Enhancement of Water Uptake in Composite Superabsorbents Based on Carboxymethyl Cellulose Through Porogen Incorporation and Lyophilization
Abstract
1. Introduction
2. Results and Discussion
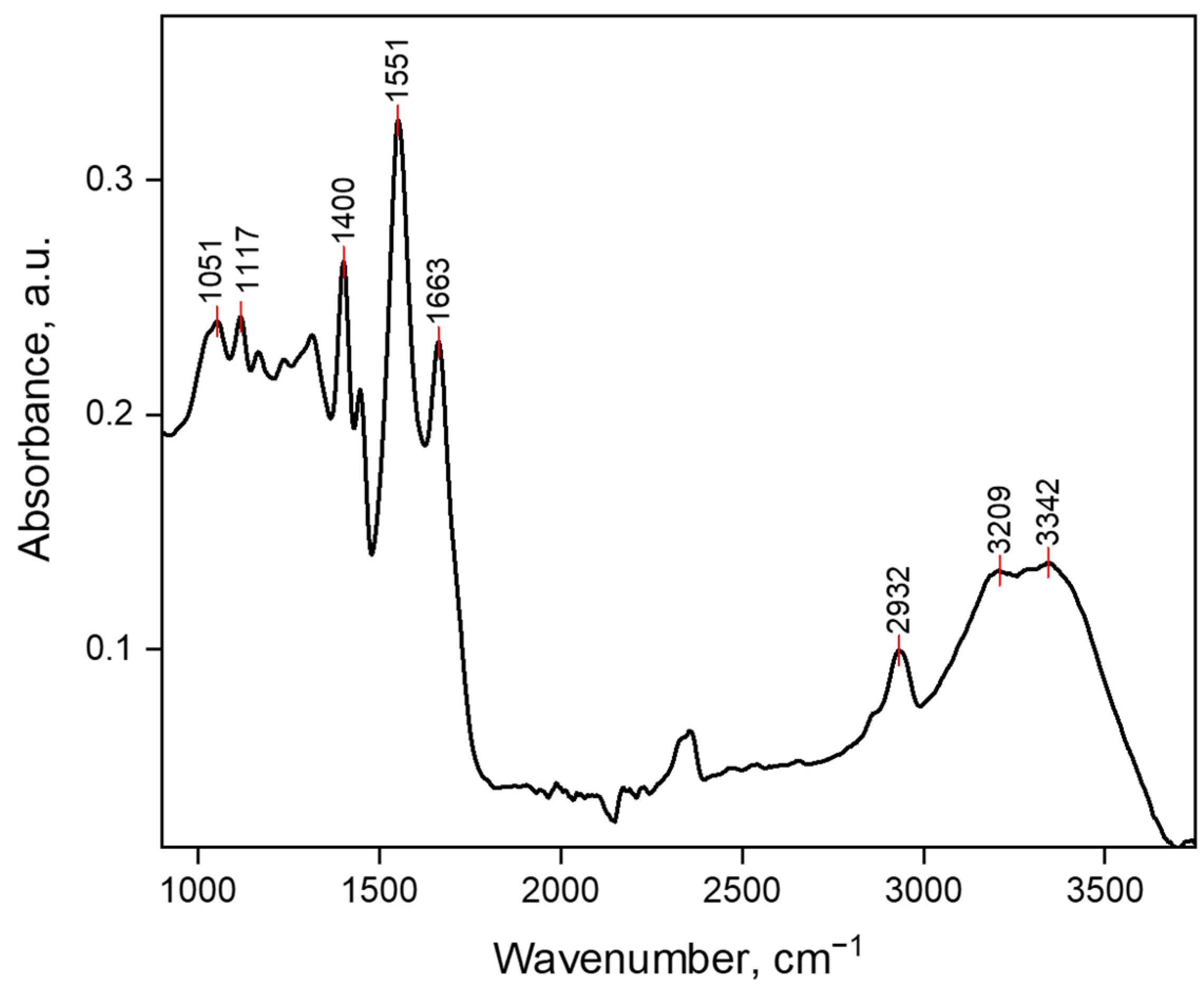
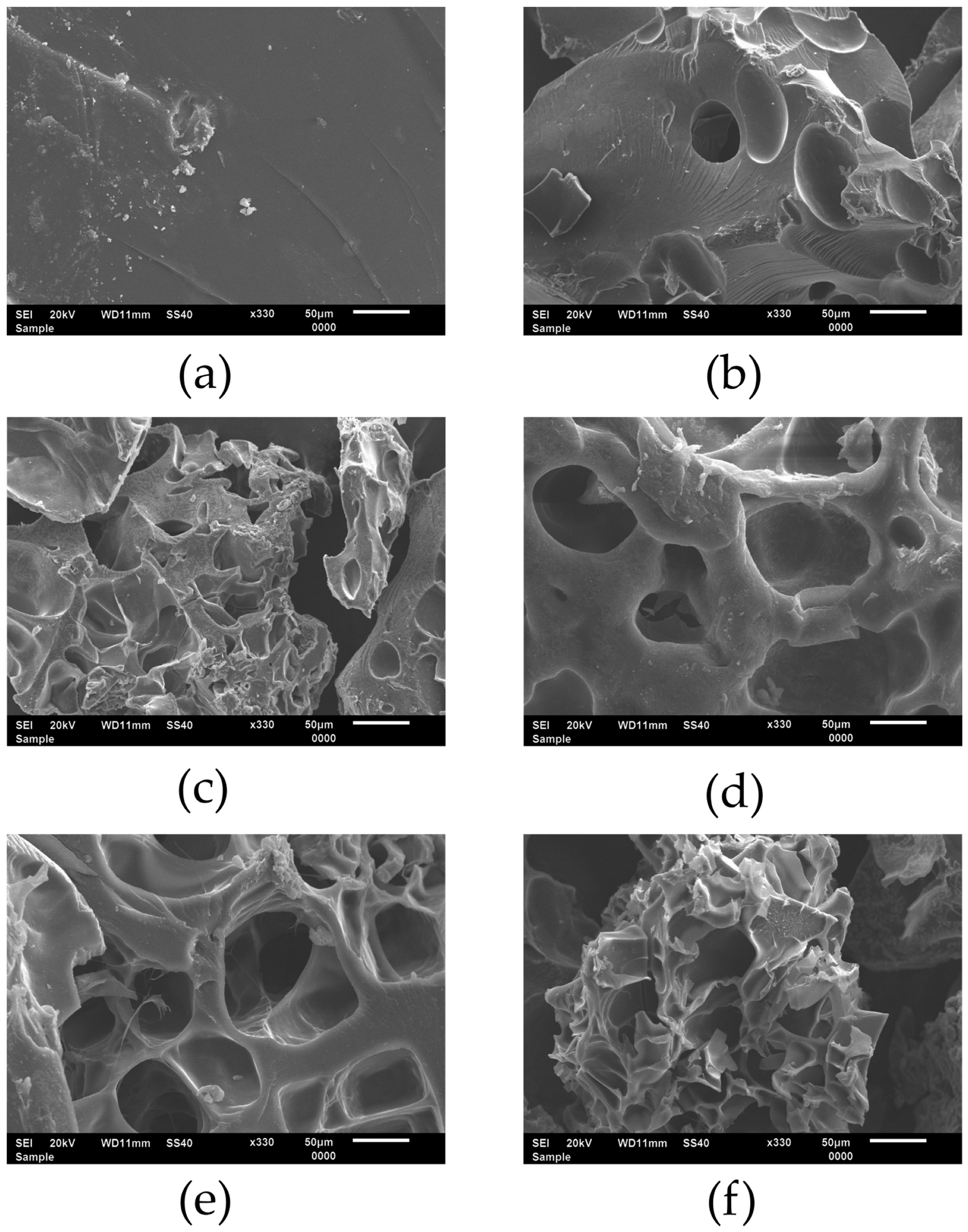
2.1. Synthesis and Characterization of the Superabsorbents Based on Carboxymethyl Cellulose Sodium Salt
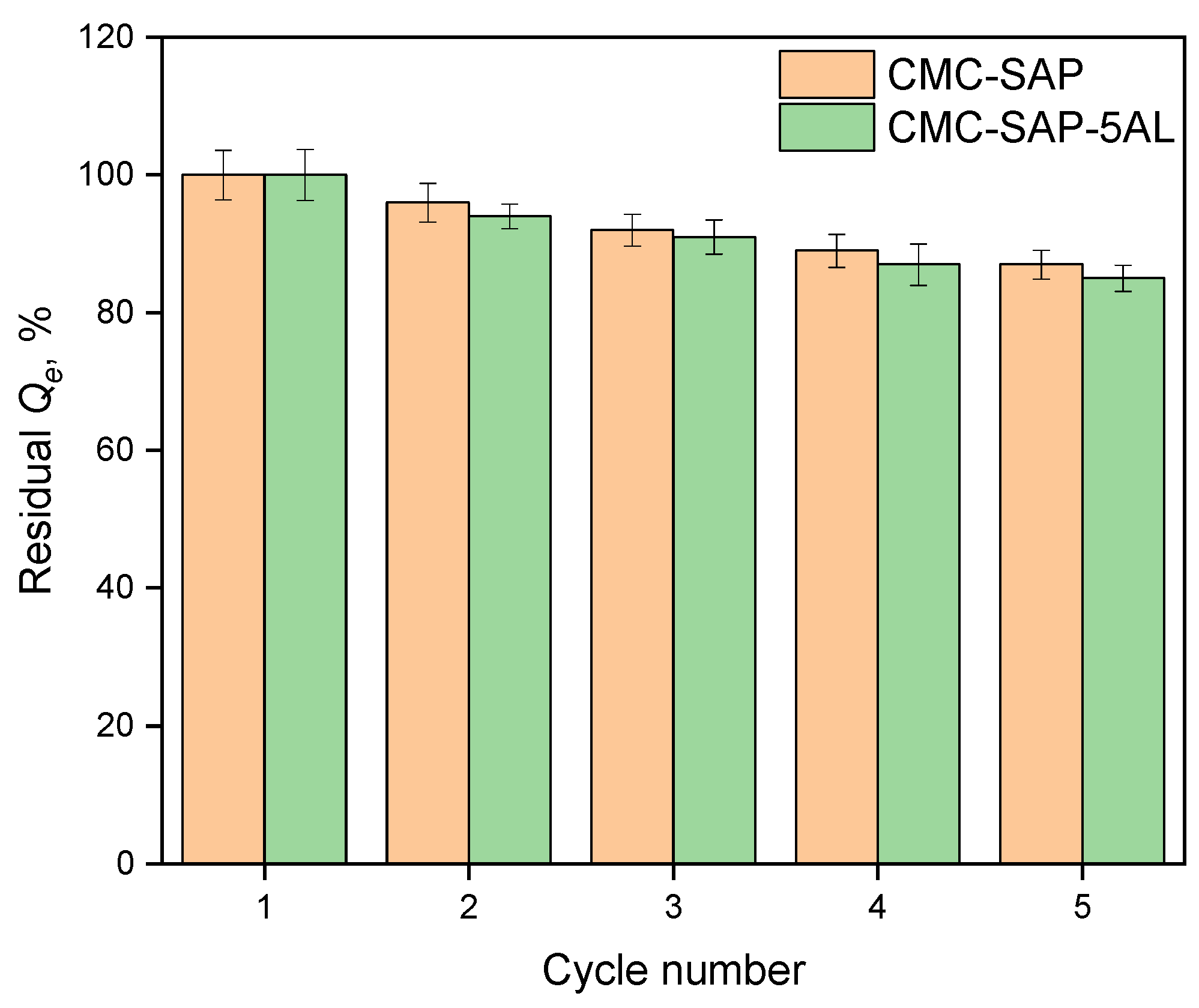
2.2. Investigation of Water Sorption
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Superabsorbent Synthesis
4.3. Instrumental Characterization
4.3.1. Fourier-Transform Infrared Spectroscopy
4.3.2. Scanning Electron Microscopy
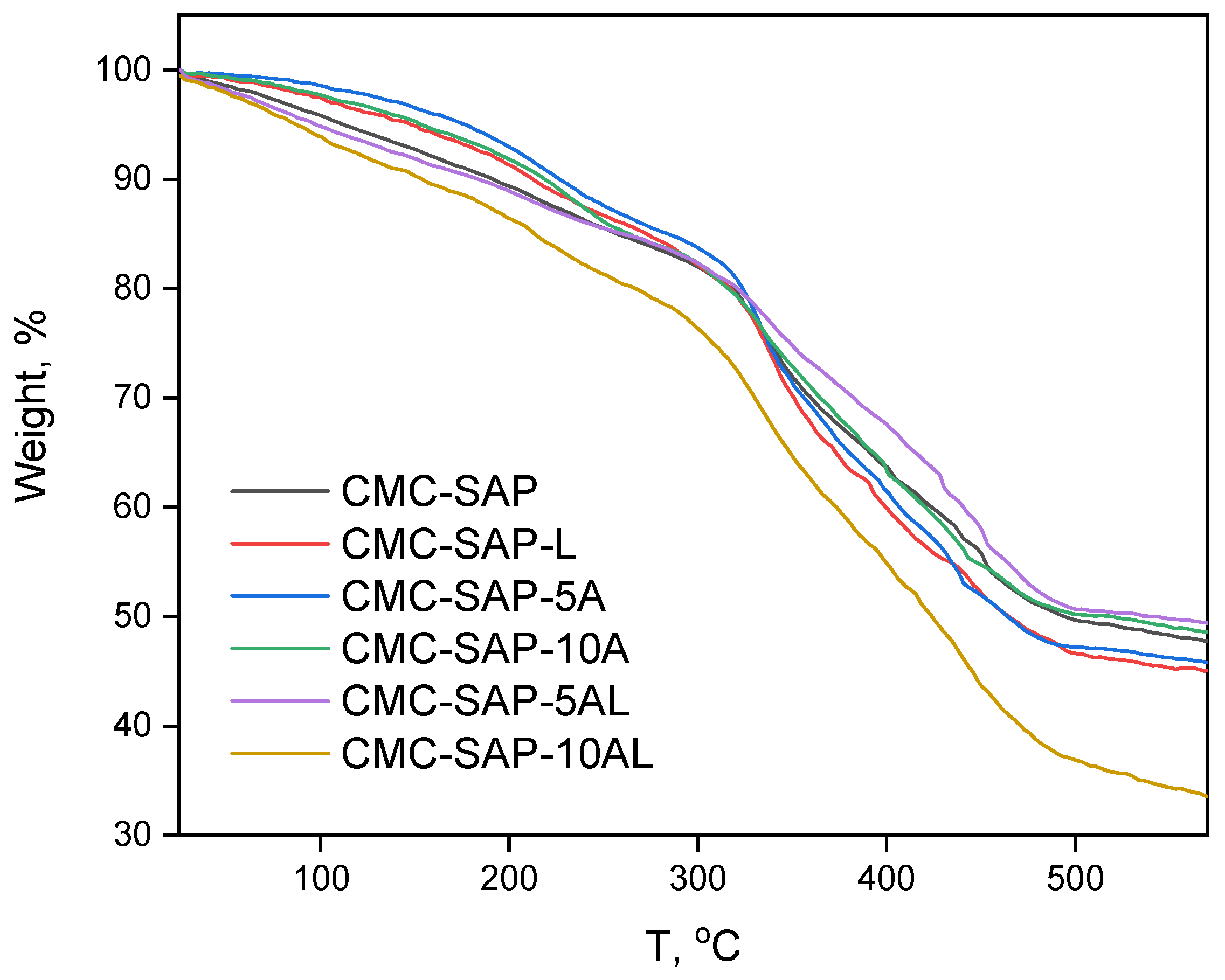
4.3.3. Thermogravimetric Analysis and Differential Scanning Calorimetry
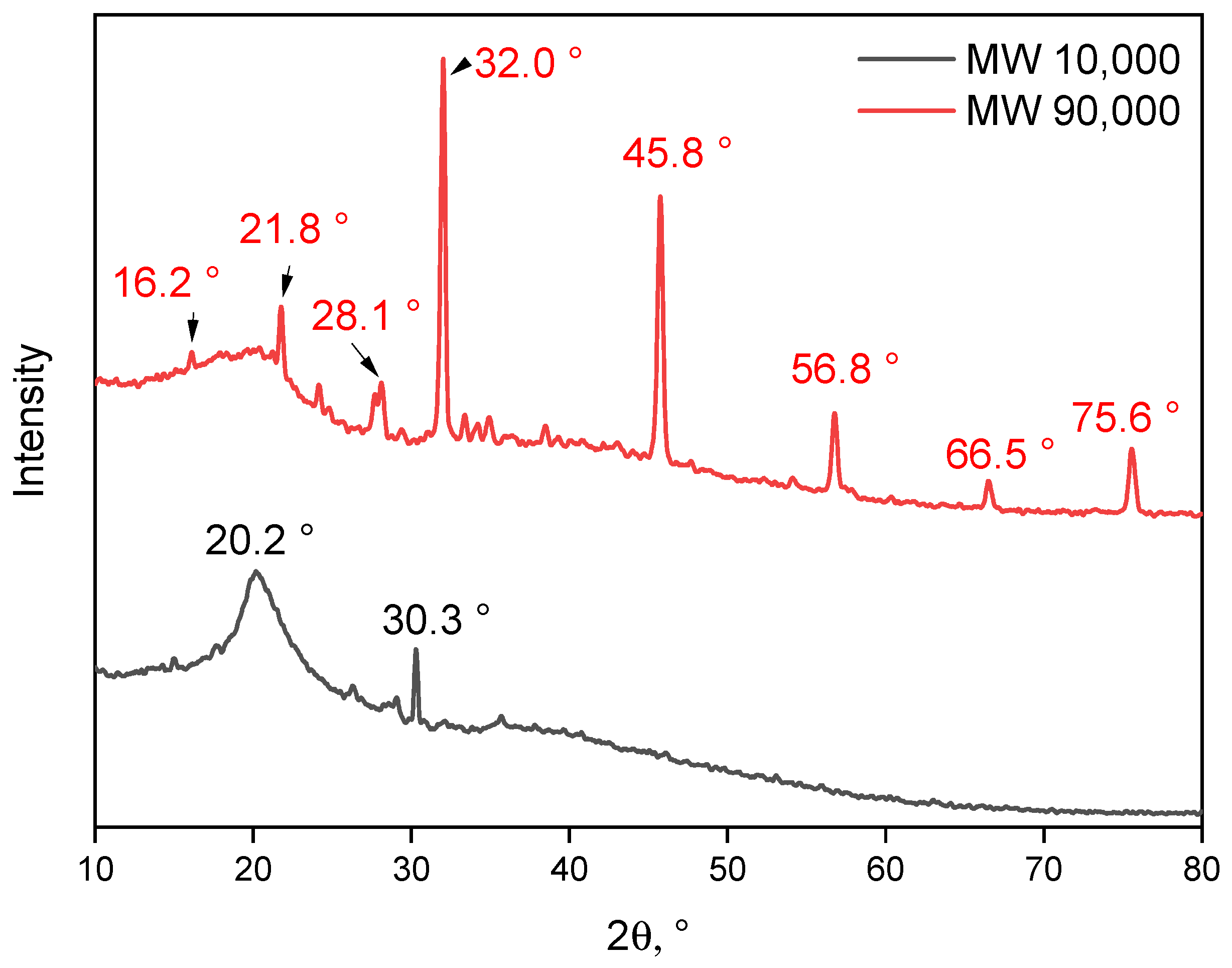
4.3.4. X-ray Diffraction
4.4. Porosity Evaluation
4.5. Swelling Research
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chelu, M. Hydrogels with Essential Oils: Recent Advances in Designs and Applications. Gels 2024, 10, 636. [Google Scholar] [CrossRef] [PubMed]
- Zanrè, E.; Dalla Valle, E.; D’Angelo, E.; Sensi, F.; Agostini, M.; Cimetta, E. Recent Advancements in Hydrogel Biomedical Research in Italy. Gels 2024, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Wang, Y. Recent Applications of Regenerated Cellulose Films and Hydrogels in Food Packaging. Curr. Opin. Food Sci. 2022, 43, 7–17. [Google Scholar] [CrossRef]
- Leyva-Jiménez, F.J.; Oliver-Simancas, R.; Castangia, I.; Rodríguez-García, A.M.; Alañón, M.E. Comprehensive Review of Natural Based Hydrogels as an Upcoming Trend for Food Packing. Food Hydrocoll. 2023, 135, 108124. [Google Scholar] [CrossRef]
- Oucif, A.; Haddadine, N.; Zakia, D.; Bouslah, N.; Benaboura, A.; Beyaz, K.; Guedouar, B.; El-Shall, M.S. Poly (Hydroxyethyl Methacrylate-Co-Hydroxyethyl Acrylate) Soft Contact Lenses for Acetazolamide Release. Polym. Bull. 2022, 79, 1535–1554. [Google Scholar] [CrossRef]
- Giri, B.R.; Jakka, D.; Sandoval, M.A.; Kulkarni, V.R.; Bao, Q. Advancements in Ocular Therapy: A Review of Emerging Drug Delivery Approaches and Pharmaceutical Technologies. Pharmaceutics 2024, 16, 1325. [Google Scholar] [CrossRef]
- Zohuriaan-Mehr, M.J.; Omidian, H.; Doroudiani, S.; Kabiri, K. Advances in Non-Hygienic Applications of Superabsorbent Hydrogel Materials. J. Mater. Sci. 2010, 45, 5711–5735. [Google Scholar] [CrossRef]
- Li, S.; Chen, G. Agricultural Waste-Derived Superabsorbent Hydrogels: Preparation, Performance, and Socioeconomic Impacts. J. Clean. Prod. 2020, 251, 119669. [Google Scholar] [CrossRef]
- Priya, E.; Sarkar, S.; Maji, P.K. A Review on Slow-Release Fertilizer: Nutrient Release Mechanism and Agricultural Sustainability. J. Environ. Chem. Eng. 2024, 12, 113211. [Google Scholar] [CrossRef]
- Eddarai, E.M.; El Mouzahim, M.; Ragaoui, B.; Eladaoui, S.; Bourd, Y.; Bellaouchou, A.; Boussen, R. Review of Current Trends in Chitosan Based Controlled and Slow-Release Fertilizer: From Green Chemistry to Circular Economy. Int. J. Biol. Macromol. 2024, 278, 134982. [Google Scholar] [CrossRef]
- El Idrissi, A.; Channab, B.; Essamlali, Y.; Zahouily, M. Superabsorbent Hydrogels Based on Natural Polysaccharides: Classification, Synthesis, Physicochemical Properties, and Agronomic Efficacy under Abiotic Stress Conditions: A Review. Int. J. Biol. Macromol. 2024, 258, 128909. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.A.; Nishat, N.; Jadoun, S.; Ansari, M.Z. Polysaccharide Based Superabsorbent Hydrogels and Their Methods of Synthesis: A Review. Carbohyd. Polym. Technol. Appl. 2020, 1, 100014. [Google Scholar] [CrossRef]
- Jayanudin; Lestari, R.S.D.; Barleany, D.R.; Pitaloka, A.B.; Yulvianti, M.; Prasetyo, D.; Anggoro, D.V.; Ruhiatna, A. Chitosan-Graft-Poly(Acrylic Acid) Superabsorbent’s Water Holding in Sandy Soils and Its Application in Agriculture. Polymers 2022, 14, 5175. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, A.V.; Lavlinskaya, M.S. The Influence of Dibutyl Sebacate on the Sorption Properties of a Composite Superabsorbent Based on Chitosan. Sorpchrom 2023, 23, 938–947. [Google Scholar] [CrossRef]
- Sorokin, A.; Lavlinskaya, M. Synthesis of the Superabsobents Enriched in Chitosan Derivatives with Excellent Water Absorption Properties. Polym. Bull. 2022, 79, 407–427. [Google Scholar] [CrossRef]
- Sorokin, A.; Sukhanov, P.; Popov, V.; Kannykin, S.; Lavlinskaya, M. A New Approach to Increasing the Equilibrium Swelling Ratio of the Composite Superabsorbents Based on Carboxymethyl Cellulose Sodium Salt. Cellulose 2022, 29, 159–173. [Google Scholar] [CrossRef]
- Mazlan, S.N.A.; Abd Rahim, S.; Ghazali, S.; Jamari, S.S. Optimization of N,N′-Methylenebis(Acrylamide), and Ammonium Persulfate Content in Carbonaceous/Acrylic Acid-Co-Acrylamide Superabsorbent Polymer. Mater. Today Proc. 2022, 57, 1088–1094. [Google Scholar] [CrossRef]
- Mohammadbagheri, Z.; Rahmati, A.; Hoshyarmanesh, P. Synthesis of a Novel Superabsorbent with Slow-Release Urea Fertilizer Using Modified Cellulose as a Grafting Agent and Flexible Copolymer. Int. J. Biol. Macromol. 2021, 182, 1893–1905. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.; Raffa, P.; Picchioni, F.; Koning, C.E. Superabsorbent Polymers: From Long-Established, Microplastics Generating Systems, to Sustainable, Biodegradable and Future Proof Alternatives. Prog. Polym. Sci. 2022, 125, 101475. [Google Scholar] [CrossRef]
- Qi, Z.; Hu, X. Water Absorbency of Super Absorbent Polymer Based on Flexible Polymeric Network. Eur. Polym. J. 2022, 166, 111045. [Google Scholar] [CrossRef]
- Hu, X.; Wang, C.; Yu, H. Study on Water-Absorbing Behaviors of Superabsorbent Polymer Enhanced by 1,2,3,4-Butane Tetracarboxylic Acid Cross-Linker. React. Funct. Polym. 2024, 194, 105776. [Google Scholar] [CrossRef]
- Jiao, Y.; Su, T.; Chen, Y.; Long, M.; Luo, X.; Xie, X.; Qin, Z. Enhanced Water Absorbency and Water Retention Rate for Superabsorbent Polymer via Porous Calcium Carbonate Crosslinking. Nanomaterials 2023, 13, 2575. [Google Scholar] [CrossRef]
- Xiao, W.; Miao, C.; Yin, X.; Zheng, Y.; Tian, M.; Li, H.; Mei, P. Effect of Urea as Pore-Forming Agent on Properties of Poly(Vinylidene Fluoride-Co-Hexafluoropropylene)-Based Gel Polymer Electrolyte. J. Power Sources 2014, 252, 14–20. [Google Scholar] [CrossRef]
- Wang, H.; Di, D.; Zhao, Y.; Yuan, R.; Zhu, Y. A Multifunctional Polymer Composite Coating Assisted with Pore-Forming Agent: Preparation, Superhydrophobicity and Corrosion Resistance. Prog. Org. Coat. 2019, 132, 370–378. [Google Scholar] [CrossRef]
- Reichelt, S.; Prager, A.; Abe, C.; Knolle, W. Tailoring the Structural Properties of Macroporous Electron-Beam Polymerized Cryogels by Pore Forming Agents and the Monomer Selection. Radiat. Phys. Chem. 2014, 94, 40–44. [Google Scholar] [CrossRef]
- Kabiri, K.; Omidian, H.; Zohuriaan-Mehr, M. Novel Approach to Highly Porous Superabsorbent Hydrogels: Synergistic Effect of Porogens on Porosity and Swelling Rate. Polym. Int. 2003, 52, 1158–1164. [Google Scholar] [CrossRef]
- Zhai, N.; Wang, B. Preparation of Fast-Swelling Porous Superabsorbent Hydrogels with High Saline Water Absorbency under Pressure by Foaming and Post Surface Crosslinking. Sci. Rep. 2023, 13, 13815. [Google Scholar] [CrossRef]
- Zhao, Q.S.; Ji, Q.X.; Xing, K.; Li, X.Y.; Liu, C.S.; Chen, X.G. Preparation and Characteristics of Novel Porous Hydrogel Films Based on Chitosan and Glycerophosphate. Carbohyd. Polym. 2009, 76, 410–416. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Goncharova, S.S.; Lavlinskaya, M.S.; Holyavka, M.G.; Faizullin, D.A.; Kondratyev, M.S.; Kannykin, S.V.; Zuev, Y.F.; Artyukhov, V.G. Carboxymethyl Cellulose-Based Polymers as Promising Matrices for Ficin Immobilization. Polymers 2023, 15, 649. [Google Scholar] [CrossRef]
- Ashraf, I.M.; El-Zahhar, A.A. Studies on the Photoelectric Properties of Crosslinked-Poly(Acrylamide Co-Acrylic Acid) for Photo Detector Applications. Results Phys. 2018, 11, 842–846. [Google Scholar] [CrossRef]
- Lee, Y.C.; Liew, C.W.; Buraidah, M.H.; Woo, H.J. Fourier Transform Infrared Studies of Gel Polymer Electrolyte Based on Poly(Acrylamide-Co-Acrylic Acid)—Ethylene Carbonate Incorporated with Water-Soluble Sodium Sulfide. Opt. Mater. 2023, 140, 113791. [Google Scholar] [CrossRef]
- Chiem, L.T.; Huynh, L.; Ralston, J.; Beattie, D.A. An In Situ ATR–FTIR Study of Polyacrylamide Adsorption at the Talc Surface. J. Colloid Interface Sci. 2006, 297, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Olad, A.; Zebhi, H.; Salari, D.; Mirmohseni, A.; Reyhanitabar, A. A Promising Porous Polymer-Nanoclay Hydrogel Nanocomposite as Water Reservoir Material: Synthesis and Kinetic Study. J. Porous Mater. 2018, 25, 665–675. [Google Scholar] [CrossRef]
- Fang, S.; Wang, G.; Xing, R.; Chen, X.; Liu, S.; Qin, Y.; Li, K.; Wang, X.; Li, R.; Li, P. Synthesis of Superabsorbent Polymers Based on Chitosan Derivative Graft Acrylic Acid-Co-Acrylamide and Its Property Testing. Int. J. Biol. Macromol. 2019, 132, 575–584. [Google Scholar] [CrossRef]
- Nam, S.; French, A.D.; Condon, B.D.; Concha, M. Segal Crystallinity Index Revisited by the Simulation of X-Ray Diffraction Patterns of Cotton Cellulose Iβ and Cellulose II. Carbohyd. Polym. 2016, 135, 1–9. [Google Scholar] [CrossRef]
- Mahon, R.; Balogun, Y.; Oluyemi, G.; Njuguna, J. Swelling Performance of Sodium Polyacrylate and Poly(Acrylamide-Co-Acrylic Acid) Potassium Salt. SN Appl. Sci. 2020, 2, 117. [Google Scholar] [CrossRef]
- Ganji, F.; Farahani, S.V.; Vasheghani-Farahani, E. Theoretical Description of Hydrogel Swelling: A Review. Iran. Polym. J. 2010, 19, 375–398. [Google Scholar]
- Peppas, N.A.; Brannon-Peppas, L. Water Diffusion and Sorption in Amorphous Macromolecular Systems and Foods. In Water in Foods; Elsevier: Amsterdam, The Netherlands, 1994; pp. 189–210. ISBN 9781858610375. [Google Scholar]
- Kim, B.; La Flamme, K.; Peppas, N.A. Dynamic Swelling Behavior of pH-sensitive Anionic Hydrogels Used for Protein Delivery. J. Appl. Polym. Sci. 2003, 89, 1606–1613. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A Simple Equation for Description of Solute Release II. Fickian and Anomalous Release from Swellable Devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Cao, Q.; Chen, J.; Wang, M.; Wang, Z.; Wang, W.; Shen, Y.; Xue, Y.; Li, B.; Ma, Y.; Yao, Y.; et al. Superabsorbent Carboxymethyl Cellulose–Based Hydrogel Fabricated by Liquid-Metal-Induced Double Crosslinking Polymerisation. Carbohyd. Polym. 2024, 331, 121910. [Google Scholar] [CrossRef] [PubMed]
- Fekete, T.; Borsa, J.; Takács, E.; Wojnárovits, L. Synthesis of Carboxymethylcellulose/Starch Superabsorbent Hydrogels by Gamma-Irradiation. Chem. Cent. J. 2017, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.M.; Tamer, T.M.; Hassan, M.E.; Khalifa, R.E.; Abd El-Monaem, E.M.; Eltaweil, A.S.; Mohy Eldin, M.S. Fabrication of Grafted Carboxymethyl Cellulose Superabsorbent Hydrogel for Water Retention and Sustained Release of Ethephon in Sandy Soil. Arab. J. Sci. Eng. 2023, 48, 561–572. [Google Scholar] [CrossRef]
- Capanema, N.S.V.; Mansur, A.A.P.; De Jesus, A.C.; Carvalho, S.M.; De Oliveira, L.C.; Mansur, H.S. Superabsorbent Crosslinked Carboxymethyl Cellulose-PEG Hydrogels for Potential Wound Dressing Applications. Int. J. Biol. Macromol. 2018, 106, 1218–1234. [Google Scholar] [CrossRef]
- Radmehr, M.; Poursattar Marjani, A.; Akhavan, A.; Nasiri, F. Synthesis and Characterization of Carboxymethyl Cellulose/Acrylic Acid Superabsorbent Hydrogel by Gamma Irradiation. Radiat. Phys. Eng. 2024, 5, 47–51. [Google Scholar] [CrossRef]
- Klinpituksa, P.; Kosaiyakanon, P. Superabsorbent Polymer Based on Sodium Carboxymethyl Cellulose Grafted Polyacrylic Acid by Inverse Suspension Polymerization. Int. J. Polym. Sci. 2017, 2017, 3476921. [Google Scholar] [CrossRef]
- Dai, H.; Huang, H. Enhanced Swelling and Responsive Properties of Pineapple Peel Carboxymethyl Cellulose-g-Poly(Acrylic Acid-Co-Acrylamide) Superabsorbent Hydrogel by the Introduction of Carclazyte. J. Agric. Food Chem. 2017, 65, 565–574. [Google Scholar] [CrossRef]
- Nie, L.; Chen, D.; Fu, J.; Yang, S.; Hou, R.; Suo, J. Macroporous Biphasic Calcium Phosphate Scaffolds Reinforced by Poly-L-Lactic Acid/Hydroxyapatite Nanocomposite Coatings for Bone Regeneration. Biochem. Eng. J. 2015, 98, 29–37. [Google Scholar] [CrossRef]
- Lagergreen, S. Zur Theorie der sogenannten Adsorption gelöster Stoffe. Z. Chem. Ind. Kolloide 1907, 2, 15. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Peppas, N.A.; Khare, A.R. Preparation, Structure and Diffusional Behavior of Hydrogels in Controlled Release. Adv. Drug Deliv. Rev. 1993, 11, 1–35. [Google Scholar] [CrossRef]

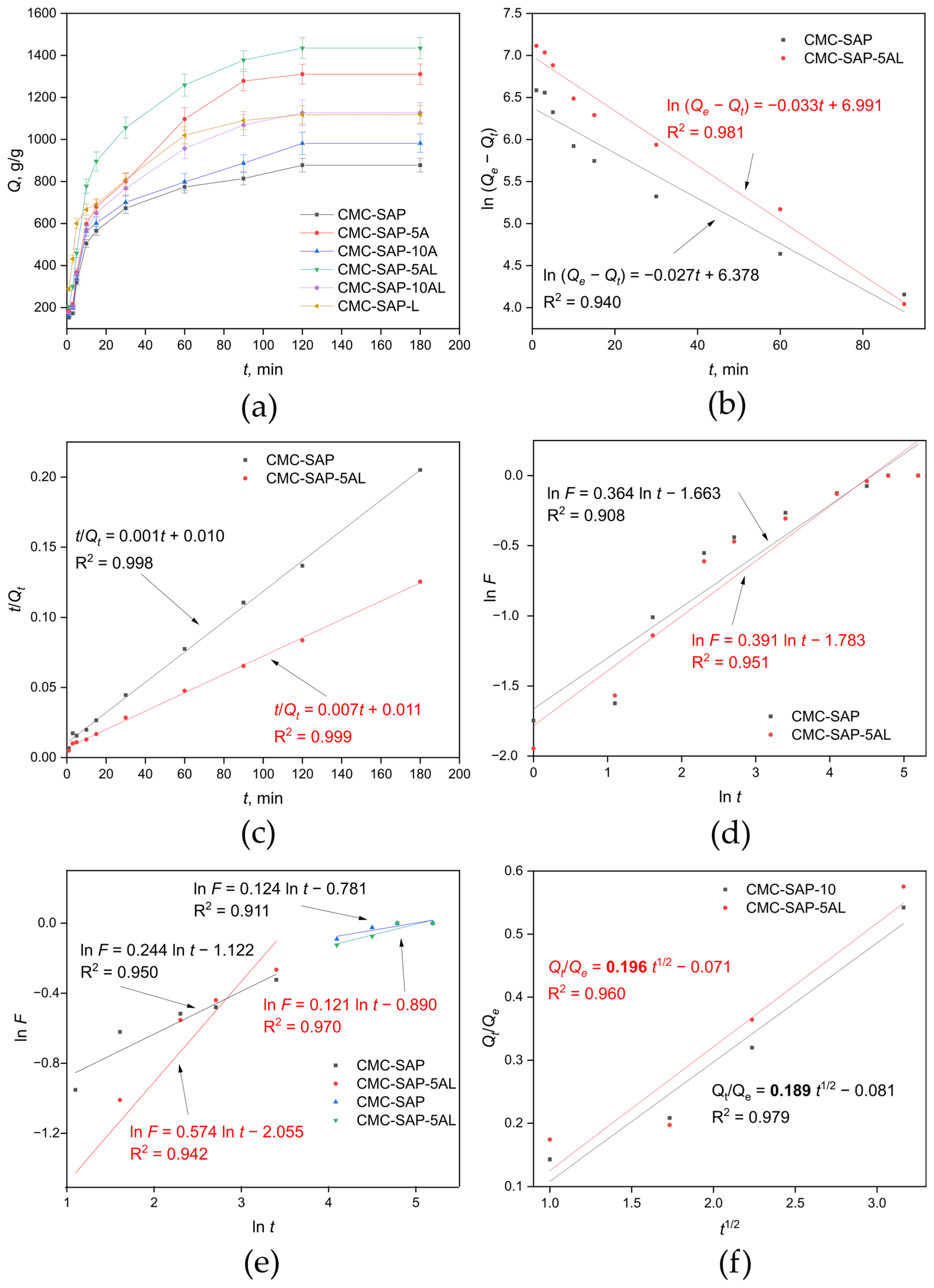
| SAP | Qe, g/g | Porosity, % | Tg, °C | |
|---|---|---|---|---|
| Distilled Water | 0.15 M NaCl | |||
| CMC-SAP | 878 ± 32 | 76 ± 2 | 9 ± 1 | 203.2 |
| CMC-SAP-5A | 1311 ± 48 | 83 ± 2 | 71 ± 2 | 203.2 |
| CMC-SAP-10A | 982 ± 49 | 79 ± 2 | 82 ± 1 | 218.3 |
| CMC-SAP-L | 1118 ± 43 | 85 ± 3 | 86 ± 3 | 215.3 |
| CMC-SAP-5AL | 1435 ± 53 | 95 ± 3 | 95 ± 2 | 205.8 |
| CMC-SAP-10AL | 1127 ± 49 | 90 ± 3 | 93 ± 4 | 213.3 |
| CMC-SAP | CMC-SAP-5AL | ||||||
|---|---|---|---|---|---|---|---|
| k1 | k2 | n | D | k1 | k2 | n | D |
| 0.027 | 0.001 | 0.364 | 0.189 | 0.033 | 0.007 | 0.391 | 0.196 |
| 0.244 1 | 0.574 1 | ||||||
| 0.124 2 | 0.121 2 | ||||||
| Composition | Initiation | Qe, g/g, Distilled Water | Application Field | Ref. |
|---|---|---|---|---|
| CMC, acrylic acid (AA), N,N′-methylene-bis-acrylamide (MBAAm), LM EGaIn (75 wt% Ga and 25 wt% In) | Ammonium persulfate | 1108 | Hygienic products | [42] |
| CMC, potato starch | γ-irradiation | 350 | - | [43] |
| CMC, 2-acrylamido-2-methylpropanesulfonic acid, MBAAm | Potassium persulfate | 178.7 | Agriculture | [44] |
| CMC, polyethylene glycol, citric acid | No | 500 | Wound dressing | [45] |
| CMC, AA | γ-irradiation | 437 | Hygienic products | [46] |
| CMC, AA, MBAAm | Potassium persulfate | 545 | - | [47] |
| CMC, AA, acrylamide (AAm), MBAAm, carclazyte | Ammonium persulfate | 515 | Slow-release fertilizer/water management/drug delivery | [48] |
| CMC, AA, AAm, MBAAm | Potassium persulfate | 1061 | - | [17] |
| CMC, AA, AAm, MBAAm, ammonium carbonate | Potassium persulfate | 1435 | Further development as a matrix for slow-release fertilizers in agricultural applications | this study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavlinskaya, M.S.; Sorokin, A.V. Enhancement of Water Uptake in Composite Superabsorbents Based on Carboxymethyl Cellulose Through Porogen Incorporation and Lyophilization. Gels 2024, 10, 797. https://doi.org/10.3390/gels10120797
Lavlinskaya MS, Sorokin AV. Enhancement of Water Uptake in Composite Superabsorbents Based on Carboxymethyl Cellulose Through Porogen Incorporation and Lyophilization. Gels. 2024; 10(12):797. https://doi.org/10.3390/gels10120797
Chicago/Turabian StyleLavlinskaya, Maria S., and Andrey V. Sorokin. 2024. "Enhancement of Water Uptake in Composite Superabsorbents Based on Carboxymethyl Cellulose Through Porogen Incorporation and Lyophilization" Gels 10, no. 12: 797. https://doi.org/10.3390/gels10120797
APA StyleLavlinskaya, M. S., & Sorokin, A. V. (2024). Enhancement of Water Uptake in Composite Superabsorbents Based on Carboxymethyl Cellulose Through Porogen Incorporation and Lyophilization. Gels, 10(12), 797. https://doi.org/10.3390/gels10120797







