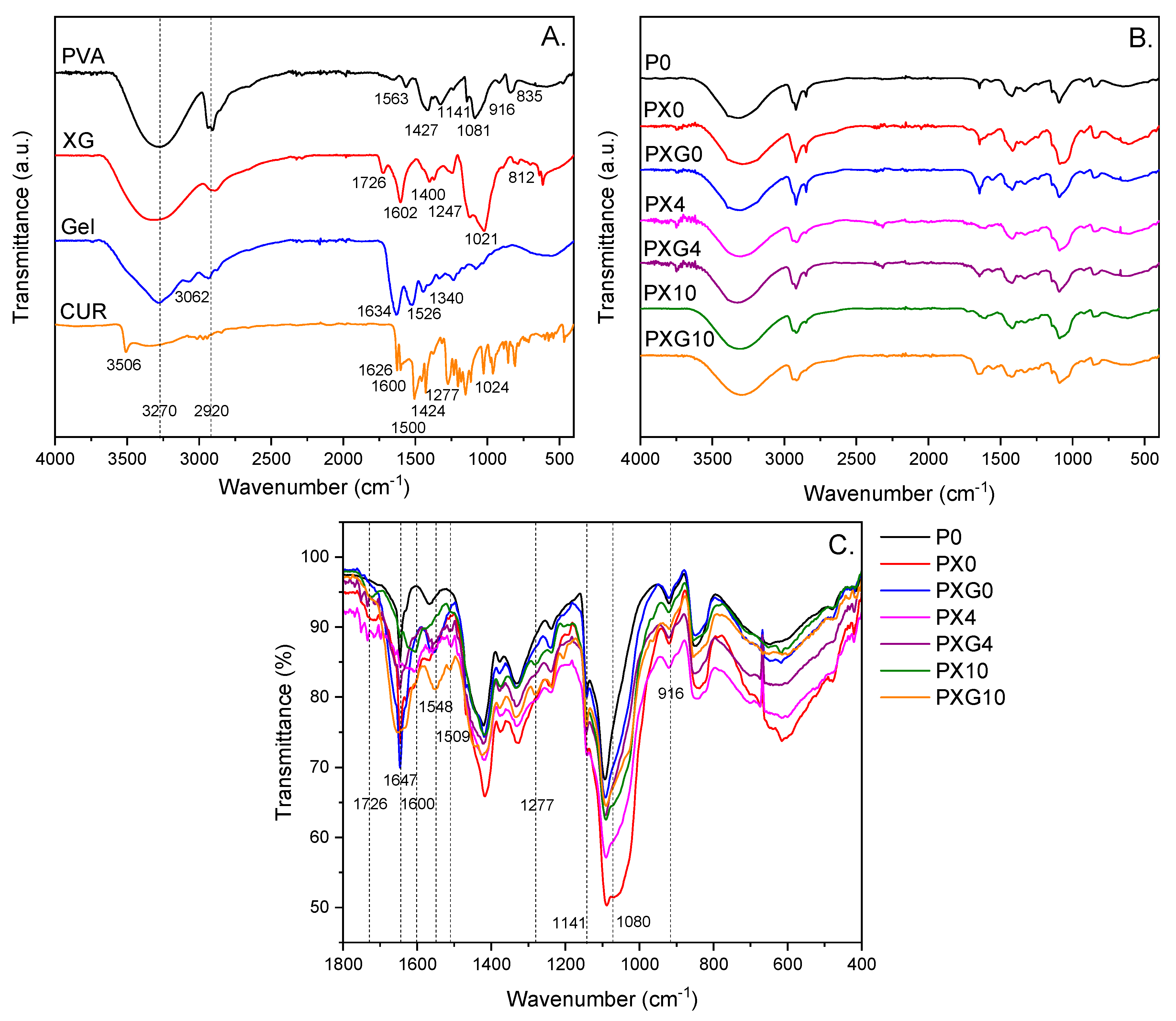
2.2.1. ATR-FTIR Spectroscopic Characterization
2.2.2. Thermal Studies
2.2.3. Mechanical Properties
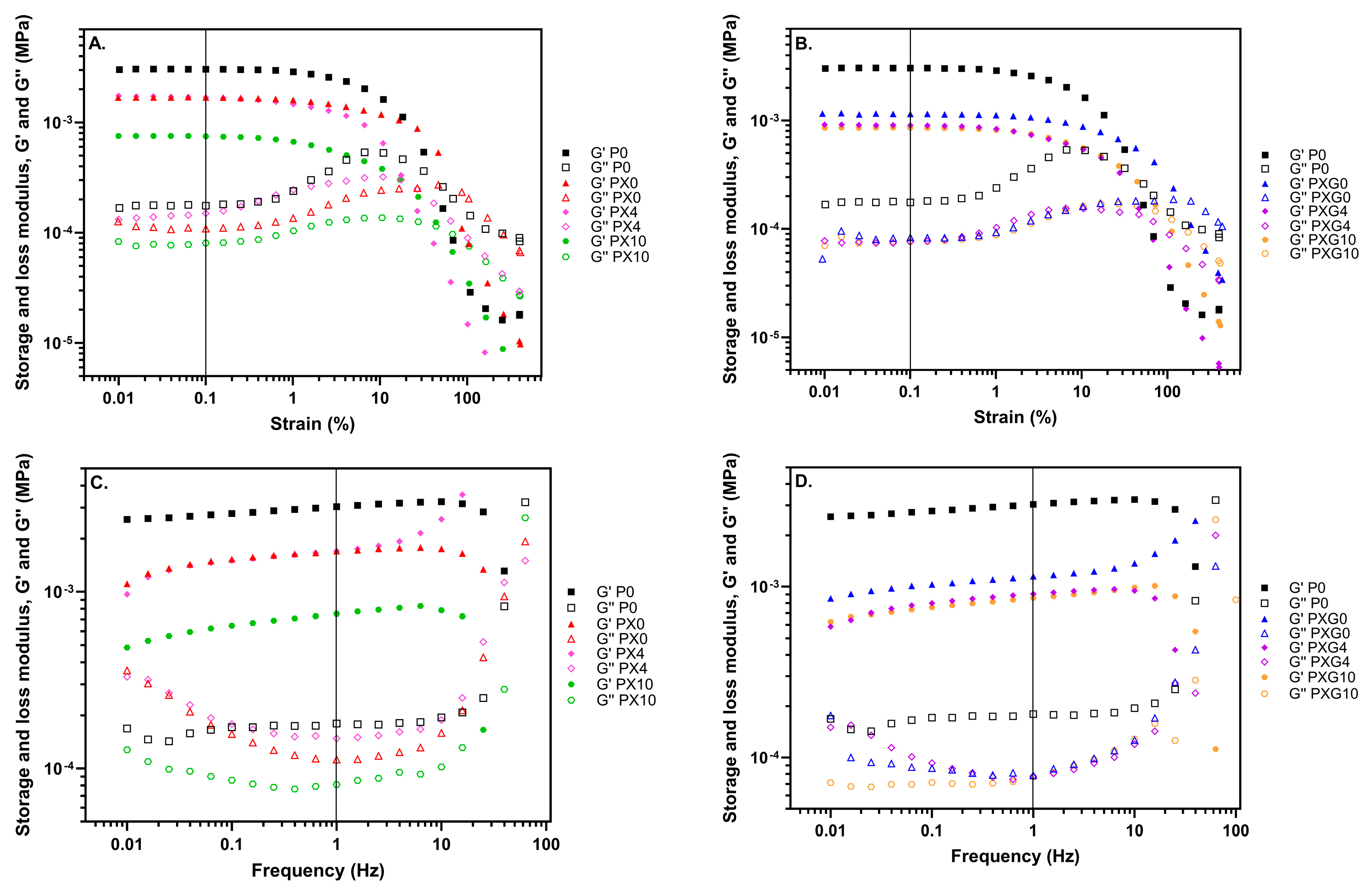
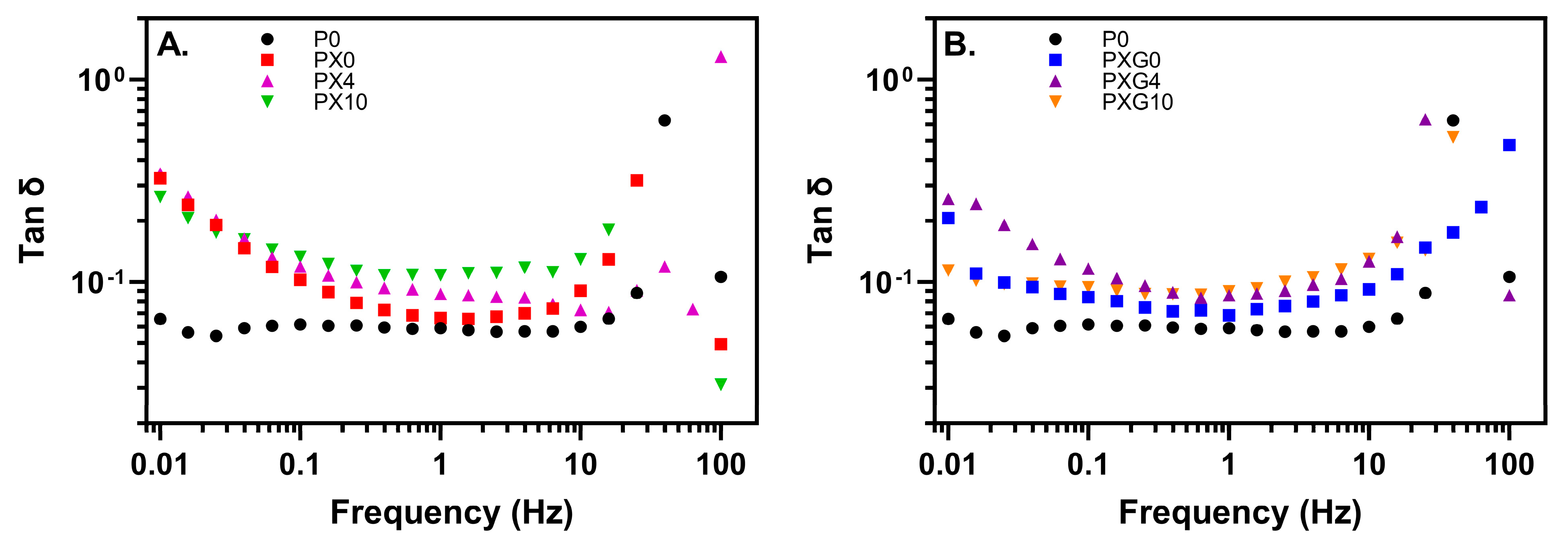
2.2.4. Rheological Properties
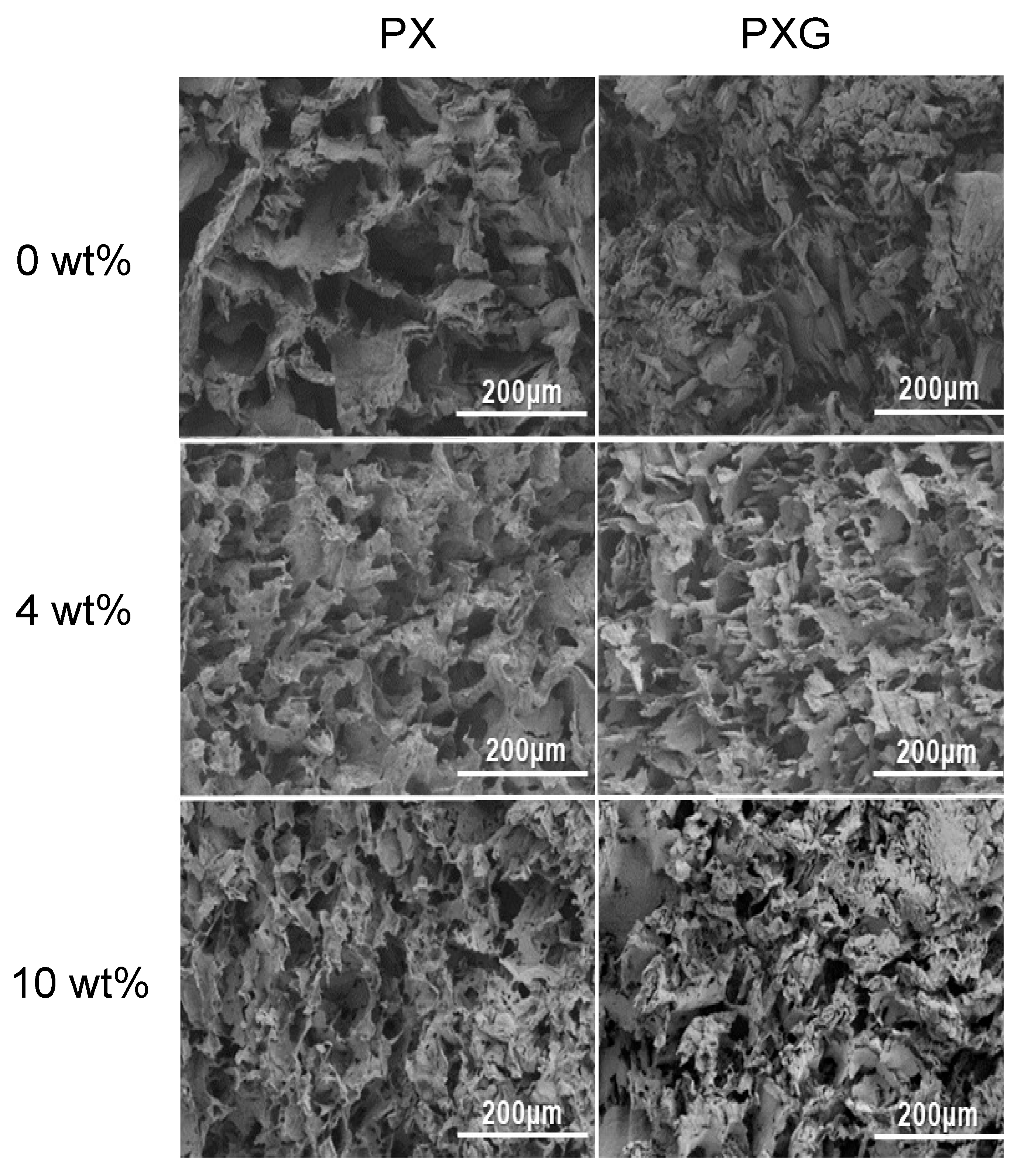
2.2.5. Morphological Analysis
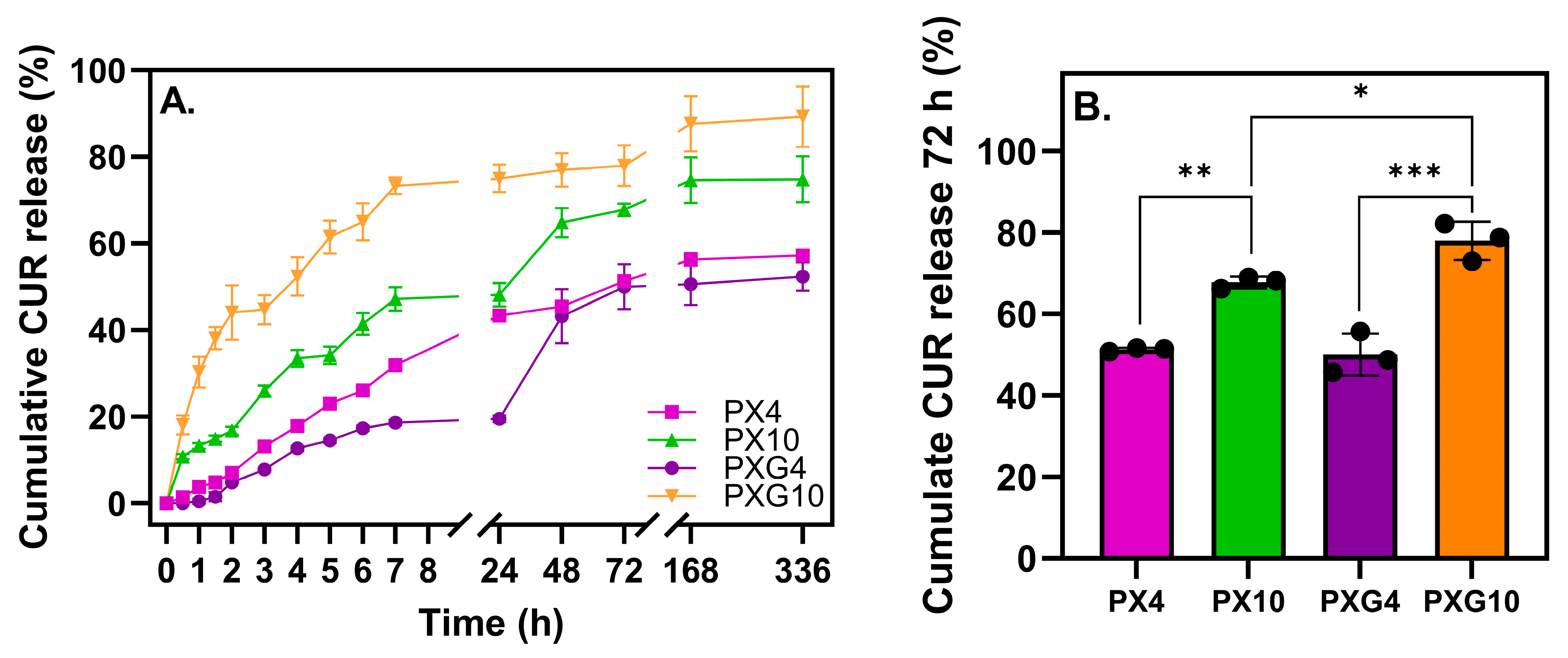
2.2.6. In Vitro Curcumin Release and Analysis of Kinetics Results
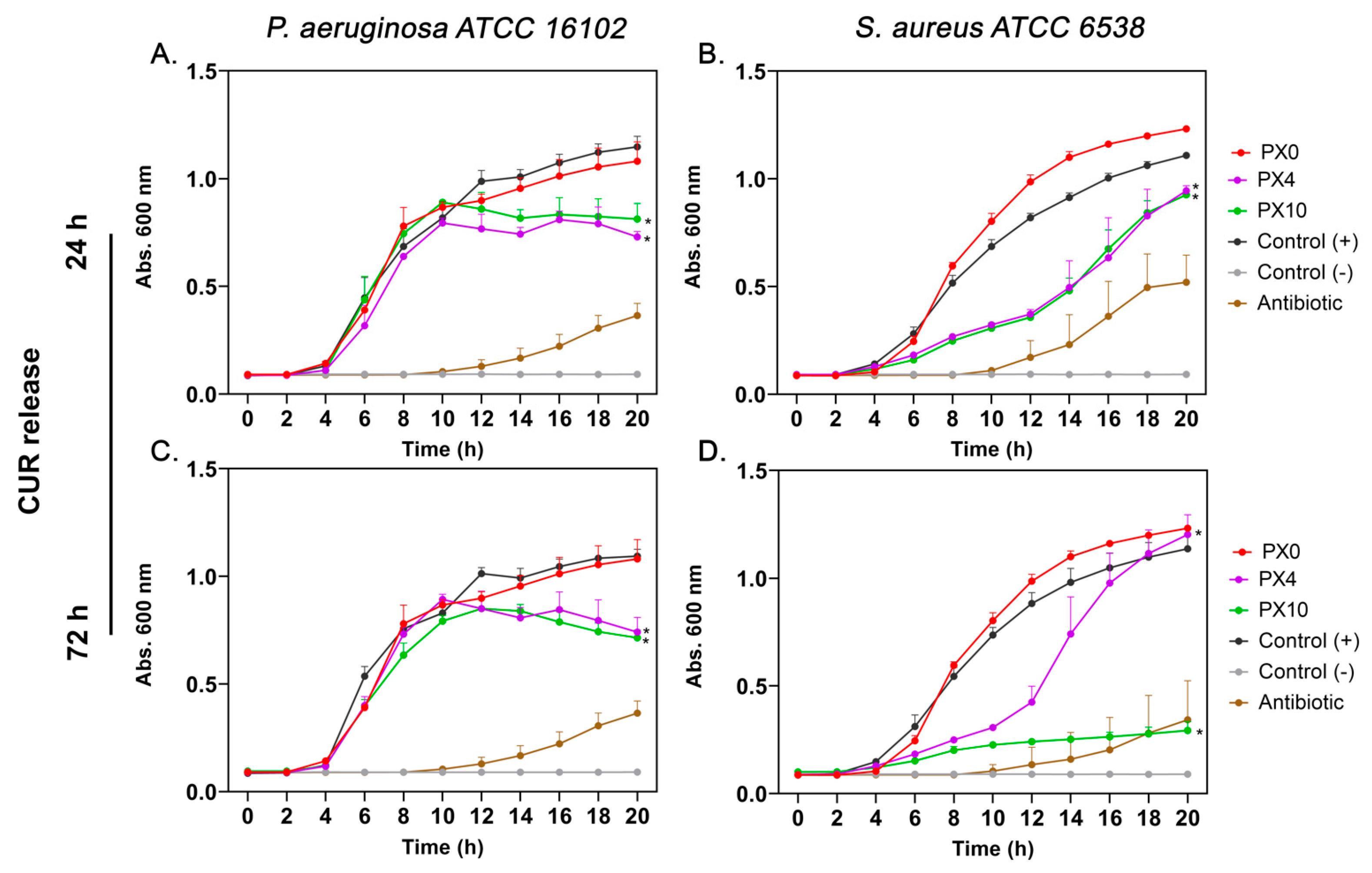
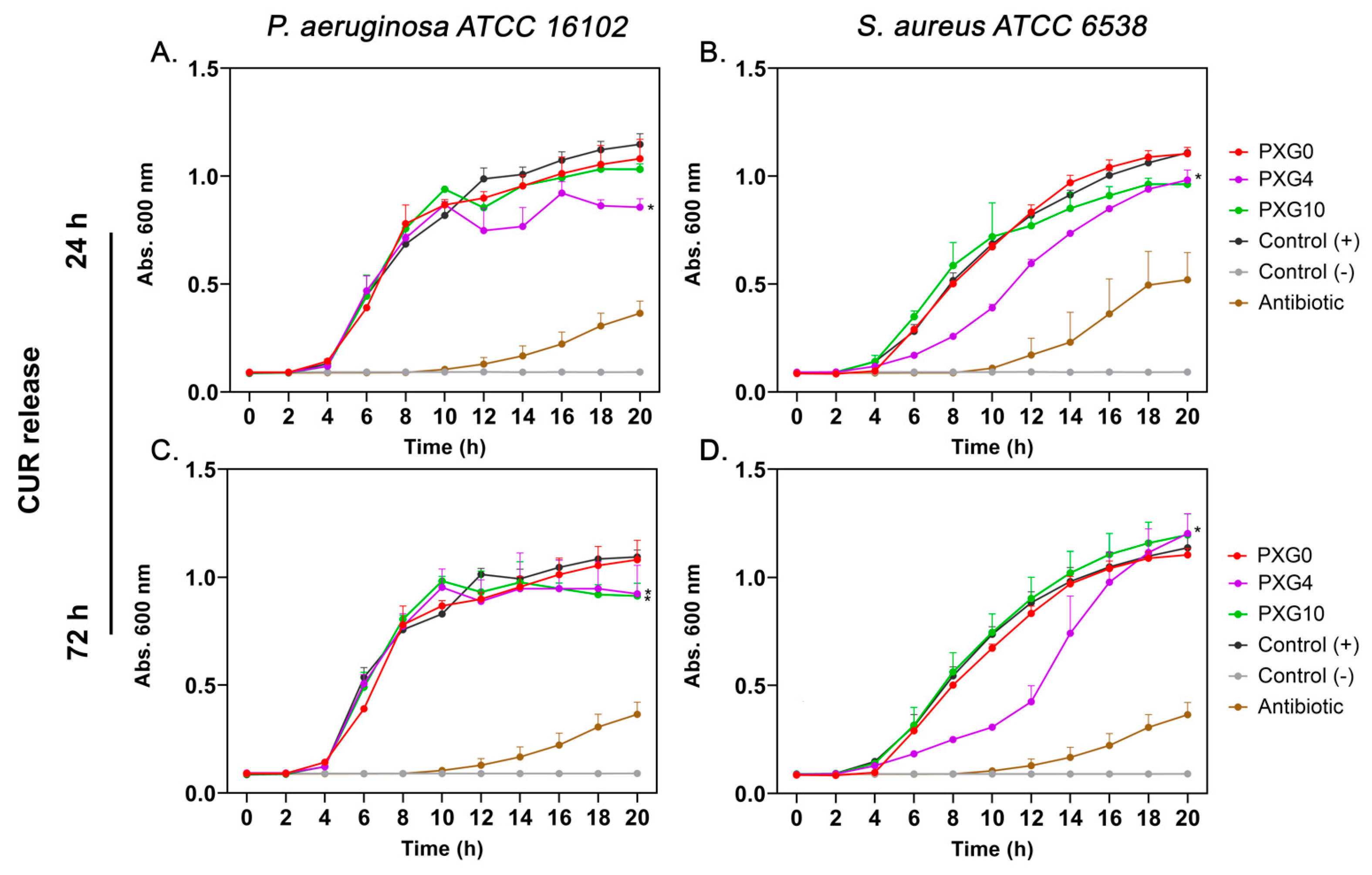
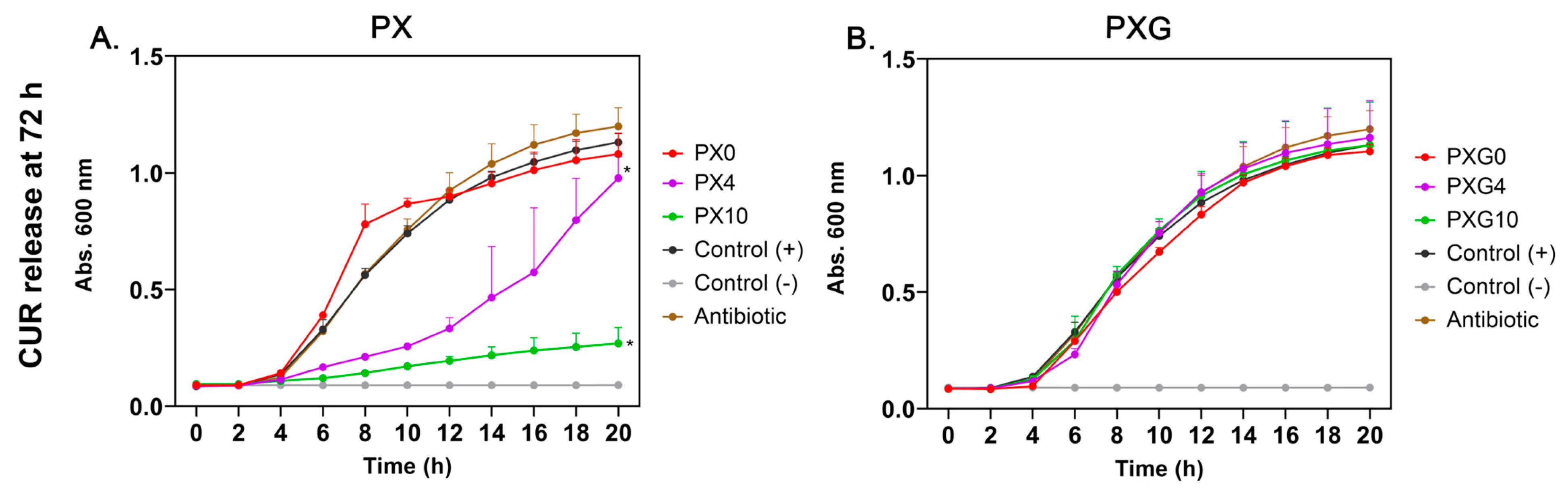
2.3. In Vitro Antibacterial Evaluation
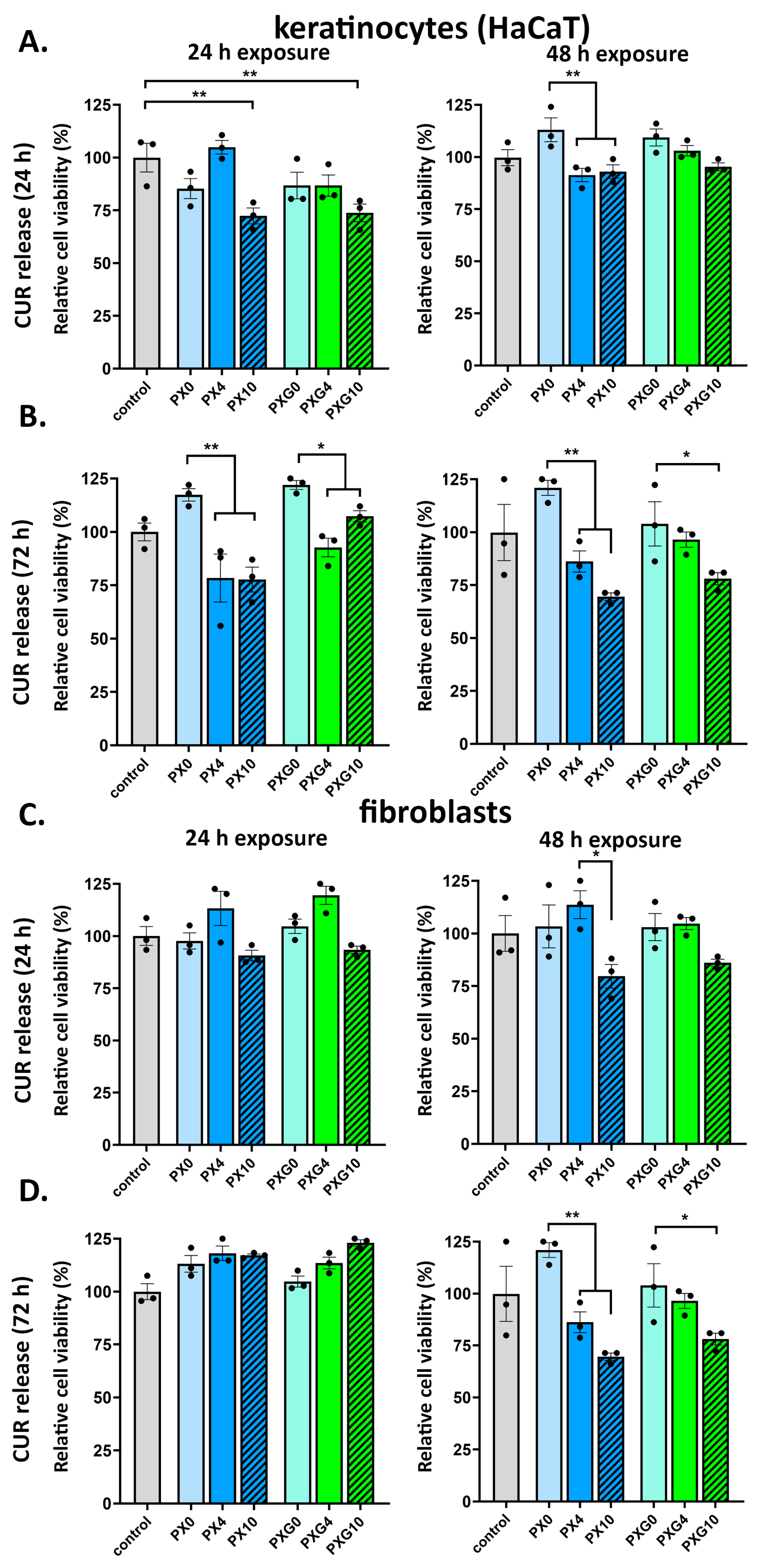
2.4. In Vitro Cell Viability Evaluation
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of PVA and Biopolymer-Based Hydrogel Dressings Loaded with Curcumin
4.3. Characterization
4.4. Swelling Ratio Determination
4.5. Study of the In Vitro Release Kinetics of Curcumin
4.6. Mechanical Properties
4.7. Rheological Properties
4.8. In Vitro Antibacterial Evaluation
4.9. In Vitro Cell Viability Evaluation
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MRSA | Methicillin-resistant Staphylococcus aureus |
| HaCaT | Immortalized human keratinocytes |
| HDFa | Adult human dermal fibroblasts |
| PVA | Polyvinyl alcohol |
| XG | Xanthan gum |
| Gel | Gelatin |
| F/T | Freeze/thaw |
| ECM | Extracellular matrix |
| CUR | Curcumin |
| DMEM | Dulbecco’s modified eagle medium |
References
- Chandra, P.K.; Soker, S.; Atala, A. Tissue Engineering: Current Status and Future Perspectives. Princ. Tissue Eng. 2020, 1–35. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/Stimuli-Responsive Hydrogels: Cutting-Edge Platforms for Tissue Engineering and Other Biomedical Applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef]
- Castelli, V.; Sastre-Escolà, E.; Puerta-Alcalde, P.; Huete-Álava, L.; Laporte-Amargós, J.; Bergas, A.; Chumbita, M.; Marín, M.; Domingo-Domenech, E.; Badia-Tejero, A.M.; et al. The Etiology, Antibiotic Therapy and Outcomes of Bacteremic Skin and Soft-Tissue Infections in Onco-Hematological Patients. Antibiotics 2023, 12, 1722. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-Resistant Staphylococcus Aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef]
- Hatami, M.; Khorasani, M.T.; Ahmadi, E.; Mohamadnia, S. Preparation and Characterization of Advancing Wound Care with PVP/QCS/DEX Hydrogels: A Multifunctional Wound Dressing Composite: In Vitro and In Vivo Assay. J. Biomater. Sci. Polym. Ed. 2025, 1–31. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Li, M.; Li, Z.; Wang, J.; Li, J.; Guo, B. Polymer Applied in Hydrogel Wound Dressing for Wound Healing: Modification/Functionalization Method and Design Strategies. ACS Biomater. Sci. Eng. 2025, 11, 1921–1944. [Google Scholar] [CrossRef]
- Malik, H.; Amir, F.; Jahan, Z.; Liaqat, U.; Andleeb, S.; Bandyopadhyay, S.; Khan Niazi, M.B. Study of Shape of Zinc Oxide Nanoparticles on the In-Vitro and In-Vivo Performance of Polymeric Hydrogels for Wound Dressing. Int. J. Pharm. 2025, 674, 125482. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Tang, Y.; Zheng, M.; Chang, Y.; Chen, H.; Wei, J.; Wu, J.; Zheng, J. Zwitterionic Hydrogels from Material Design to Wound Dressing Applications. Supramol. Mater. 2025, 4, 100108. [Google Scholar] [CrossRef]
- Gao, Y.; Li, X.; Yang, Y.; Wang, H.; Niu, X. CMCS-PVA@CA Hydrogel Dressing: A Promoter of Wound Healing with MRSA Virulence Attenuation Function. Int. J. Biol. Macromol. 2025, 295, 139614. [Google Scholar] [CrossRef]
- Rahman Khan, M.M.; Rumon, M.M.H. Synthesis of PVA-Based Hydrogels for Biomedical Applications: Recent Trends and Advances. Gels 2025, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Bercea, M. Recent Advances in Poly(Vinyl Alcohol)-Based Hydrogels. Polymers 2024, 16, 2021. [Google Scholar] [CrossRef]
- Yerra, P.; Migliario, M.; Gino, S.; Sabbatini, M.; Bignotto, M.; Invernizzi, M.; Renò, F. Polydopamine Blending Increases Human Cell Proliferation in Gelatin–Xanthan Gum 3D-Printed Hydrogel. Gels 2024, 10, 145. [Google Scholar] [CrossRef]
- Bernal-Chávez, S.A.; Alcalá-Alcalá, S.; Tapia-Guerrero, Y.S.; Magaña, J.J.; Del Prado-Audelo, M.L.; Leyva-Gómez, G. Cross-Linked Polyvinyl Alcohol-Xanthan Gum Hydrogel Fabricated by Freeze/Thaw Technique for Potential Application in Soft Tissue Engineering. RSC Adv. 2022, 12, 21713–21724. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.; Hua, S.; Wang, H.; Ping, R.; Zhang, F.; Wei, S.; Liu, J.; Wang, R.; Tian, B. Chitosan/PVA Film Containing β-Acids/HP-β-CD Inclusion Complex for MRSA-Infected Wound Healing. Int. J. Biol. Macromol. 2025, 319, 145601. [Google Scholar] [CrossRef]
- Zeng, H.; Tang, L.; Huang, L.; Yang, N.; Chen, X.; Peng, X.; Chen, Z.; Guo, J.; Weng, J.; Guo, T. A Novel Multi-Functional PVA- Alginate Hydrogel with Dynamic Bond Crosslinking for Infected Wound Repair. Carbohydr. Polym. 2025, 362, 123636. [Google Scholar] [CrossRef] [PubMed]
- Yermagambetova, A.; Tazhibayeva, S.; Takhistov, P.; Tyussyupova, B.; Tapia-Hernández, J.A.; Musabekov, K. Microbial Polysaccharides as Functional Components of Packaging and Drug Delivery Applications. Polymers 2024, 16, 2854. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.A.; Tahar, H.A.; Ali, A.E.H.; Raafat, A.I.; Elshahawy, M.F. Microbiological Evaluation and Self-Disinfecting Properties of an Electron- Beam Synthesized (Xanthan-PVA) Hydrogel Dressings Loaded with Manuka Honey and ZnO Nanoparticles. Mater. Today Commun. 2025, 47, 113216. [Google Scholar] [CrossRef]
- Serbezeanu, D.; Iftime, M.M.; Ailiesei, G.L.; Ipate, A.M.; Bargan, A.; Vlad-Bubulac, T.; Rîmbu, C.M. Evaluation of Poly(Vinyl Alcohol)–Xanthan Gum Hydrogels Loaded with Neomycin Sulfate as Systems for Drug Delivery. Gels 2023, 9, 655. [Google Scholar] [CrossRef]
- Sun, J.; Song, L.; Zhou, Y.; Wu, K.; Li, C.; Han, B.; Chang, J. Review: Advances in Multifunctional Hydrogels Based on Carbohydrate Polymer and Protein in the Treatment of Diabetic Wounds. Int. J. Biol. Macromol. 2025, 309, 142693. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Hu, F.; Gou, S.; Gao, Q.; Wang, S.; Zhang, W.; Zhang, Y.; Lu, T. Curcumin-Loaded Chitosan-Based Hydrogels Accelerating S. Aureus-Infected Wound Healing. Int. J. Biol. Macromol. 2024, 259, 129111. [Google Scholar] [CrossRef]
- Barati, S.; Yadegari, A.; Shahmohammadi, M.; Azami, F.; Tahmasebi, F.; Rouhani, M.R.; Kazemi, S.; Asl, E.R. Curcumin as a Promising Therapeutic Agent for Diabetic Neuropathy: From Molecular Mechanisms to Functional Recovery. Diabetol. Metab. Syndr. 2025, 17, 314. [Google Scholar] [CrossRef]
- Moon, D.O. Curcumin as a Dual Modulator of Pyroptosis: Mechanistic Insights and Therapeutic Potential. Int. J. Mol. Sci. 2025, 26, 7590. [Google Scholar] [CrossRef]
- Kannan, P.R.; Kumar, C.S.; Zhao, R.; Iqbal, M.Z.; Li, Y.; Kong, X. Dual-Functional Hydrogel with Curcumin-Loaded GelMA and Silk Fibroin for Wound Healing: Characterization and In Vitro Evaluation. Mater. Today Commun. 2025, 44, 112014. [Google Scholar] [CrossRef]
- Anand, R.; Collard, D.; Thomann, J.S.; Duday, D. Antimicrobial Sponge: A Polyvinyl Alcohol, Tannic Acid and Curcumin-Loaded Nanolignin Hydrogel Composite Scaffold. Gels 2025, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- Khodaei, A.; Johari, N.; Jahanmard, F.; Cecotto, L.; Khosravimelal, S.; Madaah Hosseini, H.R.; Bagheri, R.; Samadikuchaksaraei, A.; Amin Yavari, S. Particulate 3D Hydrogels of Silk Fibroin-Pluronic to Deliver Curcumin for Infection-Free Wound Healing. Biomimetics 2024, 9, 483. [Google Scholar] [CrossRef]
- Burduja, N.; Virzì, N.F.; Nocito, G.; Ginestra, G.; Saita, M.G.; Spitaleri, F.; Patanè, S.; Nostro, A.; Pittalà, V.; Mazzaglia, A. Curcumin-Laden Hydrogel Coating Medical Device for Periprosthetic Joint Infection Prevention and Control. Int. J. Pharm. 2025, 672, 125283. [Google Scholar] [CrossRef] [PubMed]
- Soto-Bustamante, F.; Bassu, G.; Fratini, E.; Laurati, M. Effect of Composition and Freeze-Thaw on the Network Structure, Porosity and Mechanical Properties of Polyvinyl-Alcohol/Chitosan Hydrogels. Gels 2023, 9, 396. [Google Scholar] [CrossRef]
- Figueroa-Pizano, M.; Vélaz, I.; Peñas, F.; Zavala-Rivera, P.; Rosas-Durazo, A.; Maldonado-Arce, A.; Martínez-Barbosa, M.E. Effect of Freeze-Thawing Conditions for Preparation of Chitosan-Poly (Vinyl Alcohol) Hydrogels and Drug Release Studies. Carbohydr. Polym. 2018, 195, 476–485. [Google Scholar] [CrossRef]
- Khorasani, M.; Joorabloo, A.; Adeli, H.; Mansoori-Moghadam, Z.; Moghaddam, A. Design and Optimization of Process Parameters of Polyvinyl (Alcohol)/Chitosan/Nano Zinc Oxide Hydrogels as Wound Healing Materials. Carbohydr. Polym. 2019, 207, 542–554. [Google Scholar] [CrossRef]
- Wu, F.; Gao, J.; Xiang, Y.; Yang, J. Enhanced Mechanical Properties of PVA Hydrogel by Low-Temperature Segment Self-Assembly vs. Freeze–Thaw Cycles. Polymers 2023, 15, 3782. [Google Scholar] [CrossRef]
- Abedini, A.A.; Pircheraghi, G.; Kaviani, A.; Hosseini, S. Exploration of Curcumin-Incorporated Dual Anionic Alginate-Quince Seed Gum Films for Transdermal Drug Delivery. Int. J. Biol. Macromol. 2023, 248, 125798. [Google Scholar] [CrossRef]
- Hamilton, A.E.; Gilbert, R.J. Curcumin Release from Biomaterials for Enhanced Tissue Regeneration Following Injury or Disease. Bioengineering 2023, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Touzout, Z.; Abdellaoui, N.; Hadj-Hamou, A.S. Conception of PH-Sensitive Calcium Alginate/Poly Vinyl Alcohol Hydrogel Beads for Controlled Oral Curcumin Delivery Systems. Antibacterial and Antioxidant Properties. Int. J. Biol. Macromol. 2024, 263, 130389. [Google Scholar] [CrossRef] [PubMed]
- De Piano, R.; Caccavo, D.; Barba, A.A.; Lamberti, G. Polyelectrolyte Hydrogels in Biological Systems: Modeling of Swelling and Deswelling Behavior. Chem. Eng. Sci. 2023, 279, 118959. [Google Scholar] [CrossRef]
- Li, J.; Yu, F.; Chen, G.; Liu, J.; Li, X.-L.; Cheng, B.; Mo, X.; Chen, C.; Pan, J.-F. Moist-Retaining, Self-Recoverable, Bioadhesive, and Transparent in Situ Forming Hydrogels to Accelerate Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 2023–2038. [Google Scholar] [CrossRef]
- Lan, Z.; Kar, R.; Chwatko, M.; Shoga, E.; Cosgriff-Hernandez, E. High Porosity PEG-Based Hydrogel Foams with Self-Tuning Moisture Balance as Chronic Wound Dressings. J. Biomed. Mater. Res. A 2023, 111, 465–477. [Google Scholar] [CrossRef]
- Nuutila, K.; Eriksson, E. Moist Wound Healing with Commonly Available Dressings. Adv. Wound Care 2021, 10, 685–698. [Google Scholar] [CrossRef]
- Ortiz, J.A.; Sepúlveda, F.A.; Flores, S.; Saavedra, M.; Sáez-Silva, S.; Jiménez, T.; Murgas, P.; Troncoso, S.; Sanhueza, C.; Ulloa, M.T.; et al. Electrospun Polyvinyl Alcohol/Sodium Alginate Nanocomposite Dressings Loaded with ZnO and Bioglass: Characterization, Antibacterial Activity, and Cytocompatibility. Polymers 2025, 17, 2185. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Y.; Cao, Z.; Miao, J.; Feng, J.; Xi, Q.; Lu, W. Structure–Property Relationship in the Evaluation of Xanthan Gum Functionality for Oral Suspensions and Tablets. Int. J. Biol. Macromol. 2023, 226, 525–534. [Google Scholar] [CrossRef]
- Ortiz, J.A.; Sepúlveda, F.A.; Panadero-Medianero, C.; Murgas, P.; Ahumada, M.; Palza, H.; Matsuhiro, B.; Zapata, P.A. Cytocompatible Drug Delivery Hydrogels Based on Carboxymethylagarose/Chitosan PH-Responsive Polyelectrolyte Complexes. Int. J. Biol. Macromol. 2022, 199, 96–107. [Google Scholar] [CrossRef]
- Skopinska-Wisniewska, J.; Tuszynska, M.; Olewnik-Kruszkowska, E. Comparative Study of Gelatin Hydrogels Modified by Various Cross-Linking Agents. Materials 2021, 14, 396. [Google Scholar] [CrossRef]
- Huang, X.; Li, J.; He, J.; Luo, J.; Cai, J.; Wei, J.; Li, P.; Zhong, H. Preparation of Curcumin-Loaded Chitosan/Polyvinyl Alcohol Intelligent Active Films for Food Packaging and Freshness Monitoring. Int. J. Biol. Macromol. 2024, 276, 133807. [Google Scholar] [CrossRef]
- Ristić, I.; Nikolić, L.; Cakić, S.; Nikolić, V.; Tanasić, J.; Zvezdanović, J.; Krstić, M. Eco-Friendly Microwave Synthesis of Sodium Alginate-Chitosan Hydrogels for Effective Curcumin Delivery and Controlled Release. Gels 2024, 10, 637. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Preparation of Antimicrobial and Antioxidant Gelatin/Curcumin Composite Films for Active Food Packaging Application. Colloids Surf. B Biointerfaces 2020, 188, 110761. [Google Scholar] [CrossRef] [PubMed]
- Kolour, A.K.; Shahrousvand, M.; Mohammadi-Rovshandeh, J.; Puppi, D.; Farzaneh, D. Absorbable and Biodegradable Enzyme-Crosslinked Gelatin/Alginate Semi-IPN Hydrogel Wound Dressings Containing Curcumin. Int. J. Biol. Macromol. 2024, 279, 134938. [Google Scholar] [CrossRef]
- Demir, G.C.; Erdemli, Ö.; Keskin, D.; Tezcaner, A. Xanthan-Gelatin and Xanthan-Gelatin-Keratin Wound Dressings for Local Delivery of Vitamin C. Int. J. Pharm. 2022, 614, 121436. [Google Scholar] [CrossRef]
- Gomaa, M.M.; Hugenschmidt, C.; Dickmann, M.; Abdel-Hady, E.E.; Mohamed, H.F.M.; Abdel-Hamed, M.O. Crosslinked PVA/SSA Proton Exchange Membranes: Correlation between Physiochemical Properties and Free Volume Determined by Positron Annihilation Spectroscopy. Phys. Chem. Chem. Phys. 2018, 20, 28287–28299. [Google Scholar] [CrossRef] [PubMed]
- Ilić-Stojanović, S.; Nikolić, L.; Nikolić, V.; Ristić, I.; Cakić, S.; Petrović, S.D. Temperature-Sensitive Hydrogels as Carriers for Modulated Delivery of Acetaminophen. Gels 2023, 9, 684. [Google Scholar] [CrossRef]
- Górska, A.; Baran, E.; Knapik-Kowalczuk, J.; Szafraniec-Szczęsny, J.; Paluch, M.; Kulinowski, P.; Mendyk, A. Physically Cross-Linked PVA Hydrogels as Potential Wound Dressings: How Freezing Conditions and Formulation Composition Define Cryogel Structure and Performance. Pharmaceutics 2024, 16, 1388. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Preparation and Optimization of Chitosan-Gelatin Films for Sustained Delivery of Lupeol for Wound Healing. Int. J. Biol. Macromol. 2018, 107, 1888–1897. [Google Scholar] [CrossRef]
- Liu, W.; Yao, C.; Wang, D.; Du, G.; Ji, Y.; Li, Q. Dynamic Double-Networked Hydrogels by Hybridizing PVA and Herbal Polysaccharides: Improved Mechanical Properties and Selective Antibacterial Activity. Gels 2024, 10, 821. [Google Scholar] [CrossRef]
- Enoch, K.; Somasundaram, A.A. Improved Mechanical Properties of Chitosan/PVA Hydrogel–A Detailed Rheological Study. Surf. Interfaces 2023, 41, 103178. [Google Scholar] [CrossRef]
- Rashid, N.; Khalid, S.H.; Ullah Khan, I.; Chauhdary, Z.; Mahmood, H.; Saleem, A.; Umair, M.; Asghar, S. Curcumin-Loaded Bioactive Polymer Composite Film of PVA/Gelatin/Tannic Acid Downregulates the Pro-Inflammatory Cytokines to Expedite Healing of Full-Thickness Wounds. ACS Omega 2023, 8, 7575–7586. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, X.; Su, T.; Lu, F.; Chang, Q.; Gao, J. Recent Advances in Macroporous Hydrogels for Cell Behavior and Tissue Engineering. Gels 2022, 8, 606. [Google Scholar] [CrossRef] [PubMed]
- Baghali, M.; Ziyadi, H.; Di Martino, A. Novel Sustainable and Thermal-Responsive Composite Hydrogel Based on Poly (Vinyl Alcohol)-Glycerin-Xanthan Gum as a Drug Carrier for Metronidazole. J. Polym. Res. 2025, 32, 148. [Google Scholar] [CrossRef]
- Raschip, I.E.; Darie-Nita, R.N.; Fifere, N.; Hitruc, G.E.; Dinu, M.V. Correlation between Mechanical and Morphological Properties of Polyphenol-Laden Xanthan Gum/Poly(Vinyl Alcohol) Composite Cryogels. Gels 2023, 9, 281. [Google Scholar] [CrossRef]
- Ninjumrat, U.; Chuysinuan, P.; Inprasit, T.; Ummartyotin, S.; Chainok, K.; Pisitsak, P. Fast-Swelling Tamarind Xyloglucan/PVA Hydrogels with Interconnected Macroporous Structures for Biomedical Applications. Polymers 2024, 16, 3457. [Google Scholar] [CrossRef]
- Azarian, M.H.; Junyusen, T.; Sutapun, W. Biogenic Vaterite Calcium Carbonate-Silver/Poly(Vinyl Alcohol) Film for Wound Dressing. ACS Omega 2024, 9, 955–969. [Google Scholar] [CrossRef]
- Rumbaugh, K.; Watters, C.; Yuan, T. Beneficial and Deleterious Bacterial-Host Interactions in Chronic Wound Pathophysiology. Chronic Wound Care Manag. Res. 2015, 2, 53–62. [Google Scholar] [CrossRef]
- Martínez-Gómez, F.; Guerrero, J.; Matsuhiro, B.; Pavez, J. Characterization of Poly-D-Mannuronate and Poly-L-Guluronate Block Fractions from Sodium Alginate and Preparation of Hydrogels with Poly(Vinylalcohol). Int. J. Biol. Macromol. 2018, 111, 935–946. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef]
- Bruschi, M.L. Mathematical Models of Drug Release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Bruschi, M.L., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 63–86. [Google Scholar]
- Kan, G.; Zi, Y.; Shi, C.; Tan, Y.; Gong, H.; Wang, X.; Zhong, J. Interaction of Curcumin with Four Types of Gelatins in Nanoparticles: Mechanism and Application for Emulsion Stabilization. Food Hydrocoll. 2024, 146, 109268. [Google Scholar] [CrossRef]
- Musso, Y.S.; Salgado, P.R.; Mauri, A.N. Smart Edible Films Based on Gelatin and Curcumin. Food Hydrocoll. 2017, 66, 8–15. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.A.; Khan, J.; Shahid, R.; Shabbir, S.; Ayoob, M.F.; Imran, M. Chitosan-Carrageenan Microbeads Containing Nano-Encapsulated Curcumin: Nano-in-Micro Hydrogels as Alternative-Therapeutics for Resistant Pathogens Associated with Chronic Wounds. Int. J. Biol. Macromol. 2024, 278, 134841. [Google Scholar] [CrossRef]
- Anbari, H.; Maghsoudi, A.; Hosseinpour, M.; Yazdian, F. Acceleration of Antibacterial Activity of Curcumin Loaded Biopolymers against Methicillin-Resistant Staphylococcus Aureus: Synthesis, Optimization, and Evaluation. Eng. Life Sci. 2022, 22, 58–69. [Google Scholar] [CrossRef]
- Hao, P.Y.; Zhou, H.Y.; Ren, L.J.; Zheng, H.J.; Tong, J.N.; Chen, Y.W.; Park, H.J. Preparation and Antibacterial Properties of Curcumin-Loaded Cyclodextrin-Grafted Chitosan Hydrogel. J. Solgel Sci. Technol. 2023, 106, 877–894. [Google Scholar] [CrossRef]
- Mohammadi, A.; Sahabi, M.; Beigi-Boroujeni, S.; Abdolvand, H.; Makvandi, P.; Pournaghshband Isfahani, A.; Gharibi, R.; Ebrahimibagha, M. Alginate Hydrogel with Enhanced Curcumin Release Through HPβCD Assisted Host-Guest Interaction. Biomater. Adv. 2022, 141, 213130. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Ding, X.; Li, J.; Guo, W.; Okoro, O.V.; Mirzaei, M.; Sun, Y.; Jiang, G.; Shavandi, A.; Nie, L. Facile Preparation of Self-Healing Hydrogels Based on Chitosan and PVA with the Incorporation of Curcumin-Loaded Micelles for Wound Dressings. Biomed. Mater. 2024, 19, 025021. [Google Scholar] [CrossRef]
- Fan, X.; Huang, J.; Zhang, W.; Su, Z.; Li, J.; Wu, Z.; Zhang, P. A Multifunctional, Tough, Stretchable, and Transparent Curcumin Hydrogel with Potent Antimicrobial, Antioxidative, Anti-Inflammatory, and Angiogenesis Capabilities for Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2024, 16, 9749–9767. [Google Scholar] [CrossRef]
- Wang, R.; Ruan, L.; Jiang, G.; Li, P.; Aharodnikau, U.E.; Yunusov, K.E.; Gao, X.; Solomevich, S.O. Fabrication of Curcumin-Loaded Silk Fibroin and Polyvinyl Alcohol Composite Hydrogel Films for Skin Wound Healing. ACS Appl. Bio Mater. 2022, 5, 4400–4412. [Google Scholar] [CrossRef]
- Cui, Z.; Yao, L.; Ye, J.; Wang, Z.; Hu, Y. Solubility Measurement and Thermodynamic Modelling of Curcumin in Twelve Pure Solvents and Three Binary Solvents at Different Temperature (T = 278.15–323.15 K). J. Mol. Liq. 2021, 338, 116795. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, L.; Zhang, J.; De Souza Rastelli, A.N.; Yang, J.; Deng, D. Anti-Inflammatory Efficacy of Curcumin as an Adjunct to Non-Surgical Periodontal Treatment: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2022, 13, 808460. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.; Panadero-Medianero, C.; Arrázola, M.S.; Ahumada, M. Mimicking the Physicochemical Properties of the Cornea: A Low-Cost Approximation Using Highly Available Biopolymers. Polymers 2024, 16, 1118. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Sun, L.; Webster, T.J. Short Communication: Selective Cytotoxicity of Curcumin on Osteosarcoma Cells Compared to Healthy Osteoblasts. Int. J. Nanomed. 2014, 9, 461–465. [Google Scholar] [CrossRef]
- Desai, N.; Finosh, G.T.; Panicker, N.G.; Ramachandran, R.; Varghese, A. Cytotoxic Effects of Curcumin at Various Concentrations and Role of Curcumin on Lipid Peroxidation and Activities of Antioxidant Enzymes of the Rat Peripheral Blood Lymphocytes. Blood 2011, 118, 4933. [Google Scholar] [CrossRef]
- Waresindo, W.X.; Luthfianti, H.R.; Priyanto, A.; Hapidin, D.A.; Edikresnha, D.; Aimon, A.H.; Suciati, T.; Khairurrijal, K. Freeze–Thaw Hydrogel Fabrication Method: Basic Principles, Synthesis Parameters, Properties, and Biomedical Applications. Mater. Res. Express 2023, 10, 024003. [Google Scholar] [CrossRef]
- Nasra, M.M.A.; Khiri, H.M.; Hazzah, H.A.; Abdallah, O.Y. Formulation, In-Vitro Characterization and Clinical Evaluation of Curcumin In-Situ Gel for Treatment of Periodontitis. Drug Deliv. 2017, 24, 133–142. [Google Scholar] [CrossRef]
- Shahriarinour, M.; Divsar, F. Release Kinetics and Antibacterial Property of Curcumin-Loaded Date Palm (Phoenix dactylifera L.) Pollen. Arab. J. Sci. Eng. 2023, 48, 7263–7272. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Y.; Wang, J.; Kang, Y.; Wang, Z.; Cao, W.; Ye, J.; Gao, C. A Tough, Antibacterial and Antioxidant Hydrogel Dressing Accelerates Wound Healing and Suppresses Hypertrophic Scar Formation in Infected Wounds. Bioact. Mater. 2024, 34, 269–281. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]


| Sample | TG Analysis | DSC Analysis | |||||
|---|---|---|---|---|---|---|---|
| T10 (°C) | Tmax (°C) | Tg (°C) | Tcc (°C) | Tm (°C) | ΔHm (J/g) | Xc (%) | |
| P0 | 240.2 | 257.0 | 75.0 | 192.2 | 224.6 | 47.2 | 34.0 |
| PX0 | 234.9 | 284.2 | 83.2 | 152.9 | 190.8 | 17.5 | 12.6 |
| PXG0 | 245.9 | 307.7 | 87.5 | 173.4 | 204.8 | 28.0 | 20.2 |
| PX4 | 237.0 | 285.7 | 87.9 | 140.8 | 192.5 | 22.7 | 16.4 |
| PXG4 | 246.5 | 299.7 | 86.0 | 173.6 | 207.3 | 31.7 | 22.9 |
| PX10 | 238.0 | 288.3 | 85.5 | 142.8 | 195.8 | 21.3 | 15.4 |
| PXG10 | 245.4 | 339.1 | 89.4 | 170.0 | 205.5 | 29.8 | 21.5 |
| Dressing | Zero Order | First Order | Higuchi | Peppas-Korsmeyer | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| K0 | R2 | K1 | R2 | KH | R2 | Kp | n | R2 | D × 104 (cm2 s−1) | |
| PX4 | 4.64 | 0.971 | 0.83 | 0.925 | 3.35 | 0.961 | 0.06 | 1.20 | 0.984 | 3.443 |
| PXG4 | 2.43 | 0.946 | 2.18 | 0.817 | 0.27 | 0.991 | 7 × 10−3 | 3.33 | 0.970 | 0.430 |
| PX10 | 6.28 | 0.944 | 0.27 | 0.957 | 14.14 | 0.999 | 0.20 | 0.54 | 0.901 | 0.225 |
| PXG10 | 13.65 | 0.804 | 0.25 | 0.735 | 38.75 | 0.995 | 0.36 | 0.49 | 0.936 | 0.424 |
| Nomenclature | PVA (wt%) | Xanthan Gum (wt%) | Gelatin (wt%) | Curcumin (wt%) |
|---|---|---|---|---|
| P0 | 3.75 | 0 | 0 | 0 |
| PX0 | 2.50 | 1.25 | 0 | 0 |
| PXG0 | 2.50 | 0.62 | 0.62 | 0 |
| PX4 | 2.40 | 1.20 | 0 | 4 |
| PXG4 | 2.40 | 0.60 | 0.60 | 4 |
| PX10 | 2.25 | 1.12 | 0 | 10 |
| PXG10 | 2.25 | 0.56 | 0.56 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera, M.J.; Cament, A.; Ahumada, M.; Corrales, T.; García, V.; Pablos, J.L.; Osorio, J.; Ramos-González, G.; Vargas-Saturno, L.; Ezquer, M.; et al. Biofunctional Polyvinyl Alcohol/Xanthan Gum/Gelatin Hydrogel Dressings Loaded with Curcumin: Antibacterial Properties and Cell Viability. Gels 2025, 11, 764. https://doi.org/10.3390/gels11100764
Rivera MJ, Cament A, Ahumada M, Corrales T, García V, Pablos JL, Osorio J, Ramos-González G, Vargas-Saturno L, Ezquer M, et al. Biofunctional Polyvinyl Alcohol/Xanthan Gum/Gelatin Hydrogel Dressings Loaded with Curcumin: Antibacterial Properties and Cell Viability. Gels. 2025; 11(10):764. https://doi.org/10.3390/gels11100764
Chicago/Turabian StyleRivera, María José, Alejandro Cament, Manuel Ahumada, Teresa Corrales, Verónica García, Jesús L. Pablos, Javiera Osorio, Giselle Ramos-González, Leslie Vargas-Saturno, Marcelo Ezquer, and et al. 2025. "Biofunctional Polyvinyl Alcohol/Xanthan Gum/Gelatin Hydrogel Dressings Loaded with Curcumin: Antibacterial Properties and Cell Viability" Gels 11, no. 10: 764. https://doi.org/10.3390/gels11100764
APA StyleRivera, M. J., Cament, A., Ahumada, M., Corrales, T., García, V., Pablos, J. L., Osorio, J., Ramos-González, G., Vargas-Saturno, L., Ezquer, M., & Ortiz, J. A. (2025). Biofunctional Polyvinyl Alcohol/Xanthan Gum/Gelatin Hydrogel Dressings Loaded with Curcumin: Antibacterial Properties and Cell Viability. Gels, 11(10), 764. https://doi.org/10.3390/gels11100764








