Aerogels of Polypyrrole/Tannic Acid with Nanofibrillated Cellulose for the Removal of Hexavalent Chromium Ions
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization
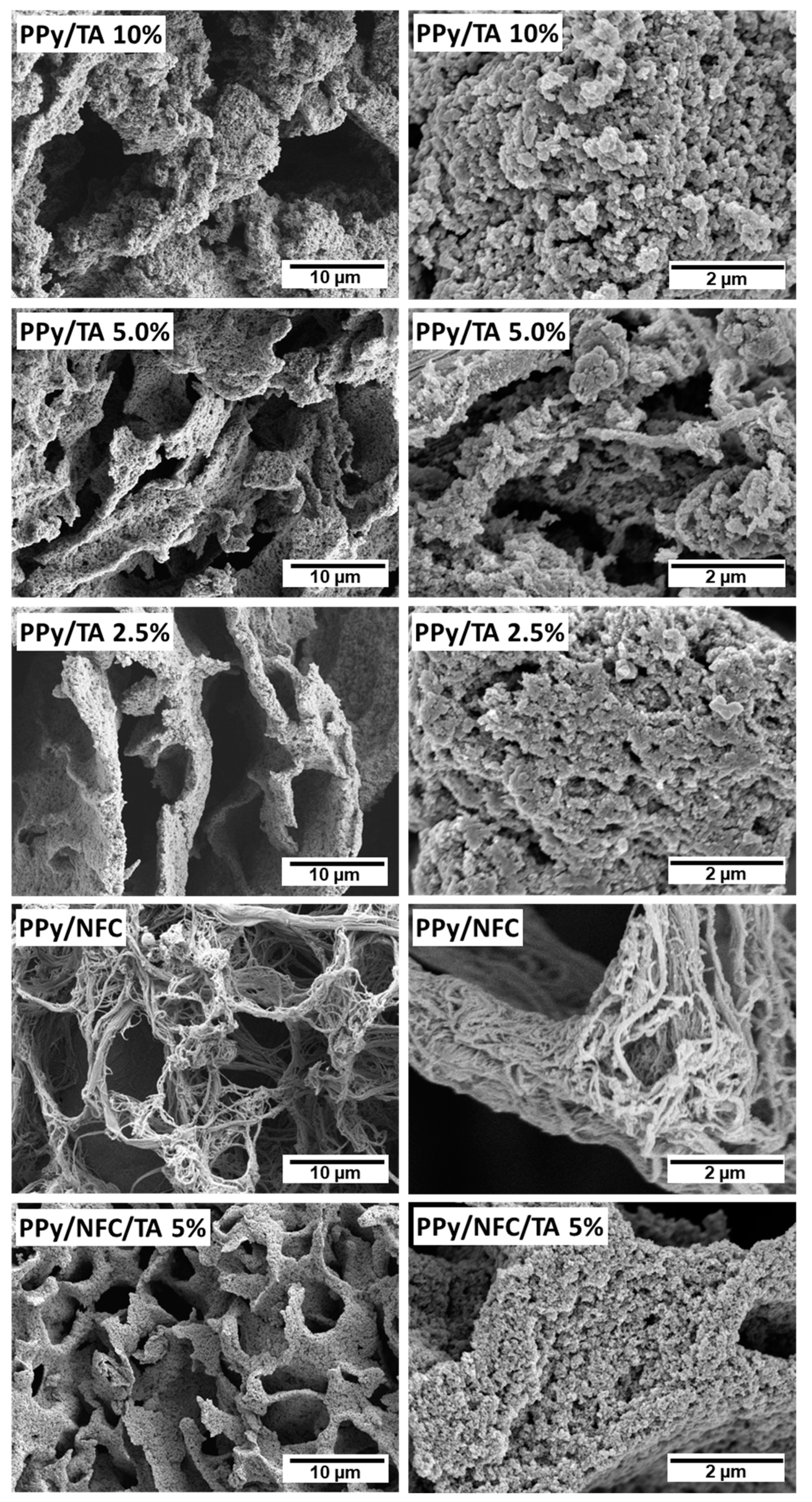
2.1.1. Morphology
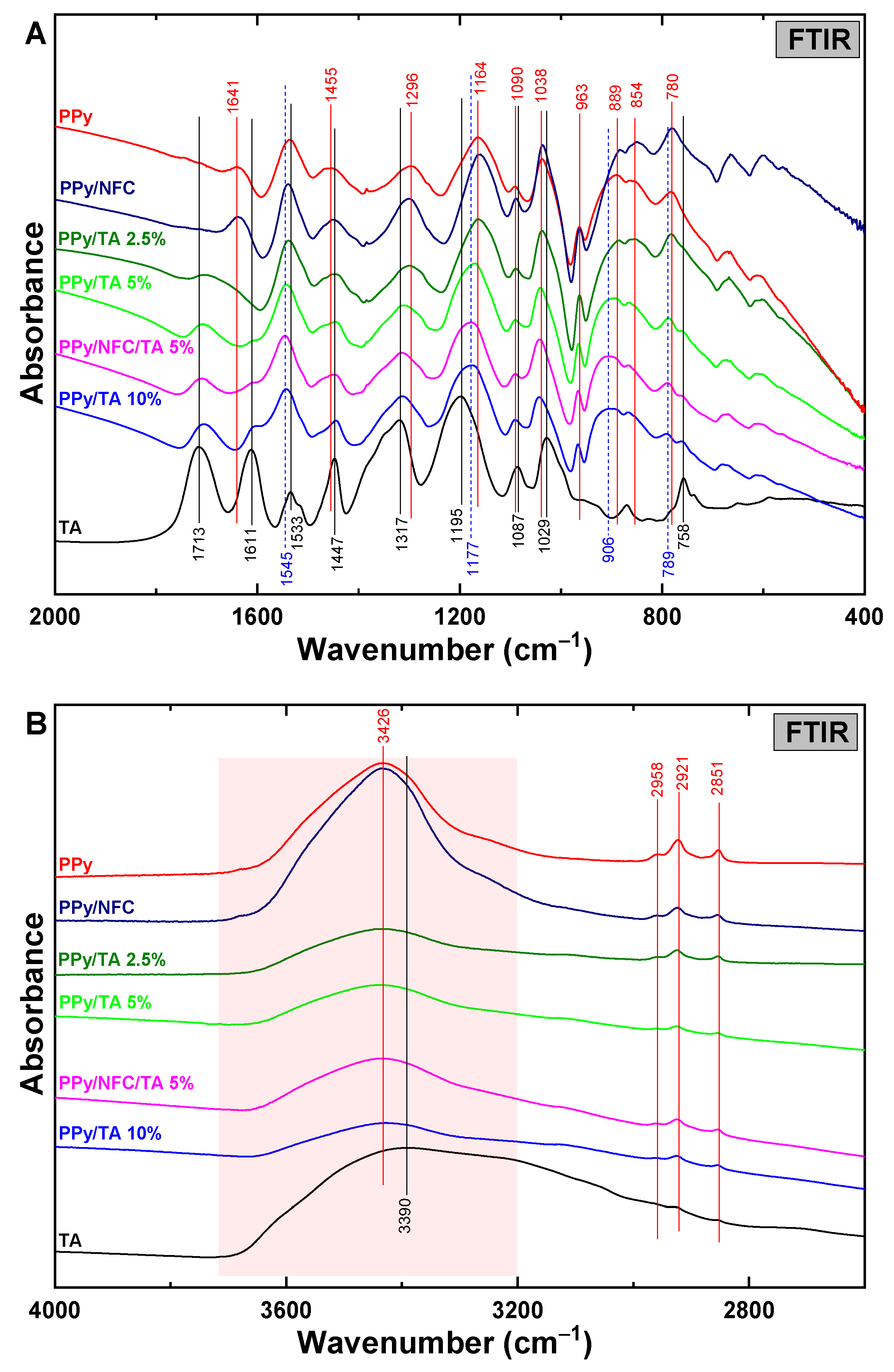
2.1.2. FTIR Spectroscopy
2.1.3. Raman Spectroscopy
2.1.4. Electrical Conductivity
2.1.5. Thermal Stability
2.2. Adsorptive Removal of Cr(VI) Ions from Aqueous Solutions
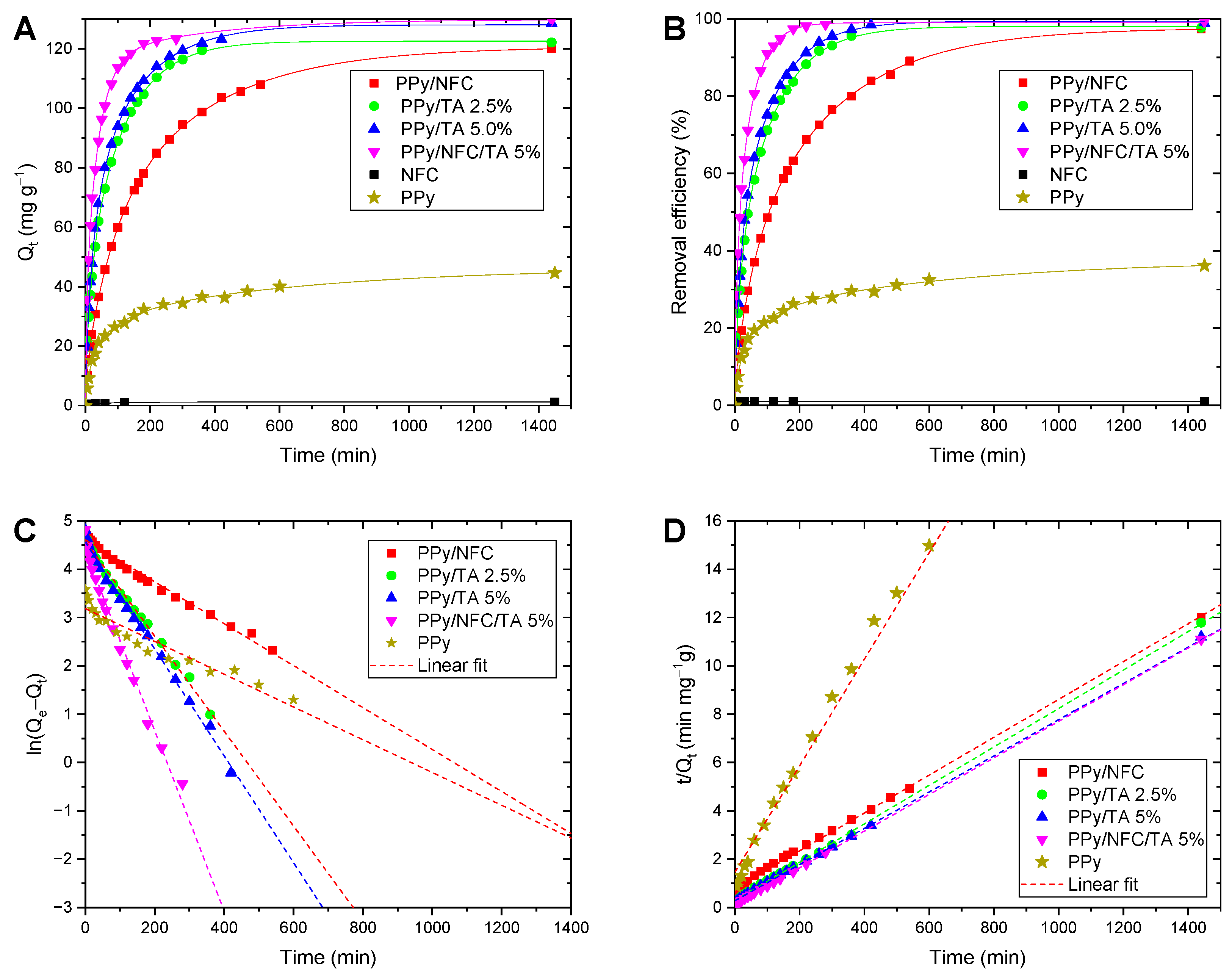
2.3. Adsorption Kinetics
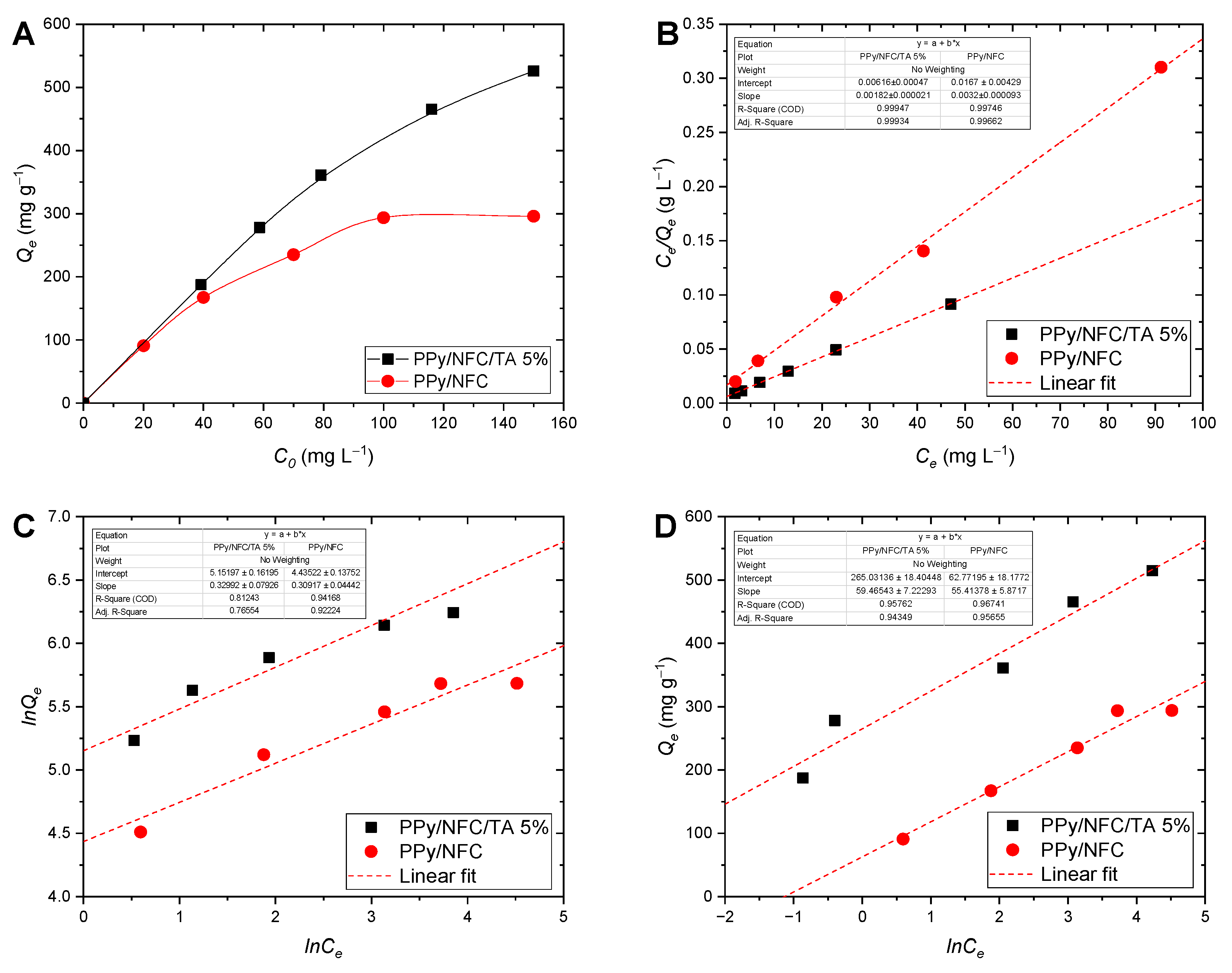
2.4. Equilibrium Adsorption Isotherms
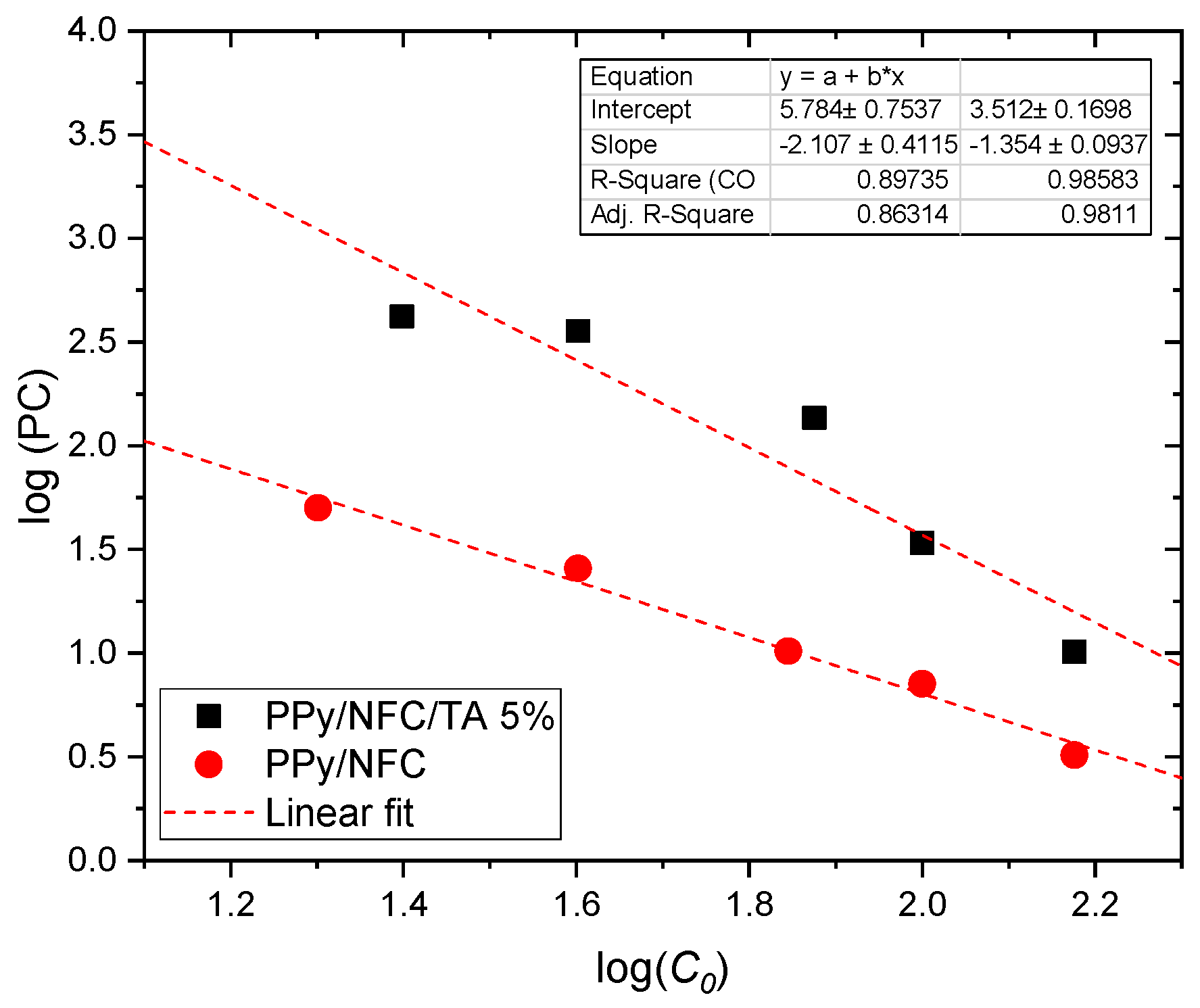
2.5. Partition Coefficient
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
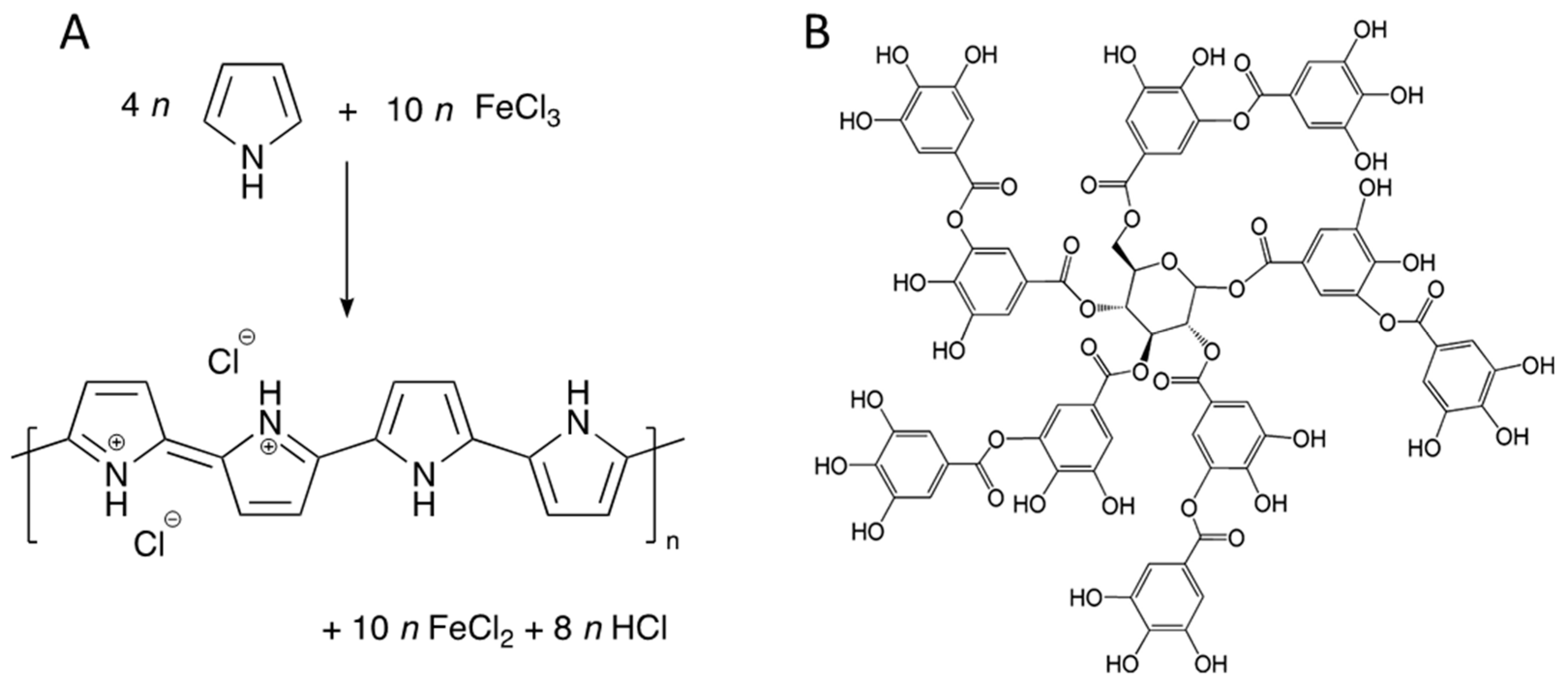
4.2. Preparation of Polypyrrole Aerogels
4.3. Characterization
4.4. Removal of Chromium Ions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- US EPA. Toxic and Priority Pollutants under the Clean Water Act/US EPA. 2021. Available online: https://www.epa.gov/eg/toxic-and-priority-pollutants-under-clean-water-act (accessed on 6 May 2024).
- Zhitkovich, A. Chromium in drinking water: Sources, metabolism, and cancer risks. Chem. Res. Toxicol. 2011, 24, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, Y.; Xin, L.; Oo, K.H.; Zheng, M.; Ma, S.; Guo, J.; Chen, Y. Near-complete recycling of real mix electroplating sludge as valuable metals via Fe/Cr co-crystallization and stepwise extraction route. J. Environ. Manag. 2024, 358, 120821. [Google Scholar] [CrossRef] [PubMed]
- Abidli, A.; Huang, Y.; Rejeb, Z.B.; Zaoui, A.; Park, C.B. Sustainable and efficient technologies for removal and recovery of toxic and valuable metals from wastewater: Recent progress, challenges, and future perspectives. Chemosphere 2022, 292, 133102. [Google Scholar] [CrossRef] [PubMed]
- Dayan, A.D.; Paine, A.J. Mechanisms of chromium toxicity, carcinogenicity and allergenicity: Review of the literature from 1985 to 2000. Hum. Exp. Toxicol. 2001, 20, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.K.; Naiya, T.K.; Mandal, S.N.; Das, S.K. Adsorption, kinetics and equilibrium studies on removal of Cr (VI) from aqueous solutions using different low-cost adsorbents. Chem. Eng. J. 2008, 137, 529–541. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, Z.; Wang, B.; Shen, J.; Zhang, J.; Li, D. Cr (VI) removal using different reducing agents combined with fly ash leachate: A comparative study of their efficiency and potential mechanisms. Chemosphere 2018, 213, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, R.D.; Su, C.; Lee, T.R.; Wilkin, R.T.; Acree, S.D.; Ross, R.R.; Keeley, A. In situ chemical reduction of Cr (VI) in groundwater using a combination of ferrous sulfate and sodium dithionite: A field investigation. Environ. Sci. Technol. 2007, 41, 5299–5305. [Google Scholar] [CrossRef] [PubMed]
- Sarbani, N.M.; Hidayat, E.; Naito, K.; Mitoma, Y.; Harada, H. Cr (VI) and Pb (II) removal using crosslinking magnetite-carboxymethyl cellulose-chitosan hydrogel beads. Gels 2023, 9, 612. [Google Scholar] [CrossRef] [PubMed]
- Striz, R.; Minisy, I.M.; Bober, P.; Taboubi, O.; Smilek, J.; Kovalcik, A. Free-standing bacterial cellulose/polypyrrole composites for eco-friendly remediation of hexavalent chromium ions. ACS Appl. Polym. Mater. 2024, 6, 6383–6392. [Google Scholar] [CrossRef]
- Minisy, I.M.; Acharya, U.; Veigel, S.; Morávková, Z.; Taboubi, O.; Hodan, J.; Breitenbach, S.; Unterweger, C.; Gindl-Altmutter, W.; Bober, P. Sponge-like polypyrrole–nanofibrillated cellulose aerogels: Synthesis and application. J. Mater. Chem. C 2021, 9, 12615–12623. [Google Scholar] [CrossRef]
- Xiang, L.; Niu, C.G.; Tang, N.; Lv, X.X.; Guo, H.; Li, Z.W.; Liu, H.Y.; Lin, L.S.; Yang, Y.Y.; Liang, C. Polypyrrole coated molybdenum disulfide composites as adsorbent for enhanced removal of Cr (VI) in aqueous solutions by adsorption combined with reduction. Chem. Eng. J. 2021, 408, 127281. [Google Scholar] [CrossRef]
- Taghizadeh, A.; Taghizadeh, M.; Jouyandeh, M.; Yazdi, M.K.; Zarrintaj, P.; Saeb, M.R.; Lima, E.C.; Gupta, V.K. Conductive polymers in water treatment: A review. J. Mol. Liq. 2020, 312, 113447. [Google Scholar] [CrossRef]
- Stejskal, J.; Trchová, M. Conducting polypyrrole nanotubes: A review. Chem. Pap. 2018, 72, 1563–1595. [Google Scholar] [CrossRef]
- Eskandari, E.; Kosari, M.; Farahani, M.H.D.A.; Khiavi, N.D.; Saeedikhani, M.; Katal, R.; Zarinejad, M. A review on polyaniline-based materials applications in heavy metals removal and catalytic processes. Sep. Purif. Technol. 2020, 231, 115901. [Google Scholar] [CrossRef]
- Minisy, I.M.; Taboubi, O.; Hromádková, J. One-step accelerated synthesis of conducting polymer/silver composites and their catalytic reduction of Cr (VI) ions and p-nitrophenol. Polymers 2023, 15, 2366. [Google Scholar] [CrossRef] [PubMed]
- Bober, P.; Minisy, I.M.; Morávková, Z.; Hlídková, H.; Hodan, J.; Hromádková, J.; Acharya, U. Polypyrrole aerogels: Efficient adsorbents of Cr (VI) ions from aqueous solutions. Gels 2023, 9, 582. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T. Systematics and health effects of chemically distinct tannins in medicinal plants. Phytochemistry 2005, 66, 2012–2031. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Ni, W.; Yang, L.; Zhang, Q. Remarkable ability of Pb (II) capture from water by self-assembled metal-phenolic networks prepared with tannic acid and ferric ions. Chem. Eng. J. 2022, 450, 138161. [Google Scholar] [CrossRef]
- Jiang, X.; Long, W.; Peng, L.; Xu, T.; He, F.; Tang, Y.; Zhang, W. Reductive immobilization of Cr (VI) in contaminated water by tannic acid. Chemosphere 2022, 297, 134081. [Google Scholar] [CrossRef]
- Du, J.; Zhang, M.; Dong, Z.; Yang, X.; Zhao, L. Facile fabrication of tannic acid functionalized microcrystalline cellulose for selective recovery of Ga(III) and In(III) from potential leaching solution. Sep. Purif. Technol. 2022, 286, 120442. [Google Scholar] [CrossRef]
- Abdi, M.M.; Azli, N.F.W.M.; Lim, H.N.; Tahir, P.M.; Karimi, G.; Hoong, Y.B.; Khorram, M. Polypyrrole/tannin biobased nanocomposite with enhanced electrochemical and physical properties. RSC Adv. 2018, 8, 2978–2985. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Fan, L.; Yi, X.; Zhou, Z.; Liu, C.; Fu, R.; Dai, C.; Wang, Z.; Chen, X.; Yu, P.; et al. Soft conducting polymer hydrogels cross-linked and doped by tannic acid for spinal cord injury repair. ACS Nano 2018, 12, 10957–10967. [Google Scholar] [CrossRef] [PubMed]
- Dinari, R.; Hosseini, S.H.; Tanzifi, M.; Mansouri, M. Comprehensive study of Acid Yellow 42 adsorption by green synthesized polypyrrole/tannic acid/iron nanocomposites. Sep. Sci. Technol. 2023, 58, 266–286. [Google Scholar] [CrossRef]
- Nam, S.; Easson, M.W.; Condon, B.D.; Hillyer, M.B.; Sun, L.; Xia, Z.; Nagarajan, R. A reinforced thermal barrier coat of a Na–tannic acid complex from the view of thermal kinetics. RSC Adv. 2019, 9, 10914–10926. [Google Scholar] [CrossRef] [PubMed]
- Ranoszek-Soliwoda, K.; Tomaszewska, E.; Socha, E.; Krzyczmonik, P.; Ignaczak, A.; Orlowski, P.; Grobelny, J. The role of tannic acid and sodium citrate in the synthesis of silver nanoparticles. J. Nanoparticle Res. 2017, 19, 273. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, J.; Trchová, M.; Ananieva, I.A.; Janča, J.; Prokeš, J.; Fedorova, S.; Sapurina, I. Poly(aniline-co-pyrrole): Powders, films, and colloids. Thermophoretic mobility of colloidal particles. Synth. Met. 2004, 146, 29–36. [Google Scholar] [CrossRef]
- Pantoja-Castro, M.A.; González-Rodríguez, H. Study by infrared spectroscopy and thermogravimetric analysis of tannins and tannic acid. Rev. Latinoam. De Química 2011, 39, 107–112. [Google Scholar]
- Espina, A.; Sanchez-Cortes, S.; Jurašeková, Z. Vibrational study (Raman, SERS, and IR) of plant gallnut polyphenols related to the fabrication of iron gall inks. Molecules 2022, 27, 279. [Google Scholar] [CrossRef] [PubMed]
- Espina, A.; Cañamares, M.V.; Jurašeková, Z.; Sanchez-Cortes, S. Analysis of iron complexes of tannic acid and other related polyphenols as revealed by spectroscopic techniques: Implications in the identification and characterization of iron gall inks in historical manuscripts. ACS Omega 2022, 7, 27937–27949. [Google Scholar] [CrossRef]
- Trchová, M.; Stejskal, J. Resonance Raman spectroscopy of conducting polypyrrole nanotubes: Disordered surface versus ordered body. J. Phys. Chem. A 2018, 122, 9298–9306. [Google Scholar] [CrossRef]
- Minisy, I.M.; Bober, P.; Acharya, U.; Trchová, M.; Hromádková, J.; Pfleger, J.; Stejskal, J. Cationic dyes as morphology-guiding agents for one-dimensional polypyrrole with improved conductivity. Polymer 2019, 174, 11–17. [Google Scholar] [CrossRef]
- Minisy, I.M.; Acharya, U.; Kobera, L.; Trchova, M.; Unterweger, C.; Breitenbach, S.; Brus, J.; Pfleger, J.; Stejskal, J.; Bober, P. Highly conducting 1-D polypyrrole prepared in the presence of safranin. J. Mater. Chem. C 2020, 8, 12140–12147. [Google Scholar] [CrossRef]
- Minisy, I.M.; Bober, P. Frozen-state polymerization as a tool in conductivity enhancement of polypyrrole. Macromol. Rapid Commun. 2020, 41, 2000364. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.S. Effect of phenolic based polymeric secondary dopants on polyaniline. Synth. Met. 2002, 126, 151–158. [Google Scholar] [CrossRef]
- Chauke, V.P.; Maity, A.; Chetty, A. High-performance towards removal of toxic hexavalent chromium from aqueous solution using graphene oxide-alpha cyclodextrin-polypyrrole nanocomposites. J. Mol. Liq. 2015, 211, 71–77. [Google Scholar] [CrossRef]
- Gong, X.; Li, W.; Wang, K.; Hu, J. Study of the adsorption of Cr (VI) by tannic acid immobilised powdered activated carbon from micro-polluted water in the presence of dissolved humic acid. Bioresour. Technol. 2013, 141, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, D.; Raeiszadeh, M.; Lewis, L.; MacLachlan, M.J.; Hatzikiriakos, S.G. Adsorptive removal of Congo red by surfactant modified cellulose nanocrystals: A kinetic, equilibrium, and mechanistic investigation. Cellulose 2020, 27, 3211–3232. [Google Scholar] [CrossRef]
- Minisy, I.M.; Salahuddin, N.A.; Ayad, M.M. Adsorption of methylene blue onto chitosan–montmorillonite/polyaniline nanocomposite. Appl. Clay Sci. 2021, 203, 105993. [Google Scholar] [CrossRef]
- Xiong, L.; Zhang, F.; Yang, Y.; Ding, Y.; Chen, S. Preparation of a novel polypyrrole/dolomite composite adsorbent for efficient removal of Cr (VI) from aqueous solution. Environ. Sci. Pollut. Res. 2024, 31, 21279–21290. [Google Scholar] [CrossRef]
- Li, L.; Yang, Q.; Shi, Y.; Zhou, L.; Li, W.; Zhao, J.; Liu, Z. Polypyrrole-modified natural eggplant aerogel with high shape recovery for simultaneous efficient clean water generation and heavy metal ion ad-sorption from wastewater. Sep. Purif. Technol. 2024, 331, 125669. [Google Scholar] [CrossRef]
- Li, D.; Liu, H.; Wang, Z.; Zhang, Z.; Wang, C.; Zhao, B.; Pan, K. Ultralight and superelastic nanofiber aerogels with in-situ loaded polypyrrole for high-efficient Cr(VI) adsorption. J. Polym. Environ. 2023, 31, 637–647. [Google Scholar] [CrossRef]
- Ahmadijokani, F.; Mohammadkhani, R.; Ahmadipouya, S.; Shokrgozar, A.; Rezakazemi, M.; Molavi, H.; Aminabhavi, T.M.; Arjmand, M. Superior chemical stability of UiO-66 metal-organic frameworks (MOFs) for selective dye adsorption. Chem. Eng. J. 2020, 399, 125346. [Google Scholar] [CrossRef]





| Sample | Conductivity, S cm−1 |
|---|---|
| PPy | 23.7 * |
| PPy/TA (2.5%) | 9.6 ± 1.7 |
| PPy/TA (5.0%) | 0.95 ± 0.13 |
| PPy/TA (10%) | 0.07 ± 0.01 |
| PPy/NFC/TA (5.0%) | 0.37 ± 0.017 |
| Adsorbent | Qe, Exp. Mg g−1 | Pseudo-First-Order | Pseudo-Second-Order | Intraparticle Diffusion | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qe, mg g−1 | k1, min−1 | R2 | Qe, mg g−1 | k2, gmg−1 min−1 | R2 | ci, mg g−1 | ki, mgg−1 min−1/2 | R2 | ||
| PPy/TA 10% | 110.2 | 69.5 | 0.019 | 0.985 | 111.2 | 9.8 × 10−4 | 0.999 | 53.40 | 4.79 | 0.972 |
| PPy/TA 5% | 124.1 | 95.6 | 0.011 | 0.994 | 133.3 | 2.0 × 10−4 | 0.999 | 45.43 | 4.83 | 0.993 |
| PPy/NFC/TA 5% | 123.5 | 89.4 | 0.021 | 0.993 | 132.4 | 4.3 × 10−4 | 0.999 | 48.66 | 6.59 | 0.986 |
| PPy/TA 2.5% | 122.2 | 97.4 | 0.010 | 0.993 | 125.6 | 2.3 × 10−4 | 0.999 | 45.33 | 4.43 | 0.992 |
| PPy/NFC | 120.1 | 100.3 | 0.004 | 0.986 | 127.7 | 7.9 × 10−5 | 0.998 | 42.90 | 2.90 | 0.988 |
| PPy | 46.61 | 24.17 | 0.0034 | 0.982 | 45.45 | 3.3 × 10−4 | 0.997 | 11.62 | 1.53 | 0.994 |
| Aerogel | Qmax, Exp., mg g−1 | Langmuir | Freundlich | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qmax, mg g−1 | KL, L mg−1 | R2 | KF, mg g−1 | 1/n | R2 | KT, L g−1 | B | R2 | ||
| PPy/NFC/TA 5% | 515.6 | 549.5 | 0.296 | 0.999 | 172.78 | 0.330 | 0.812 | 4.46 | 59.47 | 0.957 |
| PPy/NFC | 296.0 | 312.5 | 0.192 | 0.997 | 84.35 | 0.309 | 0.922 | 1.13 | 55.41 | 0.967 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minisy, I.M.; Taboubi, O.; Hromádková, J.; Bober, P. Aerogels of Polypyrrole/Tannic Acid with Nanofibrillated Cellulose for the Removal of Hexavalent Chromium Ions. Gels 2024, 10, 415. https://doi.org/10.3390/gels10070415
Minisy IM, Taboubi O, Hromádková J, Bober P. Aerogels of Polypyrrole/Tannic Acid with Nanofibrillated Cellulose for the Removal of Hexavalent Chromium Ions. Gels. 2024; 10(7):415. https://doi.org/10.3390/gels10070415
Chicago/Turabian StyleMinisy, Islam M., Oumayma Taboubi, Jiřina Hromádková, and Patrycja Bober. 2024. "Aerogels of Polypyrrole/Tannic Acid with Nanofibrillated Cellulose for the Removal of Hexavalent Chromium Ions" Gels 10, no. 7: 415. https://doi.org/10.3390/gels10070415
APA StyleMinisy, I. M., Taboubi, O., Hromádková, J., & Bober, P. (2024). Aerogels of Polypyrrole/Tannic Acid with Nanofibrillated Cellulose for the Removal of Hexavalent Chromium Ions. Gels, 10(7), 415. https://doi.org/10.3390/gels10070415









