Analysis of Structural Changes of pH–Thermo-Responsive Nanoparticles in Polymeric Hydrogels
Abstract
1. Introduction
2. Results and Discussion
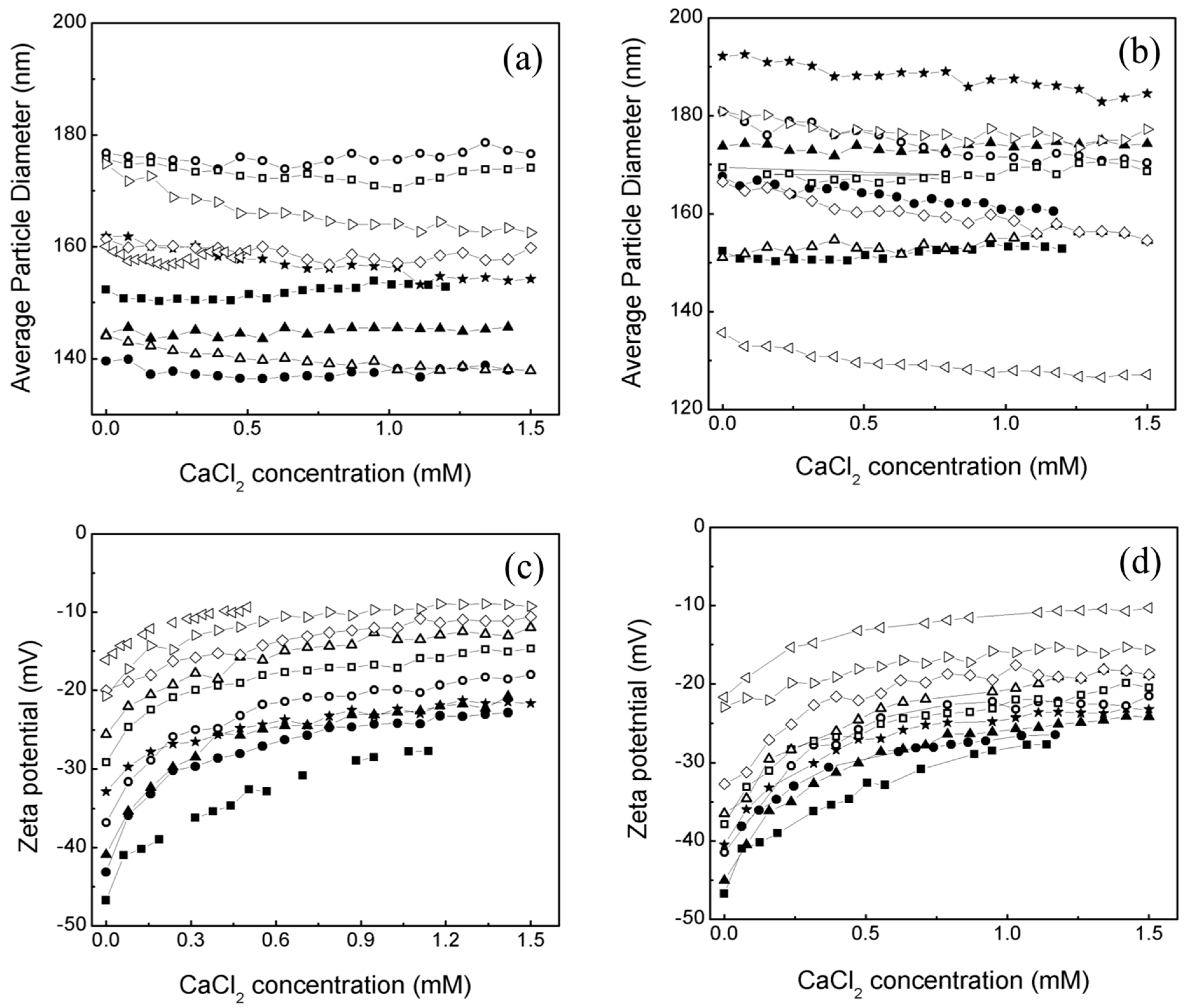
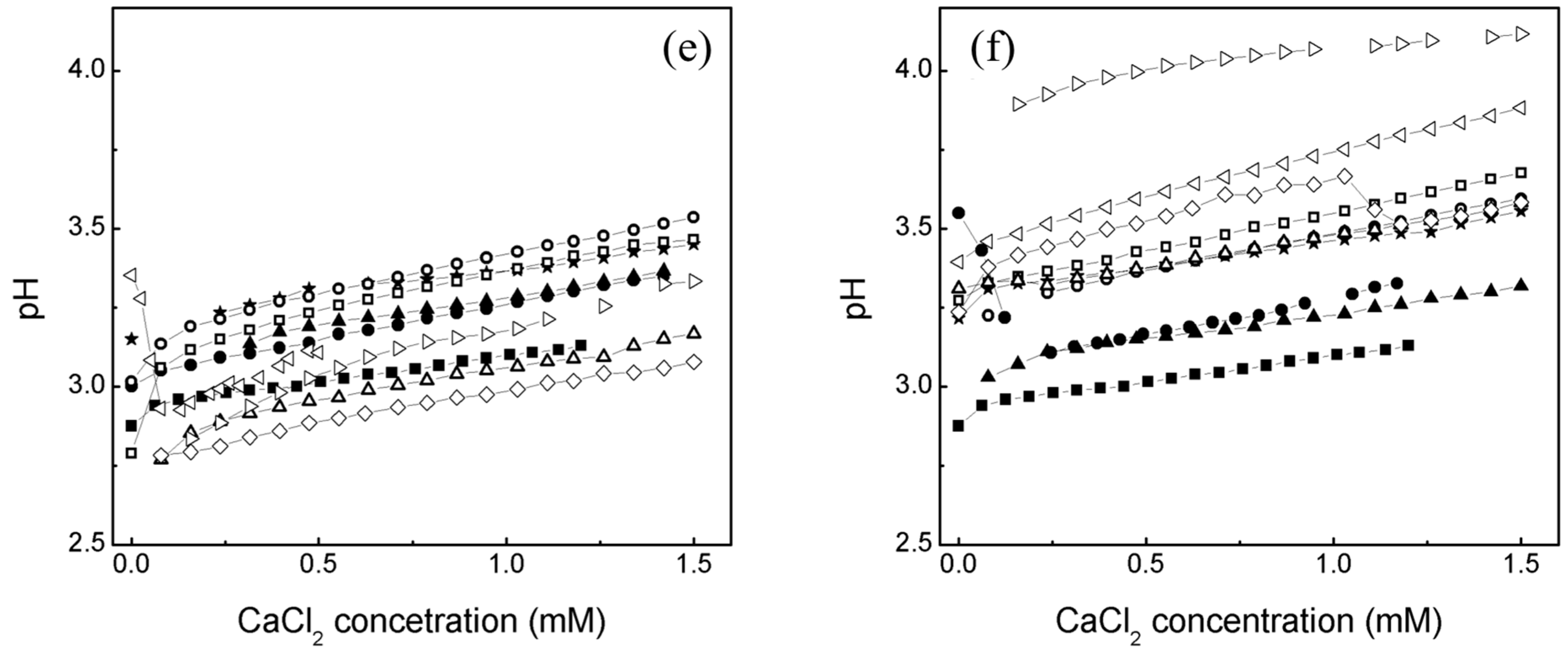
2.1. Effect of an Electrolyte on the Average Particle Diameter and Zeta Potential (ζ) of Poly Nanoparticles
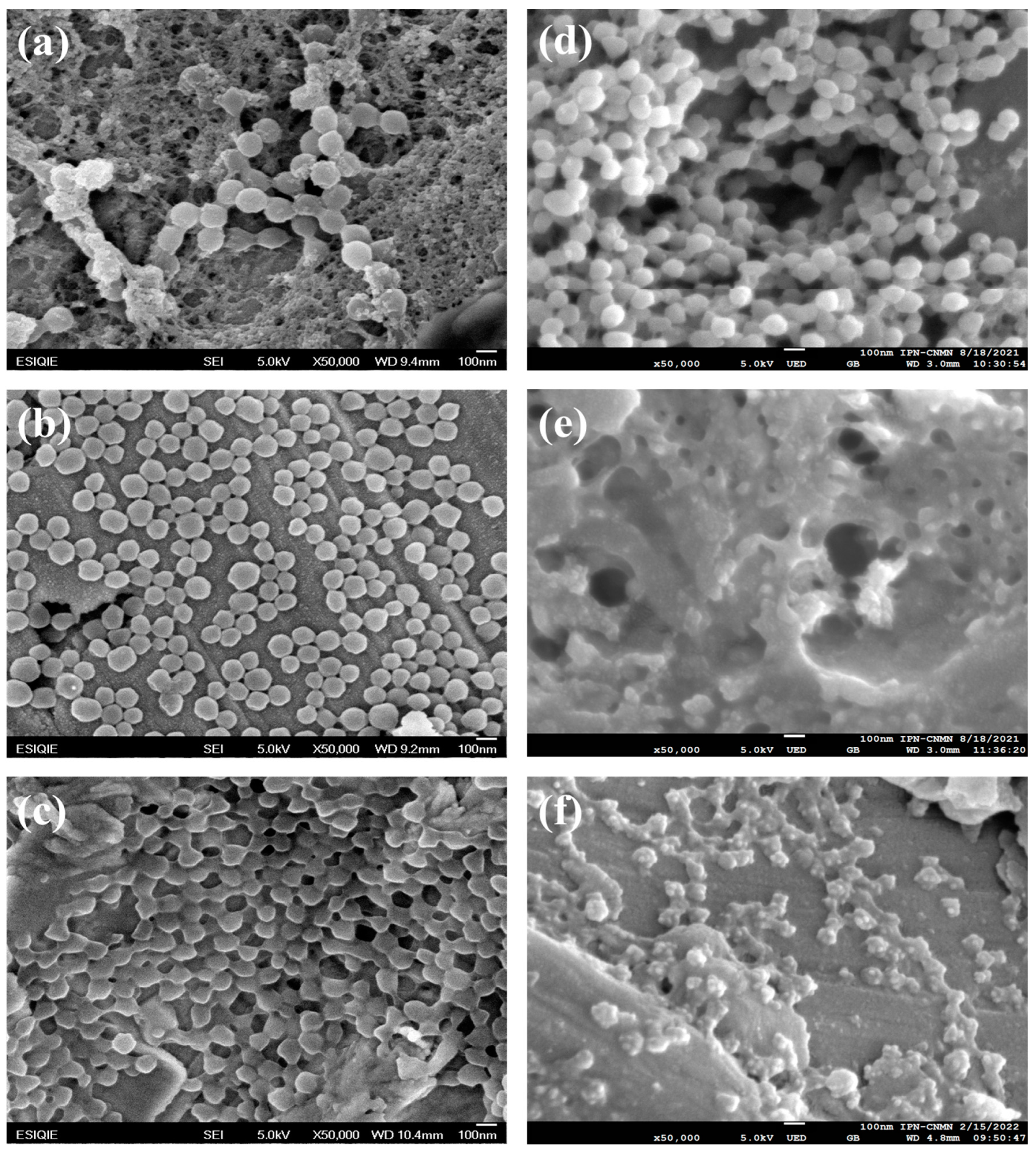
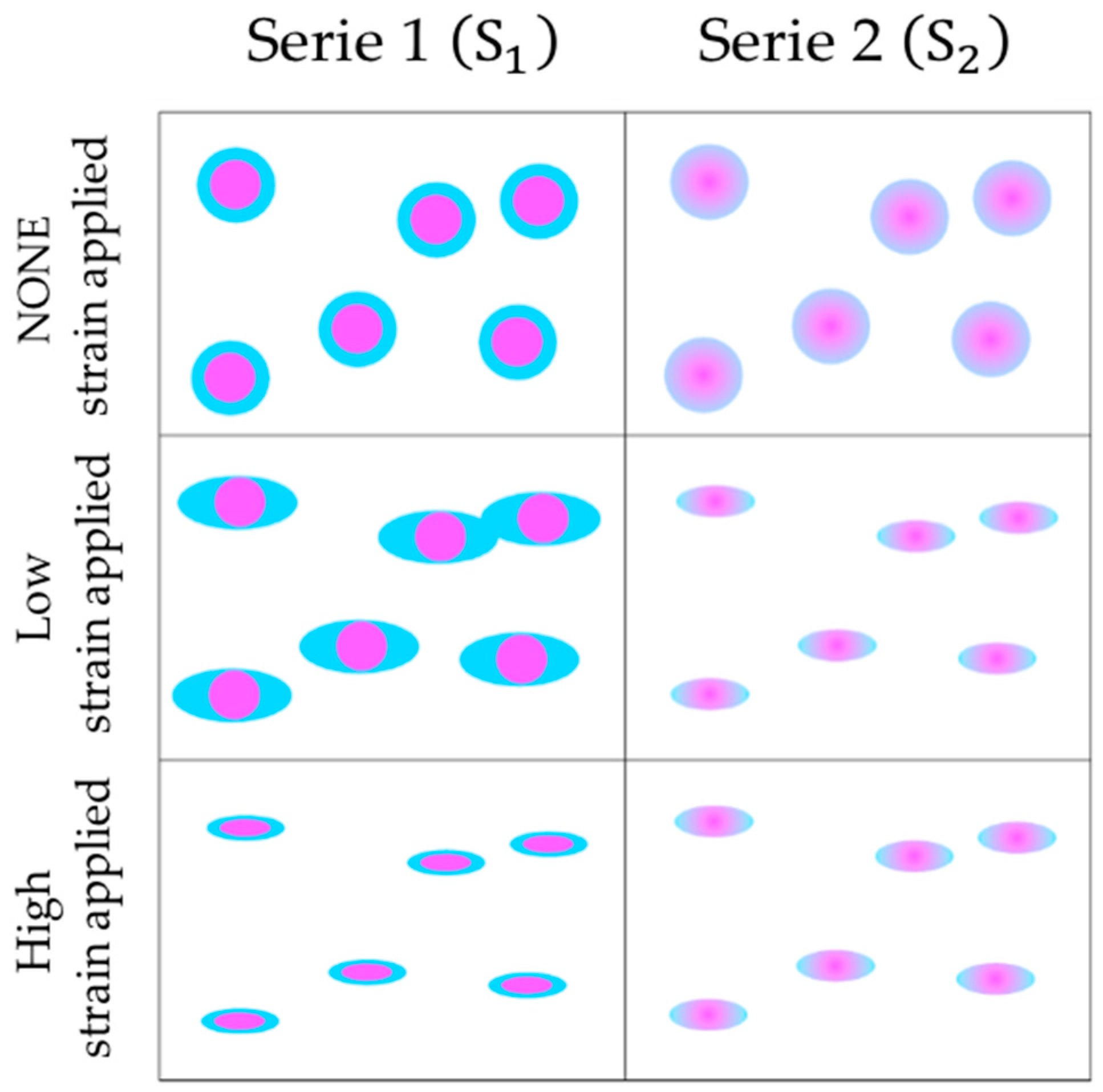
2.2. Morphology of Poly Nanoparticles
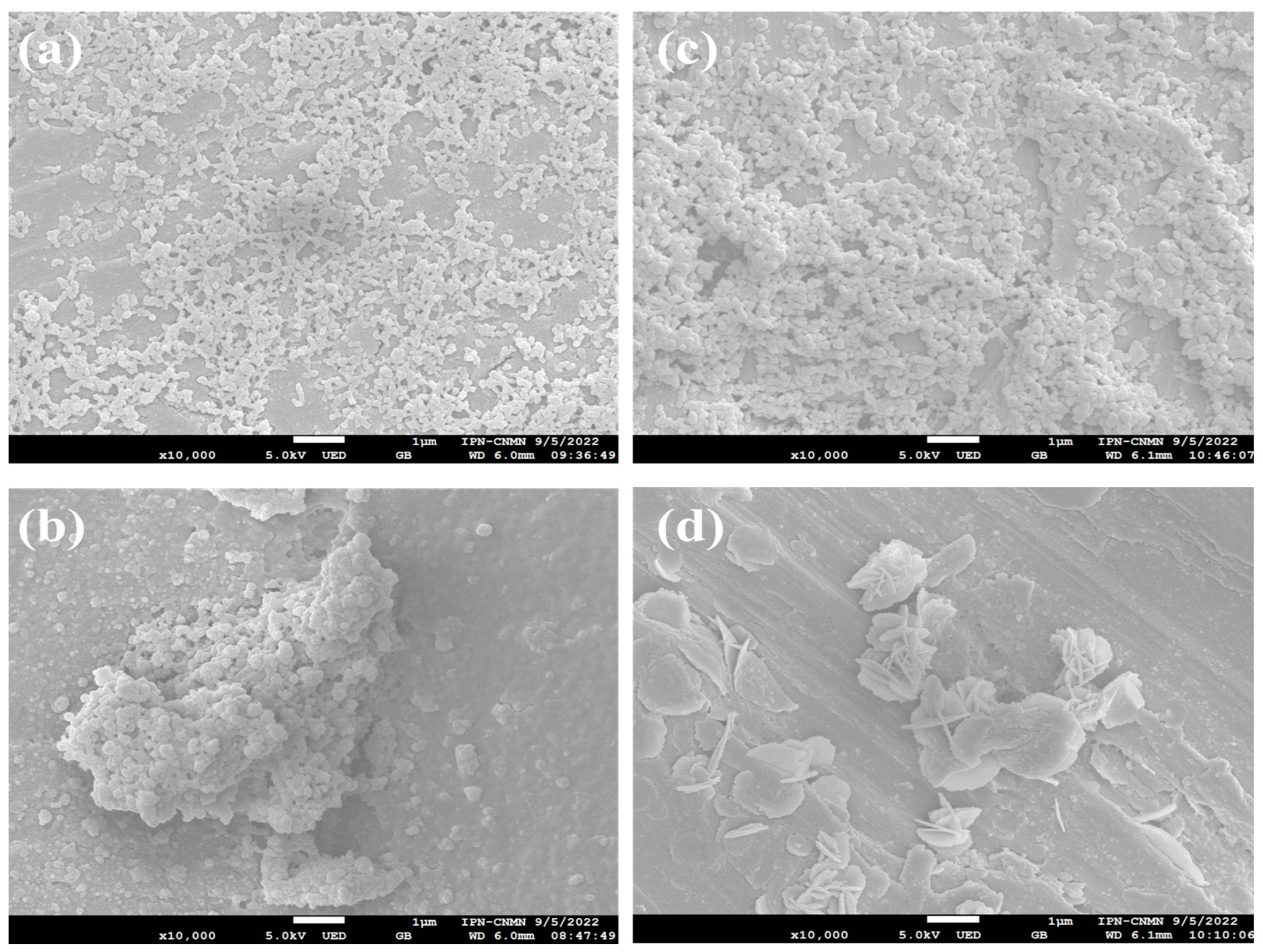
2.3. Morphology of Poly Nanoparticles Titrated with an Electrolyte
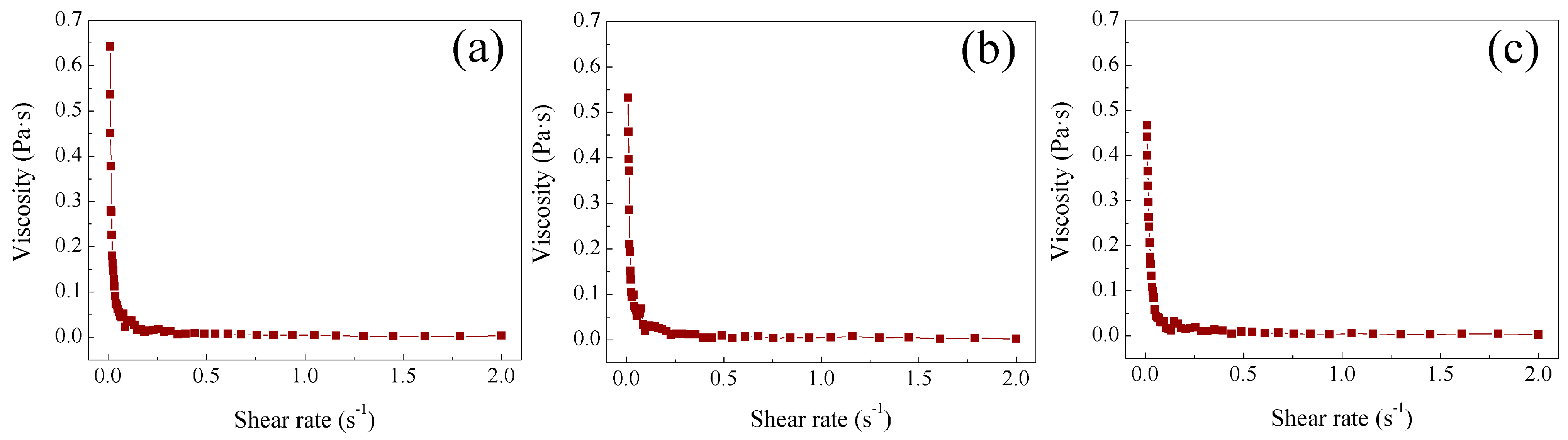
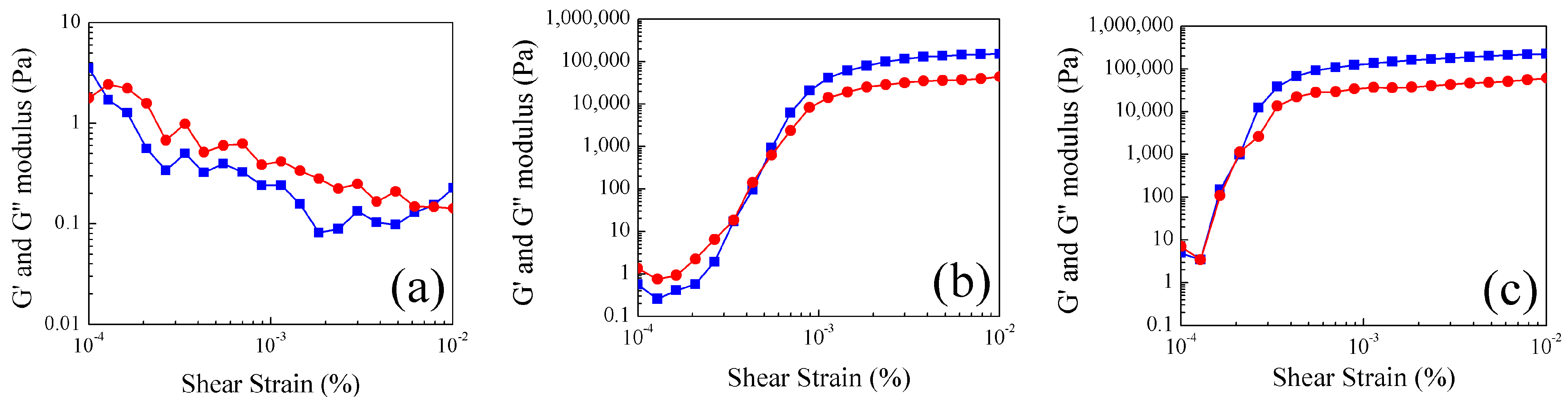
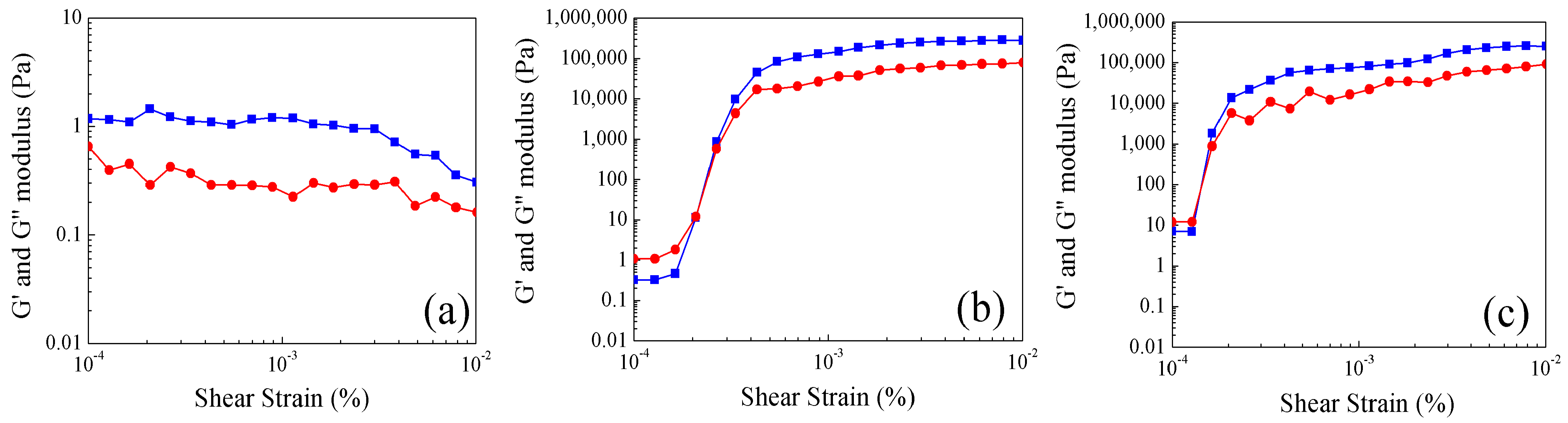
2.4. Study of Rheological Properties at Different Temperatures of Poly Nanoparticles
2.5. Analysis of Poly Nanoparticles by Nuclear Magnetic Resonance
3. Conclusions
4. Materials and Methods
4.1. Synthesis of Polymer Nanoparticles Poly
4.2. Latex Characterization
4.2.1. Dynamic Light Scattering and Zeta Potential (ζ)
4.2.2. Scanning Electron Microscopy
4.2.3. Rheology
4.2.4. Nuclear Magnetic Resonance
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nidhi, J.; Shubam, N.O.; Vaibhav, P.; Kavet, N.K. Smart materials—A state-of-the-art-review. Mater. Today Proc. 2023, 82, 381–389. [Google Scholar] [CrossRef]
- Uddeshya, S.; Kamal, G. Journey of smart material from composite to shape memory alloy (SMA), characterization and their applications-A review. Smart Mater. Med. 2023, 4, 227–242. [Google Scholar] [CrossRef]
- Gong, H.; Yu, C.; Zhang, L.; Xie, G.; Guo, D.; Luo, J. Intelligent lubricating materials: A review. Compos. Part B Eng. 2020, 202, 108450. [Google Scholar] [CrossRef]
- Prata, S.A.; Nascimiento, R.F.; Grosso, C.R.F. Designing polymeric interactions toward smart particles. Curr. Opin. Food Sci. 2022, 46, 100867. [Google Scholar] [CrossRef]
- Hoogendboom, R. Introduction to smart polymers and their applications. In Smart Polymers and Their Applications, 1st ed.; Aguilar, M.R., San Róman, J., Eds.; Elservier: Amsterdam, The Netherlands; Woodhead Publishing in Materials: Cambridge, UK, 2014; pp. 1–11. [Google Scholar] [CrossRef]
- Kocak, G.; Tuncer, C.; Bütün, V. pH-responsive polymers. Polym. Chem. 2017, 8, 144–176. [Google Scholar] [CrossRef]
- Yang, S.; Wang, F.; Han, H.; Santos, H.A.; Zhang, Y.; Zhang, H.; Wei, J.; Cai, Z. Fabricated technology of biomedical micro-nano hydrogel. Biomed. Technol. 2023, 2, 31–48. [Google Scholar] [CrossRef]
- Ofridam, F.; Tarhini, M.; Lebaz, N.; Gagnière, E.; Mangin, D.; Elaïssari, A. pH-sensitive polymers: Classification and some fine potential applications. Polym. Adv. Technol. 2021, 32, 1455–1484. [Google Scholar] [CrossRef]
- Shahbazi, M.; Jäger, H.; Ettelaie, R.; Mohammadi, A.; Kashi, P.A. Multimaterial 3D printing of self-assembling smart thermo-responsive polymers into 4D printed objects: A review. Addit. Manuf. 2023, 71, 103598. [Google Scholar] [CrossRef]
- Moreno, P.S.; De Vicente, J.; Nardecchia, S.; Marchal, J.A.; Boulaiz, H. Thermo-Sensitive Nanomaterials: Recent Advance in Synthesis and Biomedical Applications. Nanomaterials 2018, 8, 935. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, N.; Gardoni, G.; Sponchioni, M.; Moscatelli, D. Thermo-responsive polymers as surface active compounds: A review. Eur. Polym. J. 2023, 198, 112421. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, L.; Lu, L.; Wang, Q.; Benicewicz, B.C. pH and Thermal Dual-Responsive Nanoparticles for Controlled Drug Delivery with High Loading Content. ACS Omega 2017, 2, 3399–3405. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Hasirci, N. Dual-and Multistimuli-Responsive Polymers for Biomedical Applications. In Smart Polymers and Their Applications, 2nd ed.; Elservier: Amsterdam, The Netherlands; Woodhead Publishing in Materials: Cambridge, UK, 2019; pp. 255–278. [Google Scholar] [CrossRef]
- Huang, H.J.; Tsai, Y.L.; Lin, S.H.; Hsu, S.H. Smart polymers for cell therapy and precision medicine. J. Biomed. Sci. 2019, 26, 26–73. [Google Scholar] [CrossRef] [PubMed]
- Sponchionia, M.; Palmiero, U.C.; Moscatelli, D. Thermo-responsive polymers: Applications of smart materials in drug delivery and tissue engineering. Mater. Sci. Eng. C 2019, 102, 589–612. [Google Scholar] [CrossRef]
- Musarurwa, H.; Tavengwa, N.T. Recent progress in the application of pH-responsive polymers in separation science. Microchem. J. 2022, 179, 107503. [Google Scholar] [CrossRef]
- Sircar, A.; Rayavarapu, K.; Bist, N.; Yadav, K.; Singh, S. Applications of nanoparticles in enhanced oil recovery. Pet. Res. 2022, 7, 77–90. [Google Scholar] [CrossRef]
- Stefanescu, E.A. A Study of Rheological and Thermodynamic Properties of Polymer-Clay Gels and Multilayered Films. Doctoral Dissertation, Louisiana State University and Agricultural and Mechanical College, Los Angeles, CA, USA, 2008. Available online: https://repository.lsu.edu/gradschool_dissertations (accessed on 12 May 2024).
- Johns, M.L.; Hollingsworth, K.G. Characterization of emulsion systems using NMR and MRI. Prog. Nucl. Magn. Reson. Spectrosc. 2007, 50, 51–70. [Google Scholar] [CrossRef]
- Agarwal, N.; Nair, M.S.; Mazumder, A.; Poluri, K.M. Characterization of Nanomaterials Using Nuclear Magnetic Resonance Spectroscopy. In Characterization of Nanomaterials: Advances and Key Technologies, 1st. ed.; Sneha, M.B., Oluwatobi, S.O., Nandakumar, K., Sabu, T., Eds.; Elservier: Amsterdam, The Netherlands; Woodhead Publishing in Materials: Cambridge, UK, 2018; pp. 61–102. [Google Scholar] [CrossRef]
- Pamies, R. Polymer Rheology and Processing of Nano- and Micro-Composites. Materials 2022, 15, 7297. [Google Scholar] [CrossRef]
- Ruiz-Virgen, L.; Hernandez-Martinez, M.A.; Martinez-Mejia, G.; Caro-Briones, R.; Del Río, J.M.; Corea, M. Study of Thermodynamic and Rheological Properties of Sensitive Polymeric Nanoparticles as a Possible Application in the Oil Industry. J. Solut. Chem. 2024, 53, 5–27. [Google Scholar] [CrossRef]
- Cautela, J.; Stenqvist, B.; Schillen, K.; Belíc, D.; Månsson, L.K.; Hagemans, F.; Seuss, M.; Fery, A.; Crassous, J.J.; Galantini, L. Supracolloidal Atomium. ACS Nano. 2020, 14, 15748–15756. [Google Scholar] [CrossRef]
- Shymborska, Y.; Stetsyshyn, Y.; Awsiuk, K.; Raczkowska, J.; Bernasik, A.; Janiszewska, N.; Da̧bczyński, P.; Kostruba, A.; Budkowski, A. Temperature- and pH-Responsive Schizophrenic Copolymer Brush Coatings with Enhanced Temperature Response in Pure Water. ACS Appl. Mater. Interfaces 2023, 15, 8676–8690. [Google Scholar] [CrossRef]
- Seuring, J.; Agarwal, S. Polymers with Upper Critical Solution Temperature in Aqueous Solution: Unexpected Properties from Known Building Blocks. ACS Macro Lett. 2013, 2, 597–600. [Google Scholar] [CrossRef]
- Serrano-Ruiz, D.; Alonso-Cristobal, P.; Laurenti, M.; Frick, B.; López-Cabarcos, E.; Rubio-Retama, J. Influence of the inter-chain hydrogen bonds on the thermoresponsive swelling behavior of UCST-like microgels. Polymer 2013, 54, 4963–4971. [Google Scholar] [CrossRef]
- Bazban-Shotorbani, S.; Hasani-Sadrabadi, M.M.; Karkhaneh, A.; Serpooshan, V.; Jacob, K.I.; Moshaverinia, A.; Mahmoudi, M. Revisiting structure-property relationship of pH-responsive polymers for drug delivery applications. J. Control. Release 2017, 253, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Shnoudeh, A.J.; Hamad, I.; Abdo, R.W.; Qadumii, L.; Jaber, A.Y.; Surchi, H.S.; Alkelany, S.Z. Synthesis, Characterization, and Applications of Metal Nanoparticles. In Biomaterials and Bionanotechnology, 1st ed.; Tekade, R.K., Ed.; Elservier: Amsterdam, The Netherlands; Academic Press: London, UK, 2019; pp. 527–612. [Google Scholar] [CrossRef]
- Singh, J.; Nayak, P. pH-responsive polymers for drug delivery: Trends and Opportunities. J Polym. Sci. 2023, 61, 2828–2850. [Google Scholar] [CrossRef]
- Amin, M.; Lammers, T.; Ten Hagen, T.L.M. Temperature-sensitive polymers to promote heat-triggered drug release from liposomes: Towards bypassing EPR. Adv. Drug Deliv. Rev. 2022, 189, 114503. [Google Scholar] [CrossRef]
- Asadujjaman, A.; Kent, B.; Bertin, A. Phase transition and aggregation behaviour of an UCST-type copolymer poly(acrylamide-coacrylonitrile) in water: Effect of acrylonitrile content, concentration in solution, copolymer chain length and presence of electrolyte. Soft Matter. 2017, 13, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Heidari, S.; Esmaeilzadeh, F.; Mowla, D.; Ghasemi, S. Synthesis of an efficient copolymer of acrylamide and acrylic acid and determination of its swelling behavior. J. Pet. Explor. Prod. Technol. 2018, 8, 1331–1340. [Google Scholar] [CrossRef]
- Cheng, W.-M.; Hu, X.-M.; Zhao, Y.-Y.; Wu, M.-Y.; Hu, Z.-X.; Yu, X.-T. Preparation and swelling properties of poly(acrylic acid-co-acrylamide) composite hydrogels. e-Polymers 2017, 17, 95–106. [Google Scholar] [CrossRef]
- Swift, T.; Swanson, L.; Geoghegan, M.; Rimmer, S. The pH-responsive behaviour of poly(acrylic acid) in aqueous solution is dependent on molar mass. Soft Matter. 2016, 12, 2542–2549. [Google Scholar] [CrossRef]
- Li, G.; Zhang, G.; Sun, R.; Wong, C.P. Dually pH-responsive polyelectrolyte complex hydrogel composed of polyacrylic acid and poly (2-(dimthylamino) ethyl methacrylate). Polymer 2016, 107, 332–340. [Google Scholar] [CrossRef]
- Shenoy, A.V. Rheology of Filled Polymer Systems, 1st ed.; Springer: Pune, India, 1999; pp. 1–415. [Google Scholar] [CrossRef]
- Mezger, T.G. The Rheology Handbook, 4th ed.; European Coatings Library: Hanover, Germany, 2014; pp. 17–417. [Google Scholar]
- Hoogendboom, R. Temperature-responsive polymers: Properties, synthesis and applications. In Smart Polymers and Their Applications, 1st ed.; Aguilar, M.R., San Róman, J., Eds.; Elservier: Amsterdam, The Netherlands; Woodhead Publishing in Materials: Cambridge, UK, 2014; pp. 15–44. [Google Scholar] [CrossRef]
- Najafi, M.; Habibi, M.; Fokkink, R.; Hennink, W.E.; Vermonden, T. LCST polymers with UCST behavior. Soft Matter. 2021, 17, 2132–2141. [Google Scholar] [CrossRef]
- Le, M.; Huang, W.; Chen, K.F.; Lin, C.; Cai, L.; Zhang, H.; Jia, Y.G. Upper critical solution temperature polymeric drug carriers. J. Chem. Eng. 2022, 432, 134354. [Google Scholar] [CrossRef]
- Bolisettya, S.; Hoffmanna, M.; Lekkalaa, S.; Hellwega, T.; Ballauffa, M.; Harnau, L. Coupling of Rotational Motion with Shape Fluctuations of Core-shell Microgels Having Tunable Softness. Macromolecules 2009, 42, 1264–1269. [Google Scholar] [CrossRef]
- Miclotte, M.P.J.; Varlas, S.; Reynolds, C.D.; Rashid, B.; Chapman, E.; O’Reilly, R.K. Thermoresponsive Block Copolymer Core−Shell Nanoparticles with Tunable Flow Behavior in Porous Media. ACS Appl. Mater. Interfaces 2022, 14, 54182–54193. [Google Scholar] [CrossRef] [PubMed]
- Zmpitas, J.; Gross, J. Modified Stokes−Einstein Equation for Molecular Self-Diffusion Based on Entropy Scaling. Ind. Eng. Chem. Res. 2021, 60, 4453–4459. [Google Scholar] [CrossRef]
- Costigliola, L.; Heyes, D.M.; Schrøder, T.B.; Dyre, J.C. Revisiting the Stokes-Einstein relation without a hydrodynamic diameter. J. Chem. Phys. 2019, 150, 021101. [Google Scholar] [CrossRef] [PubMed]
- Pitre, L.; Plimmer, M.D.; Sparasci, F.; Himbert, M.E. Determinations of the Boltzmann constant. C. R. Phys. 2019, 20, 129–139. [Google Scholar] [CrossRef]
- Galantini, L.; Giampaolo, S.M.; Maninna, L.; Pavel, N.V.; Viel, S. Study of Intermicellar Interactions and Micellar Sizes in Ionic Micelle Solutions by Comparing Collective Diffusion and Self-Diffusion Coefficients. J. Phys. Chem. B 2004, 108, 4799–4805. [Google Scholar] [CrossRef]
- Chandran, C.V.; Heitjans, P. Solid-State NMR Studies of Lithium Ion Dynamics Across Materials Classes. In Annual Reports on NMR Spectroscopy; Webb, G.A., Ed.; Elservier: Amsterdam, The Netherlands; Academic Press: London, UK, 2016; Volume 89, pp. 1–102. [Google Scholar] [CrossRef]
- Spiechowicz, J.; Marchenko, I.G.; Hänggi, P.; Łuczka, J. Diffusion Coefficient of a Brownian Particle in Equilibrium and Nonequilibrium: Einstein Model and Beyond. Entropy 2023, 25, 42. [Google Scholar] [CrossRef]

 .
.
 .
.
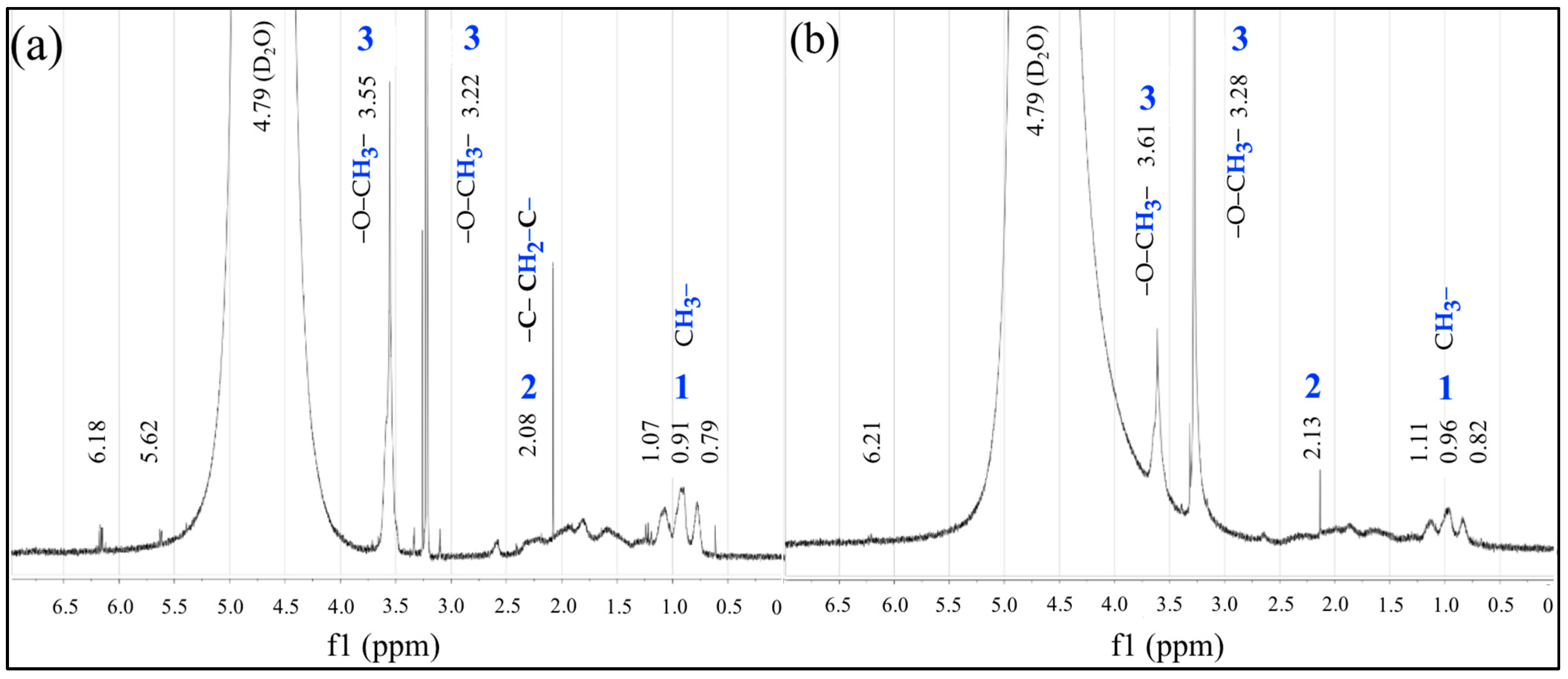

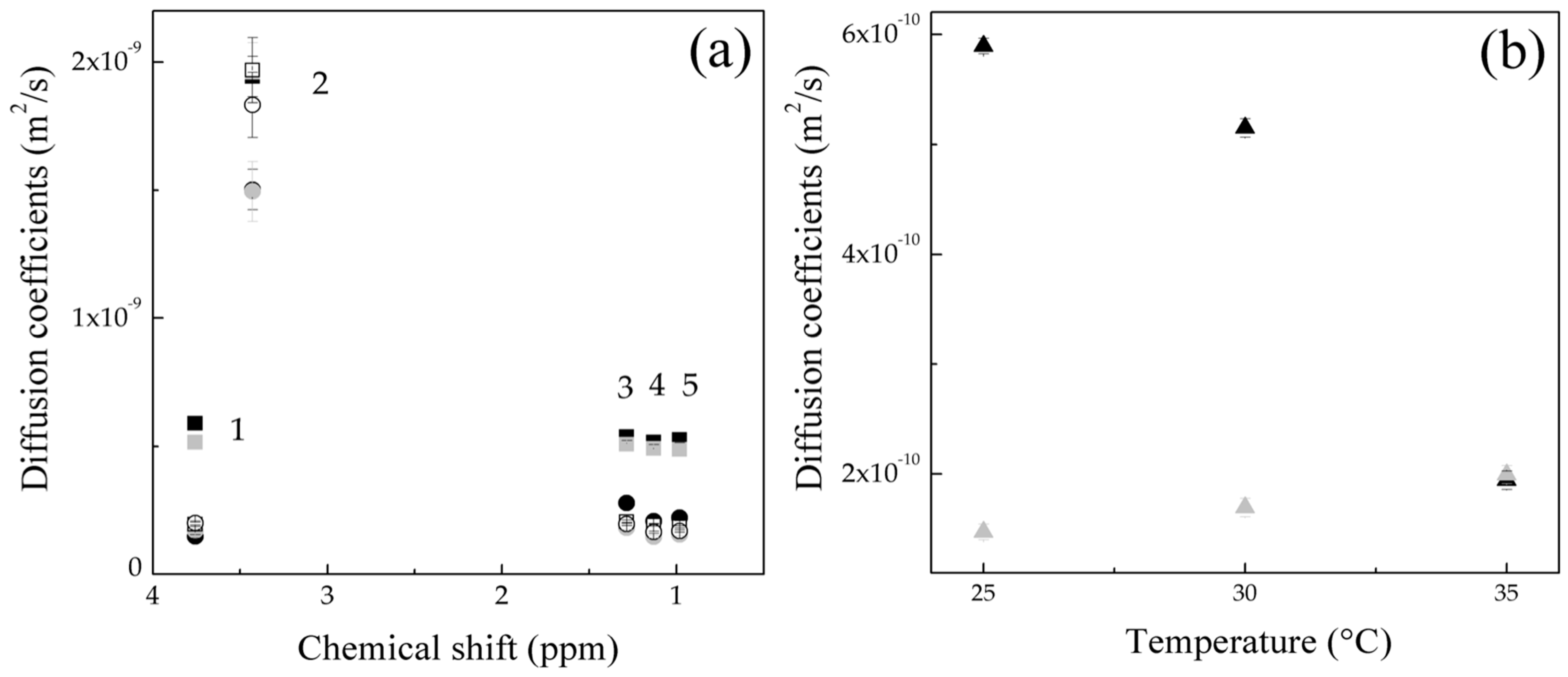
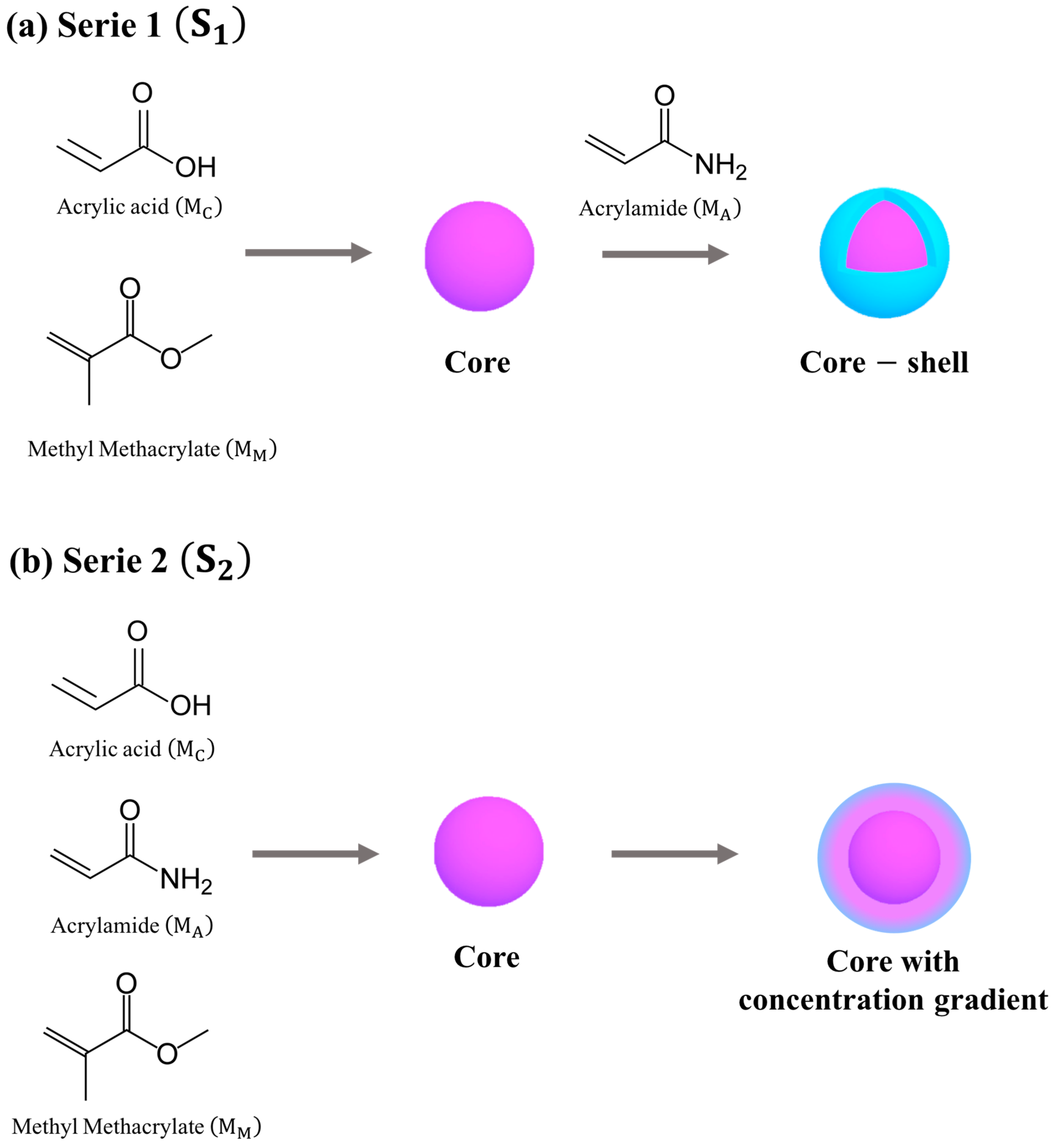
 .
.
 .
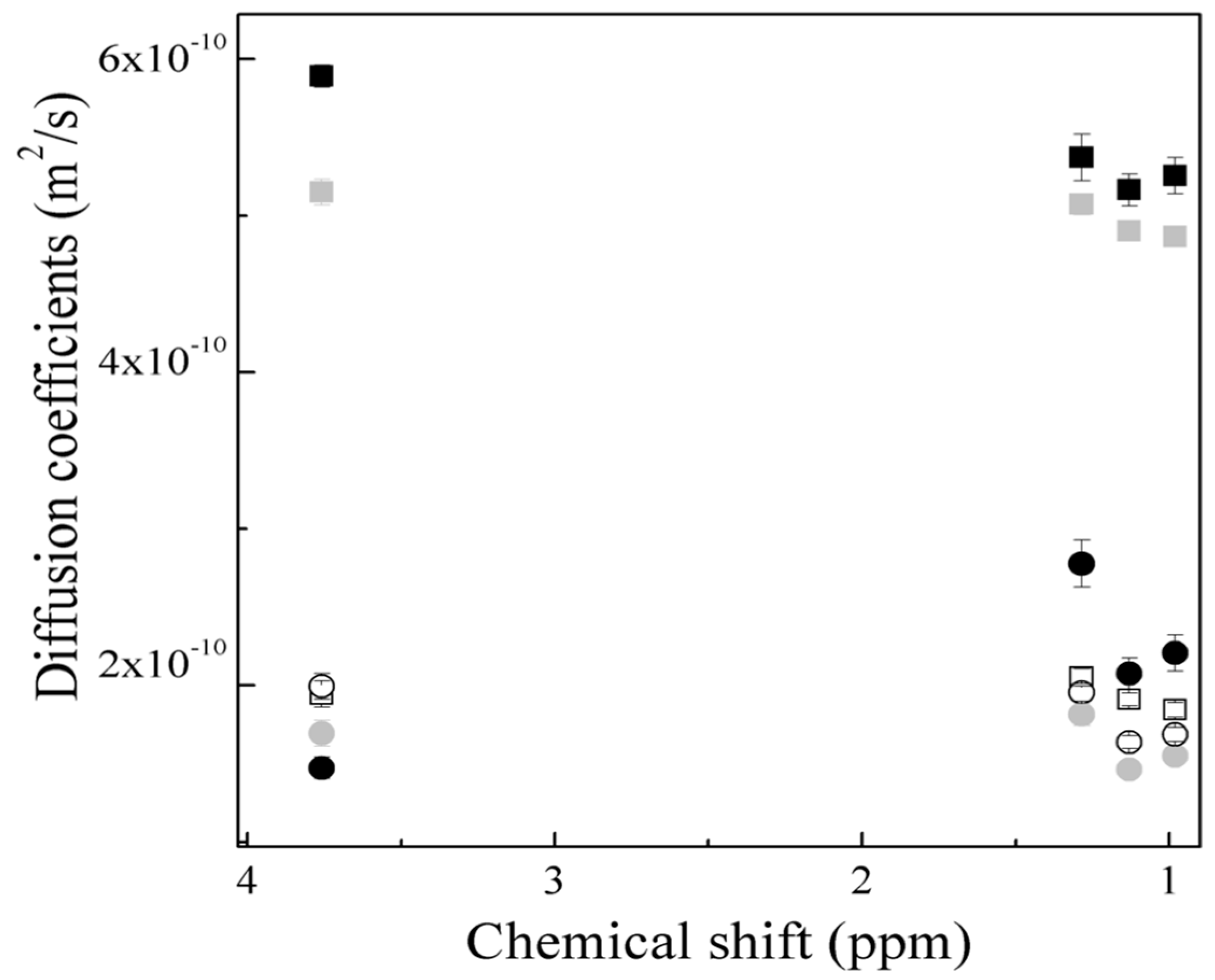
.
| T = 25 °C | T = 25 °C | |
| 1 | 1.47 × 10−10 ± 7.01 × 10−12 | 5.89 × 10−10 ± 7.01 × 10−12 |
| 2 | 1.50 × 10−9 ± 7.89 × 10−11 | 1.94 × 10−9 ± 7.89 × 10−11 |
| 3 | 2.78 × 10−10 ± 1.48 × 10−11 | 5.37 × 10−10 ± 1.48 × 10−11 |
| 4 | 2.07 × 10−10 ± 1.01 × 10−11 | 5.16 × 10−10 ± 1.01 × 10−11 |
| 5 | 2.21 × 10−10 ± 1.16 × 10−11 | 5.26 × 10−10 ± 1.16 × 10−11 |
| T = 30 °C | T = 30 °C | |
| 1 | 1.69 × 10−10 ± 8.41 × 10−12 | 5.15 × 10−10 ± 8.41 × 10−12 |
| 2 | 1.50 × 10−9 ± 1.16 × 10−10 | 1.96 × 10−9 ± 1.16 × 10−10 |
| 3 | 1.81 × 10−10 ± 6.69 × 10−12 | 5.07 × 10−10 ± 6.69 × 10−12 |
| 4 | 1.46 × 10−10 ± 4.12 × 10−12 | 4.90 × 10−10 ± 4.12 × 10−12 |
| 5 | 1.55 × 10−10 ± 5.47 × 10−12 | 4.86 × 10−10 ± 5.47 × 10−12 |
| T = 35 °C | T = 35 °C | |
| 1 | 1.99 × 10−10 ± 8.26 × 10−12 | 1.94 × 10−10 ± 8.26 × 10−12 |
| 2 | 1.83 × 10−9 ± 1.27 × 10−10 | 1.97 × 10−9 ± 1.27 × 10−10 |
| 3 | 1.96 × 10−10 ± 5.58 × 10−12 | 2.05 × 10−10 ± 5.58 × 10−12 |
| 4 | 1.64 × 10−10 ± 4.01 × 10−12 | 1.91 × 10−10 ± 4.01 × 10−12 |
| 5 | 1.68 × 10−10 ± 4.55 × 10−12 | 1.84 × 10−10 ± 4.55 × 10−12 |
| 25 | 1.50 × 10−9 a | 1.94 × 10−9 a |
| 5.82 × 10−15 b | 5.86 × 10−15 b | |
| 30 | 1.50 × 10−9 a | 1.96 × 10−9 a |
| 6.90 × 10−15 b | 6.07 × 10−15 b | |
| 35 | 1.83 × 10−9 a | 1.97 × 10−9 a |
| 8.40 × 10−15 b | 5.78 × 10−15 b | |
| Component | Source and Country | Mass Fraction Purity |
|---|---|---|
| pH-sensitive group: acrylic acid | Sigma–Aldrich (Burlington, MA, USA) | ≥98 a |
| Thermo-sensitive group: acrylamide | Sigma–Aldrich, (Shanghai, China) | ≥98 a |
| Monomer: methyl methacrylate | Poliformas Plásticas (Mexico City, Mexico) | ≥90 a |
| Initiator: sodium persulfate | Sigma–Aldrich, (Burlington, MA, USA) | ≥98 a |
| Electrolyte: calcium chloride | Sigma–Aldrich, (Tokyo, Japan) | ≥93 a |
| Deuterium oxide | Sigma–Aldrich, (Burlington, MA, USA) | ≥99.99 a |
| Surfactant: octylphenol ethoxylate | Solvay, (New York City, NY, USA) | - a |
| Destilled water | Mizu Técnica (Naucalpan de Juárez, Mexico) | - b |
| Parameter | Values |
|---|---|
| Atmosphere: Nitrogen b | ~40 |
| Temperature | ~75 |
| Mechanical stirring ( | ~250 |
| Flow rate | 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Virgen, L.; Hernandez-Martinez, M.A.; Martínez-Mejía, G.; Caro-Briones, R.; Herbert-Pucheta, E.; Río, J.M.d.; Corea, M. Analysis of Structural Changes of pH–Thermo-Responsive Nanoparticles in Polymeric Hydrogels. Gels 2024, 10, 541. https://doi.org/10.3390/gels10080541
Ruiz-Virgen L, Hernandez-Martinez MA, Martínez-Mejía G, Caro-Briones R, Herbert-Pucheta E, Río JMd, Corea M. Analysis of Structural Changes of pH–Thermo-Responsive Nanoparticles in Polymeric Hydrogels. Gels. 2024; 10(8):541. https://doi.org/10.3390/gels10080541
Chicago/Turabian StyleRuiz-Virgen, Lazaro, Miguel Angel Hernandez-Martinez, Gabriela Martínez-Mejía, Rubén Caro-Briones, Enrique Herbert-Pucheta, José Manuel del Río, and Mónica Corea. 2024. "Analysis of Structural Changes of pH–Thermo-Responsive Nanoparticles in Polymeric Hydrogels" Gels 10, no. 8: 541. https://doi.org/10.3390/gels10080541
APA StyleRuiz-Virgen, L., Hernandez-Martinez, M. A., Martínez-Mejía, G., Caro-Briones, R., Herbert-Pucheta, E., Río, J. M. d., & Corea, M. (2024). Analysis of Structural Changes of pH–Thermo-Responsive Nanoparticles in Polymeric Hydrogels. Gels, 10(8), 541. https://doi.org/10.3390/gels10080541









