Insight into CMC-PVA-fHNTs Nanocomposite Hydrogel as an Advance Carrier for Cefadroxil Monohydrate: Fabrication and Characterization/Angiogenic Potential Analysis
Abstract
1. Introduction
2. Results and Discussions
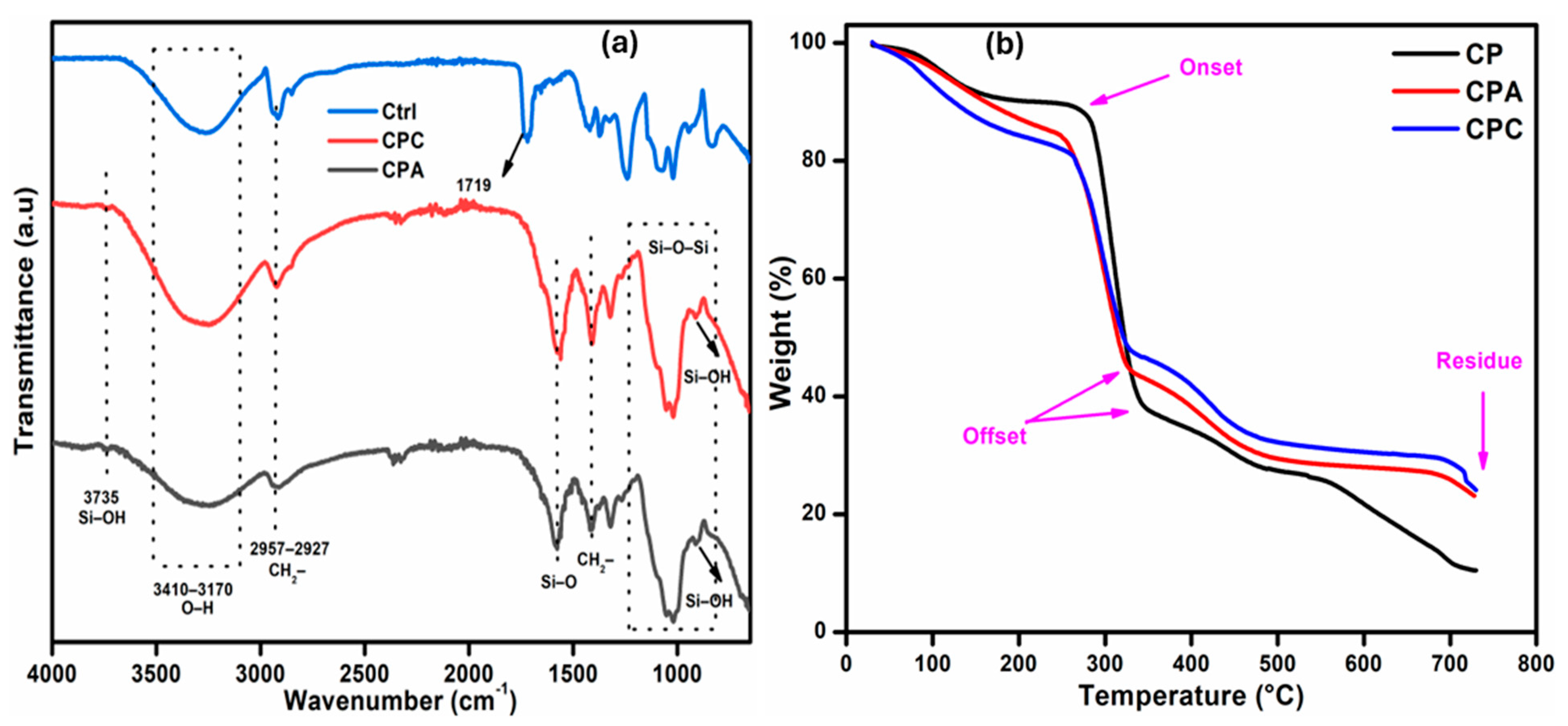
2.1. FTIR Analysis
2.2. Thermogravimetric Analysis
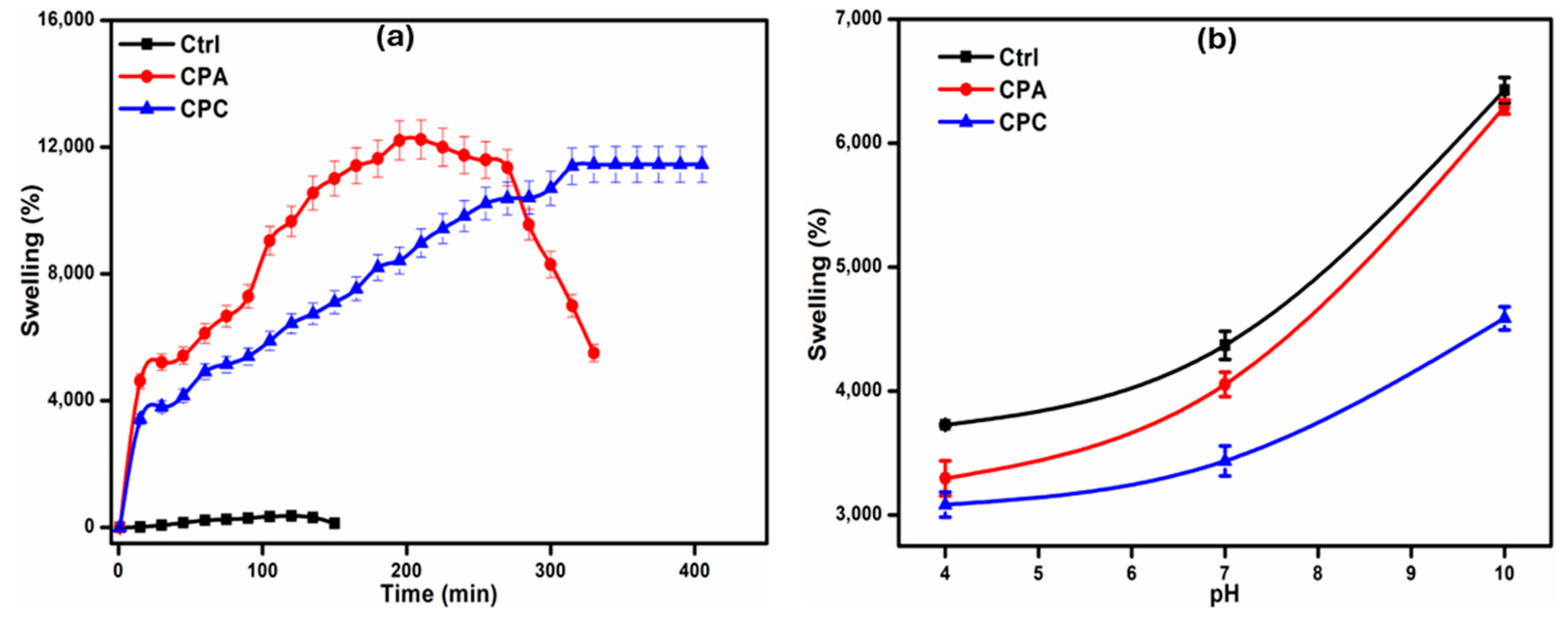
2.3. Swelling Ability of Hydrogels
2.3.1. Swelling in Water
2.3.2. Swelling in Buffer Solutions
2.4. Porosity
2.5. Hydrophilicity
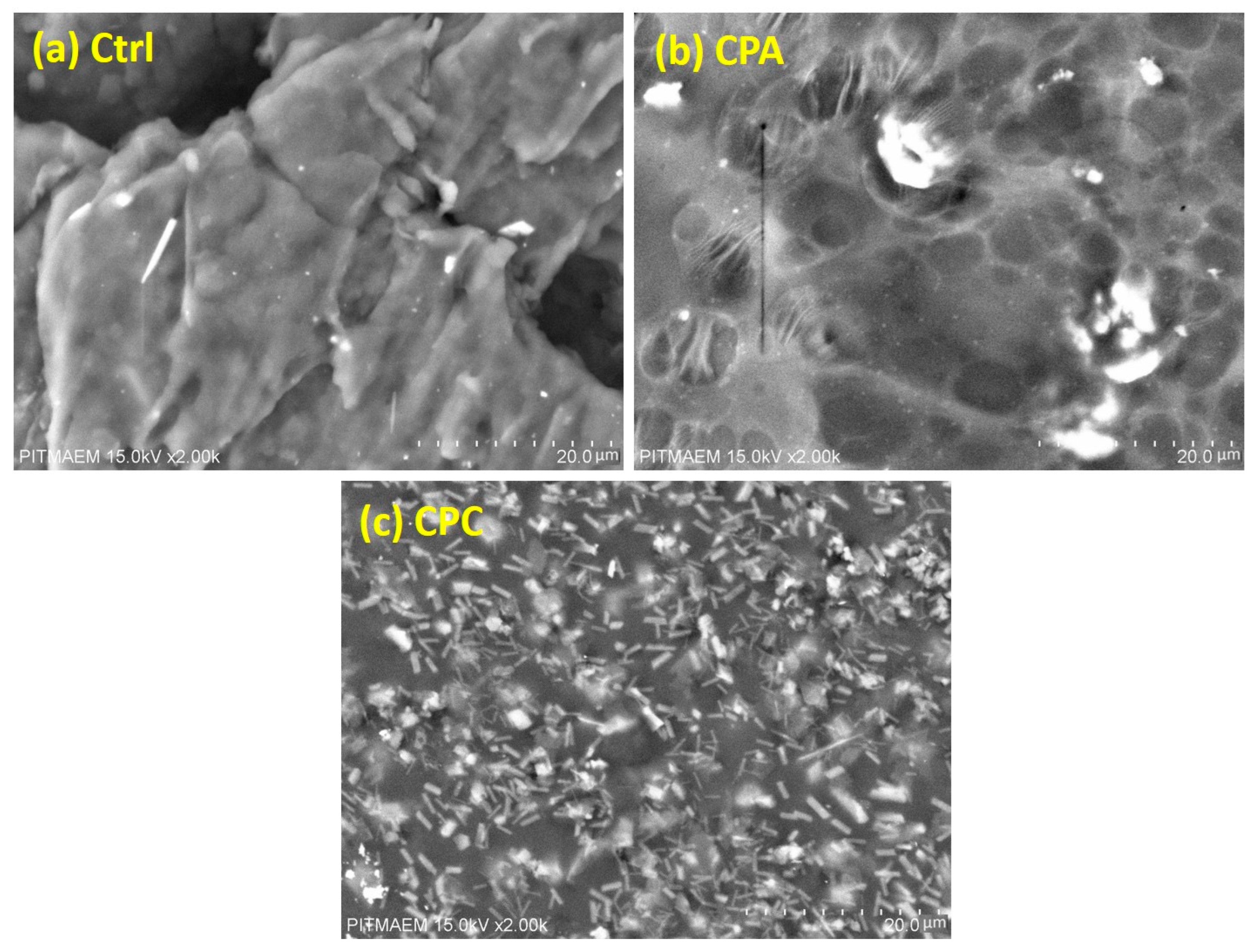
2.6. Scanning Electron Microscopy
2.7. Rheological Analysis
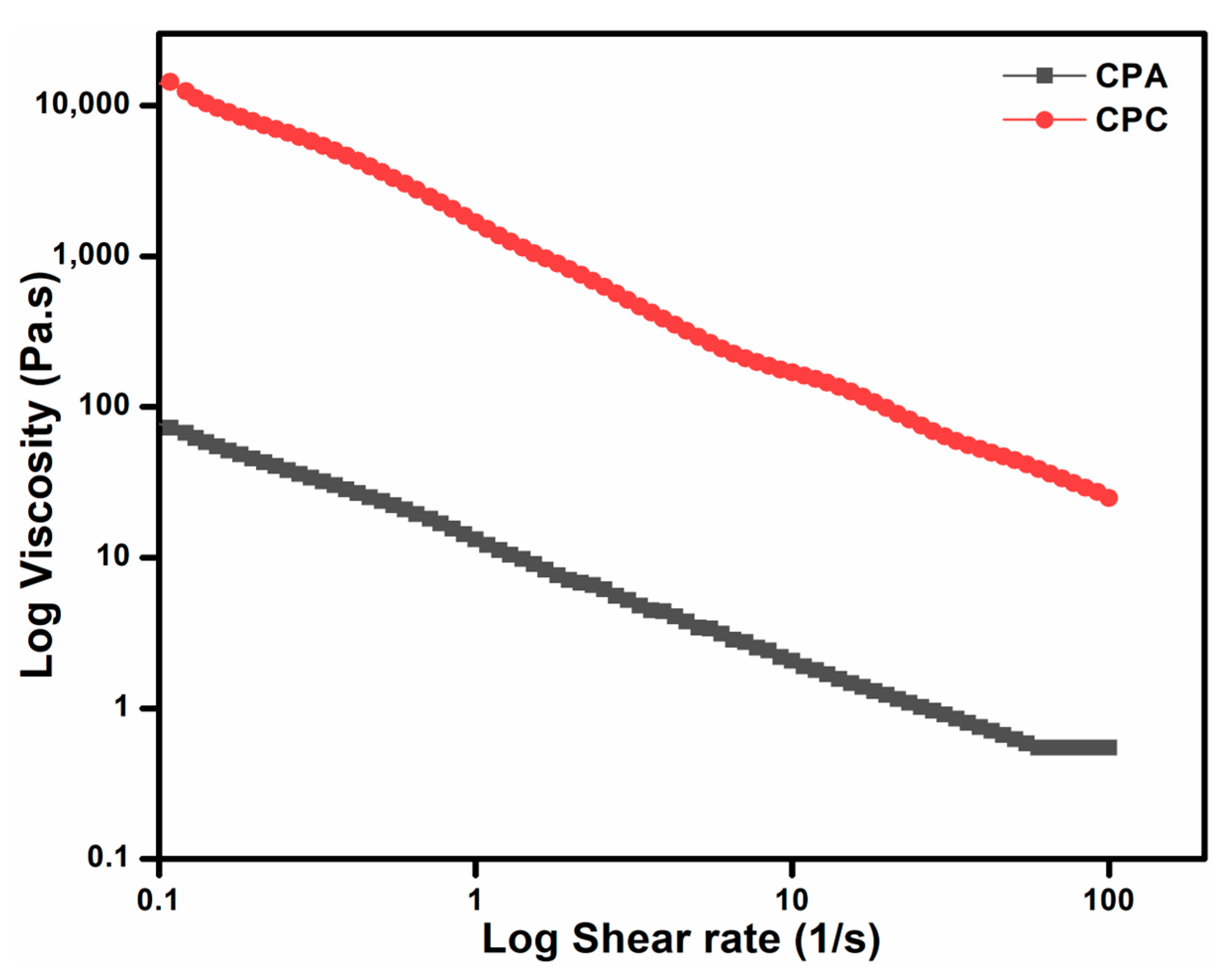
2.7.1. Steady-State Flow Behavior/Thixotropic Behavior
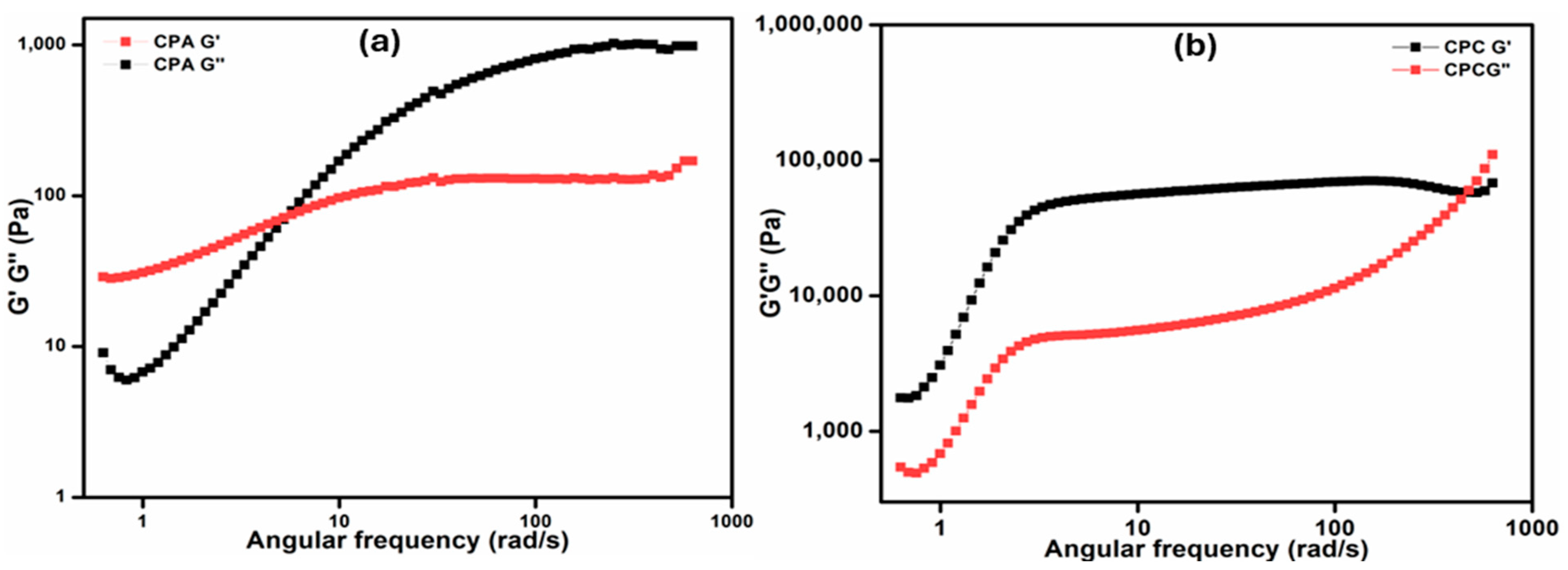
2.7.2. Frequency Sweep Test
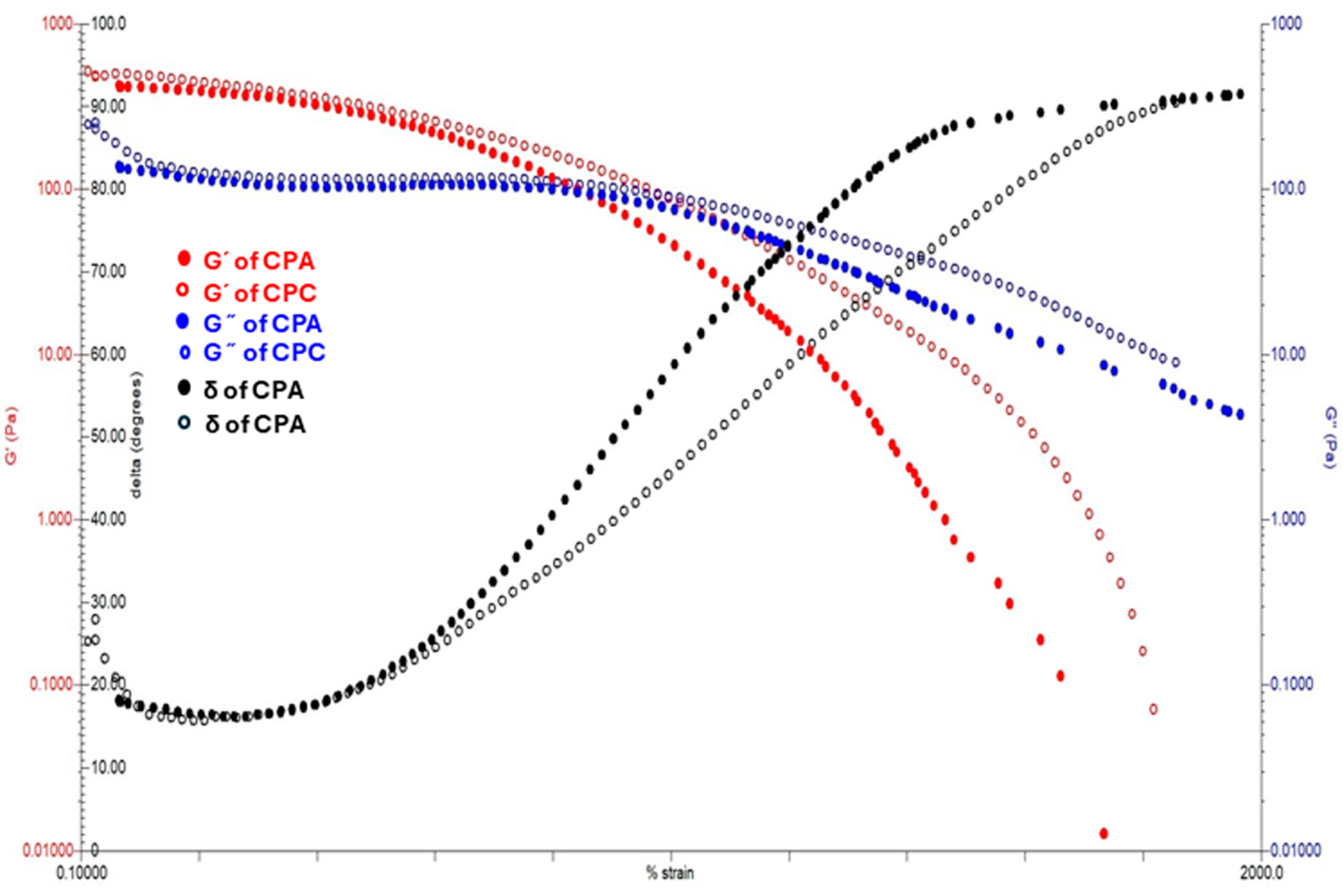
2.7.3. Strain Sweep Test
2.7.4. Rheological Models
2.8. Antibacterial Properties
2.9. Cytotoxicity
2.10. Drug Release Analysis
2.11. In Vivo Analysis
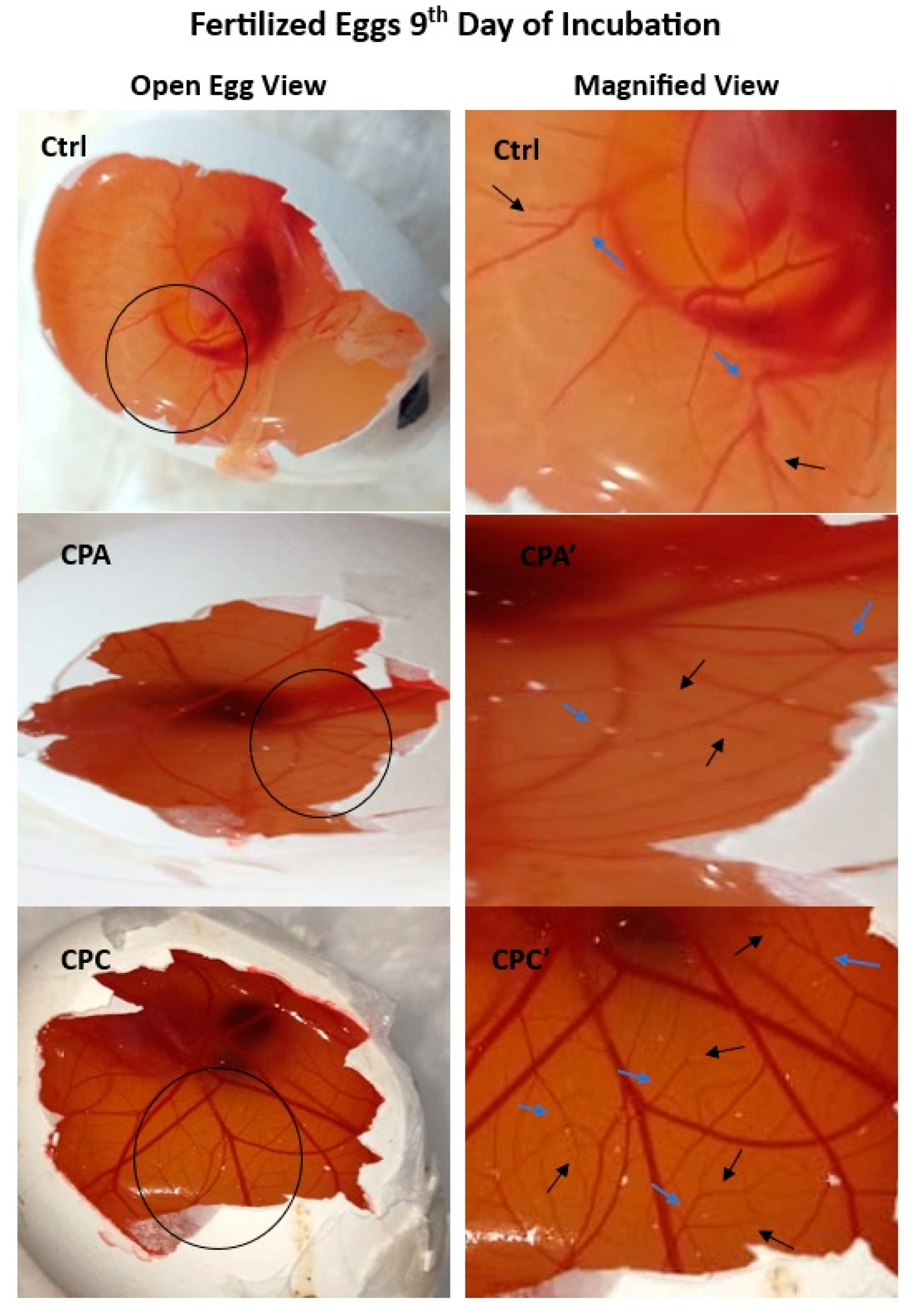
2.11.1. Angiogenic Potential of Hydrogels
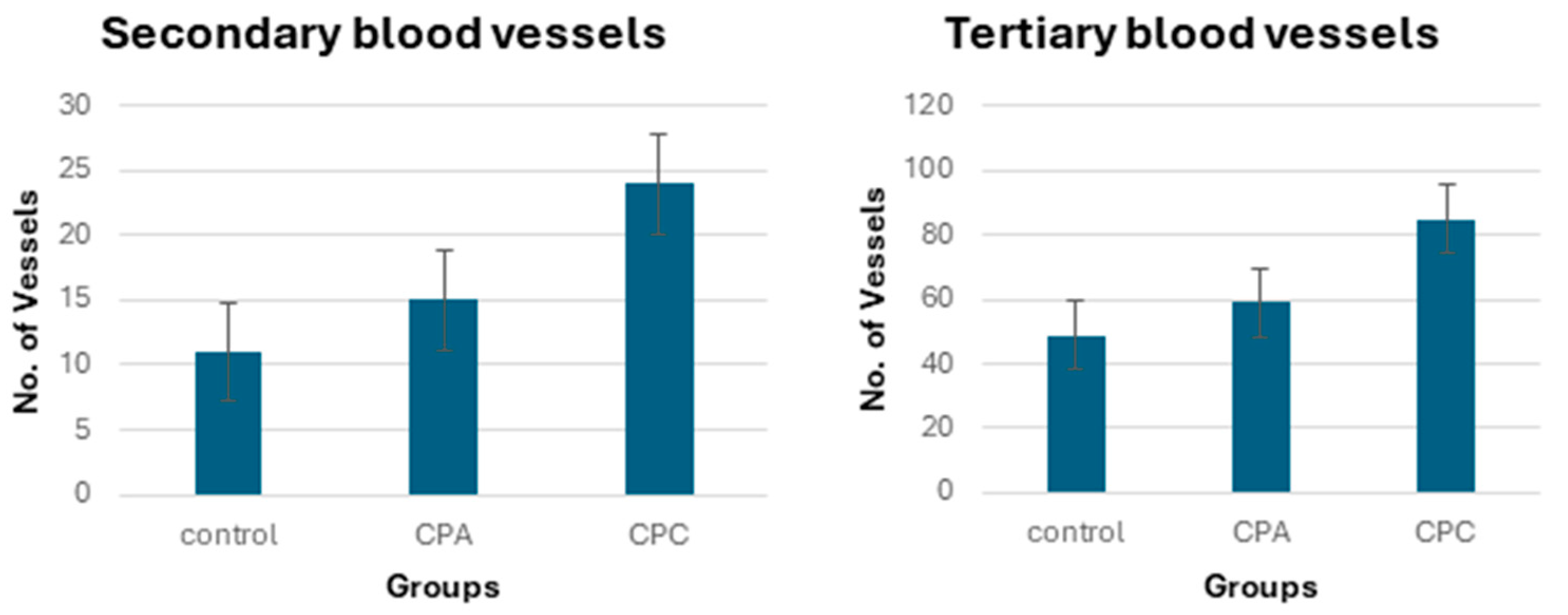
2.11.2. Quantification of Blood Vessels
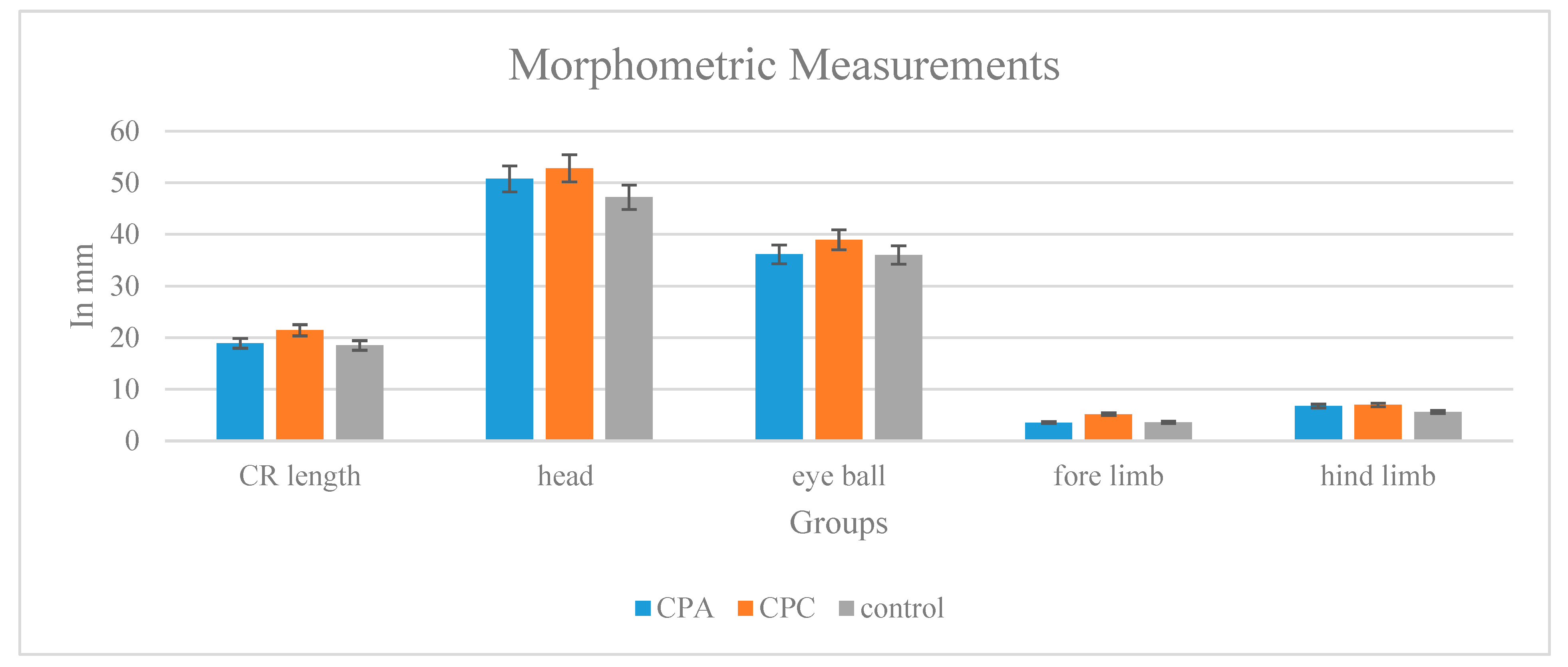
2.11.3. Toxicity Testing Morphological and Morphometric Analyses
2.11.4. Amniotic Fluid Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Method
4.2.1. Functionalization of HNTs
4.2.2. Fabrication of Nanocomposite Hydrogel
4.2.3. Fabrication of Drug-Loaded Nanocomposite Hydrogel
4.3. Fourier Transform Infrared Spectroscopy
4.4. Swelling Experiments
4.4.1. Swelling in Distilled Water
4.4.2. Swelling in Buffer Solutions
4.5. Porosity
4.6. Hydrophilicity
4.7. TGA
4.8. Scanning Electron Microscopy
4.9. Rheological Properties
4.10. Rheological Models
4.11. Bio-Assessment Tests
4.11.1. Antimicrobial Activity
4.11.2. In Vitro Cytotoxicity Analysis
4.11.3. In Vitro Drug Release Profile
4.11.4. Chorioallantoic Membrane Assay for the Assessment of Angiogenic Properties of Hydrogels and Toxico-Pathological Analyses
4.11.5. Digital Imaging, Amniotic Fluid Sampling, and Embryos Recovery
4.11.6. Enzyme Assays
4.11.7. Morphological Observations
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Gull, N.; Khan, S.M.; Khalid, S.; Zia, S.; Islam, A.; Sabir, A.; Sultan, M.; Hussain, F.; Khan, R.U.; Butt, M.T.Z. Designing of biocompatible and biodegradable chitosan based crosslinked hydrogel for in vitro release of encapsulated povidone-iodine: A clinical translation. Int. J. Biol. Macromol. 2020, 164, 4370–4380. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.G.; Hwang, S.Y.; Na, Y.H. Fabrication of a PVA-Based hydrogel microneedle patch. ACS Omega 2022, 7, 25179–25185. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, P.C.; Edgren, D. Crosslinking reaction of poly (vinyl alcohol) with glyoxal. J. Polym. Res. 2010, 17, 725–730. [Google Scholar] [CrossRef]
- Morandim-Giannetti, A.A.; Rubio, S.R.; Nogueira, R.F.; Ortega, F.D.S.; Magalhães Junior, O.; Schor, P.; Bersanetti, P.A. Characterization of PVA/glutaraldehyde hydrogels obtained using Central Composite Rotatable Design (CCRD). J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Redy Keisar, O.; Nahum, V.; Yehezkel, L.; Marcovitch, I.; Columbus, I.; Fridkin, G.; Chen, R. Active and strippable PVA/Borax/NaBO3 hydrogel for effective containment and decontamination of chemical warfare agents. ACS Omega 2021, 6, 5359–5367. [Google Scholar] [CrossRef] [PubMed]
- Varshney, N.; Sahi, A.K.; Poddar, S.; Vishwakarma, N.K.; Kavimandan, G.; Prakash, A.; Mahto, S.K. Freeze–thaw-induced physically cross-linked superabsorbent polyvinyl alcohol/soy protein isolate hydrogels for skin wound dressing: In vitro and in vivo characterization. ACS Appl. Mater. Interfaces 2022, 14, 14033–14048. [Google Scholar] [CrossRef]
- Ou, K.; Dong, X.; Qin, C.; Ji, X.; He, J. Properties and toughening mechanisms of PVA/PAM double-network hydrogels prepared by freeze-thawing and anneal-swelling. Mater. Sci. Eng. C 2017, 77, 1017–1026. [Google Scholar] [CrossRef]
- Gull, N.; Khan, S.M.; Butt, M.T.Z.; Zia, S.; Khalid, S.; Islam, A.; Sajid, I.; Khan, R.U.; King, M.W. Hybrid cross-linked hydrogels as a technology platform for in vitro release of cephradine. Polym. Adv. Technol. 2019, 30, 2414–2424. [Google Scholar] [CrossRef]
- Yu, K.; Zhou, L.; Xu, J.; Jiang, F.; Zhong, Z.; Zou, L.; Liu, W. Carboxymethyl cellulose-based water barrier coating regulated postharvest quality and ROS metabolism of pakchoi (Brassica chinensis L.). Postharvest Biol. Technol. 2021, 185, 111804. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hasan, M.S.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, M.S.; Shiddiky, M.J.; Ahmed, M.B. Recent Developments of Carboxymethyl Cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Xuan, Y.; Zhang, S. Synthesis and Applications of Carboxymethyl Cellulose Hydrogels. Gels 2022, 8, 529. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, H.; Khan, B.A.; Khan, Z.U.; Razzaq, A.; Khan, N.U.; Menaa, B.; Menaa, F. Fabrication, physical characterizations and in vitro antibacterial activity of cefadroxil-loaded chitosan/poly(vinyl alcohol) nanofibers against Staphylococcus aureus clinical isolates. Int. J. Biol. Macromol. 2019, 144, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, V.; Thakur, A. Carboxymethyl cellulose-polyvinyl alcohol based materials: A review. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Qureshi, M.A.U.R.; Arshad, N.; Rasool, A.; Rizwan, M.; Rasheed, T. Guar gum-based stimuli responsive hydrogels for sustained release of diclofenac sodium. Int. J. Biol. Macromol. 2023, 250, 126275. [Google Scholar] [CrossRef] [PubMed]
- Aloui, H.; Khwaldia, K.; Hamdi, M.; Fortunati, E.; Kenny, J.; Buonocore, G.; Lavorgna, M. Synergistic Effect of Halloysite and Cellulose Nanocrystals on Functional Properties of PVA Based Nanocomposites. ACS Sustain. Chem. Eng. 2015, 4, 794–800. [Google Scholar] [CrossRef]
- Idumah, C.I.; Hassan, A.; Ogbu, J.; Ndem, J.U.; Nwuzor, I.C. Recently emerging advancements in halloysite nanotubes polymer nanocomposites. Compos. Interfaces 2019, 26, 751–824. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, Z.; Dong, Y.; Lu, W.; Zhu, D. The Preparation and Properties of Composite Hydrogels Based on Gelatin and (3-Aminopropyl) Trimethoxysilane Grafted Cellulose Nanocrystals Covalently Linked with Microbial Transglutaminase. Gels 2022, 8, 146. [Google Scholar] [CrossRef]
- Sethi, V.; Kaur, M.; Thakur, A.; Rishi, P.; Kaushik, A. Unravelling the role of hemp straw derived cellulose in CMC/PVA hydrogel for sustained release of fluoroquinolone antibiotic. Int. J. Biol. Macromol. 2022, 222, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Ghorpade, V.S.; Dias, R.J.; Mali, K.K.; Mulla, S.I. Citric acid crosslinked carboxymethylcellulose-polyvinyl alcohol hydrogel films for extended release of water soluble basic drugs. J. Drug Deliv. Sci. Technol. 2019, 52, 421–430. [Google Scholar] [CrossRef]
- Saadiah, M.; Zhang, D.; Nagao, Y.; Muzakir, S.; Samsudin, A. Reducing crystallinity on thin film based CMC/PVA hybrid polymer for application as a host in polymer electrolytes. J. Non-Crystalline Solids 2019, 511, 201–211. [Google Scholar] [CrossRef]
- Kaplan Can, H.; Rzaev, Z.M.; Güner, A. Synthesis and characterization of new hydrogels on the basis of water-soluble maleic anhydride copolymers with γ-aminopropyltriethoxysilane. J. Appl. Polym. Sci. 2003, 90, 4009–4015. [Google Scholar] [CrossRef]
- Nambiar, A.P.; Pillai, R.; Vadikkeettil, Y.; Sanyal, M.; Shrivastav, P.S. Glutaraldehyde-crosslinked poly(vinyl alcohol)/halloysite composite films as adsorbent for methylene blue in water. Mater. Chem. Phys. 2022, 291, 126752. [Google Scholar] [CrossRef]
- Azmi, S.; Razak, S.I.A.; Kadir, M.R.A.; Iqbal, N.; Hassan, R.; Nayan, N.H.M.; Wahab, A.H.A.; Shaharuddin, S. Reinforcement of poly (vinyl alcohol) hydrogel with halloysite nanotubes as potential biomedical materials. Soft Mater 2017, 15, 45–54. [Google Scholar] [CrossRef]
- Tajeddin, B.; Ramedani, N. Preparation and Characterization (Mechanical and Water Absorption Properties) of CMC/PVA/Clay Nanocomposite Films. Iran. J. Chem. Chem. Eng. 2016, 35, 9–15. [Google Scholar]
- Farid, E.; Kamoun, E.A.; Taha, T.H.; El-Dissouky, A.; Khalil, T.E. PVA/CMC/Attapulgite Clay Composite Hydrogel Membranes for Biomedical Applications: Factors Affecting Hydrogel Membranes Crosslinking and Bio-evaluation Tests. J. Polym. Environ. 2022, 30, 4675–4689. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Q.; Tan, B.; Liu, L.; Shi, L. pH-Sensitive Poly(Vinyl Alcohol)/Sodium Carboxymethylcellulose Hydrogel Beads for Drug Delivery. J. Macromol. Sci. Part B 2011, 50, 2307–2317. [Google Scholar] [CrossRef]
- Kumar, B.; Sauraj; Negi, Y.S. To investigate the effect of ester-linkage on the properties of polyvinyl alcohol/carboxymethyl cellulose based hydrogel. Mater. Lett. 2019, 252, 308–312. [Google Scholar] [CrossRef]
- Aljar, M.A.A.; Rashdan, S.; El-Fattah, A.A. Environmentally Friendly Polyvinyl Alcohol−Alginate/Bentonite Semi-Interpenetrating Polymer Network Nanocomposite Hydrogel Beads as an Efficient Adsorbent for the Removal of Methylene Blue from Aqueous Solution. Polymers 2021, 13, 4000. [Google Scholar] [CrossRef]
- Hashmi, M.; Ullah, S.; Ullah, A.; Akmal, M.; Saito, Y.; Hussain, N.; Ren, X.; Kim, I.S. Optimized Loading of Carboxymethyl Cellulose (CMC) in Tri-component Electrospun Nanofibers Having Uniform Morphology. Polymers 2020, 12, 2524. [Google Scholar] [CrossRef]
- Patwa, R.; Saha, N.; Sáha, P. Magnetic hydrogel based shoe insoles for prevention of diabetic foot. J. Magn. Magn. Mater. 2020, 514, 167153. [Google Scholar] [CrossRef]
- Bucak, C.D.; Sahin, M.O. Super-flexible, moldable, injectable, self-healing PVA/B/CMC hydrogels synthesis and characterization, as potential water-retaining agent in agriculture. Polym. Bull. 2023, 80, 6591–6608. [Google Scholar] [CrossRef]
- Xue, F.; He, X.; Cai, S.; Nie, J.; Shi, Z.; Wang, X. Synergistic effect of graphene oxide and sodium carboxymethylcellulose on the properties of poly(vinyl alcohol) hydrogels. J. Appl. Polym. Sci. 2019, 136, 47644. [Google Scholar] [CrossRef]
- Nair, R.; Choudhury, A.R. Synthesis and rheological characterization of a novel shear thinning levan gellan hydrogel. Int. J. Biol. Macromol. 2020, 159, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Dávila, J.L.; D’ávila, M.A. Laponite as a rheology modifier of alginate solutions: Physical gelation and aging evolution. Carbohydr. Polym. 2017, 157, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jalalvandi, E.; Shavandi, A. Shear thinning/self-healing hydrogel based on natural polymers with secondary photocrosslinking for biomedical applications. J. Mech. Behav. Biomed. Mater. 2018, 90, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xu, Q.; Tao, Y.; Wang, X. Rheological and pH dependent properties of injectable and controlled release hydrogels based on mushroom hyperbranched polysaccharide and xanthan gum. Carbohydr. Polym. Technol. Appl. 2021, 2, 100063. [Google Scholar] [CrossRef]
- Kumar, L.; Deshmukh, R.K.; Hakim, L.; Gaikwad, K.K. Halloysite Nanotube as a Functional Material for Active Food Packaging Application: A Review. Food Bioprocess Technol. 2023, 17, 33–46. [Google Scholar] [CrossRef]
- Huang, W.H.; Hung, C.Y.; Chiang, P.C.; Lee, H.; Lin, I.T.; Lai, P.C.; Chan, Y.H.; Feng, S.W. Physicochemical Characterization, Biocompatibility, and Antibacterial Properties of CMC/PVA/Calendula officinalis Films for Biomedical Applications. Polymers 2023, 15, 1454. [Google Scholar] [CrossRef]
- Gull, N.; Khan, S.M.; Butt, O.M.; Islam, A.; Shah, A.; Jabeen, S.; Khan, S.U.; Khan, A.; Khan, R.U.; Butt, M.T.Z. Inflammation targeted chitosan-based hydrogel for controlled release of diclofenac sodium. Int. J. Biol. Macromol. 2020, 162, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Feng, Y.; Zhou, Y.; Tan, C.; Liu, M. Gold@Halloysite nanotubes-chitin composite hydrogel with antibacterial and hemostatic activity for wound healing. Bioact. Mater. 2022, 20, 355–367. [Google Scholar] [CrossRef]
- Sajadi-Javan, Z.S.; Varshosaz, J.; Mirian, M.; Manshaei, M.; Aminzadeh, A. Thermo-responsive hydrogels based on methylcellulose/Persian gum loaded with taxifolin enhance bone regeneration: An in vitro/in vivo study. Cellulose 2022, 29, 2413–2433. [Google Scholar] [CrossRef]
- Wang, L.; Yang, F.; Zhao, X.; Li, Y. Effects of nitro- and amino-group on the antioxidant activity of genistein: A theoretical study. Food Chem. 2019, 275, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.; Sharifi, I.; Tavakkoli, H.; Derakhshanfar, A.; Keyhani, A.R.; Salari, Z.; Mosallanejad, S.S.; Bamorovat, M. Embryonic toxico-pathological effects of meglumine antimoniate using a chick embryo model. PLoS ONE 2018, 13, e0196424. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Southon, P.D.; Liu, Z.; Kepert, C.J. Organosilane functionalization of halloysite nanotubes for enhanced loading and controlled release. Nanotechnology 2012, 23, 375705. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.V.; Shivakumar, H.G. Investigation of Swelling Behavior and Mechanical Properties of a pH-Sensitive Superporous Hydrogel Composite. Iran. J. Pharm. Res. 2012, 11, 481–493. [Google Scholar] [PubMed]
- Pena-Francesch, A.; Montero, L.; Borrós, S. Tailoring the LCST of thermosensitive hydrogel thin films deposited by iCVD. Langmuir 2014, 30, 7162–7167. [Google Scholar] [CrossRef] [PubMed]
- Bercea, M. Rheology as a Tool for Fine-Tuning the Properties of Printable Bioinspired Gels. Molecules 2023, 28, 2766. [Google Scholar] [CrossRef]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between Structure and Rheology of Hydrogels for Various Applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef]
- Gull, N.; Khan, S.M.; Butt, M.T.Z.; Khalid, S.; Shafiq, M.; Islam, A.; Asim, S.; Hafeez, S.; Khan, R.U. In vitro study of chitosan-based multi-responsive hydrogels as drug release vehicles: A preclinical study. RSC Adv. 2019, 9, 31078–31091. [Google Scholar] [CrossRef]
- Ara, C.; Jabeen, S.; Afshan, G.; Farooq, A.; Akram, M.S.; Asmatullah; Islam, A.; Ziafat, S.; Nawaz, B.; Khan, R.U. Angiogenic potential and wound healing efficacy of chitosan derived hydrogels at varied concentrations of APTES in chick and mouse models. Int. J. Biol. Macromol. 2022, 202, 177–190. [Google Scholar] [CrossRef]

| Sample Codes | Porosity (%) | Hydrophilicity (°) | Gel Strength (%) |
|---|---|---|---|
| Ctrl | 78 | 69 | 67 |
| CPA | 69 | 65 | 78 |
| CPC | 55 | 62 | 74 |
| Sample Code | Power Law | Ostwald–De | Herschel–Bulkley | Bingham | Casson | Steiger/ Ory | ||
|---|---|---|---|---|---|---|---|---|
| m | n | R2 | R2 | R2 | R2 | R2 | R2 | |
| CPA | 14.4575 | 0.28 | 0.9916 | 0.9958 | 0.9958 | 0.8507 | 0.9853 | 0.8651 |
| CPC | 1644 | 0.47 | 0.9571 | 0.9134 | 0.5330 | 0.7899 | 0.7398 | 0.9916 |
| Sample Codes | Herschel–Bulkley Parameters | Steiger/Ory Parameters | |||
|---|---|---|---|---|---|
| τ0 (Pa) | K (Pa.sn) | n | K1 | K2 | |
| CPA | 0.5 | 14.456 | 0.2823 | 0.0139 | 0.28 |
| CPC | 1.2 | 1644.6 | 0.0591 | 1644 | 0.47 |
| Sample Code | Inhibition Zone (mm) | Mortality (%) | |
|---|---|---|---|
| E. coli | S. aureus | ||
| Ctrl | 2.56 | 4.84 | 4.58 |
| CPA | 3.27 | 7.52 | 6.41 |
| CPC | 6.59 | 11.27 | 7.89 |
| Parameters | Untreated | Treated Groups (Mean ± S.E) | |
|---|---|---|---|
| Control | CPA | CPC | |
| Bilirubin (mg/dL) | 0.76 ± 0.08 a | 0.23 ± 0.08 b | 0.23 ± 0.08 b |
| ALP (U/L) | 113.00 ± 1.15 a | 143.00 ± 2.15 c | 124.00 ± 2.07 b |
| ALT (U/L) | 7.66 ± 1.20 a | 10.00 ± 1.15 b | 8.33 ± 0.88 b |
| AST (U/L) | 9.33 ± 0.88 a | 13.00 ± 1.15 b | 10.33 ± 0.88 c |
| AST (U/L) | 9.33 ± 0.88 a | 13.00 ± 1.15 b | 10.33 ± 0.88 c |
| Creatinine (mg/dL) | 0.76 ± 0.08 b | 0.56 ± 0.12 b | 0.53 ± 0.88 b |
| Sample Codes | Na-CMC (g) | PVA (g) | APTS (μL) | fHNTs (g) |
|---|---|---|---|---|
| Ctrl | 0.5 | 0.5 | 0 | 0 |
| CPA | 0.5 | 0.5 | 50 | 0 |
| CPC | 0.5 | 0.5 | 0 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zia, S.; Khan, S.M.; Butt, M.T.Z.; Gull, N. Insight into CMC-PVA-fHNTs Nanocomposite Hydrogel as an Advance Carrier for Cefadroxil Monohydrate: Fabrication and Characterization/Angiogenic Potential Analysis. Gels 2024, 10, 235. https://doi.org/10.3390/gels10040235
Zia S, Khan SM, Butt MTZ, Gull N. Insight into CMC-PVA-fHNTs Nanocomposite Hydrogel as an Advance Carrier for Cefadroxil Monohydrate: Fabrication and Characterization/Angiogenic Potential Analysis. Gels. 2024; 10(4):235. https://doi.org/10.3390/gels10040235
Chicago/Turabian StyleZia, Saba, Shahzad Maqsood Khan, Muhammad Taqi Zahid Butt, and Nafisa Gull. 2024. "Insight into CMC-PVA-fHNTs Nanocomposite Hydrogel as an Advance Carrier for Cefadroxil Monohydrate: Fabrication and Characterization/Angiogenic Potential Analysis" Gels 10, no. 4: 235. https://doi.org/10.3390/gels10040235
APA StyleZia, S., Khan, S. M., Butt, M. T. Z., & Gull, N. (2024). Insight into CMC-PVA-fHNTs Nanocomposite Hydrogel as an Advance Carrier for Cefadroxil Monohydrate: Fabrication and Characterization/Angiogenic Potential Analysis. Gels, 10(4), 235. https://doi.org/10.3390/gels10040235








