A Flexible Yet Robust 3D-Hybrid Gel Solid-State Electrolyte Based on Metal–Organic Frameworks for Rechargeable Lithium Metal Batteries
Abstract
1. Introduction
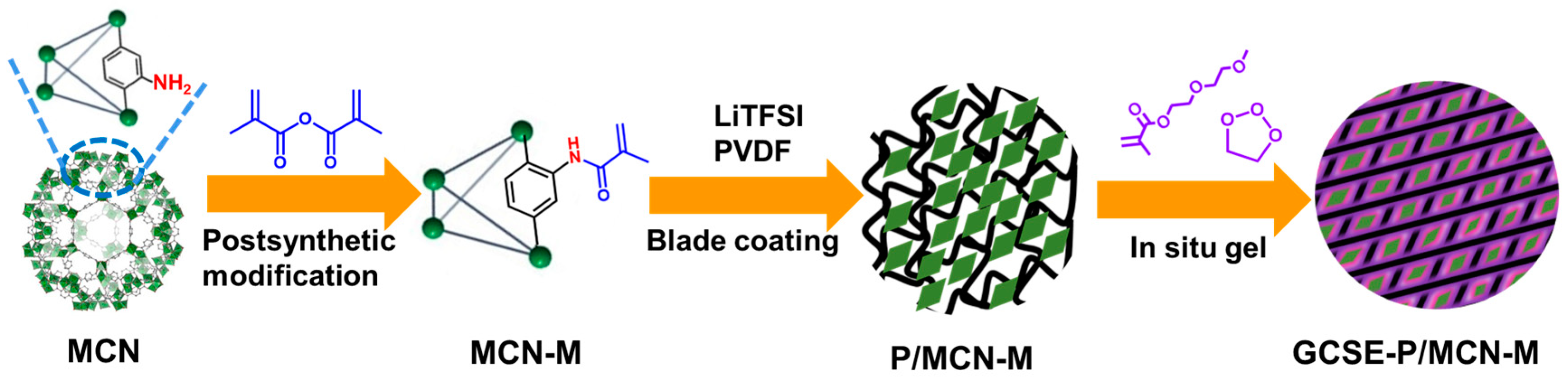
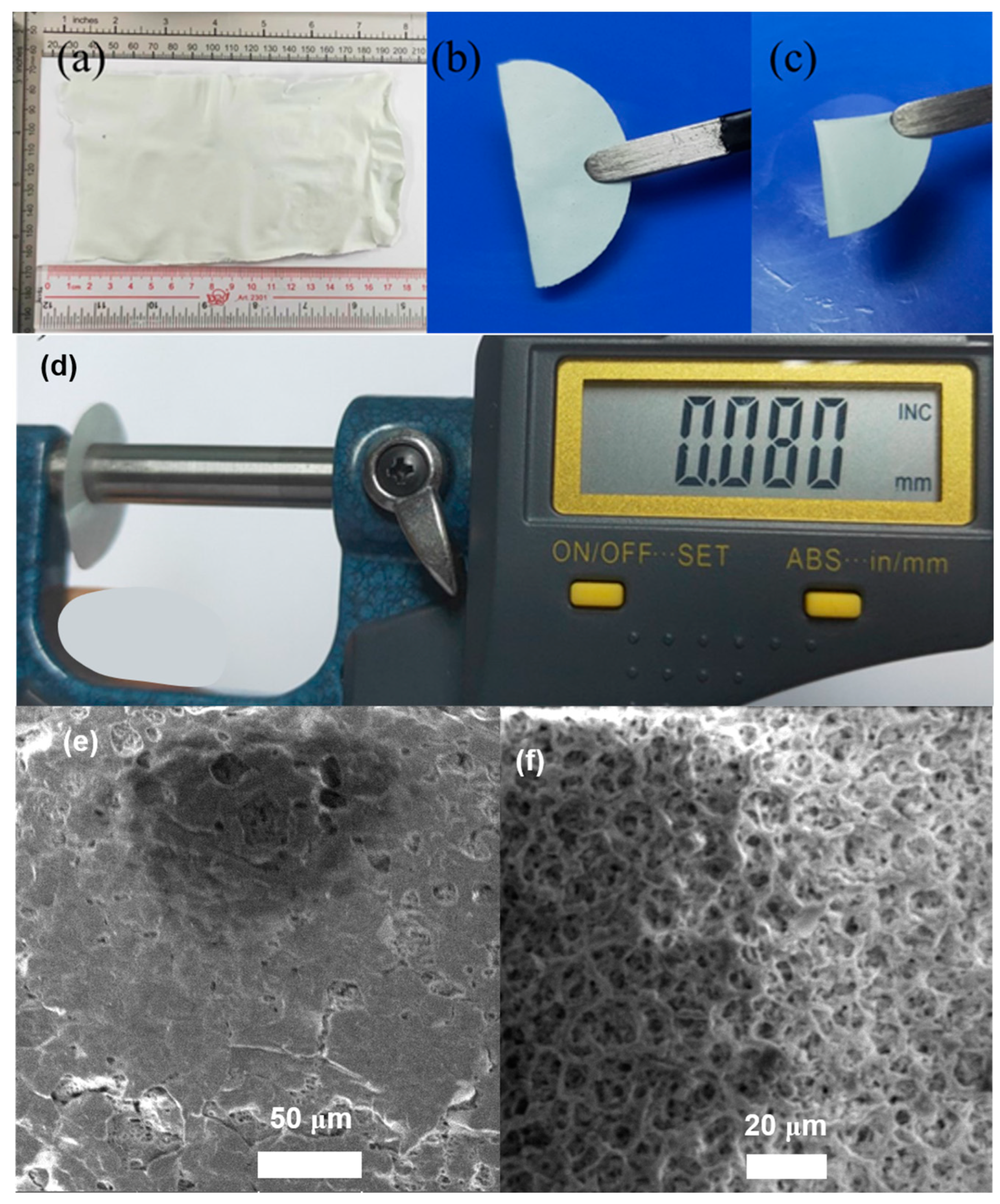
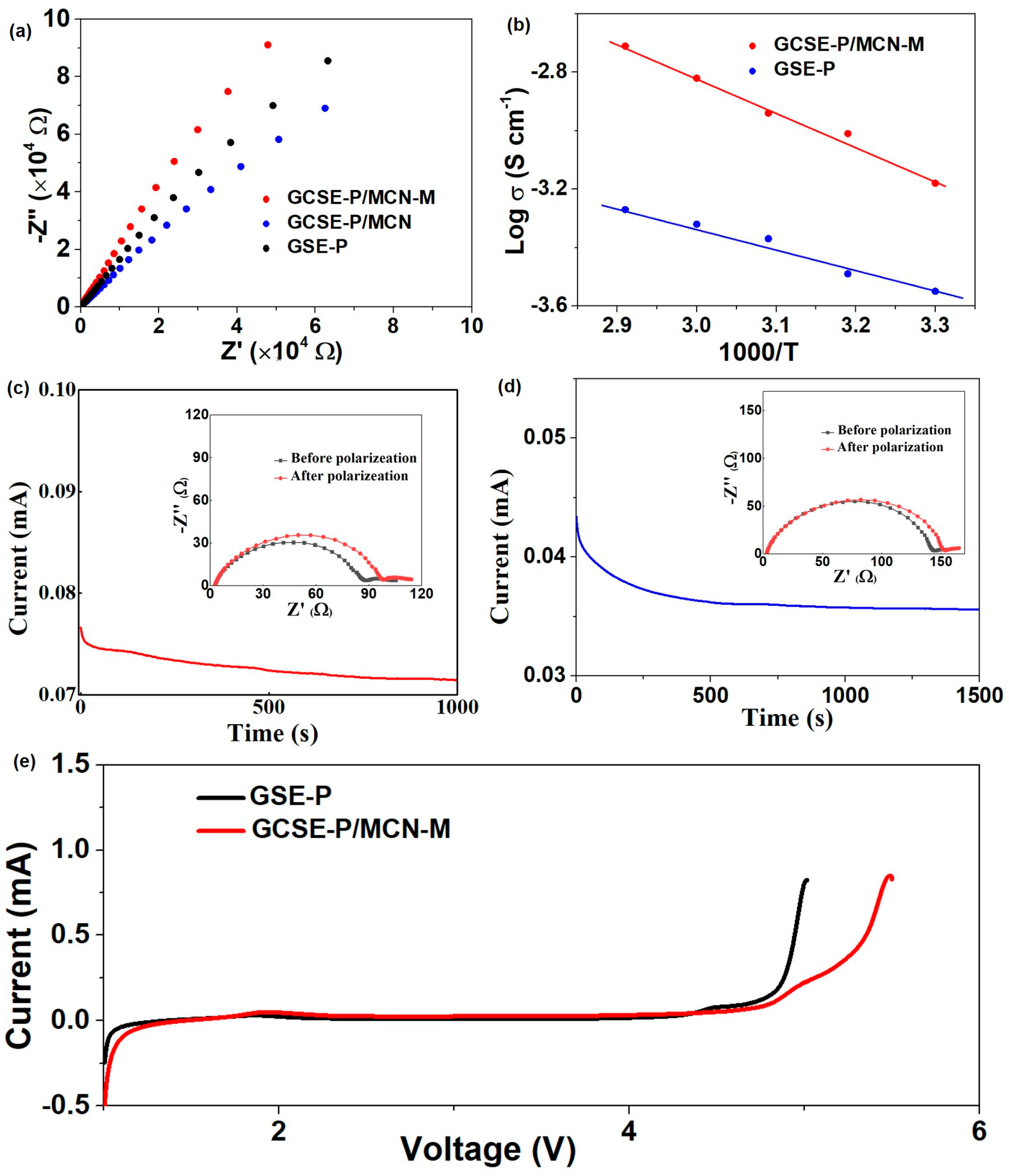
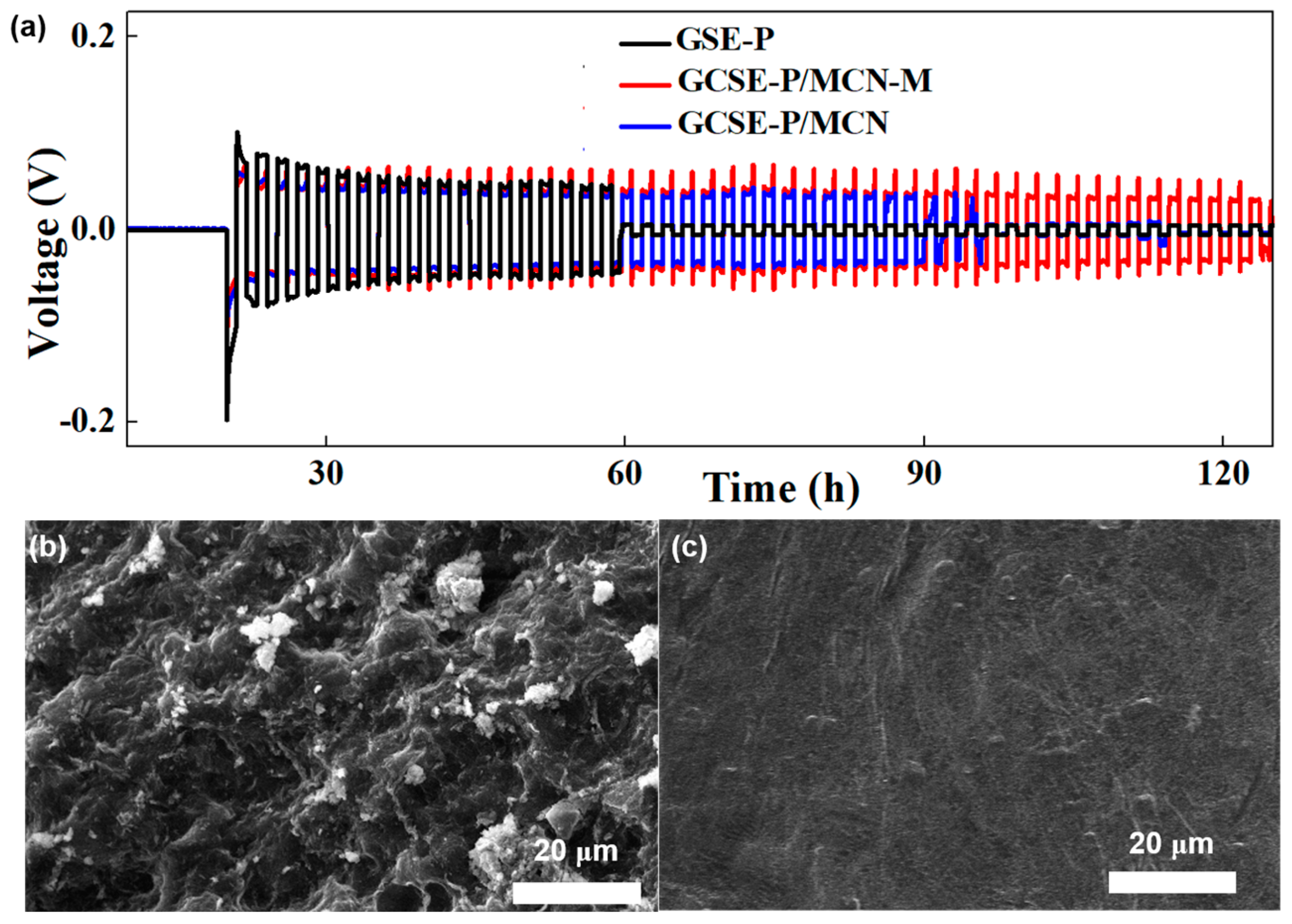
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of MOFs and Gel Composite Solid Electrolyte
4.3. Characterization
4.4. Electrochemical Measurements
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, B.; Zhang, J.-G.; Xu, W. Advancing Lithium Metal Batteries. Joule 2018, 2, 833–845. [Google Scholar] [CrossRef]
- Wang, X.; Pawar, G.; Li, Y.; Ren, X.; Zhang, M.; Lu, B.; Banerjee, A.; Liu, P.; Dufek, E.J.; Zhang, J.-G.; et al. Glassy Li metal anode for high-performance rechargeable Li batteries. Nat. Mater. 2020, 19, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, J.; Ding, F.; Chen, X.; Nasybulin, E.; Zhang, Y.; Zhang, J.-G. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 2013, 7, 513–537. [Google Scholar] [CrossRef]
- Zou, P.; Sui, Y.; Zhan, H.; Wang, C.; Xin, H.L.; Cheng, H.-M.; Kang, F.; Yang, C. Polymorph Evolution Mechanisms and Regulation Strategies of Lithium Metal Anode under Multiphysical Fields. Chem. Rev. 2021, 121, 5986–6056. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.J.; Pyo, S.; Kim, H.; Cho, J.; Yun, H.; Kim, H.; Ryu, S.; Yoo, J.; Kim, Y.S. Advanced Li metal anode by fluorinated metathesis on conjugated carbon networks. Energy Environ. Sci. 2020, 14, 940–954. [Google Scholar] [CrossRef]
- Chen, N.; Dai, Y.; Xing, Y.; Wang, L.; Guo, C.; Chen, R.; Guo, S.; Wu, F. Biomimetic ant-nest ionogel electrolyte boosts the performance of dendrite-free lithium batteries. Energy Environ. Sci. 2017, 10, 1660–1667. [Google Scholar] [CrossRef]
- Wu, W.; Luo, W.; Huang, Y. Less is more: A perspective on thinning lithium metal towards high-energy-density rechargeable lithium batteries. Chem. Soc. Rev. 2023, 52, 2553–2572. [Google Scholar] [CrossRef]
- Zheng, J.; Kim, M.S.; Tu, Z.; Choudhury, S.; Tang, T.; Archer, L.A. Regulating electrodeposition morphology of lithium: Towards commercially relevant secondary Li metal batteries. Chem. Soc. Rev. 2020, 49, 2701–2750. [Google Scholar] [CrossRef]
- Zhang, Q.-K.; Zhang, X.-Q.; Wan, J.; Yao, N.; Song, T.-L.; Xie, J.; Hou, L.-P.; Zhou, M.-Y.; Chen, X.; Li, B.-Q.; et al. Homogeneous and mechanically stable solid–electrolyte interphase enabled by trioxane-modulated electrolytes for lithium metal batteries. Nat. Energy 2023, 8, 725–735. [Google Scholar] [CrossRef]
- Huo, H.; Chen, Y.; Li, R.; Zhao, N.; Luo, J.; Pereira da Silva, J.G.; Mücke, R.; Kaghazchi, P.; Guo, X.; Sun, X. Design of a mixed conductive garnet/Li interface for dendrite-free solid lithium metal batteries. Energy Environ. Sci. 2019, 13, 127–134. [Google Scholar] [CrossRef]
- Sang, J.; Tang, B.; Pan, K.; He, Y.-B.; Zhou, Z. Current Status and Enhancement Strategies for All-Solid-State Lithium Batteries. Acc. Mater. Res. 2023, 4, 472–483. [Google Scholar] [CrossRef]
- Hu, C.; Shen, Y.; Chen, L. Recent advances in nanostructured composite solid electrolyte. Curr. Opin. Electrochem. 2020, 22, 51–57. [Google Scholar] [CrossRef]
- Shen, M.; Wang, Z.; Cheng, D.; Cheng, H.; Xu, H.; Huang, Y. Molecular regulated polymer electrolytes for solid-state lithium metal batteries: Mechanisms and future prospects. eTransportation 2023, 18, 100264. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. A review of composite polymer-ceramic electrolytes for lithium batteries. Energy Storage Mater. 2021, 34, 282–300. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, R.; Jia, D.; Cui, Y.; Liu, Q.; Liu, S.; Wu, D. Ultrathin Yet Robust Single Lithium-Ion Conducting Quasi-Solid-State Polymer-Brush Electrolytes Enable Ultralong-Life and Dendrite-Free Lithium-Metal Batteries. Adv. Mater. 2021, 33, 2100943. [Google Scholar] [CrossRef]
- Weston, J.E.; Steele, B.C.H. Effects of inert fillers on the mechanical and electrochemical properties of lithium salt-poly(ethylene oxide) polymer electrolytes. Solid State Ion. 1982, 7, 75–79. [Google Scholar] [CrossRef]
- Zheng, F.; Li, C.; Li, Z.; Cao, X.; Luo, H.; Liang, J.; Zhao, X.; Kong, J. Advanced Composite Solid Electrolytes for Lithium Batteries: Filler Dimensional Design and Ion Path Optimization. Small 2023, 19, 2206355. [Google Scholar] [CrossRef]
- Dirican, M.; Yan, C.; Zhu, P.; Zhang, X. Composite solid electrolytes for all-solid-state lithium batteries. Mater. Sci. Eng. R Rep. 2019, 136, 27–46. [Google Scholar] [CrossRef]
- Shan, Y.; Li, L.; Chen, X.; Fan, S.; Yang, H.; Jiang, Y. Gentle Haulers of Lithium-Ion–Nanomolybdenum Carbide Fillers in Solid Polymer Electrolyte. ACS Energy Lett. 2022, 7, 2289–2296. [Google Scholar] [CrossRef]
- Cui, Y.; Miao, D.; Yu, G.; Zhou, M.; Liu, R.; Liu, S.; Wu, D. Novel quasi-solid-state composite electrolytes boost interfacial Li+ transport for long-cycling and dendrite-free lithium metal batteries. Energy Storage Mater. 2023, 56, 258–266. [Google Scholar] [CrossRef]
- Cui, Y.; Yu, G.; Liu, R.; Miao, D.; Wu, D. Quasi-Solid-State Composite Electrolytes with Multifunctional 2D Molecular Brush Fillers for Long-Cycling Lithium Metal Batteries. Chin. J. Chem. 2023, 41, 2848–2854. [Google Scholar] [CrossRef]
- Pan, K.; Zhang, L.; Qian, W.; Wu, X.; Dong, K.; Zhang, H.; Zhang, S. A Flexible Ceramic/Polymer Hybrid Solid Electrolyte for Solid-State Lithium Metal Batteries. Adv. Mater. 2020, 32, 2000399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yue, B.; Shao, C.; Shao, H.; Li, L.; Dong, X.; Wang, J.; Yu, W. Suppression of lithium dendrites in all-solid-state lithium batteries by using a Janus-structured composite solid electrolyte. Chem. Eng. J. 2022, 443, 136479. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Li, Z.; Garcia, H. Catalysis and photocatalysis by metal organic frameworks. Chem. Soc. Rev. 2018, 47, 8134–8172. [Google Scholar] [CrossRef]
- Keskin, S.; Sholl, D.S. Selecting metal organic frameworks as enabling materials in mixed matrix membranes for high efficiency natural gas purification. Energy Environ. Sci. 2010, 3, 343–351. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Fu, Y. A thermoregulating separator based on black phosphorus/MOFs heterostructure for thermo-stable lithium-sulfur batteries. Chem. Eng. J. 2023, 454, 140250. [Google Scholar] [CrossRef]
- Zheng, S.; Li, Z.; Chen, L.; Huang, Y.; Shi, J.; Wang, S.; Liu, Y.; Liu, Y.; Cai, Y.-P.; Zheng, Q. MOF-Based 3D Ion-Conducting Network Enables High-Voltage All-Solid-State Lithium Metal Batteries at Room Temperature. ACS Mater. Lett. 2023, 5, 1136–1144. [Google Scholar] [CrossRef]
- Zhou, J.; Zeng, X.; Dong, L.; Chen, L.; Wei, X.; Liu, Y.; Shi, L.; Yu, L.; Fu, J. Mixed-linker MOFs-derived cross-linked copolymer electrolyte enables high lithium mobility for dendrite-free all-solid-state batteries. Chem. Eng. J. 2023, 466, 143243. [Google Scholar] [CrossRef]
- Yuan, C.; Li, J.; Han, P.; Lai, Y.; Zhang, Z.; Liu, J. Enhanced electrochemical performance of poly(ethylene oxide) based composite polymer electrolyte by incorporation of nano-sized metal-organic framework. J. Power Sources 2013, 240, 653–658. [Google Scholar] [CrossRef]
- Du, L.; Zhang, B.; Deng, W.; Cheng, Y.; Xu, L.; Mai, L. Hierarchically Self-Assembled MOF Network Enables Continuous Ion Transport and High Mechanical Strength. Adv. Energy Mater. 2022, 12, 2200501. [Google Scholar] [CrossRef]
- Ma, Y.; Qiu, Y.; Yang, K.; Lv, S.; Li, Y.; An, X.; Xiao, G.; Han, Z.; Ma, Y.; Chen, L.; et al. Competitive Li-ion coordination for constructing a three-dimensional transport network to achieve ultra-high ionic conductivity of a composite solid-state electrolyte. Energy Environ. Sci. 2024, 17, 8274–8283. [Google Scholar] [CrossRef]
- Babaee, S.; Zarei, M.; Sepehrmansourie, H.; Zolfigol, M.A.; Rostamnia, S. Synthesis of Metal–Organic Frameworks MIL-101(Cr)-NH2 Containing Phosphorous Acid Functional Groups: Application for the Synthesis of N-Amino-2-pyridone and Pyrano [2,3-c]pyrazole Derivatives via a Cooperative Vinylogous Anomeric-Based Oxidation. ACS Omega 2020, 5, 6240–6249. [Google Scholar] [CrossRef] [PubMed]
- Dapaah, M.F.; Liu, B.; Cheng, L. Adsorption of organic compounds from aqueous solution by pyridine-2-carboxaldehyde grafted MIL-101(Cr)-NH2 metal-organic frameworks. J. Environ. Chem. Eng. 2021, 9, 105275. [Google Scholar] [CrossRef]
- Xie, X.-Y.; Wu, F.; Liu, X.; Tao, W.-Q.; Jiang, Y.; Liu, X.-Q.; Sun, L.-B. Photopolymerization of metal–organic polyhedra: An efficient approach to improve the hydrostability, dispersity, and processability. Chem. Commun. 2019, 55, 6177–6180. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; He, Y.; Wang, C.; Zou, P.; Hu, E.; Yang, X.-Q.; Xu, K.; Xin, H.L. Characterization of the structure and chemistry of the solid–electrolyte interface by cryo-EM leads to high-performance solid-state Li-metal batteries. Nat. Nanotechnol. 2022, 17, 768–776. [Google Scholar] [CrossRef]
- Li, Z.; Liu, F.; Chen, S.; Zhai, F.; Li, Y.; Feng, Y.; Feng, W. Single Li ion conducting solid-state polymer electrolytes based on carbon quantum dots for Li-metal batteries. Nano Energy 2021, 82, 105698. [Google Scholar] [CrossRef]
- Zhang, T.; Li, J.; Li, X.; Wang, R.; Wang, C.; Zhang, Z.; Yin, L. A Silica-Reinforced Composite Electrolyte with Greatly Enhanced Interfacial Lithium-Ion Transfer Kinetics for High-Performance Lithium Metal Batteries. Adv. Mater. 2022, 34, 2205575. [Google Scholar] [CrossRef]
- Yang, P.; Gao, X.; Tian, X.; Shu, C.; Yi, Y.; Liu, P.; Wang, T.; Qu, L.; Tian, B.; Li, M.; et al. Upgrading Traditional Organic Electrolytes toward Future Lithium Metal Batteries: A Hierarchical Nano-SiO2-Supported Gel Polymer Electrolyte. ACS Energy Lett. 2020, 5, 1681–1688. [Google Scholar] [CrossRef]
- Jiang, D.; Keenan, L.L.; Burrows, A.D.; Edler, K.J. Synthesis and post-synthetic modification of MIL-101(Cr)-NH2 via a tandem diazotisation process. Chem. Commun. 2012, 48, 12053–12055. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Xue, J.; Xie, L.; Chen, H.; Deng, Z.; Yin, W. A Flexible Yet Robust 3D-Hybrid Gel Solid-State Electrolyte Based on Metal–Organic Frameworks for Rechargeable Lithium Metal Batteries. Gels 2024, 10, 812. https://doi.org/10.3390/gels10120812
Liu R, Xue J, Xie L, Chen H, Deng Z, Yin W. A Flexible Yet Robust 3D-Hybrid Gel Solid-State Electrolyte Based on Metal–Organic Frameworks for Rechargeable Lithium Metal Batteries. Gels. 2024; 10(12):812. https://doi.org/10.3390/gels10120812
Chicago/Turabian StyleLiu, Ruliang, Jiaqi Xue, Lijun Xie, Huirong Chen, Zhaoxia Deng, and Wei Yin. 2024. "A Flexible Yet Robust 3D-Hybrid Gel Solid-State Electrolyte Based on Metal–Organic Frameworks for Rechargeable Lithium Metal Batteries" Gels 10, no. 12: 812. https://doi.org/10.3390/gels10120812
APA StyleLiu, R., Xue, J., Xie, L., Chen, H., Deng, Z., & Yin, W. (2024). A Flexible Yet Robust 3D-Hybrid Gel Solid-State Electrolyte Based on Metal–Organic Frameworks for Rechargeable Lithium Metal Batteries. Gels, 10(12), 812. https://doi.org/10.3390/gels10120812







