Exploring the Impact of the Synthesis Variables Involved in the Polyurethane Aerogels-like Materials Design
Abstract
1. Introduction
2. Results and Discussion
2.1. Influence of the Synthesis Variables Involved in the Polyurethane Aerogel Design
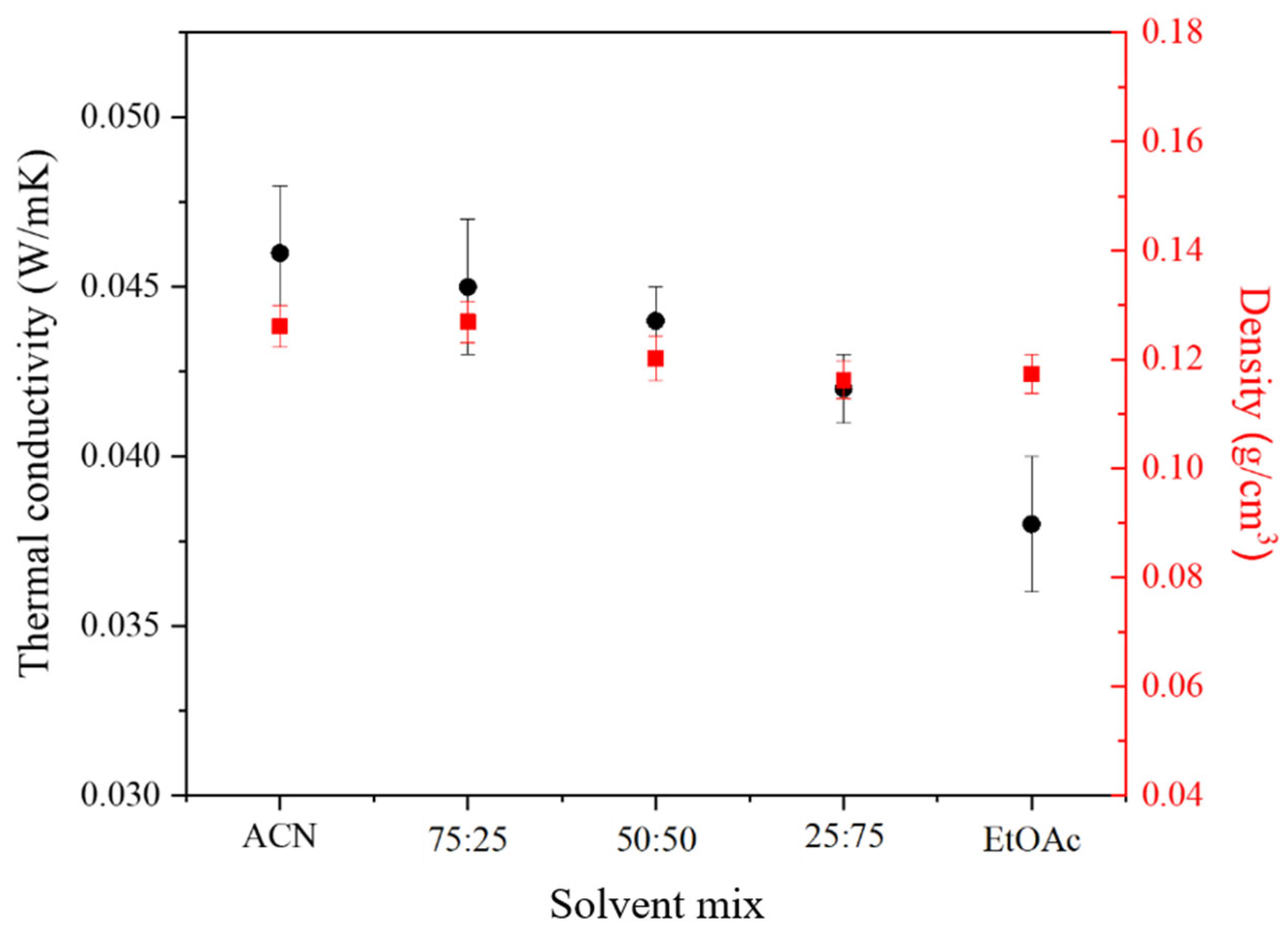
2.1.1. Influence of the Nature of the Organic Solvent
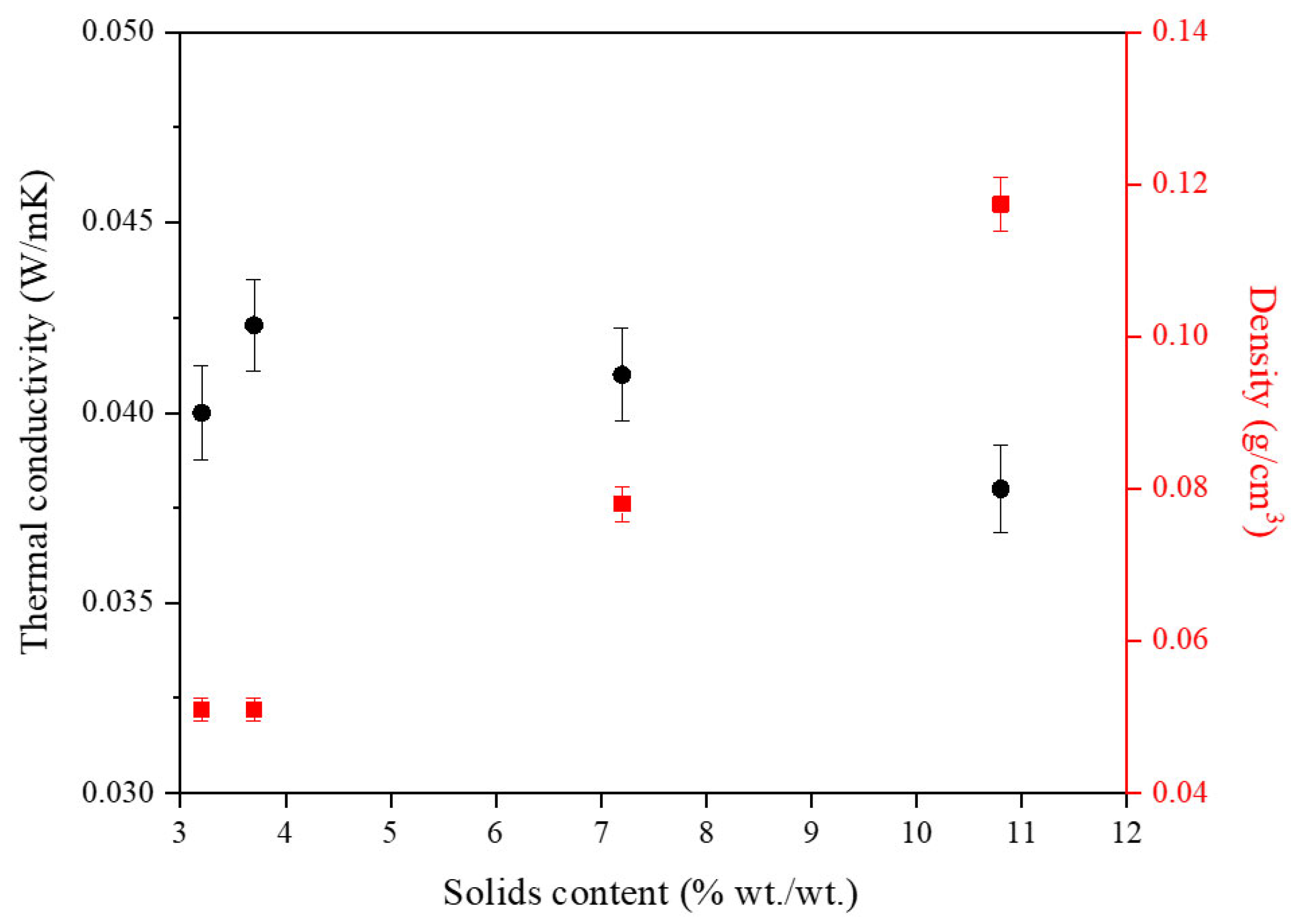
2.1.2. Influence of Solids Content
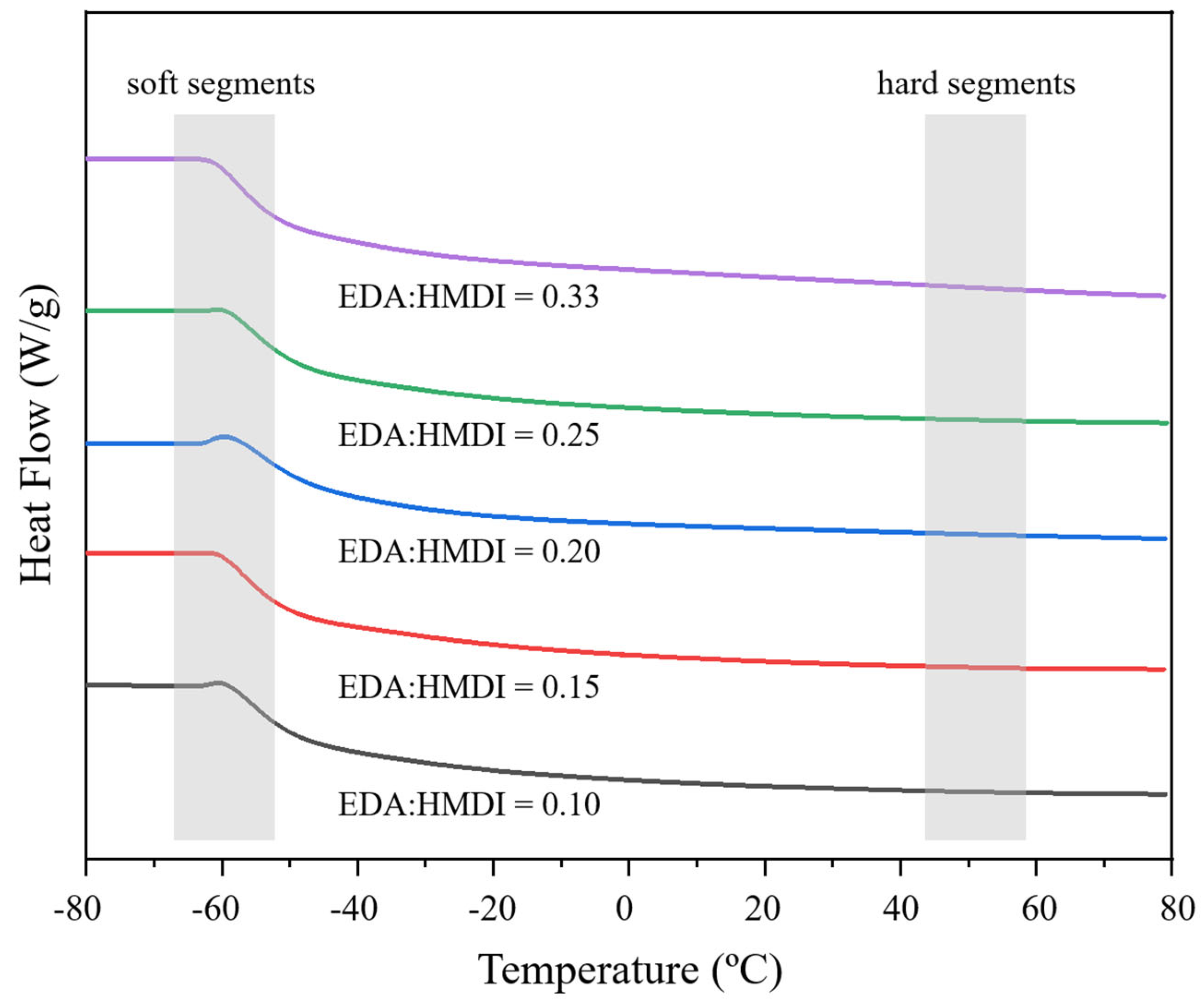
2.1.3. Influence of Chain Extender/Isocyanate Ratio
2.1.4. Influence of Polymer Dispersion Mode
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Synthesis Procedure of Waterborne Polyurethane Aerogels
4.3. Characterization Techniques of Waterborne Polyurethane Aerogels
4.3.1. Density
4.3.2. Thermal Conductivity
4.3.3. Shrinkage Ratios
4.3.4. Mechanical Characteristics
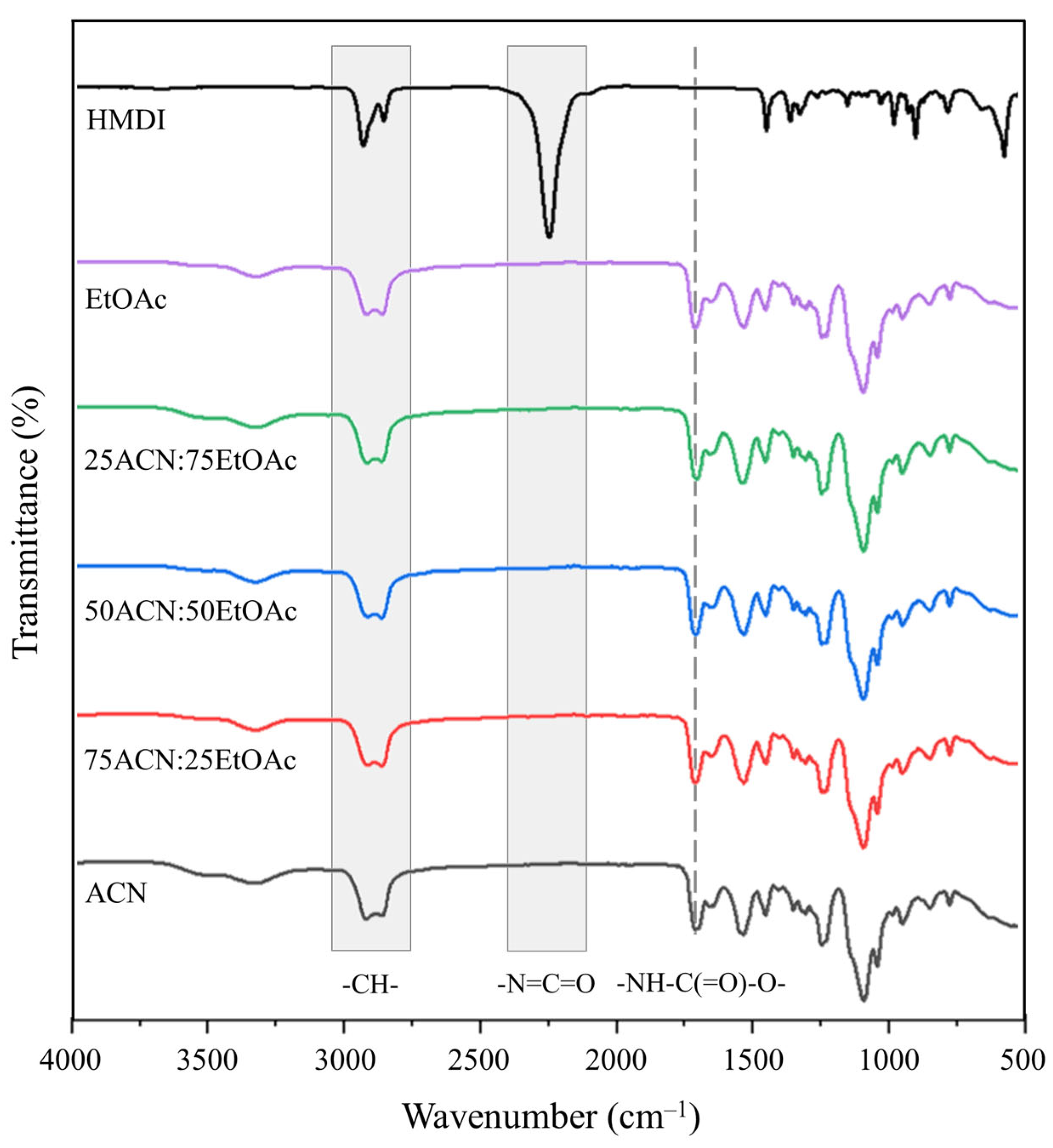
4.3.5. Chemical Structure
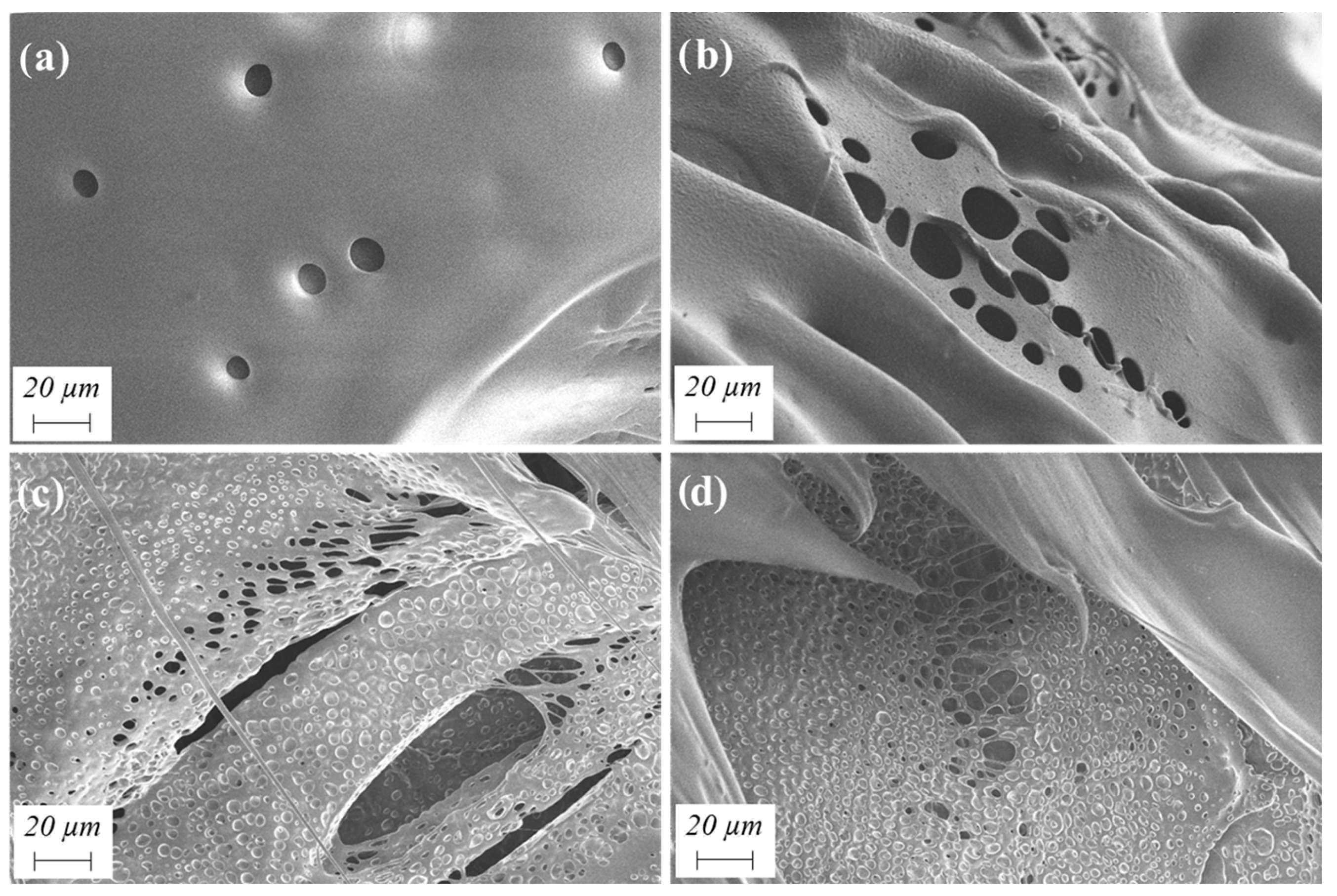
4.3.6. Morphological Structure
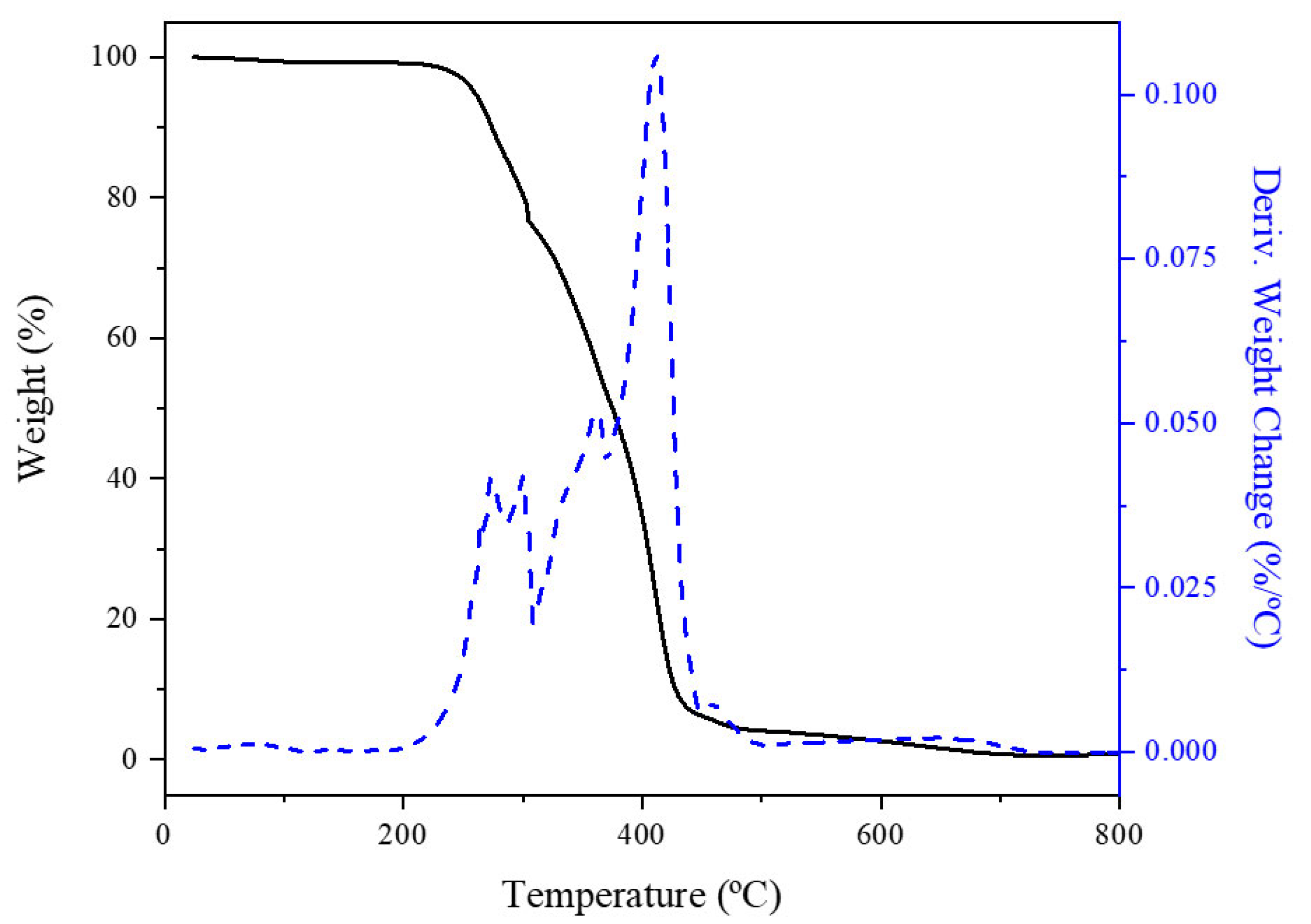
4.3.7. Thermogravimetric Analysis
4.3.8. Contact Angle
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghalandari, M.; Mukhtar, A.; Yasir, A.S.H.M.; Alkhabbaz, A.; Alviz-Meza, A.; Cárdenas-Escrocia, Y.; Le, B.N. Thermal conductivity improvement in a green building with Nano insulations using machine learning methods. Energy Rep. 2023, 9, 4781–4788. [Google Scholar] [CrossRef]
- Atbir, A.; Taibi, M.; Aouan, B.; Khabbazi, A.; Ansari, O.; Cherkaoui, M.; Cheradi, T. Physicochemical and thermomechanical performances study for Timahdite sheep wool fibers application in the building’s insulation. Sci. Rep. 2023, 13, 5038–5053. [Google Scholar] [CrossRef] [PubMed]
- Członka, S.; Bertino, M.F.; Kośny, J.; Shukla, N. Density and shrinkage as guiding criteria for the optimization of the thermal conductivity of poly(urethane)-class aerogels. J. Solgel. Sci. Technol. 2020, 93, 149–167. [Google Scholar] [CrossRef]
- Simón-Herrero, C.; Chen, X.Y.; Ortiz, M.L.; Valverde, J.L.; Sánchez-Silva, L. Linear and crosslinked polyimide aerogels: Synthesis and characterization. J. Mater. Res. Technol. 2019, 8, 2638–2648. [Google Scholar] [CrossRef]
- Członka, S.; Bertino, M.F.; Kośny, J.; Shukla, N. Freeze-drying method as a new approach to the synthesis of polyurea aerogels from isocyanate and water. J. Mater. Res. Technol. 2018, 87, 685–695. [Google Scholar] [CrossRef]
- Liu, W.; Herrmann, A.K.; Bigall, N.C.; Rodríguez, P.; Wen, D.; Oezaslan, M.; Schmidt, T.J.; Gaponik, N.; Eychmüller, A. Noble metal aerogels-synthesis, characterization, and application as electrocatalysts. Acc. Chem. Res. 2015, 48, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Ahmad, S.; Sheikh, J.N. Silica centered aerogels as advanced functional material and their applications: A review. J. Non Cryst. Solids 2023, 611, 122322–122338. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Zhang, Y.; Huang, Y.; Liu, J.; Zhang, X.; Liu, X.; Teng, H.; Zhang, X.; Zhang, J.; et al. A review of graphene-based materials/polymer composite aerogels. Polymers 2023, 15, 1888. [Google Scholar] [CrossRef]
- Basak, S.; Singhal, R.S. The potential of supercritical drying as a “green” method for the production of food-grade bioaerogels: A comprehensive critical review. Food Hydrocoll. 2023, 141, 108738–108767. [Google Scholar] [CrossRef]
- Fantucci, S.; Fenoglio, E.; Grosso, G.; Serra, V.; Perino, M.; Marino, V.; Dutto, M. Development of an aerogel-based thermal coating for the energy retrofit and the prevention of condensation risk in existing buildings. Sci. Technol. Built. Environ. 2019, 25, 1178–1186. [Google Scholar] [CrossRef]
- Rusch, P.; Schremmer, B.; Strelow, C.; Mews, A.; Dorfs, D.; Bigall, N.C. Nanocrystal aerogels with coupled or decoupled building blocks. J. Phys. Chem. Lett. 2019, 10, 7804–7810. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Snellings, G.; Koebel, M.M.; Malfait, W.J. Superinsulating polyisocyanate based aerogels: A targeted search for the optimum solvent system. ACS Appl. Mater. Interfaces 2017, 9, 18222–18230. [Google Scholar] [CrossRef]
- Dorcheh, A.S.; Abbasi, M.H. Silica aerogel; synthesis, properties and characterization. J. Mater. Process Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Gurav, J.L.; Jung, I.K.; Park, H.H.; Kang, E.S.; Nadargi, D.Y. Silica aerogel: Synthesis and applications. J. Nanomater. 2010, 2010, 409310. [Google Scholar] [CrossRef]
- Aney, S.; Schettler, J.; Schwan, M.; Milow, B.; Rege, A. Insights into the micromechanics of organic aerogels based on experimental and modeling results. Adv. Eng. Mater. 2022, 24, 2100095–2100102. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Maldonado-Hódar, F.J. Carbon aerogels for catalysis applications: An overview. Carbon 2005, 43, 455–465. [Google Scholar] [CrossRef]
- Liu, Z.; Ran, Y.; Xi, J.; Wang, J. Polymeric hybrid aerogels and their biomedical applications. J. Soft Matter 2020, 16, 9160–9175. [Google Scholar] [CrossRef]
- Biesmans, G.; Randall, D.; Francais, E.; Perrut, M. Polyurethane-based organic aerogels’ thermal performance. J. Non Cryst. Solids 1998, 225, 36–40. [Google Scholar] [CrossRef]
- Rigacci, A.; Marechal, J.C.; Repoux, M.; Moreno, M.; Achard, P. Preparation of polyurethane-based aerogels and xerogels for thermal superinsulation. J. Non Cryst. Solids 2004, 350, 372–378. [Google Scholar] [CrossRef]
- Diascorn, N.; Calas, S.; Sallée, H.; Achard, P.; Rigacci, A. Polyurethane aerogels synthesis for thermal insulation—Textural, thermal and mechanical properties. J. Supercrit. Fluids 2015, 106, 76–84. [Google Scholar] [CrossRef]
- Chidambareswarapattar, C.; McCarver, P.M.; Luo, H.; Lu, H.; Sotiriou-Leventis, C.; Leventis, N. Fractal multiscale nanoporous polyurethanes: Flexible to extremely rigid aerogels from multifunctional small molecules. Chem. Mater. 2013, 25, 3205–3224. [Google Scholar] [CrossRef]
- Meng, L.; Shi, X.; Zhang, R.; Yan, L.; Liang, Z.; Nie, Y.; Zhou, Z.; Hao, T. Preparation and properties study of waterborne polyurethane synthesized by mixing polyester diols and isocyanates. J. Appl. Polym. Sci. 2020, 137, 49314–49324. [Google Scholar] [CrossRef]
- Cakić, S.M.; Ristić, I.S.; Ristić, O.Z. Thermal analysis of polyurethane dispersions based on different polyols. In Polyurethane, 1st ed.; Zafar, F., Sharmin, E., Eds.; IntechOpen: Rijeka, Croatia, 2012; pp. 79–100. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, Y.; Cheng, X.; Yang, J.; Zhou, B.; Mi, L.; Hu, Y. Investigation on Hansen solubility parameter, solvent effect and thermodynamic properties of 3-methylflavone-8-carboxylic acid dissolved in different solvents. J. Mol. Liq. 2022, 356, 119017–119030. [Google Scholar] [CrossRef]
- Shen, Y.; Bi, Y.; Zhao, P.; Yang, X.; Zhang, Z.; Dang, D.; Wang, H.; Liu, W. Equilibrium solubility of 6-propyl-2-thiouracil in nine pure solvents: Determination, correlation, Hansen solubility parameter and thermodynamic properties. J. Indian Chem. Soc. 2023, 100, 100934–100940. [Google Scholar] [CrossRef]
- Cheng, K.; Wang, L.; Yu, Y.; Xiang, Y.; Bai, W.; Xu, L.; Li, H. Solubility and thermodynamic analysis of 1-(4-tert-butyl-2,6-dimethylphenyl)ethenone in different pure and binary solvents. J. Chem. Thermodyn. 2023, 180, 107019–107029. [Google Scholar] [CrossRef]
- Choi, S.M.; Lee, S.Y.; Lee, S.; Han, S.S.; Shin, E.J. In situ synthesis of environmentally friendly waterborne polyurethane extended with regenerated cellulose nanoparticles for enhanced mechanical performances. Polymers 2023, 15, 1541–1557. [Google Scholar] [CrossRef] [PubMed]
- Rogulska, M. The influence of diisocyanate structure on thermal stability of thermoplastic polyurethane elastomers based on diphenylmethane-derivative chain extender with sulfur atoms. Materials 2023, 16, 2618–2636. [Google Scholar] [CrossRef]
- Kayalvizhi, M.; Vakees, E.; Suresh, J.; Arun, A. Poly(urethane–urea) based on functionalized polystyrene with HMDI: Synthesis and characterization. Arab. J. Chem. 2019, 12, 2484–2491. [Google Scholar] [CrossRef]
- Kwon, Y.R.; Kim, H.C.; Moon, S.K.; Kim, J.S.; Chang, Y.W.; Kim, D.H. Facile preparation and characterization of low-gloss waterborne polyurethane coatings using amine-based chain extenders. Polym. Int. 2023, 72, 54–60. [Google Scholar] [CrossRef]
- He, R.; Xie, C.; Chen, Y.; Guo, Z.X.; Guo, B.; Tuo, B. Robust and highly resilient waterborne polyurethane-based composite aerogels prepared by blending with aramid nanofibers. Compos. Sci. Technol. 2022, 228, 109622–109630. [Google Scholar] [CrossRef]
- Vareda, J.P.; Lamy-Mendes, A.; Durães, L. A reconsideration on the definition of the term aerogel based on current drying trends. Microporous Mesoporous Mater. 2018, 258, 211–216. [Google Scholar] [CrossRef]
- Cantero, D.; Pinilla-Peñalver, E.; Romero, A.; Sánchez-Silva, L. Synthesis of waterborne polyurethane aerogels-like materials via freeze-drying: An innovative approach. J. Mater. Sci. 2023, 58, 9087–9102. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- Huang, L.J.; Lee, W.J.; Chen, Y.C. Bio-based hydrogel and aerogel composites prepared by combining cellulose solutions and waterborne polyurethane. Polymers 2022, 14, 204–218. [Google Scholar] [CrossRef] [PubMed]
- Shinko, A.; Jana, S.C.; Meador, M.A. Crosslinked polyurea-co-polyurethane aerogels with hierarchical structures and low stiffness. J. Non Cryst. Solids 2018, 487, 19–27. [Google Scholar] [CrossRef]
- Somdee, P.; Lassú-Kuknyó, T.; Kónya, C.; Szabó, T.; Marossy, K. Thermal analysis of polyurethane elastomers matrix with different chain extender contents for thermal conductive application. J. Therm. Anal. Calorim. 2019, 138, 1003–1010. [Google Scholar] [CrossRef]
- Sankar, R.M.; Meera, K.S.; Mandal, A.B.; Jaisankar, S.N. Thermoplastic polyurethane/single-walled carbon nanotube composites with low electrical resistance surfaces. High Perform. Polym. 2013, 25, 135–146. [Google Scholar] [CrossRef]
- Marlina, M.; Saiful, J.; Rahmi, R.; Saleha, S.; Nurman, S. Synthesis and characterization new polyurethane membrane from hydroxylated rubber seed oil. Orient. J. Chem. 2017, 33, 199–206. [Google Scholar] [CrossRef]
- Daneshvar, S.; Behrooz, R.; Najafi, S.K.; Sadeghi, G.M.M.M. Characterization of polyurethane wood adhesive prepared from liquefied sawdust by ethylene carbonate. Bioresources 2019, 14, 796–815. [Google Scholar] [CrossRef]
- UNE-EN 1242:2013; Adhesives. Determination of Isocyanate Content. Asociación Española de Normalización y Certificación (AENOR): Madrid, Spain. 2013. Available online: https://www.en-standard.eu/une-en-1242-2013-adhesives-determination-of-isocyanate-content/ (accessed on 13 November 2013).
- ASTM D1622-08; Standard Test Method for Apparent Density of Rigid Cellular Plastics. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2020.
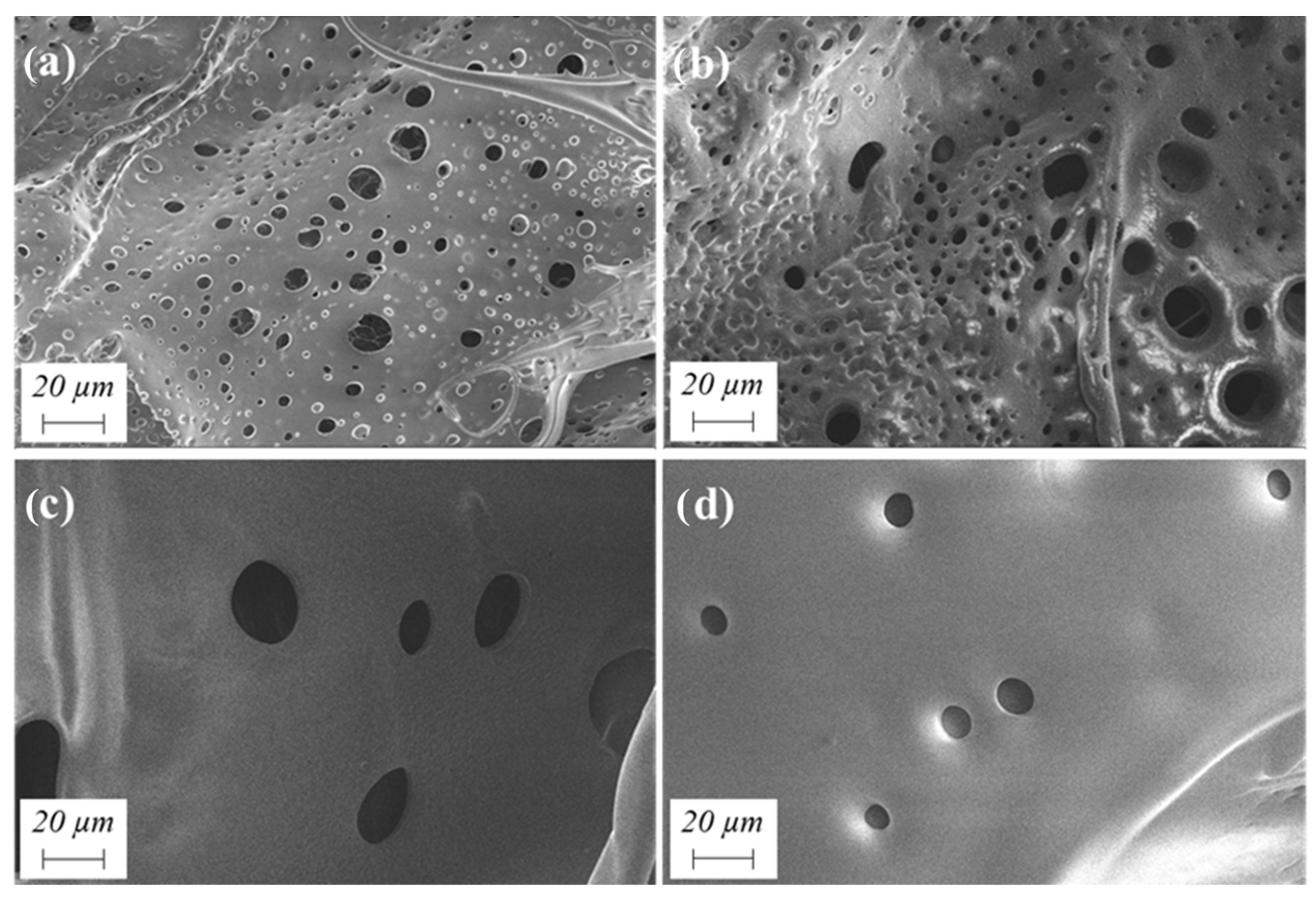
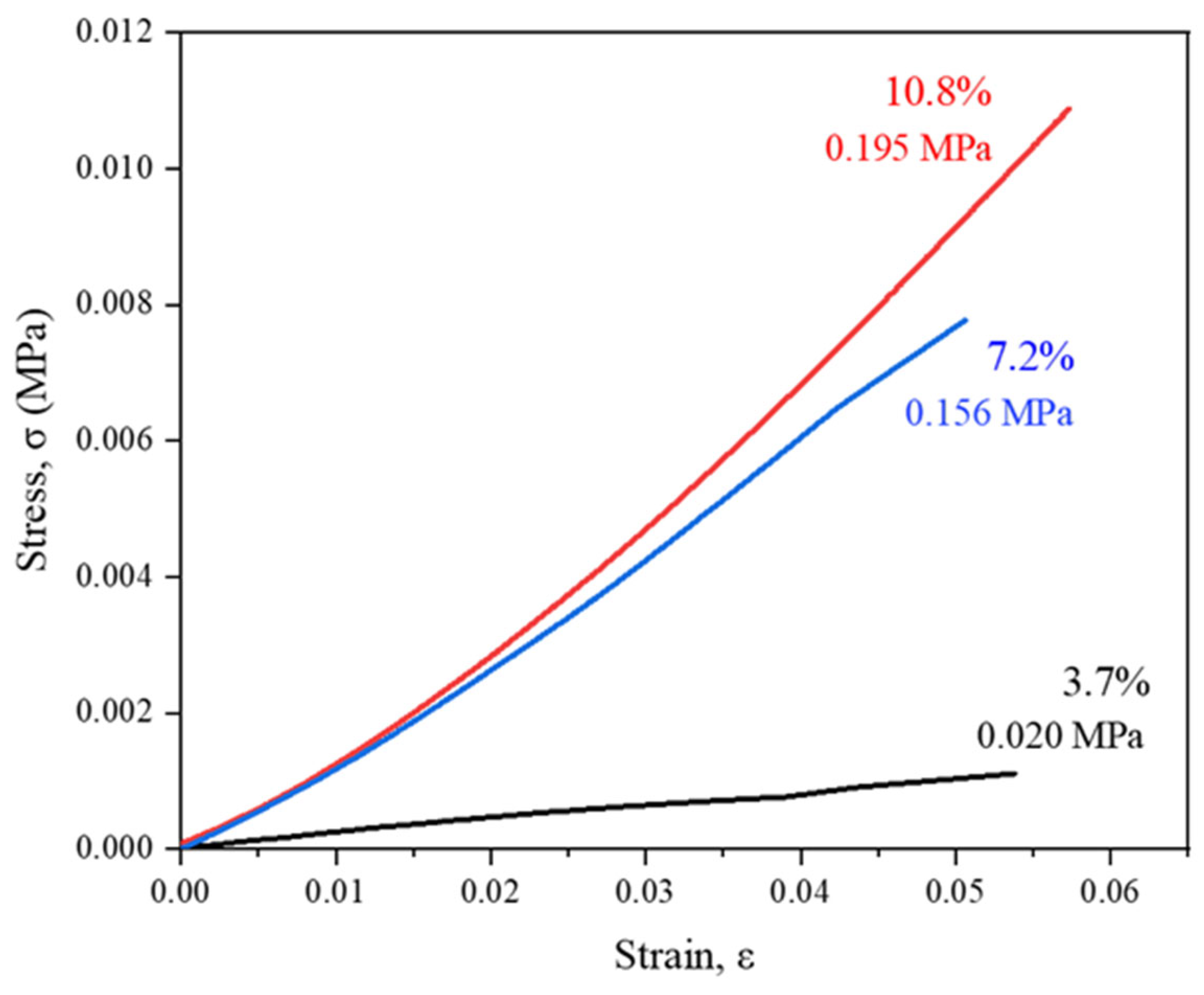
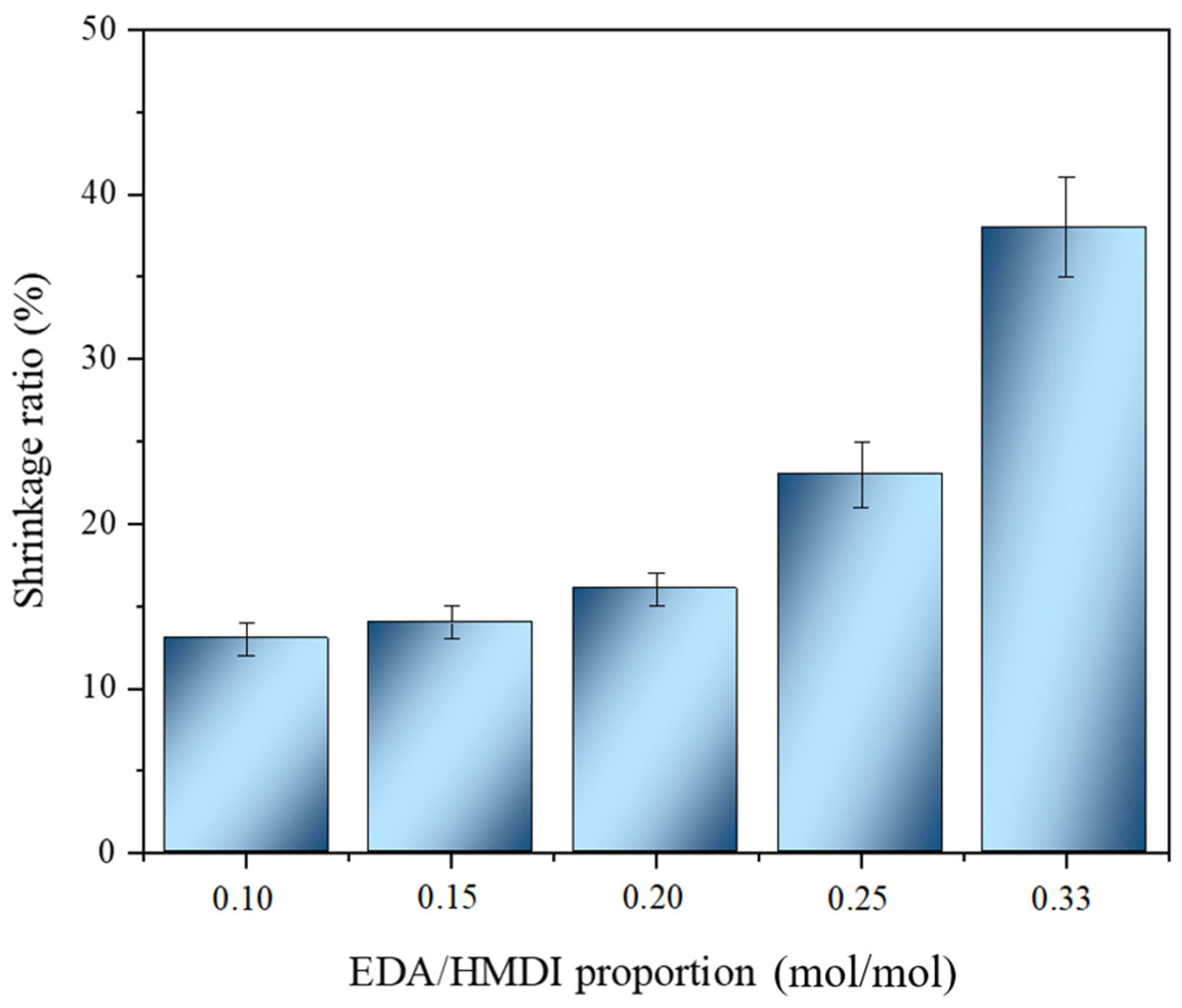

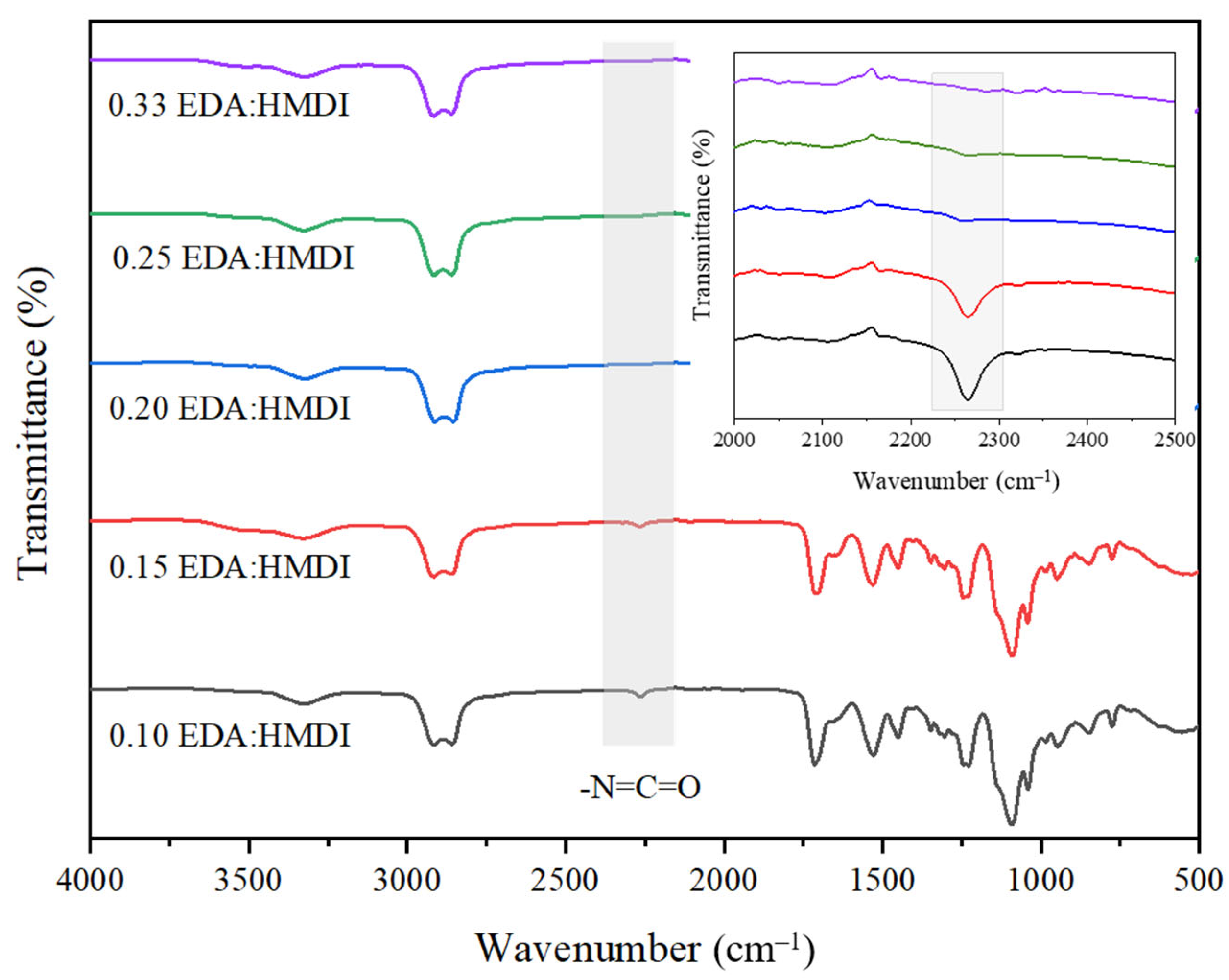



| Experiment | Organic Solvent Proportion | Solids Content (wt.%) | EDA/HMDI Ratio (mol/mol) | Dispersion Mode | |
|---|---|---|---|---|---|
| Set 1 | 1 | 100EtOAc | 10.8 | 0.33 | Mechanical agitation |
| 2 | 25ACN:75EtOAc | ||||
| 3 | 50ACN:50EtOAc | ||||
| 4 | 75ACN:25EtOAc | ||||
| 5 | 100ACN | ||||
| Set 2 | 6 | 100EtOAc | 10.8 | 0.33 | Mechanical agitation |
| 7 | 7.2 | ||||
| 8 | 3.7 | ||||
| 9 | 3.2 | ||||
| Set 3 | 10 | 100EtOAc | 3.7 | 0.33 | Mechanical agitation |
| 11 | 0.25 | ||||
| 12 | 0.20 | ||||
| 13 | 0.15 | ||||
| 14 | 0.10 | ||||
| Set 4 | 15 | 100EtOAc | 10.8 | 0.33 | Sonication |
| 16 | 7.2 | ||||
| 17 | 3.7 | ||||
| 18 | 3.2 | ||||
| Set 5 | 19 | 100EtOAc | 3.7 | 0.33 | Sonication |
| 20 | 0.25 | ||||
| 21 | 0.20 | ||||
| 22 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinilla-Peñalver, E.; Cantero, D.; Romero, A.; Sánchez-Silva, L. Exploring the Impact of the Synthesis Variables Involved in the Polyurethane Aerogels-like Materials Design. Gels 2024, 10, 209. https://doi.org/10.3390/gels10030209
Pinilla-Peñalver E, Cantero D, Romero A, Sánchez-Silva L. Exploring the Impact of the Synthesis Variables Involved in the Polyurethane Aerogels-like Materials Design. Gels. 2024; 10(3):209. https://doi.org/10.3390/gels10030209
Chicago/Turabian StylePinilla-Peñalver, Esther, Darío Cantero, Amaya Romero, and Luz Sánchez-Silva. 2024. "Exploring the Impact of the Synthesis Variables Involved in the Polyurethane Aerogels-like Materials Design" Gels 10, no. 3: 209. https://doi.org/10.3390/gels10030209
APA StylePinilla-Peñalver, E., Cantero, D., Romero, A., & Sánchez-Silva, L. (2024). Exploring the Impact of the Synthesis Variables Involved in the Polyurethane Aerogels-like Materials Design. Gels, 10(3), 209. https://doi.org/10.3390/gels10030209