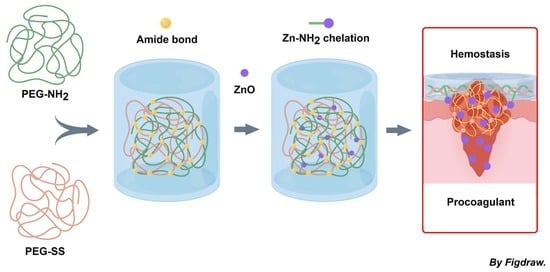
Enhanced Hemostatic and Procoagulant Efficacy of PEG/ZnO Hydrogels: A Novel Approach in Traumatic Hemorrhage Management
Abstract
1. Introduction
2. Results and Discussion
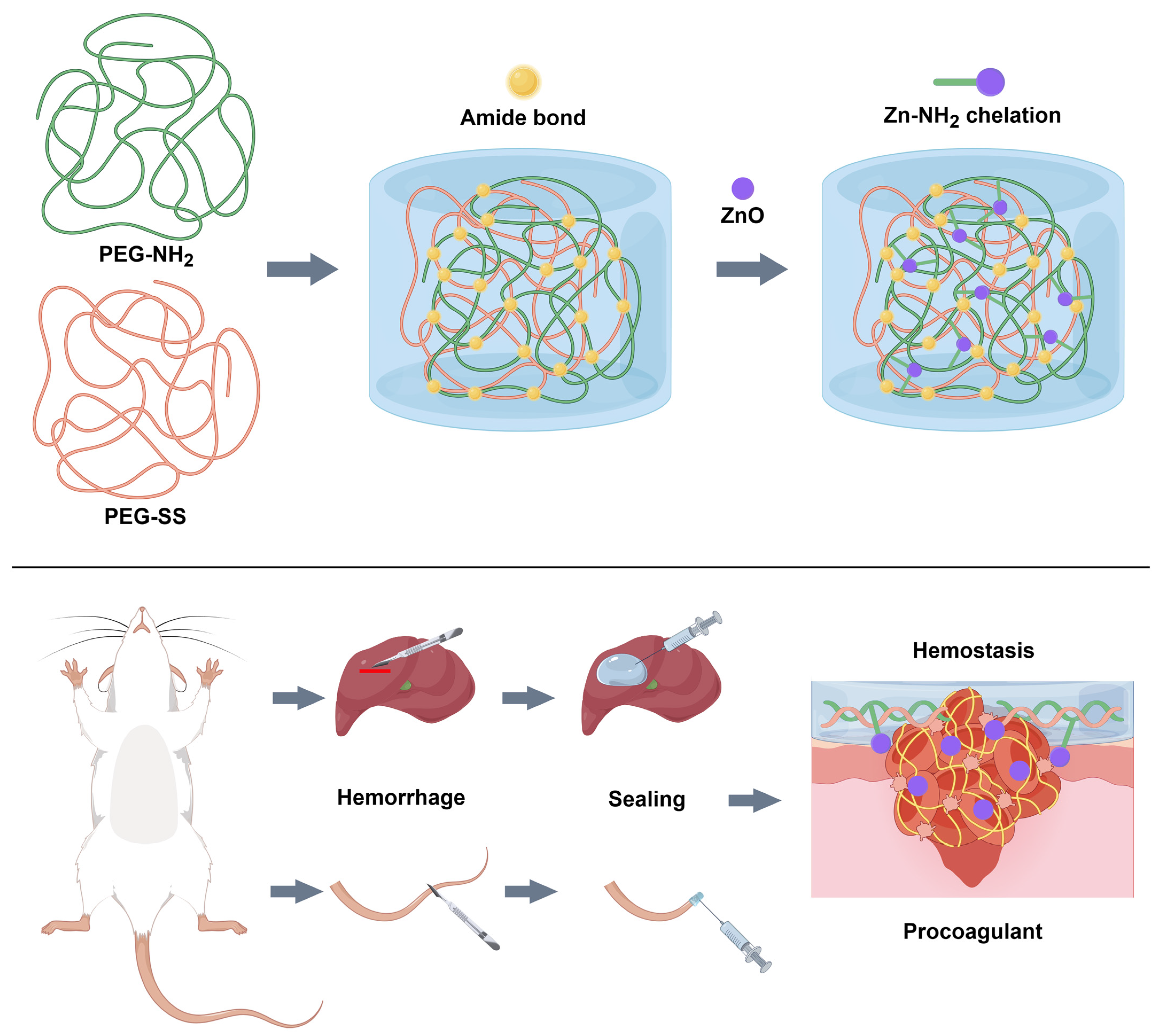
2.1. Preparation of Hydrogels
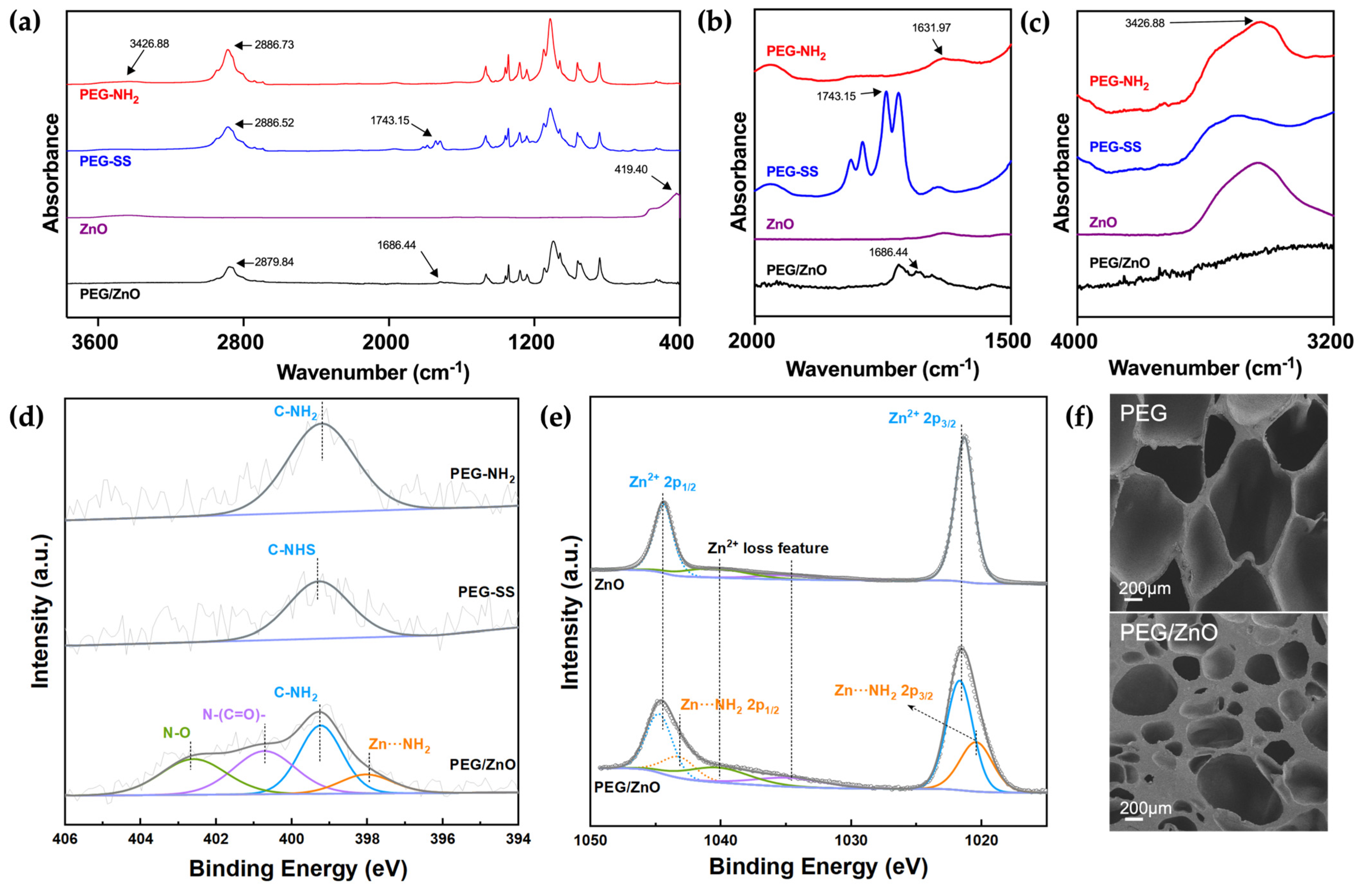
2.2. Characterization of Hydrogels
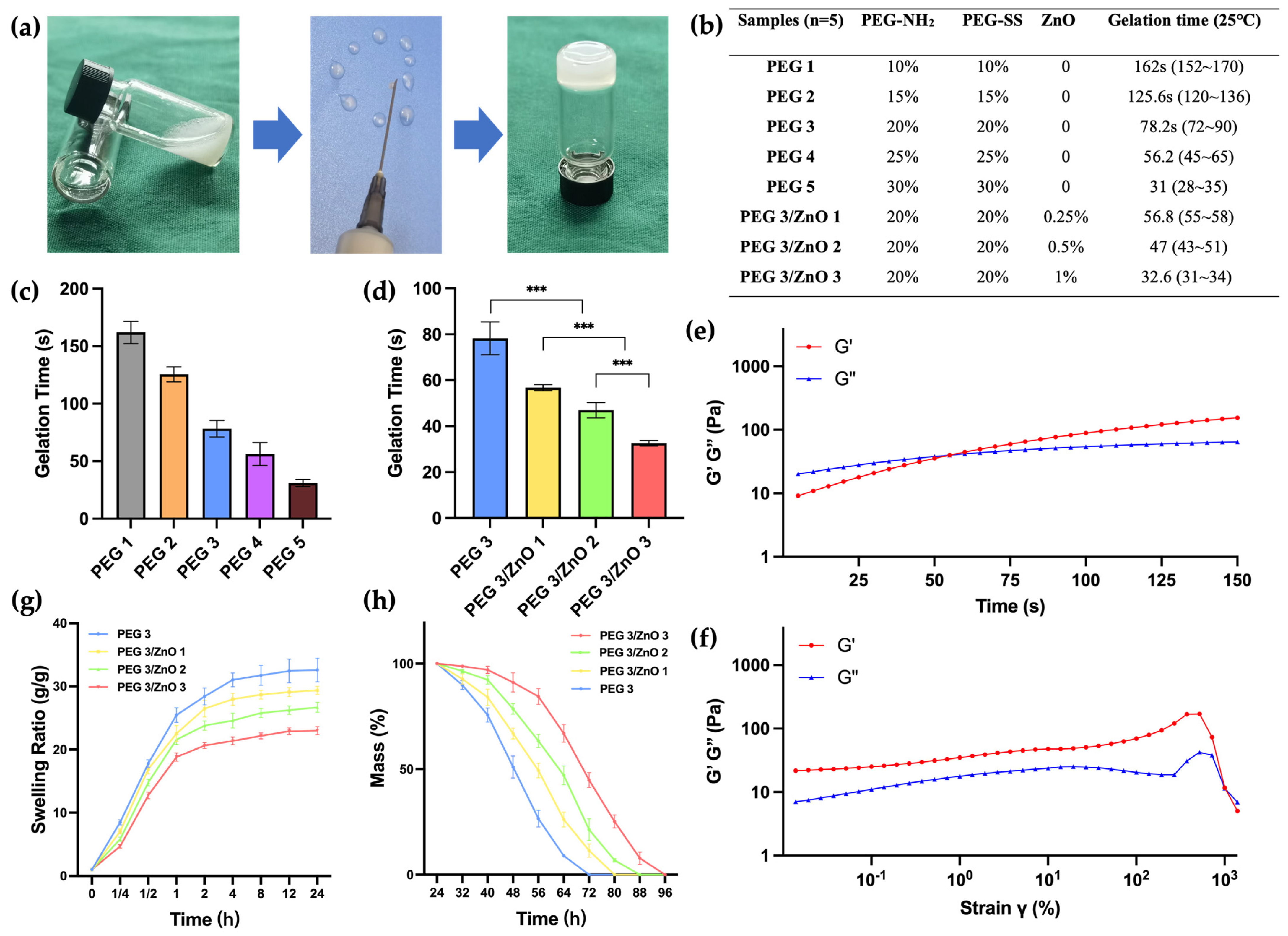
2.3. Mechanical Properties of the Hydrogels
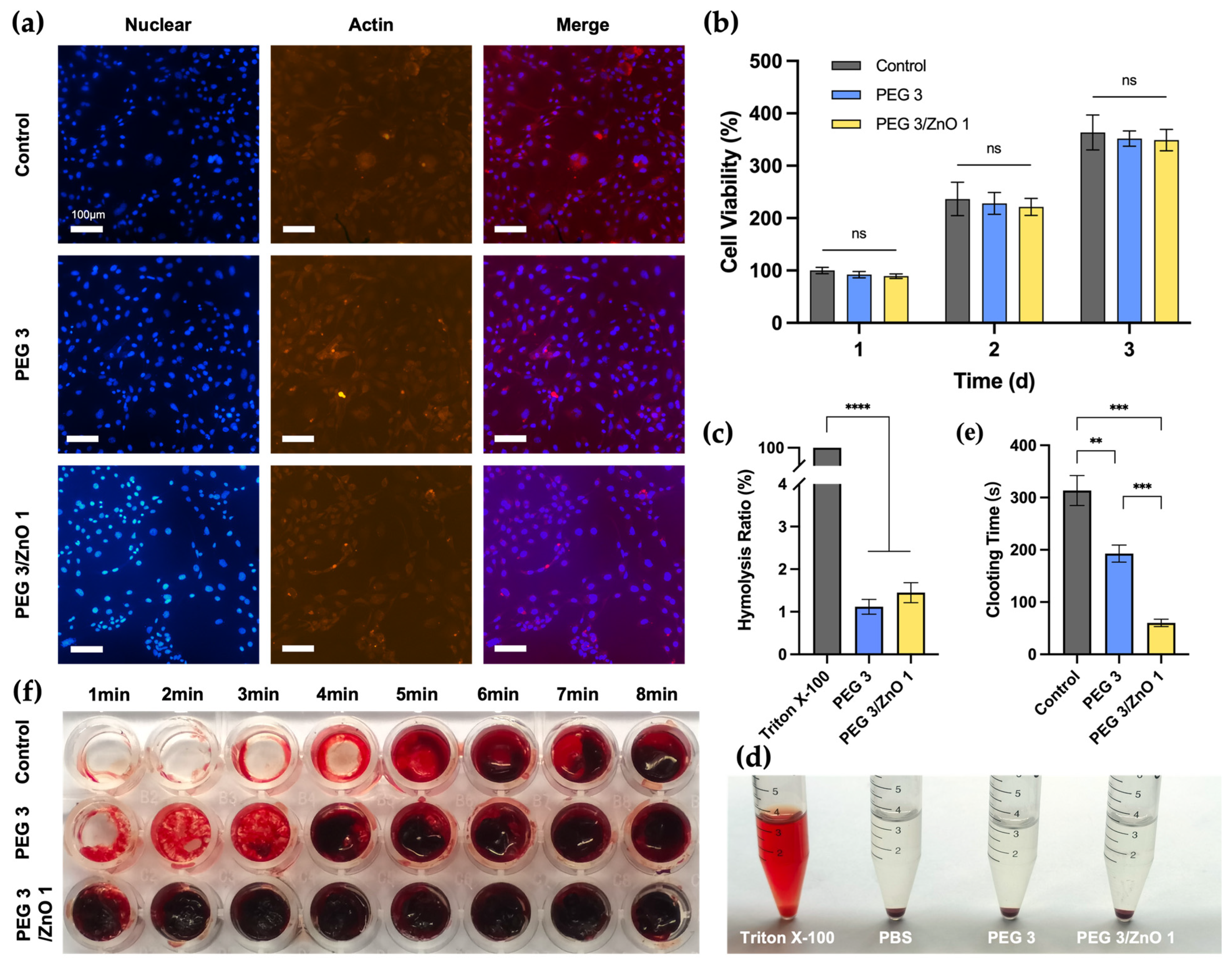
2.4. In Vitro Biocompatibility
2.5. In Vitro Hemocompatibility and Procoagulant Ability
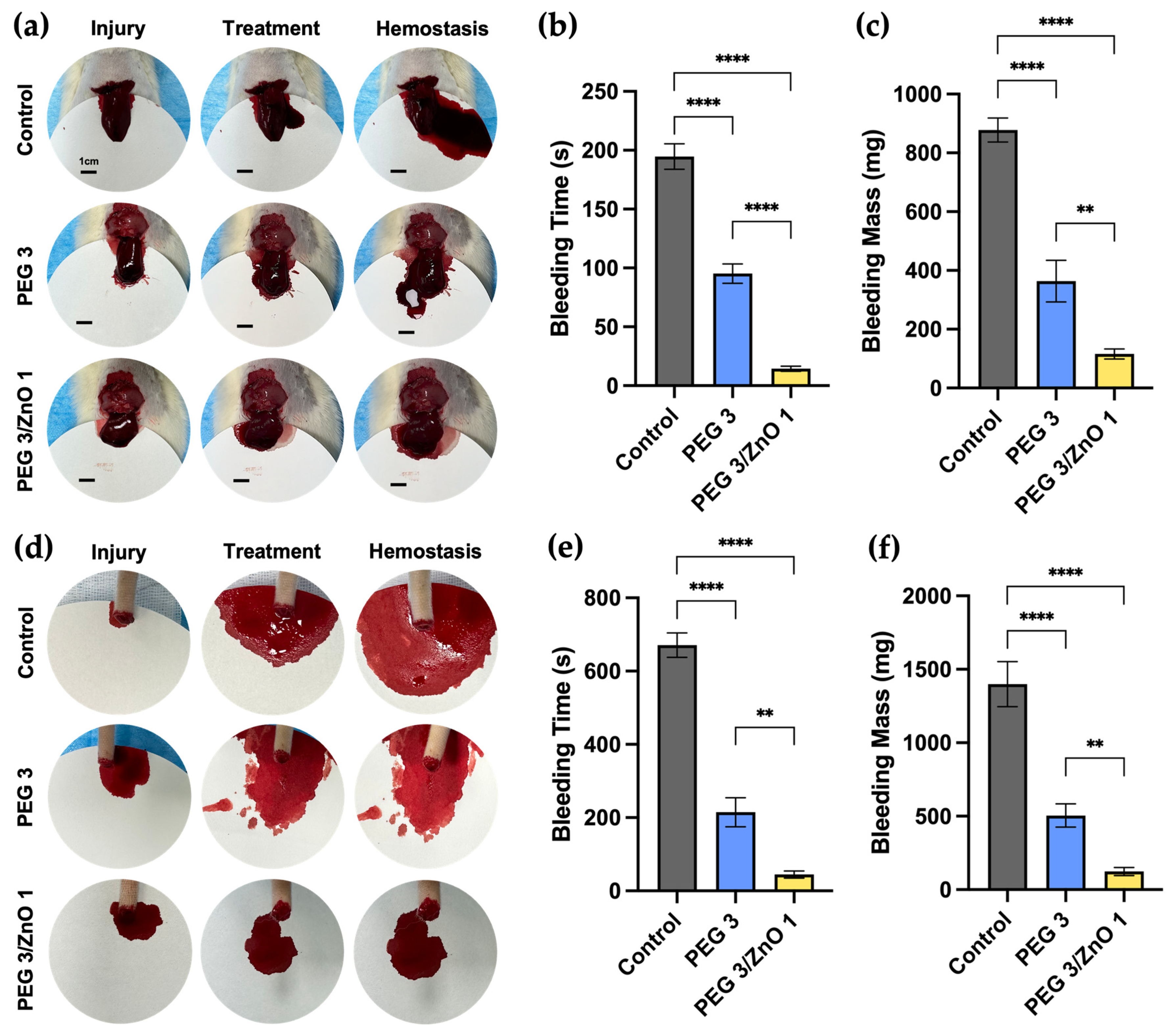
2.6. In Vivo Hemostatic Performance
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Characterization
4.3. Preparation of the Hydrogels
4.4. Gelation Time and Injectability
4.5. Swelling Ratio and Degradation of the Hydrogels
4.6. Compression Strength Test
4.7. Adhesion Strength Test
4.8. Cytocompatibility and Fluorescence Staining
4.9. Hemocompatibility
4.10. In Vitro Clotting Time
4.11. In Vivo Hemostatic Performance
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.S.; Satahoo, S.S.; Butler, F.K.; Dermer, H.; Naranjo, D.; Julien, K.; Van Haren, R.M.; Namias, N.; Blackbourne, L.H.; Schulman, C.I. An analysis of prehospital deaths: Who can we save? J. Trauma Acute Care Surg. 2014, 77, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Rhee, P.; Joseph, B.; Pandit, V.; Aziz, H.; Vercruysse, G.; Kulvatunyou, N.; Friese, R.S. Increasing Trauma Deaths in the United States. Ann. Surg. 2014, 260, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, J.L.; Charlton, N.P.; Carlson, J.N.; Flores, G.E.; Goolsby, C.A.; Hoover, A.V.; Kule, A.; Magid, D.J.; Orkin, A.M.; Singletary, E.M.; et al. 2020 American Heart Association and American Red Cross Focused Update for First Aid. Circulation 2020, 142, E287–E303. [Google Scholar] [CrossRef] [PubMed]
- Mandrioli, M.; Inaba, K.; Piccinini, A.; Biscardi, A.; Sartelli, M.; Agresta, F.; Catena, F.; Cirocchi, R.; Jovine, E.; Tugnoli, G.; et al. Advances in laparoscopy for acute care surgery and trauma. World J. Gastroenterol. 2016, 22, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Li, M.; Yang, Q.; Wan, G.; Li, Y.; Li, N.; Tang, K. Morphology-controllable cellulose/chitosan sponge for deep wound hemostasis with surfactant and pore-foaming agent. Mater. Sci. Eng. C 2021, 118, 111408. [Google Scholar] [CrossRef]
- Chen, S.; Carlson, M.A.; Zhang, Y.S.; Hu, Y.; Xie, J. Fabrication of injectable and superelastic nanofiber rectangle matrices (“peanuts”) and their potential applications in hemostasis. Biomaterials 2018, 179, 46–59. [Google Scholar] [CrossRef]
- Kilbourne, M.; Keledjian, K.; Hess, J.R.; Scalea, T.; Bochicchio, G.V. Hemostatic Efficacy of Modified Amylopectin Powder in a Lethal Porcine Model of Extremity Arterial Injury. Ann. Emerg. Med. 2009, 53, 804–810. [Google Scholar] [CrossRef]
- Du, X.; Liu, Y.; Wang, X.; Yan, H.; Wang, L.; Qu, L.; Kong, D.; Qiao, M.; Wang, L. Injectable hydrogel composed of hydrophobically modified chitosan/oxidized-dextran for wound healing. Mater. Sci. Eng. C 2019, 104, 109930. [Google Scholar] [CrossRef]
- Taghipour, Y.D.; Hokmabad, V.R.; Del Bakhshayesh, A.R.; Asadi, N.; Salehi, R.; Nasrabadi, H.T. The Application of Hydrogels Based on Natural Polymers for Tissue Engineering. Curr. Med. Chem. 2020, 27, 2658–2680. [Google Scholar] [CrossRef]
- Chen, S.L.; Fu, R.H.; Liao, S.F.; Liu, S.P.; Lin, S.Z.; Wang, Y.C. A PEG-Based Hydrogel for Effective Wound Care Management. Cell Transpl. 2018, 27, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Zhang, L.; Sun, G.; Sun, F.; Liu, J.; Yang, F.; Tang, P.; Wu, D. Tetra-PEG Based Hydrogel Sealants for In Vivo Visceral Hemostasis. Adv. Mater. 2019, 31, e1901580. [Google Scholar] [CrossRef] [PubMed]
- Strehin, I.; Nahas, Z.; Arora, K.; Nguyen, T.; Elisseeff, J. A versatile pH sensitive chondroitin sulfate–PEG tissue adhesive and hydrogel. Biomaterials 2010, 31, 2788–2797. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, N.; Yang, T.; Zhang, Y.; Jing, X.; Zhou, Y.; Long, J.; Meng, L. Amino acids and doxorubicin as building blocks for metal ion-driven self-assembly of biodegradable polyprodrugs for tumor theranostics. Acta Biomater. 2022, 147, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Glover, C.N.; Hogstrand, C. Amino acid modulation of in vivo intestinal zinc absorption in freshwater rainbow trout. J. Exp. Biol. 2002, 205, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Xia, H.; Huang, L.; Zhong, Z.; Ye, Q.; Zhang, L.; Lu, A. Biocompatible, antibacterial and anti-inflammatory zinc ion cross-linked quaternized cellulose-sodium alginate composite sponges for accelerated wound healing. Int. J. Biol. Macromol. 2021, 191, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Marx, G.; Eldor, A. The procoagulant effect of zinc on fibrin clot formation. Am. J. Hematol. 1985, 19, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.A.; Pugh, N. The contribution of zinc to platelet behaviour during haemostasis and thrombosis. Metallomics 2016, 8, 144–155. [Google Scholar] [CrossRef]
- Siebert, L.; Luna-Cerón, E.; García-Rivera, L.E.; Oh, J.; Jang, J.; Rosas-Gómez, D.A.; Pérez-Gómez, M.D.; Maschkowitz, G.; Fickenscher, H.; Oceguera-Cuevas, D.; et al. Light-controlled growth factors release on tetrapodal ZnO-incorporated 3D-printed hydrogels for developing smart wound scaffold. Adv. Funct. Mater. 2021, 31, 2007555. [Google Scholar] [CrossRef]
- Mao, C.; Xiang, Y.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Wang, X.; Chu, P.K.; Wu, S. Photo-Inspired Antibacterial Activity and Wound Healing Acceleration by Hydrogel Embedded with Ag/Ag@AgCl/ZnO Nanostructures. ACS Nano 2017, 11, 9010–9021. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, H.; Gao, J.; Liu, X.; Li, M.; Wu, D.; Liu, J.; Wang, X.; Wang, Z.; Tang, P. First-Aid Hydrogel Wound Dressing with Reliable Hemostatic and Antibacterial Capability for Traumatic Injuries. Adv. Health Mater. 2023, 12, 2300312. [Google Scholar] [CrossRef] [PubMed]
- van Dam, E.P.; Yuan, H.; Kouwer, P.H.J.; Bakker, H.J. Structure and Dynamics of a Temperature-Sensitive Hydrogel. J. Phys. Chem. B 2021, 125, 8219–8224. [Google Scholar] [CrossRef]
- Measey, T.J.; Markiewicz, B.N.; Gai, F. Amide I band and photoinduced disassembly of a peptide hydrogel. Chem. Phys. Lett. 2013, 580, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Jalili, Z.; Tayebee, R. UV-visible light-induced photochemical synthesis of benzimidazoles by coomassie brilliant blue coated on W-ZnO@NH(2) nanoparticles. RSC Adv. 2021, 11, 16359–16375. [Google Scholar] [CrossRef] [PubMed]
- Erol, I.; Hazman, Ö.; Aksu, M.; Bulut, E. Synergistic effect of ZnO nanoparticles and hesperidin on the antibacterial properties of chitosan. J. Biomater. Sci. Polym. Ed. 2022, 33, 1973–1997. [Google Scholar] [CrossRef] [PubMed]
- Ansari, K.; Chauhan, D.S.; Quraishi, M.; Mazumder, M.A.; Singh, A. Chitosan Schiff base: An environmentally benign biological macromolecule as a new corrosion inhibitor for oil & gas industries. Int. J. Biol. Macromol. 2020, 144, 305–315. [Google Scholar] [CrossRef]
- Ari, H.A.; Adewole, A.O.; Ugya, A.Y.; Asipita, O.H.; Musa, M.A.; Feng, W. Biogenic fabrication and enhanced photocatalytic degradation of tetracycline by bio structured ZnO nanoparticles. Environ. Technol. 2023, 44, 1351–1366. [Google Scholar] [CrossRef]
- Varghese, J.S.; Chellappa, N.; Fathima, N.N. Gelatin–carrageenan hydrogels: Role of pore size distribution on drug delivery process. Colloids Surf. B Biointerfaces 2014, 113, 346–351. [Google Scholar] [CrossRef]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef]
- George, L.; Bavya, M.; Rohan, K.V.; Srivastava, R. A therapeutic polyelectrolyte–vitamin C nanoparticulate system in polyvinyl alcohol–alginate hydrogel: An approach to treat skin and soft tissue infections caused by Staphylococcus aureus. Colloids Surf. B Biointerfaces 2017, 160, 315–324. [Google Scholar] [CrossRef]
- Huang, P.; Su, W.; Han, R.; Lin, H.; Yang, J.; Xu, L.; Ma, L. Physicochemical, Antibacterial Properties, and Compatibility of ZnO-NP/Chitosan/β-Glycerophosphate Composite Hydrogels. J. Microbiol. Biotechnol. 2022, 32, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, K.-I.; Nakajima, T.; Matsuda, T.; Sakai, T.; Gong, J.P. Network elasticity of a model hydrogel as a function of swelling ratio: From shrinking to extreme swelling states. Soft Matter 2018, 14, 9693–9701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Qi, Y.; Zhang, Z. Swelling Behaviour of Polystyrene Microsphere Enhanced PEG-Based Hydrogels in Seawater and Evolution Mechanism of Their Three-Dimensional Network Microstructure. Materials 2022, 15, 4959. [Google Scholar] [CrossRef] [PubMed]
- Victorelli, F.D.; Rodero, C.F.; Lutz-Bueno, V.; Chorilli, M.; Mezzenga, R. Amyloid Fibrils Enhance the Topical Bio-Adhesivity of Liquid Crystalline Mesophase-Based Drug Formulations. Adv. Health Mater. 2023, 12, e2202720. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Zhang, L.; Liu, J.; Zhang, L.; Li, T.; Shen, H.; Wang, X.; Yang, F.; Tang, P.; Wu, D. Synthesis and Properties of Hemostatic and Bacteria-Responsive in Situ Hydrogels for Emergency Treatment in Critical Situations. ACS Appl. Mater. Interfaces 2016, 8, 12674–12683. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, R.; Zheng, B.; Guo, W.; Li, C.; He, W.; Wei, Y.; Du, Y.; Wang, H.; Wu, D.; et al. Highly Stretchable, Adhesive, Biocompatible, and Antibacterial Hydrogel Dressings for Wound Healing. Adv. Sci. 2021, 8, 2003627. [Google Scholar] [CrossRef]
- Alula, K.; Adali, T.; Ebedal, O.H. Preparation characterization and blood compatibility studies of silk fibroin/gelatin/curcumin injectable hydrogels. Bio-Med. Mater. Eng. 2023, 34, 77–93. [Google Scholar] [CrossRef]
- Steuer, H.; Krastev, R.; Lembert, N. Metallic oxide nanoparticles stimulate blood coagulation independent of their surface charge. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 897–902. [Google Scholar] [CrossRef]
- Chaudhry, S.A.; Serrata, M.; Tomczak, L.; Higgins, S.; Ryu, J.; Laprise, D.; Enjyoji, K.; Bekendam, R.; Kaushik, V.; Flaumenhaft, R.; et al. Cationic zinc is required for factor XII recruitment and activation by stimulated platelets and for thrombus formation in vivo. J. Thromb. Haemost. 2020, 18, 2318–2328. [Google Scholar] [CrossRef]
- Huynh, C.T.; Liu, F.; Cheng, Y.; Coughlin, K.A.; Alsberg, E. Thiol-Epoxy “Click” Chemistry to Engineer Cytocompatible PEG-Based Hydrogel for siRNA-Mediated Osteogenesis of hMSCs. ACS Appl. Mater. Interfaces 2018, 10, 25936–25942. [Google Scholar] [CrossRef]
- Hao, Y.; Yuan, C.; Deng, J.; Zheng, W.; Ji, Y.; Zhou, Q. Injectable Self-Healing First-Aid Tissue Adhesives with Outstanding Hemostatic and Antibacterial Performances for Trauma Emergency Care. ACS Appl. Mater. Interfaces 2022, 14, 16006–16017. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Hou, W.; Liu, K.; Yang, H.; Wei, W.; Kang, H.; Dai, H. A multifunctional chitosan hydrogel dressing for liver hemostasis and infected wound healing. Carbohydr. Polym. 2022, 291, 119631. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Tian, W.; Wen, C.; Ji, X.; Diao, H.; Hou, Y.; Fan, J.; Liu, Z.; Ji, T.; Sun, F.; et al. Cellulose-Based Cryogel Microspheres with Nanoporous and Controllable Wrinkled Morphologies for Rapid Hemostasis. Nano Lett. 2022, 22, 6350–6358. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Wang, Y.; Xue, Y.; Cheng, J.; Chi, P.; Wang, Z.; Li, B.; Yan, T.; Wu, B.; Wang, Z. Enhanced Hemostatic and Procoagulant Efficacy of PEG/ZnO Hydrogels: A Novel Approach in Traumatic Hemorrhage Management. Gels 2024, 10, 88. https://doi.org/10.3390/gels10020088
Zhang C, Wang Y, Xue Y, Cheng J, Chi P, Wang Z, Li B, Yan T, Wu B, Wang Z. Enhanced Hemostatic and Procoagulant Efficacy of PEG/ZnO Hydrogels: A Novel Approach in Traumatic Hemorrhage Management. Gels. 2024; 10(2):88. https://doi.org/10.3390/gels10020088
Chicago/Turabian StyleZhang, Chuyue, Yifan Wang, Yuan Xue, Junyao Cheng, Pengfei Chi, Zhaohan Wang, Bo Li, Taoxu Yan, Bing Wu, and Zheng Wang. 2024. "Enhanced Hemostatic and Procoagulant Efficacy of PEG/ZnO Hydrogels: A Novel Approach in Traumatic Hemorrhage Management" Gels 10, no. 2: 88. https://doi.org/10.3390/gels10020088
APA StyleZhang, C., Wang, Y., Xue, Y., Cheng, J., Chi, P., Wang, Z., Li, B., Yan, T., Wu, B., & Wang, Z. (2024). Enhanced Hemostatic and Procoagulant Efficacy of PEG/ZnO Hydrogels: A Novel Approach in Traumatic Hemorrhage Management. Gels, 10(2), 88. https://doi.org/10.3390/gels10020088