A Self-Healing Gel with an Organic–Inorganic Network Structure for Mitigating Circulation Loss
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of LDH/P(AA-AMPS) NC Gels
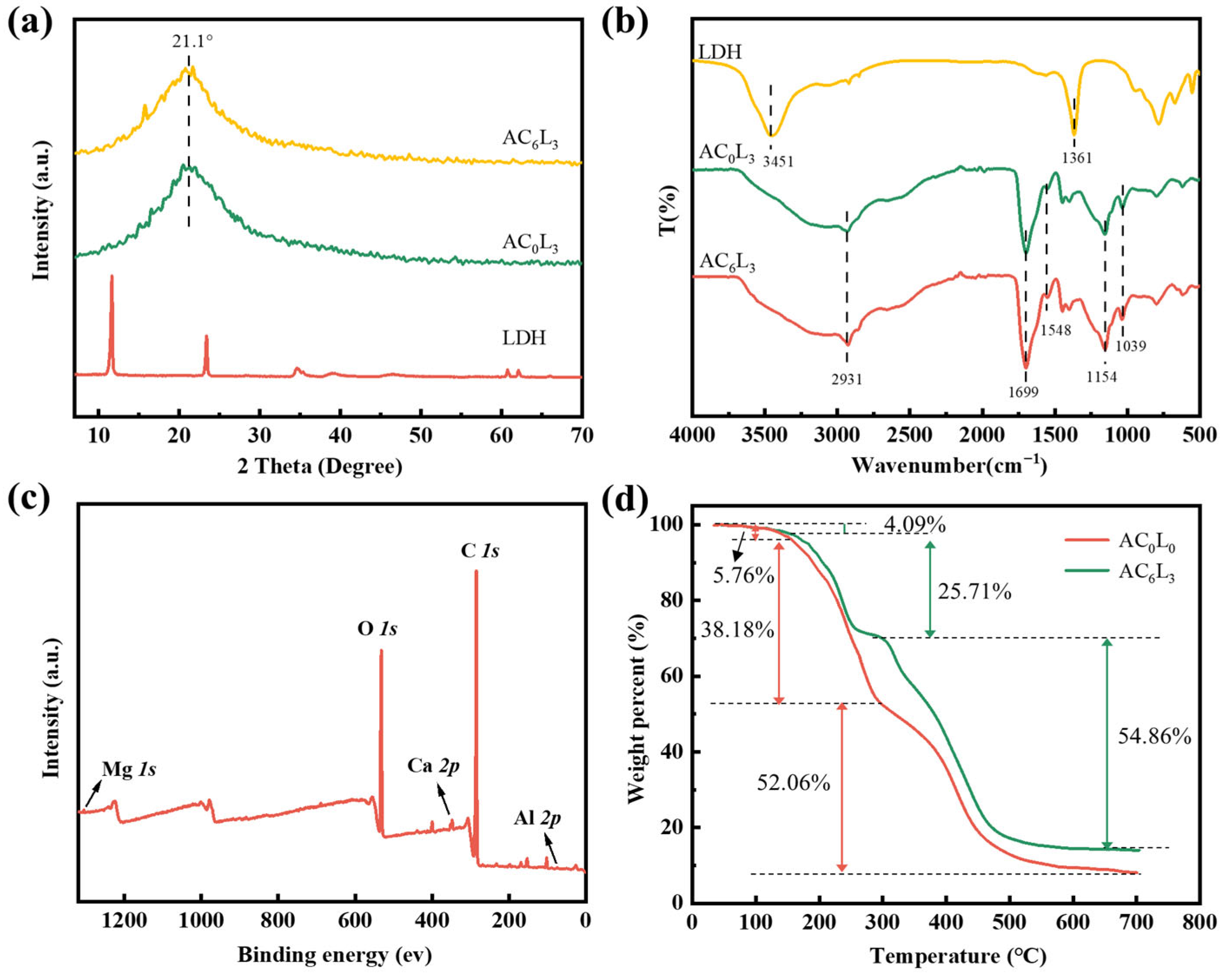
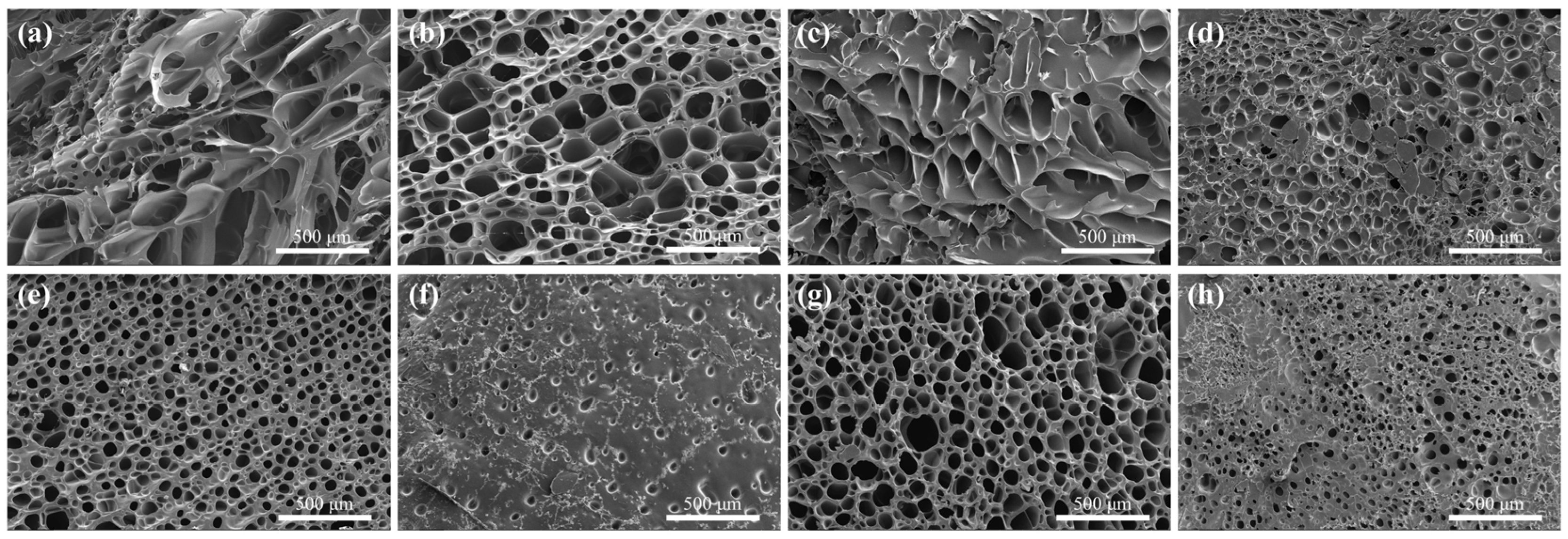
2.2. Characterization of LDH/P(AA-AMPS) NC Gels
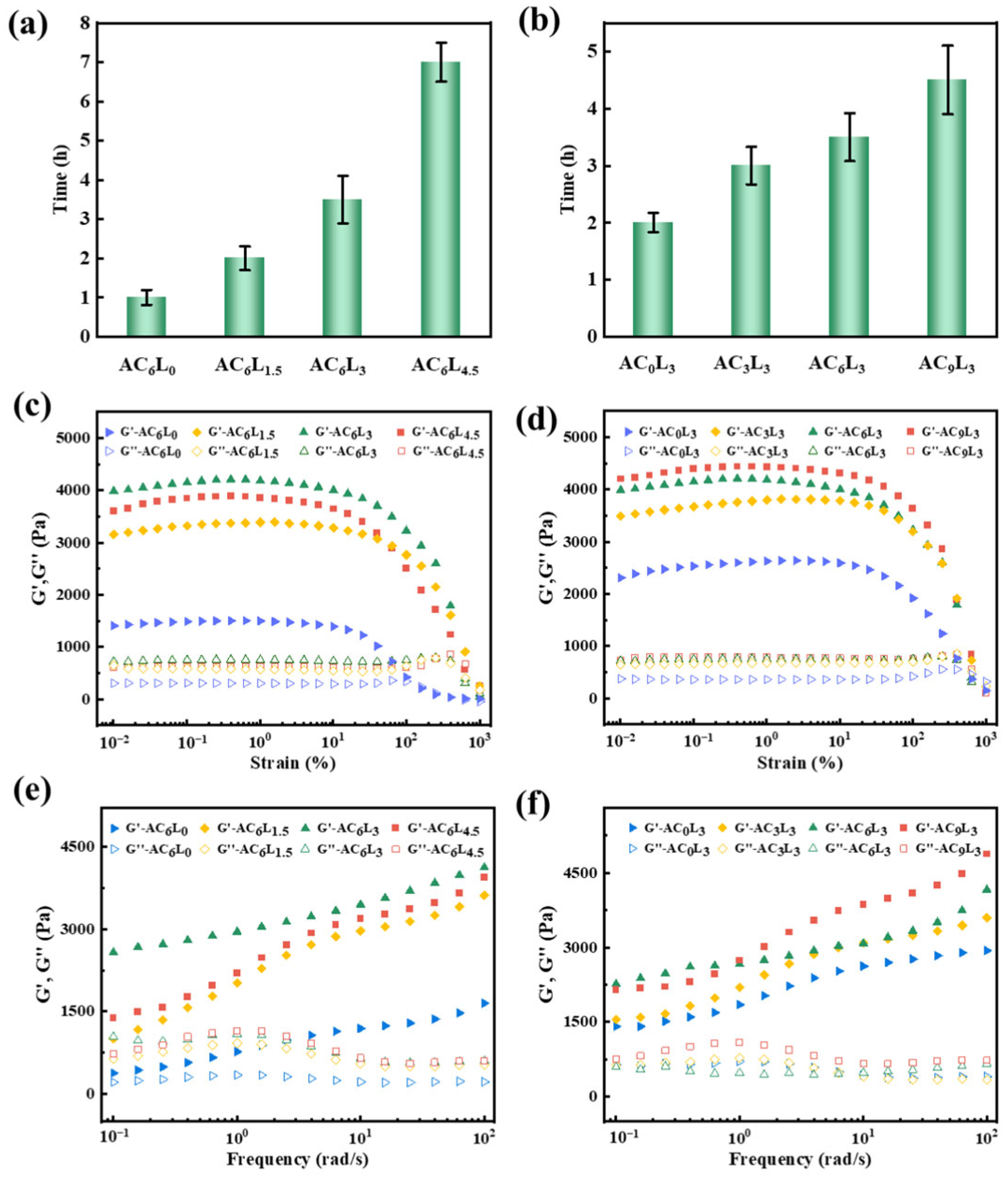
2.3. Self-Healing Properties of LDH/P(AA-AMPS) NC Gels
2.4. Swelling Behavior of LDH/P(AA-AMPS) NC Gels
2.5. Plugging Performance of LDH/P(AA-AMPS) NC Gels
3. Conclusions
4. Experimental
4.1. Materials
4.2. Preparation of LDH/P(AA-AMPS) NC Gels
4.3. Characterization
4.4. Self-Healing Performance Test
- Self-healing time test
- 2.
- Rheological performance test
- 3.
- Mechanical performance test
- 4.
- Adhesion performance test
4.5. Swelling Behaviour Test
4.6. Plugging Performance Test
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mirabbasi, S.M.; Ameri, M.J.; Biglari, F.R.; Shirzadi, A. Thermo-poroelastic wellbore strengthening modeling: An analytical approach based on fracture mechanics. J. Pet. Sci. Eng. 2020, 195, 107492. [Google Scholar] [CrossRef]
- Magzoub, M.I.; Salehi, S.; Hussein, I.A.; Nasser, M.S. Loss circulation in drilling and well construction: The significance of applications of crosslinked polymers in wellbore strengthening: A review. J. Pet. Sci. Eng. 2020, 185, 106653. [Google Scholar] [CrossRef]
- Liu, F.B.; Sun, J.S.; Wang, J.H. A Global Review of Technical Status and Development Trend of Drilling Fluids for Deep and Ultra-Deep Wells. Xinjiang Oil Gas 2023, 19, 34–39. [Google Scholar]
- Jiang, J.W.; Zhang, L.C.; Deng, Y. Problems and Its Solutions of Drilling in Wuxia Area of Junggar Basin. Xinjiang Oil Gas 2012, 8, 26–30. [Google Scholar]
- Zhao, Z.; Sun, J.S.; Liu, F.; Bai, Y.R.; Wang, R.; Geng, Y.; Li, Y.J.; Liu, C. High-Temperature-Resistant Thermal Shape Memory Polymers as Lost Circulation Materials for Fracture Formations. SPE J. 2023, 28, 2629–2641. [Google Scholar] [CrossRef]
- Yang, J.B.; Sun, J.S.; Bai, Y.R.; Lv, K.H.; Zhang, G.D.; Li, Y.H. Status and Prospect of Drilling Fluid Loss and Lost Circulation Control Technology in Fractured Formation. Gels 2022, 8, 260. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.C.; Huang, H. Technology of Prevention and Plugging Leak while Drilling in the South Margin of Junggar Basin. Xinjiang Oil Gas 2020, 16, 43–47. [Google Scholar]
- Pan, Y.; Cui, X.L.; Wang, H.; Lou, X.; Yang, S.C.; Oluwabusuyi, F.F. Research Progress of Intelligent Polymer Plugging Materials. Molecules 2023, 28, 2975. [Google Scholar] [CrossRef]
- Yang, L.L.; Xie, C.L.; Zhang, Y.W.; Jiang, G.C.; Wu, Y.P.; Liu, H.Q.; Dong, T.F.; Guo, C.P. Performance of Self-healing microgel incorporating Nano-Silica as plugging material for drilling fluid. J. Mol. Liq. 2023, 386, 122392. [Google Scholar] [CrossRef]
- Ding, X.Y.; Fan, L.; Wang, L.; Zhou, M.; Wang, Y.X.; Zhao, Y.J. Designing self-healing hydrogels for biomedical applications. Mater. Horiz. 2023, 10, 3929–3947. [Google Scholar] [CrossRef]
- Bai, Y.R.; Zhang, Q.T.; Sun, J.S.; Lv, K.H.; Shang, X.S.; Liu, C.T.; Cheng, R.C.; Wang, F. Self-healing hydrogels and their action mechanism in oil-gas drilling and development engineering: A systematic review and prospect. J. Nat. Gas Sci. Eng. 2021, 96, 104250. [Google Scholar] [CrossRef]
- Song, T.; Ahdaya, M.; Zhai, Z.M.; Schuman, T.; Bai, B.J. Comprehensive evaluation of a novel re-crosslinkable preformed particle gel for the water management of reservoir with concentrated divalent ions. Fuel 2023, 331, 125974. [Google Scholar] [CrossRef]
- Song, T.; Zhai, Z.M.; Liu, J.C.; Eriyagama, Y.; Ahdaya, M.; Alotibi, A.; Wang, Z.; Schuman, T.; Bai, B.J. Laboratory evaluation of a novel Self-healable polymer gel for CO2 leakage remediation during CO2 storage and CO2 flooding. Chem. Eng. J. 2022, 444, 136635. [Google Scholar] [CrossRef]
- Wang, C.; Sun, J.S.; Long, Y.F.; Wang, R.; Qu, Y.Z.; Peng, L.; Ren, H.; Gao, S.F. A re-crosslinkable composite gel based on curdlan for lost circulation control. J. Mol. Liq. 2023, 371, 121010. [Google Scholar] [CrossRef]
- Yang, L.; Xie, C.; Ao, T.; Cui, K.; Jiang, G.; Bai, B.; Zhang, Y.; Yang, J.; Wang, X.; Tian, W. Comprehensive evaluation of self-healing polyampholyte gel particles for the severe leakoff control of drilling fluids. J. Pet. Sci. Eng. 2022, 212, 110249. [Google Scholar] [CrossRef]
- Song, T.; Ahdaya, M.; Zhao, S.D.; Zhao, Y.; Schuman, T.; Bai, B.J. Evaluation of a Novel Recrosslinkable Hyperbranched Preformed Particle Gel for the Conformance Control of High-Temperature Reservoirs with Fractures. SPE J. 2022, 27, 3598–3610. [Google Scholar] [CrossRef]
- Ahdaya, M.; Al Brahim, A.; Bai, B.J.; Schuman, T. Low-Temperature Recrosslinkable Preformed Particle Gel as a Material for Lost Circulation Control. SPE J. 2022, 27, 2541–2551. [Google Scholar] [CrossRef]
- Bai, Y.R.; Zhang, Q.T.; Sun, J.S.; Jiang, G.C.; Lv, K.H. Double network self-healing hydrogel based on hydrophobic association and ionic bond for formation plugging. Pet. Sci. 2022, 19, 2150–2164. [Google Scholar] [CrossRef]
- Jijoe, P.S.; Yashas, S.R.; Shivaraju, H.P. Fundamentals, synthesis, characterization and environmental applications of layered double hydroxides: A review. Environ. Chem. Lett. 2021, 19, 2643–2661. [Google Scholar] [CrossRef]
- Farhan, A.; Khalid, A.; Maqsood, N.; Iftekhar, S.; Sharif, H.M.A.; Qi, F.; Sillanpaa, M.; Asif, M.B. Progress in layered double hydroxides (LDHs): Synthesis and application in adsorption, catalysis and photoreduction. Sci. Total Environ. 2024, 912, 169160. [Google Scholar] [CrossRef]
- Tong, Q.W.; Cheng, P.; Jiang, G.M.; Li, Y.M.; Wei, Y.L.; Zou, Y.; Lv, X.S.; Ao, L. Hierarchical flower-like MgAl layered double hydroxide microparticles as phosphate porter for its recovery from phosphate-contaminated water. Sep. Purif. Technol. 2024, 330, 125384. [Google Scholar] [CrossRef]
- Özgümüş, S.; Gök, M.K.; Bal, A.; Güçlü, G. Study on novel exfoliated polyampholyte nanocomposite hydrogels based on acrylic monomers and Mg–Al–Cl layered double hydroxide: Synthesis and characterization. Chem. Eng. J. 2013, 223, 277–286. [Google Scholar] [CrossRef]
- Wu, L.Y.; Gao, C.Y.; Li, Z.B.; Chen, G.M. Tunable photoluminescence from layered rare-earth hydroxide/polymer nanocomposite hydrogels by a cascaded energy transfer effect. J. Mater. Chem. C 2017, 5, 5207–5213. [Google Scholar] [CrossRef]
- Shanmugam, D.K.; Madhavan, Y.; Manimaran, A.; Kaliaraj, G.S.; Mohanraj, K.G.; Kandhasamy, N.; Mosas, K.K.A. Efficacy of Graphene-Based Nanocomposite Gels as a Promising Wound Healing Biomaterial. Gels 2023, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, G. Hydrogels containing layered double hydroxide nanosheets: Rheological behaviour and excellent stabilities. RSC Adv. 2013, 3, 12021–12025. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, H.; Lu, S. Preparation and application of layered double hydroxide nanosheets. RSC Adv. 2021, 11, 24254–24281. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, G. Novel nanocomposite hydrogels consisting of layered double hydroxide with ultrahigh tensibility and hierarchical porous structure at low inorganic content. Adv. Mater. 2014, 26, 5950–5956. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ji, J.; Li, H.; Du, N.; Song, S.; Hou, W. Synthesis of layered double hydroxide/poly(N-isopropylacrylamide) nanocomposite hydrogels with excellent mechanical and thermoresponsive performances. Soft Matter 2018, 14, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Yu, D.; Wang, S.; Fu, L.; Lin, Y. Nanosheet-hydrogel composites: From preparation and fundamental properties to their promising applications. Soft Matter 2023, 19, 1465–1481. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.; Ma, X.; Liu, Y.P.; Wu, M. Design of a highly flexible, self-healing, conductive and antimicrobial hydrogel with organic-inorganic network structure. Mater. Chem. Phys. 2023, 298, 127440. [Google Scholar] [CrossRef]
- Lei, S.Q.; Wang, S.N.; Gao, B.X.; Zhan, Y.L.; Zhao, Q.; Jin, S.; Song, G.; Lyu, X.; Zhang, Y.; Tang, Y. Ultrathin dodecyl-sulfate-intercalated Mg-Al layered double hydroxide nanosheets with high adsorption capability for dye pollution. J. Colloid Interface Sci. 2020, 577, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, H.R. Synthesis and application of a polyacrylate dispersant on the preparation of ultrafine ground calcium carbonate in a laboratory stirred media mill. Powder Technol. 2014, 266, 218–227. [Google Scholar] [CrossRef]
- Fu, L.H.; Cao, T.H.; Lei, Z.W.; Chen, H.; Shi, Y.-g.; Xu, C. Superabsorbent nanocomposite based on methyl acrylic acid-modified bentonite and sodium polyacrylate: Fabrication, structure and water uptake. Mater. Des. 2016, 94, 322–329. [Google Scholar] [CrossRef]
- Balram, D.; Lian, K.Y.; Sebastian, N.; Alharthi, S.; Al-Saidi, H.M.; Kumar, D. Nanomolar electrochemical detection of feed additive ractopamine in meat samples using spinel zinc ferrite decorated 3-dimensional graphene nanosheets to combat food fraud in livestock industries. Food Chem. 2024, 437, 137868. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Jia, F.; Peng, Z.; Zheng, Y. Development of temperature-responsive suspension stabilizer and its application in cementing slurry system. Colloids Surf. A 2023, 658, 130734. [Google Scholar] [CrossRef]
- Chang, Q.L.; Liu, G.Q.; Dong, Z.P.; Miao, X.; Hu, M.; Guo, J. Effect of poly(AMPS/DMAA/IA/SSS) intercalated Mg/Al layered double hydroxides on reducing fluid loss at 240 °C and improving early strength of oil well cement. Appl. Clay Sci. 2022, 229, 106658. [Google Scholar] [CrossRef]
- Wan, Z.Y.; He, J.H.; Yang, Y.T.; Chong, T.; Wang, J.X.; Guo, B.L.; Xue, L. Injectable adhesive self-healing biocompatible hydrogel for haemostasis, wound healing, and postoperative tissue adhesion prevention in nephron-sparing surgery. Acta Biomater. 2022, 152, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.F.; Zhao, L.Y.; Wang, J.; Wang, S.; Liu, Y.X.; Liu, X.F. High-strength and high-toughness sodium alginate/polyacrylamide double physically crosslinked network hydrogel with superior self-healing and self-recovery properties prepared by a one-pot method. Colloids Surf. A 2020, 589, 124402. [Google Scholar] [CrossRef]
- Haraguchi, K.; Uyama, K.; Tanimoto, H. Self-healing in Nanocomposite Hydrogels. Macromol. Rapid Commun. 2011, 32, 1253–1258. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Zhang, G.Z.; Li, F.B.; Zhang, Y.Q.; Lei, Y.; Xia, Y.H.; Jin, X.H.; Feng, X.Q.; Li, H.J. A self-healable and tough nanocomposite hydrogel crosslinked by novel ultrasmall aluminum hydroxide nanoparticles. Nanoscale 2017, 9, 15470–15476. [Google Scholar] [CrossRef]
- Taylor, D.L.; Panhuis, M.I.H. Self-Healing Hydrogels. Adv. Mater. 2016, 28, 9060–9093. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.P.; Zhao, X.; Hu, T.L.; Chen, B.J.; Yin, Z.H.; Ma, P.X.; Guo, B.L. Adhesive Hemostatic Conducting Injectable Composite Hydrogels with Sustained Drug Release and Photothermal Antibacterial Activity to Promote Full-Thickness Skin Regeneration During Wound Healing. Small 2019, 15, 1900046. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.Y.; Chang, H.L.; Wang, M.; Xu, F.; Yang, J. High-Strength, Tough, and Self-Healing Nanocomposite Physical Hydrogels Based on the Synergistic Effects of Dynamic Hydrogen Bond and Dual Coordination Bonds. ACS Appl. Mater. Interfaces 2017, 9, 28305–28318. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Guo, Y.; Su, F.; Huang, X.; Qian, Q.; Zhou, Y.; Pan, J. High-strength, fatigue-resistant, and fast self-healing antibacterial nanocomposite hydrogels for wound healing. Chem. Eng. J. 2023, 455, 140854. [Google Scholar] [CrossRef]
- Nie, H.; Schauser, N.S.; Self, J.L.; Tabassum, T.; Oh, S.; Geng, Z.S.; Jones, S.D.; Zayas, M.S.; Reynolds, V.G.; Chabinyc, M.L.; et al. Light-Switchable and Self-Healable Polymer Electrolytes Based on Dynamic Diarylethene and Metal-Ion Coordination. J. Am. Chem. Soc. 2021, 143, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Z.; Liang, Q.D.; Mugo, S.M.; An, L.J.; Zhang, Q.; Lu, Y.Y. Self-Healing and Shape-Editable Wearable Supercapacitors Based on Highly Stretchable Hydrogel Electrolytes. Adv. Sci. 2022, 9, 2201039. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.; Luo, Z.D.; Li, X.Y.; Fielding, L.A. 3D printable, thermo-responsive, self-healing, graphene oxide containing self-assembled hydrogels formed from block copolymer wormlike micelles. Soft Matter 2023, 19, 6513–6524. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Y.; Yu, X.Q.; Sun, X.B.; Zhu, L.; Qin, G.; Dai, Y.H.; Chen, Q. Tough and Conductive Dual Physically Cross-Linked Hydrogels for Wearable Sensors. Ind. Eng. Chem. Res. 2019, 58, 17001–17009. [Google Scholar] [CrossRef]
- Chang, M.M.; Wang, J.L.; Liu, X.X.; Lin, Q.X.; Wang, X.H.; Peng, F.; Ren, J.L. A semitransparent and tough wood-based adhesive gels with excellent adhesion both in air and underwater for emergency pipeline repair. Chem. Eng. J. 2023, 468, 143601. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, K.L.; Suo, Z.G. Photodetachable Adhesion. Adv. Mater. 2019, 31, 1806948. [Google Scholar] [CrossRef]
- Ye, L.; Lv, Q.; Sun, X.Y.; Liang, Y.Z.; Fang, P.W.; Yuan, X.Y.; Li, M.; Zhang, X.Z.; Shang, X.F.; Liang, H.Y. Fully physically cross-linked double network hydrogels with strong mechanical properties, good recovery and self-healing properties. Soft Matter 2020, 16, 1840–1849. [Google Scholar] [CrossRef]
- Li, X.Y.; Luo, F.; Sun, T.L.; Cui, K.P.; Watanabe, R.; Nakajima, T.; Gong, J.P. Effect of Salt on Dynamic Mechanical Behaviors of Polyampholyte Hydrogels. Macromolecules 2023, 56, 535–544. [Google Scholar] [CrossRef]
- Li, X.Y.; Gong, J.P. Role of dynamic bonds on fatigue threshold of tough hydrogels. Proc. Natl. Acad. Sci. USA 2022, 119, e2200678119. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Q.; Li, Y.; Yang, J.; Chen, F.; Tang, Z.; Zhu, L.; Qin, G.; Dai, Y.; Chen, Q. Nanoclay Reinforced Self-Cross-Linking Poly(N-Hydroxyethyl Acrylamide) Hydrogels with Integrated High Performances. Macromol. Mater. Eng. 2018, 303, 1800295. [Google Scholar] [CrossRef]
- Yu, B.W.; Zhao, S.D.; Long, Y.F.; Bai, B.J.; Schuman, T. Comprehensive evaluation of a high-temperature resistant re-crosslinkable preformed particle gel for water management. Fuel 2022, 309, 122086. [Google Scholar] [CrossRef]








| Samples | AA | AMPS | LDH | CaCl2 |
|---|---|---|---|---|
| AC0L0 | 30 | 3 | 0 | 0 |
| AC0L3 | 30 | 3 | 3 | 0 |
| AC6L0 | 30 | 3 | 0 | 6 |
| AC6L3 | 30 | 3 | 3 | 6 |
| AC3L3 | 30 | 3 | 3 | 3 |
| AC9L3 | 30 | 3 | 3 | 9 |
| AC6L1.5 | 30 | 3 | 1.5 | 6 |
| AC6L4.5 | 30 | 3 | 4.5 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Sun, J.; Long, Y.; Huang, H.; Song, J.; Wang, R.; Qu, Y.; Yang, Z. A Self-Healing Gel with an Organic–Inorganic Network Structure for Mitigating Circulation Loss. Gels 2024, 10, 93. https://doi.org/10.3390/gels10020093
Wang C, Sun J, Long Y, Huang H, Song J, Wang R, Qu Y, Yang Z. A Self-Healing Gel with an Organic–Inorganic Network Structure for Mitigating Circulation Loss. Gels. 2024; 10(2):93. https://doi.org/10.3390/gels10020093
Chicago/Turabian StyleWang, Cheng, Jinsheng Sun, Yifu Long, Hongjun Huang, Juye Song, Ren Wang, Yuanzhi Qu, and Zexing Yang. 2024. "A Self-Healing Gel with an Organic–Inorganic Network Structure for Mitigating Circulation Loss" Gels 10, no. 2: 93. https://doi.org/10.3390/gels10020093
APA StyleWang, C., Sun, J., Long, Y., Huang, H., Song, J., Wang, R., Qu, Y., & Yang, Z. (2024). A Self-Healing Gel with an Organic–Inorganic Network Structure for Mitigating Circulation Loss. Gels, 10(2), 93. https://doi.org/10.3390/gels10020093





