Novel, Speedy, and Eco-Friendly Carboxymethyl Cellulose-Nitrogen Doped Carbon Dots Biosensors with DFT Calculations, Molecular Docking, and Experimental Validation
Abstract
1. Introduction
2. Results and Discussion
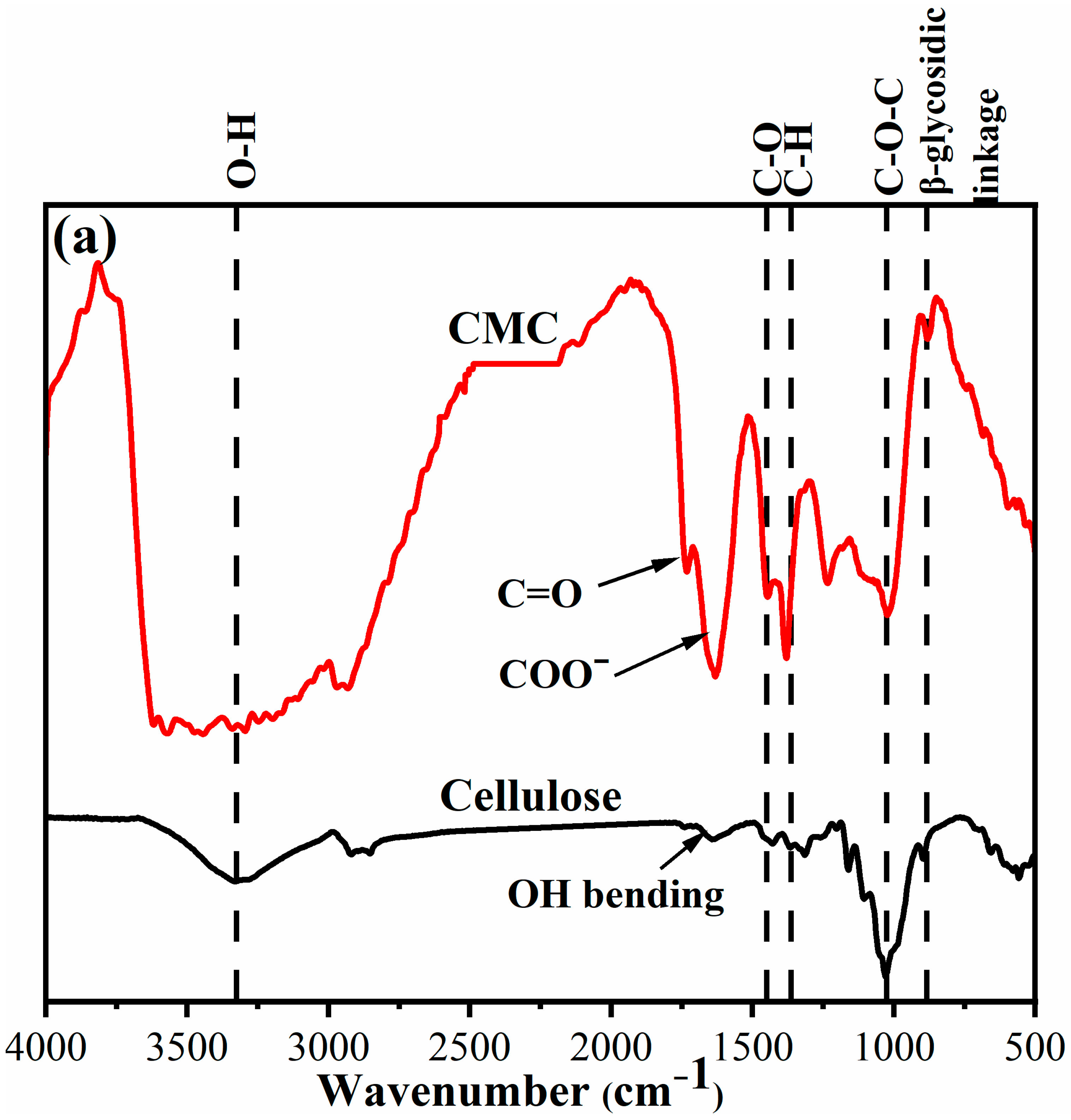
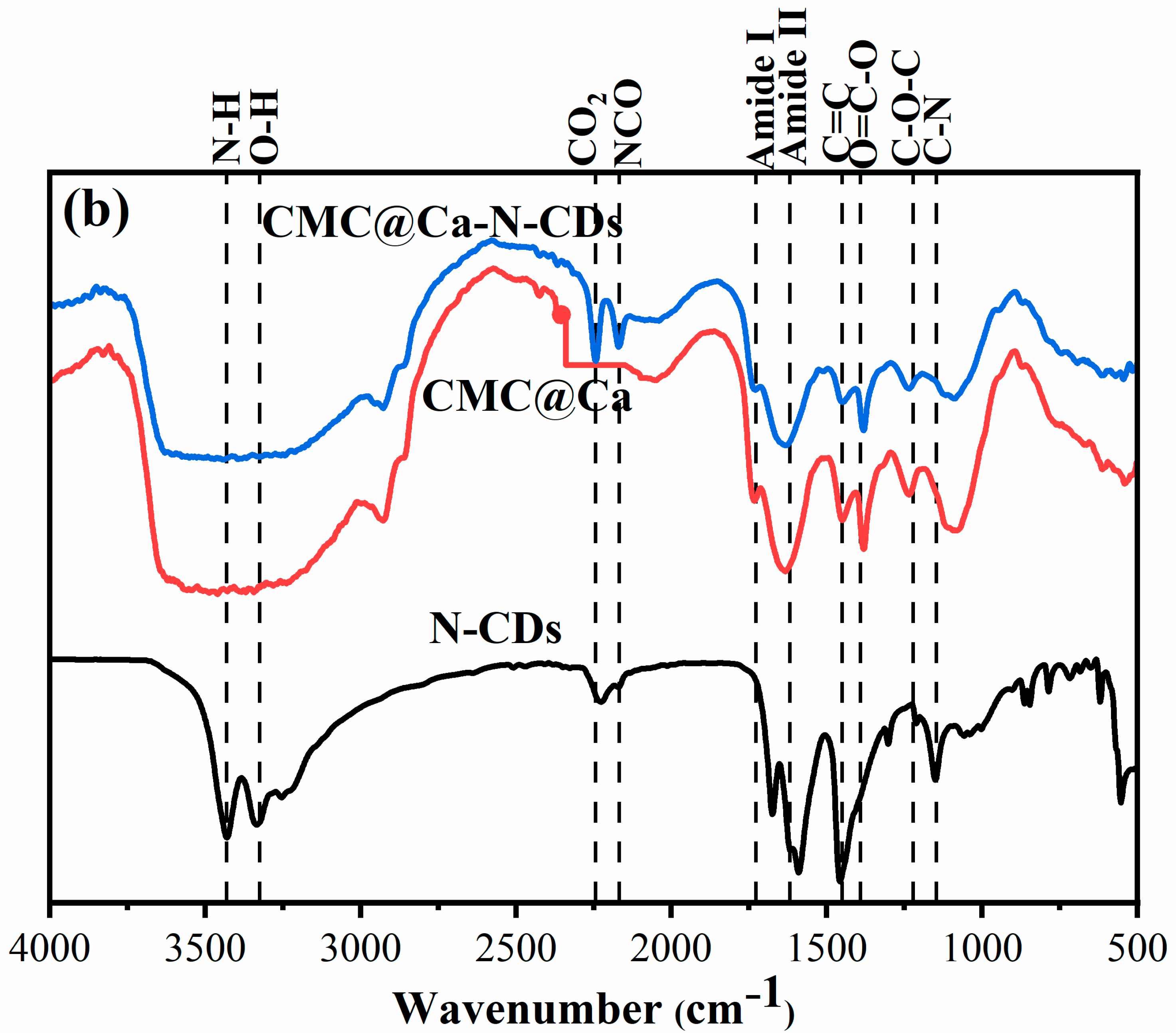
2.1. Fourier Transform Infrared Spectroscopy (FTIR) Spectra
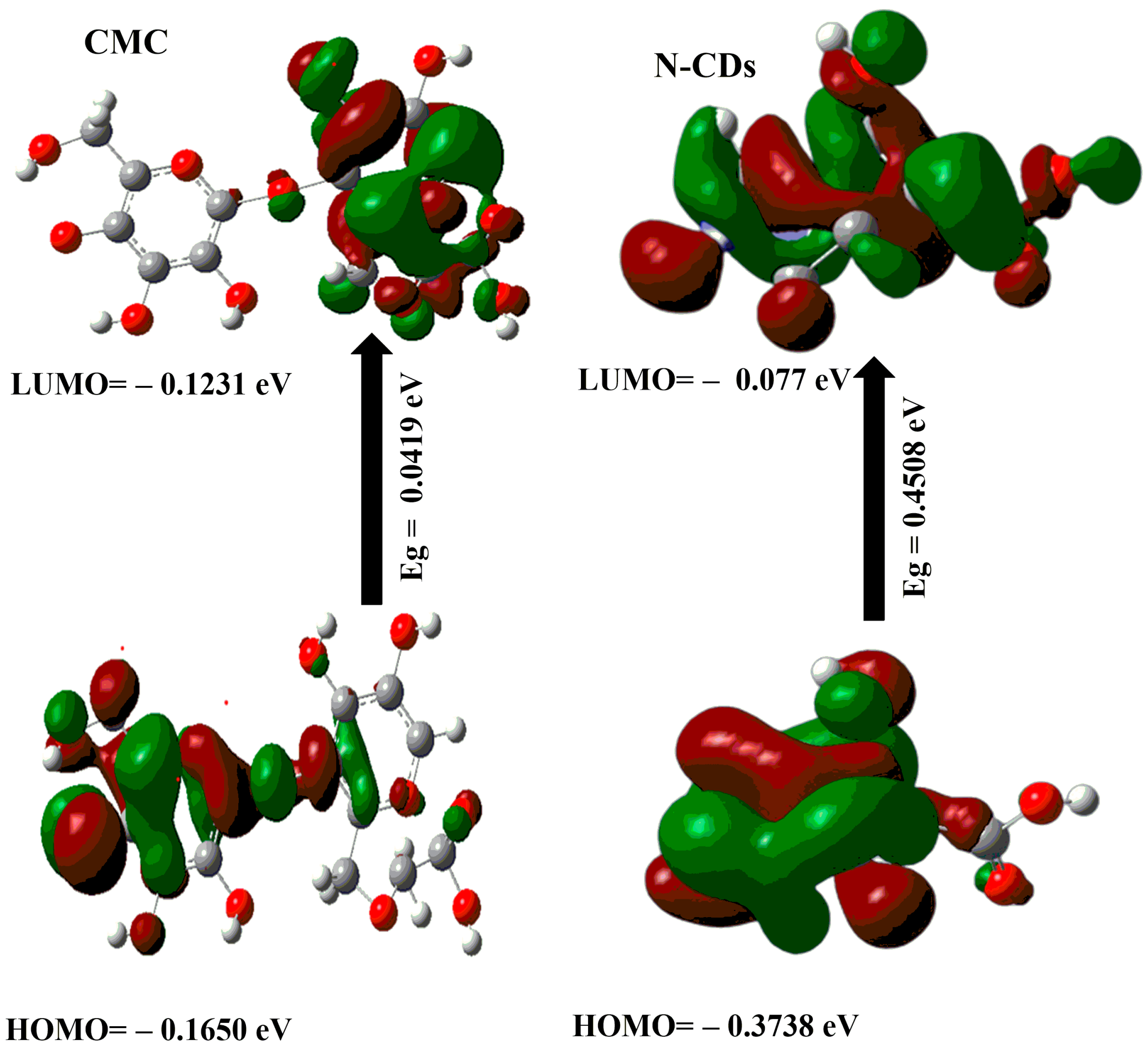
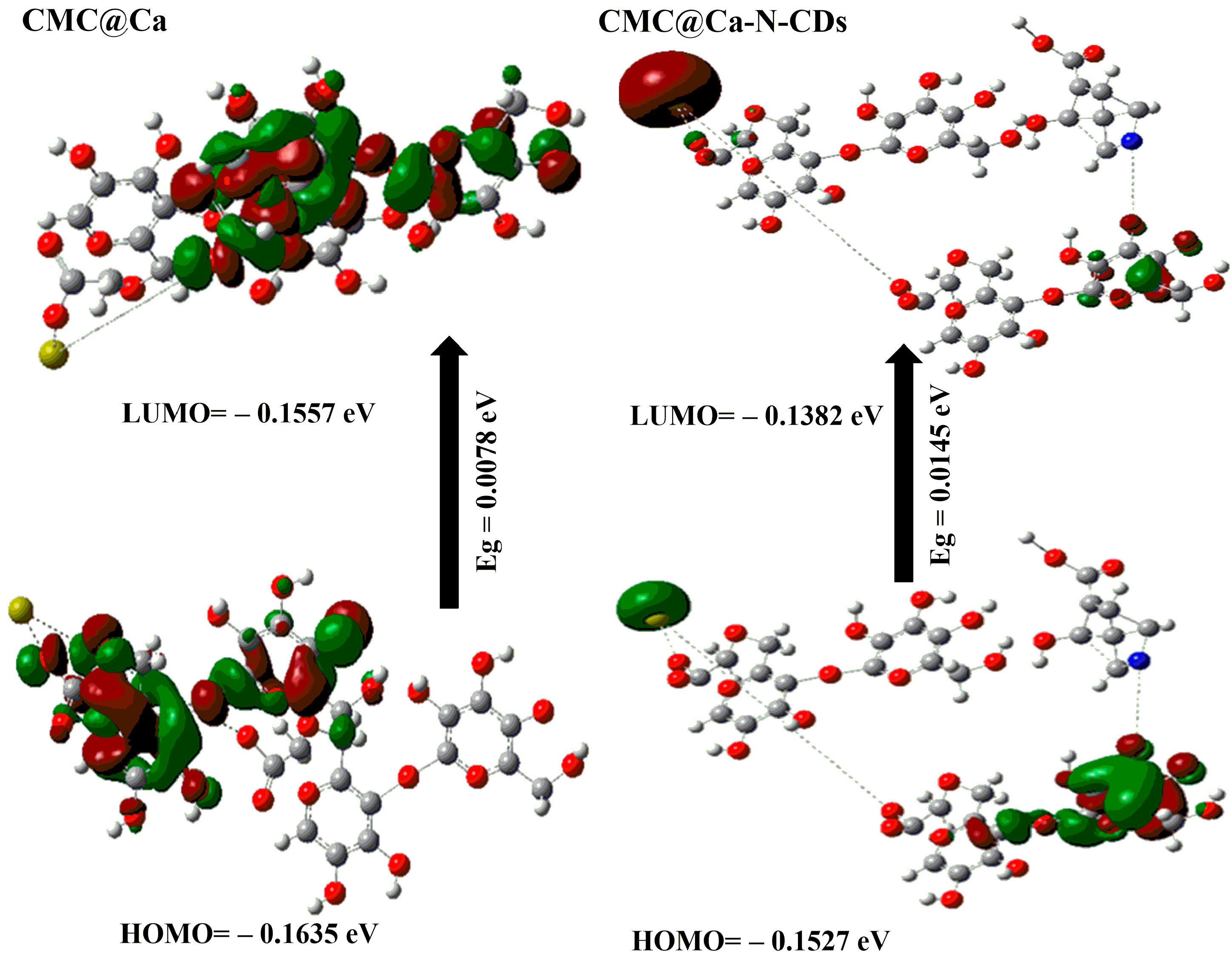
2.2. DFT Calculations
- (a)
- The μ for CMC@Ca-N–CDs (i.e., 77.763 Debye) is higher than that of CMC@Ca (i.e., 15.304 Debye). This can be attributed to the presence of more O and N atoms from N–CDs, which increase the electronegativity of CMC@Ca-N–CDs compared to CMC@Ca [51].
- (b)
- The calculated Eg for CMC@Ca and CMC@Ca-N–CDs is the lowest (i.e., 0.0078 and 0.0145 eV) compared to CMC and N–CDs (i.e., 0.0419 and 0.4508 eV). This indicates a strong chemical interaction between CMC, Ca2+, and N–CDs [1,52]. The positive charge on the nitrogen atom of N–CDs can play a role in the interactions between N–CDs and other molecules, such as the repulsive interaction with Ca2+.The lower Eg observed in CMC@Ca compared to CMC@Ca-N–CDs can be attributed to the absence of N–CDs, which in turn means the absence of repulsion with Ca2+. Without these N–CDs, the Eg in CMC@Ca may be narrower (i.e., no repulsion). However, it is important to note that the Eg of CMC@Ca-N–CDs is still lower than CMC and N–CDs. This is a good proof of the CMC@Ca-N–CD reactivity and enhanced fluorescent properties compared to N–CDs.
- (c)
- The ET of CMC@Ca-N–CDs (1.998 au) is higher than that of CMC@Ca (−3542.25 au). This can be attributed to the formation of new amide bonds between N–CDs and CMC within the CMC@Ca-N–CDs hydrogel, which introduces additional bond energies.
- (d)
- The CMC@Ca is much softer (128.205 eV) than the CMC@Ca-N–CDs (68.965 eV). This suggests that CMC@Ca likely forms a more open and flexible network due to the nature of CMC and the ionic interactions with Ca2+. The interactions between CMC chains and Ca2+ might contribute to a more elastic structure. In contrast, CMC@Ca-N–CDs contain N–CDs, which form amide bonds, leading to a stiffer hydrogel.
- (e)
- The calculated ΔNmax for CMC@Ca-N–CDs (−20.062) is higher than that of CMC@Ca (−40.923). This can be attributed to the presence of N–CDs, which introduce more electronegative atoms in the CMC@Ca-N–CDs structure.
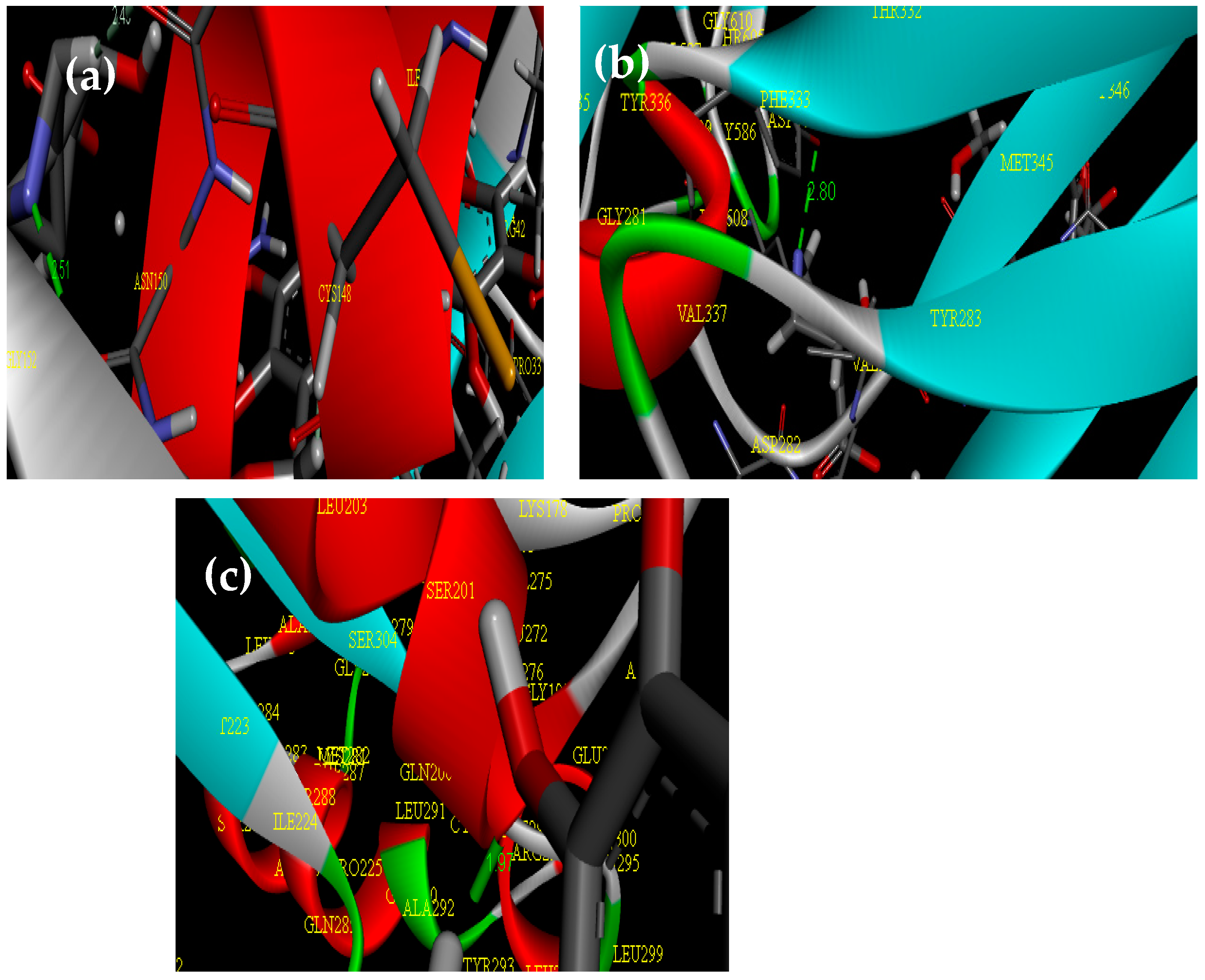
2.3. Molecular Docking Study
2.4. Antibacterial and Antifungal Activity
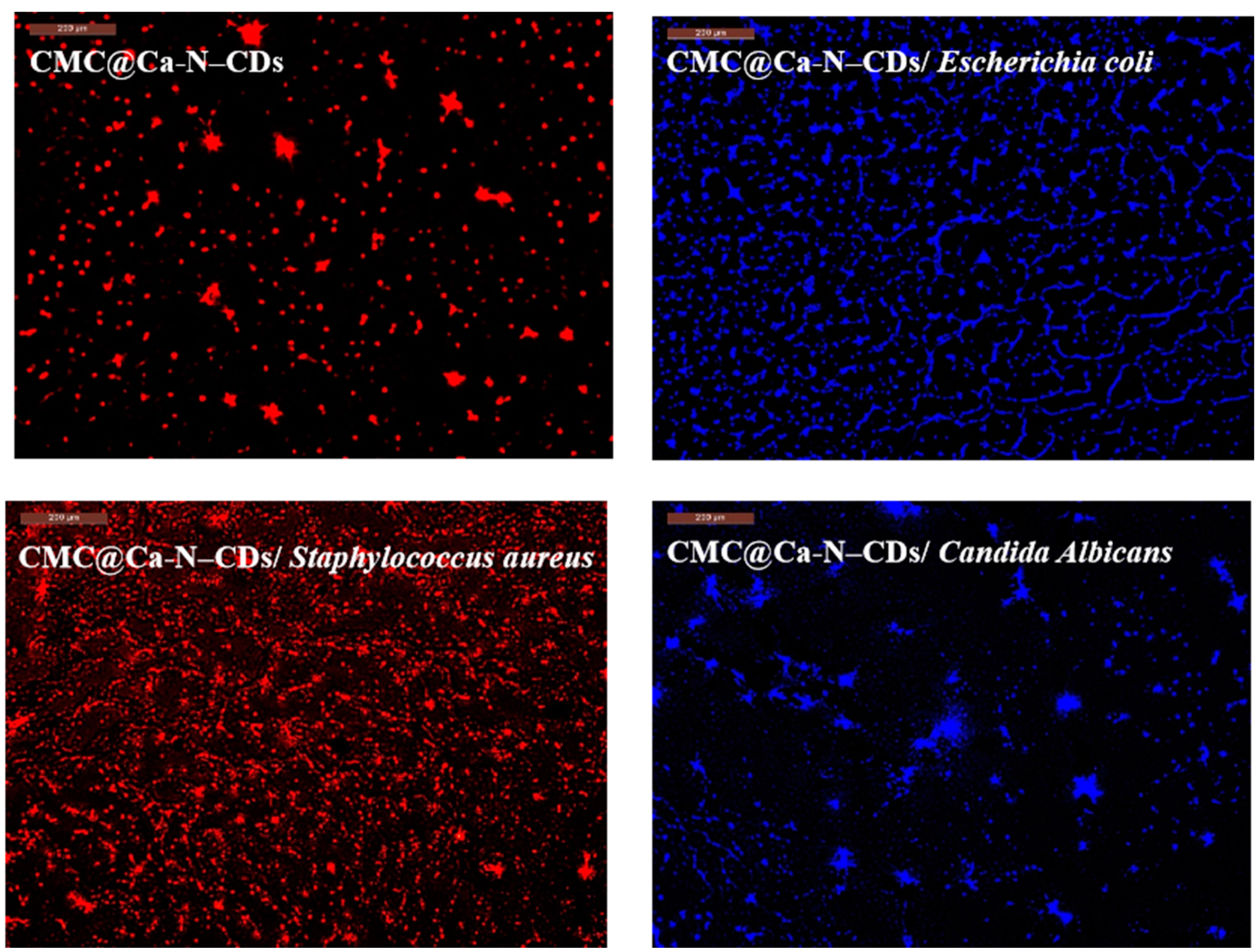
2.5. CMC@Ca-N–CD-Based Fluorescent Hydrogel as a Probe for Imaging of Bacteria and Fungi
2.6. Morphological Observations
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Cellulose
4.3. Preparation of Carboxymethyl Cellulose in Microwave
4.4. Preparation of Nitrogen Carbon Dots
4.5. Preparation of CMC@Ca-N–CDs
4.6. Carbon Dots for Bacterial and Fungal Sensing
4.7. Characterization and Analysis
4.7.1. SEM
4.7.2. Fluorescence Microscope
4.7.3. Fourier-Transform Infrared (FTIR) Spectra
4.7.4. DFT Calculations
4.7.5. Molecular Docking
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El Sayed, M.M. Production of polymer hydrogel composites and their applications. J. Polym. Environ. 2023, 31, 2855–2879. [Google Scholar] [CrossRef]
- Khalil, H.A.; Bhat, A.; Yusra, A.I. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Makhanya, F.M. Conversion of Sugarcane Bagasse into Carboxymethl Cellulose. Ph.D. Thesis, Durban University of Technology, Durban, South Africa, 2020. [Google Scholar]
- Tohamy, H.-A.S. Fluorescence ‘Turn-on’Probe for Chromium Reduction, Adsorption and Detection Based on Cellulosic Nitrogen-Doped Carbon Quantum Dots Hydrogels. Gels 2024, 10, 296. [Google Scholar] [CrossRef]
- Viscusi, G.; Mottola, S.; Tohamy, H.-A.S.; Gorrasi, G.; De Marco, I. Design of Cellulose Acetate Electrospun Membranes Loaded with N-doped Carbon Quantum Dots for Water Remediation. In Proceedings of the IWA Regional Membrane Technology Conference, Palermo, Italy, 18–21 June 2024; pp. 133–137. [Google Scholar]
- Tohamy, H.-A.S.; El-Sakhawy, M.; Hassan, E.B.; Kamel, S. Microwave-Prepared Quantum Dots and Their Potential Applications as Adsorbents and Chemosensors. Materials 2023, 16, 6722. [Google Scholar] [CrossRef]
- Abdel-Fatah, A.S.; Tohamy, H.-A.S.; Ahmed, S.I.; Youssef, M.A.; Mabrouk, M.R.; Kamel, S.; Samhan, F.A.; El-Gendi, A. Anatase-cellulose acetate for reinforced desalination membrane with antibacterial properties. BMC Chem. 2023, 17, 112. [Google Scholar] [CrossRef]
- El-Sakhawy, M.; Tohamy, H.-A.S.; AbdelMohsen, M.M.; El-Missiry, M. Biodegradable carboxymethyl cellulose based material for sustainable/active food packaging application. J. Thermoplast. Compos. Mater. 2024, 37, 2035–2050. [Google Scholar] [CrossRef]
- Mushtaq, F.; Raza, Z.A.; Batool, S.R.; Zahid, M.; Onder, O.C.; Rafique, A.; Nazeer, M.A. Preparation, properties, and applications of gelatin-based hydrogels (GHs) in the environmental, technological, and biomedical sectors. Int. J. Biol. Macromol. 2022, 218, 601–633. [Google Scholar] [CrossRef]
- Tohamy, H.-A.S.; El-Masry, H.M. Fluffy-like amphiphilic graphene oxide and its effects on improving the antibacterial activity and thermal outstanding of ethyl cellulose/polyvinyl alcohol hydrogel film. BMC Chem. 2024, 18, 116. [Google Scholar] [CrossRef]
- Tohamy, H.A.S. Oil dispersing and adsorption by carboxymethyl cellulose–oxalate nanofibrils/nanocrystals and their kinetics. J. Surfactants Deterg. 2024, 27, 147–160. [Google Scholar] [CrossRef]
- Tohamy, H.-A.S.; El-Sakhawy, M.; Elnasharty, M.M. Carboxymethyl cellulose membranes blended with carbon nanotubes/ag nanoparticles for eco-friendly safer lithium-ion batteries. Diam. Relat. Mater. 2023, 138, 110205. [Google Scholar] [CrossRef]
- Mehta, V.N.; Jha, S.; Basu, H.; Singhal, R.K.; Kailasa, S.K. One-step hydrothermal approach to fabricate carbon dots from apple juice for imaging of mycobacterium and fungal cells. Sens. Actuators B Chem. 2015, 213, 434–443. [Google Scholar] [CrossRef]
- Tyagi, V.; Thakur, A. Carboxymethyl cellulose-polyvinyl alcohol based materials: A review. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Tohamy, H.-A.S. Cellulosic nitrogen doped carbon quantum dots hydrogels with fluorescence/visco-elastic properties for pH-and temperature-sensitivity. Diam. Relat. Mater. 2023, 136, 110027. [Google Scholar] [CrossRef]
- Ehtesabi, H.; Nasri, R. Carbon dot-based materials for wound healing applications. Adv. Nat. Sci. Nanosci. Nanotechnol. 2021, 12, 025006. [Google Scholar] [CrossRef]
- Sadat, Z.; Farrokhi-Hajiabad, F.; Lalebeigi, F.; Naderi, N.; Gorab, M.G.; Cohan, R.A.; Eivazzadeh-Keihan, R.; Maleki, A. A comprehensive review on the applications of carbon-based nanostructures in wound healing: From antibacterial aspects to cell growth stimulation. Biomater. Sci. 2022, 10, 6911–6938. [Google Scholar] [CrossRef]
- Xie, F. Alginate-based nanocomposites for food preservation: Recent progress showcasing heightened material properties and functionalities. Adv. Nanocomposites 2024, 1, 248–274. [Google Scholar] [CrossRef]
- Tohamy, H.-A.S.; El-Sakhawy, M.; Kamel, S. Fullerenes and tree-shaped/fingerprinted carbon quantum dots for chromium adsorption via microwave-assisted synthesis. RSC Adv. 2024, 14, 25785–25792. [Google Scholar] [CrossRef]
- Abd El-Aziz, M.; Tohamy, H.-A.S.; Youssef, A.; El Desouky, F.G. Preparation and optimization of grafted hydroxyethyl cellulose, polypyrrole, and nitrogen-doped carbon quantum dots bionanocomposites for electrical, optical, and photoluminescence multicoloring applications. Int. J. Biol. Macromol. 2024, 278, 134965. [Google Scholar] [CrossRef]
- Zhao, X.; Tang, Q.; Zhu, S.; Bu, W.; Yang, M.; Liu, X.; Meng, Y.; Yu, W.; Sun, H.; Yang, B. Controllable acidophilic dual-emission fluorescent carbonized polymer dots for selective imaging of bacteria. Nanoscale 2019, 11, 9526–9532. [Google Scholar] [CrossRef]
- Fang, M.; Lin, L.; Zheng, M.; Liu, W.; Lin, R. Antibacterial functionalized carbon dots and their application in bacterial infections and inflammation. J. Mater. Chem. B 2023, 11, 9386–9403. [Google Scholar] [CrossRef]
- Lin, F.; Wang, Z.; Wu, F.-G. Carbon dots for killing microorganisms: An update since 2019. Pharmaceuticals 2022, 15, 1236. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Ye, Y.; Ping, J.; Sun, X. Carbon dots: Current advances in pathogenic bacteria monitoring and prospect applications. Biosens. Bioelectron. 2020, 156, 112085. [Google Scholar] [CrossRef] [PubMed]
- Lovrić, J.; Cho, S.J.; Winnik, F.M.; Maysinger, D. Unmodified cadmium telluride quantum dots induce reactive oxygen species formation leading to multiple organelle damage and cell death. Chem. Biol. 2005, 12, 1227–1234. [Google Scholar] [CrossRef]
- Cui, F.; Xi, L.; Wang, D.; Ren, L.; Tan, X.; Li, X.; Li, J.; Li, T. Advanced in carbon dot-based hydrogels for antibacterial, detection and adsorption. Coord. Chem. Rev. 2023, 497, 215457. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Wu, S.-B.; Wei, Y.-H.; Chen, Y.-C.; Tsai, M.-H.; Ho, C.-C.; Lin, S.-Y.; Yang, C.-S.; Lin, P. Cadmium-based quantum dot induced autophagy formation for cell survival via oxidative stress. Chem. Res. Toxicol. 2013, 26, 662–673. [Google Scholar] [CrossRef] [PubMed]
- MP, A.; Pardhiya, S.; Rajamani, P. Carbon dots: An excellent fluorescent probe for contaminant sensing and remediation. Small 2022, 18, 2105579. [Google Scholar]
- Tian, B.; Fu, T.; Wan, Y.; Ma, Y.; Wang, Y.; Feng, Z.; Jiang, Z. B-and N-doped carbon dots by one-step microwave hydrothermal synthesis: Tracking yeast status and imaging mechanism. J. Nanobiotechnol. 2021, 19, 456. [Google Scholar] [CrossRef]
- Yan, C.; Wang, C.; Hou, T.; Guan, P.; Qiao, Y.; Guo, L.; Teng, Y.; Hu, X.; Wu, H. Lasting tracking and rapid discrimination of live gram-positive bacteria by peptidoglycan-targeting carbon quantum dots. ACS Appl. Mater. Interfaces 2021, 13, 1277–1287. [Google Scholar] [CrossRef]
- Paul, S.; Banerjee, S.L.; Khamrai, M.; Samanta, S.; Singh, S.; Kundu, P.P.; Ghosh, A.K. Hydrothermal synthesis of gelatin quantum dots for high-performance biological imaging applications. J. Photochem. Photobiol. B Biol. 2020, 212, 112014. [Google Scholar] [CrossRef]
- Adesiji, Y.O.; Alli, O.T.; Adekanle, M.A.; Jolayemi, J.B. Prevalence of Arcobacter, Escherichia coli, Staphylococcus aureus and Salmonella species in retail raw chicken, pork, beef and goat meat in Osogbo, Nigeria. Sierra Leone J. Biomed. Res. 2011, 3, 8–12. [Google Scholar] [CrossRef]
- Nobile, C.J.; Johnson, A.D. Candida albicans biofilms and human disease. Annu. Rev. microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Borkar, S.; Shinde, K. Yeast species of diverse functionality in health sciences: A concise report. GSC Biol. Pharm. Sci. 2023, 25, 149–168. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, T.; Banerjee, D. Fungal Diseases and Their Treatment: A Holistic Approach. In Pathogenicity and Drug Resistance of Human Pathogens: Mechanism and Novel Approaches; Springer: Singapore, 2019; pp. 111–134. [Google Scholar] [CrossRef]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Gow, N.A.; Hube, B. Importance of the Candida albicans cell wall during commensalism and infection. Curr. Opin. Microbiol. 2012, 15, 406–412. [Google Scholar] [CrossRef]
- Qadri, H.; Shah, A.H.; Ahmad, S.M.; Alshehri, B.; Almilaibary, A.; Mir, M.A. Natural products and their semi-synthetic derivatives against antimicrobial-resistant human pathogenic bacteria and fungi. Saudi J. Biol. Sci. 2022, 29, 103376. [Google Scholar] [CrossRef]
- Song, C.; Wen, R.; Zhou, J.; Zeng, X.; Kou, Z.; Zhang, J.; Wang, T.; Chang, P.; Lv, Y.; Wu, R. Antibacterial and antifungal properties of a novel antimicrobial peptide GK-19 and its application in skin and soft tissue infections induced by MRSA or Candida albicans. Pharmaceutics 2022, 14, 1937. [Google Scholar] [CrossRef]
- Wang, L.; Dou, S.; Xu, J.; Liu, H.K.; Wang, S.; Ma, J.; Dou, S.X. Highly nitrogen doped carbon nanosheets as an efficient electrocatalyst for the oxygen reduction reaction. Chem. Commun. 2015, 51, 11791–11794. [Google Scholar] [CrossRef]
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.; Cai, C.; Lin, H. Red, green, and blue luminescence by carbon dots: Full-color emission tuning and multicolor cellular imaging. Angew. Chem. 2015, 127, 5450–5453. [Google Scholar] [CrossRef]
- Ding, H.; Zhou, X.-X.; Zhang, Z.-H.; Zhao, Y.-P.; Wei, J.-S.; Xiong, H.-M. Large scale synthesis of full-color emissive carbon dots from a single carbon source by a solvent-free method. Nano Res. 2022, 15, 3548–3555. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, H.; Pan, A.; Wang, Q.; Zhang, Y.; Zhou, G.; He, L. White-light-emitting melamine-formaldehyde microspheres through polymer-mediated aggregation and encapsulation of graphene quantum dots. Adv. Sci. 2019, 6, 1801432. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, Q.; Zhou, J. Recent advance in near-infrared (NIR) imaging probes for cancer theranostics. Adv. Ther. 2018, 1, 1800055. [Google Scholar] [CrossRef]
- Qu, D.; Wang, X.; Bao, Y.; Sun, Z. Recent advance of carbon dots in bio-related applications. J. Phys. Mater. 2020, 3, 022003. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Aseer, K.R.; Perumal, S.; Karthik, N.; Lee, Y.R. Highly fluorescent nitrogen-doped carbon dots derived from Phyllanthus acidus utilized as a fluorescent probe for label-free selective detection of Fe3+ ions, live cell imaging and fluorescent ink. Biosens. Bioelectron. 2018, 99, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Mowafi, S.; Tohamy, H.-A.S. Application of electro-spun nano-fibers based on agriculture cellulosic biomaterial wastes for removal of dye and heavy metal from polluted water. J. Text. Inst. 2024, 115, 1490–1499. [Google Scholar] [CrossRef]
- Riyajan, S.-A.; Nuim, J. Interaction of green polymer blend of modified sodium alginate and carboxylmethyl cellulose encapsulation of turmeric extract. Int. J. Polym. Sci. 2013, 2013, 364253. [Google Scholar] [CrossRef]
- Hemmati, A.; Emadi, H.; Nabavi, S.R. Green synthesis of sulfur-and nitrogen-doped carbon quantum dots for determination of L-DOPA using fluorescence spectroscopy and a smartphone-based fluorimeter. ACS Omega 2023, 8, 20987–20999. [Google Scholar] [CrossRef]
- Tuan Mohamood, N.F.A.-Z.; Abdul Halim, A.H.; Zainuddin, N. Carboxymethyl cellulose hydrogel from biomass waste of oil palm empty fruit bunch using calcium chloride as crosslinking agent. Polymers 2021, 13, 4056. [Google Scholar] [CrossRef]
- Akman, F. Prediction of chemical reactivity of cellulose and chitosan based on density functional theory. Cellul. Chem. Technol. 2017, 51, 253–262. [Google Scholar]
- Elsayed, G.H.; Dacrory, S.; Fahim, A.M. Anti-proliferative action, molecular investigation and computational studies of novel fused heterocyclic cellulosic compounds on human cancer cells. Int. J. Biol. Macromol. 2022, 222, 3077–3099. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, L.; Cao, F.; Leng, Y. Thermal treatment of hair for the synthesis of sustainable carbon quantum dots and the applications for sensing Hg2+. Sci. Rep. 2016, 6, 35795. [Google Scholar] [CrossRef]
- Zeng, D.; Chen, Z.; Jiang, Y.; Xue, F.; Li, B. Advances and challenges in viability detection of foodborne pathogens. Front. Microbiol. 2016, 7, 1833. [Google Scholar] [CrossRef] [PubMed]
- Hameed, S.; Xie, L.; Ying, Y. Conventional and emerging detection techniques for pathogenic bacteria in food science: A review. Trends Food Sci. Technol. 2018, 81, 61–73. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, L.; Fang, J.; Feng, X. Bio-nanoplatforms based on carbon dots conjugating with F-substituted nano-hydroxyapatite for cellular imaging. Nanoscale 2015, 7, 20033–20041. [Google Scholar] [CrossRef]
- Li, N.; Liang, X.; Wang, L.; Li, Z.; Li, P.; Zhu, Y.; Song, J. Biodistribution study of carbogenic dots in cells and in vivo for optical imaging. J. Nanopart. Res. 2012, 14, 1177. [Google Scholar] [CrossRef]
- Wang, Y.; Alenazy, R.; Gu, X.; Polyak, S.W.; Zhang, P.; Sykes, M.J.; Zhang, N.; Venter, H.; Ma, S. Design and structural optimization of novel 2H-benzo [h] chromene derivatives that target AcrB and reverse bacterial multidrug resistance. Eur. J. Med. Chem. 2021, 213, 113049. [Google Scholar] [CrossRef]
- Scheffers, D.J.; Pinho, M.G. Bacterial cell wall synthesis: New insights from localization studies. Microbiol. Mol. Biol. 2005, 69, 585–607. [Google Scholar] [CrossRef]
- Das, P.; Bose, M.; Ganguly, S.; Mondal, S.; Das, A.K.; Banerjee, S.; Das, N.C. Green approach to photoluminescent carbon dots for imaging of gram-negative bacteria Escherichia coli. Nanotechnology 2017, 28, 195501. [Google Scholar] [CrossRef]
- Huang, K.C.; Mukhopadhyay, R.; Wen, B.; Gitai, Z.; Wingreen, N.S. Cell shape and cell-wall organization in Gram-negative bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 19282–19287. [Google Scholar] [CrossRef]
- Mangoni, M.L.; Papo, N.; Barra, D.; Simmaco, M.; Bozzi, A.; Di Giulio, A.; Rinaldi, A.C. Effects of the antimicrobial peptide temporin L on cell morphology, membrane permeability and viability of Escherichia coli. Biochem. J. 2004, 380, 859–865. [Google Scholar] [CrossRef]
- Serra, D.O.; Richter, A.M.; Hengge, R. Cellulose as an architectural element in spatially structured Escherichia coli biofilms. J. Bacteriol. 2013, 195, 5540–5554. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Atchudan, R.; Karthik, N.; Xiong, D.; Lee, Y.R. Facile hydrothermal synthesis of nitrogen rich blue fluorescent carbon dots for cell bio-imaging of Candida albicans. Process Biochem. 2020, 88, 113–119. [Google Scholar] [CrossRef]
- Gouda, R.; Baishya, H.; Qing, Z. Application of mathematical models in drug release kinetics of carbidopa and levodopa ER tablets. J. Dev. Drugs 2017, 6, 171. [Google Scholar]
- Kasi Matta, S.; Zhang, C.; O’Mullane, A.P.; Du, A. Density functional theory investigation of carbon dots as hole-transport material in perovskite solar cells. ChemPhysChem 2018, 19, 3018–3023. [Google Scholar] [CrossRef]
- Hassan, H.B. Density function theory B3LYP/6-31G** calculation of geometry optimization and energies of donor-bridge-acceptor molecular system. Int. J. Curr. Eng. Technol. 2014, 4, 2342–2345. [Google Scholar]
- Chiacchio, M.A.; Legnani, L. Density functional theory calculations: A useful tool to investigate mechanisms of 1, 3-dipolar cycloaddition reactions. Int. J. Mol. Sci. 2024, 25, 1298. [Google Scholar] [CrossRef]
- Tirado-Rives, J.; Jorgensen, W.L. Performance of B3LYP density functional methods for a large set of organic molecules. J. Chem. Theory Comput. 2008, 4, 297–306. [Google Scholar] [CrossRef]
- Ibrahim, E.S.; Mustafa, H.; Abdelhalim, S. Electronic structure, global reactivity descriptors and nonlinear optical properties of some novel pyrazolyl quinolinone derivatives. DFT approach. Egypt. J. Chem. 2023, 66, 375–385. [Google Scholar] [CrossRef]
- Bulat, F.A.; Murray, J.S.; Politzer, P. Identifying the most energetic electrons in a molecule: The highest occupied molecular orbital and the average local ionization energy. Comput. Theor. Chem. 2021, 1199, 113192. [Google Scholar] [CrossRef]
- Saha, S.K.; Banerjee, P. A theoretical approach to understand the inhibition mechanism of steel corrosion with two aminobenzonitrile inhibitors. RSC Adv. 2015, 5, 71120–71130. [Google Scholar] [CrossRef]
- Hekim, S.; Azeez, Y.H.; Akpinar, S. The theoretical investigation of the HOMO, LUMO energies and chemical reactivity of C9H12 and C7F3NH5Cl molecules. J. Phys. Chem. Funct. Mater. 2019, 2, 29–31. [Google Scholar]
- Salah, A.G.; Abdelhalim, S.; Hassan, H. Synthesis, Electronic Structure, NLO Analysis and Antitumor Activity of Some Novel Quinolinones Derivatives: DFT Approach. Egypt. J. Chem. 2024, 67, 267–283. [Google Scholar] [CrossRef]



| DFT B3LYP/6–31G (d) | CMC | N–CDs | CMC@Ca | CMC@Ca-N–CDs |
|---|---|---|---|---|
| ELUMO (eV) | −0.1231 | 0.077 | −0.1557 | −0.1382 |
| EHOMO (eV) | −0.1650 | −0.3738 | −0.1635 | −0.1527 |
| Eg (eV) | 0.0419 | 0.4508 | 0.0078 | 0.0145 |
| ET (au) | −1134.555 | −244.29 | −3542.25 | 1.998 |
| χ (eV) | 0.144 | 0.148 | 0.1596 | 0.1454 |
| μ (Debye) | 11.14 | 2.98 | 15.304 | 77.763 |
| η (eV) | 0.0209 | 0.2254 | 0.0039 | 0.00725 |
| σ (eV) | 47.732 | 4.436 | 256.41 | 137.931 |
| S (eV) | 23.866 | 2.218 | 128.205 | 68.965 |
| ΔNmax | −6.675 | −0.6583 | −40.923 | −20.062 |
| Microbe | CMC@Ca | Average | CMC@Ca-N–CDs | Average |
|---|---|---|---|---|
| Escherichia coli | 10, 12 | 11 | 15, 15 | 15 |
| Staphylococcus aureus | – | – | 12, 14 | 13 |
| Candida albicans | 14, 16 | 15 | 16, 18 | 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tohamy, H.-A.S. Novel, Speedy, and Eco-Friendly Carboxymethyl Cellulose-Nitrogen Doped Carbon Dots Biosensors with DFT Calculations, Molecular Docking, and Experimental Validation. Gels 2024, 10, 686. https://doi.org/10.3390/gels10110686
Tohamy H-AS. Novel, Speedy, and Eco-Friendly Carboxymethyl Cellulose-Nitrogen Doped Carbon Dots Biosensors with DFT Calculations, Molecular Docking, and Experimental Validation. Gels. 2024; 10(11):686. https://doi.org/10.3390/gels10110686
Chicago/Turabian StyleTohamy, Hebat-Allah S. 2024. "Novel, Speedy, and Eco-Friendly Carboxymethyl Cellulose-Nitrogen Doped Carbon Dots Biosensors with DFT Calculations, Molecular Docking, and Experimental Validation" Gels 10, no. 11: 686. https://doi.org/10.3390/gels10110686
APA StyleTohamy, H.-A. S. (2024). Novel, Speedy, and Eco-Friendly Carboxymethyl Cellulose-Nitrogen Doped Carbon Dots Biosensors with DFT Calculations, Molecular Docking, and Experimental Validation. Gels, 10(11), 686. https://doi.org/10.3390/gels10110686







