Manufacture and Initial Characterisation of RAPIDTM Biodynamic Haematogel, an Autologous Platelet and Leukocyte-Rich Plasma Gel for Diabetic Foot Ulcers
Abstract
1. Introduction
1.1. Healthcare Burden of Diabetic Foot Ulcers
1.2. Need for More Effective Treatments
1.3. RAPID Gel as a Point-of-Care Therapeutic Product
1.4. Challenges Associated with RAPID Gel
1.5. Quality Considerations for RAPID Gel
1.6. Aims and Objectives
2. Results and Discussion
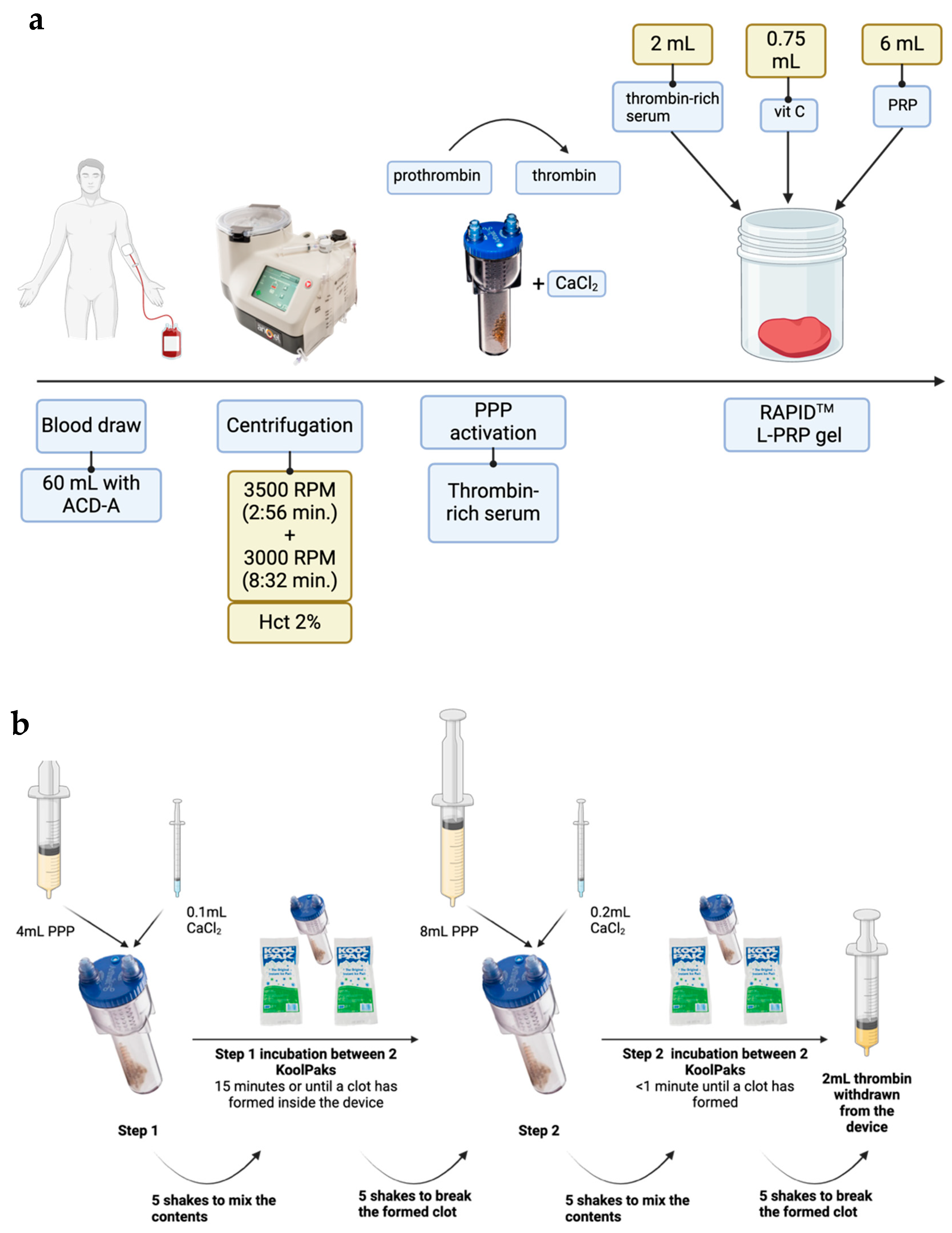
2.1. Reagent Preparation: L-PRP and Thrombin-Rich Serum
2.2. RAPID—Gel Formation and Characterisation
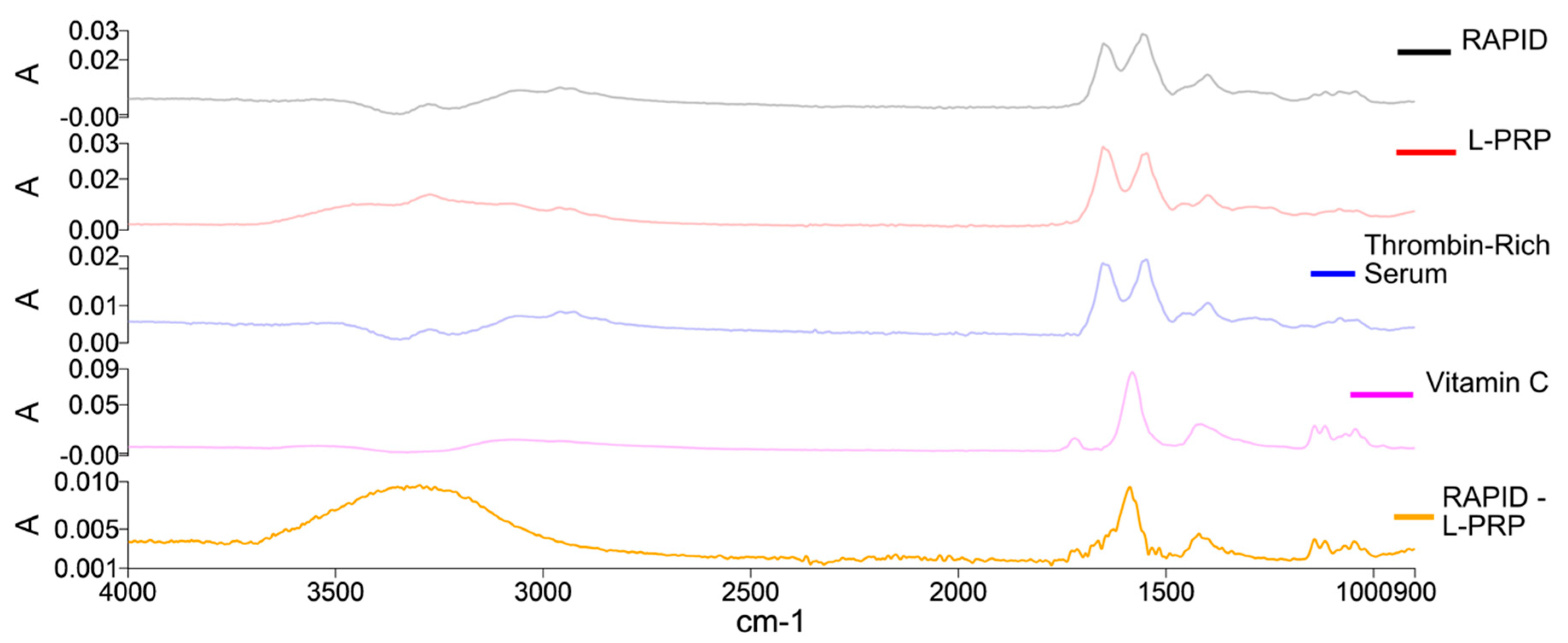
2.3. Gel Formation: FTIR Spectral Analysis
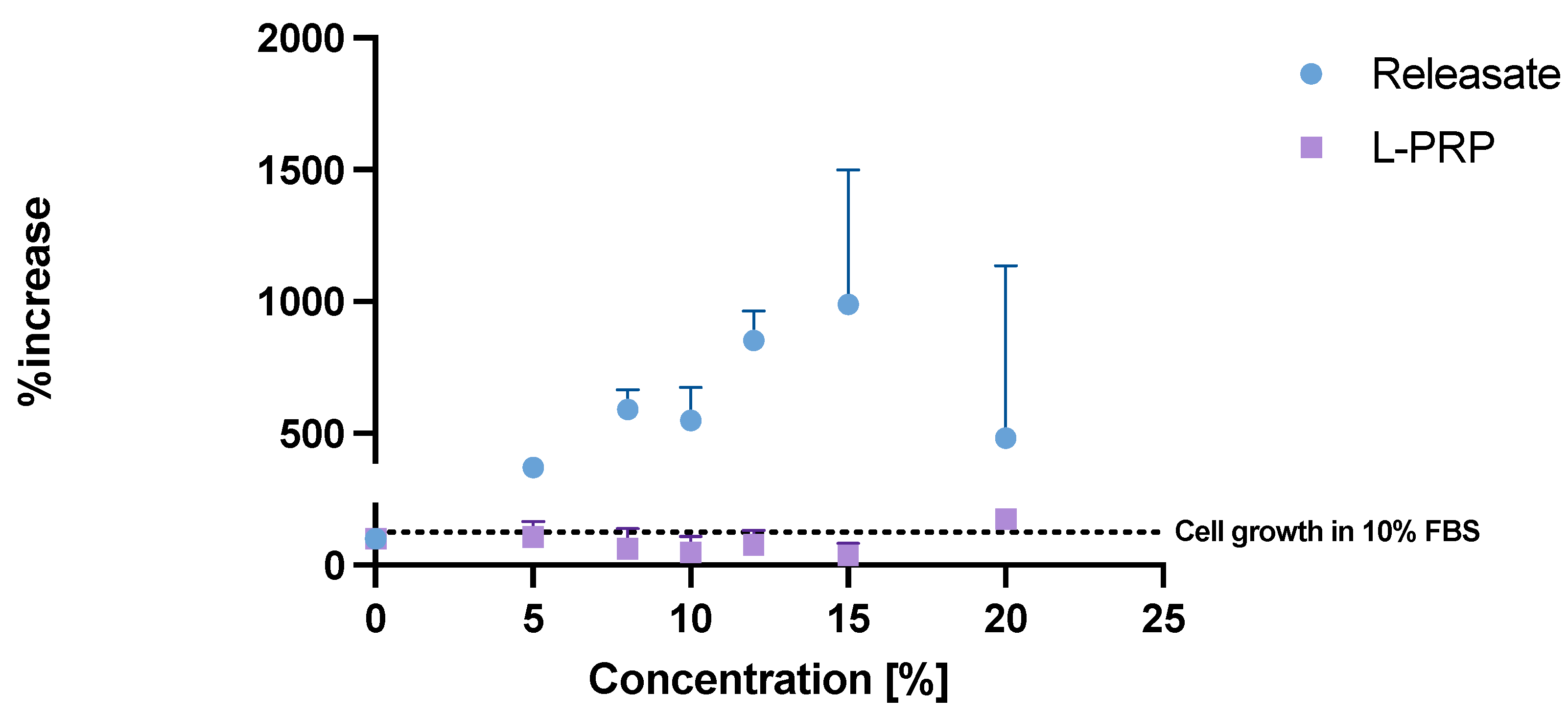
2.4. Growth Factors in Releasate
2.5. HaCat Cell Proliferation over Time
2.6. Future Work
2.7. A Note on Regulatory Framework for POC Products
3. Conclusions
4. Materials and Methods
4.1. Blood Collection and Cell Counts
4.2. RAPIDTM Gel Manufacture
4.3. RAPID L-PRP Gel Characterisation
4.3.1. Exudation of Releasate and Growth Factor Measurement
4.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
4.4. Keratinocyte Proliferation Assay
4.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.W.; Hoffstad, O.J.; Sullivan, M.O.; Margolis, D.J. Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabet. Med. 2016, 33, 1493–1498. [Google Scholar] [CrossRef]
- Guest, J.F.; Fuller, G.W.; Vowden, P. Cohort study evaluating the burden of wounds to the UK’s National Health Service in 2017/2018: Update from 2012/2013. BMJ Open 2020, 10, e045253. [Google Scholar] [CrossRef] [PubMed]
- CDC. Diabetes. 2024. National Diabetes Statistics Report. Available online: https://www.cdc.gov/diabetes/php/data-research/index.html (accessed on 14 July 2024).
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef]
- International Diabetes Federation [Internet]. Pakistan. Available online: https://idf.org/our-network/regions-and-members/middle-east-and-north-africa/members/pakistan/ (accessed on 14 July 2024).
- Akhtar, S.; Ali, A.; Ahmad, S.; Khan, M.I.; Shah, S.; Hassan, F. The Prevalence of Foot Ulcers in Diabetic Patients in Pakistan: A Systematic Review and Meta-Analysis. Front Public Health [Internet]. 25 October 2022. Available online: https://www.frontiersin.org/journals/public-health/articles/10.3389/fpubh.2022.1017201/full (accessed on 14 July 2024).
- Butt, M.D.; Ong, S.C.; Wahab, M.U.; Rasool, M.F.; Saleem, F.; Hashmi, A.; Sajjad, A.; Chaudhry, F.A.; Babar, Z.U. Cost of Illness Analysis of Type 2 Diabetes Mellitus: The Findings from a Lower-Middle Income Country. IJERPH 2022, 19, 12611. [Google Scholar] [CrossRef]
- Everett, E.; Mathioudakis, N. Update on management of diabetic foot ulcers. Ann. N. Y. Acad. Sci. 2018, 1411, 153–165. [Google Scholar] [CrossRef]
- Boulton, A.J.M.; Armstrong, D.G.; Löndahl, M.; Frykberg, R.G.; Game, F.L.; Edmonds, M.E.; Orgill, D.P.; Kramer, K.; Gurtner, G.C.; Januszyk, M.; et al. New Evidence-Based Therapies for Complex Diabetic Foot Wounds [Internet]. Arlington (VA): American Diabetes Association. 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK581559/ (accessed on 17 January 2024).
- Yang, L.; Rong, G.C.; Wu, Q.N. Diabetic foot ulcer: Challenges and future. World J. Diabetes 2022, 13, 1014–1034. [Google Scholar] [CrossRef]
- Greenhalgh, D.G. The Role of Growth Factors in Wound Healing. J. Trauma Acute Care Surg. 1996, 41, 159. [Google Scholar] [CrossRef]
- Martinez-Zapata, M.J.; Martí-Carvajal, A.J.; Solà, I.; Expósito, J.A.; Bolíbar, I.; Rodríguez, L.; Garcia, J.; Zaror, C. Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst. Rev. 2016, 5, CD006899. [Google Scholar] [CrossRef]
- OuYang, H.; Tang, Y.; Yang, F.; Ren, X.; Yang, J.; Cao, H.; Yin, Y. Platelet-Rich Plasma for the Treatment of Diabetic Foot Ulcer: A Systematic Review. Frontiers in Endocrinology [Internet]. 2023. Available online: https://www.frontiersin.org/articles/10.3389/fendo.2023.1256081 (accessed on 18 January 2024).
- Su, Y.N.; Li, J.; Feng, D.H.; Lu, R.R.; Dong, G.X.; Zhao, D.Y. Efficacy and safety of autologous platelet-rich plasma for diabetic foot ulcers: A systematic review and meta-analysis. J. Wound Care 2023, 32, 773–786. [Google Scholar] [CrossRef]
- Martin Kyriakides, C.K.; Sarkar, S. Platelet Rich Plasma (PRP) is an Adjunct for the Accelerated Closure of High-Risk Diabetic Foot Wounds. Br. J. Surg. 2015, 102, 24. [Google Scholar]
- Overview|3C Patch for Treating Diabetic Foot Ulcers|Guidance|NICE [Internet]. 2022. Available online: https://www.nice.org.uk/guidance/mtg66 (accessed on 14 July 2024).
- Overview|Platelet-Rich Plasma Injections for Knee Osteoarthritis|Guidance|NICE [Internet]. 2019. Available online: https://www.nice.org.uk/guidance/ipg637 (accessed on 14 July 2024).
- Centers for Medicare & Medicaid Services. Autologous Platelet-Rich Plasma [Internet]. Available online: https://www.cms.gov/medicare/coverage/evidence/plasma (accessed on 14 July 2024).
- Degen, R.M.; Bernard, J.A.; Oliver, K.S.; Dines, J.S. Commercial Separation Systems Designed for Preparation of Platelet-Rich Plasma Yield Differences in Cellular Composition. HSS J. 2017, 13, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Arthrex [Internet]. Angel® System. Available online: https://www.arthrex.com/orthobiologics/arthrex-angel-system (accessed on 18 January 2024).
- Bloemen, S.; Hemker, H.C.; Dieri, R.A. Large inter-individual variation of the pharmacodynamic effect of anticoagulant drugs on thrombin generation. Haematologica 2013, 98, 549–554. [Google Scholar] [CrossRef]
- Danforth, C.M.; Orfeo, T.; Everse, S.J.; Mann, K.G.; Brummel-Ziedins, K.E. Defining the Boundaries of Normal Thrombin Generation: Investigations into Hemostasis. PLoS ONE 2012, 7, e30385. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.A.M.; Knape, J.T.A.; Weibrich, G.; Schönberger, J.P.A.M.; Hoffmann, J.; Overdevest, E.P.; Box, H.A.; Van Zundert, A. Platelet-rich plasma and platelet gel: A review. J. Extracorpor. Technol. 2006, 38, 174–187. [Google Scholar] [CrossRef]
- Dhall, T.Z.; Shah, G.A.; Ferguson, I.A.; Dhall, D.P. Fibrin network structure: Modification by platelets. Thromb. Haemost. 1983, 49, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Paarakh, M.P.; Jose, P.A.; Setty, C.; Peter, G.V. Release Kinetics—Concepts and Applications. Int. J. Pharm. Res. Technol. 2018, 8, 12–20. [Google Scholar]
- Bruschi, M.L. (Ed.) 5—Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems [Internet]; Woodhead Publishing: New Delhi, Delhi, 2015; pp. 63–86. Available online: https://www.sciencedirect.com/science/article/pii/B9780081000922000059 (accessed on 18 January 2024).
- Wolkers, W.F.; Oldenhof, H. In situ FTIR studies on mammalian cells. Spectroscopy 2010, 24, 576151. [Google Scholar]
- Yang, H.S.; Shin, J.; Bhang, S.H.; Shin, J.Y.; Park, J.; Im, G.I.; Kim, C.S.; Kim, B.S. Enhanced skin wound healing by a sustained release of growth factors contained in platelet-rich plasma. Exp. Mol. Med. 2011, 43, 622–629. [Google Scholar] [CrossRef]
- Zheng, C.; Zhu, Q.; Liu, X.; Huang, X.; He, C.; Jiang, L.; Quan, D. Improved Peripheral Nerve Regeneration Using Acellular Nerve Allografts Loaded with Platelet-Rich Plasma. Tissue Eng. Part A 2014, 20, 3228–3240. [Google Scholar] [CrossRef]
- Wang, X.; Fok, M.R.; Pelekos, G.; Jin, L.; Tonetti, M.S. In Vitro and Ex Vivo Kinetic Release Profile of Growth Factors and Cytokines from Leucocyte- and Platelet-Rich Fibrin (L-PRF) Preparations. Cells 2022, 11, 2089. [Google Scholar] [CrossRef]
- Miroshnychenko, O.; Chalkley, R.J.; Leib, R.D.; Everts, P.A.; Dragoo, J.L. Proteomic analysis of platelet-rich and platelet-poor plasma. Regen. Ther. 2020, 15, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.; Zhang, N.; Li, T.; Zhou, X.; Jia, J.; Liang, Y.; Sun, X.; Chen, H. The Effects of Platelet-Rich and Platelet-Poor Plasma on Biological Characteristics of BM-MSCs In Vitro. Anal. Cell. Pathol. 2020, 2020, 8546231. [Google Scholar] [CrossRef] [PubMed]
- Beitzel, K.; Allen, D.; Apostolakos, J.; Russell, R.P.; McCarthy, M.B.; Gallo, G.J.; Cote, M.P.; Mazzocca, A.D. US Definitions, Current Use, and FDA Stance on Use of Platelet-Rich Plasma in Sports Medicine. J. Knee Surg. 2015, 28, 29–34. [Google Scholar] [CrossRef]
- Sebbagh, P.; Cannone, A.; Gremion, G.; Gremeaux, V.; Raffoul, W.; Hirt-Burri, N.; Michetti, M.; Abdel-Sayed, P.; Laurent, A.; Wardé, N.; et al. Current Status of PRP Manufacturing Requirements & European Regulatory Frameworks: Practical Tools for the Appropriate Implementation of PRP Therapies in Musculoskeletal Regenerative Medicine. Bioengineering 2023, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Evaluation of the EU Blood and Tissues and Cells Legislation [Internet]. Available online: https://ec.europa.eu/health/blood-tissues-cells-and-organs/overview/evaluation-eu-blood-and-tissues-and-cells-legislation_en (accessed on 29 March 2022).
- Medicines & Healthcare Products Regulatory Agency. Consultation on Point of Care Manufacturing [Internet]. 2021. Available online: https://www.gov.uk/government/consultations/point-of-care-consultation/consultation-on-point-of-care-manufacturing (accessed on 31 March 2022).
- Dean, L. Table 1, Complete Blood Count [Internet]. National Center for Biotechnology Information (US). 2005. Available online: https://www.ncbi.nlm.nih.gov/books/NBK2263/table/ch1.T1/ (accessed on 27 July 2022).



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olszewska, A.; Duan, J.; Javorovic, J.; Chan, K.L.A.; Rickard, J.; Pitchford, S.; Forbes, B. Manufacture and Initial Characterisation of RAPIDTM Biodynamic Haematogel, an Autologous Platelet and Leukocyte-Rich Plasma Gel for Diabetic Foot Ulcers. Gels 2024, 10, 572. https://doi.org/10.3390/gels10090572
Olszewska A, Duan J, Javorovic J, Chan KLA, Rickard J, Pitchford S, Forbes B. Manufacture and Initial Characterisation of RAPIDTM Biodynamic Haematogel, an Autologous Platelet and Leukocyte-Rich Plasma Gel for Diabetic Foot Ulcers. Gels. 2024; 10(9):572. https://doi.org/10.3390/gels10090572
Chicago/Turabian StyleOlszewska, Aleksandra, Jiajing Duan, Jana Javorovic, K. L. Andrew Chan, James Rickard, Simon Pitchford, and Ben Forbes. 2024. "Manufacture and Initial Characterisation of RAPIDTM Biodynamic Haematogel, an Autologous Platelet and Leukocyte-Rich Plasma Gel for Diabetic Foot Ulcers" Gels 10, no. 9: 572. https://doi.org/10.3390/gels10090572
APA StyleOlszewska, A., Duan, J., Javorovic, J., Chan, K. L. A., Rickard, J., Pitchford, S., & Forbes, B. (2024). Manufacture and Initial Characterisation of RAPIDTM Biodynamic Haematogel, an Autologous Platelet and Leukocyte-Rich Plasma Gel for Diabetic Foot Ulcers. Gels, 10(9), 572. https://doi.org/10.3390/gels10090572






