Experimental Evaluation of a Recrosslinkable CO2-Resistant Micro-Sized Preformed Particle Gel for CO2 Sweep Efficiency Improvement in Reservoirs with Super-K Channels
Abstract
1. Introduction
2. Results and Discussion
3. Conclusions
- The micro-sized CO2-BRPPG demonstrates improved stability and effectiveness in high-salinity and varying-pH environments. At ambient conditions, the CO2-BRPPG can swell up to 76 times its original volume. This swelling ratio decreases with increasing salinity and remains relatively stable at low pH levels, demonstrating the gel’s excellent resistance to the acidic conditions resulting from CO2 flooding;
- The micro-sized CO2-BRPPG exhibits shear-thinning behavior, with viscosity decreasing as the shear rate increases. This characteristic ensures that the gel can be easily injected under high-shear conditions near the wellbore but retains sufficient viscosity under low-shear conditions farther from wellbore to effectively block high-permeability channels;
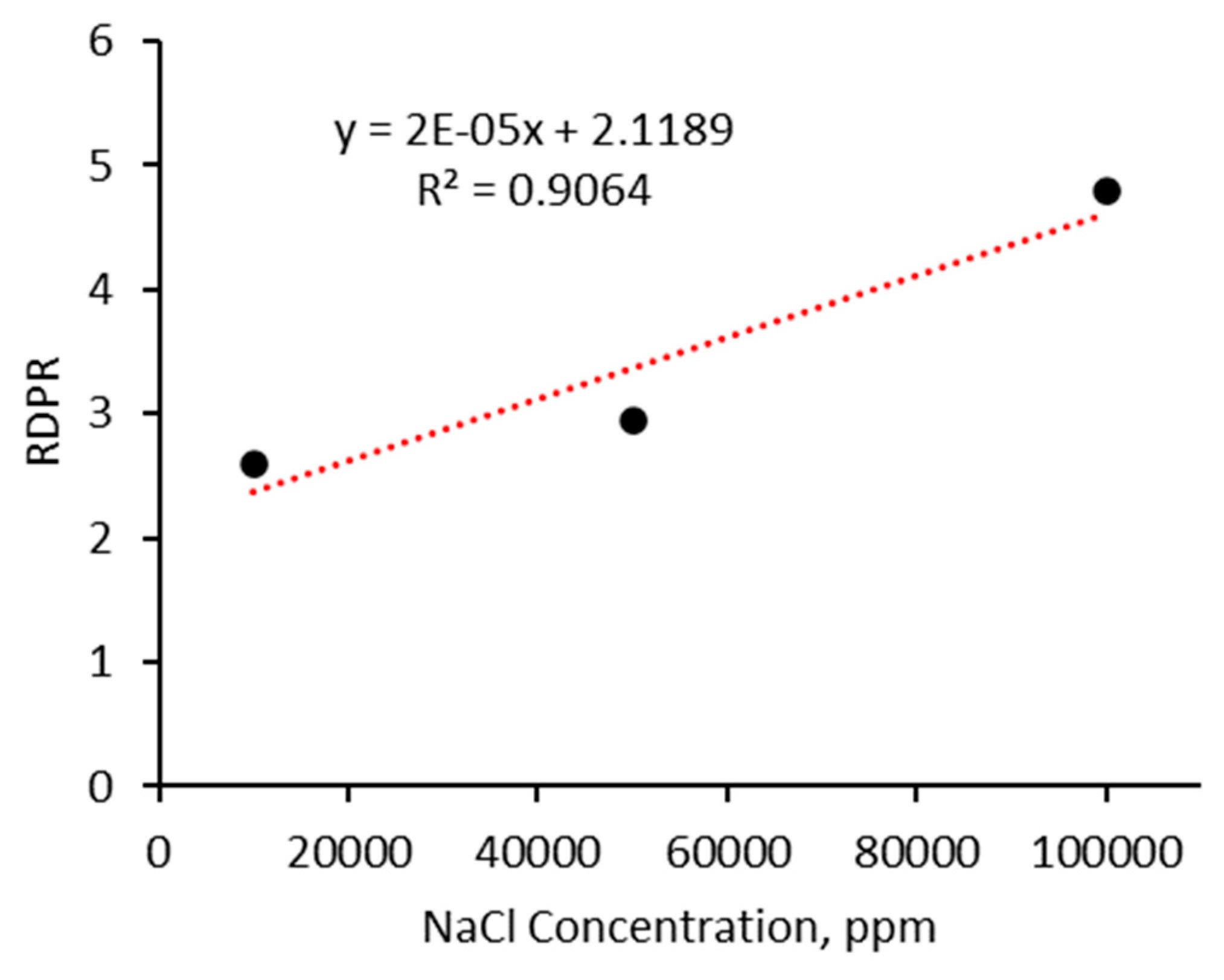
- The strength of the micro-sized CO2-BRPPG increases with higher salinity levels, with the storage modulus rising from 113 Pa at 1% NaCl to 145 Pa at 10% NaCl. This demonstrates that a reservoir with higher salinity improves the mechanical stability and rigidity of the gel, which enhances its effectiveness in blocking CO2 flow;
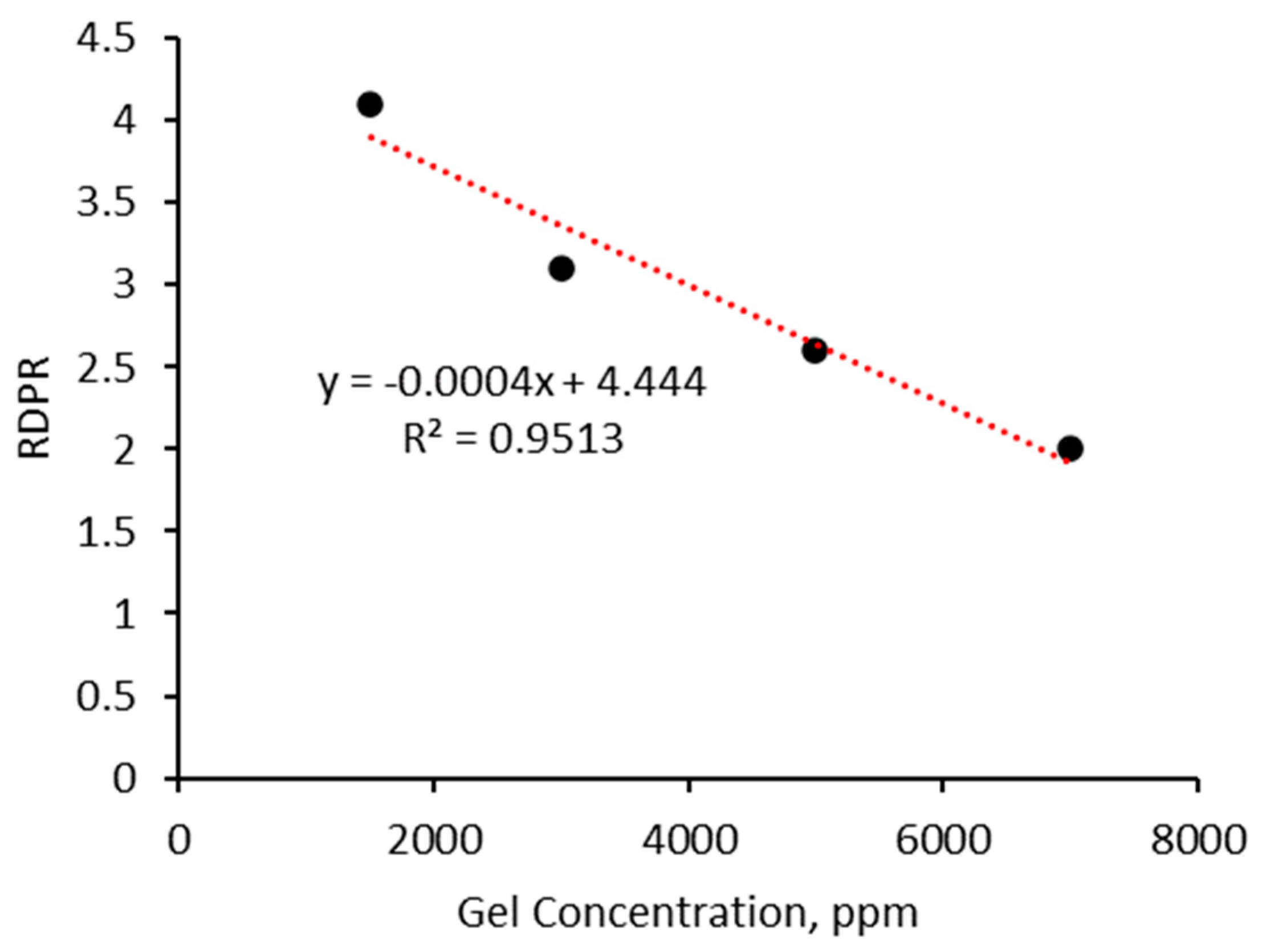
- The injectivity of the micro-sized CO2-BRPPG can be controlled by adjusting the gel concentration and particle size. Low concentrations result in low injection pressure, which is suitable for deep injection treatments. For approximately equal-permeability media, highly swollen particles showed lower injection pressures than less swollen ones, likely due to their lower strength and better deformability;
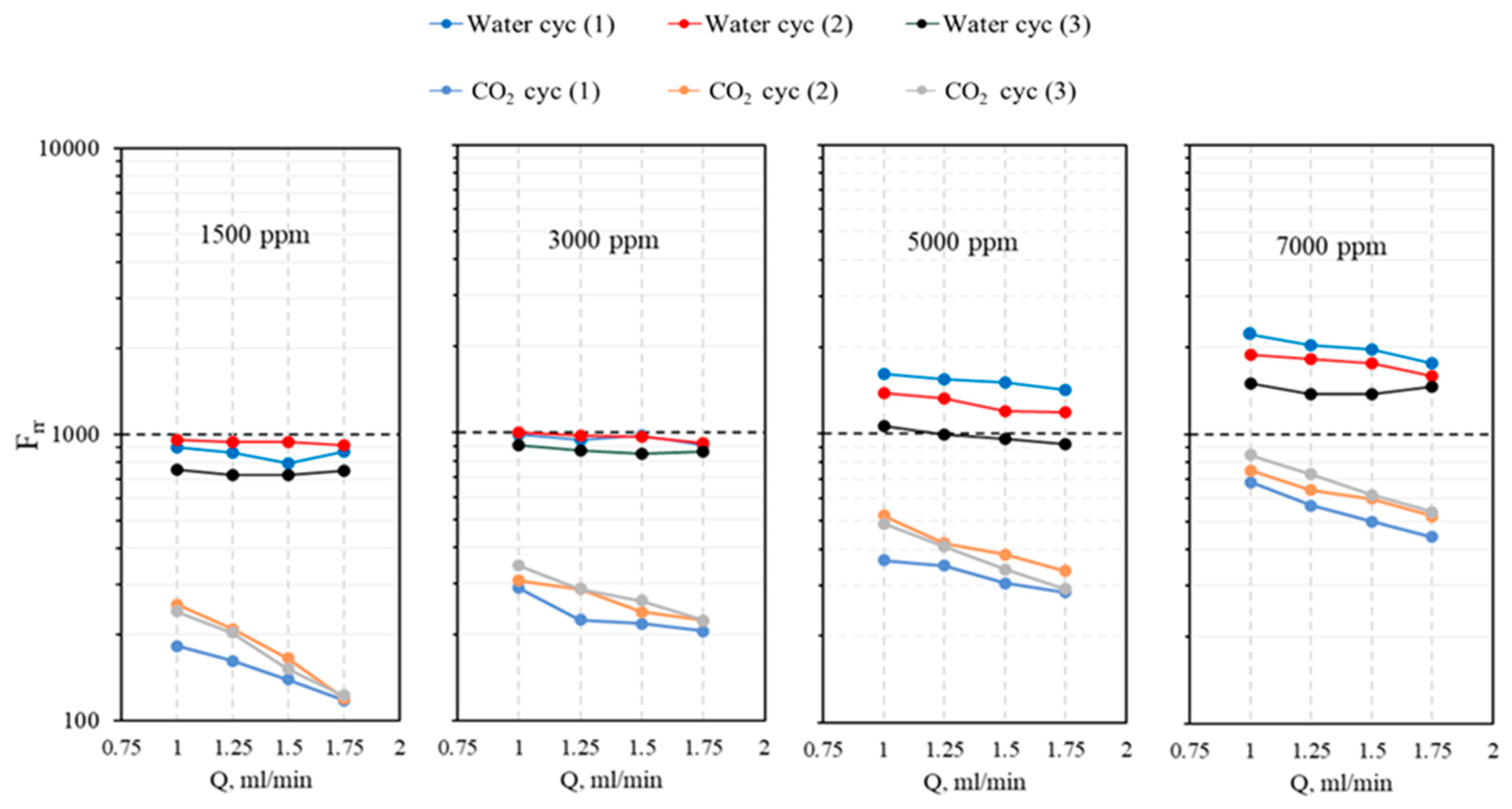
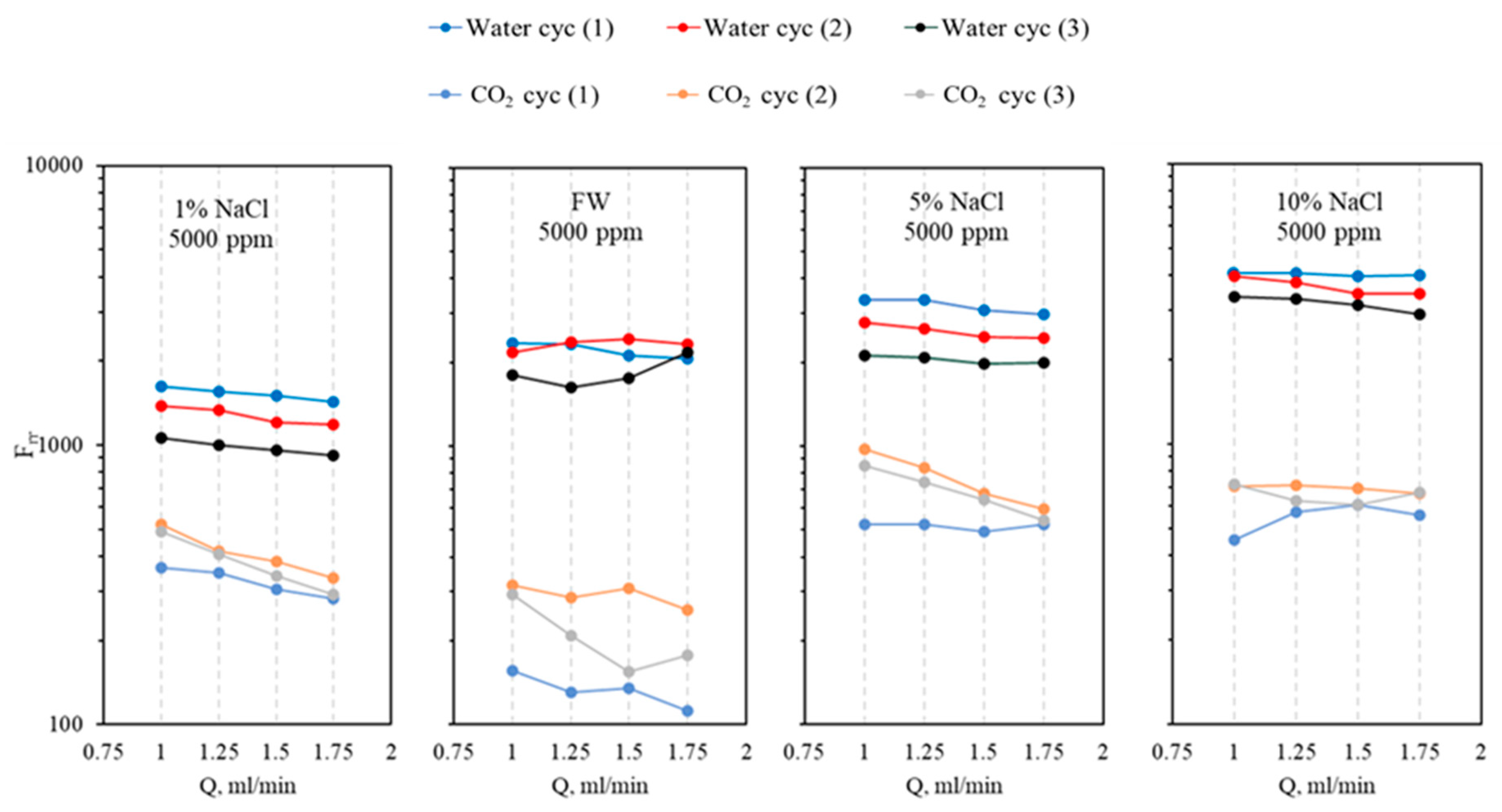
- The micro-sized CO2-BRPPG exhibits selective plugging performance, reducing water permeability more effectively than CO2. The ratio of Frrw to Frrg increases with higher brine concentrations and decreases with higher gel concentrations, indicating that gel strength significantly impacts this parameter.
4. Materials and Methods
4.1. Materials
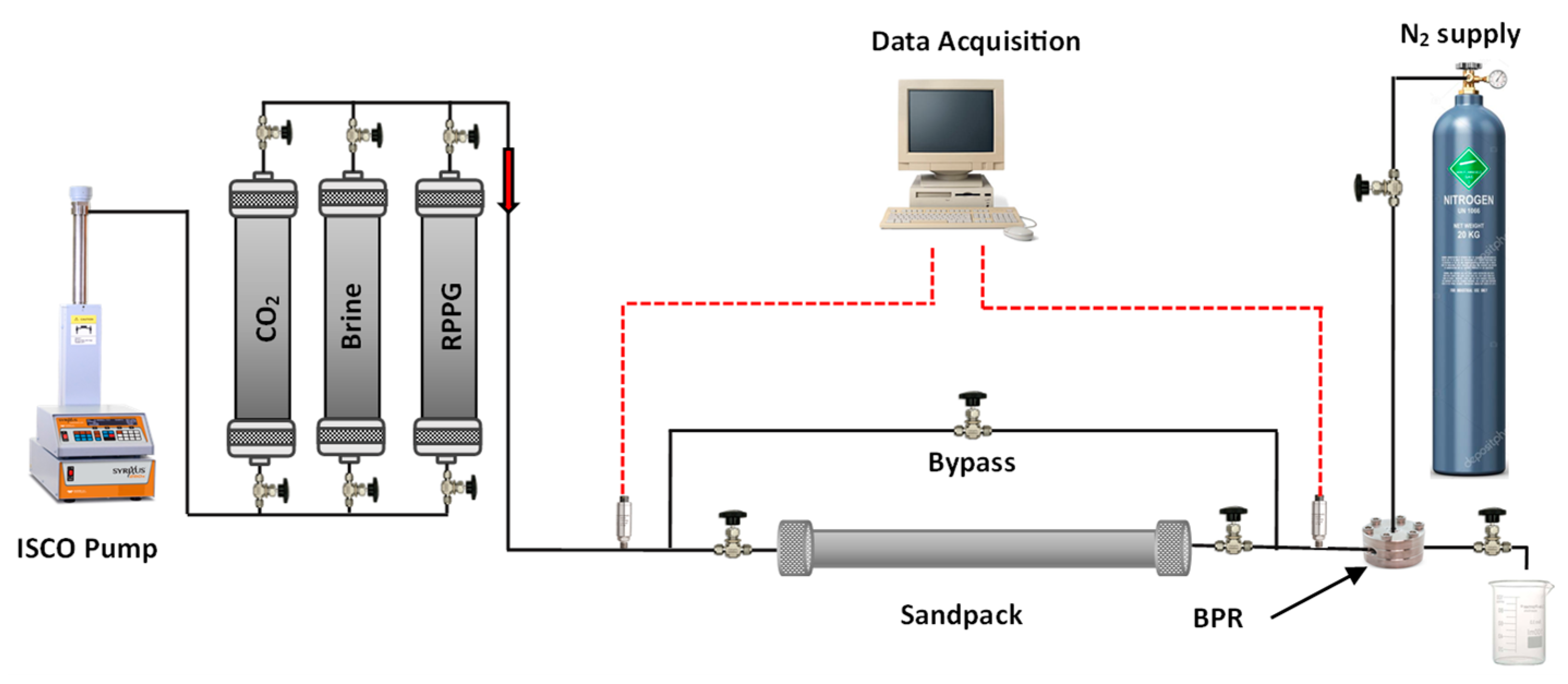
4.2. Methods
- The system’s initial permeabilities were evaluated by injecting brine and CO2 at different flow rates and calculating the permeability using the Darcy equation;
- A suspension of the CO2-BRPPG (170/230 mesh) was prepared by immersing dry gel particles in brine until the equilibrium swelling ratio was reached. The swollen particles were then placed in a high-pressure magnetic stirrer accumulator to ensure a homogeneous suspension during the injection. The gel suspension was injected at a flow rate of 2 mL/min until gel breakthrough occurred and a stable injection pressure was achieved. The gel injectivity was evaluated by calculating the resistance factor (Fr) during the injection process using Equation (2), where is the water mobility ratio, is the gel mobility ratio, is the differential pressure of the injected gel in psi, and is the water differential pressure in psi;
- The system was shut in for three days, allowing the gel to recrosslink. The tubes and fittings connecting the sandpack with accumulators and the backpressure regulator were cleaned with water and air;
- The CO2–water flooding process was carried out by injecting CO2 first to evaluate the gas breakthrough pressure in pressure stepwise mode. Three water-alternating-CO2 cycles were injected for the entire process. For both brine and CO2, the injection flow rates were 1, 1.25, 1.5, and 1.75 mL/min. The flow rate was changed once a stable injection pressure was achieved. The residual resistance factor (Frr) for both water and CO2 for each cycle was calculated using Equation (3), and the proportional permeability reduction was evaluated using Equation (4), where is the initial permeability of the system in mD, is the permeability after gel treatment in mD, and is the disproportional permeability reduction ratio.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, L.B.; Li, X.; Wei, N. CO2-EOR in China: A comparative review. Int. J. Greenh. Gas Control. 2020, 103, 103173. [Google Scholar] [CrossRef]
- Ran, X.; Zhao, Y.; Liao, X. An assessment of a CO2 flood for EOR and sequestration benefits in the Ordos Basin, Northwest China. In Proceedings of the Carbon Management Technology Conference, Orlando, FL, USA, 7–9 February 2012; p. CMTC-150272. [Google Scholar] [CrossRef]
- Sun, X.; Long, Y.; Bai, B.; Wei, M.; Suresh, S. Evaluation and plugging performance of carbon dioxide-resistant particle gels for conformance control. SPE J. 2020, 25, 1745–1760. [Google Scholar] [CrossRef]
- Li, F.; Luo, Y.; Luo, X.; Wang, L.; Nagre, R. Experimental study on a new plugging agent during CO2 flooding for heterogeneous oil reservoirs: A case study of Block G89-1 of Shengli oilfield. J. Pet. Sci. Eng. 2016, 146, 103–110. [Google Scholar] [CrossRef]
- Martin, F.D.; Kovarik, F.S.; Chang, P.-W.; Goldman, I.M.; Philips, J.C. Gels for CO2 profile modification. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 16–21 April 1988; p. SPE-17330. [Google Scholar] [CrossRef]
- Smith, D.D.; Giraud, M.J.; Kemp, C.C.; McBee, M.S.; Taitano, J.A.; Winfield, M.; Portwood, J.T.; Everett, D.M. The successful evolution of Anton Irish conformance efforts. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 24–27 September 2006; p. SPE-103044. [Google Scholar] [CrossRef]
- Durucan, S.; Korre, A.; Shi, J.-Q.; Govindan, R.; Mosleh, M.H.; Syed, A. The use of polymer-gel solutions for CO2 flow diversion and mobility control within storage sites. Energy Procedia 2016, 86, 450–459. [Google Scholar] [CrossRef]
- Christensen, J.R.; Stenby, E.H.; Skauge, A. Review of WAG field experience. SPE Reserv. Eval. Eng. 2001, 4, 97–106. [Google Scholar] [CrossRef]
- Zhou, D.; Yan, M.; Calvin, W.M. Optimization of a mature CO2 flood—From continuous injection to WAG. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 14–18 April 2012; p. SPE-154181. [Google Scholar] [CrossRef]
- Al-Ali, A.H.; Schechter, D.S.; Lane, R.H. Application of polymer gels as conformance control agents for carbon dioxide EOR WAG floods. In Proceedings of the SPE International Conference on Oilfield Chemistry, The Woodlands, TX, USA, 8–10 April 2013; p. SPE-164096. [Google Scholar] [CrossRef]
- Talebian, S.H.; Masoudi, R.; Tan, I.M.; Zitha, P.L.J. Foam assisted CO2-EOR: A review of concept, challenges, and future prospects. J. Pet. Sci. Eng. 2014, 120, 202–215. [Google Scholar] [CrossRef]
- Yang, H.; Lv, Z.; Wang, L.; Feng, C.; Wang, J.; Xu, Z.; Huang, Y.; Li, Z.; Kang, W. Stability mechanism of controlled acid-resistant hydrophobic polymer nanospheres on CO2 foam. Fuel 2023, 346, 128332. [Google Scholar] [CrossRef]
- Heller, J.P.; Dandge, D.K.; Card, R.J.; Donaruma, L.G. Direct thickeners for mobility control of CO2 floods. Soc. Pet. Eng. J. 1985, 25, 679–686. [Google Scholar] [CrossRef]
- Enick, R.M.; Beckman, E.J.; Shi, C.; Huang, Z.; Xu, J.; Kilic, S. Direct thickeners for carbon dioxide. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, KO, USA, 3–5 April 2000; p. SPE-59325. [Google Scholar] [CrossRef]
- Al Brahim, A.; Eriyagama, Y.; Bai, B.; Schuman, T. Feasibility Study of Recrosslinkable Preformed Particle Gels for Natural Gas Injection Profile Control. SPE J. 2023, 28, 1829–1841. [Google Scholar] [CrossRef]
- Seright, R.S. Reduction of gas and water permeabilities using gels. SPE Prod. Facil. 1995, 10, 103–108. [Google Scholar] [CrossRef]
- Qiu, Y.; Wu, F.; Wei, M.; Kang, W.; Li, B. Lessons learned from applying particle gels in mature oilfields. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 12–16 April 2014; p. SPE-169161. [Google Scholar]
- Esfahlan, M.S.; Khodapanah, E.; Tabatabaei-Nezhad, S.A. Comprehensive review on the research and field application of preformed particle gel conformance control technology. J. Pet. Sci. Eng. 2021, 202, 108440. [Google Scholar] [CrossRef]
- Martin, F.D.; Kovarik, F.S. Chemical gels for diverting CO2: baseline experiments. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 27–30 September 1987; p. SPE-16728. [Google Scholar] [CrossRef]
- Raje, M.; Asghari, K.; Vossoughi, S.; Green, D.W.; Willhite, G.P. Gel systems for controlling CO2 mobility in carbon dioxide miscible flooding. SPE Reserv. Eval. Eng. 1999, 2, 205–210. [Google Scholar] [CrossRef]
- Hou, Y.; Yue, X. Research on a novel composite gel system for CO2 breakthrough. Pet. Sci. 2010, 7, 245–250. [Google Scholar] [CrossRef][Green Version]
- Sun, X.; Bai, B.; Alhuraishawy, A.K.; Zhu, D. Understanding the plugging performance of HPAM-Cr (III) polymer gel for CO2 conformance control. SPE J. 2021, 26, 3109–3118. [Google Scholar] [CrossRef]
- Bai, B.; Wang, Q.; Du, Y.; Liu, Y. Factors affecting in-depth gel treatment for reservoirs with thick heterogeneous oil layers. In Proceedings of the PETSOC Canadian International Petroleum Conference, Calgary, AB, Canada, 8–10 June 2004; p. PETSOC-2004. [Google Scholar] [CrossRef]
- Chauveteau, G.; Omari, A.; Tabary, R.; Renard, M.; Veerapen, J.; Rose, J. New size-controlled microgels for oil production. In Proceedings of the SPE International Conference on Oilfield Chemistry, Houston, TX, USA, 13–16 February 2001; p. SPE-64988. [Google Scholar]
- Zhao, Y.; Wen, X.; Rui, Z.; Ding, Y. A novel dual-network CO2-responsive particle gel for mitigating CO2 channeling and leakage in hydrocarbon recovery and carbon storage. Chem. Eng. J. 2024, 498, 155187. [Google Scholar] [CrossRef]
- Gary, T.; Gallo, C.; Smith, D.; Huang, C.-K.; Autry, S.; Peirce, J.; Li, B. Case history of conformance solutions for west sak wormhole/void space conduit with a new reassembling preformed particle gel RPPG. In Proceedings of the SPE Annual Technical Conference and Exhibition, Virtual, 26–29 October 2020; p. D031S029R007. [Google Scholar]
- Nakajima, T.; Hoshino, K.-I.; Guo, H.; Kurokawa, T.; Gong, J.P. Experimental verification of the balance between elastic pressure and ionic osmotic pressure of highly swollen charged gels. Gels 2021, 7, 39. [Google Scholar] [CrossRef]
- Zhu, Y.; Bai, Y.; Sun, J.; Lv, K. Experimental Study of the Swelling and Rheological Properties of a High-Strength Preformed Particle Gel Lost Circulation Materia. SPE J. 2024, 29, 3924–3941. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; He, H.; Yuan, F.; Liu, H.; Chen, Y. Insights into the effects of salinity on the transport behavior of polymer-enhanced branched-preformed particle gel suspension in porous media. Energy Fuels 2021, 35, 1104–1112. [Google Scholar] [CrossRef]
- Vuuren, R.D.J.-V.; Naficy, S.; Ramezani, M.; Cunningham, M.; Jessop, P. CO2-responsive gels. Chem. Soc. Rev. 2023, 52, 3470–3542. [Google Scholar] [CrossRef]
- Yang, H.; Iqbal, M.W.; Lashari, Z.A.; Cao, C.; Tang, X.; Kang, W. Experimental research on amphiphilic polymer/organic chromium gel for high salinity reservoirs. Colloids Surf. A Physi-Cochemical Eng. Asp. 2019, 582, 123900. [Google Scholar] [CrossRef]
- Mousavi Moghadam, A.; Vafaie Sefti, M.; Baghban Salehi, M.; Dadvand Koohi, A. Preformed particle gel: evaluation and optimization of salinity and pH on equilibrium swelling ratio. J. Pet. Explor. Prod. Technol. 2012, 2, 85–91. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef] [PubMed]
- Asghari, K.; Mirjafari, P.; Mahinpey, N.; Dong, M. Reducing the Permeability of Sandstone Porous Media to Water and CO2: Application of Bovine Carbonic Anhydrase Enzyme. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 22–26 April 2006; p. SPE-99787. [Google Scholar] [CrossRef]
- Song, T.; Zhai, Z.; Liu, J.; Eriyagama, Y.; Ahdaya, M.; Alotibi, A.; Wang, Z.; Schuman, T.; Bai, B. Laboratory evaluation of a novel Self-healable polymer gel for CO2 leakage remediation during CO2 storage and CO2 flooding. Chem. Eng. J. 2022, 444. [Google Scholar] [CrossRef]
- Wang, H.; Lin, M.; Chen, D.; Dong, Z.; Yang, Z.; Zhang, J. Research on the rheological properties of cross-linked polymer microspheres with different microstructures. Powder Technol. 2018, 331, 310–321. [Google Scholar] [CrossRef]

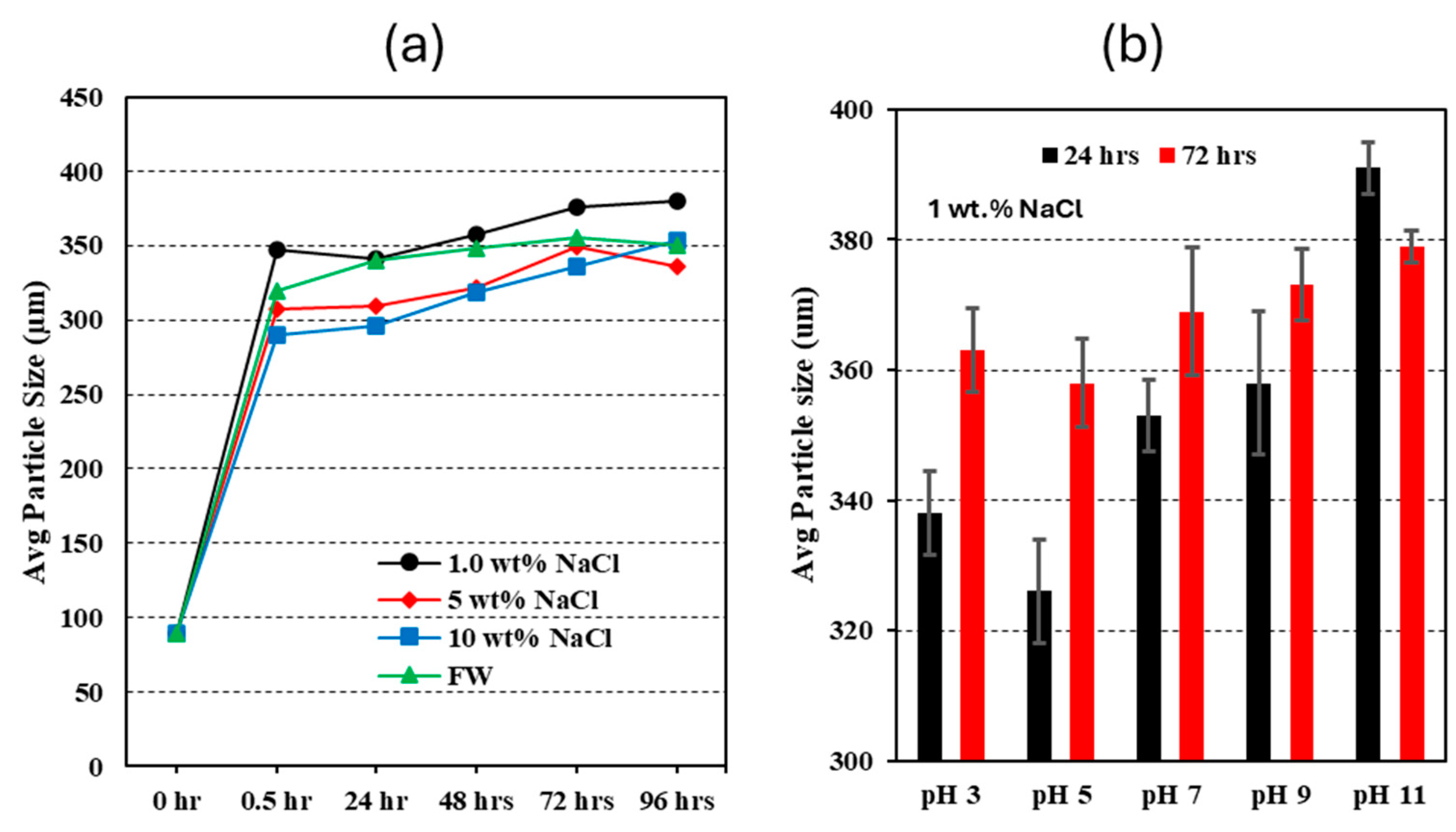
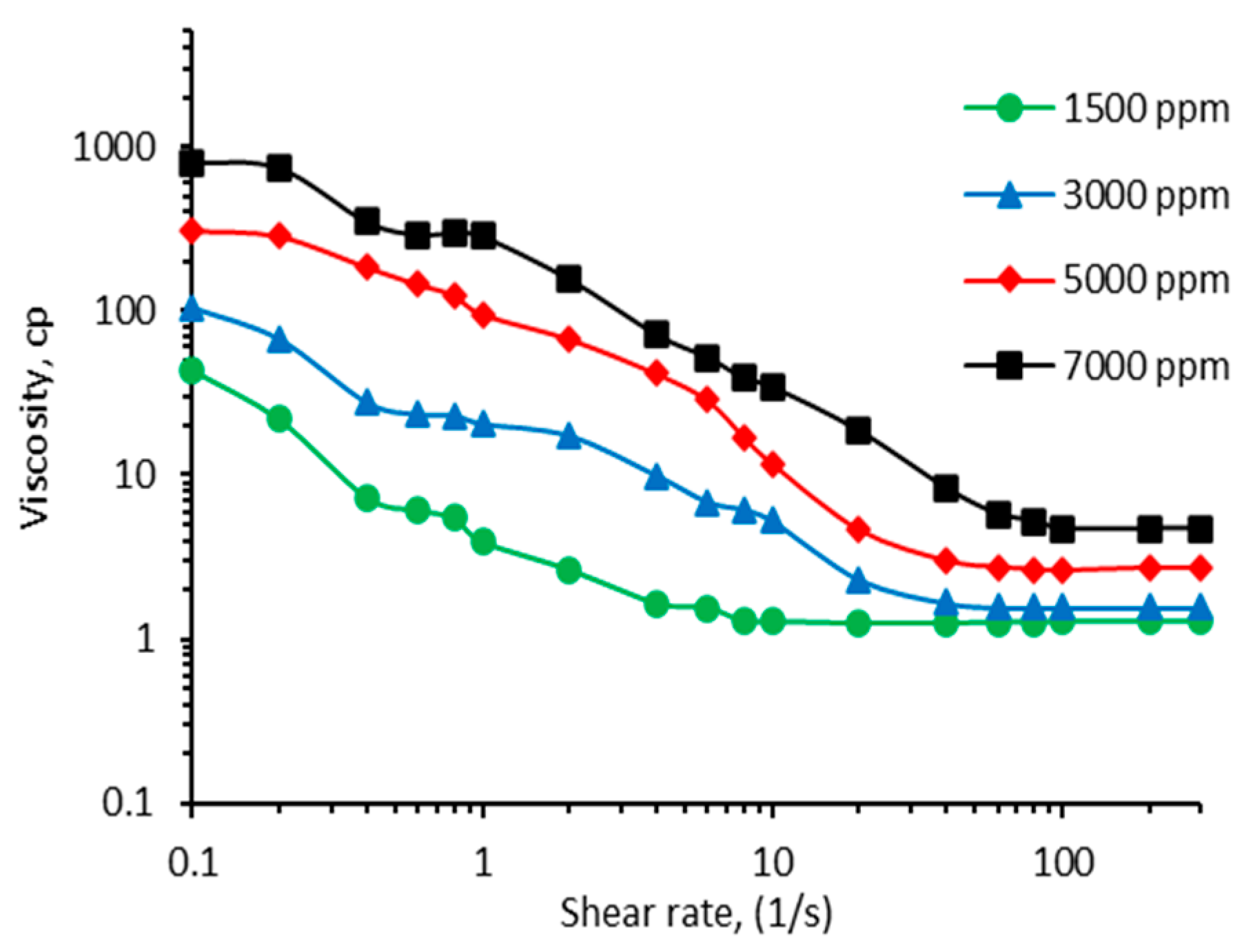
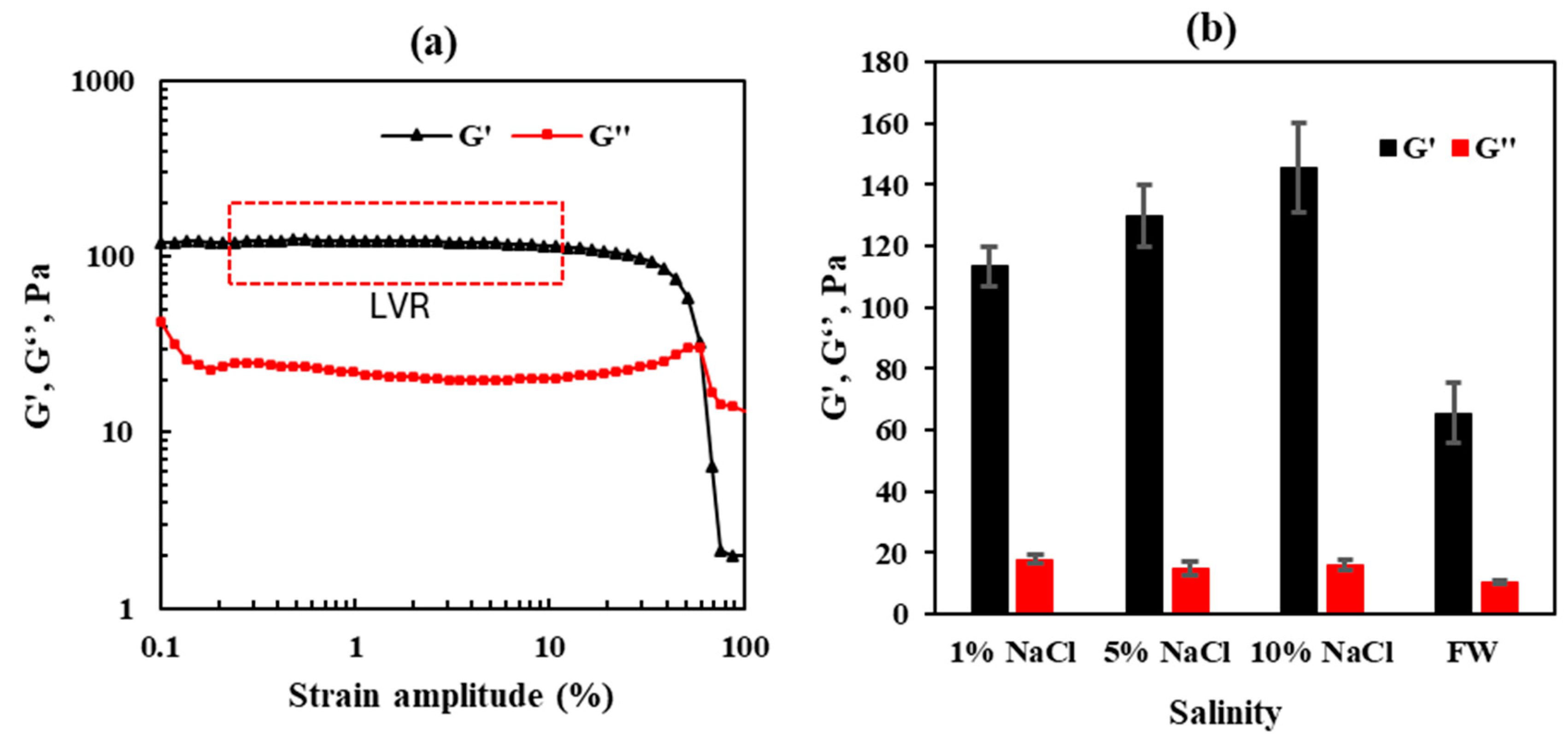
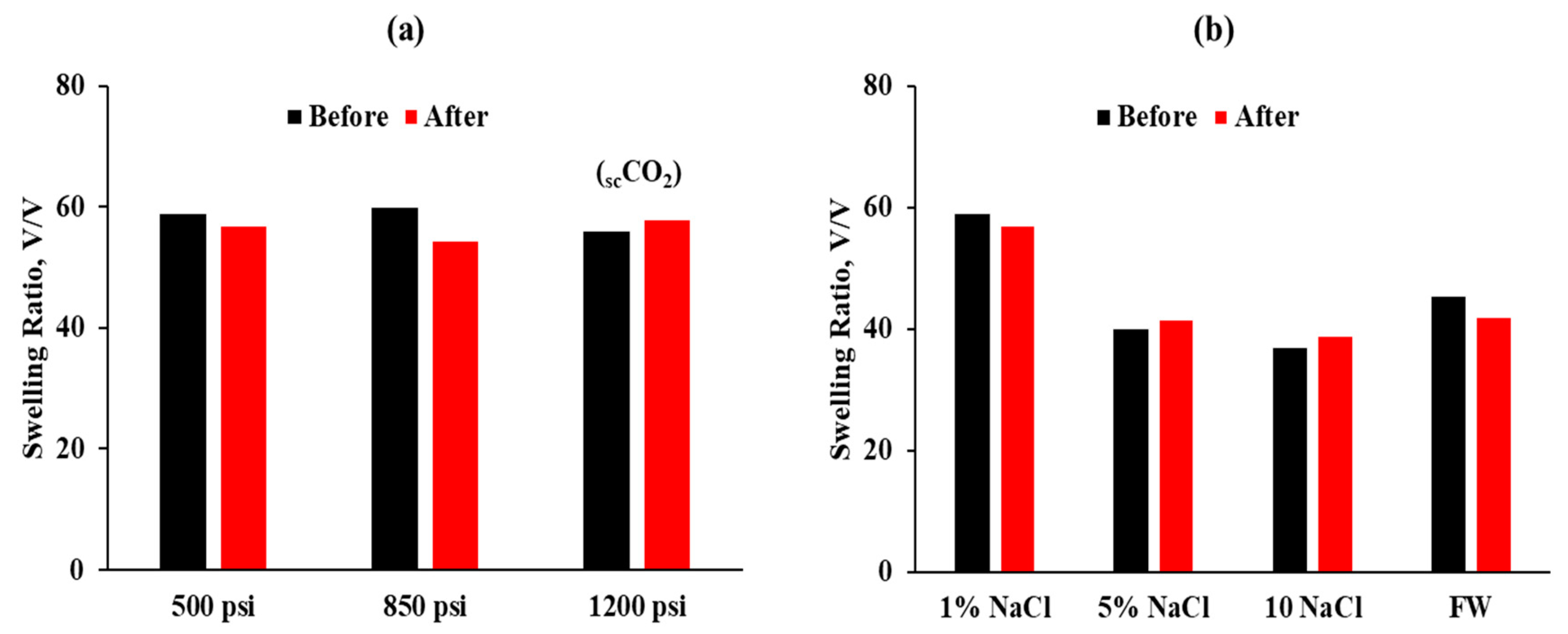
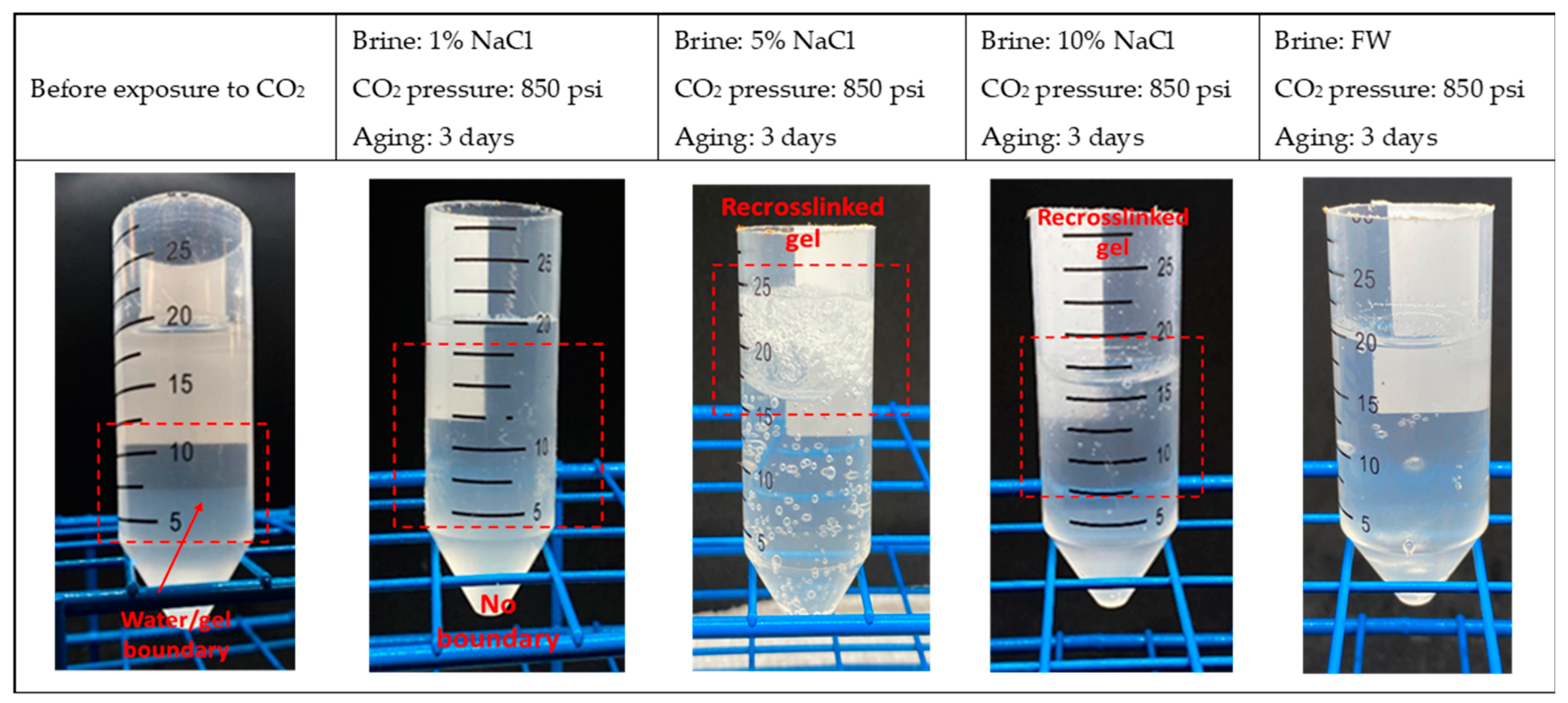


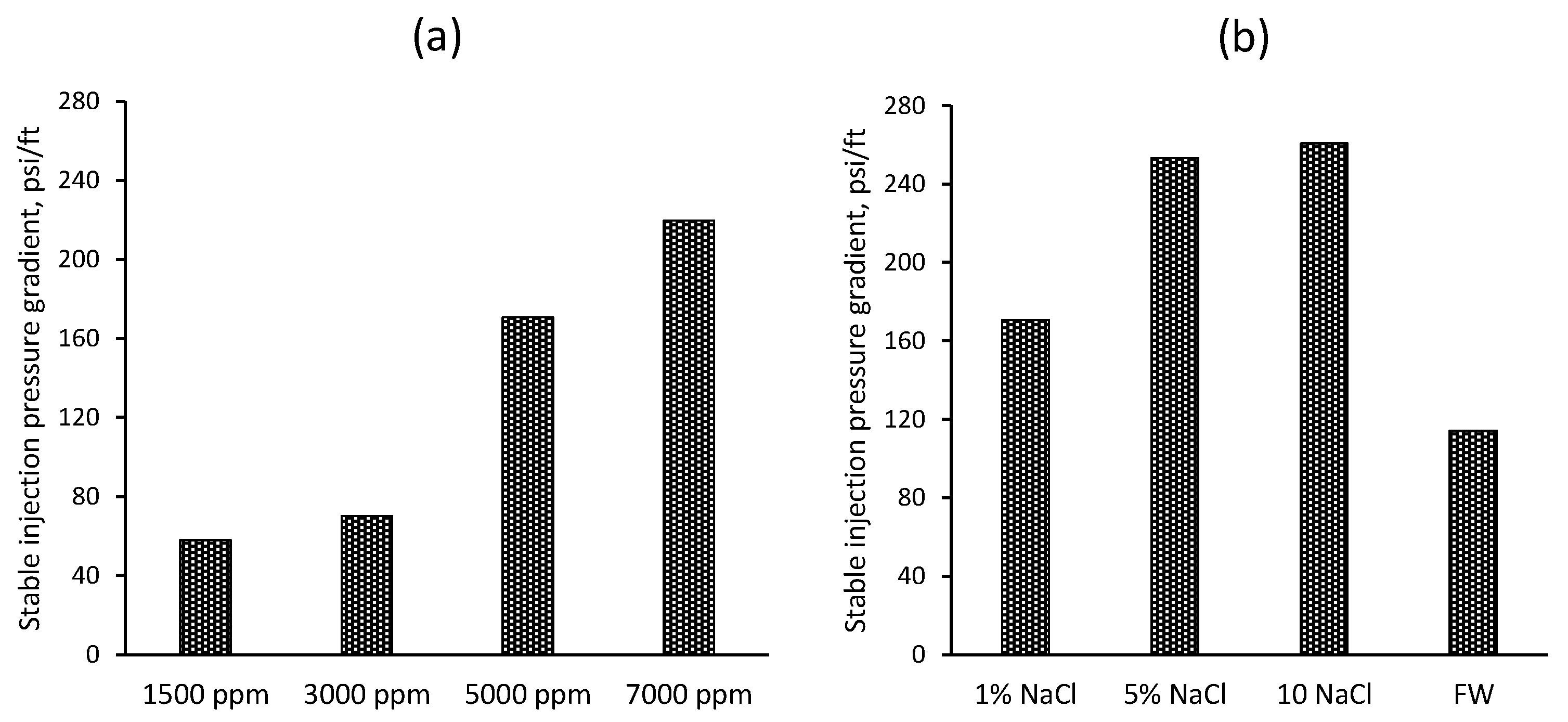
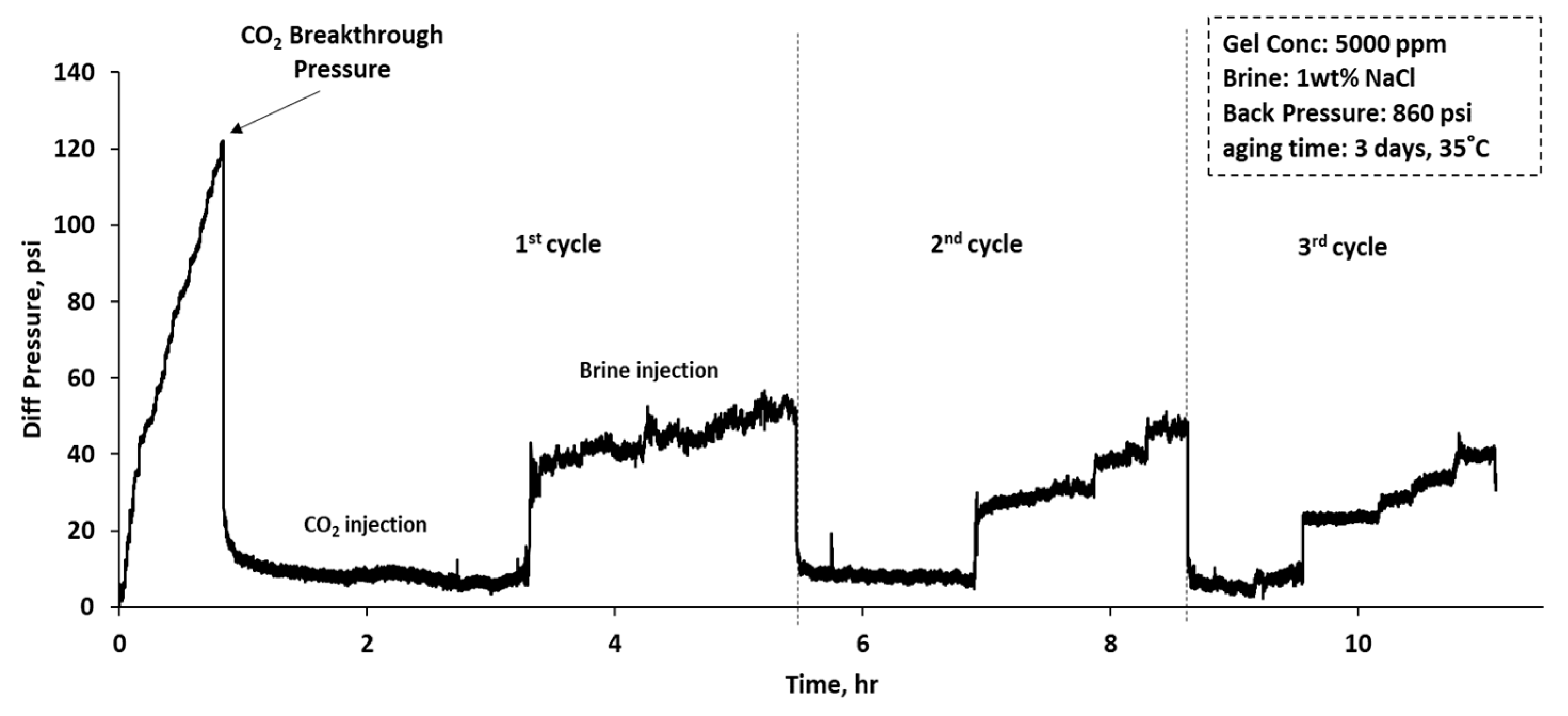
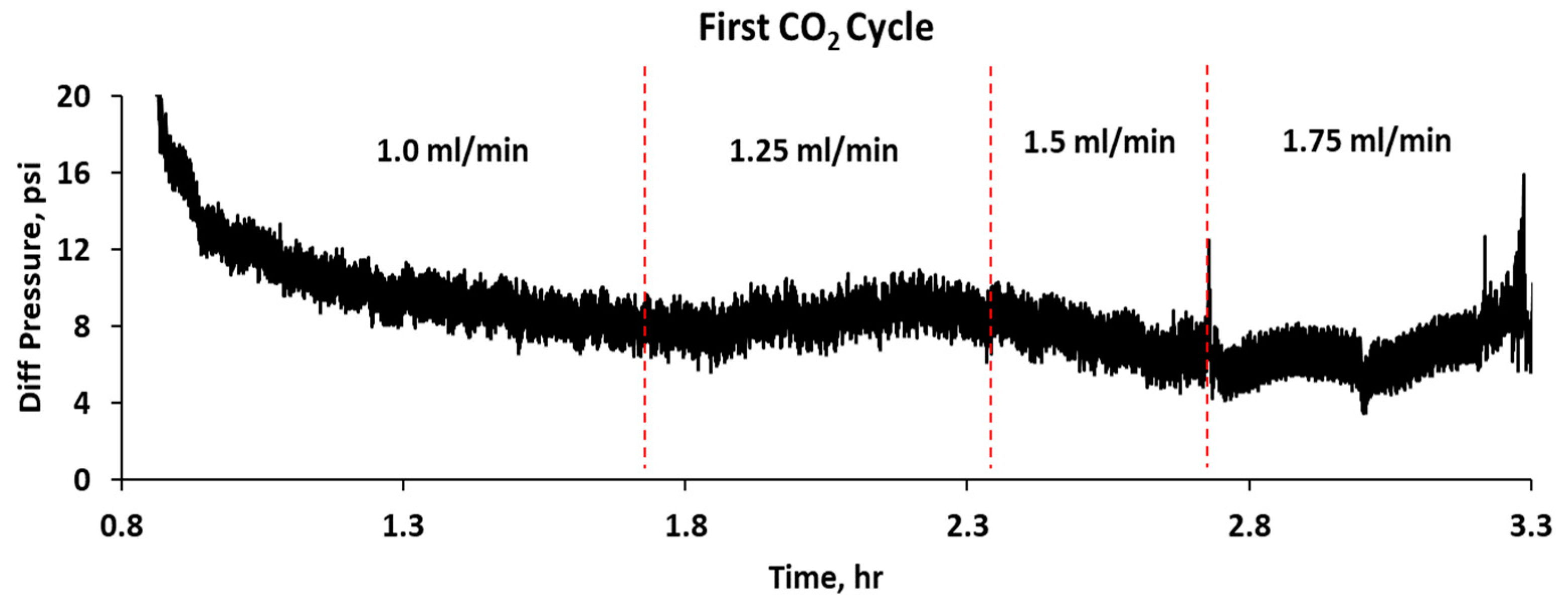


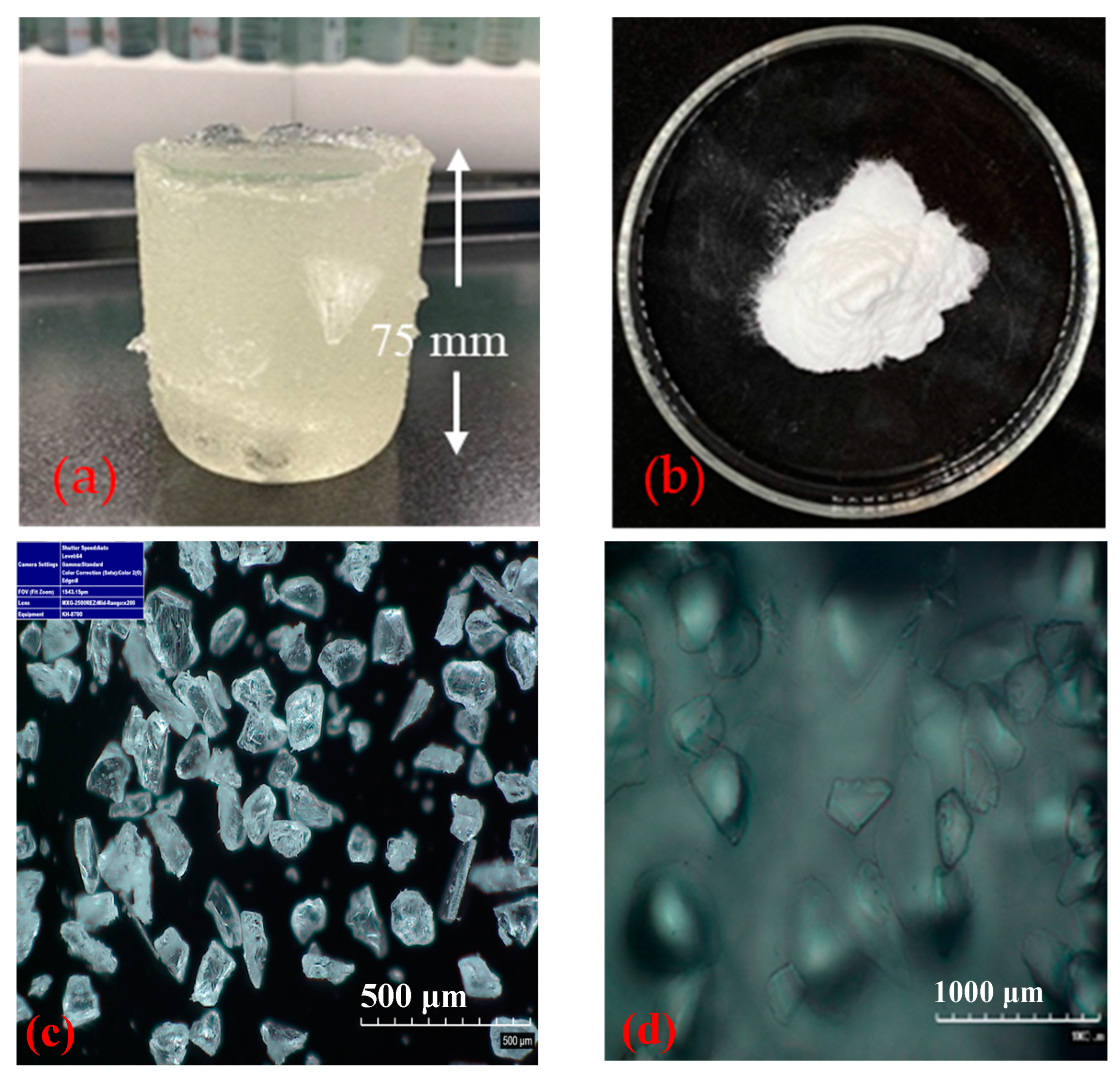

| Sample # | Brine | pH | ESR (V/V) |
|---|---|---|---|
| 1 | 1 NaCl% | ≈7 | 76 |
| 2 | 5 NaCl% | ≈7 | 52.4 |
| 3 | 10 NaCl% | ≈7 | 60.9 |
| 4 | FW | ≈7 | 59.6 |
| 5 | 1 NaCl% | ≈3.0 | 66 |
| 6 | 1 NaCl% | ≈5.0 | 68 |
| 7 | 1 NaCl% | ≈9.0 | 72 |
| 8 | 1 NaCl% | ≈11.0 | 76 |
| Exp No. | Gel Conc (ppm) | Brine | Fr |
|---|---|---|---|
| 1 | 1500 | 1 NaCl% | 1318 |
| 2 | 3000 | 1 NaCl% | 1707 |
| 3 | 5000 | 1 NaCl% | 3977 |
| 4 | 7000 | 1 NaCl% | 5116 |
| 5 | 5000 | 5 NaCl% | 5750 |
| 6 | 5000 | 10 NaCl% | 6214 |
| 7 | 5000 | Formation water | 2714 |
| Composition | Na+/K+ | Ca2+ | Mg2+ | Cl− | HCO3− | SO42− | Total |
|---|---|---|---|---|---|---|---|
| Concentration (ppm) | 20,043 | 2172 | 658 | 28,916 | 3063 | 2977 | 57,829 |
| Exp No. | Permeability (md) | Porosity (%) | PV (mL) | Gel Conc (ppm) | Brine |
|---|---|---|---|---|---|
| 1 | 45 | 34.2 | 33 | 1500 | 1 NaCl% |
| 2 | 49 | 32.8 | 35 | 3000 | 1 NaCl% |
| 3 | 47 | 34.4 | 32.5 | 5000 | 1 NaCl% |
| 4 | 49 | 34.7 | 32.5 | 7000 | 1 NaCl% |
| 5 | 45 | 33.9 | 34 | 5000 | 5 NaCl% |
| 6 | 48 | 34.4 | 35 | 5000 | 10 NaCl% |
| 7 | 47 | 34.6 | 35 | 5000 | Formation water |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alotibi, A.; Song, T.; Al Brahim, A.; Bai, B.; Schuman, T. Experimental Evaluation of a Recrosslinkable CO2-Resistant Micro-Sized Preformed Particle Gel for CO2 Sweep Efficiency Improvement in Reservoirs with Super-K Channels. Gels 2024, 10, 765. https://doi.org/10.3390/gels10120765
Alotibi A, Song T, Al Brahim A, Bai B, Schuman T. Experimental Evaluation of a Recrosslinkable CO2-Resistant Micro-Sized Preformed Particle Gel for CO2 Sweep Efficiency Improvement in Reservoirs with Super-K Channels. Gels. 2024; 10(12):765. https://doi.org/10.3390/gels10120765
Chicago/Turabian StyleAlotibi, Adel, Tao Song, Ali Al Brahim, Baojun Bai, and Thomas Schuman. 2024. "Experimental Evaluation of a Recrosslinkable CO2-Resistant Micro-Sized Preformed Particle Gel for CO2 Sweep Efficiency Improvement in Reservoirs with Super-K Channels" Gels 10, no. 12: 765. https://doi.org/10.3390/gels10120765
APA StyleAlotibi, A., Song, T., Al Brahim, A., Bai, B., & Schuman, T. (2024). Experimental Evaluation of a Recrosslinkable CO2-Resistant Micro-Sized Preformed Particle Gel for CO2 Sweep Efficiency Improvement in Reservoirs with Super-K Channels. Gels, 10(12), 765. https://doi.org/10.3390/gels10120765







