Development of High-Efficiency Fertilizer by Hydrogels Obtained from Cassava Starch and Citric Acid for Slow Release of Ammonium and Potassium
Abstract
1. Introduction
2. Results and Discussion
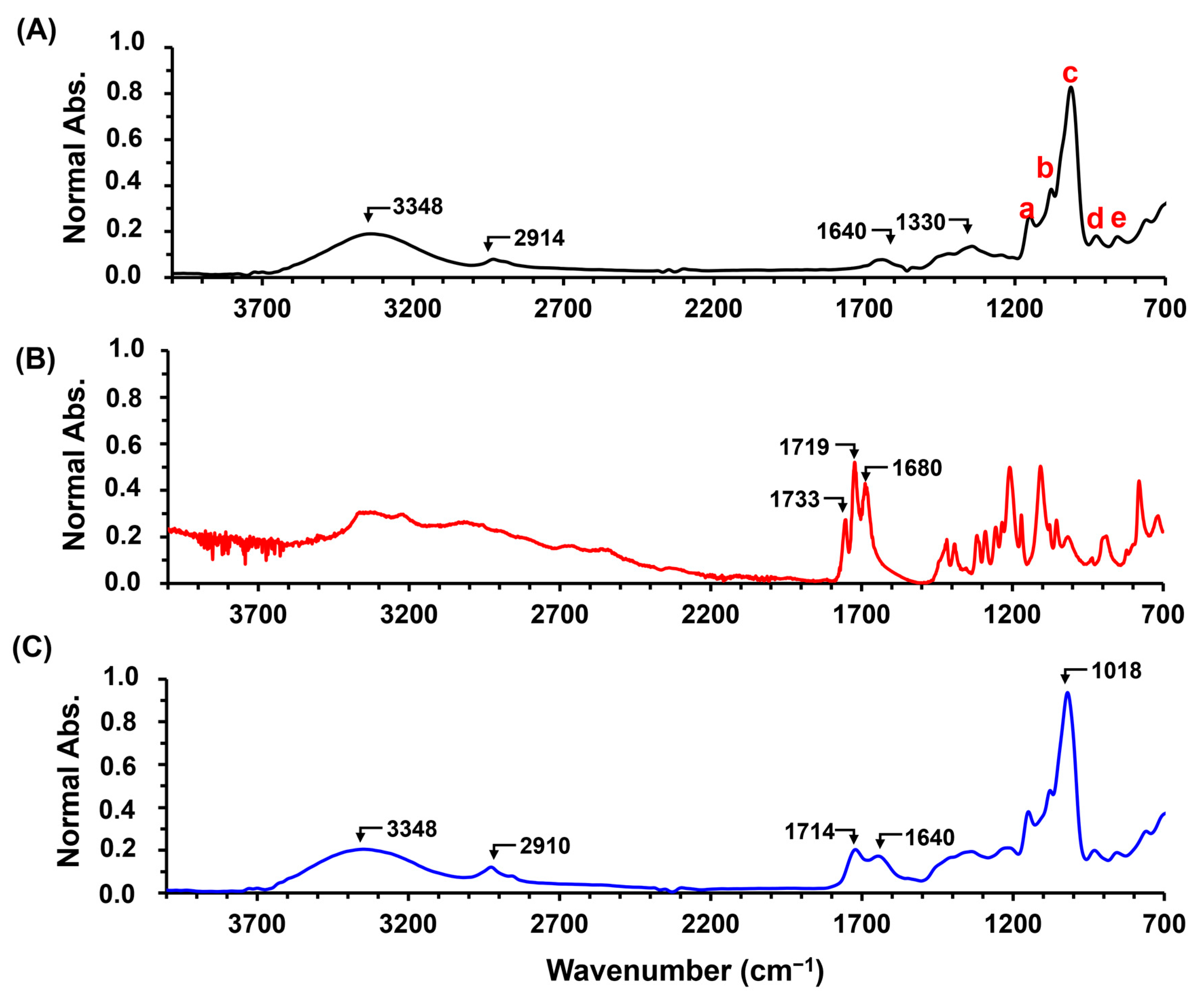
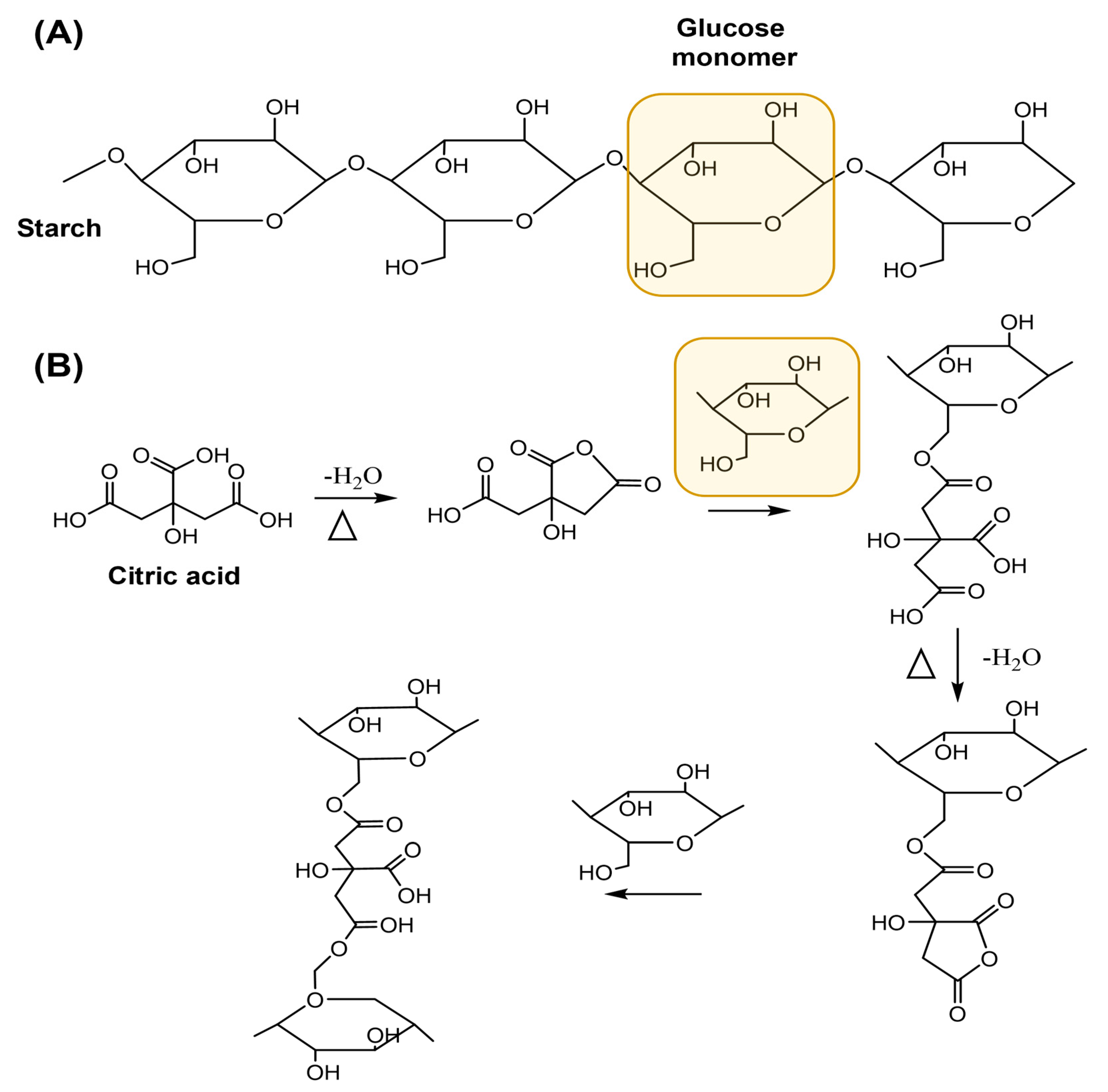
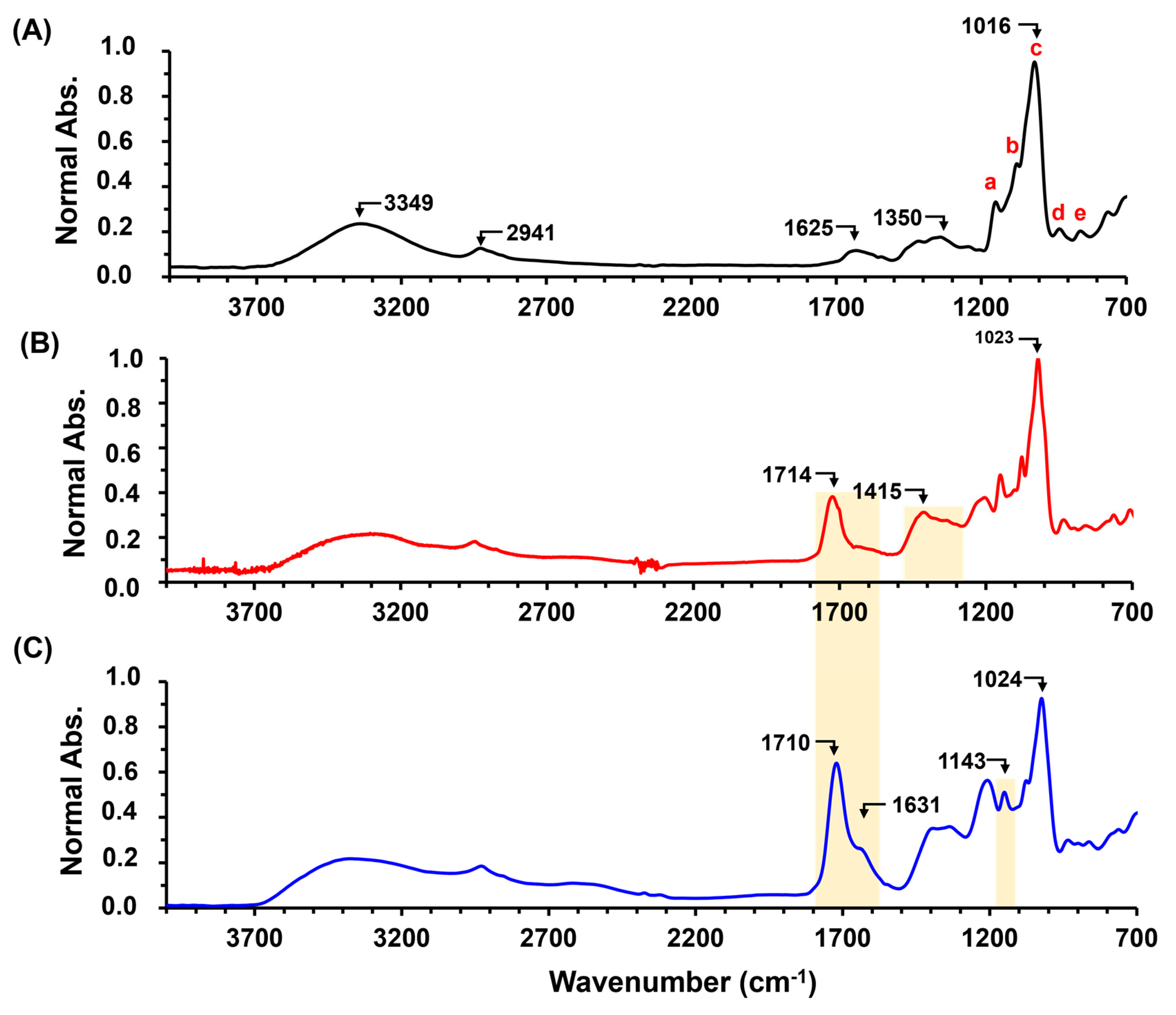
2.1. Hydrogel Characterization
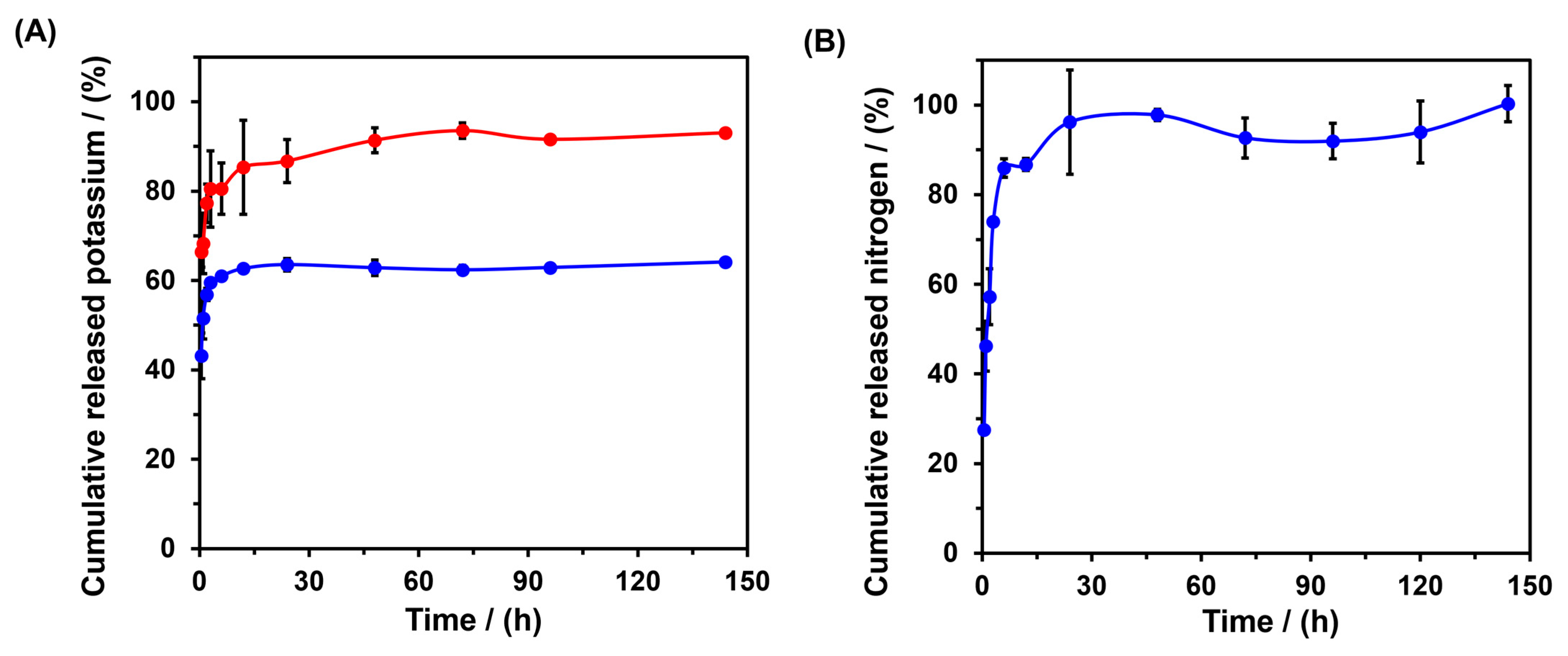
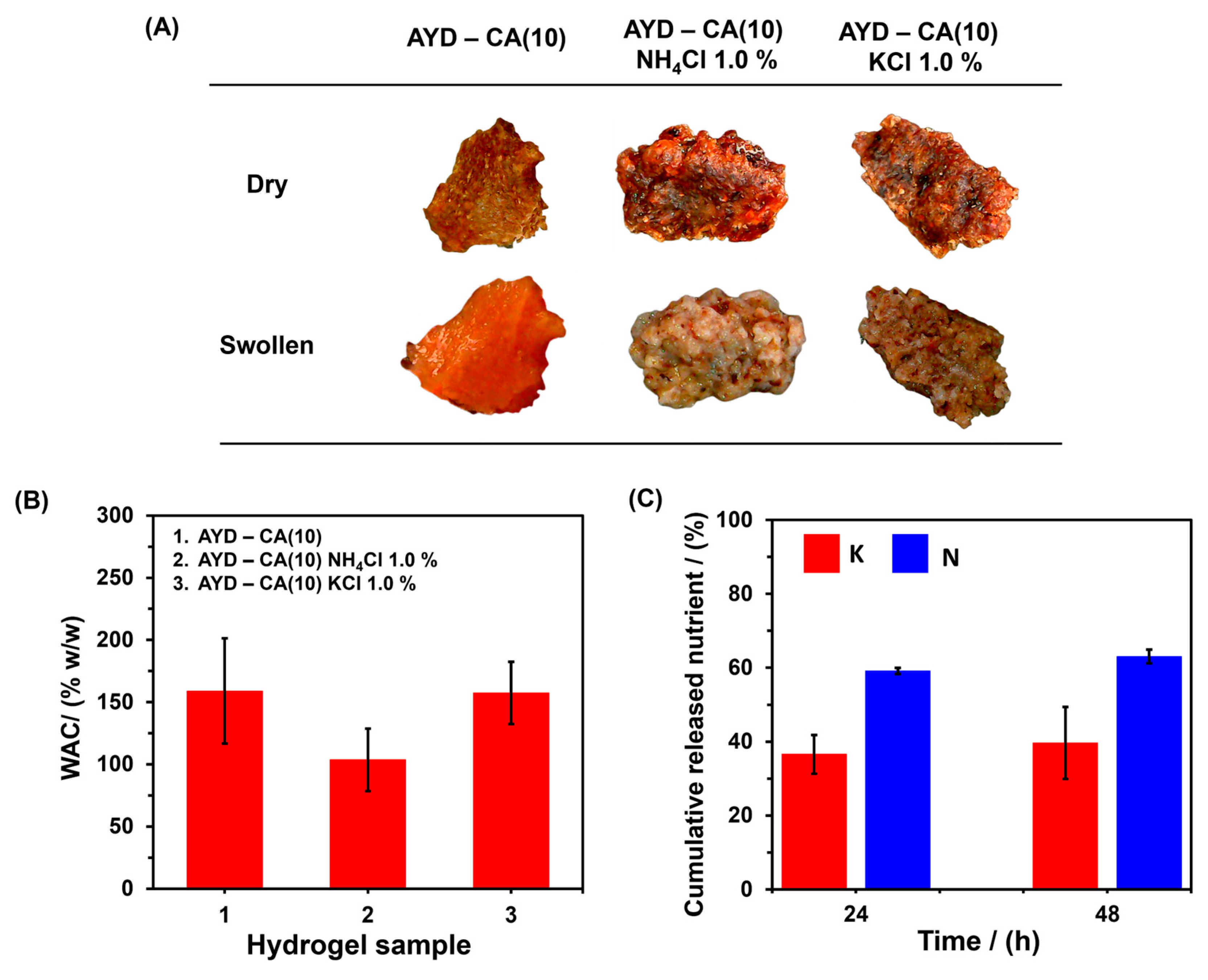
2.2. Release Studies In Vitro of Potassium and Nitrogen
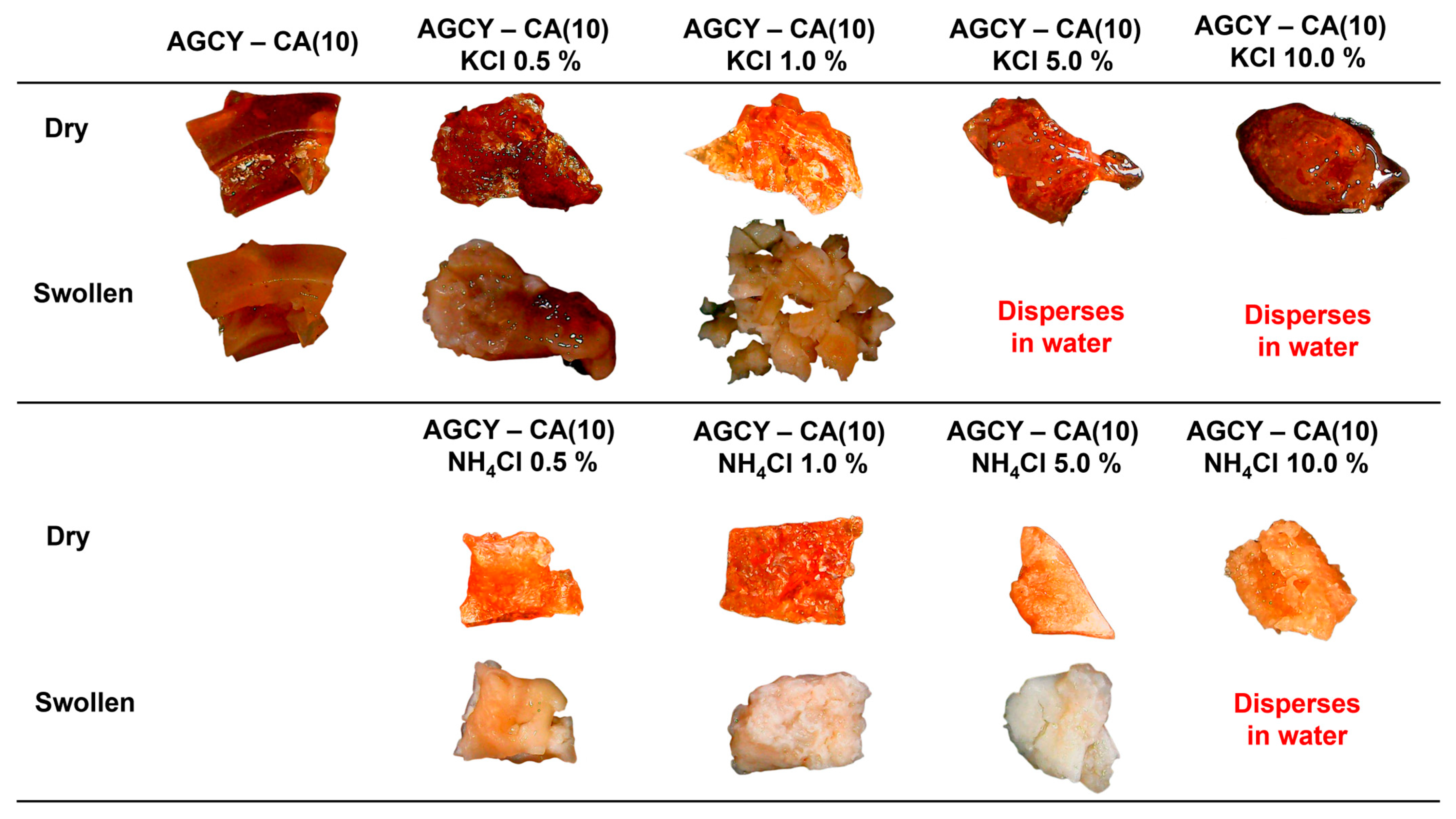
2.3. Production and Application of Economic Starch—CA Hydrogel
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Starch-CA Hydrogels
4.3. Swelling Studies
4.4. Physicochemical and Morphological Characterizations of the Hydrogels
4.5. Preparation of AGCY-CA with Potassium and Nitrogen Nutrients Entrapped
4.6. Nutrients Release Kinetics—In Vitro Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations, World Population Prospects 2019: Highlights. 2019. Available online: https://population.un.org/wpp/Publications/Files/WPP2019_10KeyFindings.pdf (accessed on 22 May 2024).
- Chakraborty, R.; Mukhopadhyay, A.; Sarkar, P.; Mukhopadhyay, R. Nanocomposite-based smart fertilizers: A boon to agricultural and environmental sustainability. Sci. Total Environ. 2023, 863, 160859. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.T.; Kennedy, I.R. Nitrogen Fertilizer Losses from Rice Soils and Control of Environmental Pollution Problems. Commun. Soil Sci. Plant Anal. 2005, 36, 1625. [Google Scholar] [CrossRef]
- Li, M.; Gao, L.; White, J.C.; Haynes, C.L.; O’Keefe, T.L.; Rui, Y.; Zhang, P. Nano-enabled strategies to enhance biological nitrogen fixation. Nat. Nanotechnol. 2023, 18, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Amodeo, G.; Giacometti, R.; Spagnoletti, F.; Santagapita, P.R.; Perullini, M. Eco-friendly routes for obtaining nanoparticles and their application in agro-industry. In Nano-Enabled Agrochemicals in Agriculture; Ghorbanpour, M., Muhammad Shahid, M.A., Eds.; Elsevier: London, UK, 2022; pp. 49–62. [Google Scholar] [CrossRef]
- Narjary, B.; Aggarwal, P.; Singh, A.; Chakraborty, D.; Singh, R. Water availability in different soils in relation to hydrogel application. Geoderma 2012, 187, 94. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Aouada, F.A.; Fajardo, A.R.; Martins, A.F.; Paulino, A.T.; Davi, M.F.; Muniz, E.C. Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: A review. Eur. Polym. J. 2015, 72, 365. [Google Scholar] [CrossRef]
- Liu, M.; Liang, R.; Zhan, F.; Liu, Z.; Niu, A. Preparation of superabsorbent slow-release nitrogen fertilizer by inverse suspension polymerization. Polym. Int. 2007, 56, 729. [Google Scholar] [CrossRef]
- Tyliszczak, B.; Polaczek, J.; Pielichowski, J.; Pielichowski, K. Preparation and Properties of Biodegradable Slow-Release PAA Superabsorbent Matrixes for Phosphorus Fertilizers. Macromol. Symp. 2009, 279, 236. [Google Scholar] [CrossRef]
- Ramli, R.A. Slow-release fertilizer hydrogels: A review. Polym. Chem. 2019, 10, 6073. [Google Scholar] [CrossRef]
- Zhao, L.Z.; Zhou, C.H.; Wang, J.; Tong, D.S.; Yu, W.H.; Wang, H. Recent advances in clay mineral-containing nanocomposite hydrogels. J. Soft Matter 2015, 11, 9229. [Google Scholar] [CrossRef]
- Michalik, R.; Wandzik, I.A. Mini-Review on Chitosan-Based Hydrogels with Potential for Sustainable Agricultural Applications. Polymers 2020, 12, 2425. [Google Scholar] [CrossRef]
- Klein, M.; Poverenov, E. Natural biopolymer-based hydrogels for use in food and agriculture. J. Sci. Food Agric. 2020, 100, 2337. [Google Scholar] [CrossRef]
- Alharbi, K.; Ghoneim, A.; Ebid, A.; El-Hamshary, H.; El-Newehy, M.H. Controlled release of phosphorous fertilizer bound to carboxymethyl starch-g-polyacrylamide and maintaining a hydration level for the plant. Int. J. Biol. Macromol. 2018, 116, 224. [Google Scholar] [CrossRef]
- Ismail, H.; Irani, M.; Ahmad, Z. Starch-Based Hydrogels: Present Status and Applications. Int. J. Polym. Mater. 2013, 62, 411. [Google Scholar] [CrossRef]
- Qamruzzaman, M.; Ahmed, F.; Mondal, M.I.H. An Overview on Starch-Based Sustainable Hydrogels: Potential Applications and Aspects. J. Environ. Polym. Degrad. 2022, 30, 190. [Google Scholar] [CrossRef]
- Garces, V.; García-Quintero, A.; Lerma, T.; Palencia, M.; Combatt, E. Characterization of Cassava Starch and Its Structural Changes Resulting of Thermal Stress by Functionally-Enhanced Derivative Spectroscopy (FEDS). Polysaccharides 2021, 2, 866–877. [Google Scholar] [CrossRef]
- Nikonenko, N.; Buslov, D.; Sushiko, N.; Zhbankov, R. Investigation of stretching vibrations of glycosidic linkages in disaccharides and polysaccarides with use of IR spectra deconvolution. Biopolymers 2000, 57, 257. [Google Scholar] [CrossRef]
- Ghorpade, V.S.; Yadav, A.V.; Dias, R.J. Citric acid crosslinked cyclodextrin/hydroxypropylmethylcellulose hydrogel films for hydrophobic drug delivery. Int. J. Biol. Macromol. 2016, 93, 75. [Google Scholar] [CrossRef] [PubMed]
- Uliniuc, A.; Hamaide, T.; Popa, M.; Băcăiță, S. Modified Starch-Based Hydrogels Cross-Linked with Citric Acid and their use as Drug Delivery Systems for Levofloxacin. Soft Mater. 2013, 11, 483–493. [Google Scholar] [CrossRef]
- Hassan, M.M.; Tucker, N.; Le Guen, M.J. Thermal, mechanical and viscoelastic properties of citric acid-crosslinked starch/cellulose composite foams. Carbohydr. Polym. 2020, 230, 115675. [Google Scholar] [CrossRef]
- Seligra, P.G.; Jaramillo, C.M.; Famá, L.; Goyanes, S. Biodegradable and non-retrogradable eco-films based on starch–glycerol with citric acid as crosslinking agent. Carbohydr. Polym. 2016, 138, 66. [Google Scholar] [CrossRef]
- Zhou, J.; Tong, J.; Su, X.; Ren, L. Hydrophobic starch nanocrystals preparations through crosslinking modification using citric acid. Int. J. Biol. Macromol. 2016, 91, 1186. [Google Scholar] [CrossRef]
- Shen, L.; Xu, H.; Kong, L.; Yang, Y. Non-Toxic Crosslinking of Starch Using Polycarboxylic Acids: Kinetic Study and Quantitative Correlation of Mechanical Properties and Crosslinking Degrees. J. Environ. Polym. Degrad. 2015, 23, 588. [Google Scholar] [CrossRef]
- Golachowski, A.; Drożdż, W.; Golachowska, M.; Kapelko-Żeberska, M.; Raszewski, B. Production and Properties of Starch Citrates—Current Research. Foods 2020, 9, 1311. [Google Scholar] [CrossRef]
- Xie, X.; Liu, Q. Development and Physicochemical Characterization of New Resistant Citrate Starch from Different Corn Starches. Starch-Stärke 2004, 56, 364. [Google Scholar] [CrossRef]
- Nusrat, Z.; Tahira, M.A.; Abid, H. Comparative study on citric acid modified instant starches (alcoholic alkaline treated) isolated from white sorghum and corn grains. Int. J. Biol. Macromol. 2020, 150, 1331. [Google Scholar] [CrossRef]
- Kapelko-Żeberska, M.; Zięba, T.; Pietrzak, W.; Gryszkin, A. Effect of citric acid esterification conditions on the properties of the obtained resistant starch. Int. J. Food Sci. Technol. 2016, 51, 1647. [Google Scholar] [CrossRef]
- Mei, J.Q.; Zhou, D.N.; Jin, Z.Y.; Xu, X.M.; Chen, H.Q. Effects of citric acid esterification on digestibility, structural and physicochemical properties of cassava starch. Food Chem. 2015, 187, 378. [Google Scholar] [CrossRef]
- Pajarito, B.B.; Llorens, C.; Tsuzuki, T. Effects of ammonium chloride on the yield of carbon nanofiber aerogels derived from cellulose nanofibrils. Cellulose 2019, 26, 7727. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, J.; Luo, S.J.; Liu, C.M.; Liu, W. Effect of food additives on starch retrogradation: A review. Starch-Stärke 2015, 67, 69. [Google Scholar] [CrossRef]
- Beck, B.M.; Jekle, M.; Becker, T. Starch re-crystallization kinetics as a function of various cations. Starch-Stärke 2011, 63, 792. [Google Scholar] [CrossRef]
- Oosten, J. Tentative Hypothesis to Explain How Electrolytes Affect the Gelatinization Temperature of Starches in Water. Starch-Stärke 1982, 34, 233. [Google Scholar] [CrossRef]
- León, O.; Soto, D.; Antúnez, A.; Fernández, R.; González, J.; Piña, C.; Fernandez-García, M. Hydrogels based on oxidized starches from different botanical sources for release of fertilizers. Int. J. Biol. Macromol. 2019, 136, 813. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. JCR 1987, 5, 23. [Google Scholar] [CrossRef]
- Ganji, F.; Vasheghani, F.S.; Vasheghani, F.E. Theoretical Description of Hydrogel Swelling: A Review. Iran. Polym. J. 2010, 19, 375. [Google Scholar]
- Langer, R.S.; Peppas, N.A. Present and future applications of biomaterials in controlled drug delivery systems. Biomaterials 1981, 2, 201. [Google Scholar] [CrossRef]
- Wang, J.; Wu, W.; Lin, Z.J. Kinetics and thermodynamics of the water sorption of 2-hydroxyethyl methacrylate/styrene copolymer hydrogels. Appl. Polym. Sci. 2008, 109, 3018. [Google Scholar] [CrossRef]
- Palencia, M.; Mora, M.; Palencia, S. Biodegradable Polymer Hydrogels Based in Sorbitol and Citric Acid for Controlled Release of Bioactive Substances from Plants (Polyphenols). Curr. Chem. Biol. 2017, 11, 36. [Google Scholar] [CrossRef]
- The Colombian Institute of Technical Standards and Certification, ICONTEC, Soil Quality. Quantitative Methods to Determine Water Soluble Potassium, in Fertilizers and Material Sources for Fertilizers Production, NTC 202. 2001. Available online: https://tienda.icontec.org/gp-metodos-cuantitativos-para-la-determinacion-de-potasio-soluble-en-agua-en-abonos-o-fertilizantes-y-fuentes-de-materias-para-su-fabricacion-ntc202-2001.html (accessed on 23 May 2024).
- The Colombian Institute of Technical Standards and Certification, ICONTEC, Soil Quality. Determination of Cationic Exchange Capacity, NTC 5268. 2014. Available online: https://tienda.icontec.org/ (accessed on 23 May 2024).
- Bauli, C.R.; Lima, G.F.; de Souza, A.G.; Ferreira, R.R.; Rosa, D.S. Eco-friendly carboxymethyl cellulose hydrogels filled with nanocellulose or nanoclays for agriculture applications as soil conditioning and nutrient carrier and their impact on cucumber growing. Coll. Surf. A 2021, 623, 126771. [Google Scholar] [CrossRef]
- Lakshani, N.; Wijerathne, H.S.; Sandaruwan, C.; Kottegoda, N.; Karunarathne, V. Release Kinetic Models and Release Mechanisms of Controlled-Release and Slow-Release Fertilizers. ACS Agric. Sci. Technol. 2023, 3, 939–956. [Google Scholar] [CrossRef]



| Mathematical Models | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fertilizer/Concentration | Zero-Order | First-Order | Higuchi | Power-Law | |||||
| k0 () | R2 | k1 () | R2 | kH () | R2 | K () | R2 | n | |
| KCl/0.5% | 3.4 | 0.7747 | 7.0 | 0.8157 | 1.0 | 0.9005 | 7.81 | 0.9447 | 0.13 |
| KCl/1.0% | 1.7 | 0.2154 | 1.5 | 0.2662 | 0.7 | 0.3958 | 1.49 | 0.995 | 0.14 |
| NH4Cl/5% | 1.2 | 0.8564 | 2.7 | 0.8907 | 3.92 | 0.8986 | 4.21 | 0.9791 | 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamorro, A.F.; Palencia, M.; Arrieta, Á.A. Development of High-Efficiency Fertilizer by Hydrogels Obtained from Cassava Starch and Citric Acid for Slow Release of Ammonium and Potassium. Gels 2024, 10, 434. https://doi.org/10.3390/gels10070434
Chamorro AF, Palencia M, Arrieta ÁA. Development of High-Efficiency Fertilizer by Hydrogels Obtained from Cassava Starch and Citric Acid for Slow Release of Ammonium and Potassium. Gels. 2024; 10(7):434. https://doi.org/10.3390/gels10070434
Chicago/Turabian StyleChamorro, Andrés F., Manuel Palencia, and Álvaro A. Arrieta. 2024. "Development of High-Efficiency Fertilizer by Hydrogels Obtained from Cassava Starch and Citric Acid for Slow Release of Ammonium and Potassium" Gels 10, no. 7: 434. https://doi.org/10.3390/gels10070434
APA StyleChamorro, A. F., Palencia, M., & Arrieta, Á. A. (2024). Development of High-Efficiency Fertilizer by Hydrogels Obtained from Cassava Starch and Citric Acid for Slow Release of Ammonium and Potassium. Gels, 10(7), 434. https://doi.org/10.3390/gels10070434







