The Role of WO3 Nanoparticles on the Properties of Gelatin Films
Abstract
1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Synthesis of WO3 Nanoparticles
4.2. Film Preparation
4.3. Film Characterization
4.4. Photochromic Properties
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.R.; Sadiq, M.B. Importance of gelatin, nanoparticles and their interactions in the formulation of biodegradable composite films: A review. Polym. Bull. 2021, 78, 4047–4073. [Google Scholar] [CrossRef]
- Shyni, K.; Hema, G.; Ninan, G.; Mathew, S.; Joshy, C.; Lakshmanan, P. Isolation and characterization of gelatin from the skins of skipjack tuna (Katsuwonus pelamis), dog shark (Scoliodon sorrakowah), and rohu (Labeo rohita). Food Hydrocoll. 2014, 39, 68–76. [Google Scholar] [CrossRef]
- Rather, J.A.; Akhter, N.; Ashraf, Q.S.; Mir, S.A.; Makroo, H.A.; Majid, D.; Barba, F.J.; Khaneghah, A.M.; Dar, B.N. A comprehensive review on gelatin: Understanding impact of the sources, extraction methods, and modifications on potential packaging applications. Food Packag. Shelf Life 2022, 34, 100945. [Google Scholar] [CrossRef]
- Gomez-Guillen, M.C.; Gimenez, B.; Lopez-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Amadori, S.; Torricelli, P.; Rubini, K.; Fini, M.; Panzavolta, S.; Bigi, A. Effect of sterilization and crosslinking on gelatin films. J. Mater. Sci. Mater. Med. 2015, 26, 9. [Google Scholar] [CrossRef]
- Rubini, K.; Boanini, E.; Parmeggiani, S.; Bigi, A. Curcumin-functionalized gelatin films: Antioxidant materials with modulated physico-chemical properties. Polymers 2021, 13, 1824. [Google Scholar] [CrossRef]
- Tschon, M.; Boanini, E.; Sartori, M.; Salamanna, F.; Panzavolta, S.; Bigi, A.; Fini, M. Antiosteoporotic nanohydroxyapatite zoledronate scaffold seeded with bone marrow mesenchymal stromal cells for bone regeneration: A 3D in vitro model. Int. J. Mol. Sci. 2022, 23, 5988. [Google Scholar] [CrossRef]
- Matloob, A.; Ayub, H.; Mohsin, M.; Ambreen, S.; Khan, F.A.; Oranab, S.; Rahim, M.A.; Khalid, W.; Nayik, G.A.; Ramniwas, S.; et al. A review on edible coatings and films: Advances, composition, production methods, and safety concerns. ACS Omega 2023, 8, 28932–28944. [Google Scholar] [CrossRef] [PubMed]
- Mikhailov, O.V. Gelatin as it is: History and modernity. Int. J. Mol. Sci. 2023, 24, 3583. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillén, M.; Pérez-Mateos, M.; Gómez-Estaca, J.; López-Caballero, E.; Giménez, B.; Montero, P. Fish gelatin: A renewable material for developing active biodegradable films. Trends Food Sci. Technol. 2009, 20, 3–16. [Google Scholar] [CrossRef]
- Sung, H.W.; Huang, D.M.; Chang, W.H.; Huang, R.N.; Hsu, J.C. Evalution of gelatin hydrogel crosslinked with various crosslinking agents as bioadhesives: In vitro study. J. Biomed. Mater. Res. 1999, 46, 520–530. [Google Scholar] [CrossRef]
- Boanini, E.; Rubini, K.; Panzavolta, S.; Bigi, A. Chemico-physical characterization of gelatin films modified with oxidized alginate. Acta Biomater. 2010, 6, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Derkach, S.R.; Ilyin, S.O.; Maklakova, A.A.; Kulichikhin, V.G.; Malkin, A.Y. The rheology of gelatin hydrogels modified by κ-carrageenan. LWT-Food Sci. Technol. 2015, 63, 612–619. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Ahmed, J.; Hiremath, N.; Auras, R.; Joseph, A. Thermo-mechanical, rheological, structural and antimicrobial properties of bionanocomposite films based on fish skin gelatin and silver–copper nanoparticles. Food Hydrocoll. 2017, 62, 191–202. [Google Scholar] [CrossRef]
- Sahraee, S.; Ghanbarzadeh, B.; Milani, J.M.; Hamishehkar, H. Development of gelatin bionanocomposite films containing chitin and ZnO nanoparticles. Food Bioprocess Technol. 2017, 10, 1441–1453. [Google Scholar] [CrossRef]
- Lou, L.; Chen, H. Functional modification of gelatin based biodegradable composite films: A review. Food Addit. Contam. Part A 2023, 40, 928–949. [Google Scholar] [CrossRef]
- Jorge, M.F.C.; Alexandre, E.M.C.; Flaker, C.H.C.; Bittante, A.M.Q.B.; Sobral, P.J.A. Biodegradable films based on gelatin and montmorillonite produced by spreading. Int. J. Polym. Sci. 2015, 2015, 806791. [Google Scholar] [CrossRef]
- Kumar, S.; Mitra, A.; Halder, D. Centella asiatica leaf mediated synthesis of silver nanocolloid and its application as filler in gelatin based antimicrobial nanocomposite film. LWT 2017, 75, 293–300. [Google Scholar] [CrossRef]
- Santos-López, G.; Soto-Castro, D.; León-Martínez, F.M.; Hernández-Martínez, Á.R.; Gutiérrez, M.C. Characterization of gelatin films functionalized with silver nanoparticles or polysorbate 20 as UV–vis light controllers. Food Packag. Shelf Life 2023, 40, 101208. [Google Scholar] [CrossRef]
- Mohanraj, M.; Ashraf, I.M.; Shkir, M.; Reddy, V.R.M.; Kim, W.K. Investigation on the microstructural, optical, electrical, and photocatalytic properties of WO3 nanoparticles: An effect of Ce doping concentrations. J. Mater. Sci. Mater. Electron. 2023, 34, 1961. [Google Scholar] [CrossRef]
- Li, J.; Wu, J.; Yu, Y. Applied surface science DFT exploration of sensor performances of two-dimensional WO3 to ten small gases in terms of work function and band gap changes and I–V responses. Appl. Surf. Sci. 2021, 546, 149104. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, C.; Sun, H.; Liu, Z.; Liu, Z.; Li, K.; Zhu, L.; Qin, G.; Sun, G.; Li, Y.; et al. Tough, transparent, and anti-freezing nanocomposite organohydrogels with photochromic properties. ACS Appl. Mater. Interfaces 2021, 13, 31180–31192. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yao, Y.; Chen, Z.; Zhang, Z.; Zhang, P.; Cheng, Z.; Gao, Y. WO3 quantum dot photochromical film. Sol. Energy Mater. Sol. Cells 2022, 239, 111664. [Google Scholar] [CrossRef]
- Zhao, H.; Cun, Y.; Bai, X.; Xiao, D.; Qiu, J.; Song, Z.; Liao, J.; Yang, Z. Entirely reversible photochromic glass with high coloration and luminescence contrast for 3D optical storage. ACS Energy Lett. 2022, 7, 2060–2069. [Google Scholar] [CrossRef]
- Silingardi, F.; Bonvicini, F.; Cassani, M.C.; Mazzaro, R.; Rubini, K.; Gentilomi, G.A.; Bigi, A.; Boanini, E. Hydroxyapatite decorated with tungsten oxide nanoparticles: New composite materials against bacterial growth. J. Funct. Biomater. 2022, 13, 88. [Google Scholar] [CrossRef]
- Popov, A.L.; Han, B.; Ermakov, A.M.; Savintseva, I.V.; Ermakova, O.N.; Popova, N.R.; Shcherbakov, A.B.; Shekunova, T.O.; Ivanova, O.S.; Kozlov, D.A.; et al. PVP-stabilized tungsten oxide nanoparticles: pH sensitive anti-cancer platform with high cytotoxicity. Mater. Sci. Eng. C 2020, 108, 110494. [Google Scholar] [CrossRef] [PubMed]
- Gioffrè, M.; Torricelli, P.; Panzavolta, S.; Rubini, K.; Bigi, A. Role of pH on stability and mechanical properties of gelatin films. J. Bioact. Compat. Polym. 2012, 27, 67–77. [Google Scholar] [CrossRef]
- Kozlov, D.A.; Shcherbakov, A.B.; Kozlova, T.O.; Angelov, B.; Kopitsa, G.P.; Garshev, A.V.; Baranchikov, A.E.; Ivanova, O.S.; Ivanov, V.K. Photochromic and photocatalytic properties of ultra-small PVP-stabilized WO3 nanoparticles. Molecules 2020, 25, 154. [Google Scholar] [CrossRef]
- Kundu, S.; Das, A.; Basu, A.; Abdullah, M.F.; Mukherjee, A. Guar gum benzoate nanoparticle reinforced gelatin films for enhanced thermal insulation, mechanical and antimicrobial properties. Carbohydr. Polym. 2017, 170, 89–98. [Google Scholar] [CrossRef]
- Bigi, A.; Panzavolta, S.; Rubini, K. Relationship between triple helix content and mechanical properties of gelatin films. Biomaterials 2004, 25, 5675–5680. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Roveri, N.; Rubini, K. Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials 2001, 22, 763–768. [Google Scholar] [CrossRef]
- Tonda-Turo, C.; Gentile, P.; Saracino, S.; Chiono, V.; Nandagiri, V.K.; Muzio, G.; Canuto, R.A.; Ciardelli, G. Comparative analysis of gelatin scaffolds crosslinked by genipin and silane coupling agent. Int. J. Biol. Macromol. 2011, 49, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, K. Revisiting the molecular structure of collagen. Connect. Tissue Res. 2008, 49, 299–310. [Google Scholar] [CrossRef]
- Staroszczyk, H.; Sztuka, K.; Wolska, J.; Wojtasz-Pająk, A.; Kołodziejska, I. Interactions of fish gelatin and chitosan in uncrosslinked and crosslinked with EDC films: FT-IR study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 117, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Mireles, L.K.; Wu, M.R.; Saadeh, N.; Yahia, L.; Sacher, E. Physicochemical characterization of polyvinyl pyrrolidone: A tale of two polyvinyl pyrrolidones. ACS Omega 2020, 5, 30461–30467. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Kaur, N.; Savita; Sharma, A. Enhanced photochromic properties of 1D h-WO3 embedded in bio-degradable polymer for fabrication of smart windows. Mater. Sci. Eng. B 2024, 299, 116931. [Google Scholar] [CrossRef]
- He, T.; Yao, J. Photochromic materials based on tungsten oxide. J. Mater. Chem. 2007, 17, 4547–4557. [Google Scholar] [CrossRef]
- Wang, S.; Fan, W.; Liu, Z.; Yu, A.; Jiang, X. Advances on tungsten oxide based photochromic materials: Strategies to improve their photochromic properties. J. Mater. Chem. C 2018, 6, 191. [Google Scholar] [CrossRef]
- Li, R.; Zhou, Y.; Shao, Z.; Zhao, S.; Chang, T.; Huang, A.; Li, N.; Ji, S.; Jin, P. Enhanced coloration/bleaching photochromic performance of WO3 based on PVP/PU composite matrix. ChemistrySelect 2019, 4, 9817. [Google Scholar] [CrossRef]
- Yamazaki, S.; Shimizu, D.; Tani, S.; Honda, K.; Sumimoto, M.; Komaguchi, K. Effect of dispersants on photochromic behavior of tungsten oxide nanoparticles in methylcellulose. ACS Appl. Mater. Interfaces 2018, 10, 19889. [Google Scholar] [CrossRef]
- Pardo, R.; Zayat, M.; Levy, D. Photochromic organic–inorganic hybrid materials. Chem. Soc. Rev. 2011, 40, 672–687. [Google Scholar] [CrossRef] [PubMed]
- Zhana, Y.; Yanga, Z.; Xua, Z.; Hua, Z.; Baia, X.; Rena, Y.; Lia, M.; Ullaha, A.; Khana, I.; Qiua, J.; et al. Electrochromism induced reversible upconversion luminescence modulation of WO3:Yb3+, Er3+ inverse opals for optical storage application. Chem. Eng. J. 2020, 394, 124967. [Google Scholar] [CrossRef]
- Ruan, J.; Yang, Z.; Huang, A.; Zhang, H.; Qiu, J.; Song, Z. Thermochromic reaction-induced reversible upconversion emission modulation for switching devices and tunable upconversion emission based on defect engineering of WO3:Yb3+,Er3+ phosphor. ACS Appl. Mater. Interfaces 2018, 10, 14941–14947. [Google Scholar] [CrossRef] [PubMed]
- Giménez, B.; Gómez-Estaca, J.; Alemán, A.; Gómez-Guillén, M.C.; Montero, P. Physico-chemical and film forming properties of giant squid (Dosidicus gigas) gelatin. Food Hydrocoll. 2009, 23, 585–592. [Google Scholar] [CrossRef]
- E96-93; Standard Test Methods for Water-Vapor Transmission of Materials. American Society for Testing and Materials: Philadelphia, PA, USA, 1993; Volume 4, pp. 701–708.
- Bozdemir, O.A.; Tutas, M. Plasticiser effect on water vapour permeability properties of locust bean gum based edible film. Turk. J. Chem. 2003, 27, 773–782. Available online: https://journals.tubitak.gov.tr/chem/vol27/iss6/14 (accessed on 17 May 2024).
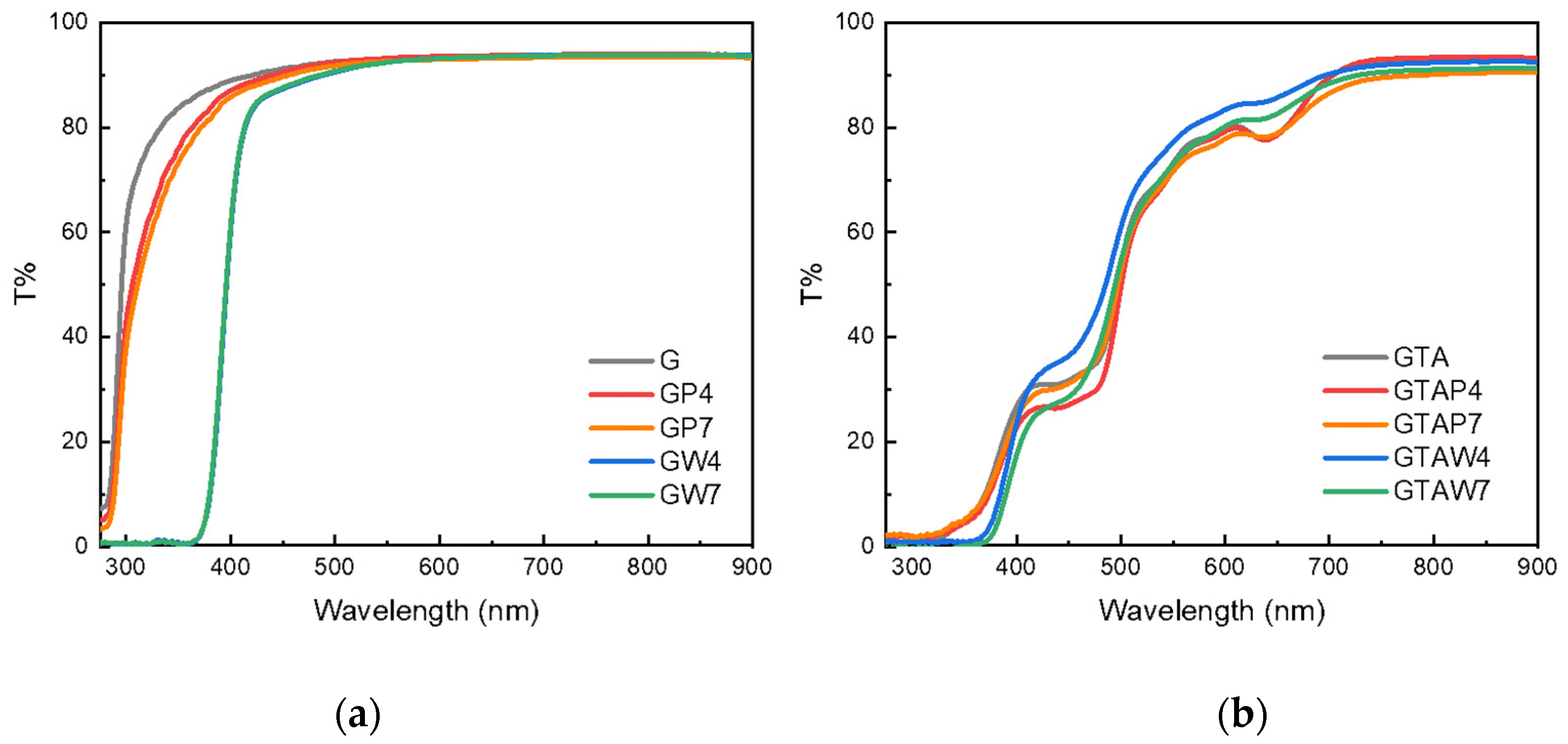
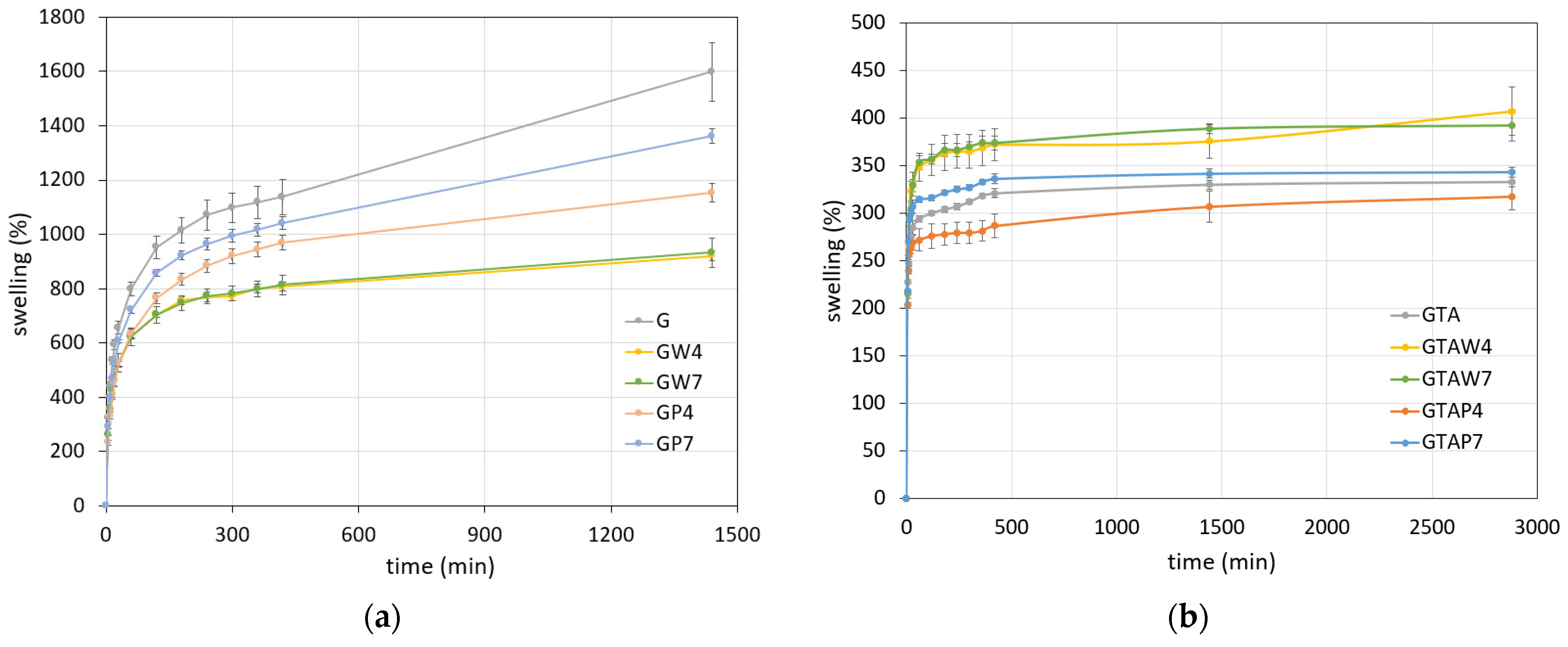
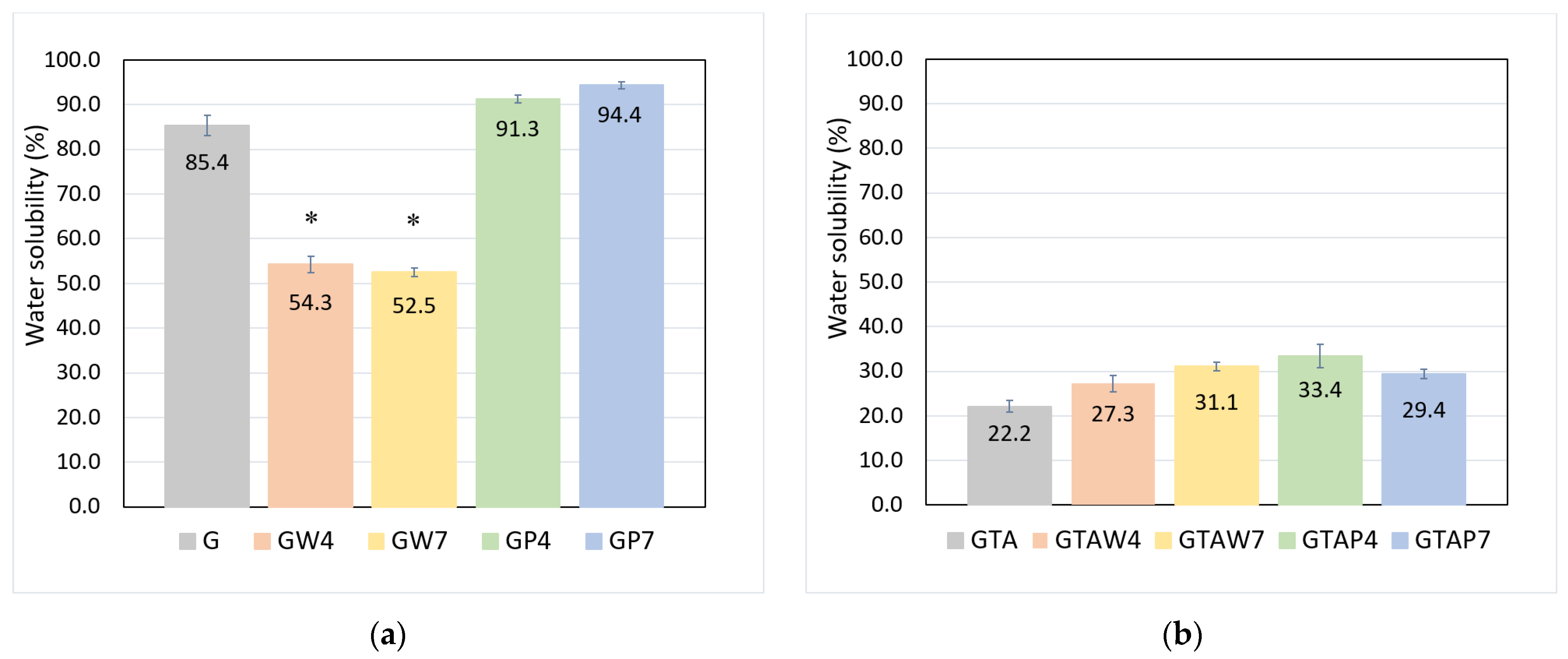
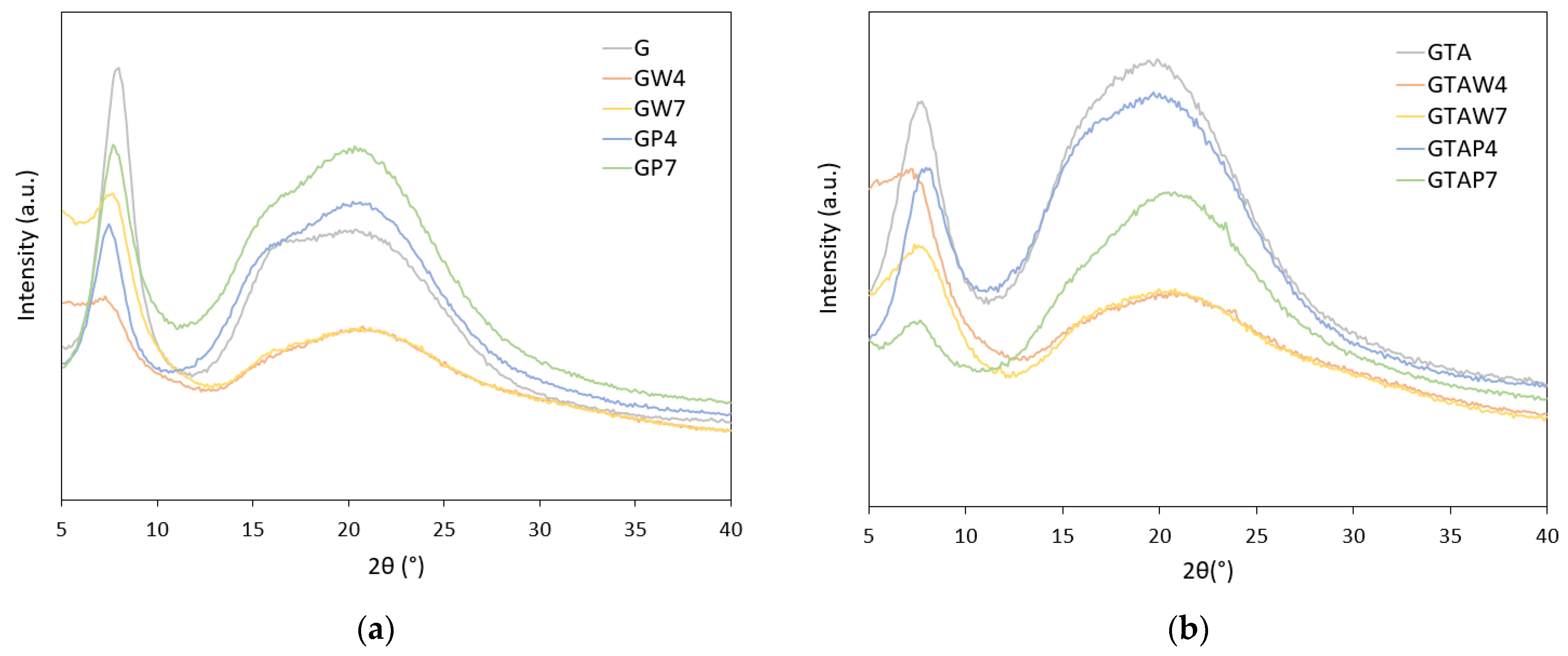
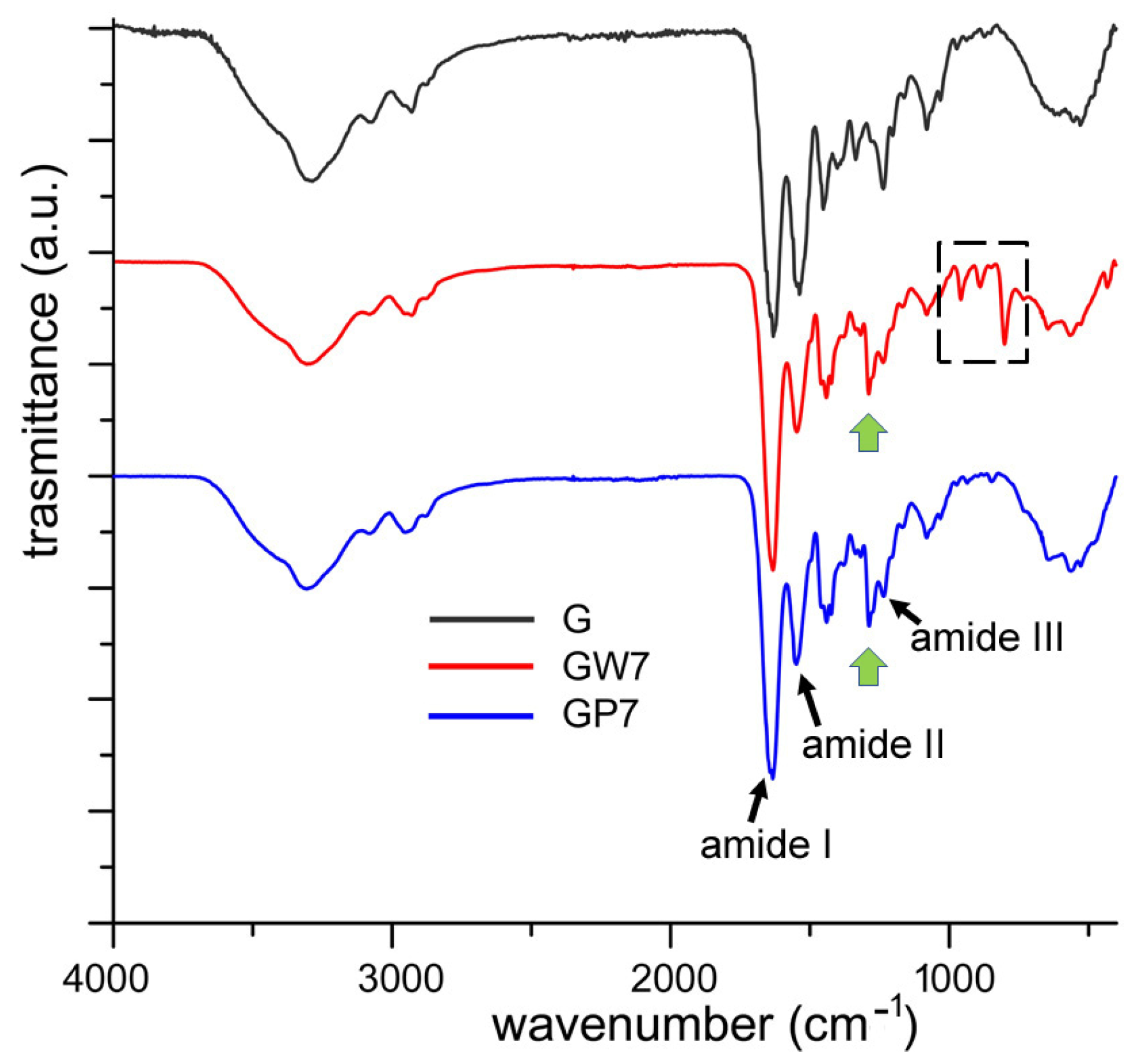
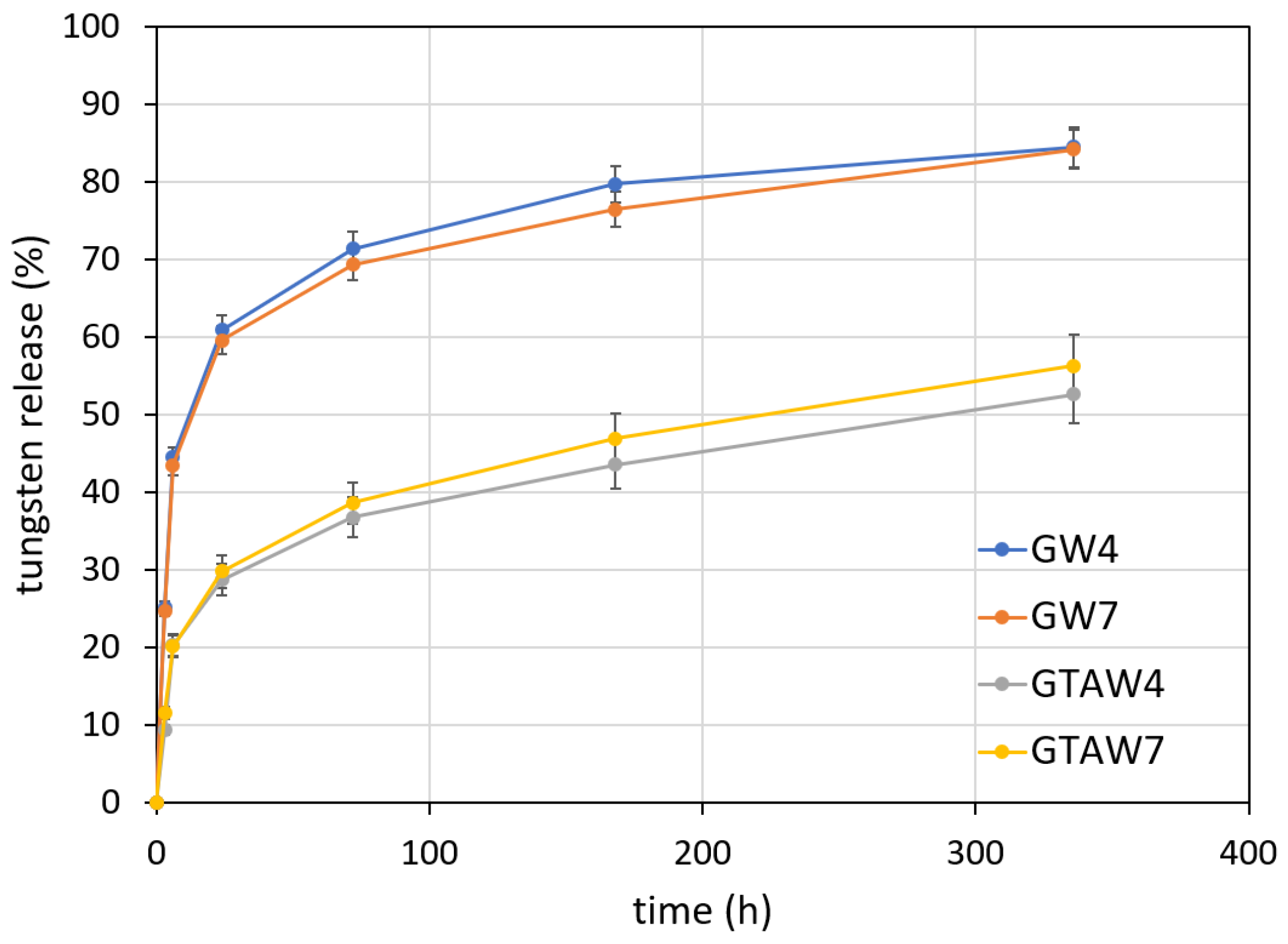
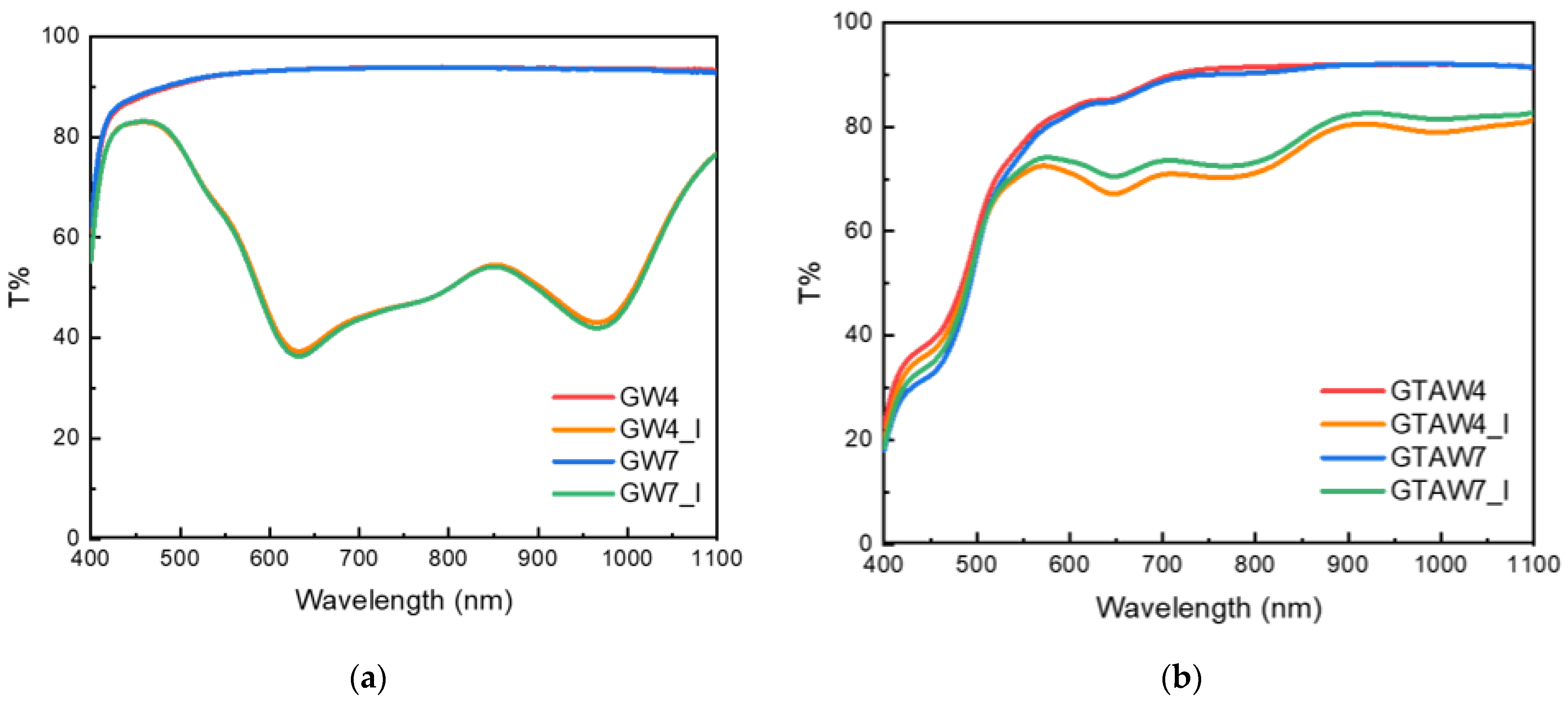

| Sample | Thickness × 10−4 (m) | WVTR (g h−1 m−2) | WVP × 10−10 (g s−1 m−1 Pa−1) | Sample | Thickness × 10−4 (m) | WVTR (g h−1 m−2) | WVP × 10−10 (g s−1 m−1 Pa−1) |
|---|---|---|---|---|---|---|---|
| G | 1.13 ± 0.07 | 6.52 ± 0.26 | 1.23 ± 0.07 | GTA | 1.28 ± 0.06 | 4.49 ± 0.22 | 0.95 ± 0.05 |
| GP4 | 1.86 ± 0.05 (*) | 3.45 ± 0.07 (*) | 1.07 ± 0.03 | GTAP4 | 1.53 ± 0.07 | 4.32 ± 0.16 | 1.10 ± 0.04 |
| GP7 | 1.77 ± 0.07 (*) | 3.61 ± 0.09 (*) | 1.06 ± 0.02 | GTAP7 | 1.52 ± 0.07 | 4.40 ± 0.09 | 1.11 ± 0.04 |
| GW4 | 1.80 ± 0.08 (*) | 3.08 ± 0.25 (*) | 0.92 ± 0.06 (*) | GTAW4 | 1.32 ± 0.05 | 4.40 ± 0.11 | 0.97 ± 0.02 |
| GW7 | 1.87 ± 0.05 (*) | 2.73 ± 1.22 (*) | 0.85 ± 0.04 (*) | GTAW7 | 1.47 ± 0.08 | 3.82 ± 0.09 | 0.93 ± 0.02 |
| Sample | Contact Angle (°) | Sample | Contact Angle (°) |
|---|---|---|---|
| G | 76.1 ± 1.4 | GTA | 78.3 ± 1.7 |
| GP4 | 68.1 ± 0.8 (*) | GTAP4 | 65.6 ± 1.5 (***) |
| GP7 | 65.5 ± 1.3 (**) | GTAP7 | 65.1 ± 1 (***) |
| GW4 | 68.4 ± 1 (*) | GTAW4 | 67.2 ± 1.3 (***) |
| GW7 | 70.1 ± 1 (*) | GTAW7 | 69.2 ± 0.4 (***) |
| Sample | TD (°C) | ΔHD (J/g) | Sample | TD (°C) | ΔHD (J/g) |
|---|---|---|---|---|---|
| G | 93 ± 0.3 | 22.8 ± 0.4 | GTA | 95.3 ± 0.1 | 15.2 ± 0.4 |
| GP4 | 91.6 ± 0.1 | 13.3 ± 0.2 (*) | GTAP4 | 95 ± 0.2 | 13.4 ± 0.1 |
| GP7 | 90.8 ± 0.5 | 14.2 ± 0.3 (*) | GTAP7 | 94.6 ± 0.1 | 13.0 ± 0.4 |
| GW4 | 92.1 ± 0.1 | 14.6 ± 0.2 (*) | GTAW4 | 94.7 ± 0.1 | 15.2 ± 0.1 |
| GW7 | 90.4 ± 0.3 | 16.4 ± 0.2 (*) | GTAW7 | 94.3 ± 0.1 | 14.5 ± 0.4 |
| Sample | E (MPa) | σb (MPa) | εb (%) |
|---|---|---|---|
| G | 2.0 ± 0.4 | 2.5 ± 0.3 | 152.3 ± 28 |
| GP4 | 1.3 ± 0.3 | 2.1 ± 0.2 | 155.6 ± 39 |
| GP7 | 1.3 ± 0.4 | 1.9 ± 0.3 | 147.9 ± 36 |
| GW4 | 0.7 ± 0.08 (*) | 1.4 ± 0.2 (*) | 151.8 ± 29 |
| GW7 | 0.7 ± 0.08 (*) | 1.7 ± 0.2 (*) | 152.3 ± 35 |
| Samples | E (MPa) | σb (MPa) | εb (%) |
|---|---|---|---|
| GTA | 4.9 ± 0.6 | 3.1 ± 0.9 | 20 ± 0.9 |
| GTAP4 | 4.1 ± 1 | 2.8 ± 0.3 | 17.4 ± 0.9 |
| GTAP7 | 4.0 ± 0.9 | 3.0 ± 0.6 | 19.7 ± 1.2 |
| GTAW4 | 3.3 ± 0.4 (*) | 2.4 ± 0.9 | 20.9 ± 1.8 |
| GTAW7 | 3.2 ± 0.2 (*) | 2.5 ± 0.5 | 21.3 ± 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubini, K.; Menichetti, A.; Cassani, M.C.; Montalti, M.; Bigi, A.; Boanini, E. The Role of WO3 Nanoparticles on the Properties of Gelatin Films. Gels 2024, 10, 354. https://doi.org/10.3390/gels10060354
Rubini K, Menichetti A, Cassani MC, Montalti M, Bigi A, Boanini E. The Role of WO3 Nanoparticles on the Properties of Gelatin Films. Gels. 2024; 10(6):354. https://doi.org/10.3390/gels10060354
Chicago/Turabian StyleRubini, Katia, Arianna Menichetti, Maria Cristina Cassani, Marco Montalti, Adriana Bigi, and Elisa Boanini. 2024. "The Role of WO3 Nanoparticles on the Properties of Gelatin Films" Gels 10, no. 6: 354. https://doi.org/10.3390/gels10060354
APA StyleRubini, K., Menichetti, A., Cassani, M. C., Montalti, M., Bigi, A., & Boanini, E. (2024). The Role of WO3 Nanoparticles on the Properties of Gelatin Films. Gels, 10(6), 354. https://doi.org/10.3390/gels10060354









